Divergent Risks of Hematologic Malignancies Associated with GLP-1 Receptor Agonists and SGLT2 Inhibitors: Preliminary Findings from a Pilot Network Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Analytical Framework
2.2. Literature Search Strategy
2.3. Eligibility Criteria
2.4. Risk of Bias Assessment
2.5. Outcome Definitions and Dose Categorization
2.6. Data Extraction and Handling
2.7. Statistical Methodology
2.8. Sensitivity and Subgroup Analyses
2.9. Ethics
3. Results
3.1. Study Selection and Characteristics
3.2. Primary Outcome: Incidence of Overall Hematologic Malignancies
3.3. Subgroup Analysis: Specific Hematologic Cancer Types
3.4. Safety Profile: Dropout Rate
3.5. Robustness Evaluation: Bayesian-Based Sensitivity Analysis
3.6. Risk of Bias and Inconsistency Assessment
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avogaro, A.; de Kreutzenberg, S.V.; Morieri, M.L.; Fadini, G.P.; Del Prato, S. Glucose-lowering drugs with cardiovascular benefits as modifiers of critical elements of the human life history. Lancet Diabetes Endocrinol. 2022, 10, 882–889. [Google Scholar] [CrossRef]
- Hung, C.M.; Zeng, B.Y.; Hsu, C.W.; Chen, P.H.; Sun, C.K.; Carvalho, A.F.; Stubbs, B.; Chen, Y.W.; Chen, T.Y.; Lei, W.T.; et al. The different colorectal tumor risk related to GLP-1 receptor agonists and SGLT2 inhibitors use: A network meta-analysis of 68 randomized controlled trials. Int. J. Surg. 2025. [Google Scholar] [CrossRef]
- Gong, I.Y.; Cheung, M.C.; Read, S.; Na, Y.; Lega, I.C.; Lipscombe, L.L. Association between diabetes and haematological malignancies: A population-based study. Diabetologia 2021, 64, 540–551. [Google Scholar] [CrossRef]
- Wuthikraikun, C.; Panja, P.; Decha-Umphai, C.; Sananpanichkul, N.; Lawsereevanich, P.; Kobkarnsakul, T.; Ngaosuwan, K. Impact of diabetes and glycemic control during cancer care on mortality in non-metastatic solid and hematologic malignancies. Ann. Med. 2025, 57, 2575108. [Google Scholar] [CrossRef]
- Yang, Z.; Lv, Y.; Yu, M.; Mei, M.; Xiang, L.; Zhao, S.; Li, R. GLP-1 receptor agonist-associated tumor adverse events: A real-world study from 2004 to 2021 based on FAERS. Front. Pharmacol. 2022, 13, 925377. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Huang, C.; Ma, K.S. Sodium Glucose Cotransporter-2 Inhibitor Is Associated with Lower Risk of Lymphoid Malignancy: Result from Large Real-World Cohorts. Blood 2023, 142, 379. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Zhong, Q.Z.; Yang, Y.; Wu, T.; Chen, S.Y.; Chen, B.; Song, Y.W.; Fang, H.; Wang, S.L.; et al. Disparities in mortality risk after diagnosis of hematological malignancies in 185 countries: A global data analysis. Cancer Lett. 2024, 595, 216793. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B., Sr.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Wang, S.D. Opportunities and challenges of clinical research in the big-data era: From RCT to BCT. J. Thorac. Dis. 2013, 5, 721–723. [Google Scholar] [CrossRef]
- Zhang, Z. Big data and clinical research: Perspective from a clinician. J. Thorac. Dis. 2014, 6, 1659–1664. [Google Scholar] [CrossRef]
- Higgins, J.P.; Welton, N.J. Network meta-analysis: A norm for comparative effectiveness? Lancet 2015, 386, 628–630. [Google Scholar] [CrossRef]
- Tseng, P.T.; Zeng, B.Y.; Hsu, C.W.; Hung, C.M.; Carvalho, A.F.; Stubbs, B.; Chen, Y.W.; Chen, T.Y.; Lei, W.T.; Chen, J.J.; et al. The pharmacodynamics-based prophylactic benefits of GLP-1 receptor agonists and SGLT2 inhibitors on neurodegenerative diseases: Evidence from a network meta-analysis. BMC Med. 2025, 23, 197. [Google Scholar] [CrossRef]
- Hsu, C.W.; Zeng, B.S.; Liang, C.S.; Zeng, B.Y.; Hung, C.M.; Stubbs, B.; Chen, Y.W.; Lei, W.T.; Chen, J.J.; Chen, P.H.; et al. The Preventive Effects of GLP-1 Receptor Agonists and SGLT2 Inhibitors on Cancer Metastasis: A Network Meta-Analysis of 67 Randomized Controlled Trials. Int. J. Mol. Sci. 2025, 26, 8202. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Hsu, C.W.; Hung, C.M.; Liang, C.S.; Su, K.P.; Carvalho, A.F.; Stubbs, B.; Chen, Y.W.; Chen, T.Y.; Lei, W.T.; et al. Risk of Hearing Loss in Patients Treated with Exendin-4 Derivatives: A Network Meta-Analysis of Glucagon-like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors. Pharmaceuticals 2025, 18, 735. [Google Scholar] [CrossRef] [PubMed]
- Peryer, G.; Golder, S.; Junqueira, D.; Vohra, S.; Loke, Y.K.; on Behalf of the Cochrane Adverse Effects Methods Group. Chapter 19: Adverse effects. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2023. [Google Scholar]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Phillips, R.; Hazell, L.; Sauzet, O.; Cornelius, V. Analysis and reporting of adverse events in randomised controlled trials: A review. BMJ Open 2019, 9, e024537. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2; The Cochrane Collaboration: London, UK, 2009. [Google Scholar]
- Keykhaei, M.; Masinaei, M.; Mohammadi, E.; Azadnajafabad, S.; Rezaei, N.; Saeedi Moghaddam, S.; Rezaei, N.; Nasserinejad, M.; Abbasi-Kangevari, M.; Malekpour, M.R.; et al. A global, regional, and national survey on burden and Quality of Care Index (QCI) of hematologic malignancies; global burden of disease systematic analysis 1990–2017. Exp. Hematol. Oncol. 2021, 10, 11. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Chaimani, A.; Caldwell, D.M.; Li, T.; Higgins, J.P.T.; Salanti, G. Chapter 11: Undertaking network meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2018; Volume 7. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.K.; Bradbury, N.; Xin, Y.; Cooper, N.; Sutton, A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res. Synth. Methods 2019, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Pullenayegum, E.; Marshall, J.K.; Iorio, A.; Thabane, L. Impact of including or excluding both-armed zero-event studies on using standard meta-analysis methods for rare event outcome: A simulation study. BMJ Open 2016, 6, e010983. [Google Scholar] [CrossRef]
- Brockhaus, A.C.; Bender, R.; Skipka, G. The Peto odds ratio viewed as a new effect measure. Stat. Med. 2014, 33, 4861–4874. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M. Converting Among Effect Sizes. Available online: https://www.meta-analysis.com/downloads/Meta-analysis%20Converting%20among%20effect%20sizes.pdf (accessed on 30 December 2024).
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef]
- Nevill, C.R.; Cooper, N.J.; Sutton, A.J. A multifaceted graphical display, including treatment ranking, was developed to aid interpretation of network meta-analysis. J. Clin. Epidemiol. 2023, 157, 83–91. [Google Scholar] [CrossRef]
- Dias, S.; Ades, A.E.; Welton, N.J.; Jansen, J.P.; Sutton, A.J. Chapter 3: Model fit, model comparison and outlier detection. In Network Meta-Anlaysis for Decision-Making; Scott, M., Barnett, V., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2002; pp. 59–92. [Google Scholar]
- Brignardello-Petersen, R.; Izcovich, A.; Rochwerg, B.; Florez, I.D.; Hazlewood, G.; Alhazanni, W.; Yepes-Nunez, J.; Santesso, N.; Guyatt, G.H.; Schunemann, H.J.; et al. GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ 2020, 371, m3907. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Bohm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef]
- Bailey, C.J.; Gross, J.L.; Pieters, A.; Bastien, A.; List, J.F. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 375, 2223–2233. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Rosenstock, J.; Sesti, G.; Schmidt, W.E.; Montanya, E.; Brett, J.H.; Zychma, M.; Blonde, L.; for the LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009, 374, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Ferrannini, E.; Umpierrez, G.E.; Peters, A.L.; Rosenstock, J.; Powell, D.R.; Davies, M.J.; Banks, P.; Agarwal, R. Efficacy and safety of sotagliflozin in patients with type 2 diabetes and stage 3 chronic kidney disease. Diabetes Obes. Metab. 2023, 25, 1646–1657. [Google Scholar] [CrossRef]
- Danne, T.; Cariou, B.; Banks, P.; Brandle, M.; Brath, H.; Franek, E.; Kushner, J.A.; Lapuerta, P.; McGuire, D.K.; Peters, A.L.; et al. HbA(1c) and Hypoglycemia Reductions at 24 and 52 Weeks with Sotagliflozin in Combination with Insulin in Adults with Type 1 Diabetes: The European inTandem2 Study. Diabetes Care 2018, 41, 1981–1990. [Google Scholar] [CrossRef]
- Davies, M.; Faerch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): A randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Berk, A.; Hantel, S.; Pinnetti, S.; Hach, T.; Woerle, H.J.; Broedl, U.C. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: An active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 2013, 36, 4015–4021. [Google Scholar] [CrossRef]
- Frias, J.P.; Davies, M.J.; Rosenstock, J.; Perez Manghi, F.C.; Fernandez Lando, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; for the SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Gallo, S.; Charbonnel, B.; Goldman, A.; Shi, H.; Huyck, S.; Darekar, A.; Lauring, B.; Terra, S.G. Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial. Diabetes Obes. Metab. 2019, 21, 1027–1036. [Google Scholar] [CrossRef]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Ryden, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Sattar, N.; Rosenstock, J.; Ramasundarahettige, C.; Pratley, R.; Lopes, R.D.; Lam, C.S.P.; Khurmi, N.S.; Heenan, L.; Del Prato, S.; et al. Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 896–907. [Google Scholar] [CrossRef]
- Giorgino, F.; Benroubi, M.; Sun, J.H.; Zimmermann, A.G.; Pechtner, V. Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients With Type 2 Diabetes on Metformin and Glimepiride (AWARD-2). Diabetes Care 2015, 38, 2241–2249. [Google Scholar] [CrossRef]
- Grunberger, G.; Camp, S.; Johnson, J.; Huyck, S.; Terra, S.G.; Mancuso, J.P.; Jiang, Z.W.; Golm, G.; Engel, S.S.; Lauring, B. Ertugliflozin in Patients with Stage 3 Chronic Kidney Disease and Type 2 Diabetes Mellitus: The VERTIS RENAL Randomized Study. Diabetes Ther. 2018, 9, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, S.; Rosenstock, J.; Meinicke, T.; Woerle, H.J.; Broedl, U.C. Initial Combination of Empagliflozin and Metformin in Patients With Type 2 Diabetes. Diabetes Care 2016, 39, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Home, P.D.; Ahren, B.; Reusch, J.E.B.; Rendell, M.; Weissman, P.N.; Cirkel, D.T.; Miller, D.; Ambery, P.; Carr, M.C.; Nauck, M.A. Three-year data from 5 HARMONY phase 3 clinical trials of albiglutide in type 2 diabetes mellitus: Long-term efficacy with or without rescue therapy. Diabetes Res. Clin. Pract. 2017, 131, 49–60. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kaku, K.; Yamada, Y.; Watada, H.; Abiko, A.; Nishida, T.; Zacho, J.; Kiyosue, A. Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: A randomized trial. Diabetes Obes. Metab. 2018, 20, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Lock, J.P. Bexagliflozin Efficacy and Safety Trial (BEST). Available online: https://clinicaltrials.gov/study/NCT02558296?cond=NCT02558296&rank=1 (accessed on 28 October 2024).
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jodar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Nauck, M.; Frid, A.; Hermansen, K.; Shah, N.S.; Tankova, T.; Mitha, I.H.; Zdravkovic, M.; During, M.; Matthews, D.R.; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: The LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009, 32, 84–90. [Google Scholar] [CrossRef]
- Nauck, M.; Rizzo, M.; Johnson, A.; Bosch-Traberg, H.; Madsen, J.; Cariou, B. Once-Daily Liraglutide Versus Lixisenatide as Add-on to Metformin in Type 2 Diabetes: A 26-Week Randomized Controlled Clinical Trial. Diabetes Care 2016, 39, 1501–1509. [Google Scholar] [CrossRef]
- Nauck, M.A.; Stewart, M.W.; Perkins, C.; Jones-Leone, A.; Yang, F.; Perry, C.; Reinhardt, R.R.; Rendell, M. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia 2016, 59, 266–274. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Kober, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Ridderstrale, M.; Andersen, K.R.; Zeller, C.; Kim, G.; Woerle, H.J.; Broedl, U.C.; on Behalf of the EMPA-REG H2H-SU Trial Investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: A 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014, 2, 691–700. [Google Scholar] [CrossRef]
- Rosenstock, J.; Allison, D.; Birkenfeld, A.L.; Blicher, T.M.; Deenadayalan, S.; Jacobsen, J.B.; Serusclat, P.; Violante, R.; Watada, H.; Davies, M.; et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults With Type 2 Diabetes Uncontrolled With Metformin Alone or With Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA 2019, 321, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Fonseca, V.A.; Gross, J.L.; Ratner, R.E.; Ahren, B.; Chow, F.C.; Yang, F.; Miller, D.; Johnson, S.L.; Stewart, M.W.; et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: A comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care 2014, 37, 2317–2325. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; The EMPA-KIDNEY Collaborative Group; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Levin, A.; Nangaku, M.; Kadowaki, T.; Agarwal, R.; Hauske, S.J.; Elsasser, A.; Ritter, I.; Steubl, D.; Wanner, C.; et al. Safety of Empagliflozin in Patients With Type 2 Diabetes and Chronic Kidney Disease: Pooled Analysis of Placebo-Controlled Clinical Trials. Diabetes Care 2022, 45, 1445–1452. [Google Scholar] [CrossRef]
- Umpierrez, G.; Tofe Povedano, S.; Perez Manghi, F.; Shurzinske, L.; Pechtner, V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014, 37, 2168–2176. [Google Scholar] [CrossRef]
- Wada, T.; Mori-Anai, K.; Takahashi, A.; Matsui, T.; Inagaki, M.; Iida, M.; Maruyama, K.; Tsuda, H. Effect of canagliflozin on the decline of estimated glomerular filtration rate in chronic kidney disease patients with type 2 diabetes mellitus: A multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase III study in Japan. J. Diabetes Investig. 2022, 13, 1981–1989. [Google Scholar] [CrossRef]
- Wason, S. Efficacy and Safety of Sotagliflozin Versus Placebo in Participants with Type 2 Diabetes Mellitus Who Have Inadequate Glycemic Control While Taking Insulin Alone or with Other Oral Antidiabetic Agents (SOTA-INS). Available online: https://clinicaltrials.gov/study/NCT03285594?cond=NCT03285594&rank=1 (accessed on 28 October 2024).
- Weissman, P.N.; Carr, M.C.; Ye, J.; Cirkel, D.T.; Stewart, M.; Perry, C.; Pratley, R. HARMONY 4: Randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia 2014, 57, 2475–2484. [Google Scholar] [CrossRef]
- Wilding, J.P.; Woo, V.; Soler, N.G.; Pahor, A.; Sugg, J.; Rohwedder, K.; Parikh, S.; for the Dapagliflozin 006 Study Group. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: A randomized trial. Ann. Intern. Med. 2012, 156, 405–415. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Xiao, Y.; Xie, X.; Meng, N.; Qi, Q.; Xu, J.; Jiang, X.; Zhang, Z.; Teng, Z.; Lv, P. Dulaglutide Improves Gliosis and Suppresses Apoptosis/Autophagy Through the PI3K/Akt/mTOR Signaling Pathway in Vascular Dementia Rats. Neurochem. Res. 2023, 48, 1561–1579. [Google Scholar] [CrossRef] [PubMed]
- Vemula, H.; Villanueva, F.S.; Nguyen, H.D.; Mohan, A.; Potluri, S. Medullary Carcinoma of Thyroid Due to GLP-1 Receptor Agonist. J. Endocr. Soc. 2021, 5, A893. [Google Scholar] [CrossRef]
- Tatsch, J.M.; Furman, D.P.; Nobre, R.M.; Wurzer, K.M.; da Silva, L.C.; Picheth, G.F.; Ramos, E.A.; Acco, A.; Klassen, G. Dulaglutide as a demethylating agent to improve the outcome of breast cancer. Epigenomics 2023, 15, 1309–1322. [Google Scholar] [CrossRef]
- Zebardast, S.; Sahmani, M.; Mohammadi, S.; Foroughi, F.; Dehghani Fard, A.; Mohammadi, Z.; Khojastepour, S.; Azad, M. The Gene Expression Profile and DNA Methylation Pattern of CDH1 and DNMT1 Genes in Acute Promyelocytic Leukemia (APL). Rep. Biochem. Mol. Biol. 2020, 8, 454–457. [Google Scholar]
- Ma, Z.; Jin, K.; Yue, M.; Chen, X.; Chen, J. Research Progress on the GIP/GLP-1 Receptor Coagonist Tirzepatide, a Rising Star in Type 2 Diabetes. J. Diabetes Res. 2023, 2023, 5891532. [Google Scholar] [CrossRef]
- Willard, F.S.; Douros, J.D.; Gabe, M.B.; Showalter, A.D.; Wainscott, D.B.; Suter, T.M.; Capozzi, M.E.; van der Velden, W.J.; Stutsman, C.; Cardona, G.R.; et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020, 5, e140532. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Ji, Q.; Li, Q. The role and mechanism of beta-arrestins in cancer invasion and metastasis (Review). Int. J. Mol. Med. 2018, 41, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Deng, B.; Shen, X.; John, C.; Haag, J.; Sinha, N.; Lee, D.; Sun, W.; Chen, S.; Zhang, H.; et al. Tirzepatide as an innovative treatment strategy in a pre-clinical model of obesity-driven endometrial cancer. Gynecol. Oncol. 2024, 191, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Leahy, D.J. Structure of the extracellular region of HER3 reveals an interdomain tether. Science 2002, 297, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [CrossRef]
- Hicks, B.; Kaye, J.A.; Azoulay, L.; Kristensen, K.B.; Habel, L.A.; Pottegard, A. The application of lag times in cancer pharmacoepidemiology: A narrative review. Ann. Epidemiol. 2023, 84, 25–32. [Google Scholar] [CrossRef]

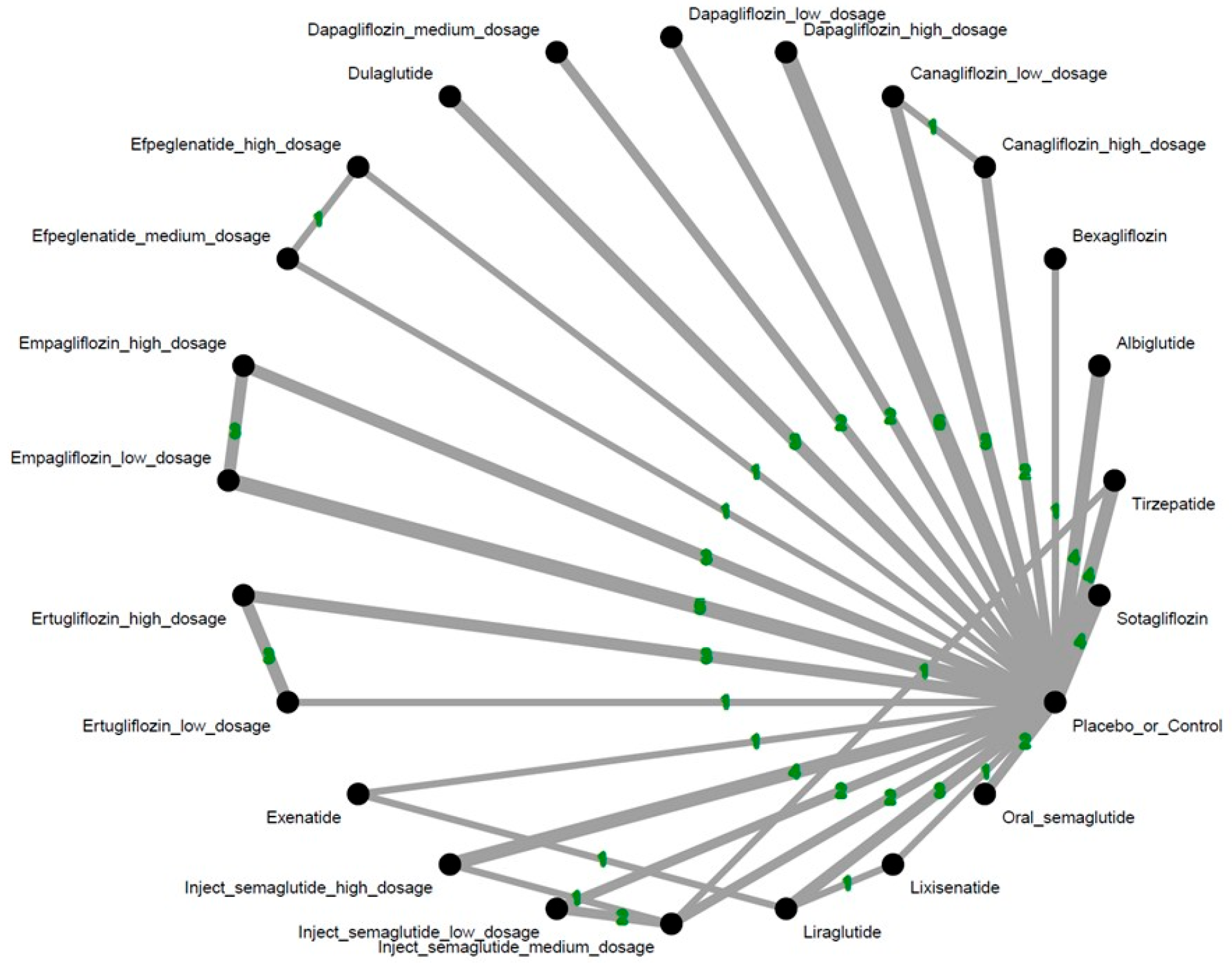

| Tirzepatide | 0.11 [0.00; 2.72] | * 0.19 [0.04; 0.90] | . | . | . | . | . | . | . | . | . | . | . | . | |||||||||
| 0.37 [0.05; 2.85] | Inject_ semaglutide_ low_dosage | 0.95 [0.14; 6.52] | 0.34 [0.07; 1.59] | . | . | . | . | . | . | . | . | . | . | . | . | ||||||||
| 0.33 [0.06; 1.85] | 0.91 [0.16; 5.19] | Inject_ semaglutide_ medium_dosage | 0.41 [0.10; 1.64] | 0.20 [0.01; 4.17] | . | . | . | . | . | . | . | . | . | . | . | ||||||||
| 0.44 [0.03; 6.50] | 1.21 [0.08; 18.32] | 1.33 [0.10; 17.31] | Dapagliflozin_ medium_dosage | 0.32 [0.03; 3.09] | . | . | . | . | . | . | . | . | . | . | . | . | |||||||
| 0.43 [0.03; 6.35] | 1.18 [0.08; 17.88] | 1.30 [0.10; 16.90] | 0.98 [0.04; 24.14] | Dapagliflozin_ low_dosage | 0.33 [0.03; 3.16] | . | . | . | . | . | . | . | . | . | . | . | . | ||||||
| 0.31 [0.05; 2.13] | 0.86 [0.12; 6.08] | 0.94 [0.17; 5.37] | 0.71 [0.05; 9.50] | 0.73 [0.05; 9.73] | Canagliflozin_ high_dosage | 0.75 [0.17; 3.36] | 0.56 [0.14; 2.17] | . | . | . | . | . | . | . | . | . | . | . | . | ||||
| 0.24 [0.04; 1.53] | 0.65 [0.10; 4.39] | 0.72 [0.13; 3.85] | 0.54 [0.04; 6.94] | 0.55 [0.04; 7.11] | 0.76 [0.14; 4.27] | Lixisenatide | 0.33 [0.01; 8.19] | 0.67 [0.19; 2.36] | . | . | . | . | . | . | . | . | . | . | . | . | |||
| 0.20 [0.03; 1.22] | 0.55 [0.09; 3.50] | 0.61 [0.12; 3.05] | 0.46 [0.04; 5.63] | 0.47 [0.04; 5.77] | 0.64 [0.18; 2.35] | 0.85 [0.17; 4.20] | Canagliflozin_ low_dosage | 0.87 [0.28; 2.68] | . | . | . | . | . | . | . | . | . | . | . | . | |||
| 0.19 [0.03; 1.22] | 0.53 [0.08; 3.50] | 0.58 [0.11; 3.07] | 0.44 [0.03; 5.57] | 0.45 [0.04; 5.70] | 0.62 [0.11; 3.40] | 0.81 [0.16; 4.23] | 0.96 [0.20; 4.63] | Empagliflozin_ high_dosage | 0.59 [0.15; 2.28] | . | . | . | . | . | . | . | . | . | 0.37 [0.11; 1.33] | . | . | ||
| * 0.17 [0.04; 0.79] | 0.45 [0.09; 2.28] | 0.50 [0.13; 1.90] | 0.38 [0.04; 3.92] | 0.39 [0.04; 4.01] | 0.53 [0.13; 2.13] | 0.70 [0.19; 2.52] | 0.82 [0.24; 2.81] | 0.86 [0.24; 3.14] | Liraglutide | 0.85 [0.47; 1.56] | . | 0.33 [0.01; 8.08] | . | . | . | . | . | . | . | . | . | . | |
| 0.16 [0.02; 1.16] | 0.44 [0.06; 3.30] | 0.48 [0.08; 2.93] | 0.36 [0.03; 5.09] | 0.37 [0.03; 5.21] | 0.51 [0.08; 3.24] | 0.68 [0.11; 4.05] | 0.80 [0.14; 4.46] | 0.83 [0.14; 4.90] | 0.97 [0.22; 4.19] | Oral_ semaglutide | 0.88 [0.23; 3.36] | . | . | . | . | . | . | . | . | . | . | . | . |
| * 0.14 [0.03; 0.60] | 0.39 [0.09; 1.73] | 0.42 [0.13; 1.41] | 0.32 [0.03; 3.09] | 0.33 [0.03; 3.16] | 0.45 [0.13; 1.59] | 0.59 [0.18; 1.93] | 0.70 [0.24; 2.05] | 0.73 [0.23; 2.32] | 0.85 [0.47; 1.52] | 0.88 [0.23; 3.36] | Placebo_ or_Control | 0.99 [0.56; 1.75] | 0.95 [0.53; 1.71] | 0.83 [0.25; 2.73] | 0.87 [0.58; 1.30] | 0.66 [0.03; 16.34] | 0.81 [0.28; 2.35] | 0.62 [0.21; 1.88] | 0.33 [0.01; 8.20] | 0.61 [0.22; 1.70] | * 0.52 [0.28; 0.99] | 0.20 [0.01; 4.18] | * 0.46 [0.24; 0.88] |
| * 0.14 [0.03; 0.63] | 0.37 [0.07; 1.83] | 0.41 [0.11; 1.48] | 0.31 [0.03; 3.18] | 0.31 [0.03; 3.26] | 0.43 [0.11; 1.72] | 0.57 [0.15; 2.11] | 0.67 [0.20; 2.27] | 0.70 [0.19; 2.54] | 0.82 [0.36; 1.84] | 0.84 [0.20; 3.63] | 0.96 [0.55; 1.69] | Inject_ semaglutide_ high_dosage | . | . | . | . | . | . | . | . | . | . | . |
| * 0.13 [0.03; 0.62] | 0.36 [0.07; 1.78] | 0.39 [0.10; 1.49] | 0.30 [0.03; 3.07] | 0.30 [0.03; 3.14] | 0.42 [0.10; 1.67] | 0.55 [0.15; 2.04] | 0.65 [0.19; 2.20] | 0.68 [0.19; 2.46] | 0.79 [0.35; 1.76] | 0.81 [0.19; 3.50] | 0.93 [0.52; 1.65] | 0.96 [0.43; 2.16] | Exenatide | . | . | . | . | . | . | . | . | . | . |
| * 0.12 [0.02; 0.73] | 0.32 [0.05; 2.10] | 0.36 [0.07; 1.83] | 0.27 [0.02; 3.36] | 0.28 [0.02; 3.44] | 0.38 [0.07; 2.03] | 0.50 [0.10; 2.53] | 0.59 [0.12; 2.77] | 0.61 [0.12; 3.06] | 0.71 [0.20; 2.51] | 0.74 [0.13; 4.22] | 0.84 [0.28; 2.56] | 0.87 [0.25; 3.05] | 0.91 [0.26; 3.18] | Ertugliflozin_ low_dosage | . | . | . | . | . | 0.71 [0.27; 1.91] | . | . | . |
| * 0.12 [0.03; 0.55] | 0.34 [0.07; 1.59] | 0.37 [0.10; 1.31] | 0.28 [0.03; 2.78] | 0.28 [0.03; 2.85] | 0.39 [0.10; 1.47] | 0.52 [0.15; 1.80] | 0.61 [0.19; 1.92] | 0.64 [0.19; 2.16] | 0.74 [0.36; 1.50] | 0.76 [0.19; 3.10] | 0.87 [0.58; 1.30] | 0.90 [0.45; 1.81] | 0.94 [0.47; 1.90] | 1.04 [0.32; 3.38] | Dapagliflozin_ high_dosage | . | . | . | . | . | . | . | . |
| 0.09 [0.00; 3.15] | 0.26 [0.01; 8.80] | 0.28 [0.01; 8.63] | 0.21 [0.00; 10.74] | 0.22 [0.00; 11.01] | 0.30 [0.01; 9.35] | 0.39 [0.01; 11.97] | 0.46 [0.02; 13.62] | 0.49 [0.02; 14.62] | 0.56 [0.02; 14.63] | 0.58 [0.02; 18.78] | 0.66 [0.03; 16.34] | 0.69 [0.03; 17.85] | 0.72 [0.03; 18.59] | 0.79 [0.03; 23.46] | 0.76 [0.03; 19.25] | Bexagliflozin | . | . | . | . | . | . | . |
| * 0.12 [0.02; 0.69] | 0.31 [0.05; 1.97] | 0.35 [0.07; 1.71] | 0.26 [0.02; 3.18] | 0.27 [0.02; 3.26] | 0.37 [0.07; 1.90] | 0.48 [0.10; 2.36] | 0.57 [0.13; 2.58] | 0.59 [0.12; 2.85] | 0.69 [0.21; 2.32] | 0.71 [0.13; 3.95] | 0.81 [0.28; 2.35] | 0.85 [0.25; 2.81] | 0.88 [0.26; 2.94] | 0.97 [0.21; 4.50] | 0.93 [0.30; 2.91] | 1.22 [0.04; 35.71] | Sotagliflozin | . | . | . | . | . | . |
| * 0.09 [0.01; 0.54] | 0.24 [0.04; 1.55] | 0.27 [0.05; 1.35] | 0.20 [0.02; 2.48] | 0.20 [0.02; 2.54] | 0.28 [0.05; 1.50] | 0.37 [0.07; 1.86] | 0.44 [0.09; 2.04] | 0.46 [0.09; 2.25] | 0.53 [0.15; 1.85] | 0.55 [0.10; 3.11] | 0.62 [0.21; 1.88] | 0.65 [0.19; 2.24] | 0.67 [0.19; 2.34] | 0.74 [0.15; 3.56] | 0.72 [0.22; 2.32] | 0.94 [0.03; 27.76] | 0.77 [0.17; 3.55] | Albiglutide | . | . | . | . | . |
| 0.05 [0.00; 1.58] | 0.13 [0.00; 4.42] | 0.14 [0.00; 4.33] | 0.11 [0.00; 5.39] | 0.11 [0.00; 5.52] | 0.15 [0.00; 4.69] | 0.20 [0.01; 6.01] | 0.23 [0.01; 6.84] | 0.24 [0.01; 7.34] | 0.28 [0.01; 7.34] | 0.29 [0.01; 9.43] | 0.33 [0.01; 8.20] | 0.35 [0.01; 8.96] | 0.36 [0.01; 9.33] | 0.40 [0.01; 11.77] | 0.38 [0.02; 9.66] | 0.50 [0.01; 46.50] | 0.41 [0.01; 11.97] | 0.53 [0.02; 15.80] | Efpeglenatide_ high_dosage | . | . | 0.60 [0.08; 4.55] | . |
| * 0.08 [0.01; 0.47] | 0.22 [0.04; 1.35] | 0.24 [0.05; 1.17] | 0.18 [0.02; 2.19] | 0.19 [0.02; 2.24] | 0.26 [0.05; 1.30] | 0.34 [0.07; 1.61] | 0.40 [0.09; 1.75] | 0.42 [0.09; 1.94] | 0.49 [0.15; 1.56] | 0.50 [0.09; 2.70] | 0.57 [0.21; 1.57] | 0.60 [0.19; 1.89] | 0.62 [0.19; 1.98] | 0.68 [0.26; 1.79] | 0.66 [0.22; 1.95] | 0.86 [0.03; 24.76] | 0.70 [0.16; 3.05] | 0.92 [0.21; 4.09] | 1.72 [0.06; 49.25] | Ertugliflozin_ high_dosage | . | . | . |
| * 0.08 [0.02; 0.36] | 0.21 [0.04; 1.05] | * 0.23 [0.06; 0.88] | 0.17 [0.02; 1.79] | 0.17 [0.02; 1.84] | * 0.24 [0.06; 0.98] | 0.32 [0.08; 1.20] | 0.37 [0.11; 1.30] | 0.39 [0.13; 1.19] | 0.45 [0.19; 1.07] | 0.47 [0.11; 2.06] | 0.53 [0.29; 1.00] | 0.55 [0.24; 1.29] | 0.58 [0.25; 1.35] | 0.64 [0.18; 2.28] | 0.61 [0.29; 1.29] | 0.80 [0.03; 20.98] | 0.66 [0.19; 2.25] | 0.86 [0.24; 3.04] | 1.60 [0.06; 41.73] | 0.93 [0.28; 3.05] | Empagliflozin_ low_dosage | . | . |
| * 0.03 [0.00; 0.82] | 0.08 [0.00; 2.29] | 0.09 [0.00; 2.23] | 0.06 [0.00; 2.84] | 0.07 [0.00; 2.90] | 0.09 [0.00; 2.42] | 0.12 [0.00; 3.09] | 0.14 [0.01; 3.52] | 0.15 [0.01; 3.78] | 0.17 [0.01; 3.75] | 0.18 [0.01; 4.87] | 0.20 [0.01; 4.18] | 0.21 [0.01; 4.58] | 0.22 [0.01; 4.77] | 0.24 [0.01; 6.06] | 0.23 [0.01; 4.93] | 0.30 [0.00; 24.90] | 0.25 [0.01; 6.15] | 0.32 [0.01; 8.13] | 0.60 [0.08; 4.55] | 0.35 [0.01; 8.59] | 0.38 [0.02; 8.34] | Efpeglenatide_ medium_dosage | . |
| * 0.07 [0.01; 0.31] | * 0.18 [0.03; 0.91] | * 0.19 [0.05; 0.76] | 0.15 [0.01; 1.55] | 0.15 [0.01; 1.59] | * 0.21 [0.05; 0.85] | 0.27 [0.07; 1.05] | 0.32 [0.09; 1.13] | 0.34 [0.09; 1.26] | * 0.39 [0.16; 0.94] | 0.40 [0.09; 1.79] | * 0.46 [0.24; 0.88] | 0.48 [0.20; 1.13] | 0.50 [0.21; 1.18] | 0.55 [0.15; 1.98] | 0.53 [0.25; 1.13] | 0.69 [0.03; 18.12] | 0.56 [0.16; 1.96] | 0.73 [0.20; 2.65] | 1.37 [0.05; 36.05] | 0.80 [0.24; 2.66] | 0.86 [0.35; 2.12] | 2.29 [0.10; 51.12] | Dulaglutide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.-Y.; Zeng, B.-Y.; Hsu, C.-W.; Suen, M.-W.; Hung, C.-M.; Stubbs, B.; Chen, Y.-W.; Chen, T.-Y.; Lei, W.-T.; Chen, J.-J.; et al. Divergent Risks of Hematologic Malignancies Associated with GLP-1 Receptor Agonists and SGLT2 Inhibitors: Preliminary Findings from a Pilot Network Meta-Analysis. Biomolecules 2025, 15, 1622. https://doi.org/10.3390/biom15111622
Lin P-Y, Zeng B-Y, Hsu C-W, Suen M-W, Hung C-M, Stubbs B, Chen Y-W, Chen T-Y, Lei W-T, Chen J-J, et al. Divergent Risks of Hematologic Malignancies Associated with GLP-1 Receptor Agonists and SGLT2 Inhibitors: Preliminary Findings from a Pilot Network Meta-Analysis. Biomolecules. 2025; 15(11):1622. https://doi.org/10.3390/biom15111622
Chicago/Turabian StyleLin, Pao-Yen, Bing-Yan Zeng, Chih-Wei Hsu, Mein-Woei Suen, Chao-Ming Hung, Brendon Stubbs, Yen-Wen Chen, Tien-Yu Chen, Wei-Te Lei, Jiann-Jy Chen, and et al. 2025. "Divergent Risks of Hematologic Malignancies Associated with GLP-1 Receptor Agonists and SGLT2 Inhibitors: Preliminary Findings from a Pilot Network Meta-Analysis" Biomolecules 15, no. 11: 1622. https://doi.org/10.3390/biom15111622
APA StyleLin, P.-Y., Zeng, B.-Y., Hsu, C.-W., Suen, M.-W., Hung, C.-M., Stubbs, B., Chen, Y.-W., Chen, T.-Y., Lei, W.-T., Chen, J.-J., Zeng, B.-S., Su, K.-P., Liang, C.-S., & Tseng, P.-T. (2025). Divergent Risks of Hematologic Malignancies Associated with GLP-1 Receptor Agonists and SGLT2 Inhibitors: Preliminary Findings from a Pilot Network Meta-Analysis. Biomolecules, 15(11), 1622. https://doi.org/10.3390/biom15111622








