Assessment of [125I]a-Bungarotoxin Binding to a7 Nicotinic Acetylcholinergic Receptors in Hippocampus-Subiculum of Postmortem Human Parkinson’s Disease Brain
Abstract
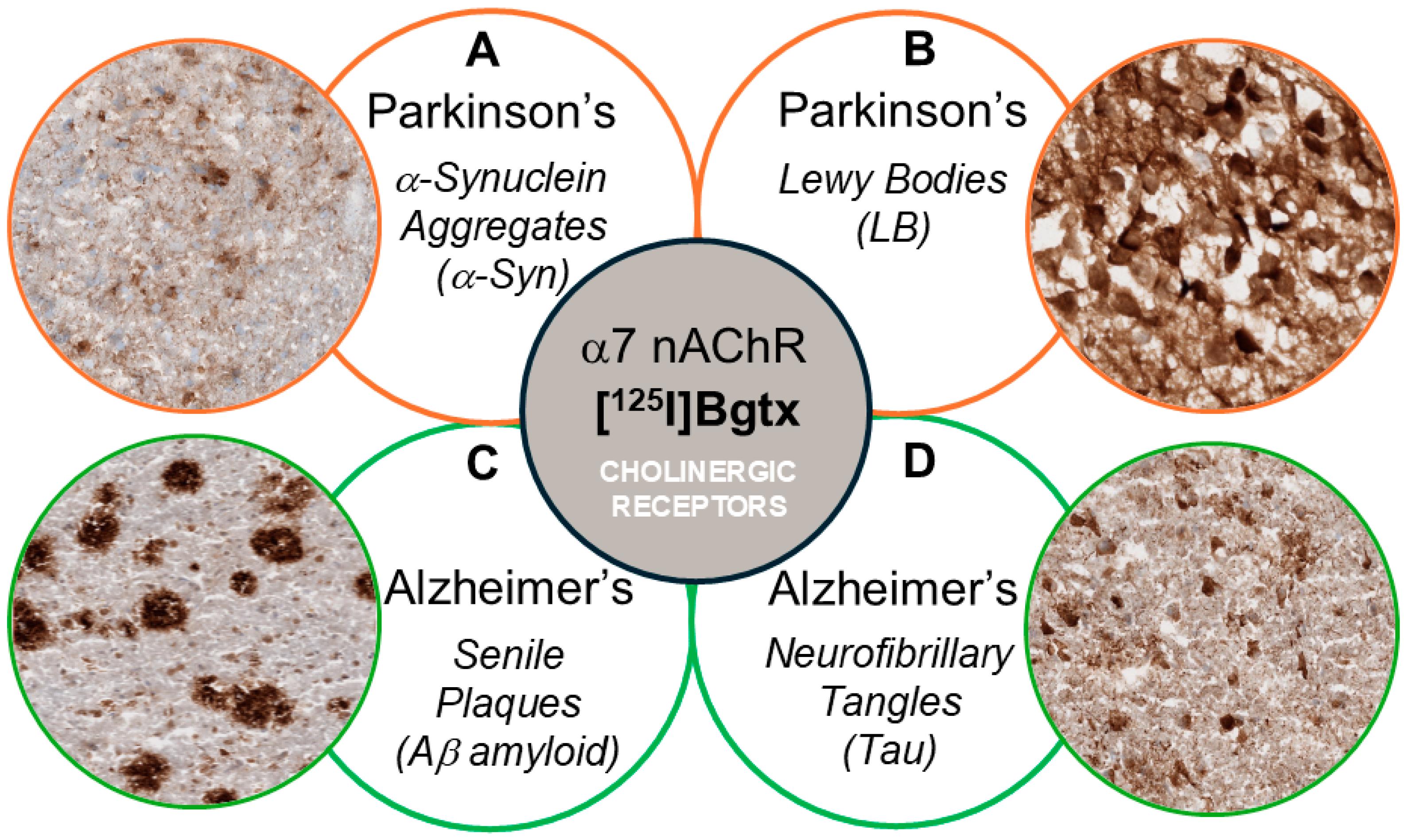
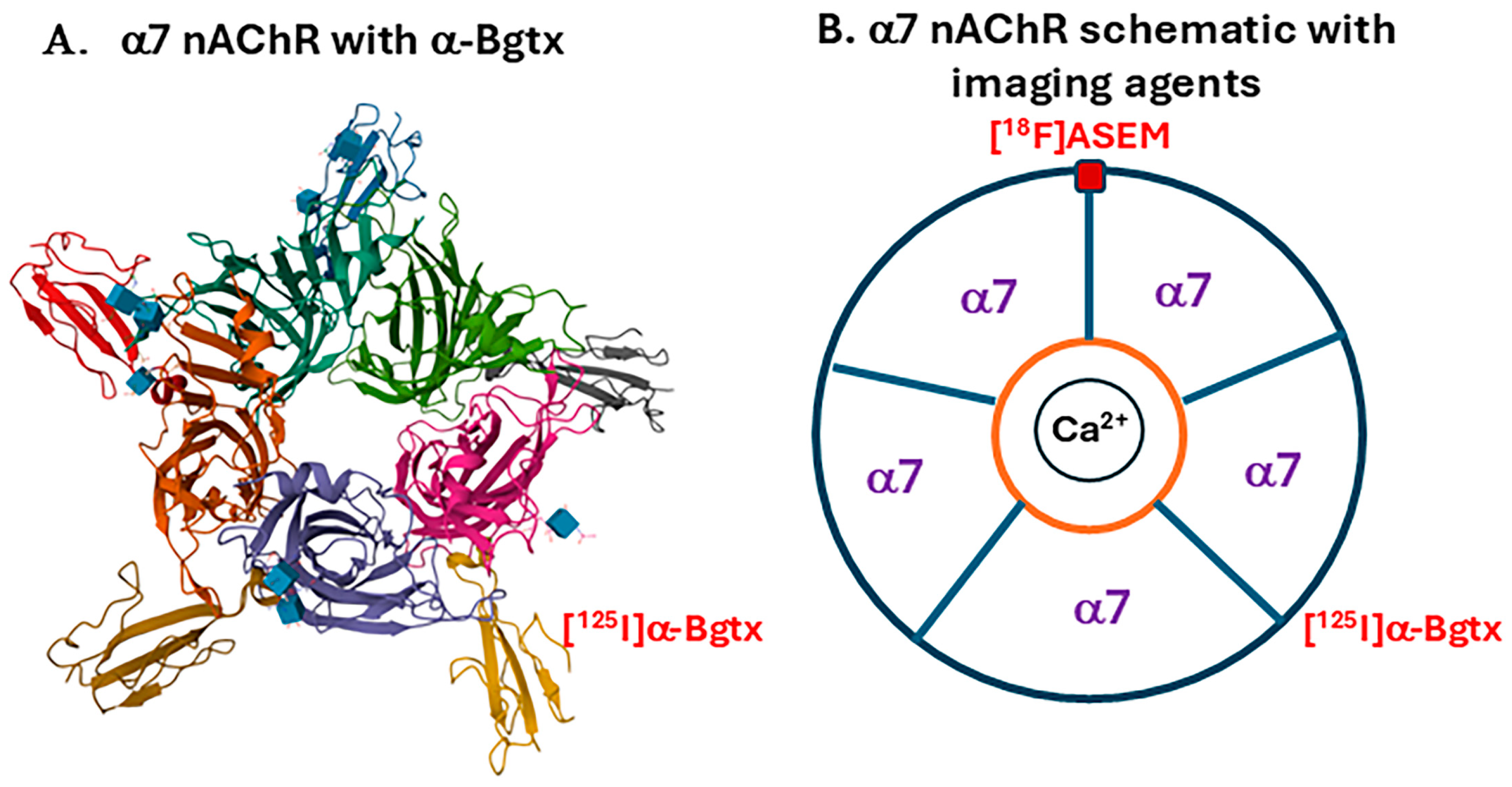
1. Introduction
2. Materials and Methods
2.1. Postmortem Human Brain
2.2. Radiopharmaceuticals
2.3. [125I]α-Bgtx Autoradiography
2.4. Immunohistochemistry
2.5. Statistical Analysis
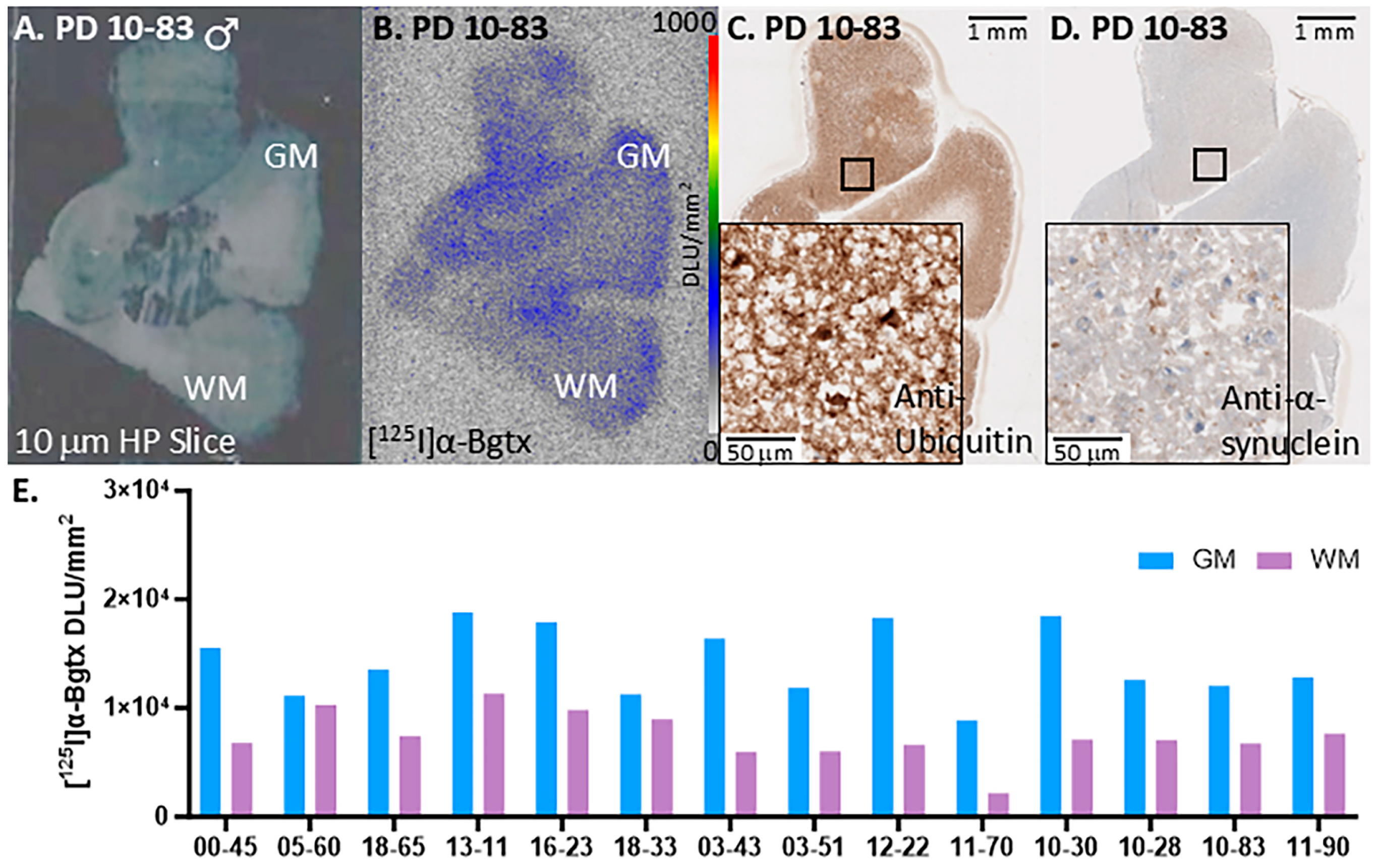
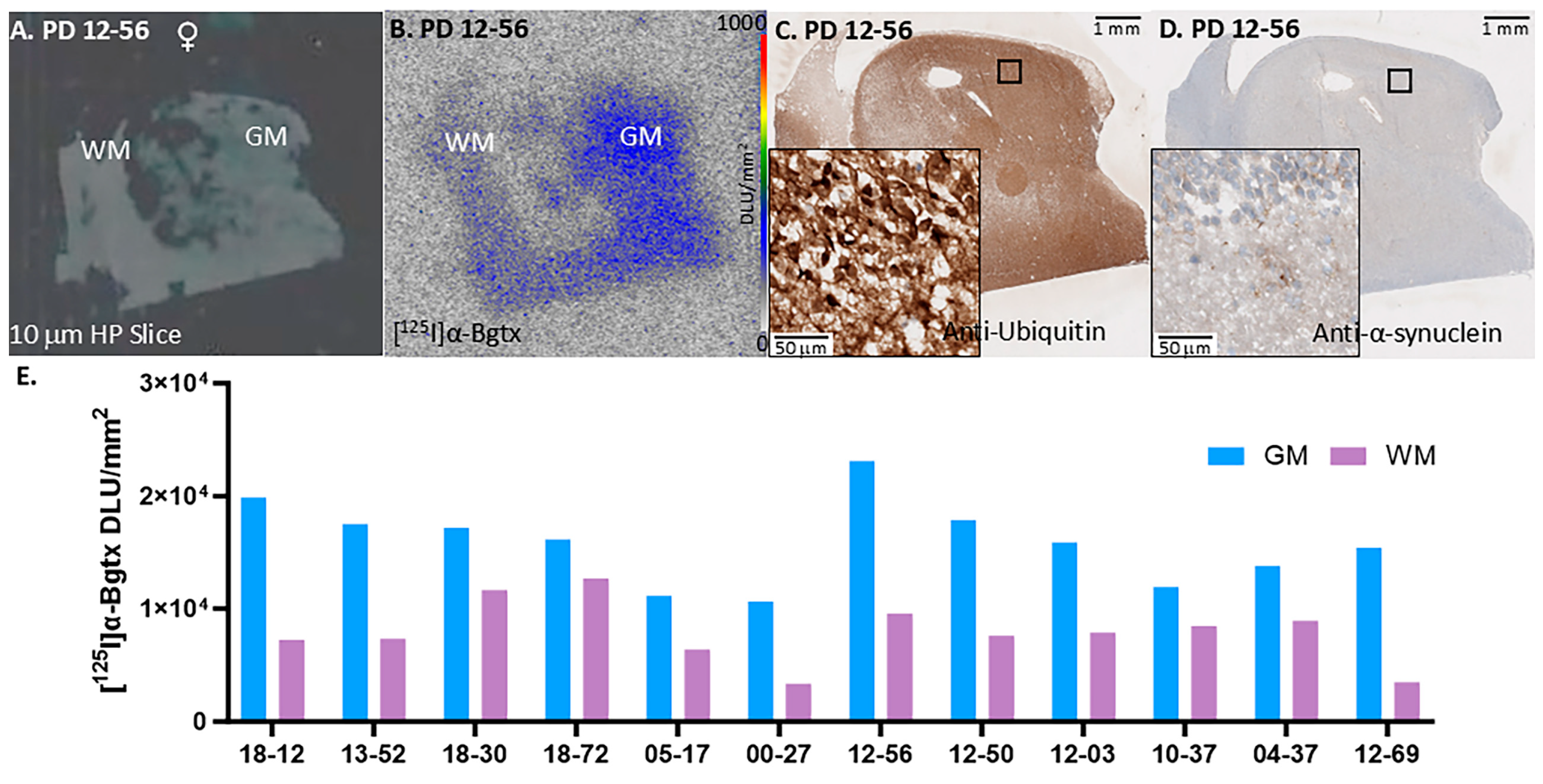
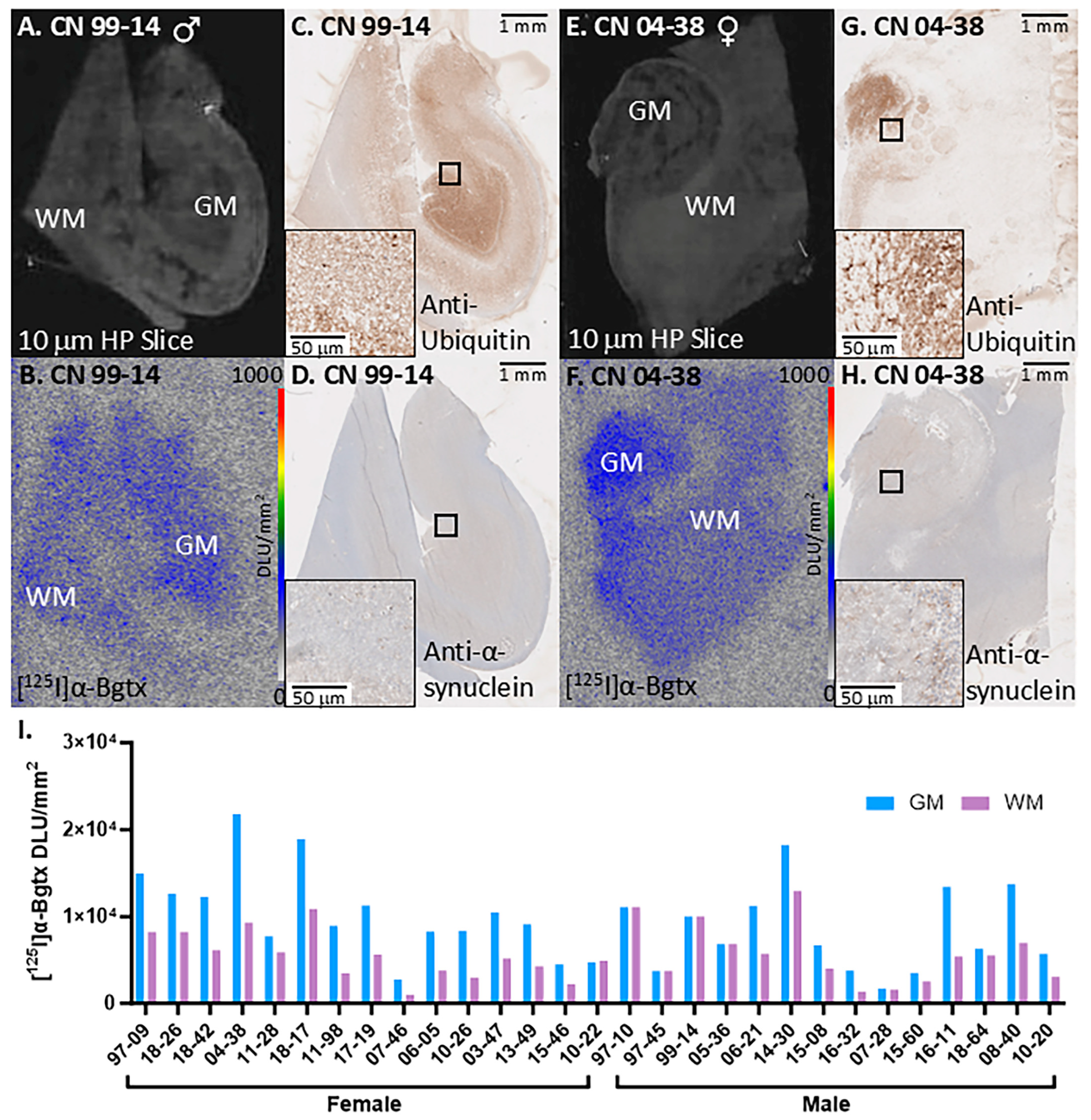
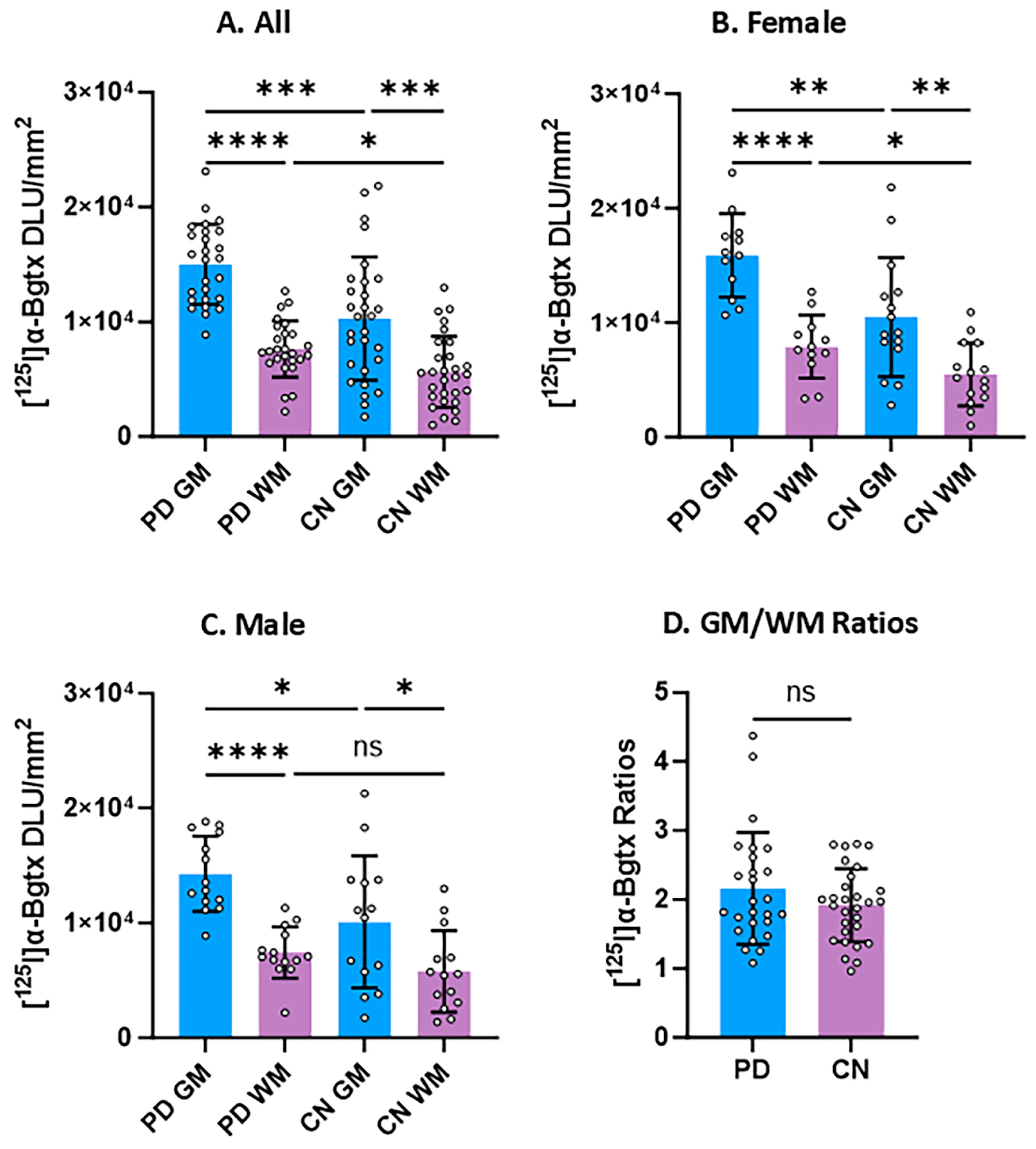
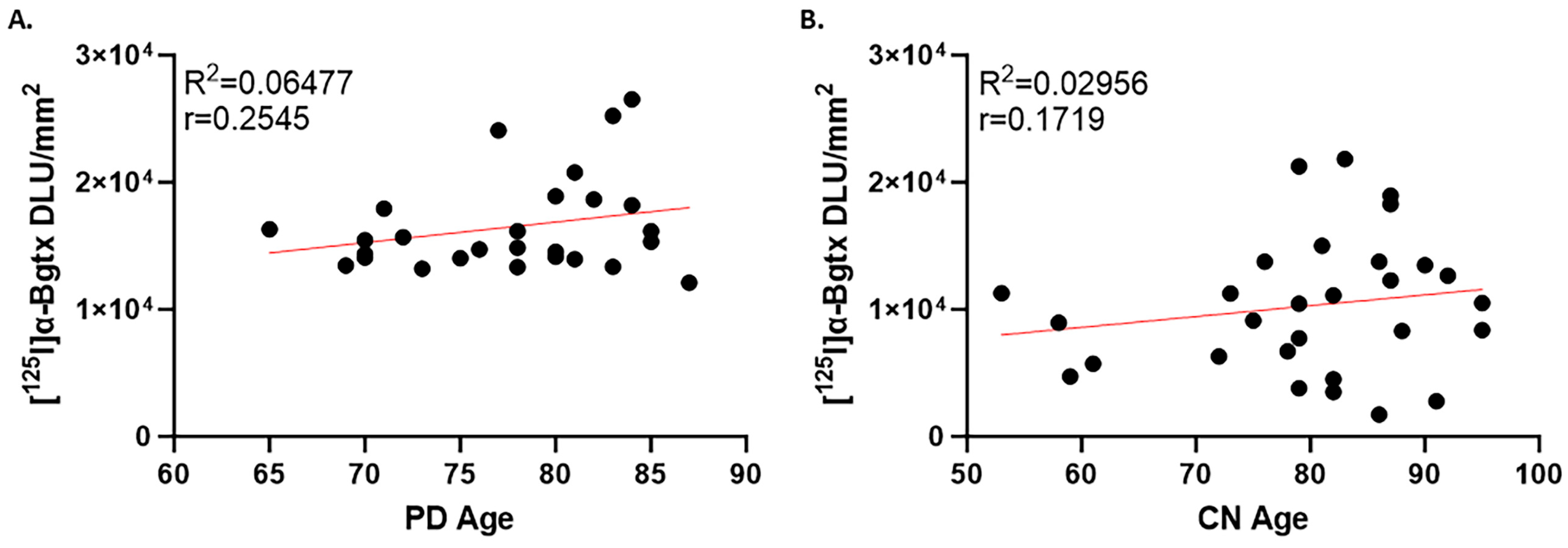
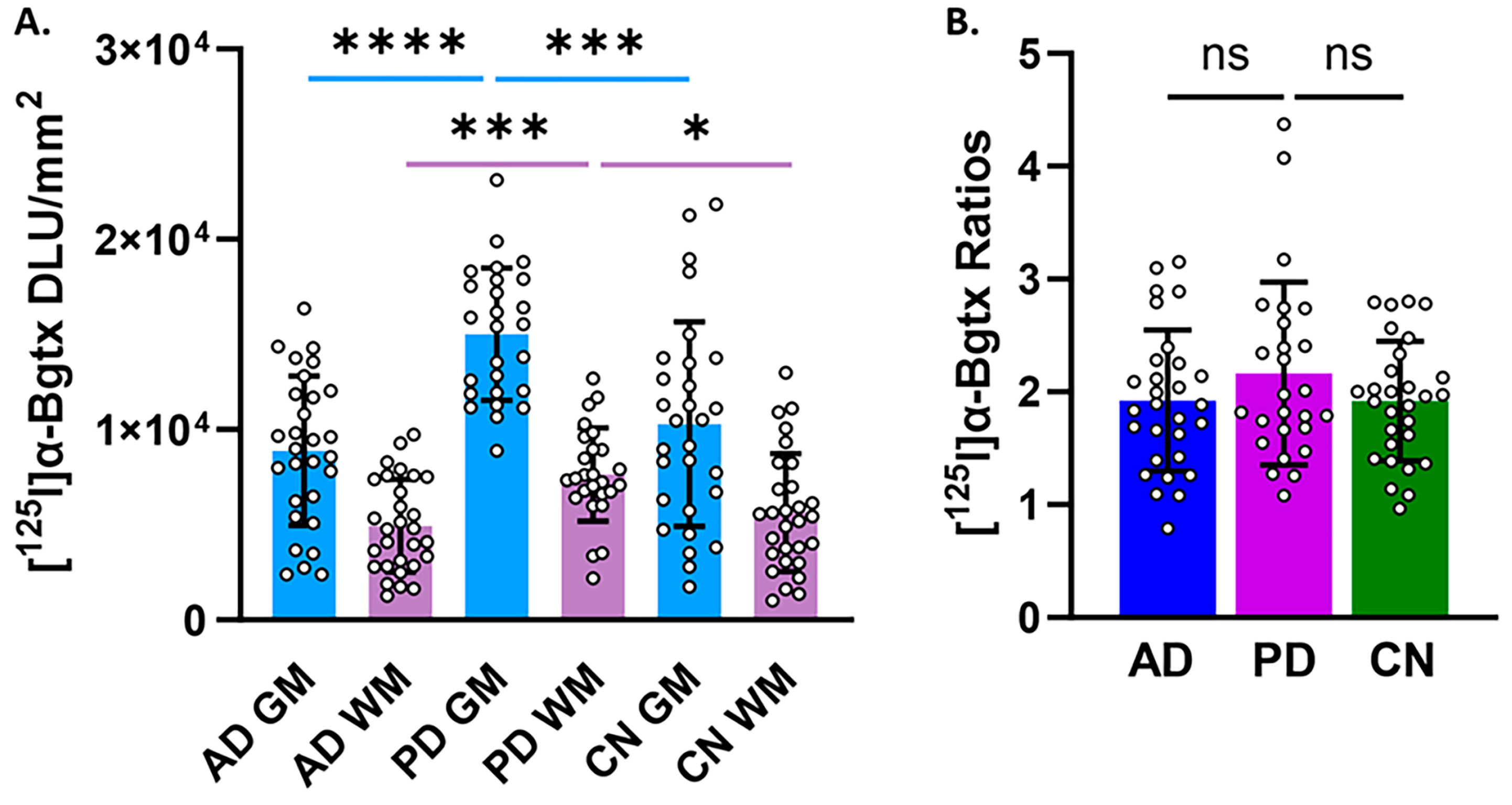
3. Results
3.1. PD Cases
3.2. CN Cases
3.3. Group Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sveinbjornsdottir, S. The Clinical Symptoms of Parkinson’s Disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathology of Parkinson Disease. Park. Relat. Disord. 2018, 46, S30–S33. [Google Scholar] [CrossRef]
- Mahul-Mellier, A.-L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The Process of Lewy Body Formation, Rather than Simply α-Synuclein Fibrillization, Is One of the Major Drivers of Neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial Dysfunction in Parkinson’s Disease—A Key Disease Hallmark with Therapeutic Potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef]
- Levigoureux, E.; Bouillot, C.; Baron, T.; Zimmer, L.; Lancelot, S. PET imaging of the influence of physiological and pathological α-synuclein on dopaminergic and serotonergic neurotransmission in mouse models. CNS Neurosci. Ther. 2019, 25, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Tofaris, G.K.; Razzaq, A.; Ghetti, B.; Lilley, K.S.; Spillantini, M.G. Ubiquitination of α-Synuclein in Lewy Bodies Is a Pathological Event Not Associated with Impairment of Proteasome Function *. J. Biol. Chem. 2003, 278, 44405–44411. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.J.; Tolosa, E.; Campdelacreu, J. Clinical overview of the synucleinopathies. Mov. Disord. 2003, 18, 21–27. [Google Scholar] [CrossRef]
- Kuzuhara, S.; Mori, H.; Izumiyama, N.; Yoshimura, M.; Ihara, Y. Lewy Bodies Are Ubiquitinated. Acta Neuropathol. 1988, 75, 345–353. [Google Scholar] [CrossRef]
- Ngo, A.; Karim, F.; Keerthisinghe, O.V.; Danh, T.B.; Liang, C.; Mukherjee, J. Evaluation of [125I]α-Bungarotoxin Binding to A7 Nicotinic Acetylcholinergic Receptors in Hippocampus–Subiculum of Postmortem Human Alzheimer’s Disease Brain. Receptors 2025, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Barrantes, F.J. Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. Park. Dis. 2016, 2, 16001. [Google Scholar] [CrossRef]
- Quik, M.; Bordia, T.; Zhang, D.; Perez, X.A. Nicotine and nicotinic receptor drugs: Potential for Parkinson’s disease and drug-induced movement disorders. Int. Rev. Neurobiol. 2015, 124, 247–271. [Google Scholar]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; León, R.; Lopez, M.G. Anti-Inflammatory Role of Microglial Alpha7 nAChRs and Its Role in Neuroprotection. Biochem. Pharmacol. 2015, 97, 463–472. [Google Scholar] [CrossRef]
- Kalkman, H.O.; Feuerbach, D. Modulatory Effects of A7 nAChRs on the Immune System and Its Relevance for CNS Disorders. Cell. Mol. Life Sci. 2016, 73, 2511–2530. [Google Scholar] [CrossRef]
- Hawrot, E.; Wilson, P.T.; Gershoni, J.M.; Reese, J.H.; Lentz, T.L. Alpha-bungarotoxin binding to a high molecular weight component from lower vertebrate brain identified on dodecyl sulfate protein-blots. Brain Res. 1986, 373, 227–234. [Google Scholar] [CrossRef]
- Love, R.A.; Stroud, R.M. Love RA, Stroud RM. The crystal structure of a-bungarotoxin at 2.5A resolution: Relation to solution structure and binding to acetylcholine receptor. Protein Eng. 1986, 1, 37–46. [Google Scholar] [CrossRef]
- Rasmussen, B.A.; Perry, D.C. An Autoradiographic analysis of [125I]α-bungarotoxin binding in rat brain after chronic nicotine exposure. Neurosci. Lett. 2006, 404, 9–14. [Google Scholar] [CrossRef]
- Singh, K.; Ngo, A.; Keerthisinghe, O.V.; Patel, K.K.; Liang, C.; Mukherjee, J. Synthesis and Evaluation of Compound Targeting α7 and β2 Subunits in Nicotinic Acetylcholinergic Receptor. Molecules 2023, 28, 8128. [Google Scholar] [CrossRef]
- Donat, C.K.; Hansen, H.H.; Hansen, H.D.; Mease, R.C.; Horti, A.G.; Pomper, M.G.; L’Estrade, E.T.; Herth, M.M.; Peters, D.; Knudsen, G.M.; et al. In Vitro and In Vivo Characterization of Dibenzothiophene Derivatives [125I]Iodo-ASEM and [18F]ASEM as Radiotracers of Homo- and Heteromeric α7 Nicotinic Acetylcholine Receptors. Molecules 2020, 25, 1425. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Cheng, K.; Gomoto, R.; Bren, N.; Huang, S.; Sine, S.; Chen, L. Alpha7 nicotinic receptor chimera and its complex with Alpha bungarotoxin. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Horti, A.G.; Gao, Y.; Kuwabara, H.; Wang, Y.; Abazyan, S.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Sahibzada, N.; Holt, D.P.; et al. 18F-ASEM, a Radiolabeled Antagonist for Imaging the A7-Nicotinic Acetylcholine Receptor with PET. J. Nucl. Med. 2014, 55, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kellar, K.J.; Yasuda, R.P.; Tran, T.; Xiao, Y.; Dannals, R.F.; Horti, A.G. Derivatives of Dibenzothiophene for Positron Emission Tomography Imaging of A7-Nicotinic Acetylcholine Receptors. J. Med. Chem. 2013, 56, 7574–7589. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.F.; Kuwabara, H.; Pomper, M.; Holt, D.P.; Brasic, J.R.; George, N.; Frolov, B.; Willis, W.; Gao, Y.; Valentine, H.; et al. Human Brain Imaging of A7 nAChR with [18F]ASEM: A New PET Radiotracer for Neuropsychiatry and Determination of Drug Occupancy. Mol. Imaging Biol. 2014, 16, 730–738. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.H.; Jung, J.H.; Kim, S.-Y.; Ko, N.; Lee, S.J.; Oh, S.J.; Ryu, J.-S.; Ko, D.; Kim, W.; et al. 18F-ASEM PET/MRI Targeting Alpha7-Nicotinic Acetylcholine Receptor Can Reveal Skeletal Muscle Denervation. EJNMMI Res. 2024, 14, 8. [Google Scholar] [CrossRef]
- Tang, T.; Wang, D.; Chen, X.; Liang, Y.; Guo, F.; Wu, C.; Jia, L.; Hou, Z.; Li, W.; He, Z.; et al. 18F-ASEM imaging for evaluating atherosclerotic plaques linked to a-7 nicotinic acetylcholine receptor. Front. Bioeng. Biotechnol. 2021, 9, 684221. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Rubin, L.H.; Du, Y.; Rowe, S.P.; Crawford, J.L.; Rosenthal, H.B.; Frey, S.M.; Marshall, E.S.; Shinehouse, L.K.; Chen, A.; et al. High Availability of the A7-Nicotinic Acetylcholine Receptor in Brains of Individuals with Mild Cognitive Impairment: A Pilot Study Using 18F-ASEM PET. J. Nucl. Med. 2020, 61, 423–426. [Google Scholar] [CrossRef]
- Wong, N.R.; Rubin, L.H.; Harrington, C.K.; Jenkins, K.R.; Shinehouse, L.K.; Yoon, M.; Kilgore, J.J.; Soule, A.R.; Lesniak, W.G.; Rowe, S.P.; et al. Hippocampal availability of the a7 nicotinic acetylcholine receptor in recent-onset psychosis. JAMA 2024, 7, e2427163. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Du, Y.; Crawford, J.L.; Rubin, L.H.; Azad, B.B.; Lesniak, W.G.; Horti, A.G.; Schretlen, D.J.; Sawa, A.; Pomper, M.G. Use of 18F-ASEM PET to determine the availability of the a7-nicotinic acetylcholine receptor in recent-onset psychosis. J. Nucl. Med. 2019, 60, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Vetel, S.; Vercouillie, J.; Buron, F.; Vergote, J.; Tauber, C.; Busson, J.; Chicheri, G.; Routier, S.; Sérrière, S.; Chalon, S. Longitudinal PET Imaging of A7 Nicotinic Acetylcholine Receptors with [18F]ASEM in a Rat Model of Parkinson’s Disease. Mol. Imaging Biol. 2020, 22, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Kulak, J.M.; Schneider, J.S. Differences in A7 Nicotinic Acetylcholine Receptor Binding in Motor Symptomatic and Asymptomatic MPTP-Treated Monkeys. Brain Res. 2004, 999, 193–202. [Google Scholar] [CrossRef]
- Meyer, P.M.; Tiepolt, S.; Barthel, H.; Hesse, S.; Sabri, O. Radioligand imaging of a4b2* nicotinic acetylcholine receptors in Alzheimer’s disease and Parkinson’s disease. Q. J. Nucl. Med. Mol. Imaging 2014, 58, 376–386. [Google Scholar]
- Schmaljohann, J.; Gundisch, D.; Minnerop, M.; Bucerius, J.; Joe, A.; Reinhardt, M.; Guhlke, S.; Biersack, H.-J.; Wullner, U. In vitro evaluation of nicotinic acetylcholine receptors with 2-[18F]F-A85380 in Parkinson’s disease. Nucl. Med. Biol. 2006, 33, 305–309. [Google Scholar] [CrossRef]
- Campoy, A.-D.T.; Liang, C.; Ladwa, R.M.; Patel, K.K.; Patel, I.H.; Mukherjee, J. [18F]Nifene PET/CT imaging in mice models: Improved methods and preliminary studies of α4β2* nicotinic acetylcholinergic receptors in transgenic A53T mouse model of α-synucleinopathy and post-mortem human Parkinson’s disease. Molecules 2021, 26, 7360. [Google Scholar] [CrossRef]
- Letsinger, A.C.; Gu, Z.; Yakel, J.L. α7 nicotinc acetylcholine receptors in the hippocampal circuit: Taming complexity. Trends Neurosci. 2022, 45, 145–157. [Google Scholar] [CrossRef]
- Lin, X.; Li, Q.; Pu, M.; Dong, H.; Zhang, Q. Significance of nicotine and nicotinic acetylcholine receptors in Parkinson’s disease. Front. Aging Neurosci. 2025, 17, 1535310. [Google Scholar] [CrossRef]
- Mizrachi, T.; Vaknin-Dembinsky, A.; Brenner, T.; Treinin, M. Neuroinflammation modulation via a7 nicotinic acetylcholine receptor and its chaperone, RIC-3. Molecules 2021, 26, 6139. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Ladwa, R.M.; Liang, C.; Syed, A.U. Elevated monoamine oxidase-A in anterior cingulate of postmortem human Parkinson’s disease: A potential surrogate biomarker for Lewy bodies? Cells 2022, 11, 4000. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Serrano, G.; Shill, H.A.; Walker, D.G.; Lue, L.; Roher, A.E.; Dugger, B.N.; Maarouf, C.; et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology 2015, 35, 354–389. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Adler, C.H.; Lue, L.; Sue, L.I.; Bachalakuri, J.; Henry-Watson, J.; Sasse, J.; Boyer, S.; Shirohi, S.; Brooks, R.; et al. Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009, 117, 613–634. [Google Scholar] [CrossRef] [PubMed]
- Shimohama, S.; Kawamata, J. Roles of Nicotinic Acetylcholine Receptors in the Pathology and Treatment of Alzheimer’s and Parkinson’s Diseases. In Nicotinic Acetylcholine Receptor Signaling in Neuroprotection; Akaike, A., Shimohama, S., Misu, Y., Eds.; Springer: Singapore, 2018; pp. 137–158. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Li, Y.; Xu, S.; Tao, T.; Hua, Y.; Zhang, J.; Fan, Y. Activation of A7-nAChRs Promotes the Clearance of α-Synuclein and Protects Against Apoptotic Cell Death Induced by Exogenous α-Synuclein Fibrils. Front. Cell Dev. Biol. 2021, 9, 637319. [Google Scholar] [CrossRef]
- Burghaus, L.; Schütz, U.; Krempel, U.; Lindstrom, J.; Schröder, H. Loss of nicotinic acetylcholine receptor subunits α4 and α7 in the cerebral cortex of Parkinson patients. Park. Relat. Disord. 2003, 9, 243–246. [Google Scholar] [CrossRef]
- Quik, M.; Vailati, S.; Bordia, T.; Kulak, J.M.; Fan, H.; McIntosh, J.M.; Clementi, F.; Gotti, C. Subunit composition of nicotinic receptors in monkey striatum: Effect of treatments with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine or L-DOPA. Mol. Pharmacol. 2005, 67, 32–41. [Google Scholar] [CrossRef]
- Guan, Z.Z.; Nordberg, A.; Mousavi, M.; Rinne, J.O.; Hellström-Lindahl, E. Selective changes in the levels of nicotinic acetylcholine receptor protein and of corresponding mRNA species in the brains of patients with Parkinson’s disease. Brain Res. 2002, 956, 358–366. [Google Scholar] [CrossRef]
- Hou, X.; Fiesel, F.C.; Truban, D.; Castanedes Casey, M.; Lin, W.; Soto, A.I.; Tacik, P.; Rousseau, L.G.; Diehl, N.N.; Heckman, M.G.; et al. Age- and Disease-Dependent Increase of the Mitophagy Marker Phospho-Ubiquitin in Normal Aging and Lewy Body Disease. Autophagy 2018, 14, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, S.; Ohuchi, K.; Fujimaki, A.; Ito, T.; Murakami, T.; Kurita, H.; Inden, M. Effects of α7 nicotinic acetylcholine receptor agonist against α-synuclein-induced neurotoxicity. Neurosci. Lett. 2024, 823, 137654. [Google Scholar] [CrossRef]
- Shah, N.; Ghazaryan, N.; Gonzaga, N.L.; Paclibar, C.G.; Biju, A.P.; Liang, C.; Mukherjee, J. Glutamate’s effects on N-methyl-D-aspartate (NMDA) receptor ion-channel in Alzheimer’s disease: Challenges for PET radiotracer development for imaging the NMDA ion-channel. Molecules 2024, 29, 20. [Google Scholar] [CrossRef]
- Gonzaga, N.; Karim, F.; Liang, C.; Mukherjee, J. [18F]Mefway: Imaging serotonin 5HT1A receptors in human post-mortem Parkinson’s and Alzheimer’s disease anterior cingulate. Potential applications to human positron emission tomography studies. Biomolecules 2025, 15, 592. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, C.; Spetsieri, P.G.; Dhawan, V.; Eidelberg, D. Abnormal metabolic network activity in Parkinson’s disease: Test-retest reproducibility. J. Cerebr. Blood Flow Metab. 2007, 27, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.C.; Lerman, H.; Lukic, A.; Andrews, R.D.; Mirelman, A.; Wernick, M.N.; Giladi, N.; Strother, S.C.; Evans, K.C.; Cedarbaum, J.M.; et al. FDG PET Parkinson’s disease-related pattern as a biomarker for clinical trials in early stage disease. Neuroimage Clin. 2018, 20, 572–579. [Google Scholar] [CrossRef]
- Buchert, R.; Buhmann, C.; Apostolova, I.; Meyer, P.T.; Gallinat, J. Nuclear Imaging in the Diagnosis of Clinically Uncertain Parkinsonian Syndromes. Dtsch. Arztebl. Int. 2019, 116, 747. [Google Scholar] [CrossRef]
- Trošt, M.; Perovnik, M.; Pirtošek, Z. Correlations of neuropsychological and metabolic brain changes in Parkinson’s disease and other α-synucleinopathies. Front. Neurol. 2019, 10, 1204. [Google Scholar] [CrossRef]
- Walker, Z.; Gandolfo, F.; Orini, S.; Garibotto, V.; Agosta, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Nestor, P.; Boccardi, M.; et al. Clinical utility of FDG PET in Parkinson’s disease and a typical parkinsonism associated with dementia. Eur. J. Nucl. Med. Mol. I. 2018, 45, 1534–1545. [Google Scholar] [CrossRef]
- Lu, J.; Wang, M.; Wu, P.; Yakushev, I.; Zhang, H.; Ziegler, S.; Jiang, J.; Förster, S.; Wang, J.; Schwaiger, M.; et al. Adjustment for the Age- and Gender-Related Metabolic Changes Improves the Differential Diagnosis of Parkinsonism. Phenomics 2023, 3, 50–63. [Google Scholar] [CrossRef]
- Karim, F.; Ngo, A.; Danh, T.B.; Delaney, B.D.; Liang, C.; Serrano, G.E.; Beach, T.G.; Mukherjee, J. Human hippocampal [18F]nifene binding to nicotinic acetylcholinergic α4β2* receptors in hippocampus-subiculum of postmortem Alzheimer’s disease brains. BRAIN Res. 2025, 1857, 149600. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, Y.K.; Bath, H.S.; Shergill, J.; Liang, C.; Syed, A.U.; Ngo, A.; Karim, F.; Serrano, G.E.; Beach, T.G.; Mukherjee, J. [18F]Flotaza for Ab plaque diagnostic imaging: Evaluation in postmortem human Alzheimer’s disease brain hippocampus and PET/CT imaging in 5xFAD transgenic mice. Int. J. Mol. Sci. 2024, 25, 7890. [Google Scholar] [CrossRef] [PubMed]
- Karim, F.; Delaney, B.A.; Mondal, R.; Liang, C.; Serrano, G.E.; Beach, T.G.; Mukherjee, J. [125I]IPPI for Tau imaging: Binding studies in postmortem human Alzheimer’s disease hippocampus and evaluation of drug effects. Synapse 2025, 79, e70024. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.B.; Schwartz, R.D.; Paul, S.M.; Pert, C.B.; Pert, A. Nicotinic binding in rat brain: Autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J. Neurosci. Off. J. Soc. Neurosci. 1985, 5, 1307–1315. [Google Scholar] [CrossRef]
- Samuel, N.; Wonnacott, S.; Lindstrom, J.; Futerman, A.H. Parallel increases in [alpha-125I]bungarotoxin binding and alpha 7 nicotinic subunit immunoreactivity during the development of rat hippocampal neurons in culture. Neurosci. Lett. 1997, 222, 179–182. [Google Scholar] [CrossRef]
| Cases, n | CERAD Pathology 1 | Gender | Age Range, Mean ± SD | PMI, Hrs 2 | Brain Region 3 | Plaque Total | Tangle Total | LB 4 | Braak Score |
|---|---|---|---|---|---|---|---|---|---|
| 12 | PD | Male | 69–85 (76.8 ± 5.03) | 1.83–4.37 | HP | 0–1.5 | 0–6.5 | III–IV | II–IV |
| 14 | PD | Female | 65–87 (78.1 ± 6.84) | 2–5 | HP | 0–13 | 0.5–6.5 | III–IV | I–IV |
| 14 | CN | Male | 61–90 (79.3 ± 7.44) | 2–5.42 | HP | 0–5.5 | 0–6 | 0 | I–III |
| 15 | CN | Female | 53–95 (80.3 ± 13.5) | 2.07–4.83 | HP | 0–10 | 0.5–6.5 | 0–II | I–III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, F.; Ngo, A.; Tucker, T.E.; Coronel, A.D.L.; Mukherjee, J. Assessment of [125I]a-Bungarotoxin Binding to a7 Nicotinic Acetylcholinergic Receptors in Hippocampus-Subiculum of Postmortem Human Parkinson’s Disease Brain. Biomolecules 2025, 15, 1686. https://doi.org/10.3390/biom15121686
Karim F, Ngo A, Tucker TE, Coronel ADL, Mukherjee J. Assessment of [125I]a-Bungarotoxin Binding to a7 Nicotinic Acetylcholinergic Receptors in Hippocampus-Subiculum of Postmortem Human Parkinson’s Disease Brain. Biomolecules. 2025; 15(12):1686. https://doi.org/10.3390/biom15121686
Chicago/Turabian StyleKarim, Fariha, Allyson Ngo, Titus E. Tucker, Ashlee D. L. Coronel, and Jogeshwar Mukherjee. 2025. "Assessment of [125I]a-Bungarotoxin Binding to a7 Nicotinic Acetylcholinergic Receptors in Hippocampus-Subiculum of Postmortem Human Parkinson’s Disease Brain" Biomolecules 15, no. 12: 1686. https://doi.org/10.3390/biom15121686
APA StyleKarim, F., Ngo, A., Tucker, T. E., Coronel, A. D. L., & Mukherjee, J. (2025). Assessment of [125I]a-Bungarotoxin Binding to a7 Nicotinic Acetylcholinergic Receptors in Hippocampus-Subiculum of Postmortem Human Parkinson’s Disease Brain. Biomolecules, 15(12), 1686. https://doi.org/10.3390/biom15121686








