1. Introduction
Sepsis is a life-threatening condition marked by a dysfunctional immune response to infection. It can advance to septic shock, multiple organ failure, and death, especially without early diagnosis and treatment [
1]. Sepsis remains a significant challenge for global health systems and is the leading cause of death among critically ill patients. Intensive care admission and extensive medical resources are often required, particularly within the first 48 h after presentation, and mortality can reach up to 46.4% [
2]. Additionally, the prolonged use of life support measures (such as invasive mechanical ventilation and venous, arterial, and urinary catheters) increases the risk of nosocomial infections, which are common complications in intensive care units [
3,
4]. The number of severe sepsis cases is expected to rise from 2.3 million in 2020 to 2.8 million by 2030, driven by an aging population, a higher burden of comorbidities, and increasing microbial resistance to antibiotics [
5,
6,
7].
Although antimicrobial resistance (AMR) is not the primary focus of this review, it further highlights the urgent need for rapid and precise diagnostic biomarkers. Earlier identification of sepsis could reduce the unnecessary use of broad-spectrum antimicrobials, prevent treatment delays, and improve patient outcomes.
The pathophysiology of sepsis is complex, involving a network of interactions between the innate immune system, endothelium, coagulation, and cellular metabolism. Activation of Toll-like receptors (TLRs) by pathogens triggers an inflammatory cascade via the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway. This causes a “cytokine storm,” the massive release of pro-inflammatory cytokines, such as Tumor Necrosis Factor Alpha (TNF-α), interleukin (IL)-1β, and IL-6 [
8,
9].
Sepsis involves a complex and dysregulated immune response where pro-inflammatory and counteracting anti-inflammatory mechanisms happen at the same time, leading to endothelial dysfunction, blood clotting issues, and metabolic problems that contribute to multiple organ failure [
8,
10]. In this pathophysiological state, early detection of patients in the initial stages of sepsis is essential for improving outcomes.
Traditional biomarkers such as C-reactive protein (CRP) and procalcitonin (PCT) have been widely used for diagnosing and monitoring sepsis; however, their clinical performance remains limited. CRP indicates overall inflammation rather than infection-specificity, whereas PCT exhibits variable kinetics depending on the infectious source and the presence of organ dysfunction. Although these markers aid in early suspicion and antibiotic stewardship, their diagnostic accuracy and prognostic value remain limited, especially in differentiating sepsis from sterile inflammation or non-infectious organ failure [
11]. Therefore, the search for novel, more specific, and dynamically regulated biomarkers, such as circulating microRNAs (miRNAs) and plasma gelsolin (pGSN), is of growing clinical and research interest. These short, non-coding RNA molecules are stable in biological fluids and accurately reflect the molecular disturbances of the immune and inflammatory response in sepsis [
12,
13].
miRNAs regulate various molecular pathways involved in sepsis, including those that trigger inflammatory responses, oxidative stress, apoptosis, and angiogenesis. Several studies have reported altered levels of miRNAs in sepsis patients, such as miR-150, miR-146a, and miR-223. These changes have been linked to disease severity, organ failure, and risk of death.
Given the major clinical impact of sepsis—and the complexity of the pathophysiological mechanisms involved—the early and accurate identification of high-risk patients is vital to reduce mortality, complications, and the economic burden associated with critical care [
14]. The use of molecular biomarkers, especially
miRNAs, not only offers the potential for early diagnosis and more precise risk stratification, but could also help guide therapeutic decision-making tailored to individual patient immune profiles [
15,
16].
Besides
microRNAs, plasma gelsolin (pGSN) has emerged as a significant circulating biomarker of the host’s inflammatory and immune response in sepsis. pGSN is a multifunctional actin-binding protein involved in extracellular actin scavenging, endothelial stabilization, and modulation of leukocyte activation. Lower plasma levels of pGSN have been linked to increased disease severity, organ failure, and worse outcomes in septic patients [
17,
18].
In contrast,
miRNAs regulate many of the same pathways at the post-transcriptional level, implying that pGSN and
miRNAs could serve as complementary markers that reflect different yet interconnected aspects of the host response and tissue injury resolution. Among emerging molecular biomarkers,
microRNAs (
miRNAs) and plasma gelsolin (pGSN) have garnered increasing attention for their complementary roles in immune regulation and tissue injury during sepsis.
miRNAs are small, non-coding RNAs that regulate gene expression post-transcriptionally, influencing inflammatory and immune signaling pathways [
14,
15]. Circulating miRNAs are stable and reliably detectable in plasma, making them promising diagnostic and prognostic biomarkers. Similarly, plasma gelsolin (pGSN) is an actin-binding protein with extracellular anti-inflammatory and cytoprotective properties; its levels decrease significantly during sepsis and correlate with disease severity and adverse outcomes [
17,
18]. Together, these molecules offer complementary insights into immune dysregulation and cellular damage, which are supported by their combined evaluation in this narrative review and precede the methodological overview.
2. Methods
This work was designed as a narrative, integrative review of the literature on the diagnostic and prognostic value of circulating microRNAs (miRNAs) and plasma gelsolin (pGSN) in sepsis and critical illness. The integrative review approach was chosen to synthesize findings from both clinical and experimental studies, combining molecular evidence with translational perspectives relevant to biomarker validation and clinical application.
A comprehensive search was conducted in PubMed, Embase, and Web of Science databases for studies published between 2008 and 2025, using the following keywords and Boolean combinations: “microRNA” OR “miRNA” OR “plasma gelsolin” AND “sepsis” OR “septic shock” OR “critical illness.” Only peer-reviewed articles published in English were included.
Additionally, the search strategy was expanded to include the literature on the use of artificial intelligence (AI), machine learning (ML), and computational modeling to combine and predict sepsis outcomes with miRNA and pGSN data. The following additional keywords were used: “artificial intelligence,” “machine learning,” “predictive model,” “biomarker integration,” and “data-driven diagnosis.” This ensured the inclusion of translational and computational research focused on AI-based biomarker modeling and multi-omics integration.
Studies were eligible if they reported experimental or clinical data on miRNA or pGSN expression in the context of sepsis, systemic inflammation, or organ dysfunction. Exclusion criteria included non-original publications (e.g., editorials, letters, conference abstracts), studies lacking biomarker quantification, and animal models unrelated to sepsis.
Data extraction covered the study population, the biomarker(s) assessed, the diagnostic criteria (Sepsis-2 or Sepsis-3), the severity scores, the main outcomes, and the biological mechanisms proposed. The quality assessment evaluated the study design, sample size, and biomarker validation methods.
3. Pathophysiology of Sepsis
3.1. Risk Factors and Susceptibility to Infections
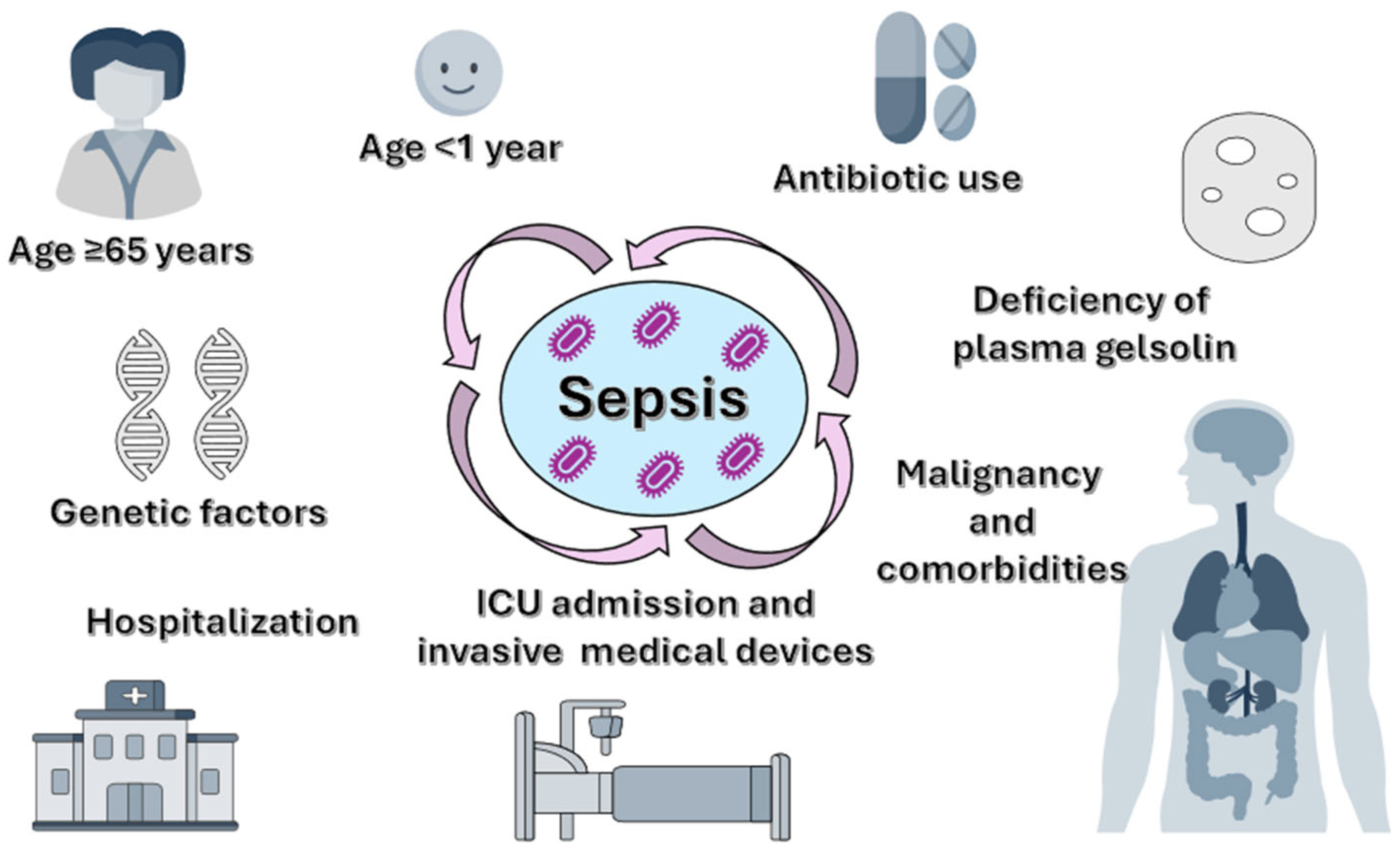
While even an apparently minor infection can cause sepsis, severe cases typically occur in vulnerable patients with weakened immune systems (
Figure 1). Older patients (≥65 years) are among the most affected groups due to immunosenescence, decreased physiological reserves, and related comorbidities such as diabetes mellitus, chronic kidney failure, or chronic obstructive pulmonary disease [
19,
20,
21].
Newborns and children under one year old are also highly vulnerable because their immune systems are still developing, limiting their ability to mount an effective response to infections. Additionally, both younger and older age groups face increased risks of hospitalization and exposure to invasive medical devices, which can lead to the development of nosocomial infections [
22]. Other risk groups include pregnant women in the postpartum period (due to pregnancy-specific immune changes); patients with chronic conditions (such as diabetes, cancer, liver, kidney, or lung diseases); those with immunosuppression (congenital, iatrogenic, or acquired, including Human Immunodeficiency Virus (HIV), chemotherapy, or corticosteroids); and individuals who have undergone recent major surgeries or prolonged hospital stays [
19,
23]. Moreover, survivors of a previous episode of sepsis are at higher risk of recurrence and death during the first year after discharge, with nearly 50% requiring rehospitalization [
19].
Prolonged intensive care unit (ICU) stays are linked to a higher risk of nosocomial infections due to exposure to invasive devices, such as venous and urinary catheters, parenteral nutrition, and mechanical ventilation [
22].
Besides clinical factors, genetic variants have been identified that impact the immune response at the molecular level, increasing susceptibility to sepsis and its severe forms. Genetic polymorphisms in genes encoding pathogen recognition receptors, such as Toll-like receptors (TLR-4 or TLR-2), can change immune cell responses to bacterial lipopolysaccharides (LPS), influencing the development of the inflammatory response [
24,
25]. For instance, the TLR4 polymorphism Asp299Gly is associated with a hyporeactive response to LPS, leading to delayed and insufficient activation of innate immunity, thereby increasing the risk of severe sepsis [
26].
Similarly, the variability of human leukocyte antigen (HLA) haplotypes involved in antigen presentation influences immune system recognition of pathogens, determining individual differences in susceptibility and disease severity [
27,
28].
Recent studies have identified rare mutations or altered expression levels in genes such as FER (Fps/Fes related tyrosine kinase) [
29], Vacuolar Protein Sorting 13 Homolog A (VPS13A [
30], and IRAK4 (Interleukin-1 Receptor-Associated Kinase 4) [
31], which modulate essential inflammatory pathways, such as NF-kB or JAK/STAT (Janus kinase/Signal Transducers and Activators of Transcription). These changes influence the immunological phenotype and prognosis of patients [
32]. Genome-wide association studies have helped decipher these genetic correlations and have supported the advancement of personalized medicine for sepsis [
33].
FER is a cytoplasmic tyrosine kinase involved in maintaining vascular integrity, regulating cell adhesion, and coordinating innate immune responses. Recent genomic studies have identified a variant (rs4957796) significantly associated with 28-day survival in patients with pneumococcal sepsis [
29], suggesting a protective role in regulating excessive inflammation and maintaining endothelial homeostasis [
29,
34,
35]. Functionally, FER influences neutrophil chemotaxis and stabilizes the endothelial barrier, most likely through indirect regulation of the NF-kB signaling pathway [
34]. Inactivation of FER in murine models led to unbalanced cytokine production, increased capillary permeability, and reduced responsiveness to bacterial endotoxins such as LPS [
36].
3.2. Dysregulated Host Immune Response: Early Triggers and Signaling Pathways
Sepsis is characterized by complex systemic immune dysfunction, including early imbalances between proinflammatory hyperactivation and profound, progressive immunosuppression [
37] (
Figure 2). The early inflammatory response is triggered by the Pathogen-Associated Molecular Patterns (PAMPs) and endogenous products released from damaged cells—damage-associated molecular patterns (DAMPs)—via pattern-recognition receptors (PRRs), especially toll-Like Receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) [
38]. The activation of these pathways leads to the nuclear translocation of the transcription factor NF-kB and the initiation of the cytokine storm through the massive release of proinflammatory mediators (TNF-α, IL-1β, IL-6); this results in fever, tachycardia, and changes in tissue perfusion [
39]. At the same time, inflammation activates the complement and coagulation systems, leading to immunothrombosis and cellular ischemia.
3.3. Endothelial Dysfunction, Immunothrombosis, and Sepsis-Related Coagulopathy
Systemic inflammation synergistically activates the complement and coagulation cascades (
Figure 2). Anaphylatoxins C3a and C5a help recruit and activate leukocytes, while also stimulating parallel platelet aggregation and tissue factor expression on endothelial cell surfaces. This concurrent activation leads to the formation of intravascular microthrombi, a process called immunothrombosis, which impairs microcirculatory flow and contributes to tissue ischemia and cellular hypoxia [
40]. Subsequent cell necrosis further amplifies the inflammatory response through the additional release of DAMPs [
41].
3.4. Acquired Immunosuppression and Immunometabolic Reprogramming
Paradoxically, early inflammatory hyperactivation occurs in parallel with a state of immunosuppression, marked by widespread lymphocyte apoptosis, reduced expression of the human leukocyte antigen (HLA)-DR molecule on monocytes, and functional depletion of regulatory T lymphocytes and suppressor myeloid cells [
42]. Additionally, the activation of certain immune pathways (Programmed Cell Death Protein-1 (PD-1)/Programmed Death-Ligand 1 (PD-L1)) exacerbates immune dysfunction and increases susceptibility to secondary infections [
43].
An essential aspect of immune dysfunction in sepsis is the metabolic reprogramming of immune cells, which switch from oxidative phosphorylation to aerobic glycolysis (the Warburg effect) [
44]. Simultaneous mitochondrial dysfunction, lactate buildup, and oxidative stress contribute to worsening organ damage and subsequent multiple organ failure.
4. Emerging Biomarkers in Sepsis
4.1. miRNAs: Epigenetic Regulators and Emerging Biomarkers in Sepsis
miRNAs are essential for the post-transcriptional regulation of gene expression. In sepsis, their expression profile is significantly changed, impacting both inflammatory hyperactivation and immune response suppression [
44].
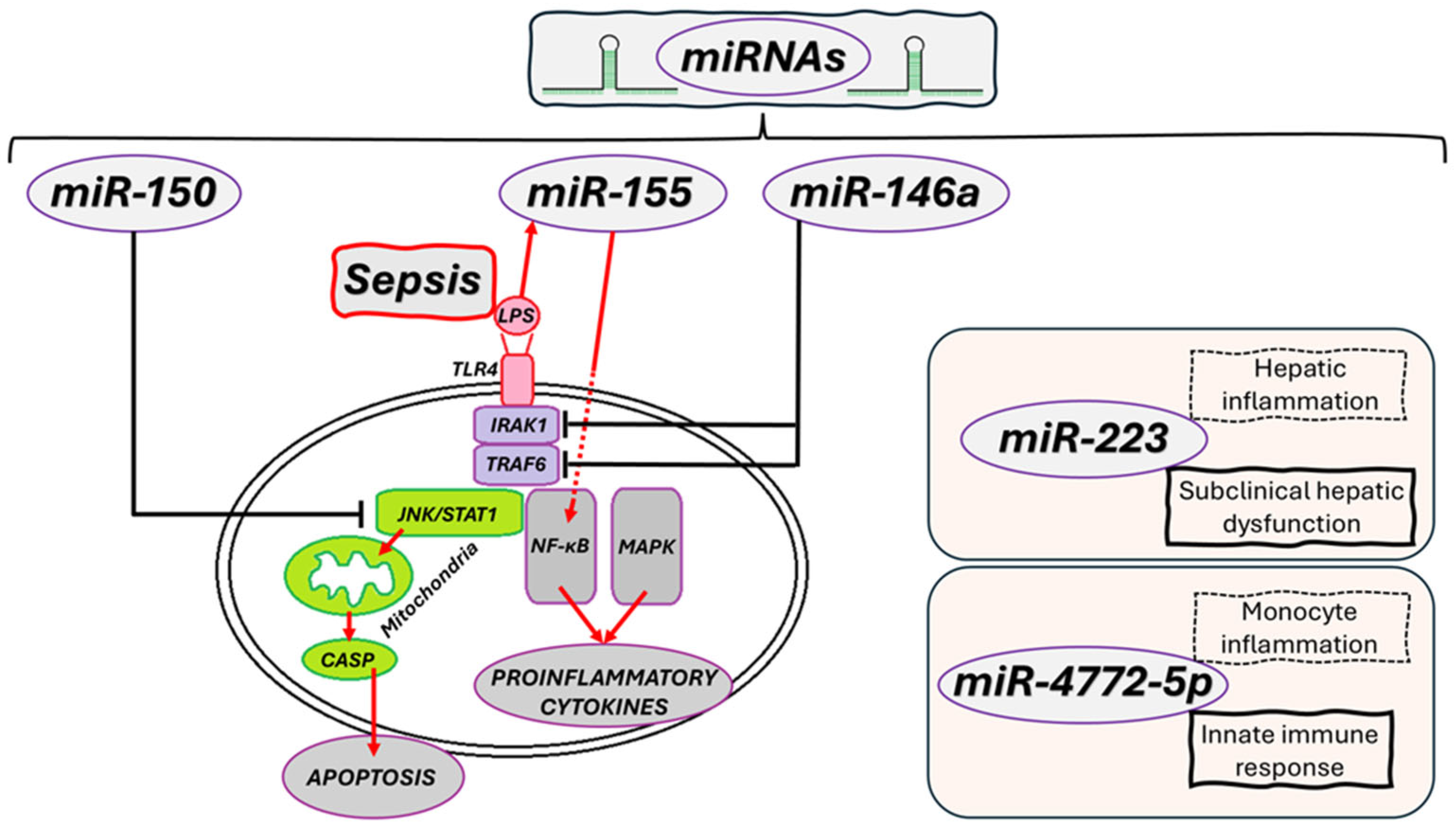
Figure 2 summarizes the dual inflammatory and immunosuppressive responses in sepsis, showing how dysregulated activation of innate immune pathways causes cytokine storm, immunothrombosis, and multi-organ damage.
For example, miR-146a inhibits TLR4 expression and reduces NF-κB activation, exerting an anti-inflammatory effect; meanwhile,
miR-155 mediates pro-inflammatory effects by stimulating the production of pro-inflammatory cytokines and worsening lung dysfunction [
45,
46].
Low
miR-150 levels are linked to poor prognosis, and
miR-122 signals early liver dysfunction. Other miRNAs, such as miR-223, have a protective role in lung inflammation. These epigenetic signatures support the use of
miRNAs as biomarkers for immunological and prognostic stratification in sepsis [
47]. For instance, low levels of miR-150 have been associated with increased mortality, making it a potential diagnostic and prognostic biomarker [
48].
The pathophysiology of sepsis involves complex interactions between uncontrolled inflammation and progressive immunosuppression, with metabolic, genetic, and epigenetic factors playing a role. Understanding these integrated mechanisms is the necessary basis for developing personalized treatments and optimizing early interventions in critical care. Besides sepsis,
miRNAs have also been extensively studied in other critical syndromes, such as acute respiratory distress syndrome (ARDS), acute pancreatitis, major trauma, and systemic inflammatory response syndrome (SIRS) [
49]. For example, miR-155 contributes to the amplification of inflammation and LPS-induced lung injury in ARDS [
50]. Conversely, a protective role has recently been confirmed for
miR-223 in models of post-traumatic or transfusion-induced lung injury [
51].
In both trauma-induced and sepsis-associated acute respiratory distress syndrome (ARDS),
miR-223 plays a key role in regulating pulmonary inflammation. Elevated levels of miR-223 have been observed in bronchoalveolar lavage fluid from patients and experimental models of ARDS after severe trauma, transfusion, or infection [
51].
In murine models, genetic removal of
miR-223 causes increased neutrophil infiltration and pulmonary edema, while administering
miR-223 externally decreases alveolar epithelial inflammation and reduces tissue damage. These results show that
miR-223 has a protective role in different ARDS causes—whether from trauma or sepsis—by lowering excessive inflammation and maintaining alveolar structure [
13,
52].
The specificity of the epigenetic response in sepsis—marked by the low expression of miR-150 and the increase of
miR-122—reflects the imbalance between inflammation and immunosuppression and supports the utility of these biomarkers as immunological stratification tools [
13,
52]. The immune imbalance in sepsis results from a chaotic response that is simultaneously pro-inflammatory and immunosuppressive, producing an exaggerated inflammatory response that is worsened by metabolic and epigenetic disturbances. Understanding these integrative mechanisms provides the basis for personalized medicine in intensive care.
miRNAs consist of approximately 20–24 nucleotides and regulate gene expression through
mRNA degradation and/or translational repression.
miRNAs act by binding to target
mRNA sequences via base pairing [
15]. Through this mechanism,
miRNAs precisely and specifically modulate fundamental biological processes such as inflammation, immune response, cellular metabolism, and apoptosis [
53]. In sepsis, immune homeostasis is severely disrupted, and the expression of certain circulating
miRNAs is profoundly altered, correlating with disease severity, organ dysfunction, and patient mortality. These molecules reflect a patient’s immunological status, and some directly influence key signaling pathways such as NF-kB, TLR4, Mitogen-Activated Protein Kinase (MAPK), or the systemic inflammatory response [
52]. Recent studies also show that several
microRNAs influence innate immune signaling beyond traditional NF-κB and TLR4 pathways. Specifically,
miR-223,
miR-146a, and
miR-155 have been linked to the regulation of cytosolic nucleic acid sensing and interferon signaling through the STING (Stimulator of Interferon Genes) pathway [
54].
These data establish the empirical basis for the mechanistic models and molecular interactions discussed below and summarized in
Figure 3 and
Table 1.
By fine-tuning STING expression and downstream type I interferon responses, these miRNAs may affect the balance between antimicrobial defense and hyperinflammation in sepsis.
This emerging link underscores the complex interplay between epigenetic regulation and innate immune activation, suggesting that
miRNA–STING interactions could serve as new therapeutic targets for immunomodulation in critical illness. For this reason, miRNAs are being increasingly studied as diagnostic biomarkers, predictors of severity and survival, and as potential targets for personalized therapies. A detailed overview of clinical and preclinical studies examining circulating microRNAs (
miRNAs) and plasma gelsolin (pGSN) in sepsis is provided later in
Table 2 and
Table 3.
4.1.1. miR-146a—Negative Regulator of Inflammation
miR-146a plays a crucial role in inhibiting the TLR4/NF-kB pathway by suppressing the expression of tumor necrosis factor receptor-associated factor 6 (TRAF6) and Interleukin-1 Receptor-Associated Kinase 1 (IRAK1), two essential amplifiers of inflammatory signaling [
55]. This mechanism results in a decrease in proinflammatory cytokine production (
Figure 3), limiting the expansion of the immune response. In the early stages of sepsis,
miR-146a overexpression has a protective effect, helping attenuate the excessive inflammatory response. However, in later phases, persistently elevated levels can promote a state of profound immunosuppression, impairing the host’s ability to fight secondary infection [
56].
As a dynamic biomarker, serum
miR-146a levels can indicate the shift from systemic inflammation to immunosuppression, offering an opportunity for early immunological stratification of patients [
57]. Therapeutic mimetics of
miR-146a have shown the ability to significantly reduce proinflammatory cytokine production in preclinical models, indicating their potential for translation [
58]. Currently,
miR-146a is primarily used as a circulating marker of immune response dynamics (from hyperinflammation to immunosuppression); its clinical application, on the other hand, remains unproven, and caution is advised due to the risk of immunosuppression [
59].
4.1.2. miR-150—Prognostic Biomarker and Anti-Inflammatory Regulator
miR-150 is well-studied in the context of sepsis, known for its role in reducing inflammation and protecting endothelial tissues. Its prognostic value is supported by a clinical study involving 223 critically ill patients—including 138 with sepsis—which revealed low plasma levels of
miR-150 to be significantly linked to liver and kidney dysfunction, as well as long-term mortality, with predictive power comparable to the Acute Physiology and Chronic Health Evaluation (APACHE) score [
59,
60]. Although it does not have proven diagnostic value in distinguishing septic from non-septic patients, miR-150 modulates NF-kB1 expression, leading to the suppression of TNFα, IL-6, Intercellular Adhesion Molecule 1 (ICAM1), Vascular Cell Adhesion Molecule 1 (VCAM1), and E-selectin, resulting in anti-inflammatory and protective effects in endothelial cells such as Human Umbilical Vein Endothelial Cells (HUVEC) and supporting vascular barrier integrity (
Figure 3) [
15]. In animal models, overexpression of miR-150 reduced endoplasmic reticulum (ER) stress and improved cardiac function in septic cardiomyopathy, through Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1)/NF-kB and Signal Transducer and Activator of Transcription 1 (STAT1)/c-Jun N-terminal Kinase (JNK)-dependent mechanisms [
59]. Furthermore, overexpression of
miR-150-5p in murine models of septic cardiomyopathy also showed protective effects, such as decreased apoptosis and enhanced cardiac function [
59]. In human clinical trials, lower levels of
miR–150 have been directly associated with increased mortality risk in critically ill patients, indicating its potential as a reliable prognostic biomarker [
61]. Currently,
miR-150 mainly has prognostic utility; low plasma levels correlate with adverse outcomes and organ dysfunction. Dynamic measurement at 0, 24, and 48 h from plasma via Reverse Transcription Quantitative Polymerase Chain Reaction/Digital PCR (RT-qPCR/Dpcr) is recommended, with integration of standard biomarkers and Sequential Organ Failure Assessment (SOFA) and APACHE II scores. Clinical application has not yet been demonstrated; main challenges include the targeting of delivery, determining the optimal timing of administration considering the hyperinflammation-immunosuppression transition, and safety concerns [
62].
4.1.3. miR-155—Inflammatory Response Enhancer
miR-155 is a proinflammatory microRNA induced primarily by LPS exposure, involved in activating the NF-κB pathway and regulating the synthesis of TNF-α and IL-6 (
Figure 3) [
63]. Its expression steadily increases in severe forms of sepsis and correlates with endothelial dysfunction, extensive tissue damage, and the cytokine storm [
64].
Pharmacological inhibition of
miR-155 in animal models has resulted in reduced systemic inflammation and improved survival [
65,
66]. In clinical context,
miR-155 has been proposed as an indicator of hyperinflammatory severity, but its therapeutic targeting remains at the preclinical stage [
67].
Although experimental data suggest that modulation of
miRNA expression could attenuate hyperinflammatory responses, these strategies remain at an early stage and have not yet been validated in human sepsis. Pharmacological inhibition with modified anti-
miR-155 locked nucleic acid has been shown to reduce inflammation and improve survival in preclinical models, but has not yet been clinically validated in humans [
67,
68,
69]. Therefore, the therapeutic potential of
miRNAs in sepsis should currently be viewed as experimental, pending further clinical research development.
4.1.4. miR-223—Fine Regulator of Inflammatory Homeostasis
miR-223 regulates the balance between inflammation activation and resolution, playing a role in controlling neutrophil recruitment and maintaining immune homeostasis. Although a defined clinical use is yet to be established, preclinical studies on models of ARDS and trauma suggest a protective role in lung inflammation and tissue damage prevention. Currently,
miR-223 is mainly used as a marker of an inflammatory lung phenotype (measured by serum or bronchoalveolar lavage (BAL) RT-qPCR, without a point-of-care test or standard clinical application (
Figure 3). Pulmonary delivery of
miR-223 for lower alveolar inflammation, potentially inhibiting NOD-like Receptor Family Pyrin Domain Containing 3 (NLRP3), is a potential direction. At present, evidence is limited to preclinical studies, with no validation in humans [
68].
4.1.5. miR-122 as a Biomarker of Hepatic Dysfunction
miR-122 is mainly expressed in hepatocytes and plays a vital role in regulating lipid metabolism and liver inflammation. In sepsis, circulating levels rise significantly before typical changes detected by liver tests, indicating subclinical liver damage [
69]. Due to this sensitivity,
miR-122 can serve as an early biomarker for liver dysfunction and enhance the prognostic profiling of critically ill patients (
Figure 3) [
70]. In sepsis, plasma
miR-122 increases early, predicting subclinical hepatic dysfunction; it is independently linked to 30-day mortality. Its inclusion could improve prognostic accuracy (through RT-qPCR/dPCR measurement, without the option for point-of-care testing) [
71,
72].
4.1.6. miR-4772-5p—The Emerging Epigenetic Signature
miR-4772-5p has recently been recognized for its role in regulating monocyte activation and associated inflammatory pathways. Emerging studies show that its expression varies significantly between bacterial and fungal infections, as well as between patients with favorable and poor outcomes [
73]. This variation highlights its potential as a marker for immunological stratification within sepsis, and as part of multi-
miRNA signatures used to identify endophenotypes [
74]. In sepsis,
miR-4772-5p is an emerging marker for stratification: it increases in cluster of differentiation (CD)14 monocytes (including after TLR stimulation) and can differentiate SIRS from sepsis, specifically for bacterial and fungal infections. Its clinical application remains experimental (determination by RT-qPCR/dPCR). Currently, there are no validated targets or therapies, and the absence of a murine homologue limits preclinical evaluation [
67].
Although promising diagnostic and prognostic correlations exist, most circulating
miRNAs (including
miR-223 and
miR-4772-5p) have only been validated in preclinical or retrospective human studies. Their measurement still depends on quantitative reverse-transcription PCR (RT-qPCR) or digital PCR (dPCR) conducted in centralized labs, with no point-of-care tests available for real-time clinical use [
67,
68,
69].
The lack of standardized pre-analytical handling, extraction, normalization, and reference materials is a significant obstacle to the routine application of these processes. Developing harmonized protocols and calibration across laboratories will be essential to ensure reproducibility and comparability before moving forward with prospective clinical validation.
An overview of clinical and preclinical studies examining circulating microRNAs (
miRNAs) and plasma gelsolin (pGSN) in sepsis is provided in
Table 2 and
Table 3.
Table 2 summarizes human clinical research on the diagnostic and prognostic performance of circulating
miRNAs (e.g.,
miR-150,
miR-122) and pGSN, while
Table 3 displays experimental and translational models that explore mechanistic pathways and therapeutic potential.
Table 2.
Human studies investigating circulating microRNAs (miRNAs) and plasma gelsolin (pGSN) in sepsis and severe infection.
Table 2.
Human studies investigating circulating microRNAs (miRNAs) and plasma gelsolin (pGSN) in sepsis and severe infection.
| Study (Author, Year) | Biomarker(s) | Population/Setting | N (If Stated) | Sepsis Type/Diagnostic Criteria | Severity Scores Used | Organ System Focus | Outcomes (Direction) | Notes |
|---|
| Vasilescu et al., 2009 [49] | miR-150 (plasma) | Adult ICU patients with severe sepsis and septic shock | 30 sepsis + 20 healthy controls | Sepsis-2 criteria; infection confirmed microbiologically and ≥2 SIRS criteria | NR | Predominantly pulmonary and abdominal infections | ↓ miR-150 levels significantly associated with ↑ mortality; inverse correlation with IL-18 and TNF-α | First clinical study identifying miR-150 as a prognostic plasma biomarker in sepsis; expression measured by RT-qPCR |
| Roderburg et al., 2013 [59] | miR-150 (serum) | Critically ill adults; subset with sepsis | 223 total patients (138 with sepsis) + 76 healthy controls | Sepsis diagnosed according to ACCP/SCCM (Sepsis-2) criteria | APACHE II and SOFA scores | Mixed etiologies —mainly abdominal and pulmonary sepsis | ↓ miR-150 correlated with ↑ SOFA and APACHE II scores and higher mortality; low miR-150 = poor prognosis | Independent validation of miR-150 as a prognostic marker in critical illness and sepsis; confirmed inverse relation with inflammatory cytokines and organ failure scores |
| Ma et al., 2018 [61] | miR-150 | Adult ICU patients diagnosed with sepsis; plasma samples analyzed and compared with healthy controls | 40 sepsis + 30 healthy controls | Sepsis-3 criteria (infection + organ dysfunction) | SOFA | Systemic/vascular endothelial dysfunction | ↓ miR-150 associated with ↑ mortality and ↑ inflammatory cytokines; in vitro overexpression suppressed LPS-induced NF-κB1 activation and endothelial apoptosis | Combined clinical + HUVEC experimental evidence showing miR-150 down-regulation contributes to sepsis-related endothelial injury and poor outcome |
| Zhou et al., 2024 [51] | miR-223, miR-146a miR-155 | Patients with severe polytrauma; pulmonary tissue and plasma samples analyzed | 24 trauma patients + control samples | systemic inflammatory response following polytrauma (sepsis-like immune dysregulation) | NR | Pulmonary/systemic inflammation | Combined C5 and CD14 inhibition induced an anti-inflammatory miRNA expression profile; ↑ miR-146a and ↓ miR-155 correlated with reduced cytokine release | Translational study linking trauma-related systemic inflammation and pulmonary miRNA regulation; supports mechanistic parallels with sepsis-induced lung injury and ARDS |
| Abou El-Khier et al., 2019 [73] | miR-122 (plasma) | Adult ICU patients with sepsis compared to healthy controls | 40 sepsis + 20 controls | Sepsis diagnosed according to Sepsis-2 (SIRS + infection criteria) | APACHE II | Hepatic dysfunction | ↑ miR-122 levels in sepsis vs. controls; expression correlated positively with disease severity and mortality | Supports miR-122 as a diagnostic and prognostic biomarker reflecting liver dysfunction and systemic inflammation in sepsis |
| Self et al., 2019 [17] | Plasma gelsolin (pGSN) | Adults hospitalized with community-acquired pneumonia (CAP); prospective, multicenter cohort in U.S. hospitals | 655 patients | CAP; subset fulfilling Sepsis-3 criteria analyzed | NR in manuscript | Respiratory/systemic | ↓ pGSN at admission predicted higher risk of ICU admission, need for mechanical ventilation, and 30-day mortality | Demonstrated prognostic value of low plasma gelsolin as an early biomarker of severe outcomes in infection-related sepsis; supports link between pGSN depletion, inflammation, and poor prognosis |
Most clinical studies examining circulating
miRNAs in sepsis used the Sepsis-3 diagnostic criteria proposed by Singer et al. (2016) [
1] and evaluated disease severity with the Sequential Organ Failure Assessment (SOFA) or APACHE II scoring systems.
The study populations mainly consisted of adult patients with sepsis of pulmonary or abdominal origin, often linked to Gram-negative bacterial infections (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa).
These clinical variables were considered in interpreting biomarker correlations, particularly the prognostic roles of
miR-122,
miR-150, and
miR-146a in relation to organ dysfunction and outcome severity. Additionally,
miR-155, although mainly studied in experimental CLP and LPS models, has consistently been linked to amplifying systemic inflammation through NF-κB and STAT3 activation, highlighting its importance in sepsis pathophysiology [
1].
The consistent use of standardized diagnostic and scoring systems enhances the comparability of results across studies, even within the narrative synthesis approach of this review.
Table 3.
Experimental and preclinical studies on circulating microRNAs (miRNAs) and plasma gelsolin (pGSN) in sepsis.
Table 3.
Experimental and preclinical studies on circulating microRNAs (miRNAs) and plasma gelsolin (pGSN) in sepsis.
| Study (Author, Year) | Biomarker(s) | Model/Species | Experimental Design | Organ System Focus | Key Findings |
|---|
| Feng et al., 2017 [68] | miR-223 | Mouse model of mitochondrial DAMP–induced acute lung injury (ALI) | Sepsis lung inflammation induced by mitochondrial damage–associated molecular patterns (mtDAMP-induced ALI) | Lung (ARDS-like injury) | Neutrophil-derived miR-223 inhibits NLRP3 activation, reduces IL-1β release, and attenuates ALI (ARDS-like) injury. |
| An et al., 2018 [56] | miR-146a | Mouse model of LPS-induced sepsis and cardiomyocyte culture | Endotoxin-induced sepsis (LPS 10 mg/kg, i.p.) | Heart (septic cardiomyopathy) | miR-146a overexpression suppresses IRAK1/TRAF6, decreases NF-κB signaling, reduces myocardial injury, and improves function. |
| Cao et al., 2021 [66] | miR-155 | Mouse model of cecal ligation and puncture (CLP)-induced sepsis | CLP-induced polymicrobial sepsis | Intestine/systemic inflammation | Improved barrier integrity; ↓ NF-κB activity; ↓ inflammation |
| Lee et al., 2007 [75] | pGSN | Mouse model of cecal ligation and puncture (CLP)–induced sepsis | CLP-induced polymicrobial sepsis and endotoxin challenge | Systemic/multi-organ | Demonstrated that pGSN depletion contributes to sepsis mortality; exogenous pGSN supplementation restored immune homeostasis and improved outcomes, suggesting therapeutic potential |
| Zhi et al., 2024 [39] | pGSN | Mouse model of SARS-CoV-2 spike protein–induced cytokine storm and acute lung injury (ALI) | Recombinant pGSN administration | Lung systemic | Demonstrated the anti-inflammatory and cytoprotective effects of exogenous gelsolin in cytokine storm and ALI; findings parallel therapeutic potential in sepsis-induced ARDS |
Collectively, these clinical and preclinical data support the translational importance of miRAs and pGSN as biomarkers that indicate immune dysregulation and tissue damage in sepsis, providing a conceptual link to the diagnostic and prognostic implications discussed in the next section.
Although circulating
miRNAs show strong promise as immunoregulatory biomarkers and potential therapeutic targets, their clinical application is still limited by several technical and biological barriers. These include low in vivo stability and delivery efficiency of synthetic mimics or inhibitors, non-specific tissue distribution, and possible off-taret gene interactions that could disrupt normal signaling networks [
67,
68].
Moreover, immune responses to oligonucleotide-based therapies and the absence of standardized delivery systems—such as exosome-based or lipid nanoparticle methods—pose significant translational challenges. Consequently,
miRNA-focused treatments for sepsis should currently be considered experimental and need further validation through multicenter prospective studies before they can be widely used in clinical settings [
67,
68].
4.2. Cytokine–miRNA Regulatory Axis in Sepsis: Roles of miRNA-509-3p, miRNA-145-5p and miRNA-494
A detailed overview of clinical and preclinical studies examining circulating microRNAs (
miRNAs) and plasma gelsolin (pGSN) in sepsis is provided later in
Table 2 and
Table 3. These data establish the empirical foundation for the mechanistic models and molecular interactions discussed below and summarized in
Figure 3 and
Table 1.
Experimental studies have demonstrated that
miR-509-3p and
miR-494 are transcriptionally activated by pro-inflammatory cytokines such as TNF-α and IL-1β, leading to the suppression of cystic fibrosis transmembrane conductance regulator (CFTR)
mRNA and protein expression in epithelial cells [
76,
77]. CFTR down-regulation impairs epithelial ion and fluid transport, increases surface viscosity, and reduces the clearance of inflammatory mediators—mechanisms that, when activated in the lung during systemic inflammation, may contribute to alveolar fluid buildup, impaired gas exchange, and heightened susceptibility to secondary infections [
78].
These molecular changes are not exclusive to genetic cystic fibrosis but can also happen as an acquired response in severe inflammation, including sepsis-induced lung injury (SILI) [
79]. The cytokine–
miRNA–CFTR pathway shows how systemic inflammation can cause local barrier dysfunction through post-transcriptional mechanisms.
Meanwhile,
miR-145-5p plays a crucial regulatory role in vascular smooth muscle and endothelial cells, influencing cytoskeletal structure and intercellular junction proteins. Inflammatory cytokines like IL-6 and TGF-β have been shown to increase
miR-145-5p, which in turn promotes endothelial contraction, actin stress-fiber formation, and increased permeability—features of microcirculatory dysfunction in sepsis [
80].
These data collectively support the existence of a cytokine-driven
miRNA regulatory network that affects both epithelial and endothelial compartments in sepsis. The induction of
miR-509-3p and
miR-494 links inflammatory signaling to failure of epithelial ion transport, while
miR-145-5p contributes to vascular leak and endothelial instability [
76,
77,
78]. This integrated cytokine–
miRNA–barrier axis provides a clear mechanism for sepsis-related organ dysfunction and highlights potential biomarkers and therapeutic targets for future translational research.
4.3. pGSN in Sepsis: Diagnostic and Prognostic Implications
Beyond established clinical and genetic factors, pGSN deficiency has emerged as a new risk factor for severe infections, sepsis, and ARDS. Although plasma gelsolin (pGSN) and circulating microRNAs (miRNAs) are different biomarker types, both indicate the severity and regulation of the host response in sepsis.
Several miRNAs involved in cytoskeletal remodeling and inflammation, such as miR-21, miR-146a, and miR-155, target signaling pathways that also regulate pGSN expression and actin dynamics.
Therefore, pGSN may act as a complementary indicator to miRNA profiles, offering insights into cellular injury, immune dysregulation, and endothelial dysfunction.
pGSN has multiple biological roles: it regulates inflammation, clears extracellular actin, stabilizes the endothelial barrier, and neutralizes bacterial toxins. In sepsis, pGSN levels drop sharply, reaching 25–30% of normal values within the first 6 h, a drop linked to extracellular actin presence and the exaggerated inflammatory response [
39].
Preclinical studies in murine models using LPS and cecal ligation and puncture (CLP) techniques have demonstrated that exogenous pGSN supplementation boosts survival, reduces cytokine storms, and increases IL-10 levels, producing an anti-inflammatory effect.
Additionally, pGSN can bind and neutralize various bacterial endotoxins, including LPS, lipoteichoic acid, and platelet-activating factor. It also inhibits neutrophil and platelet activation and stabilizes the vascular barrier, reducing permeability and edema [
39].
The therapeutic potential of pGSN has been demonstrated in Streptococcus pneumoniae models, where replenishing pGSN boosted Nitric Oxide Synthase 3 (NOS3)-mediated phagocytosis, lowered bacterial load, and significantly improved survival, even without antibiotics. In chemically induced ARDS models, pGSN administration lowered pulmonary leukocyte infiltration, alveolar permeability, and mortality, indicating a direct protective effect on the lungs [
39].
In humans, low plasma pGSN levels at ICU admission have been linked to severe sepsis and ARDS, and serve as an independent predictor of mortality. Survivors showed a gradual recovery in pGSN levels, which correlated with clinical improvement.
In 2024, a Phase II study was launched to assess the safety and efficacy of recombinant human pGSN as an adjunctive treatment in patients with moderate to severe ARDS [
81]. These data suggest that monitoring pGSN levels and administering the exogenous form [
75] as therapy can play a crucial role in preventing severe progression and improving patient outcomes.
Taken together, circulating miRNAs and pGSN reflect complementary aspects of the host response in sepsis—the former regulating gene expression and signaling pathways, and the latter indicating cytoskeletal integrity and extracellular actin clearance.
This complementarity provides the foundation for integrative molecular profiling, which will be discussed in the next section.
5. Integrating miRNA and pGSN Profiles for Personalized Medicine in Sepsis
Advances in systems biology (multi-omics) demonstrate the integration of transcriptomic, proteomic, and epigenetic data including miRNA expression to define distinct molecular subtypes of sepsis (e.g., hyperinflammatory, immunosuppressive, or metabolically deregulated phenotypes) [
73].
Such integrative approaches support a personalized approach to treating critically ill patients, where
miRNA signatures could guide the selection of immunotherapies such as anti-PD-1, IL-7 or be used for monitoring treatment effectiveness, response, or early adjustments to life support strategies [
53].
This strategy paves the way toward individualized management of critically ill patients, combining molecular markers like
miRNA and pGSN with computational modeling and AI-based prediction to enhance diagnostic precision and prognostic accuracy [
66].
6. Integrative Perspectives: Artificial Intelligence and Biomarker-Guided Sepsis Management
6.1. Early Diagnosis and Prognostic Stratification with Artificial Intelligence
Artificial intelligence (AI) algorithms, especially those based on machine learning (ML) and deep learning (DL), have shown significant potential for early sepsis detection and prognostic stratification. By processing large-scale data from electronic health records (EHRs), vital signs, and laboratory results, these models can identify subtle multidimensional patterns preceding clinical diagnosis, often outperforming traditional systems such as NEWS2 or qSOFA in sensitivity and timeliness [
82].
The Targeted Real-time Early Warning System (TREWS), developed at Johns Hopkins, has been shown to predict sepsis onset up to six hours before clinical recognition, reducing mortality by approximately 20% in selected groups. Likewise, advanced recurrent neural network (RNN)-based architectures, such as Multi-task Gaussian Process (MGP)-RNNs and Sepsis Finger, achieved areas under the receiver operating characteristic curve (AUROC) values greater than 0.85 [
83,
84].
Despite these advances, current AI models rely almost exclusively on clinical and physiological variables. The integration of molecular and epigenetic biomarkers—particularly circulating microRNAs (miRNAs) and plasma gelsolin (pGSN)—remains conceptual and exploratory, without prospective clinical validation.
Such integration could theoretically improve immune phenotyping and early detection of high-risk subgroups, but it requires standardized molecular assays, harmonized data infrastructures, and regulatory validation before being used clinically.
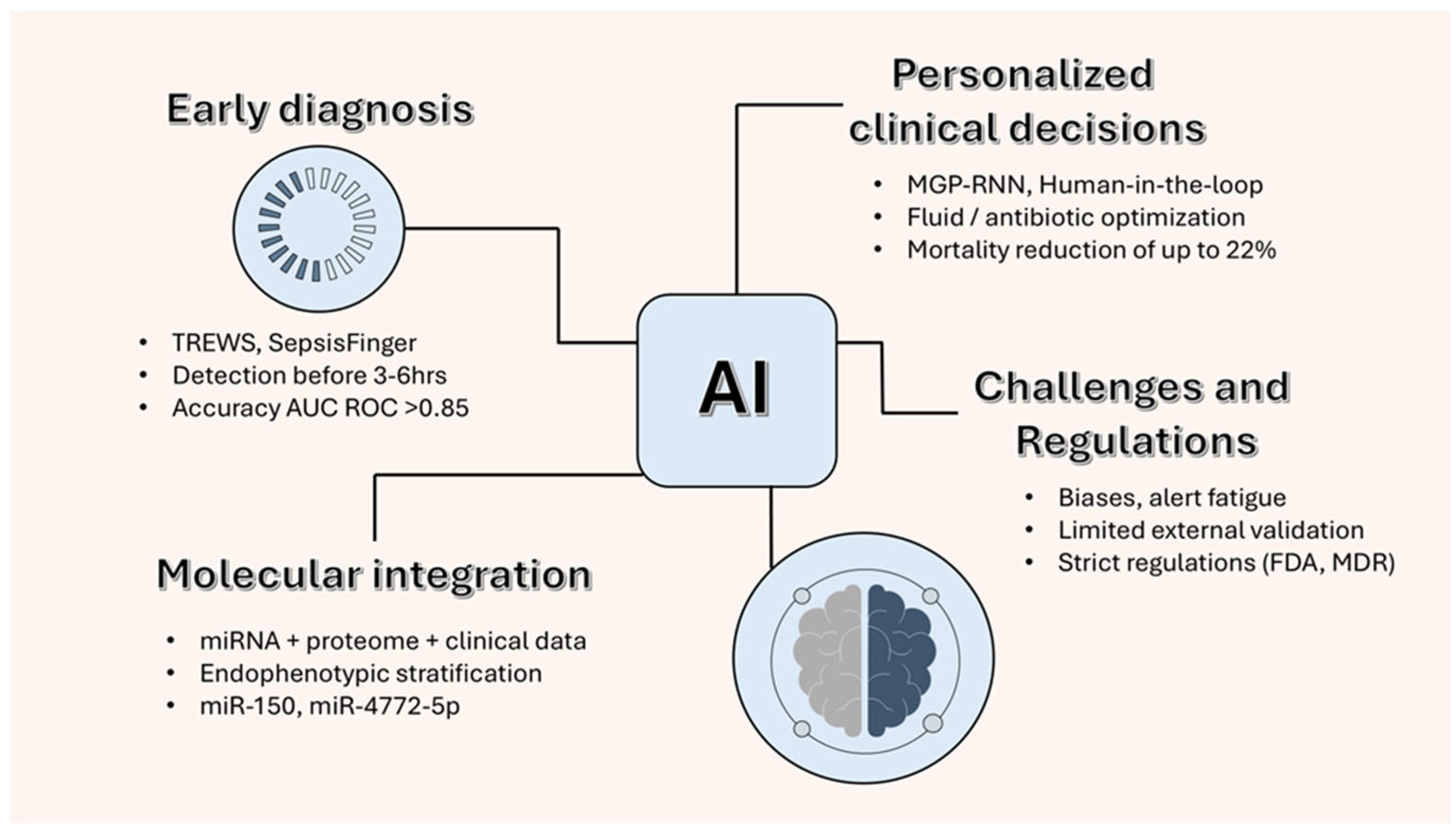
Figure 4 illustrates how integrating circulating
miRNAs and plasma gelsolin (pGSN) data with AI-based analytic models may enhance early sepsis detection and personalized treatment decisions. This conceptual framework highlights the translational potential of combining molecular and computational approaches, while acknowledging that such integration remains at a preclinical, exploratory stage.
6.2. AI for Clinical Decision Support and Real-Time Treatment Monitoring
In intensive care settings, AI-assisted clinical decision-support systems are increasingly used to optimize fluid therapy, antibiotic dosing, and life-sustaining interventions. Retrospective analyses (e.g., MIMIC-III) reported reductions in mortality of up to 20% following ML-guided treatment adjustments, along with fewer prescription errors and shorter ICU stays [
30,
85,
86].
Recent advances in multi-omics have facilitated the integration of transcriptomic, proteomic, and epigenetic data, including miRNA and pGSN signatures, into AI-driven predictive models. These multimodal frameworks can distinguish different immunological endotypes, such as hyperinflammatory and immunosuppressive phenotypes, thereby enhancing diagnostic accuracy and prognostic stratification [
12,
87].
Although promising, such approaches remain at a conceptual and preclinical stage, requiring multicenter validation, algorithm transparency, and reproducibility before clinical adoption.
7. Future Directions: AI and Biomarkers for Personalized Immunotherapy
Advances in precision medicine are transforming sepsis management by incorporating molecular biomarkers and Artificial Intelligence (AI) tools to enable personalized treatment approaches.
While microRNAs (
miRNAs) such as
miR-155 and
miR-146a have shown the ability to modulate inflammatory and endothelial pathways in preclinical models, their translation into therapy remains difficult due to pharmacokinetic barriers, molecular instability, and potential off-target effects [
60,
88].
Strategies using antagomirs or miRNA mimics are still in the experimental phase, needing optimized delivery methods, enhanced safety profiles, and thorough clinical testing before being used in humans.
In parallel, AI and machine learning (ML) technologies may eventually assist in personalizing immunotherapy by integrating molecular, clinical, and physiological data to predict individual treatment responses and optimize timing and dosage [
89].
This integration could enable precise immunomodulation, such as identifying candidates for IL-7 supplementation, immune checkpoint inhibition (PD-1/PD-L1 blockade), or epigenetic therapies targeting dysfunctional immune states [
13,
90].
Nonetheless, these applications remain theoretical. Translational success will depend on multicenter validation, algorithm reproducibility, and ethical data management frameworks that promote transparency and clinical safety.
The convergence of omics biomarkers (miRNA, pGSN) with AI-guided analytics is an emerging yet promising approach in personalized sepsis care, where computational and biological insights could eventually support tailored, mechanism-based treatments.
8. Challenges in Translating AI and Biomarkers into Clinical Practice
Although the technical performance shows promise, the clinical use of AI in sepsis remains limited. Issues include selection biases, alert fatigue among doctors, data variability, and lack of external validation [
91]. A prominent example is the algorithm developed by Epic Systems, which, despite heavy promotion, performed poorly in real-world settings. As reported in a 2021 study by Wong et al., the system missed approximately 67% of true sepsis cases and generated a substantial number of false alerts [
92].
Meanwhile, the clinical use of
miRNAs faces challenges due to the lack of standardization, cross-platform differences, and the need for their regulation as diagnostic tools. Incorporating a quick bedside detection panel in intensive care units, such as for miR-150 and miR-146a, could facilitate early immune profiling and personalized therapy adjustments [
89,
90]. For both AI and epigenetic biomarkers, the move from research to clinical practice demands a responsible, regulated, and patient-focused approach [
90].
Despite encouraging findings, several practical barriers hinder the integration of molecular biomarkers and AI tools into routine sepsis care. First, the economic burden of implementing omics-based diagnostics and AI-driven platforms is significant; costs include not only those of assays but also ongoing investment in data infrastructure, cybersecurity, and technical support. This presents a challenge for low- and middle-income healthcare systems, where sepsis has the greatest impact. Second, the need for standardized assays and multicenter validation remains critical. Most candidate biomarkers, including circulating miRNAs and pGSN, have been studied in relatively small, diverse cohorts. Without harmonized protocols and reproducibility across different patient populations, their translation into clinical practice will remain limited.
Finally, the absence of pilot clinical models that combine biomarker-guided decision-making with AI-based tools represents a major gap. Prospective studies and pragmatic clinical trials are essential to determine whether such integrated approaches enhance patient-centered outcomes, decrease inappropriate antibiotic use, and are practical within current intensive care infrastructures.
9. Strengths and Limitations
This report should be considered a narrative synthesis rather than a systematic review, which may affect reproducibility. However, it is one of the first comprehensive efforts to highlight the complementary role of emerging biomarkers, such as circulating
microRNAs and pGSN, alongside AI tools for sepsis management. Previous reviews have focused either on the diagnostic and prognostic value of
miRNAs [
13], the clinical use of AI-based models in critical care, or the application of AI-driven models in sepsis [
89,
91].
From a clinical perspective, circulating microRNAs (miRNAs) and plasma gelsolin (pGSN) may support precision strategies for managing sepsis. First, specific miRNA signatures (e.g., miR-150, miR-146a, miR-122) could help identify patients at higher risk of sepsis or organ dysfunction. Second, early changes in miRNA and pGSN levels may complement established clinical scores (SOFA, APACHE II) for rapid diagnosis and severity assessment. Third, sequential monitoring of these biomarkers could help evaluate treatment response and recovery. Incorporating such molecular data into AI-driven decision systems is still experimental but may ultimately improve personalized therapy and outcome prediction in critical care.
By combining these perspectives, our article uniquely emphasizes how molecular biomarkers paired with AI analytics could lead to earlier diagnosis, better immune stratification, and personalized treatment strategies in sepsis.
10. Conclusions
Circulating miRNAs and pGSN are emerging as promising biomarkers for early diagnosis, risk stratification, and outcome prediction in sepsis. Their biological relevance and consistent association with disease severity support further investigation. However, translation into clinical practice remains limited by the absence of large-scale multicenter validation, the high cost and low availability of rapid bedside assays, and the lack of standardized measurement protocols.
AI-based approaches may enhance diagnostic accuracy and facilitate personalized treatments, but their implementation requires robust digital infrastructure, interoperability across clinical systems, and external validation to ensure reproducibility and safety.
Future work should focus on multicenter prospective studies to validate biomarker panels across diverse intensive care populations and develop standardized rapid assays, and pilot trials conducted to determine whether biomarker-guided or AI-assisted strategies enhance patient-centered outcomes.
In summary, although circulating miRNAs and pGSN show great potential, their use as practical tools should be treated with cautious optimism, requiring validation and gradual implementation before they can enable precision medicine in sepsis.
Author Contributions
Conceptualization, M.S., C.B. and A.S.; methodology, M.S. and C.B.; software, D.-F.B.; validation, M.S. and A.S.; formal analysis, C.B. and L.A.; investigation, M.S.; resources, M.S.; data cu-ration, D.-F.B. and S.R.B.; writing—original draft preparation, M.S.; writing—review and editing, M.S.; visualization, L.A.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureș, Research Grant number 170/1/09.01.2024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors gratefully acknowledge the financial support of George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures, Research grant number 170/1/09.01.2024. This paper was published with the support of George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Targu Mures.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| ARDS | Acute Respiratory Distress Syndrome |
| ICU | Intensive Care Unit |
| IL | Interleukin |
| IL-1β | Interleukin 1 Beta |
| IL-6 | Interleukin 6 |
| LPS | Lipopolysaccharide |
| miRNA | microRNA |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells |
| NLRP3 | NOD-like Receptor Family Pyrin Domain Containing 3 |
| PCT | Procalcitonin |
| PD-1 | Programmed Cell Death Protein-1 |
| PD-L1 | Programmed Death-Ligand 1 |
| pGSN | Plasma Gelsolin |
| Rhu-pGSN | Recombinant Human Plasma Gelsolin |
| RT-qPCR | Reverse Transcription Quantitative PCR |
| RT-qPCR/dPCR | Reverse Transcription Quantitative Polymerase Chain Reaction/Digital PCR |
| SIRS | Systemic Inflammatory Response Syndrome |
| TLR/TLR4 | Toll-Like Receptors/Toll-Like Receptor 4 |
| VCAM1 | Vascular Cell Adhesion Molecule 1 |
| SOFA | Sequential Organ Failure Assessment |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor Necrosis Factor-α |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- van den Berg, M.; van Beuningen, F.E.; Ter Maaten, J.C.; Bouma, H.R. Hospital-related costs of sepsis around the world: A systematic review exploring the economic burden of sepsis. J. Crit. Care 2022, 71, 154096, ISSN 0883-9441. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Azamfirei, L.; Andone, A.; Văsieșiu, A.M.; Stîngaciu, A.; Huțanu, A.; Bândilă, S.R.; Dobru, D.; Manea, A.; Stoian, A. Incidence and Risk Factors of Secondary Infections in Critically Ill SARS-CoV-2 Patients: A Retrospective Study in an Intensive Care Unit. Biomedicines 2025, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Andone, A.; Bândilă, S.R.; Onișor, D.; Laszlo, S.Ș.; Lupu, G.; Danielescu, A.; Baba, D.F.; Văsieșiu, A.M.; Manea, A.; et al. Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units. Antibiotics 2025, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- GlobalData. Severe Sepsis and Septic Shock—Epidemiology Forecast to 2030. Available online: https://www.globaldata.com/store/report/severe-sepsis-and-septic-shock-epidemiology-analysi (accessed on 15 July 2025).
- Patel, J.; Harant, A.; Fernandes, G.; Mwamelo, A.J.; Hein, W.; Dekker, D.; Sridhar, D. Measuring the global response to antimicrobial resistance, 2020–2021: A systematic governance analysis of 114 countries. Lancet Infect. Dis. 2023, 23, 706–718. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464, ISSN 1074-7613. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int. Immunopharmacol. 2020, 89 Pt B, 107087. [Google Scholar] [CrossRef]
- Tavaci, T.; Akgun, N. Sepsis: Immunopathology, Immunotherapies, and Future Perspectives. Eurasian J. Med. 2022, 54 (Suppl. 1), 127–132. [Google Scholar] [CrossRef]
- Ghatak, T.; Sajjad, S.A.; Das, A.; Kumar, A.; Halemani, K.; Rochwerg, B. The Diagnostic Accuracy of Serum Procalcitonin for Sepsis in Adult Patients in the Emergency Department: A Systematic Review and Meta-Analysis. Chest Crit. Care 2025, 3, 100179, ISSN 2949-7884. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Huang, Y.; Tong, J.; Zhang, L.; Zhang, Z.; Yu, W.; Qiu, Y. Accuracy of circulating microRNAs in diagnosis of sepsis: A systematic review and meta-analysis. J. Intensive Care 2020, 8, 84. [Google Scholar] [CrossRef]
- Formosa, A.; Turgeon, P.; dos Santos, C.C. Role of miRNA dysregulation in sepsis. Mol. Med. 2022, 28, 99. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.S.S.; Tamayo-Giraldo, F.J.; Bejarano-Zuleta, A.; Nati-Castillo, H.A.; Quintero, D.A.; Ospina-Mejía, M.J.; Salazar-Santoliva, C.; Suárez-Sangucho, I.; Ortiz-Prado, E.; Izquierdo-Condoy, J.S. Sepsis and post-sepsis syndrome: A multisystem challenge requiring comprehensive care and management—A review. Front. Med. 2025, 12, 1560737. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, Y.; Li, Y.; Zheng, X. Significances of miRNAs for predicting sepsis mortality: A meta-analysis. Front. Microbiol. 2025, 16, 1472124. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Ahmad, W.; Gull, B.; Baby, J.; Panicker, N.G.; Khader, T.A.; Baki, H.A.; Rehman, E.; Salim, A.M.; Ahmed, R.L.G.; et al. miRNA biomarkers for prognosis and therapy monitoring in a multi-ethnic cohort with SARS-CoV-2 infection. Sci. Rep. 2025, 15, 30815. [Google Scholar] [CrossRef]
- Self, W.H.; Wunderink, R.G.; DiNubile, M.J.; Stossel, T.P.; Levinson, S.L.; Williams, D.J.; Anderson, E.J.; Bramley, A.M.; Jain, S.; Edwards, K.M.; et al. Low Admission Plasma Gelsolin Concentrations Identify Community-acquired Pneumonia Patients at High Risk for Severe Outcomes. Clin. Infect. Dis. 2019, 69, 1218–1225. [Google Scholar] [CrossRef]
- Piktel, E.; Levental, I.; Durnaś, B.; Janmey, P.A.; Bucki, R. Plasma Gelsolin: Indicator of Inflammation and Its Potential as a Diagnostic Tool and Therapeutic Target. Int. J. Mol. Sci. 2018, 19, 2516. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery From Sepsis: A Review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock. JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Stoian, M.; Andone, A.; Bândilă, S.R.; Onișor, D.; Babă, D.F.; Niculescu, R.; Stoian, A.; Azamfirei, L. Personalized Nutrition Strategies for Patients in the Intensive Care Unit: A Narrative Review on the Future of Critical Care Nutrition. Nutrients 2025, 17, 1659. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240010789 (accessed on 10 August 2025).
- Chantratita, N.; Tandhavanant, S.; Seal, S.; Wikraiphat, C.; Wongsuvan, G.; Ariyaprasert, P.; Suntornsut, P.; Teerawattanasook, N.; Jutrakul, Y.; Srisurat, N.; et al. TLR4 genetic variation is associated with inflammatory responses in Gram-positive sepsis. Clin. Microbiol. Infect. 2017, 23, 47.e1–47.e10. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Nadel, S. Impact of Inherited Genetic Variants on Critically Ill Septic Children. Pathogens 2022, 11, 96. [Google Scholar] [CrossRef]
- Lundberg, A.; Wikberg, L.A.; Ilonen, J.; Vaarala, O.; Böttcher, M.F. Lipopolysaccharide-induced immune responses in relation to the TLR4(Asp299Gly) gene polymorphism. Clin. Vaccine Immunol. 2008, 15, 1878–1883. [Google Scholar] [CrossRef]
- Migliorini, F.; Torsiello, E.; Spiezia, F.; Oliva, F.; Tingart, M.; Maffulli, N. Association between HLA genotypes and COVID-19 susceptibility, severity and progression: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 84. [Google Scholar] [CrossRef]
- Crux, N.B.; Elahi, S. Human Leukocyte Antigen (HLA) and Immune Regulation: How Do Classical and Non-Classical HLA Alleles Modulate Immune Response to Human. Immunodeficiency Virus and Hepatitis C Virus Infections? Front. Immunol. 2017, 8, 832. [Google Scholar] [CrossRef]
- Rautanen, A.; Mills, T.C.; Gordon, A.C.; Hutton, P.; Steffens, M.; Nuamah, R.; Chiche, J.D.; Parks, T.; Chapman, S.J.; Davenport, E.E.; et al. ESICM/ECCRN GenOSept Investigators. Genome-wide association study of survival from sepsis due to pneumonia: An observational cohort study. Lancet Respir. Med. 2015, 3, 53–60. [Google Scholar] [CrossRef]
- Wong, H.R.; Cvijanovich, N.Z.; Anas, N.; Allen, G.L.; Thomas, N.J.; Bigham, M.T.; Weiss, S.L.; Fitzgerald, J.; Checchia, P.A.; Meyer, K.; et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am. J. Respir. Crit. Care Med. 2015, 191, 309–315. [Google Scholar] [CrossRef]
- Picard, C.; Puel, A.; Bonnet, M.; Ku, C.L.; Bustamante, J.; Yang, K.; Soudais, C.; Dupuis, S.; Feinberg, J.; Fieschi, C.; et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 2003, 299, 2076–2079. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Sig. Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Hernandez-Beeftink, T.; Guillen-Guio, B.; Lorenzo-Salazar, J.M.; Corrales, A.; Suarez-Pajes, E.; Feng, R.; Rubio-Rodríguez, L.A.; Paynton, M.L.; Cruz, R.; García-Laorden, M.I.; et al. Genetics of Sepsis (GEN-SEP) Network. A genome-wide association study of survival in patients with sepsis. Crit. Care 2022, 26, 341. [Google Scholar] [CrossRef]
- Rogne, T.; Damås, J.K.; Flatby, H.M.; Åsvold, B.O.; DeWan, A.T.; Solligård, E. The Role of FER rs4957796 in the Risk of Developing and Dying from a Bloodstream Infection: A 23-Year Follow-up of the Population-based Nord-Trøndelag Health Study. Clin. Infect. Dis. 2021, 73, e297–e303. [Google Scholar] [CrossRef] [PubMed]
- Hinz, J.; Büttner, B.; Kriesel, F.; Steinau, M.; Frederik Popov, A.; Ghadimi, M.; Beissbarth, T.; Tzvetkov, M.; Bergmann, I.; Mansur, A. The FER rs4957796 TT genotype is associated with unfavorable 90-day survival in Caucasian patients with severe ARDS due to pneumonia. Sci. Rep. 2017, 7, 9887. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, D.M.; Craig, A.W.; Senis, Y.A.; Greer, P.A. Absence of Fer protein-tyrosine kinase exacerbates leukocyte recruitment in response to endotoxin. J. Immunol. 2002, 168, 4930–4935. [Google Scholar] [CrossRef] [PubMed]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Zhi, J.; Zhao, K.X.; Liu, J.H.; Yang, D.; Deng, X.M.; Xu, J.; Zhang, H. The therapeutic potential of gelsolin in attenuating cytokine storm, ARDS, and ALI in severe COVID-19. Front. Pharmacol. 2024, 15, 1447403. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef]
- Rittirsch, D.; Flierl, M.A.; Ward, P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008, 8, 776–787. [Google Scholar] [CrossRef]
- Schuermans, S.; Kestens, C.; Marques, P.E. Systemic mechanisms of necrotic cell debris clearance. Cell Death Dis. 2024, 15, 557. [Google Scholar] [CrossRef]
- King, H.A.D.; Lewin, S.R. Immune checkpoint inhibitors in infectious disease. Immunol. Rev. 2024, 328, 350–371. [Google Scholar] [CrossRef] [PubMed]
- Papaneophytou, C. The Warburg Effect: Is it Always an Enemy? Front. Biosci. 2024, 29, 402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, L.; Wang, K.; Qi, Q.; Wang, M.; Yang, L.; Sun, P.; Mu, H. MicroRNA-146a inhibits NF-κB activation and pro-inflammatory cytokine production by regulating IRAK1 expression in THP-1 cells. Exp. Ther. Med. 2019, 18, 3078–3084. [Google Scholar] [CrossRef]
- Zhou, J.; Chaudhry, H.; Zhong, Y.; Ali, M.M.; Perkins, L.A.; Owens, W.B.; Morales, J.E.; McGuire, F.R.; Zumbrun, E.E.; Zhang, J.; et al. Dysregulation in microRNA expression in peripheral blood mononuclear cells of sepsis patients is associated with immunopathology. Cytokine 2015, 71, 89–100. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef]
- Wang, X.; Kan, Y.; Chen, L.; Ge, P.; Ding, T.; Zhai, Q.; Yu, Y.; Wang, X.; Zhao, Z.; Yang, H.; et al. miR-150 is a negative independent prognostic biomarker for primary gastrointestinal diffuse large B-cell lymphoma. Oncol. Lett. 2020, 19, 3487–3494. [Google Scholar] [CrossRef]
- Vasilescu, C.; Rossi, S.; Shimizu, M.; Tudor, S.; Veronese, A.; Ferracin, M.; Nicoloso, M.S.; Barbarotto, E.; Popa, M.; Stanciulea, O.; et al. MicroRNA fingerprints identify miR-150 as a plasma prognostic marker in patients with sepsis. PLoS ONE 2009, 4, e7405. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Zhou, N.; Groven, R.V.M.; Horst, K.; Mert, Ü.; Greven, J.; Mollnes, T.E.; Huber-Lang, M.; van Griensven, M.; Hildebrand, F.; Balmayor, E.R. Pulmonary miRNA expression after polytrauma depends on the surgical invasiveness and displays an anti-inflammatory pattern by the combined inhibition of C5 and CD14. Front. Immunol. 2024, 15, 1402571. [Google Scholar] [CrossRef]
- de Kerckhove, M.; Tanaka, K.; Umehara, T.; Okamoto, M.; Kanematsu, S.; Hayashi, H.; Yano, H.; Nishiura, S.; Tooyama, S.; Matsubayashi, Y.; et al. Targeting miR-223 in neutrophils enhances the clearance of Staphylococcus aureus in infected wounds. EMBO Mol. Med. 2018, 10, e9024. [Google Scholar] [CrossRef]
- Bannazadeh Baghi, H.; Bayat, M.; Mehrasa, P.; Alavi, S.M.A.; Lotfalizadeh, M.H.; Memar, M.Y.; Taghavi, S.P.; Zarepour, F.; Hamblin, M.R.; Nahand, J.S.; et al. Regulatory role of microRNAs in virus-mediated inflammation. J. Inflamm. 2024, 21, 43. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Liu, Y.; Ying, H.; Zeng, X. MicroRNAs regulate the STING pathway: Implications for immune homeostasis and inflammatory diseases. Cell Death Differ. 2025, 32, 1295–1307. [Google Scholar]
- Niazi, S.K.; Magoola, M. MicroRNA Nobel Prize: Timely Recognition and High Anticipation of Future Products-A Prospective Analysis. Int. J. Mol. Sci. 2024, 25, 12883. [Google Scholar] [CrossRef]
- An, R.; Feng, J.; Xi, C.; Xu, J.; Sun, L. miR-146a Attenuates Sepsis-Induced Myocardial Dysfunction by Suppressing IRAK1 and TRAF6 via Targeting ErbB4 Expression. Oxid. Med. Cell Longev. 2018, 2018, 7163057. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Gao, J.; Wang, L.; Hao, P.; Chen, X.; Wang, Y.; Jiang, Z.; Jiang, L.; Wang, T.; Zhu, L.; et al. MicroRNA-146a negatively regulates inflammation via the IRAK1/TRAF6/NF-κB signaling pathway in dry eye. Sci. Rep. 2023, 13, 11192. [Google Scholar] [CrossRef] [PubMed]
- Saba, R.; Sorensen, D.L.; Booth, S.A. MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune Response. Front. Immunol. 2014, 5, 578. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Luedde, M.; Vargas Cardenas, D.; Vucur, M.; Scholten, D.; Frey, N.; Koch, A.; Trautwein, C.; Tacke, F.; Luedde, T. Circulating microRNA-150 serum levels predict survival in patients with critical illness and sepsis. PLoS ONE 2013, 8, e54612. [Google Scholar] [CrossRef]
- Gronau, L.; Duecker, R.P.; Jerkic, S.-P.; Eickmeier, O.; Trischler, J.; Chiocchetti, A.G.; Blumchen, K.; Zielen, S.; Schubert, R. Dual Role of microRNA-146a in Experimental Inflammation in Human Pulmonary Epithelial and Immune Cells and Expression in Inflammatory Lung Diseases. Int. J. Mol. Sci. 2024, 25, 7686. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Hou, H.; Yao, Y.; Meng, H. MiR-150 predicts survival in patients with sepsis and inhibits LPS-induced inflammatory factors and apoptosis by targeting NF-κB1 in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2018, 500, 828–837. [Google Scholar] [CrossRef]
- What will it take to get miRNA therapies to market? Nat. Biotechnol. 2024, 42, 1623–1624. [CrossRef]
- Valsamaki, A.; Vazgiourakis, V.; Mantzarlis, K.; Stamatiou, R.; Makris, D. MicroRNAs in Sepsis. Biomedicines 2024, 12, 2049. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef]
- Cao, Y.-Y.; Wang, Z.; Wang, Z.-H.; Jiang, X.-G.; Lu, W.-H. Inhibition of miR-155 alleviates sepsis-induced inflammation and intestinal barrier dysfunction by inactivating NF-κB signaling. Int. Immunopharmacol. 2021, 90, 107218, ISSN 1567-5769. [Google Scholar] [CrossRef] [PubMed]
- Bindayna, K. MicroRNA as Sepsis Biomarkers: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 6476. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Qi, S.; Zhang, Y.; Qi, Z.; Yan, L.; Zhou, J.; He, F.; Li, Q.; Yang, Y.; Chen, Q.; et al. Ly6G+ neutrophil-derived miR-223 inhibits the NLRP3 inflammasome in mitochondrial DAMP-induced acute lung injury. Cell Death Dis. 2017, 8, e3170. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.; Chakraborty, A.; Khan, I.; Monts, J. Relevance of miR-223 as Potential Diagnostic and Prognostic Markers in Cancer. Biology 2022, 11, 249. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Baltimore, D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. USA 2009, 106, 7113–7118. [Google Scholar] [CrossRef]
- Butt, A.M.; Raja, A.J.; Siddique, S.; Khan, J.S.; Shahid, M.; Tayyab, G.U.; Minhas, Z.; Umar, M.; Idrees, M.; Tong, Y. Parallel expression profiling of hepatic and serum microRNA-122 associated with clinical features and treatment responses in chronic hepatitis C patients. Sci. Rep. 2016, 6, 21510. [Google Scholar] [CrossRef]
- He, R.R.; Yue, G.L.; Dong, M.L.; Wang, J.Q.; Cheng, C. Sepsis Biomarkers: Advancements and Clinical Applications-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9010. [Google Scholar] [CrossRef]
- Abou El-Khier, N.T.; Zaki, M.E.; Alkasaby, N.M. Study of MicroRNA-122 as a Diagnostic Biomarker of Sepsis. Egypt. J. Immunol. 2019, 26, 105–116. [Google Scholar] [PubMed]
- Pennisi, F.; Pinto, A.; Ricciardi, G.E.; Signorelli, C.; Gianfredi, V. Artificial intelligence in antimicrobial stewardship: A systematic review and meta-analysis of predictive performance and diagnostic accuracy. Eur. J. Clin. Microbiol. Infect. Dis. 2025, 44, 463–513. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Waxman, A.B.; Cotich, K.L.; Chung, S.W.; Perrella, M.A.; Stossel, T.P. Plasma gelsolin is a marker and therapeutic agent in animal sepsis. Crit. Care Med. 2007, 35, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Karp, P.H.; Osterhaus, S.R.; Jiang, P.; Wohlford-Lenane, C.; Lennox, K.A.; Jacobi, A.M.; Praekh, K.; Rose, S.D.; Behlke, M.A.; et al. Post-transcriptional regulation of cystic fibrosis transmembrane conductance regulator expression function by microRNAs. Am. J. Respir. Cell Mol. Biol. 2013, 49, 544–551. [Google Scholar] [CrossRef]
- Megiorni, F.; Cialfi, S.; Dominici, C.; Quattrucci, S.; Pizzuti, A. Synergistic post-transcriptional regulation of the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) by miR-101 and miR-494 specific binding. PLoS ONE 2011, 6, e26601. [Google Scholar] [CrossRef]
- Hook, J.L.; Kuebler, W.M. CFTR as a therapeutic target for severe lung infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2025, 328, L229–L238. [Google Scholar] [CrossRef]
- Eisenhut, M.; Wallace, H. Acquired cystic fibrosis transmembrane conductance regulator dysfunction. Pflugers Arch-Eur. J. Physiol. 2025. [Google Scholar] [CrossRef]
- Li, L.; Mao, D.; Li, C.; Li, M. miR-145-5p Inhibits Vascular Smooth Muscle Cells (VSMCs) Proliferation and Migration by Dysregulating the Transforming Growth Factor-b Signaling Cascade. Med. Sci. Monit. 2018, 24, 4894–4904. [Google Scholar] [CrossRef]
- October 17, 2024: BioAegis Therapeutics Enrolls First Patient in Phase 2 Clinical Trial of Gelsolin, an lmmune Regulator, for the Treatment of Acute Respiratory Distress Syndrome(ARDS). Available online: https://www.bioaegistherapeutics.com/news/october-17-2024-bioaegis-therapeutics-enrolls-first-patient-in-phase-2-clinical-trial-of-gelsolin-an-immune-regulator-for-the-treatment-of-acute-respiratory-distress-syndrome-ards/?utm_source=chatgpt.com (accessed on 10 August 2025).
- Wu, M.; Du, X.; Gu, R.; Wei, J. Artificial Intelligence for Clinical Decision Support in Sepsis. Front. Med. 2021, 8, 665464. [Google Scholar] [CrossRef]
- Qayyum, S.N.; Ullah, I.; Rehan, M.; Noori, S. AI integration in sepsis care: A step towards improved health and quality of life outcomes. Ann. Med. Surg. 2024, 86, 2411–2412. [Google Scholar] [CrossRef]
- ran-The, T.; Heo, E.; Lim, S.; Suh, Y.; Heo, K.N.; Lee, E.E.; Lee, H.Y.; Kim, E.S.; Lee, J.Y.; Jung, S.Y. Development of machine learning algorithms for scaling-up antibiotic stewardship. Int. J. Med. Inform. 2024, 181, 105300. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, H.; Wu, R.; Zhou, Z. AI-driven multi-omics profiling of sepsis immunity in the digestive system. Front. Immunol. 2025, 16, 1590526. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Artificial Intelligence and Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan; FDA: Silver Spring, ML, USA, 2021. Available online: https://www.fda.gov/media/145022/download (accessed on 10 August 2025).
- Yang, X.; Luo, B.; Tian, J.; Wang, Y.; Lu, X.; Ni, J.; Yang, Y.; Jiang, L.; Ren, S. Biomarkers and ImmuneScores in lung cancer: Predictive insights for immunotherapy and combination treatment strategies. Biol. Proced. Online 2025, 27, 25. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Danesh, F.R. Promises and challenges of miRNA therapeutics. Am. J. Physiol. Renal Physiol. 2022, 323, F673–F674. [Google Scholar] [CrossRef] [PubMed]
- Bignami, E.G.; Berdini, M.; Panizzi, M.; Domenichetti, T.; Bezzi, F.; Allai, S.; Damiano, T.; Bellini, V. Artificial Intelligence in Sepsis Management: An Overview for Clinicians. J. Clin. Med. 2025, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Garduno, A.; Cusack, R.; Leone, M.; Einav, S.; Martin-Loeches, I. Multi-Omics Endotypes in ICU Sepsis-Induced Immunosuppression. Microorganisms 2023, 11, 1119. [Google Scholar] [CrossRef]
- Adams, R.; Henry, K.E.; Sridharan, A.; Soleimani, H.; Zhan, A.; Rawat, N.; Johnson, L.; Hager, D.N.; Cosgrove, S.E.; Markowski, A.; et al. Prospective, multi-site study of patient outcomes after implementation of the TREWS machine learning-based early warning system for sepsis. Nat. Med. 2022, 28, 1455–1460. [Google Scholar] [CrossRef]
- Wong, A.; Otles, E.; Donnelly, J.P.; Krumm, A.; McCullough, J.; DeTroyer-Cooley, O.; Pestrue, J.; Phillips, M.; Konye, J.; Penoza, C.; et al. External Validation of a Widely Implemented Proprietary Sepsis Prediction Model in Hospitalized Patients. JAMA Intern Med. 2021, 181, 1065–1070, Erratum in JAMA Intern Med. 2021, 181, 1144. https://doi.org/10.1001/jamainternmed.2021.3907. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).