Alternative Splicing Dysregulation in Retinitis Pigmentosa: Pathogenic Mechanisms and Therapeutic Opportunities
Abstract
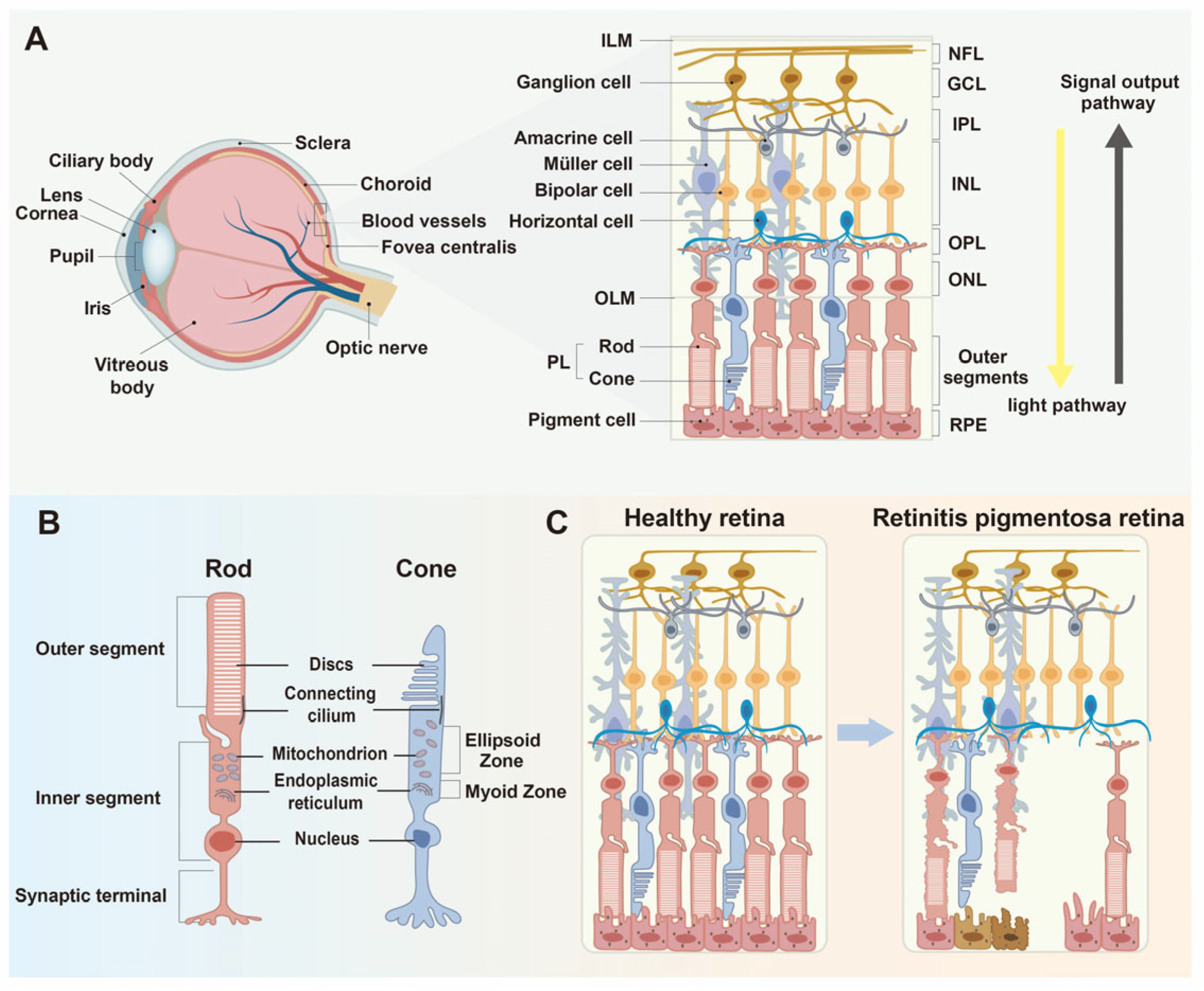
1. The Pathogenesis of Retinitis Pigmentosa
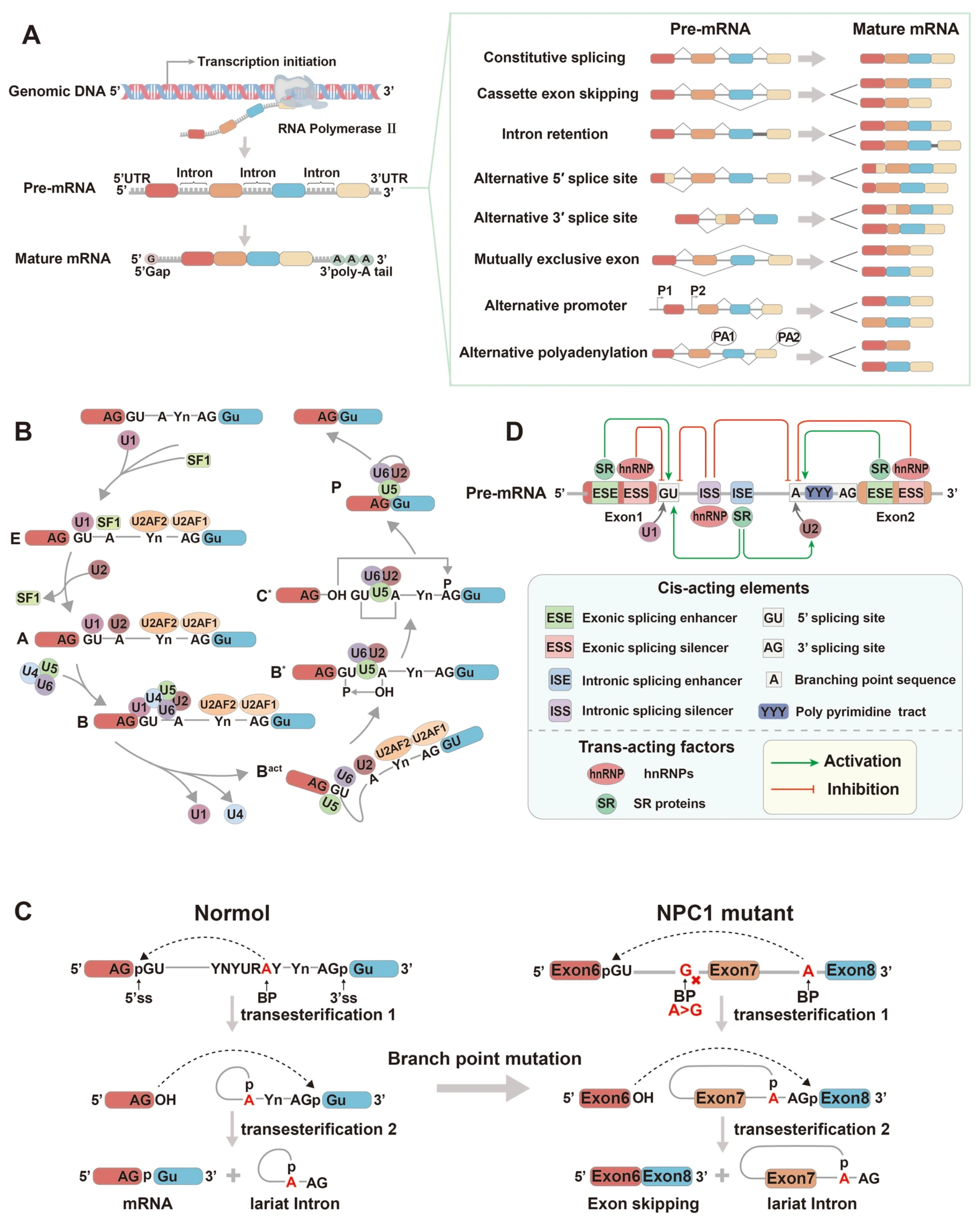
2. The Mechanism of Alternative Splicing
3. The Mechanisms of Alternative Splicing in Retinitis Pigmentosa
3.1. Splicing Factors in the Mechanisms of Retinitis Pigmentosa
3.1.1. U4 snRNP Specific Protein-PRPF31
3.1.2. U4/U6 snRNP Specific Proteins-PRPF3 and PRPF4
3.1.3. U5 snRNP Specific Proteins—PRPF8, PRPF6, and SNRNP200
3.1.4. Non-snRNP Splicing Regulatory Factors: PAP-1 (RP9), DHX38, and CWC27
3.2. Disease Modeling Using iPSC-Derived Retinal Pigment Epithelium and Organoids
3.3. The Mechanism of Cis-Regulatory Element Mutations in Retinitis Pigmentosa
4. The Clinical Significance and Therapeutic Implications of Alternative Splicing in Retinitis Pigmentosa: Exploring Novel Treatment Strategies
4.1. Gene Supplementation Therapy
4.2. Antisense Oligonucleotide (ASO) Therapy
4.3. U1 snRNA Engineering
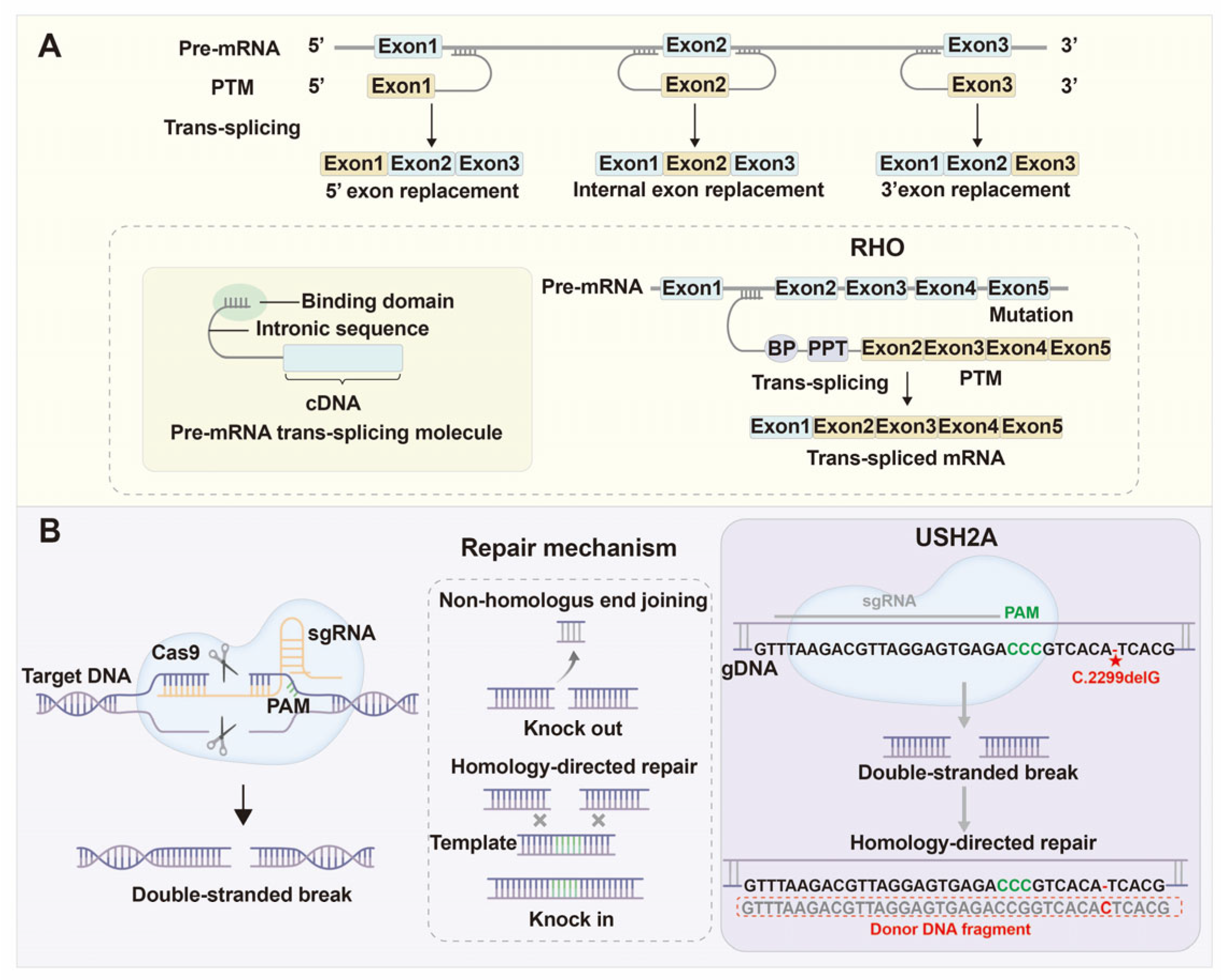
4.4. Spliceosome-Mediated RNA Trans-Splicing (SMaRT)
4.5. Genome Editing Technologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| adRP | Autosomal Dominant Retinitis Pigmentosa. |
| arRP | Autosomal Recessive Retinitis Pigmentosa. |
| ASOs | Antisense Oligonucleotides. |
| AAV | Adeno-Associated Virus. |
| ABCA4 | ATP-binding cassette transporter A4. |
| ABE | adenine base editor. |
| BPS | Branch point sequence. |
| Brr2 | Bad Response to Refrigeration 2 (also known as SNRNP200). |
| BBS2 | Bardet–Biedl syndrome 2. |
| PSC | Posterior Subcapsular Cataract. |
| CME | Cystoid Macular Edema. |
| CNOT3 | CCR4-NOT transcription complex subunit 3. |
| CCR4-NOT | carbon catabolite repression 4-negative on TATA-less. |
| CRISPR/Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-Associated Protein 9. |
| CWC22 | complexed with CEF1 protein 22. |
| CWC27 | complexed with CEF1 protein 27. |
| CLRN1 | Clarin 1. |
| CEP290 | centrosomal protein 290. |
| CBE | cytosine base editor. |
| DExD/H-box | aspartate-glutamate-X-aspartate/histidine box. |
| DHX38 | DEAH-box helicase 38. |
| di-snRNP | di-small nuclear ribonucleoprotein. |
| ESEs | exonic splicing enhancers. |
| ESSs | exonic splicing silencers. |
| eQTL | expression quantitative trait locus. |
| EFTUD2 | elongation factor Tu GTP-binding domain containing 2. |
| eIF4A3 | eukaryotic initiation factor 4A3. |
| EJC | exon junction complex. |
| ER | endoplasmic reticulum. |
| ERG | electroretinography. |
| eDCas9 | enhanced dead Cas9. |
| eSpCas9 | enhanced specificity Cas9. |
| FSCN2 | fascin actin-bundling protein 2. |
| FDA | Food and Drug Administration. |
| GCL | Ganglion cell layer. |
| GNAT1 | guanine nucleotide-binding protein alpha transducing 1. |
| GNAT2 | guanine nucleotide-binding protein alpha transducing 2. |
| GPKOW | G-patch domain and KOW motifs. |
| GEF | guanine nucleotide exchange factor. |
| hnRNPs | heterogeneous nuclear ribonucleoprotein particles. |
| ROs | retinal organoids. |
| HSP70 | heat shock protein 70. |
| hSnu114 | human small nuclear U114 (alternative name for EFTUD2). |
| HBB | hemoglobin subunit beta. |
| HDR | homology-directed repair. |
| IPL | Inner plexiform layer. |
| INL | Inner nuclear layer. |
| ISSs | intronic splicing silencers. |
| IFT88 | intraflagellar transport protein 88. |
| IFT122 | intraflagellar transport protein 122. |
| iPSC | induced pluripotent stem cell. |
| Jab1/MPN | Jun activation domain-binding protein 1/Mpr1-Pad1 N-terminal. |
| LSm | like-Sm proteins. |
| LCA | Leber congenital amaurosis. |
| mRNA | messenger RNA. |
| MSR1 | macrophage scavenger receptor 1. |
| MOs | morpholino oligonucleotides. |
| NHEJ | non-homologous end joining. |
| OPL | Outer plexiform layer. |
| ONL | Outer nuclear layer. |
| OMCS | Oliver–McFarlane Syndrome. |
| PHARC | Polyneuropathy, Hearing loss, Ataxia, Retinitis pigmentosa, and Cataract syndrome. |
| PCARP | Photoreceptor Ciliopathy And Retinal Pigmentation. |
| PPT | Polypyrimidine tract. |
| pre-mRNA | precursor messenger RNA. |
| PTBP1 | polypyrimidine tract-binding protein 1. |
| PRPF3 | pre-mRNA processing factor 3. |
| PRPF4 | pre-mRNA processing factor 4. |
| PRPF6 | pre-mRNA processing factor 6. |
| PRPF8 | pre-mRNA processing factor 8. |
| PRPF31 | pre-mRNA processing factor 31. |
| PAP-1 | polyadenylation specificity factor 1. |
| PPIase | peptidyl-prolyl cis-trans isomerase. |
| PDE6B | Phosphodiesterase 6B. |
| PTMs | pre-mRNA trans-splicing molecules. |
| RPE | Retinal pigment epithelium. |
| RP | Retinitis Pigmentosa. |
| RBM20 | RNA-binding motif protein 20. |
| RPGR | retinitis pigmentosa GTPase regulator. |
| RPCs | retinal progenitor cells. |
| RHO | rhodopsin. |
| RPGRIP1 | retinitis pigmentosa GTPase regulator interacting protein 1. |
| RPE65 | Retinal Pigment Epithelium 65. |
| rAAV | recombinant adeno-associated virus. |
| RAB37 | Ras-related protein 37. |
| REVeRT | mRNA trans-splicing recombinant technology. |
| PPIH | peptidyl prolyl isomerase H. |
| Sm | Smith antigens. |
| snRNA | small nuclear RNA. |
| snRNPs | small nuclear ribonucleoprotein complexes. |
| SF1 | Splicing Factor 1. |
| SR | serine/arginine-rich. |
| SNRNP200 | small nuclear ribonucleoprotein 200. |
| siRNA | small interfering RNA. |
| SC35 | serine/arginine-rich splicing factor 2. |
| SaCas9 | Staphylococcus aureus Cas9. |
| sgRNA | single guide RNA. |
| SMaRT | spliceosome-mediated RNA trans-splicing. |
| tri-snRNP | U4/U6.U5 tri-snRNP complex. |
| TPR | tetratricopeptide repeat. |
| TTLL3 | tubulin tyrosine ligase like 3. |
| U snRNAs | uridine-rich small nuclear RNAs. |
| U2AF1 | U2 Auxiliary Factor 35 kDa subunit. |
| U2AF2 | U2 Auxiliary Factor 65 kDa subunit. |
| ULK1 | unc-51 like autophagy activating kinase 1. |
| USH2A | Usherin 2A. |
| U1_asRNA | U1 antisense small nuclear RNA. |
| WD40 | WD repeat domain 40. |
| XLRP | X-Linked Retinitis Pigmentosa. |
| 5′ ss | 5′ splice site. |
| 3′ ss | 3′ splice site. |
References
- Tomkins-Netzer, O.; Niederer, R.; Greenwood, J.; Fabian, I.D.; Serlin, Y.; Friedman, A.; Lightman, S. Mechanisms of blood-retinal barrier disruption related to intraocular inflammation and malignancy. Prog. Retin. Eye Res. 2024, 99, 101245. [Google Scholar] [CrossRef]
- Diacou, R.; Nandigrami, P.; Fiser, A.; Liu, W.; Ashery-Padan, R.; Cvekl, A. Cell fate decisions, transcription factors and signaling during early retinal development. Prog. Retin. Eye Res. 2022, 91, 101093. [Google Scholar] [CrossRef]
- Seo, H.; Chung, W.G.; Kwon, Y.W.; Kim, S.; Hong, Y.-M.; Park, W.; Kim, E.; Lee, J.; Lee, S.; Kim, M.; et al. Smart Contact Lenses as Wearable Ophthalmic Devices for Disease Monitoring and Health Management. Chem. Rev. 2023, 123, 11488–11558. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, S.; Ha, T.; Kim, S.; Lim, S.; You, H.; Kim, J.W. Retinoid Metabolism in the Degeneration of Pten-Deficient Mouse Retinal Pigment Epithelium. Mol. Cells 2021, 44, 613–622. [Google Scholar] [CrossRef]
- Barret, D.C.A.; Kaupp, U.B.; Marino, J. The structure of cyclic nucleotide-gated channels in rod and cone photoreceptors. Trends Neurosci. 2022, 45, 763–776. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, N.K. Inflammation and retinal degenerative diseases. Neural Regen. Res. 2023, 18, 513–518. [Google Scholar] [CrossRef]
- Patrizi, C.; Llado, M.; Benati, D.; Iodice, C.; Marrocco, E.; Guarascio, R.; Surace, E.M.; Cheetham, M.E.; Auricchio, A.; Recchia, A. Allele-specific editing ameliorates dominant retinitis pigmentosa in a transgenic mouse model. Am. J. Hum. Genet. 2021, 108, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Cepko, C.L. Gene Therapies for Retinitis Pigmentosa that Target Glucose Metabolism. Cold Spring Harb. Perspect. Med. 2024, 14, a041289. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, L.; Jiao, X.; Riazuddin, S.; Riazuddin, S.A.; Fielding Hetmancik, J. A novel LRAT mutation affecting splicing in a family with early onset retinitis pigmentosa. Hum. Genom. 2018, 12, 35. [Google Scholar] [CrossRef]
- Georgiou, M.; Shakarchi, A.F.; Elhusseiny, A.M.; Michaelides, M.; Sallam, A.B. Cataract Surgery Outcomes in Retinitis Pigmentosa A Comparative Clinical Database Study. Am. J. Ophthalmol. 2024, 262, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kuppuraj, R.L.; Srividya, N.; Mathangi, S.; Pandian, A.J.; Adithya, V.; Rajiv, R. Phenotypic heterogeneity in family members of patients with retinitis pigmentosa. Indian. J. Ophthalmol. 2023, 71, 2504–2511. [Google Scholar] [CrossRef]
- Donato, L.; Abdalla, E.M.; Scimone, C.; Alibrandi, S.; Rinaldi, C.; Nabil, K.M.; D’Angelo, R.; Sidoti, A. Impairments of Photoreceptor Outer Segments Renewal and Phototransduction Due to a Peripherin Rare Haplotype Variant: Insights from Molecular Modeling. Int. J. Mol. Sci. 2021, 22, 3484. [Google Scholar] [CrossRef]
- Ellingford, J.M.; Barton, S.; Bhaskar, S.; O’Sullivan, J.; Williams, S.G.; Lamb, J.A.; Panda, B.; Sergouniotis, P.I.; Gillespie, R.L.; Daiger, S.P.; et al. Molecular findings from 537 individuals with inherited retinal disease. J Med Genet 2016, 53, 761–767. [Google Scholar] [CrossRef]
- Kamde, S.P.; Anjankar, A. Retinitis Pigmentosa: Pathogenesis, Diagnostic Findings, and Treatment. Cureus 2023, 15, e48006. [Google Scholar] [CrossRef] [PubMed]
- Wawrocka, A.; Walczak-Sztulpa, J.; Kuszel, L.; Niedziela-Schwartz, Z.; Skorczyk-Werner, A.; Bernardczyk-Meller, J.; Krawczynski, M.R. Coexistence of Retinitis Pigmentosa and Ataxia in Patients with PHARC, PCARP, and Oliver-McFarlane Syndromes. Int. J. Mol. Sci. 2024, 25, 5759. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Abdalla, E.M.; Nabil, K.M.; D’Angelo, R.; Sidoti, A. New Omics-Derived Perspectives on Retinal Dystrophies: Could Ion Channels-Encoding or Related Genes Act as Modifier of Pathological Phenotype? Int. J. Mol. Sci. 2020, 22, 70. [Google Scholar] [CrossRef]
- Gao, Y.; Lin, K.-T.; Jiang, T.; Yang, Y.; Rahman, M.A.; Gong, S.; Bai, J.; Wang, L.; Sun, J.; Sheng, L.; et al. Systematic characterization of short intronic splicing-regulatory elements in SMN2 pre-mRNA. Nucleic Acids Res. 2022, 50, 731–749. [Google Scholar] [CrossRef]
- Hu, H.; Tang, J.; Wang, H.; Guo, X.; Tu, C.; Li, Z. The crosstalk between alternative splicing and circular RNA in cancer: Pathogenic insights and therapeutic implications. Cell. Mol. Biol. Lett. 2024, 29, 142. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Q.; Wang, H.; Yang, X.; Mu, H. Alternative splicing and related RNA binding proteins in human health and disease. Signal Transduct. Target. Ther. 2024, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Zhang, B.H.; Lu, Y.C.; Li, Z.Q.; Chen, C.Y.; Yang, Y.C.; Chen, Y.J.; Ma, D. A novel 16-gene alternative mRNA splicing signature predicts tumor relapse and indicates immune activity in stage I-III hepatocellular carcinoma. Front. Pharmacol. 2022, 13, 939912. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Wan, R.; Wang, L.; Xu, K.; Zhang, Q.; Lei, J.; Shi, Y. Structure of the activated human minor spliceosome. Science 2021, 371, eabg0879. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Parada, G.E.; Wong, H.Y.; Medhi, R.; Furlan, G.; Munita, R.; Miska, E.A.; Kwok, C.K.; Hemberg, M. Alternative splicing modulation by G-quadruplexes. Nat. Commun. 2022, 13, 2404. [Google Scholar] [CrossRef]
- Olthof, A.M.; White, A.K.; Mieruszynski, S.; Doggett, K.; Lee, M.F.; Chakroun, A.; Abdel Aleem, A.K.; Rousseau, J.; Magnani, C.; Roifman, C.M.; et al. Disruption of exon-bridging interactions between the minor and major spliceosomes results in alternative splicing around minor introns. Nucleic Acids Res. 2021, 49, 3524–3545. [Google Scholar] [CrossRef]
- Wilkinson, M.E.; Charenton, C.; Nagai, K. RNA Splicing by the Spliceosome. Annu. Rev. Biochem. 2020, 89, 359–388. [Google Scholar] [CrossRef]
- Beusch, I.; Madhani, H.D. Understanding the dynamic design of the spliceosome. Trends Biochem. Sci. 2024, 49, 583–595. [Google Scholar] [CrossRef]
- Martínez-Lumbreras, S.; Morguet, C.; Sattler, M. Dynamic interactions drive early spliceosome assembly. Curr. Opin. Struct. Biol. 2024, 88, 102907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Zhan, X.; Bai, R.; Lei, J.; Yan, C.; Shi, Y. Structural insights into human exon-defined spliceosome prior to activation. Cell Res. 2024, 34, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Lu, Y.; Shi, Y. Molecular basis for the activation of human spliceosome. Nat. Commun. 2024, 15, 6348. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, Y.; Zhang, X.; Yan, C.; Shi, Y. Mechanism of exon ligation by human spliceosome. Mol. Cell 2022, 82, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Klimešová, K.; Vojáčková, J.; Radivojević, N.; Vandermoere, F.; Bertrand, E.; Verheggen, C.; Staněk, D. TSSC4 is a component of U5 snRNP that promotes tri-snRNP formation. Nat. Commun. 2021, 12, 3646. [Google Scholar] [CrossRef]
- Tang, Q.; Liao, D.; Zheng, B. DBR1 orchestrates the fate of lariat RNA: Debranching-dependent turnover and function. Nucleic Acids Res. 2025, 53, gkaf639. [Google Scholar] [CrossRef]
- Di Leo, E.; Panico, F.; Tarugi, P.; Battisti, C.; Federico, A.; Calandra, S. A point mutation in the lariat branch point of intron 6 of NPC1 as the cause of abnormal pre-mRNA splicing in Niemann-Pick type C disease. Hum. Mutat. 2004, 24, 440. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X. Aberrant alternative splicing in cancer: Splicing events and their regulatory mechanisms (Review). Int. J. Oncol. 2024, 65, 90. [Google Scholar] [CrossRef]
- Sarkar, A.; Panati, K.; Narala, V.R. Code inside the codon: The role of synonymous mutations in regulating splicing machinery and its impact on disease. Mutat. Res. Rev. Mutat. Res. 2022, 790, 108444. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, Q.; Zhang, J. SFRS8 Regulates Memory by Modulating RNA Splicing of Synaptic Genes. Mol. Neurobiol. 2025, 62, 12523–12538. [Google Scholar] [CrossRef]
- Jones, A.N.; Graß, C.; Meininger, I.; Geerlof, A.; Klostermann, M.; Zarnack, K.; Krappmann, D.; Sattler, M. Modulation of pre-mRNA structure by hnRNP proteins regulates alternative splicing of MALT1. Sci. Adv. 2022, 8, eabp9153. [Google Scholar] [CrossRef]
- Fochi, S.; Lorenzi, P.; Galasso, M.; Stefani, C.; Trabetti, E.; Zipeto, D.; Romanelli, M.G. The Emerging Role of the RBM20 and PTBP1 Ribonucleoproteins in Heart Development and Cardiovascular Diseases. Genes 2020, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Beutler, B.; Zhang, D. Emerging roles of spliceosome in cancer and immunity. Protein Cell 2022, 13, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Pan, J.-Q.; Wan, J.-F.; Ren, C.-Y.; Xu, Z.-H.; Pan, X.-B.; Gao, R.-N.; Liu, S.-Q.; Zhang, J.-L.; Yao, Q.-H.; et al. A novel missense mutation of RPGR identified from retinitis pigmentosa affects splicing of the ORF15 region and causes loss of transcript heterogeneity. Biochem. Biophys. Res. Commun. 2020, 531, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Cong, P.-K.; He, T.; Yu, X.-F.; Huo, Y.-N. A novel pathogenic splicing mutation of RPGR in a Chinese family with X-linked retinitis pigmentosa verified by minigene splicing assay. Int. J. Ophthalmol. 2023, 16, 1595–1600. [Google Scholar] [CrossRef]
- Atkinson, R.; Georgiou, M.; Yang, C.; Szymanska, K.; Lahat, A.; Vasconcelos, E.J.R.; Ji, Y.; Moya Molina, M.; Collin, J.; Queen, R.; et al. PRPF8-mediated dysregulation of hBrr2 helicase disrupts human spliceosome kinetics and 5′-splice-site selection causing tissue-specific defects. Nat. Commun. 2024, 15, 3138. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; McClements, M.E.; Whitfield, J.; Shanks, M.; Clouston, P.; MacLaren, R.E. Association of a Novel Intronic Variant in RPGR with Hypomorphic Phenotype of X-Linked Retinitis Pigmentosa. JAMA Ophthalmol. 2020, 138, 1151–1158. [Google Scholar] [CrossRef]
- Nguyen, T.H.D.; Galej, W.P.; Bai, X.-c.; Savva, C.G.; Newman, A.J.; Scheres, S.H.W.; Nagai, K. The architecture of the spliceosomal U4/U6.U5 tri-snRNP. Nature 2015, 523, 47–52. [Google Scholar] [CrossRef]
- Yang, C.; Georgiou, M.; Atkinson, R.; Collin, J.; Al-Aama, J.; Nagaraja-Grellscheid, S.; Johnson, C.; Ali, R.; Armstrong, L.; Mozaffari-Jovin, S.; et al. Pre-mRNA Processing Factors and Retinitis Pigmentosa: RNA Splicing and Beyond. Front. Cell Dev. Biol. 2021, 9, 700276. [Google Scholar] [CrossRef]
- Fritze, J.S.; Stiehler, F.F.; Wolfrum, U. Pathogenic Variants in USH1G/SANS Alter Protein Interaction with Pre-RNA Processing Factors PRPF6 and PRPF31 of the Spliceosome. Int. J. Mol. Sci. 2023, 24, 17608. [Google Scholar] [CrossRef] [PubMed]
- Makarova, O.V.; Makarov, E.M.; Liu, S.; Vornlocher, H.-P.; Lührmann, R. Protein 61K, encoded by a gene (PRPF31) linked to autosomal dominant retinitis pigmentosa, is required for U4/U6*U5 tri-snRNP formation and pre-mRNA splicing. EMBO J. 2002, 21, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Vithana, E.N.; Abu-Safieh, L.; Allen, M.J.; Carey, A.; Papaioannou, M.; Chakarova, C.; Al-Maghtheh, M.; Ebenezer, N.D.; Willis, C.; Moore, A.T.; et al. A human homolog of yeast pre-mRNA splicing gene, PRP31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13.4 (RP11). Mol. Cell 2001, 8, 375–381. [Google Scholar] [CrossRef]
- Lisbjerg, K.; Grønskov, K.; Bertelsen, M.; Møller, L.B.; Kessel, L. Genetic Modifiers of Non-Penetrance and RNA Expression Levels in PRPF31-Associated Retinitis Pigmentosa in a Danish Cohort. Genes 2023, 14, 435. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Lv, Y.; Sun, K.; Zhao, Y.; Reilly, J.; Zhang, Y.; Tu, J.; Yu, S.; Liu, X.; et al. Prpf31 is essential for the survival and differentiation of retinal progenitor cells by modulating alternative splicing. Nucleic Acids Res. 2021, 49, 2027–2043. [Google Scholar] [CrossRef]
- Azizzadeh Pormehr, L.; Ahmadian, S.; Daftarian, N.; Mousavi, S.A.; Shafiezadeh, M. PRPF31 reduction causes mis-splicing of the phototransduction genes in human organotypic retinal culture. Eur. J. Hum. Genet. EJHG 2020, 28, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Buskin, A.; Zhu, L.; Chichagova, V.; Basu, B.; Mozaffari-Jovin, S.; Dolan, D.; Droop, A.; Collin, J.; Bronstein, R.; Mehrotra, S.; et al. Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nat. Commun. 2018, 9, 4234. [Google Scholar] [CrossRef]
- Valdés-Sánchez, L.; Calado, S.M.; de la Cerda, B.; Aramburu, A.; García-Delgado, A.B.; Massalini, S.; Montero-Sánchez, A.; Bhatia, V.; Rodríguez-Bocanegra, E.; Diez-Lloret, A.; et al. Retinal pigment epithelium degeneration caused by aggregation of PRPF31 and the role of HSP70 family of proteins. Mol. Med. 2019, 26, 1. [Google Scholar] [CrossRef]
- Georgiou, M.; Yang, C.; Atkinson, R.; Pan, K.-T.; Buskin, A.; Molina, M.M.; Collin, J.; Al-Aama, J.; Goertler, F.; Ludwig, S.E.J.; et al. Activation of autophagy reverses progressive and deleterious protein aggregation in PRPF31 patient-induced pluripotent stem cell-derived retinal pigment epithelium cells. Clin. Transl. Med. 2022, 12, e759. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Peng, C.; Yu, J.; Jiang, W.; Yang, J. Identification of two novel PRPF31 mutations in Chinese families with non-syndromic autosomal dominant retinitis pigmentosa. Mol. Genet. Genomic. Med. 2020, 8, e1537. [Google Scholar] [CrossRef]
- Xie, D.; Peng, K.; Yi, Q.; Liu, W.; Yang, Y.; Sun, K.; Zhu, X.; Lu, F. Targeted Next Generation Sequencing Revealed Novel PRPF31 Mutations in Autosomal Dominant Retinitis Pigmentosa. Genet. Test Mol Biomark. 2018, 22, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, H.-L.; Li, J.-K.; Xu, L.; Tellier, L.; Li, X.-L.; Huang, X.-Y.; Li, W.; Niu, T.-T.; Yang, H.-M.; et al. A novel mutation in PRPF31, causative of autosomal dominant retinitis pigmentosa, using the BGISEQ-500 sequencer. Int. J. Ophthalmol. 2018, 11, 31–35. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, M.; Li, M.; Huang, H.; Liao, J.; Lu, A.; Guo, K.; Ma, N.; Lin, J.; Duan, J.; et al. Mutation Analysis of Pre-mRNA Splicing Genes PRPF31, PRPF8, and SNRNP200 in Chinese Families with Autosomal Dominant Retinitis Pigmentosa. Curr. Mol. Med. 2018, 18, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; You, B.; Chen, Y.-N.; Li, Y.; Li, W.; Wei, W.-B. Whole-exome sequencing identified genes known to be responsible for retinitis pigmentosa in 28 Chinese families. Mol. Vis. 2022, 28, 96–113. [Google Scholar]
- Birtel, J.; Gliem, M.; Mangold, E.; Müller, P.L.; Holz, F.G.; Neuhaus, C.; Lenzner, S.; Zahnleiter, D.; Betz, C.; Eisenberger, T.; et al. Next-generation sequencing identifies unexpected genotype-phenotype correlations in patients with retinitis pigmentosa. PLoS ONE 2018, 13, e0207958. [Google Scholar] [CrossRef]
- Koyanagi, Y.; Akiyama, M.; Nishiguchi, K.M.; Momozawa, Y.; Kamatani, Y.; Takata, S.; Inai, C.; Iwasaki, Y.; Kumano, M.; Murakami, Y.; et al. Genetic characteristics of retinitis pigmentosa in 1204 Japanese patients. J. Med. Genet. 2019, 56, 662–670. [Google Scholar] [CrossRef]
- Sun, H.-N.; Du, K.-L.; Sun, Y.; Liu, C.; Xue, J.-H.; Wang, X.-X.; Liu, Y.; Yu, H.-H.; Ge, J.-Y.; Rong, J.; et al. Unveiling the Genetic and Phenotypic Landscape of a Chinese Cohort with Retinitis Pigmentosa. Mol. Genet. Genomic. Med. 2025, 13, e70011. [Google Scholar] [CrossRef]
- Ali-Nasser, T.; Zayit-Soudry, S.; Banin, E.; Sharon, D.; Ben-Yosef, T. Autosomal dominant retinitis pigmentosa with incomplete penetrance due to an intronic mutation of the PRPF31 gene. Mol. Vis. 2022, 28, 359–368. [Google Scholar] [PubMed]
- Martin-Merida, I.; Sanchez-Alcudia, R.; Fernandez-San Jose, P.; Blanco-Kelly, F.; Perez-Carro, R.; Rodriguez-Jacy da Silva, L.; Almoguera, B.; Garcia-Sandoval, B.; Lopez-Molina, M.I.; Avila-Fernandez, A.; et al. Analysis of the PRPF31 Gene in Spanish Autosomal Dominant Retinitis Pigmentosa Patients: A Novel Genomic Rearrangement. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1045–1053. [Google Scholar] [CrossRef]
- Thorsteinsson, D.A.; Stefansdottir, V.; Eysteinsson, T.; Thorisdottir, S.; Jonsson, J.J. Molecular genetics of inherited retinal degenerations in Icelandic patients. Clin. Genet. 2021, 100, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Bodenbender, J.-P.; Bethge, L.; Stingl, K.; Mazzola, P.; Haack, T.; Biskup, S.; Wissinger, B.; Weisschuh, N.; Kohl, S.; Kühlewein, L. Clinical and Genetic Findings in a Cohort of Patients with PRPF31-Associated Retinal Dystrophy. Am. J. Ophthalmol. 2024, 267, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Grainok, J.; Pitout, I.L.; Chen, F.K.; McLenachan, S.; Heath Jeffery, R.C.; Mitrpant, C.; Fletcher, S. A Precision Therapy Approach for Retinitis Pigmentosa 11 Using Splice-Switching Antisense Oligonucleotides to Restore the Open Reading Frame of PRPF31. Int. J. Mol. Sci. 2024, 25, 3391. [Google Scholar] [CrossRef]
- Xiao, X.; Cao, Y.; Zhang, Z.; Xu, Y.; Zheng, Y.; Chen, L.J.; Pang, C.P.; Chen, H. Novel Mutations in PRPF31 Causing Retinitis Pigmentosa Identified Using Whole-Exome Sequencing. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6342–6350. [Google Scholar] [CrossRef]
- Dan, H.; Huang, X.; Xing, Y.; Shen, Y. Application of targeted panel sequencing and whole exome sequencing for 76 Chinese families with retinitis pigmentosa. Mol. Genet. Genom. Med. 2020, 8, e1131. [Google Scholar] [CrossRef]
- Wheway, G.; Nazlamova, L.; Meshad, N.; Hunt, S.; Jackson, N.; Churchill, A. A Combined in silico, in vitro and Clinical Approach to Characterize Novel Pathogenic Missense Variants in PRPF31 in Retinitis Pigmentosa. Front. Genet. 2019, 10, 248. [Google Scholar] [CrossRef]
- de la Cerda, B.; Díez-Lloret, A.; Ponte, B.; Vallés-Saiz, L.; Calado, S.M.; Rodríguez-Bocanegra, E.; Garcia-Delgado, A.B.; Moya-Molina, M.; Bhattacharya, S.S.; Díaz-Corrales, F.J. Generation and characterization of the human iPSC line CABi001-A from a patient with retinitis pigmentosa caused by a novel mutation in PRPF31 gene. Stem Cell Res. 2019, 36, 101426. [Google Scholar] [CrossRef]
- Bryant, L.; Lozynska, O.; Marsh, A.; Papp, T.E.; van Gorder, L.; Serrano, L.W.; Gai, X.; Maguire, A.M.; Aleman, T.S.; Bennett, J. Identification of a novel pathogenic missense mutation in PRPF31 using whole exome sequencing: A case report. Br. J. Ophthalmol. 2019, 103, 761–767. [Google Scholar] [CrossRef]
- Bhatia, S.; Goyal, S.; Singh, I.R.; Singh, D.; Vanita, V. A novel mutation in the PRPF31 in a North Indian adRP family with incomplete penetrance. Doc. Ophthalmol. 2018, 137, 103–119. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Gao, M.; Liu, Y.; Wu, Y.; Wang, Y.; Gong, Y.; Yu, S.; Liu, W.; Wan, X.; et al. Comprehensive analysis of the PRPF31 gene in retinitis pigmentosa patients: Four novel Alu-mediated copy number variations at the PRPF31 locus. Hum. Mutat. 2022, 43, 2279–2294. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, Y.; Qiao, Y.; Xu, Q.; Zhai, R.; Sun, X.; Wu, J.; Chen, X. A 69 kb Deletion in chr19q13.42 including PRPF31 Gene in a Chinese Family Affected with Autosomal Dominant Retinitis Pigmentosa. J. Clin. Med. 2022, 11, 6682. [Google Scholar] [CrossRef]
- Ruberto, F.P.; Balzano, S.; Namburi, P.; Kimchi, A.; Pescini-Gobert, R.; Obolensky, A.; Banin, E.; Ben-Yosef, T.; Sharon, D.; Rivolta, C. Heterozygous deletions of noncoding parts of the PRPF31 gene cause retinitis pigmentosa via reduced gene expression. Mol. Vis. 2021, 27, 107–116. [Google Scholar] [PubMed]
- Scott, H.A.; Larson, A.; Rong, S.S.; Mehrotra, S.; Butcher, R.; Chao, K.R.; Wiggs, J.L.; Place, E.M.; Pierce, E.A.; Bujakowska, K.M. A hidden structural variation in a known IRD gene: A cautionary tale of two new disease candidate genes. Cold Spring Harb. Mol. Case Stud. 2022, 8, a006131. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mozaffari-Jovin, S.; Wollenhaupt, J.; Santos, K.F.; Theuser, M.; Dunin-Horkawicz, S.; Fabrizio, P.; Bujnicki, J.M.; Lührmann, R.; Wahl, M.C. A composite double-/single-stranded RNA-binding region in protein Prp3 supports tri-snRNP stability and splicing. eLife 2015, 4, e07320. [Google Scholar] [CrossRef]
- Chakarova, C.F.; Hims, M.M.; Bolz, H.; Abu-Safieh, L.; Patel, R.J.; Papaioannou, M.G.; Inglehearn, C.F.; Keen, T.J.; Willis, C.; Moore, A.T.; et al. Mutations in HPRP3, a third member of pre-mRNA splicing factor genes, implicated in autosomal dominant retinitis pigmentosa. Hum. Mol. Genet. 2002, 11, 87–92. [Google Scholar] [CrossRef]
- Gonzalez-Santos, J.M.; Cao, H.; Duan, R.C.; Hu, J. Mutation in the splicing factor Hprp3p linked to retinitis pigmentosa impairs interactions within the U4/U6 snRNP complex. Hum. Mol. Genet. 2008, 17, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Rajiv, C.; Jackson, S.R.; Cocklin, S.; Eisenmesser, E.Z.; Davis, T.L. The spliceosomal proteins PPIH and PRPF4 exhibit bi-partite binding. Biochem. J. 2017, 474, 3689–3704. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Sheng, X.; Tam, P.O.S.; Zhao, K.; Chen, X.; Rong, W.; Liu, Y.; Liu, X.; Pan, X.; et al. PRPF4 mutations cause autosomal dominant retinitis pigmentosa. Hum. Mol. Genet. 2014, 23, 2926–2939. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Hirmer, A.; Gal, A.; Rüther, K.; Bolz, H.J.; Winkler, C.; Laggerbauer, B.; Fischer, U. Identification of a PRPF4 loss-of-function variant that abrogates U4/U6.U5 tri-snRNP integration and is associated with retinitis pigmentosa. PLoS ONE 2014, 9, e111754. [Google Scholar] [CrossRef]
- Galej, W.P.; Nguyen, T.H.D.; Newman, A.J.; Nagai, K. Structural studies of the spliceosome: Zooming into the heart of the machine. Curr. Opin. Struct. Biol. 2014, 25, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Arzalluz-Luque, Á.; Cabrera, J.L.; Skottman, H.; Benguria, A.; Bolinches-Amorós, A.; Cuenca, N.; Lupo, V.; Dopazo, A.; Tarazona, S.; Delás, B.; et al. Mutant PRPF8 Causes Widespread Splicing Changes in Spliceosome Components in Retinitis Pigmentosa Patient iPSC-Derived RPE Cells. Front. Neurosci. 2021, 15, 636969. [Google Scholar] [CrossRef]
- Bergfort, A.; Preußner, M.; Kuropka, B.; Ilik, İ.A.; Hilal, T.; Weber, G.; Freund, C.; Aktaş, T.; Heyd, F.; Wahl, M.C. A multi-factor trafficking site on the spliceosome remodeling enzyme BRR2 recruits C9ORF78 to regulate alternative splicing. Nat. Commun. 2022, 13, 1132. [Google Scholar] [CrossRef]
- Malinová, A.; Cvačková, Z.; Matějů, D.; Hořejší, Z.; Abéza, C.; Vandermoere, F.; Bertrand, E.; Staněk, D.; Verheggen, C. Assembly of the U5 snRNP component PRPF8 is controlled by the HSP90/R2TP chaperones. J. Cell Biol. 2017, 216, 1579–1596. [Google Scholar] [CrossRef]
- Shakhmantsir, I.; Dooley, S.J.; Kishore, S.; Chen, D.; Pierce, E.; Bennett, J.; Sehgal, A. RNA Splicing Factor Mutations That Cause Retinitis Pigmentosa Result in Circadian Dysregulation. J. Biol. Rhythm. 2020, 35, 72–83. [Google Scholar] [CrossRef]
- Xu, G.; Li, T.; Chen, J.; Li, C.; Zhao, H.; Yao, C.; Dong, H.; Wen, K.; Wang, K.; Zhao, J.; et al. Autosomal dominant retinitis pigmentosa-associated gene PRPF8 is essential for hypoxia-induced mitophagy through regulating ULK1 mRNA splicing. Autophagy 2018, 14, 1818–1830. [Google Scholar] [CrossRef]
- Ruiz-Justiz, A.J.; Molina Thurin, L.J.; Izquierdo, N. A New Variant in the PRPF6 Gene Leading to Retinitis Pigmentosa: A Case Report. Cureus 2023, 15, e48489. [Google Scholar] [CrossRef] [PubMed]
- Tanackovic, G.; Ransijn, A.; Ayuso, C.; Harper, S.; Berson, E.L.; Rivolta, C. A missense mutation in PRPF6 causes impairment of pre-mRNA splicing and autosomal-dominant retinitis pigmentosa. Am. J. Hum. Genet. 2011, 88, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tan, F.; Sun, X.; Cui, Z.; Gu, J.; Mao, S.; Chan, H.F.; Tang, S.; Chen, J. Aberrant Retinal Pigment Epithelial Cells Derived from Induced Pluripotent Stem Cells of a Retinitis Pigmentosa Patient with the PRPF6 Mutation. Int. J. Mol. Sci. 2022, 23, 9049. [Google Scholar] [CrossRef]
- Nazlamova, L.; Villa Vasquez, S.S.; Lord, J.; Karthik, V.; Cheung, M.-K.; Lakowski, J.; Wheway, G. Microtubule modification defects underlie cilium degeneration in cell models of retinitis pigmentosa associated with pre-mRNA splicing factor mutations. Front. Genet. 2022, 13, 1009430. [Google Scholar] [CrossRef]
- Guidarelli Mattioli, F.; Saltalamacchia, A.; Magistrato, A. Tracing Allostery in the Spliceosome Ski2-like RNA Helicase Brr2. J. Phys. Chem. Lett. 2024, 15, 3502–3508. [Google Scholar] [CrossRef]
- Růžičková, Š.; Staněk, D. Mutations in spliceosomal proteins and retina degeneration. RNA Biol. 2017, 14, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Zimmann, F.; McNicoll, F.; Thakur, P.K.; Blažíková, M.; Kubovčiak, J.; Hernández Cañás, M.C.; Nováková, Z.; Bařinka, C.; Kolář, M.; Staněk, D.; et al. Retinitis pigmentosa-linked mutations impair the snRNA unwinding activity of SNRNP200 and reduce pre-mRNA binding of PRPF8. Cell. Mol. Life Sci. CMLS 2025, 82, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Bai, J.; Zhang, X.; Zheng, X.; Lu, N.; Liang, Z.; Lin, L.; Chen, Y. SNRNP200 Mutations Cause Autosomal Dominant Retinitis Pigmentosa. Front. Med. 2020, 7, 588991. [Google Scholar] [CrossRef] [PubMed]
- Maita, H.; Harada, Y.; Nagakubo, D.; Kitaura, H.; Ikeda, M.; Tamai, K.; Takahashi, K.; Ariga, H.; Iguchi-Ariga, S.M. PAP-1, a novel target protein of phosphorylation by pim-1 kinase. Eur. J. Biochem. 2000, 267, 5168–5178. [Google Scholar] [CrossRef]
- Maita, H.; Kitaura, H.; Keen, T.J.; Inglehearn, C.F.; Ariga, H.; Iguchi-Ariga, S.M.M. PAP-1, the mutated gene underlying the RP9 form of dominant retinitis pigmentosa, is a splicing factor. Exp. Cell Res. 2004, 300, 283–296. [Google Scholar] [CrossRef]
- Lv, J.-N.; Zhou, G.-H.; Chen, X.; Chen, H.; Wu, K.-C.; Xiang, L.; Lei, X.-L.; Zhang, X.; Wu, R.-H.; Jin, Z.-B. Targeted RP9 ablation and mutagenesis in mouse photoreceptor cells by CRISPR-Cas9. Sci. Rep. 2017, 7, 43062. [Google Scholar] [CrossRef]
- Yang, D.; Yao, Q.; Li, Y.; Xu, Y.; Wang, J.; Zhao, H.; Liu, F.; Zhang, Z.; Liu, Y.; Bie, X.; et al. A c.544_618del75bp mutation in the splicing factor gene PRPF31 is involved in non-syndromic retinitis pigmentosa by reducing the level of mRNA expression. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. (Optom.) 2020, 40, 289–299. [Google Scholar] [CrossRef]
- Chung, C.-S.; Wai, H.L.; Kao, C.-Y.; Cheng, S.-C. An ATP-independent role for Prp16 in promoting aberrant splicing. Nucleic Acids Res. 2023, 51, 10815–10828. [Google Scholar] [CrossRef]
- Ajmal, M.; Khan, M.I.; Neveling, K.; Khan, Y.M.; Azam, M.; Waheed, N.K.; Hamel, C.P.; Ben-Yosef, T.; De Baere, E.; Koenekoop, R.K.; et al. A missense mutation in the splicing factor gene DHX38 is associated with early-onset retinitis pigmentosa with macular coloboma. J. Med. Genet. 2014, 51, 444–448. [Google Scholar] [CrossRef]
- Sun, K.; Han, Y.; Li, J.; Yu, S.; Huang, Y.; Zhang, Y.; Reilly, J.; Tu, J.; Gao, P.; Jia, D.; et al. The splicing factor DHX38 enables retinal development through safeguarding genome integrity. iScience 2023, 26, 108103. [Google Scholar] [CrossRef] [PubMed]
- Busetto, V.; Barbosa, I.; Basquin, J.; Marquenet, É.; Hocq, R.; Hennion, M.; Paternina, J.A.; Namane, A.; Conti, E.; Bensaude, O.; et al. Structural and functional insights into CWC27/CWC22 heterodimer linking the exon junction complex to spliceosomes. Nucleic Acids Res. 2020, 48, 5670–5683. [Google Scholar] [CrossRef]
- Bertrand, R.E.; Wang, J.; Li, Y.; Cheng, X.; Wang, K.; Stoilov, P.; Chen, R. Cwc27, associated with retinal degeneration, functions as a splicing factor in vivo. Hum. Mol. Genet. 2022, 31, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xie, Y.A.; Abouzeid, H.; Gordon, C.T.; Fiorentino, A.; Sun, Z.; Lehman, A.; Osman, I.S.; Dharmat, R.; Riveiro-Alvarez, R.; et al. Mutations in the Spliceosome Component CWC27 Cause Retinal Degeneration with or without Additional Developmental Anomalies. Am. J. Hum. Genet. 2017, 100, 592–604. [Google Scholar] [CrossRef]
- Rodrigues, A.; Slembrouck-Brec, A.; Nanteau, C.; Terray, A.; Tymoshenko, Y.; Zagar, Y.; Reichman, S.; Xi, Z.; Sahel, J.A.; Fouquet, S.; et al. Modeling PRPF31 retinitis pigmentosa using retinal pigment epithelium and organoids combined with gene augmentation rescue. npj Regen. Med. 2022, 7, 39. [Google Scholar] [CrossRef]
- Chen, X.; Xu, N.; Li, J.; Zhao, M.; Huang, L. Stem cell therapy for inherited retinal diseases: A systematic review and meta-analysis. Stem Cell Res. Ther. 2023, 14, 286. [Google Scholar] [CrossRef]
- Ullah, M.; Rehman, A.U.; Folcher, M.; Ullah, A.; Usman, F.; Rashid, A.; Khan, B.; Quinodoz, M.; Ansar, M.; Rivolta, C. A Novel Intronic Deletion in PDE6B Causes Autosomal Recessive Retinitis Pigmentosa by Interfering with RNA Splicing. Ophthalmic Res. 2023, 66, 878–884. [Google Scholar] [CrossRef]
- Li, Y.; Furhang, R.; Ray, A.; Duncan, T.; Soucy, J.; Mahdi, R.; Chaitankar, V.; Gieser, L.; Poliakov, E.; Qian, H.; et al. Aberrant RNA splicing is the major pathogenic effect in a knock-in mouse model of the dominantly inherited c.1430A>G human RPE65 mutation. Hum. Mutat. 2019, 40, 426–443. [Google Scholar] [CrossRef]
- Riedmayr, L.M.; Böhm, S.; Biel, M.; Becirovic, E. Enigmatic rhodopsin mutation creates an exceptionally strong splice acceptor site. Hum. Mol. Genet. 2020, 29, 295–304. [Google Scholar] [CrossRef]
- Jung, H.; Lee, K.S.; Choi, J.K. Comprehensive characterisation of intronic mis-splicing mutations in human cancers. Oncogene 2021, 40, 1347–1361. [Google Scholar] [CrossRef]
- Elasal, M.A.; Khateb, S.; Panneman, D.M.; Roosing, S.; Cremers, F.P.M.; Banin, E.; Sharon, D.; Sarma, A.S. A Leaky Deep Intronic Splice Variant in CLRN1 Is Associated with Non-Syndromic Retinitis Pigmentosa. Genes 2024, 15, 1363. [Google Scholar] [CrossRef]
- Slijkerman, R.; Goloborodko, A.; Broekman, S.; de Vrieze, E.; Hetterschijt, L.; Peters, T.; Gerits, M.; Kremer, H.; van Wijk, E. Poor Splice-Site Recognition in a Humanized Zebrafish Knockin Model for the Recurrent Deep-Intronic c.7595-2144A>G Mutation in USH2A. Zebrafish 2018, 15, 597–609. [Google Scholar] [CrossRef]
- Su, B.-N.; Shen, R.-J.; Liu, Z.-L.; Li, Y.; Jin, Z.-B. Global spectrum of USH2A mutation in inherited retinal dystrophies: Prompt message for development of base editing therapy. Front. Aging Neurosci. 2022, 14, 948279. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.; Lin, S.; Jurkute, N.; Oprych, K.; Estramiana Elorrieta, L.; Schiff, E.; Malka, S.; Wright, G.; Michaelides, M.; Mahroo, O.A.; et al. Investigating Splice Defects in USH2A Using Targeted Long-Read Sequencing. Cells 2024, 13, 1261. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Munir, A.; Zahid, M.; Ullah, M.; Rehman, A.U. Exome sequencing identifies a homozygous splice site variant in RP1 as the underlying cause of autosomal recessive retinitis pigmentosa in a Pakistani family. Ann. Med. 2025, 57, 2470953. [Google Scholar] [CrossRef]
- Liu, X.; Jia, R.; Meng, X.; Wang, L.; Yang, L. Analysis of RPGR gene mutations in 41 Chinese families affected by X-linked inherited retinal dystrophy. Front. Genet. 2022, 13, 999695. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Vanita, V. A missense mutation in TTC8/BBS8 affecting mRNA splicing in patients with non-syndromic retinitis pigmentosa. Mol Genet. Genom. 2022, 297, 1439–1449. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Li, S.; Yang, Y.; Yang, M.; Yang, Z.; Zhu, X.; Zhang, L. Targeted next-generation sequencing reveals that a compound heterozygous mutation in phosphodiesterase 6a gene leads to retinitis pigmentosa in a Chinese family. Ophthalmic Genet. 2018, 39, 487–491. [Google Scholar] [CrossRef]
- Bodenbender, J.-P.; Marino, V.; Bethge, L.; Stingl, K.; Haack, T.B.; Biskup, S.; Kohl, S.; Kühlewein, L.; Dell’Orco, D.; Weisschuh, N. Biallelic Variants in TULP1 Are Associated with Heterogeneous Phenotypes of Retinal Dystrophy. Int. J. Mol. Sci. 2023, 24, 2709. [Google Scholar] [CrossRef]
- Owczarek-Lipska, M.; Song, F.; Jakšić, V.; Neidhardt, J. Compound heterozygous RPE65 mutations associated with an early onset autosomal recessive retinitis pigmentosa. J. Gene Med. 2020, 22, e3211. [Google Scholar] [CrossRef] [PubMed]
- Olivier, G.; Corton, M.; Intartaglia, D.; Verbakel, S.K.; Sergouniotis, P.I.; Le Meur, G.; Dhaenens, C.-M.; Naacke, H.; Avila-Fernández, A.; Hoyng, C.B.; et al. Pathogenic variants in IMPG1 cause autosomal dominant and autosomal recessive retinitis pigmentosa. J. Med. Genet. 2021, 58, 570–578. [Google Scholar] [CrossRef]
- Bouzidi, A.; Charif, M.; Bouzidi, A.; Amalou, G.; Kandil, M.; Barakat, A.; Lenaers, G. Clinical and genetic investigations of three Moroccan families with retinitis pigmentosa phenotypes. Mol. Vis. 2021, 27, 17–25. [Google Scholar]
- Fiorentino, A.; Yu, J.; Arno, G.; Pontikos, N.; Halford, S.; Broadgate, S.; Michaelides, M.; Carss, K.J.; Raymond, F.L.; Cheetham, M.E.; et al. Novel homozygous splicing mutations in ARL2BP cause autosomal recessive retinitis pigmentosa. Mol. Vis. 2018, 24, 603–612. [Google Scholar] [PubMed]
- Maggi, J.; Hanson, J.V.M.; Kurmann, L.; Koller, S.; Feil, S.; Gerth-Kahlert, C.; Berger, W. Retinal Dystrophy Associated with Homozygous Variants in NRL. Genes 2024, 15, 1594. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, C.; Xiao, Y.; Liu, H. Novel splice receptor-site mutation of RPGR in a Chinese family with X-linked retinitis pigmentosa. Medicine 2018, 97, e12779. [Google Scholar] [CrossRef]
- Cheng, J.; Fu, J.; Zhou, Q.; Xiang, X.; Wei, C.; Yang, L.; Fu, S.; Khan, M.A.; Lv, H.; Fu, J. A novel splicing mutation in the PRPH2 gene causes autosomal dominant retinitis pigmentosa in a Chinese pedigree. J. Cell. Mol. Med. 2019, 23, 3776–3780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-D.; Huang, S.-S.; Li, M.; Lek, M.; Song, D.-Y.; Tan, D.-D.; Chen, X.-Y.; Zhang, H.; Liu, J.-Y.; Chang, X.-Z.; et al. A new phenotype of syndromic retinitis pigmentosa with myopathy is caused by mutations in retinol dehydrogenase 11. Clin. Genet. 2022, 101, 448–453. [Google Scholar] [CrossRef]
- Dawood, M.; Lin, S.; Din, T.U.; Shah, I.U.; Khan, N.; Jan, A.; Marwan, M.; Sultan, K.; Nowshid, M.; Tahir, R.; et al. Novel mutations in PDE6A and CDHR1 cause retinitis pigmentosa in Pakistani families. Int. J. Ophthalmol. 2021, 14, 1843–1851. [Google Scholar] [CrossRef]
- Gray, J.M.; Orlans, H.O.; Shanks, M.; Clouston, P.; MacLaren, R.E. Slowly progressive retinitis pigmentosa caused by two novel mutations in the MAK gene. Ophthalmic Genet. 2018, 39, 508–511. [Google Scholar] [CrossRef]
- Fadaie, Z.; Whelan, L.; Dockery, A.; Li, C.H.Z.; van den Born, L.I.; Hoyng, C.B.; Gilissen, C.; Corominas, J.; Rowlands, C.; Megaw, R.; et al. BBS1 branchpoint variant is associated with non-syndromic retinitis pigmentosa. J. Med. Genet. 2022, 59, 438–444. [Google Scholar] [CrossRef]
- Pomares, E.; Riera, M.; Castro-Navarro, J.; Andrés-Gutiérrez, A.; Gonzàlez-Duarte, R.; Marfany, G. Identification of an intronic single-point mutation in RP2 as the cause of semidominant X-linked retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5107–5114. [Google Scholar] [CrossRef]
- Tatour, Y.; Tamaiev, J.; Shamaly, S.; Colombo, R.; Bril, E.; Rabinowitz, T.; Yaakobi, A.; Mezer, E.; Leibu, R.; Tiosano, B.; et al. A novel intronic mutation of PDE6B is a major cause of autosomal recessive retinitis pigmentosa among Caucasus Jews. Mol. Vis. 2019, 25, 155–164. [Google Scholar] [PubMed]
- Kortüm, F.; Kieninger, S.; Mazzola, P.; Kohl, S.; Wissinger, B.; Prokisch, H.; Stingl, K.; Weisschuh, N. X-Linked Retinitis Pigmentosa Caused by Non-Canonical Splice Site Variants in RPGR. Int. J. Mol. Sci. 2021, 22, 850. [Google Scholar] [CrossRef] [PubMed]
- Koller, S.; Beltraminelli, T.; Maggi, J.; Wlodarczyk, A.; Feil, S.; Baehr, L.; Gerth-Kahlert, C.; Menghini, M.; Berger, W. Functional Analysis of a Novel, Non-Canonical RPGR Splice Variant Causing X-Linked Retinitis Pigmentosa. Genes 2023, 14, 934. [Google Scholar] [CrossRef] [PubMed]
- Reurink, J.; Oostrik, J.; Aben, M.; Ramos, M.G.; van Berkel, E.; Ołdak, M.; van Wijk, E.; Kremer, H.; Roosing, S.; Cremers, F.P.M. Minigene-Based Splice Assays Reveal the Effect of Non-Canonical Splice Site Variants in USH2A. Int. J. Mol. Sci. 2022, 23, 13343. [Google Scholar] [CrossRef]
- Jurkute, N.; Cancellieri, F.; Pohl, L.; Li, C.H.Z.; Heaton, R.A.; Reurink, J.; Bellingham, J.; Quinodoz, M.; Yioti, G.; Stefaniotou, M.; et al. Biallelic variants in coenzyme Q10 biosynthesis pathway genes cause a retinitis pigmentosa phenotype. npj Genom. Med. 2022, 7, 60. [Google Scholar] [CrossRef]
- Covello, G.; Ibrahim, G.H.; Bacchi, N.; Casarosa, S.; Denti, M.A. Exon Skipping Through Chimeric Antisense U1 snRNAs to Correct Retinitis Pigmentosa GTPase-Regulator (RPGR) Splice Defect. Nucleic Acid Ther. 2022, 32, 333–349. [Google Scholar] [CrossRef]
- Webb, T.R.; Parfitt, D.A.; Gardner, J.C.; Martinez, A.; Bevilacqua, D.; Davidson, A.E.; Zito, I.; Thiselton, D.L.; Ressa, J.H.C.; Apergi, M.; et al. Deep intronic mutation in OFD1, identified by targeted genomic next-generation sequencing, causes a severe form of X-linked retinitis pigmentosa (RP23). Hum. Mol. Genet. 2012, 21, 3647–3654. [Google Scholar] [CrossRef]
- Pan, W.W.; Wubben, T.J.; Besirli, C.G. Photoreceptor metabolic reprogramming: Current understanding and therapeutic implications. Commun. Biol. 2021, 4, 245. [Google Scholar] [CrossRef] [PubMed]
- Izarbe, A.-M.; Rocío, G.-A.; Serena, M.; Gemma, M. The Alter Retina: Alternative Splicing of Retinal Genes in Health and Disease. Int. J. Mol. Sci. 2021, 22, 1855. [Google Scholar] [CrossRef] [PubMed]
- Matalkah, F.; Jeong, B.; Sheridan, M.; Horstick, E.; Stoilov, P.; Visvanathan Ramamurthy. The Musashi proteins direct post-transcriptional control of protein expression and alternate exon splicing in vertebrate photoreceptors. Commun. Biol. 2022, 5, 1011. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, L.; Mantica, F.; López-Blanch, L.; Permanyer, J.; Rodriguez-Marín, C.; Zang, J.; Cianferoni, D.; Jiménez-Delgado, S.; Bonnal, S.; Miravet-Verde, S.; et al. Specialization of the photoreceptor transcriptome by Srrm3 -dependent microexons is required for outer segment maintenance and vision. Proc. Natl. Acad. Sci. USA 2022, 119, e2117090119. [Google Scholar] [CrossRef]
- Jiao, L.; Gong, M.; Yang, X.; Li, M.; Shao, Y.; Wang, Y.; Li, H.; Yu, Q.; Sun, L.; Xuan, L.; et al. NAD+ attenuates cardiac injury after myocardial infarction in diabetic mice through regulating alternative splicing of VEGF in macrophages. Vasc. Pharmacol. 2022, 147, 107126. [Google Scholar] [CrossRef]
- Nanamatsu, A.; Rhodes, G.J.; LaFavers, K.A.; Micanovic, R.; Lazar, V.; Khan, S.; Barwinska, D.; Makino, S.; Zollman, A.; Cheng, Y.-H.; et al. Alternative splicing of uromodulin enhances mitochondrial metabolism for adaptation to stress in kidney epithelial cells. J. Clin. Investig. 2025, 135, e183343. [Google Scholar] [CrossRef]
- Hartong, D.T.; Dange, M.; McGee, T.L.; Berson, E.L.; Dryja, T.P.; Colman, R.F. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat. Genet. 2008, 40, 1230–1234. [Google Scholar] [CrossRef]
- Ducloyer, J.-B.; Le Meur, G.; Cronin, T.; Adjali, O.; Weber, M. Gene therapy for retinitis pigmentosa. Med. Sci. 2020, 36, 607–615. [Google Scholar] [CrossRef]
- Gange, W.S.; Sisk, R.A.; Besirli, C.G.; Lee, T.C.; Havunjian, M.; Schwartz, H.; Borchert, M.; Sengillo, J.D.; Mendoza, C.; Berrocal, A.M.; et al. Perifoveal Chorioretinal Atrophy after Subretinal Voretigene Neparvovec-rzyl for RPE65-Mediated Leber Congenital Amaurosis. Ophthalmol. Retin. 2022, 6, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Vats, A.; Sahel, J.-A.; Chen, Y.; Byrne, L.C. Gene augmentation prevents retinal degeneration in a CRISPR/Cas9-based mouse model of PRPF31 retinitis pigmentosa. Nat. Commun. 2022, 13, 7695. [Google Scholar] [CrossRef] [PubMed]
- Brydon, E.M.; Bronstein, R.; Buskin, A.; Lako, M.; Pierce, E.A.; Fernandez-Godino, R. AAV-Mediated Gene Augmentation Therapy Restores Critical Functions in Mutant PRPF31+/- iPSC-Derived RPE Cells. Mol. Ther. Methods Clin. Dev. 2019, 15, 392–402. [Google Scholar] [CrossRef]
- Michaelides, M.; Besirli, C.G.; Yang, Y.; De Guimaraes, T.A.C.; Wong, S.C.; Huckfeldt, R.M.; Comander, J.I.; Sahel, J.-A.; Shah, S.M.; Tee, J.J.L.; et al. Phase 1/2 AAV5-hRKp.RPGR (Botaretigene Sparoparvovec) Gene Therapy: Safety and Efficacy in RPGR-Associated X-Linked Retinitis Pigmentosa. Am. J. Ophthalmol. 2024, 267, 122–134. [Google Scholar] [CrossRef]
- Yang, P.; Birch, D.; Lauer, A.; Sisk, R.; Anand, R.; Pennesi, M.E.; Iannaccone, A.; Yaghy, A.; Scaria, A.; Jung, J.A.; et al. Subretinal Gene Therapy Drug AGTC-501 for XLRP Phase 1/2 Multicenter Study (HORIZON): 24-Month Safety and Efficacy Results. Am. J. Ophthalmol. 2025, 271, 268–285. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K. RNA-based therapeutics for neurological diseases. RNA Biol. 2022, 19, 176–190. [Google Scholar] [CrossRef]
- Kolesnik, V.V.; Nurtdinov, R.F.; Oloruntimehin, E.S.; Karabelsky, A.V.; Malogolovkin, A.S. Optimization strategies and advances in the research and development of AAV-based gene therapy to deliver large transgenes. Clin. Transl. Med. 2024, 14, e1607. [Google Scholar] [CrossRef]
- Xue, K.; MacLaren, R.E. Antisense oligonucleotide therapeutics in clinical trials for the treatment of inherited retinal diseases. Expert. Opin. Investig. Drugs 2020, 29, 1163–1170. [Google Scholar] [CrossRef]
- Dulla, K.; Slijkerman, R.; van Diepen, H.C.; Albert, S.; Dona, M.; Beumer, W.; Turunen, J.J.; Chan, H.L.; Schulkens, I.A.; Vorthoren, L.; et al. Antisense oligonucleotide-based treatment of retinitis pigmentosa caused by USH2A exon 13 mutations. Mol. Ther. 2021, 29, 2441–2455. [Google Scholar] [CrossRef]
- García-Bohórquez, B.; Barberán-Martínez, P.; Aller, E.; Jaijo, T.; Mínguez, P.; Rodilla, C.; Fernández-Caballero, L.; Blanco-Kelly, F.; Ayuso, C.; Sanchis-Juan, A.; et al. Exploring non-coding variants and evaluation of antisense oligonucleotides for splicing redirection in Usher syndrome. Mol. Ther. Nucleic Acids 2024, 35, 102374. [Google Scholar] [CrossRef]
- Schellens, R.T.W.; Broekman, S.; Peters, T.; Graave, P.; Malinar, L.; Venselaar, H.; Kremer, H.; De Vrieze, E.; Van Wijk, E. A protein domain-oriented approach to expand the opportunities of therapeutic exon skipping for USH2A-associated retinitis pigmentosa. Mol. Ther. Nucleic Acids 2023, 32, 980–994. [Google Scholar] [CrossRef]
- Slijkerman, R.W.; Vaché, C.; Dona, M.; García-García, G.; Claustres, M.; Hetterschijt, L.; Peters, T.A.; Hartel, B.P.; Pennings, R.J.; Millan, J.M.; et al. Antisense Oligonucleotide-based Splice Correction for USH2A-associated Retinal Degeneration Caused by a Frequent Deep-intronic Mutation. Mol. Ther. Nucleic Acids 2016, 5, e381. [Google Scholar] [CrossRef]
- Panagiotopoulos, A.-L.; Karguth, N.; Pavlou, M.; Böhm, S.; Gasparoni, G.; Walter, J.; Graf, A.; Blum, H.; Biel, M.; Riedmayr, L.M.; et al. Antisense Oligonucleotide- and CRISPR-Cas9-Mediated Rescue of mRNA Splicing for a Deep Intronic CLRN1 Mutation. Mol. Ther. Nucleic Acids 2020, 21, 1050–1061. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Gao, F.-J.; Ju, Y.-Q.; Guo, L.-Y.; Duan, C.; Chang, Q.; Zhang, T.; Xu, G.-Z.; Du, H.; Zong, Y.; et al. Clinical and mutational signatures of CRB1-associated retinopathies: A multicentre study. J. Med. Genet. 2024, 62, 6–14. [Google Scholar] [CrossRef]
- Lopes da Costa, B.; Kolesnikova, M.; Levi, S.R.; Cabral, T.; Tsang, S.H.; Maumenee, I.H.; Quinn, P.M.J. Clinical and Therapeutic Evaluation of the Ten Most Prevalent CRB1 Mutations. Biomedicines 2023, 11, 385. [Google Scholar] [CrossRef]
- Collotta, D.; Bertocchi, I.; Chiapello, E.; Collino, M. Antisense oligonucleotides: A novel Frontier in pharmacological strategy. Front. Pharmacol. 2023, 14, 1304342. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.; Santos, J.I.; Coutinho, M.F.; Matos, L.; Alves, S. Development of Engineered-U1 snRNA Therapies: Current Status. Int. J. Mol. Sci. 2023, 24, 14617. [Google Scholar] [CrossRef] [PubMed]
- Jüschke, C.; Klopstock, T.; Catarino, C.B.; Owczarek-Lipska, M.; Wissinger, B.; Neidhardt, J. Autosomal dominant optic atrophy: A novel treatment for OPA1 splice defects using U1 snRNA adaption. Mol. Ther. Nucleic Acids 2021, 26, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Tanner, G.; Glaus, E.; Barthelmes, D.; Ader, M.; Fleischhauer, J.; Pagani, F.; Berger, W.; Neidhardt, J. Therapeutic strategy to rescue mutation-induced exon skipping in rhodopsin by adaptation of U1 snRNA. Hum. Mutat. 2009, 30, 255–263. [Google Scholar] [CrossRef]
- Glaus, E.; Schmid, F.; Da Costa, R.; Berger, W.; Neidhardt, J. Gene therapeutic approach using mutation-adapted U1 snRNA to correct a RPGR splice defect in patient-derived cells. Mol. Ther. 2011, 19, 936–941. [Google Scholar] [CrossRef]
- Yokomori, R.; Kusakabe, T.G.; Nakai, K. Characterization of trans-spliced chimeric RNAs: Insights into the mechanism of trans-splicing. NAR Genom. Bioinform. 2024, 6, lqae067. [Google Scholar] [CrossRef] [PubMed]
- Riedmayr, L.M. SMaRT for Therapeutic Purposes. In Chimeric RNA: Methods and Protocols; Springer: New York, NY, USA, 2020; Volume 2079, pp. 219–232. [Google Scholar] [CrossRef]
- Berger, A.; Lorain, S.; Joséphine, C.; Desrosiers, M.; Peccate, C.; Voit, T.; Garcia, L.; Sahel, J.-A.; Bemelmans, A.-P. Repair of rhodopsin mRNA by spliceosome-mediated RNA trans-splicing: A new approach for autosomal dominant retinitis pigmentosa. Mol. Ther. 2015, 23, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Ying, R.; Li, C.; Li, H.; Zou, J.; Hu, M.; Hong, Q.; Shen, Y.; Hou, L.; Cheng, H.; Zhou, R. RPGR is a guanine nucleotide exchange factor for the small GTPase RAB37 required for retinal function via autophagy regulation. Cell Rep. 2024, 43, 114010. [Google Scholar] [CrossRef] [PubMed]
- Riedmayr, L.M.; Hinrichsmeyer, K.S.; Thalhammer, S.B.; Mittas, D.M.; Karguth, N.; Otify, D.Y.; Böhm, S.; Weber, V.J.; Bartoschek, M.D.; Splith, V.; et al. mRNA trans-splicing dual AAV vectors for (epi)genome editing and gene therapy. Nat. Commun. 2023, 14, 6578. [Google Scholar] [CrossRef]
- Hong, E.M.; Ingemarsdotter, C.K.; Lever, A.M.L. Therapeutic applications of trans-splicing. Br. Med. Bull. 2020, 136, 4–20. [Google Scholar] [CrossRef]
- Mou, H.; Smith, J.L.; Peng, L.; Yin, H.; Moore, J.; Zhang, X.-O.; Song, C.-Q.; Sheel, A.; Wu, Q.; Ozata, D.M.; et al. CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion. Genome Biol. 2017, 18, 108. [Google Scholar] [CrossRef]
- Sanjurjo-Soriano, C.; Erkilic, N.; Baux, D.; Mamaeva, D.; Hamel, C.P.; Meunier, I.; Roux, A.-F.; Kalatzis, V. Genome Editing in Patient iPSCs Corrects the Most Prevalent USH2A Mutations and Reveals Intriguing Mutant mRNA Expression Profiles. Mol. Ther. Methods Clin. Dev. 2020, 17, 156–173. [Google Scholar] [CrossRef]
- Gumerson, J.D.; Alsufyani, A.; Yu, W.; Lei, J.; Sun, X.; Dong, L.; Wu, Z.; Li, T. Restoration of RPGR expression in vivo using CRISPR/Cas9 gene editing. Gene Ther. 2022, 29, 81–93. [Google Scholar] [CrossRef]
- Pierce, E.A.; Aleman, T.S.; Jayasundera, K.T.; Ashimatey, B.S.; Kim, K.; Rashid, A.; Jaskolka, M.C.; Myers, R.L.; Lam, B.L.; Bailey, S.T.; et al. Gene Editing for CEP290-Associated Retinal Degeneration. N. Engl. J. Med. 2024, 390, 1972–1984. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, W.; Zhang, S.; Feng, Y.; Xu, W.; Qi, J.; Zhang, Q.; Xu, C.; Liu, S.; Zhang, J.; et al. Vision rescue via unconstrained in vivo prime editing in degenerating neural retinas. J. Exp. Med. 2023, 220, e20220776. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Choi, E.H.; Raguram, A.; Liu, D.R.; Palczewski, K. Precision genome editing in the eye. Proc. Natl. Acad. Sci. USA 2022, 119, e2210104119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wei, L.; Chen, Y. From bench to bedside: Developing CRISPR/Cas-based therapy for ocular diseases. Pharmacol. Res. 2025, 213, 107638. [Google Scholar] [CrossRef]



| Mutation Type | Nucleotide Mutation | Protein/RNA Splicing Changes | Affected Exon | Reference |
|---|---|---|---|---|
| Frameshift | c.849_855del | p.Pro284Ilefs*35 | exon8 | [54] |
| c.1226_1227insA | p.T410Dfs*65 | exon12 | [55] | |
| c.357_358delAA | p.Ser119Serfs*5 | exon5 | [56] | |
| c.1115_1125del | p.Arg372Glnfs*99 | exon11 | [51] | |
| c.1035_1036insGC | p.Pro346Argfs*18 | exon10 | [57] | |
| c.1224dupG | p.Gln409Alafs*66 | exon12 | ||
| c.967_968delGA | p.E323Dfs*151 | exon10 | [58] | |
| c.327_330delCATC | p.H111Sfs*86 | exon5 | ||
| c.816_830delCTACATCTACCACAG | p.Tyr273_Ser277del | exon8 | [59] | |
| c.1142delG | p.Gly381fs | exon11 | [60] | |
| c.1168_1169insGATTCAGCCTGGCC | p.Glu390Glyfs*28 | exon12 | [61] | |
| Splicing | c.1146+5G>T | p.Tyr359Serfs*29 | Intron11 | [62] |
| c.855+5G>A | p.Glu333ArgAspfs*33 | Intron8 | [54] | |
| c.1146+2T>A | - | Intron11 | [63] | |
| c.322+1G>A | p.Val80_Leu107del | Intron4 | [57] | |
| c.527+2T>G | p.Gln177Tyrfs*10/ p.Glu141Alafs*102 | Intron6 | ||
| c.1073+5G>A | - | Intron10 | [64] | |
| c.-9+1G>A | - | exon1 | [65] | |
| Nonsense | c.1205C>A | p.Ser402* | exon12 | [66] |
| c.1060C>T | p. Arg354* | exon10 | [67] | |
| c.1015C>T | p.Glu339* | exon10 | [55] | |
| c.220C>T | p.Gln74* | exon3 | [68] | |
| c.1168G>T | p.Glu390* | exon 12 | [65] | |
| Missense | c.341T>A | p.Ile114Asn | exon5 | [69] |
| c.165G>A | p.Gly55Asp | exon2 | [70] | |
| c.590T>C | p.Leu197Pro | exon7 | [71] | |
| c.646G>C | p.A216Pro | exon7 | [52] | |
| c.896G>A | p.Cys299Tyr | exon8 | [72] | |
| c.839T>G | p.Val280Gly | exon8 | [59] | |
| Large-scale deletion | chr19:54117745–54125389 (7645 bp) | - | exon 2–8 | [73] |
| chr19:54110458–54130356 (19,899 bp) | - | exon 1–13 | ||
| chr19:54109148–54133219 (24,072 bp) | - | Exon 1–14+TFPT E1–E3 | ||
| chr19:54043540–54132981 (89,442 bp) | - | Exon 1–14+large-scale gene deletion | ||
| chr19:54048499–54118055 (69 kb) | - | Exon 1+5 upstream genes | [74] | |
| chr19:54113356–54116922 | - | Promoter and 5′ untranslated region (5′ UTR) | [75] | |
| chr19:54113882–54116394 | - | |||
| duplication | chr19:54621606–54626745 (5.1 kb) | - | Tandem repeat of exons 2–5 | [63] |
| c.73_166dup | p.Asp56GlyfsTer33 | exon 2 | [76] |
| Mutation Type | Gene | Nucleotide Mutation | Exon | Reference |
|---|---|---|---|---|
| Canonical splice donor site | RP1 | c.615+1G>A | Intron2 | [117] |
| RPGR | c.619+1G>C | Intron6 | [40] | |
| c.310+3A>G | Intron4 | [118] | ||
| c.619+2T>A | Intron6 | |||
| TTC8/BBS8 | c.1347G>C | Exon13 | [119] | |
| PDE6A | c.1407+1G>C | Intron12 | [120] | |
| TULP1 | c.1495+1G>A | Intron14 | [121] | |
| RPE65 | c.1338+1G>A | Intron12 | [122] | |
| IMPG1 | c.1824+1G>A | Intron13 | [123] | |
| PDE6B | c.1920+2T>C | Intron15 | [124] | |
| ARL2BP | c.207+1G>A | Intron3 | [125] | |
| NRL | c.-41_-28+23del | Exon1 | [126] | |
| Canonical splice acceptor site | PDE6B | c.1921-20_1921-3del | Intron15 | [109] |
| RPGR | c.470-1G>A | Intron5 | [127] | |
| c.29-2A>T | Intron1 | [118] | ||
| c.1754-3C>G | Intron14 | |||
| RHO | c.620 T > G | Exon3 | [111] | |
| PRPH2 | c.582-2A>T | Exon3 | [128] | |
| RDH11 | c.75-3C>A | Intron2 | [129] | |
| CDHR1 | c.1168-1G>A | Intron11 | [130] | |
| MAK | c.279-2A>G | Intron3 | [131] | |
| Branch point | BBS1 | c.592-21A>T | Intron7 | [132] |
| Polypyrimidine tract | RP2 | c.1073-9T>A | Intron3 | [133] |
| Cryptic splice site | LRAT | c.541-15T>G | Intron2 | [9] |
| PDE6B | c.1921–9C>G | Intron15 | [134] | |
| RPE65 | c.1430A>G | Exon13 | [110] | |
| Non-Canonical Splice Site | RPGR | c.247+5G>A | Intron3 | [135] |
| c.154+3_154+6del | Intron2 | |||
| c.779-5T>G | Intron7 | |||
| c.1573-12A>G | Intron13 | |||
| c.1415-9A>G | Intron11 | [136] | ||
| USH2A | c.5776G>A | Exon 28 | [137] | |
| c.10182G>A | Exon 51 | |||
| c.15519+2dup | Intron 71 | |||
| COQ5 | c.682-7T>G | Intron 5 | [138] | |
| Deep Intronic Splice Variant | CLRN1 | c.254-643G>T | Intron1 | [113] |
| RPGR | c.1059+363G>A | Intron9 | [139] | |
| USH2A | c.8682-654C>G | Intron43 | [116] | |
| OFD1 | c.IVS9+706A>G | Intron9 | [140] |
| Therapeutic Strategy | Representative Target/Disease Model | Clinical Trial Phase | Major Findings | Key Challenges |
|---|---|---|---|---|
| Gene supplementation (AAV-based) | RPE65, RPGR, PRPF31 | Luxturna (RPE65): FDA-approved (Phase IV); RPGR: Phase III (NCT03116113); PRPF31: Preclinical | Restored visual function in RPE65-LCA; structural and functional rescue in RPGR models; partial recovery in PRPF31-deficient RPE cells | Limited AAV cargo (~4.7 kb); inefficient for dominant-negative alleles; variable transduction efficiency |
| Antisense oligonucleotide (ASO) therapy | USH2A, PRPF31, CLRN1, CEP290 | QR-421a (USH2A): Phase I/II (NCT03780257); Sepofarsen or QR-110 (CEP290): Phase II/III for LCA10 (NCT03913143) | Corrected exon skipping or pseudoexon inclusion; restored functional protein expression and retinal function in models | Short duration, requires repeated intravitreal injection; delivery heterogeneity; mutation-specific design |
| U1 snRNA modification | RHO c.936G>A, RPGR c.1245+3A>T | Preclinical | Restored correct splicing and reduced exon skipping in RHO and RPGR mutations | Mutation-limited applicability; risk of overexpression or off-target binding; delivery optimization required |
| Spliceosome-mediated RNA trans-splicing (SMaRT) | RHO, RPGR, ABCA4 | Preclinical | Generated hybrid mRNA restoring correct reading frame and protein localization; functional recovery in animal models | Low efficiency and reproducibility; risk of off-target chimeric transcripts; difficult stoichiometric control |
| Genome editing (CRISPR/Cas9, base/prime editing) | CEP290, PRPF31, USH2A, RPGR, Pde6b | EDIT-101 (CEP290): Phase I/II (NCT03872479); others preclinical | Permanent correction of splice mutations; partial functional recovery in RP models | Off-target risks, low HDR efficiency in post-mitotic cells, immune response to Cas proteins, ethical concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Liu, X.; Fu, J.; Wu, Y.; Yu, S.; Yao, K. Alternative Splicing Dysregulation in Retinitis Pigmentosa: Pathogenic Mechanisms and Therapeutic Opportunities. Biomolecules 2025, 15, 1624. https://doi.org/10.3390/biom15111624
Jiang Y, Liu X, Fu J, Wu Y, Yu S, Yao K. Alternative Splicing Dysregulation in Retinitis Pigmentosa: Pathogenic Mechanisms and Therapeutic Opportunities. Biomolecules. 2025; 15(11):1624. https://doi.org/10.3390/biom15111624
Chicago/Turabian StyleJiang, Yuxin, Xuyu Liu, Jie Fu, Yican Wu, Shanshan Yu, and Kai Yao. 2025. "Alternative Splicing Dysregulation in Retinitis Pigmentosa: Pathogenic Mechanisms and Therapeutic Opportunities" Biomolecules 15, no. 11: 1624. https://doi.org/10.3390/biom15111624
APA StyleJiang, Y., Liu, X., Fu, J., Wu, Y., Yu, S., & Yao, K. (2025). Alternative Splicing Dysregulation in Retinitis Pigmentosa: Pathogenic Mechanisms and Therapeutic Opportunities. Biomolecules, 15(11), 1624. https://doi.org/10.3390/biom15111624








