The Anticonvulsant Effects of Different Cannabis Extracts in a Zebrafish Model of Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Pure CBD
2.3. Cannabis Extractions
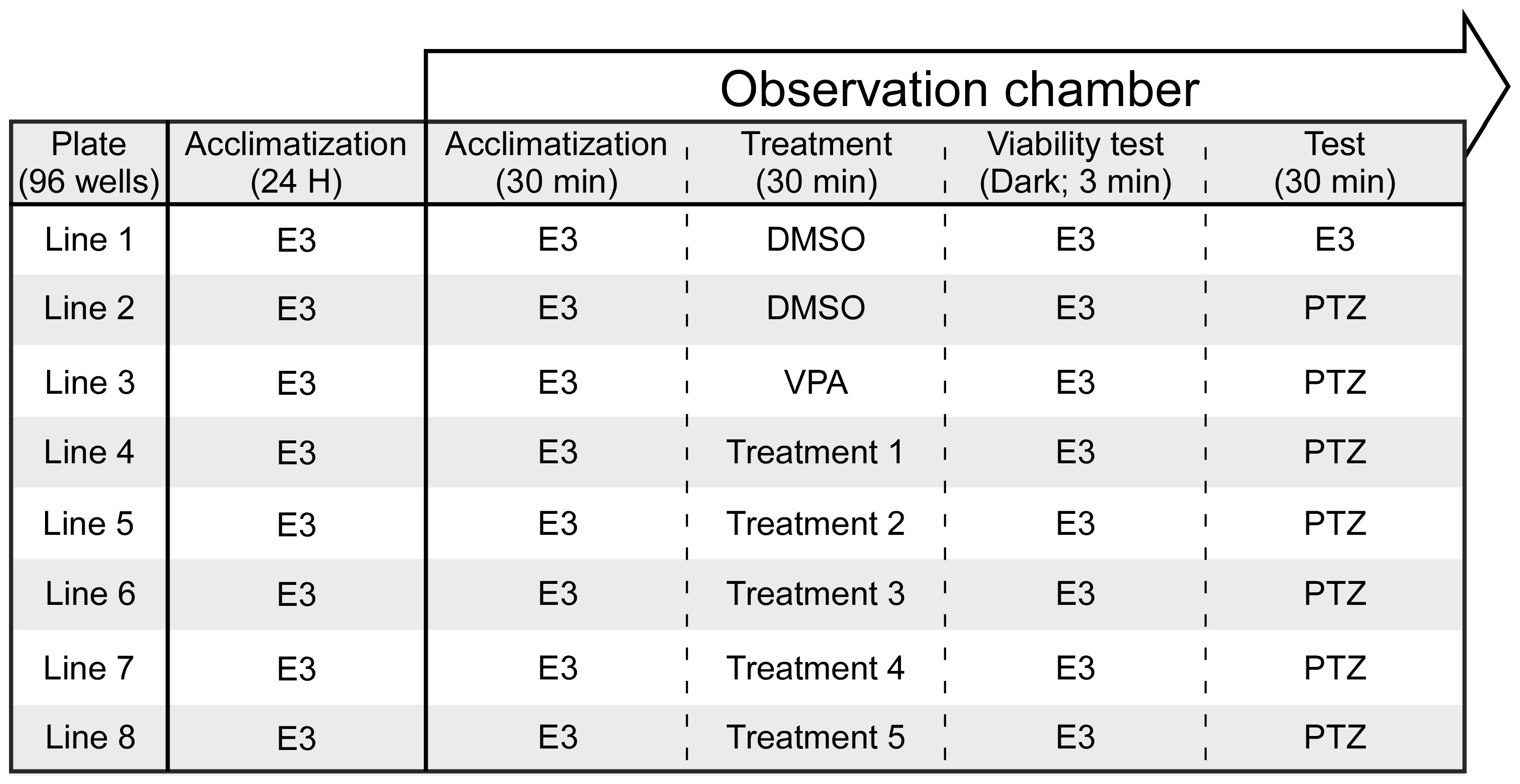
2.4. Seizure Assay
2.5. Video Tracking and Analysis
2.6. Characterizing Extracts’ Chemical Compositions
2.7. Statistical Analysis
3. Results
3.1. Behavioral Effects
3.1.1. Lack of Sedative Effects
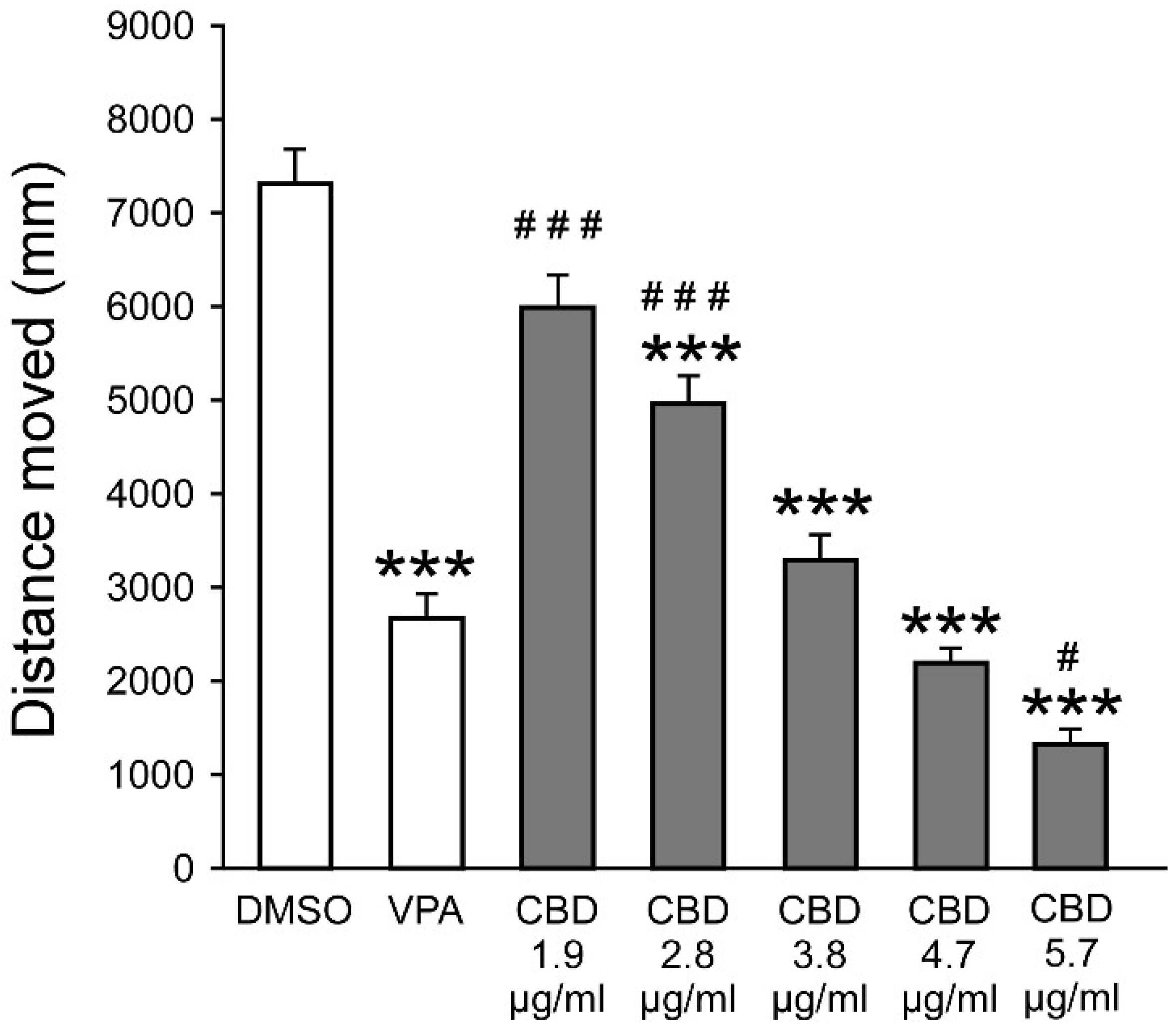
3.1.2. CBD Reduces PTZ-Induced Hyperactivity
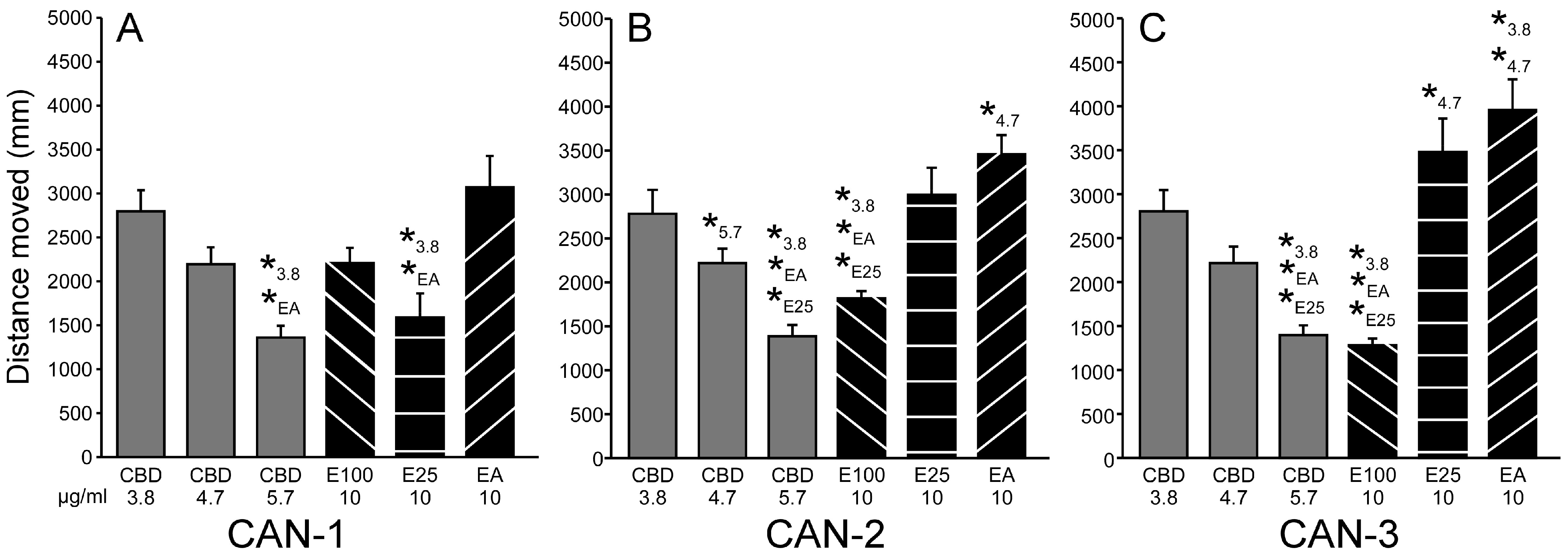
3.1.3. Extracts Reduce PTZ-Induced Hyperactivity
3.1.4. Comparing Effective CBD and Extract Concentrations
3.2. Extract Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fine, A.; Wirrell, E.C. Seizures in Children. Pediatr. Rev. 2020, 41, 321–347. [Google Scholar] [CrossRef] [PubMed]
- WHO. Epilepsy: A Public Health Imperative. Available online: https://www.who.int/publications/i/item/epilepsy-a-public-health-imperative (accessed on 29 November 2024).
- Singh, G.; Sander, J.W. The Global Burden of Epilepsy Report: Implications for Low- and Middle-Income Countries. Epilepsy Behav. 2020, 105, 106949. [Google Scholar] [CrossRef]
- Engel, J. Seizures and Epilepsy; Oxford University Press: New York, NY, USA, 2013; ISBN 978-0-19-532854-7. [Google Scholar]
- Sirven, J.I. Epilepsy: A Spectrum Disorder. Cold Spring Har. Perspec. Med. 2015, 5, a022848. [Google Scholar] [CrossRef]
- Guerrini, R.; Marini, C.; Mantegazza, M. Genetic Epilepsy Syndromes Without Structural Brain Abnormalities: Clinical Features and Experimental Models. Neurotherapeutics 2014, 11, 269–285. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; de Curtis, M.; Perucca, P. Epilepsy (Primer). Nat. Rev. Dis. Primers 2018, 4, 18024. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.-M.; Britsch, S. Molecular Genetics of Acquired Temporal Lobe Epilepsy. Biomolecules 2024, 14, 669. [Google Scholar] [CrossRef]
- Gierbolini, J.; Giarratano, M.; Benbadis, S.R. Carbamazepine-Related Antiepileptic Drugs for the Treatment of Epilepsy—A Comparative Review. Expert Opin. Pharmacother. 2016, 17, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Werner, F.-M.; Coveñas, R. Naturally Occurring and Exogenous Benzodiazepines in Epilepsy: An Update. In Naturally Occurring Benzodiazepines, Endozepines, and their Receptors; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-367-81437-3. [Google Scholar]
- Rogawski, M.A. Reduced Efficacy and Risk of Seizure Aggravation When Cannabidiol Is Used without Clobazam. Epilepsy Behav. 2020, 103, 106506. [Google Scholar] [CrossRef]
- Abdelsayed, M.; Sokolov, S. Voltage-Gated Sodium Channels: Pharmaceutical Targets via Anticonvulsants to Treat Epileptic Syndromes. Channels 2013, 7, 146–152. [Google Scholar] [CrossRef]
- Löscher, W. Basic Pharmacology of Valproate. CNS Drugs 2002, 16, 669–694. [Google Scholar] [CrossRef]
- Iftinca, M. Neuronal T–Type Calcium Channels: What’s New? Iftinca: T–Type Channel Regulation. J. Med. Life 2011, 4, 126–138. [Google Scholar] [PubMed]
- Espinosa-Jovel, C.; Valencia, N. The Current Role of Valproic Acid in the Treatment of Epilepsy: A Glimpse into the Present of an Old Ally. Curr. Treat. Options Neurol. 2024, 26, 393–410. [Google Scholar] [CrossRef]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients with Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef]
- Fattorusso, A.; Matricardi, S.; Mencaroni, E.; Dell’Isola, G.B.; Di Cara, G.; Striano, P.; Verrotti, A. The Pharmacoresistant Epilepsy: An Overview on Existent and New Emerging Therapies. Front. Neurol. 2021, 12, 674483. [Google Scholar] [CrossRef]
- Akyüz, E.; Köklü, B.; Ozenen, C.; Arulsamy, A.; Shaikh, M.F. Elucidating the Potential Side Effects of Current Anti-Seizure Drugs for Epilepsy. Curr. Neuropharmacol. 2021, 19, 1865–1883. [Google Scholar] [CrossRef]
- Roullet, F.I.; Lai, J.K.Y.; Foster, J.A. In Utero Exposure to Valproic Acid and Autism—A Current Review of Clinical and Animal Studies. Neurotoxicol. Teratol. 2013, 36, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Scaparrotta, A.; Cofini, M.; Chiarelli, F.; Tiboni, G.M. Developmental Neurotoxicity and Anticonvulsant Drugs: A Possible Link. Reprod. Toxicol. 2014, 48, 72–80. [Google Scholar] [CrossRef]
- Zimmermann, F.F.; Gaspary, K.V.; Leite, C.E.; De Paula Cognato, G.; Bonan, C.D. Embryological Exposure to Valproic Acid Induces Social Interaction Deficits in Zebrafish (Danio rerio): A Developmental Behavior Analysis. Neurotoxicol. Teratol. 2015, 52, 36–41. [Google Scholar] [CrossRef]
- Babiec, L.; Wilkaniec, A.; Adamczyk, A. Prenatal Exposure to Valproic Acid Induces Alterations in the Expression and Activity of Purinergic Receptors in the Embryonic Rat Brain. Folia Neuropathol. 2022, 60, 390–402. [Google Scholar] [CrossRef]
- Corrales-Hernández, M.G.; Villarroel-Hagemann, S.K.; Mendoza-Rodelo, I.E.; Palacios-Sánchez, L.; Gaviria-Carrillo, M.; Buitrago-Ricaurte, N.; Espinosa-Lugo, S.; Calderon-Ospina, C.-A.; Rodríguez-Quintana, J.H. Development of Antiepileptic Drugs throughout History: From Serendipity to Artificial Intelligence. Biomedicines 2023, 11, 1632. [Google Scholar] [CrossRef]
- Lessman, C.A. The Developing Zebrafish (Danio Rerio): A Vertebrate Model for High-Throughput Screening of Chemical Libraries. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 268–280. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef] [PubMed]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole Induced Changes in Zebrafish Behavior, Neural Activity and c-Fos Expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Afrikanova, T.; Serruys, A.-S.K.; Buenafe, O.E.M.; Clinckers, R.; Smolders, I.; de Witte, P.A.M.; Crawford, A.D.; Esguerra, C.V. Validation of the Zebrafish Pentylenetetrazol Seizure Model: Locomotor versus Electrographic Responses to Antiepileptic Drugs. PLoS ONE 2013, 8, e54166. [Google Scholar] [CrossRef] [PubMed]
- Barker-Haliski, M.; Pitsch, J.; Galanopoulou, A.S.; Köhling, R. A Companion to the Preclinical Common Data Elements for Phenotyping Seizures and Epilepsy in Rodent Models. A Report of the TASK3-WG1C: Phenotyping Working Group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2022. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, P.; Yan, F.; Luo, Y.; Zhao, G. Animal Models of Epilepsy: A Phenotype-Oriented Review. Aging Dis. 2022, 13, 215–231. [Google Scholar] [CrossRef]
- Kundap, U.P.; Kumari, Y.; Othman, I.; Shaikh, M.F. Zebrafish as a Model for Epilepsy-Induced Cognitive Dysfunction: A Pharmacological, Biochemical and Behavioral Approach. Front. Pharmacol. 2017, 8, 515. [Google Scholar] [CrossRef]
- Turrini, L.; Fornetto, C.; Marchetto, G.; Müllenbroich, M.C.; Tiso, N.; Vettori, A.; Resta, F.; Masi, A.; Mannaioni, G.; Pavone, F.S.; et al. Optical Mapping of Neuronal Activity during Seizures in Zebrafish. Sci. Rep. 2017, 7, 3025. [Google Scholar] [CrossRef]
- Liu, J.; Baraban, S.C. Network Properties Revealed during Multi-Scale Calcium Imaging of Seizure Activity in Zebrafish. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Burrows, D.R.W.; Samarut, É.; Liu, J.; Baraban, S.C.; Richardson, M.P.; Meyer, M.P.; Rosch, R.E. Imaging Epilepsy in Larval Zebrafish. Eur. J. Paediatr. Neurol. 2020, 24, 70–80. [Google Scholar] [CrossRef]
- Milder, P.C.; Zybura, A.S.; Cummins, T.R.; Marrs, J.A. Neural Activity Correlates With Behavior Effects of Anti-Seizure Drugs Efficacy Using the Zebrafish Pentylenetetrazol Seizure Model. Front. Pharmacol. 2022, 13, 836573. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Boiti, A.; Sovrano, V.A.; Sgadò, P. Micromolar Valproic Acid Doses Preserve Survival and Induce Molecular Alterations in Neurodevelopmental Genes in Two Strains of Zebrafish Larvae. Biomolecules 2020, 10, 1364. [Google Scholar] [CrossRef] [PubMed]
- Torres-Hernández, B.A.; Colón, L.R.; Rosa-Falero, C.; Torrado, A.; Miscalichi, N.; Ortíz, J.G.; González-Sepúlveda, L.; Pérez-Ríos, N.; Suárez-Pérez, E.; Bradsher, J.N.; et al. Reversal of Pentylenetetrazole-Altered Swimming and Neural Activity-Regulated Gene Expression in Zebrafish Larvae by Valproic Acid and Valerian Extract. Psychopharmacology 2016, 233, 2533–2547. [Google Scholar] [CrossRef]
- Chitolina, R.; Gallas-Lopes, M.; Reis, C.G.; Benvenutti, R.; Stahlhofer-Buss, T.; Calcagnotto, M.E.; Herrmann, A.P.; Piato, A. Chemically-Induced Epileptic Seizures in Zebrafish: A Systematic Review. Epilepsy Res. 2023, 197, 107236. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Kannan, R.R. Zebrafish: A Potential Preclinical Model for Neurological Research in Modern Biology. In Zebrafish Model for Biomedical Research; Bhandari, P.R., Bharani, K.K., Khurana, A., Eds.; Springer Nature: Singapore, 2022; pp. 321–345. ISBN 978-981-16-5217-2. [Google Scholar]
- Sierra, A.; Gröhn, O.; Pitkänen, A. Imaging Microstructural Damage and Plasticity in the Hippocampus during Epileptogenesis. Neuroscience 2015, 309, 162–172. [Google Scholar] [CrossRef]
- Kollipara, R.; Langille, E.; Tobin, C.; French, C.R. Phytocannabinoids Reduce Seizures in Larval Zebrafish and Affect Endocannabinoid Gene Expression. Biomolecules 2023, 13, 1398. [Google Scholar] [CrossRef]
- Thornton, C.; Dickson, K.E.; Carty, D.R.; Ashpole, N.M.; Willett, K.L. Cannabis Constituents Reduce Seizure Behavior in Chemically-Induced and Scn1a-Mutant Zebrafish. Epilepsy Behav. 2020, 110, 107152. [Google Scholar] [CrossRef]
- Samarut, É.; Nixon, J.; Kundap, U.P.; Drapeau, P.; Ellis, L.D. Single and Synergistic Effects of Cannabidiol and Δ-9-Tetrahydrocannabinol on Zebrafish Models of Neuro-Hyperactivity. Front. Pharmacol. 2019, 10, 226. [Google Scholar] [CrossRef]
- Leo, A.; Russo, E.; Elia, M. Cannabidiol and Epilepsy: Rationale and Therapeutic Potential. Pharmacol. Res. 2016, 107, 85–92. [Google Scholar] [CrossRef]
- Lazarini-Lopes, W.; Do Val-da Silva, R.A.; da Silva-Júnior, R.M.P.; Leite, J.P.; Garcia-Cairasco, N. The Anticonvulsant Effects of Cannabidiol in Experimental Models of Epileptic Seizures: From Behavior and Mechanisms to Clinical Insights. Neurosci. Biobehav. Rev. 2020, 111, 166–182. [Google Scholar] [CrossRef]
- Lachowicz, J.; Szopa, A.; Ignatiuk, K.; Świąder, K.; Serefko, A. Zebrafish as an Animal Model in Cannabinoid Research. Int. J. Mol. Sci. 2023, 24, 10455. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in Patients with Seizures Associated with Lennox-Gastaut Syndrome (GWPCARE4): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Subbanna, S. Unveiling the Potential of Phytocannabinoids: Exploring Marijuana’s Lesser-Known Constituents for Neurological Disorders. Biomolecules 2024, 14, 1296. [Google Scholar] [CrossRef]
- Caprioglio, D.; Amin, H.I.M.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research. Biomolecules 2022, 12, 1084. [Google Scholar] [CrossRef]
- Silva-Reis, R.; Silva, A.M.S.; Oliveira, P.A.; Cardoso, S.M. Antitumor Effects of Cannabis Sativa Bioactive Compounds on Colorectal Carcinogenesis. Biomolecules 2023, 13, 764. [Google Scholar] [CrossRef]
- Peeri, H.; Koltai, H. Cannabis Biomolecule Effects on Cancer Cells and Cancer Stem Cells: Cytotoxic, Anti-Proliferative, and Anti-Migratory Activities. Biomolecules 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Abyadeh, M.; Gupta, V.; Paulo, J.A.; Gupta, V.; Chitranshi, N.; Godinez, A.; Saks, D.; Hasan, M.; Amirkhani, A.; McKay, M.; et al. A Proteomic View of Cellular and Molecular Effects of Cannabis. Biomolecules 2021, 11, 1411. [Google Scholar] [CrossRef]
- André, R.; Gomes, A.P.; Pereira-Leite, C.; Marques-da-Costa, A.; Monteiro Rodrigues, L.; Sassano, M.; Rijo, P.; Costa, M.d.C. The Entourage Effect in Cannabis Medicinal Products: A Comprehensive Review. Pharmaceuticals 2024, 17, 1543. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.-H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An Entourage Effect: Inactive Endogenous Fatty Acid Glycerol Esters Enhance 2-Arachidonoyl-Glycerol Cannabinoid Activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Scott, J.R.; Litinas, E.; Sisley, S.; Clauw, D.J.; Goesling, J.; Williams, D.A. Cannabis Use Preferences and Decision-Making Among a Cross-Sectional Cohort of Medical Cannabis Patients with Chronic Pain. J. Pain 2019, 20, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Gagnier, J.J.; Matallana, L.; Williams, D.A. Cannabidiol Product Dosing and Decision-Making in a National Survey of Individuals with Fibromyalgia. J. Pain 2022, 23, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kvamme, S.L.; Pedersen, M.M.; Rømer Thomsen, K.; Thylstrup, B. Exploring the Use of Cannabis as a Substitute for Prescription Drugs in a Convenience Sample. Harm Reduct. J. 2021, 18, 72. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Stella, N. THC and CBD: Similarities and Differences between Siblings. Neuron 2023, 111, 302–327. [Google Scholar] [CrossRef]
- Licitra, R.; Damiani, D.; Naef, V.; Fronte, B.; Vecchia, S.D.; Sangiacomo, C.; Marchese, M.; Santorelli, F.M. Cannabidiol Mitigates Valproic Acid-Induced Developmental Toxicity and Locomotor Behavioral Impairment in Zebrafish. J. Biol. Regul. Homeost. Agents 2023, 37, 4935–4946. [Google Scholar] [CrossRef]
- Pamplona, F.A.; da Silva, L.R.; Coan, A.C. Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-Analysis. Front. Neurol. 2018, 9, 759. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Martorell, M.; Sharopov, F.; Tumer, T.B.; Kurt, B.; Lankatillake, C.; Docea, A.O.; Moreira, A.C.; et al. A Pharmacological Perspective on Plant-Derived Bioactive Molecules for Epilepsy. Neurochem. Res. 2021, 46, 2205–2225. [Google Scholar] [CrossRef]
- Challal, S.; Skiba, A.; Langlois, M.; Esguerra, C.V.; Wolfender, J.-L.; Crawford, A.D.; Saklikar-Woźniak, K. Natural Product-Derived Therapies for Treating Drug-Resistant Epilepsies: From Ethnopharmacology to Evidence-Based Medicine. J. Ethnopharmacol. 2023, 317, 116740. [Google Scholar] [CrossRef]
- Nutt, D.J.; Phillips, L.D.; Barnes, M.P.; Brander, B.; Curran, H.V.; Fayaz, A.; Finn, D.P.; Horsted, T.; Moltke, J.; Sakal, C.; et al. A Multicriteria Decision Analysis Comparing Pharmacotherapy for Chronic Neuropathic Pain, Including Cannabinoids and Cannabis-Based Medical Products. Cannabis Cannabinoid Res. 2022, 7, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Stueber, A.; Cuttler, C. A Large-Scale Survey of Cannabis Use for Sleep: Preferred Products and Perceived Effects in Comparison to over-the-Counter and Prescription Sleep Aids. Explor. Med. 2023, 4, 709–719. [Google Scholar] [CrossRef]
- Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, M.; Uliel-Siboni, S.; Linder, I.; Kramer, U.; Epstein, O.; Menascu, S.; Nissenkorn, A.; Yosef, O.B.; Hyman, E.; Granot, D.; et al. CBD-Enriched Medical Cannabis for Intractable Pediatric Epilepsy: The Current Israeli Experience. Seizure 2016, 35, 41–44. [Google Scholar] [CrossRef]
- Ross-Munro, E.; Isikgel, E.; Fleiss, B. Evaluation of the Efficacy of a Full-Spectrum Low-THC Cannabis Plant Extract Using In Vitro Models of Inflammation and Excitotoxicity. Biomolecules 2024, 14, 1434. [Google Scholar] [CrossRef]
- Christensen, C.; Rose, M.; Cornett, C.; Allesø, M. Decoding the Postulated Entourage Effect of Medicinal Cannabis: What It Is and What It Isn’t. Biomedicines 2023, 11, 2323. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, S.; Holdsworth, C.J.; Meza Santoscoy, P.L.; Harrison, M.R.M.; Fox, J.; Parkin, C.A.; Ingham, P.W.; Cunliffe, V.T. Identification of Compounds with Anti-Convulsant Properties in a Zebrafish Model of Epileptic Seizures. Dis. Models Mech. 2012, 5, 773–784. [Google Scholar] [CrossRef]
- Rahn, J.J.; Bestman, J.E.; Josey, B.J.; Inks, E.S.; Stackley, K.D.; Rogers, C.E.; Chou, C.J.; Chan, S.S.L. Novel Vitamin K Analogues Suppress Seizures in Zebrafish and Mouse Models of Epilepsy. Neuroscience 2014, 259, 142–154. [Google Scholar] [CrossRef]

| Strain | CAN-1 | CAN-2 | CAN-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Extract | E25 | E100 | EA | E25 | E100 | EA | E25 | E100 | EA |
| CBDA | 0.032 | 0.017 | 0.026 | 4.545 | 1.557 | 4.791 | 4.055 | 2.199 | 4.164 |
| CBD | 0.015 | 0.024 | 0.012 | 0.763 | 5.174 | 0.884 | 0.201 | 2.661 | 0.215 |
| THCA | 5.722 | 0.740 | 4.571 | 0.259 | 0.133 | 0.274 | 2.135 | 0.491 | 2.166 |
| Δ9-THC | 0.816 | 5.149 | 0.604 | 0.097 | 0.852 | 0.101 | 0.299 | 2.343 | 0.291 |
| CBGA | 0.417 | 0.124 | 0.304 | 0.083 | 0.040 | 0.092 | 0.086 | 0.057 | 0.087 |
| CBG | 0.045 | 0.169 | 0.035 | 0.033 | 0.117 | 0.036 | 0.041 | 0.079 | 0.042 |
| CBCA | 0.438 | 0.120 | 0.382 | 0.359 | 0.077 | 0.378 | 0.236 | 0.106 | 0.247 |
| CBC | 0.033 | 0.230 | 0.025 | 0.063 | 0.302 | 0.069 | 0.033 | 0.149 | 0.035 |
| CBNA | 0.112 | 0.024 | 0.083 | 0.010 | 0.010 | 0.011 | 0.066 | 0.029 | 0.058 |
| CBN | 0.027 | 0.070 | 0.022 | 0.016 | 0.028 | 0.017 | 0.029 | 0.066 | 0.029 |
| CBDV | 0 | 0 | 0 | 0.018 | 0.028 | 0.018 | 0.026 | 0.037 | 0.026 |
| THCV | 0.020 | 0.071 | 0.017 | 0 | 0.016 | 0 | 0.022 | 0.039 | 0.022 |
| Strain | CAN-1 | CAN-2 | CAN-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Extract | E25 | E100 | EA | E25 | E100 | EA | E25 | E100 | EA |
| Alpha Bisabolol | 0.0063 | 0.0076 | 0.0068 | 0.0151 | 0.0126 | 0.0140 | - | - | - |
| Alpha Cedrene | 0.0087 | 0.0024 | 0.0090 | 0.0036 | 0.0007 | 0.0042 | 0.0017 | 0.0011 | 0.0035 |
| Alpha Humulene | 0.0044 | 0.0017 | 0.0045 | 0.0018 | 0.0007 | 0.0019 | 0.0011 | 0.0009 | 0.0020 |
| Alpha Pinene | - | - | - | - | - | 0.0008 | - | - | 0.0010 |
| Alpha Terpineol | 0.0012 | 0.0005 | 0.0012 | 0.0010 | - | 0.0010 | 0.0010 | - | 0.0018 |
| Beta Caryophyllene | 0.0109 | 0.0030 | 0.0113 | 0.0045 | 0.0008 | 0.0052 | 0.0021 | 0.0013 | 0.0044 |
| Beta Eudesmol | 0.0039 | 0.0042 | 0.0045 | 0.0047 | 0.0040 | 0.0044 | 0.0012 | 0.0030 | 0.0046 |
| Beta Pinene | 0.0001 | 0.00007 | 0.0003 | - | 0.00007 | 0.0004 | 0.0002 | - | 0.0005 |
| Borneol | 0.0019 | 0.0024 | 0.0022 | 0.0027 | 0.0027 | 0.0045 | 0.0026 | 0.0050 | 0.0054 |
| Cedrol | 0.0009 | 0.0008 | 0.0010 | 0.0008 | 0.0007 | 0.0008 | 0.0007 | 0.0012 | 0.0013 |
| Fenchol | 0.0010 | 0.0004 | 0.0010 | 0.0008 | - | 0.0008 | 0.0008 | - | 0.0016 |
| (-) Guaiol | 0.0034 | 0.0040 | 0.0036 | 0.0047 | 0.0035 | 0.0044 | 0.0020 | 0.0030 | 0.0039 |
| Limonene (2) | 0.0005 | 0.0002 | 0.0008 | - | - | 0.0002 | - | - | 0.0005 |
| Myrcene | 0.0012 | 0.0005 | 0.0033 | 0.0006 | - | 0.0024 | 0.0007 | - | 0.0022 |
| Terpinolene | 0.0006 | 0.0003 | 0.0006 | 0.0006 | - | 0.0006 | 0.0006 | - | 0.0010 |
| Trans Nerolidol | - | - | - | 0.0021 | 0.0018 | 0.0021 | 0.0008 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, K.; Shabat-Simon, M.; Bar-On, J.; Steckler, R.; Khatib, S.; Tamir, S.; Pitashny, P.A. The Anticonvulsant Effects of Different Cannabis Extracts in a Zebrafish Model of Epilepsy. Biomolecules 2025, 15, 654. https://doi.org/10.3390/biom15050654
Jackson K, Shabat-Simon M, Bar-On J, Steckler R, Khatib S, Tamir S, Pitashny PA. The Anticonvulsant Effects of Different Cannabis Extracts in a Zebrafish Model of Epilepsy. Biomolecules. 2025; 15(5):654. https://doi.org/10.3390/biom15050654
Chicago/Turabian StyleJackson, Karen, Maytal Shabat-Simon, Jonathan Bar-On, Rafi Steckler, Soliman Khatib, Snait Tamir, and Paula Adriana Pitashny. 2025. "The Anticonvulsant Effects of Different Cannabis Extracts in a Zebrafish Model of Epilepsy" Biomolecules 15, no. 5: 654. https://doi.org/10.3390/biom15050654
APA StyleJackson, K., Shabat-Simon, M., Bar-On, J., Steckler, R., Khatib, S., Tamir, S., & Pitashny, P. A. (2025). The Anticonvulsant Effects of Different Cannabis Extracts in a Zebrafish Model of Epilepsy. Biomolecules, 15(5), 654. https://doi.org/10.3390/biom15050654









