High-Throughput Antibody Profiling Identifies Targets of Protective Immunity against P. falciparum Malaria in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Samples and Ethical Statements
2.2. Production of P. falciparum Biotinylated Protein Library Using Wheat Germ Cell-Free System (WGCFS)
2.3. Quantitation of Protein-Specific Antibodies Using AlphaScreen
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Malaria Participants
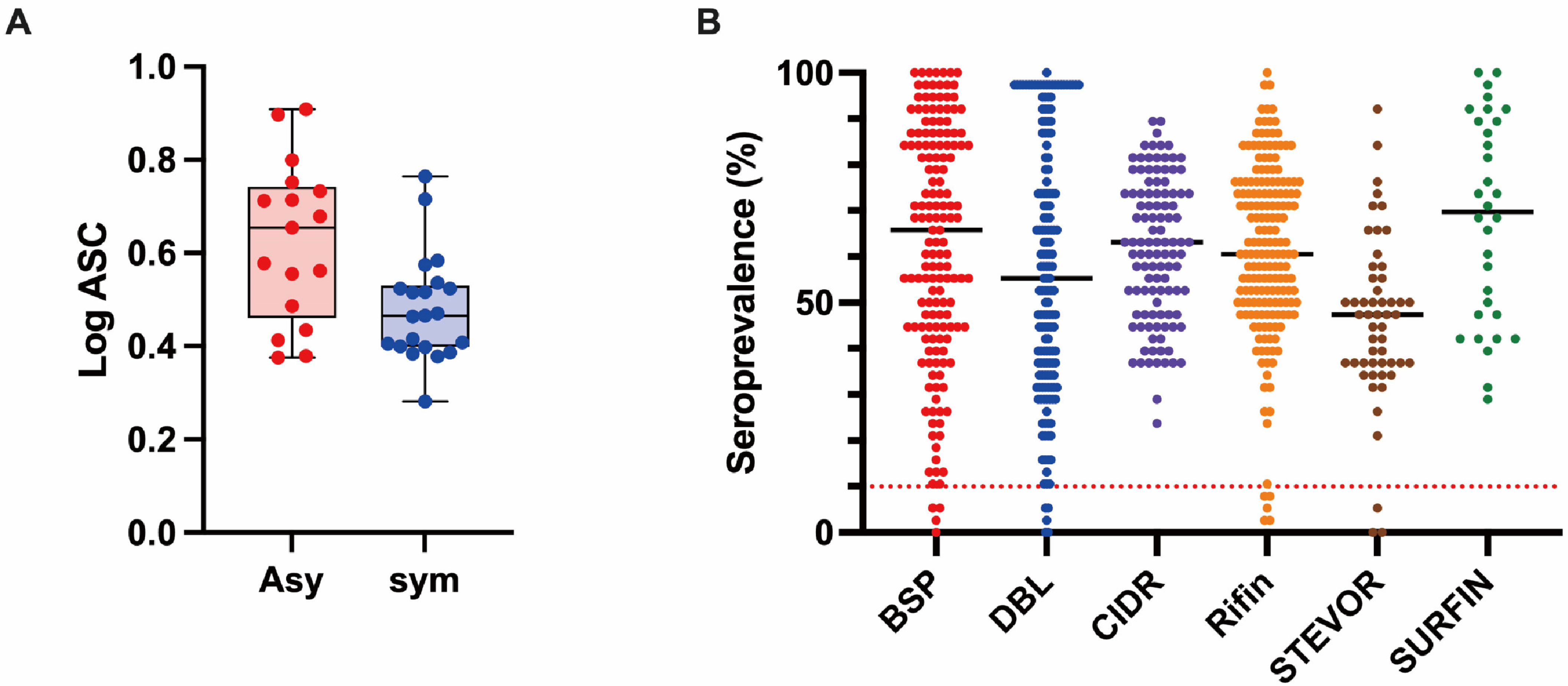
3.2. Profiling of the Human IgG Responses to Plasmodium Falciparum Proteins
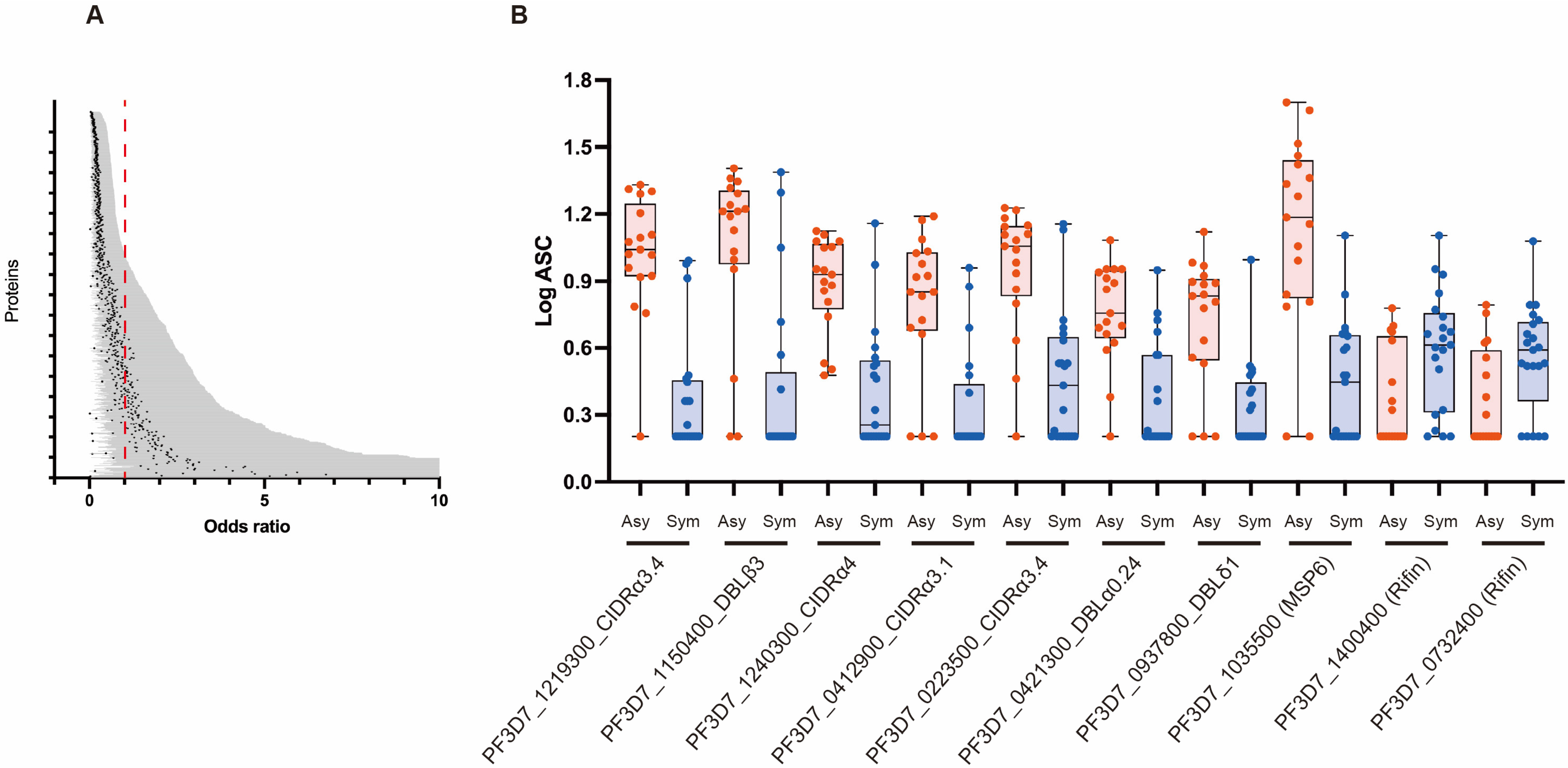
3.3. Association between Antibody Responses and Risk of Clinical Malaria
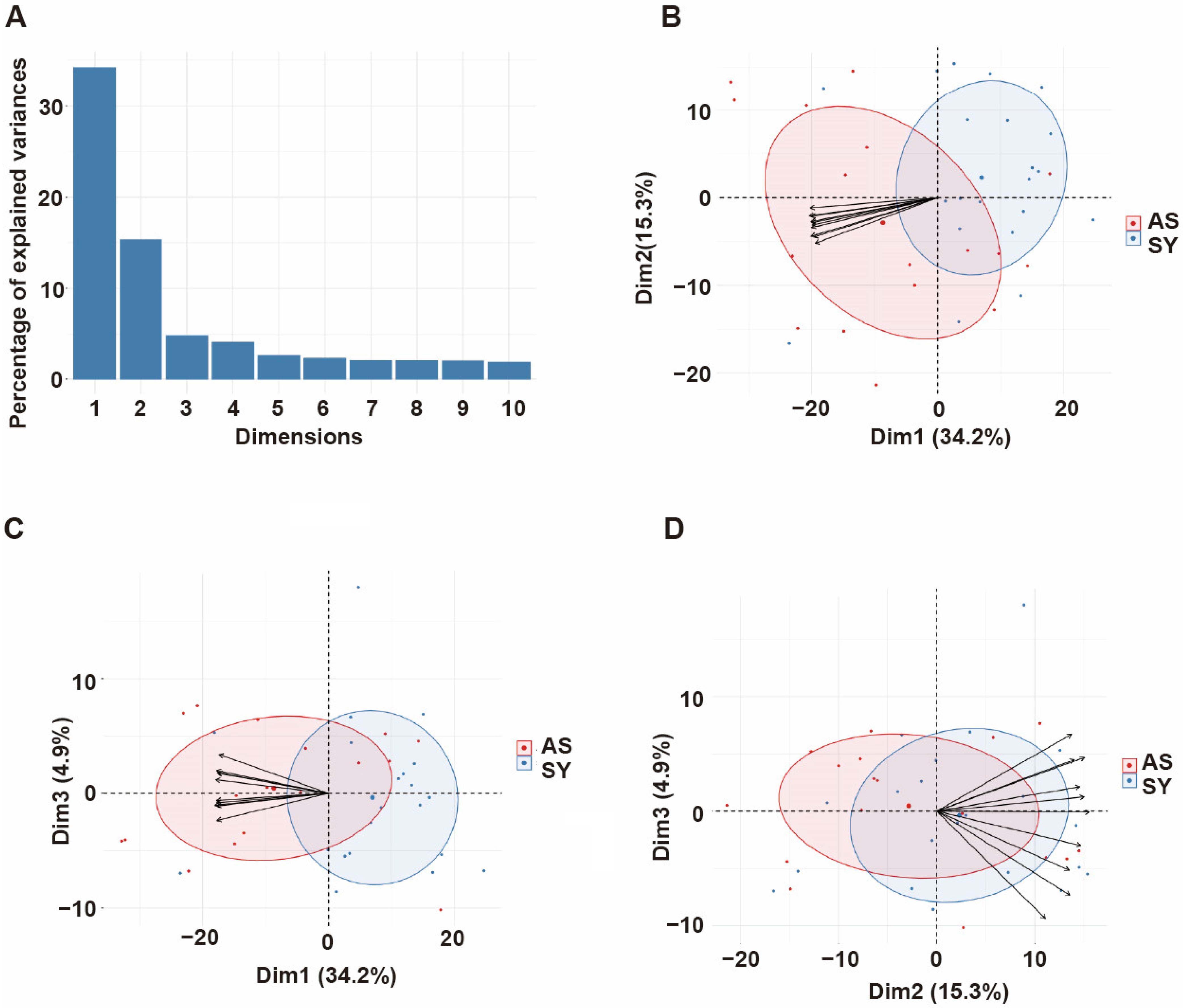
3.4. Principal Component Analysis of Antibody Responses
3.5. Comparison of Seropositivity between Thai and Ugandan Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Cockburn, I.A.; Seder, R.A. Malaria prevention: From immunological concepts to effective vaccines and protective antibodies. Nat. Immunol. 2018, 19, 1199–1211. [Google Scholar] [CrossRef]
- Gupta, S.; Snow, R.W.; Donnelly, C.A.; Marsh, K.; Newbold, C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 1999, 5, 340–343. [Google Scholar] [CrossRef]
- Fowkes, F.J.; Richards, J.S.; Simpson, J.A.; Beeson, J.G. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010, 7, e1000218. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, S.J.; Reyes, R.A.; Braddom, A.E.; Batugedara, G.; Bol, S.; Bunnik, E.M. Naturally acquired humoral immunity against Plasmodium falciparum malaria. Front. Immunol. 2020, 11, 594653. [Google Scholar] [CrossRef] [PubMed]
- Good, M.F.; Miller, L.H. Interpreting challenge data from early phase malaria blood stage vaccine trials. Expert Rev. Vaccines 2018, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Horii, T.; Bzik, D.J.; Inselburg, J. Characterization of antigen-expressing Plasmodium falciparum cDNA clones that are reactive with parasite inhibitory antibodies. Mol. Biochem. Parasitol. 1988, 30, 9–18. [Google Scholar] [CrossRef]
- Borre, M.B.; Dziegiel, M.; Høgh, B.; Petersen, E.; Rieneck, K.; Riley, E.; Meis, J.F.; Aikawa, M.; Nakamura, K.; Harada, M.; et al. Primary structure and localization of a conserved immunogenicPlasmodium falciparum glutamate rich protein (GLURP) expressed in both the preerythrocytic and erythrocytic stages of the vertebrate life cycle. Mol. Biochem. Parasitol. 1991, 49, 119–131. [Google Scholar] [CrossRef]
- Ntege, E.H.; Takashima, E.; Morita, M.; Nagaoka, H.; Ishino, T.; Tsuboi, T. Blood-stage malaria vaccines: Post-genome strategies for the identification of novel vaccine candidates. Expert Rev. Vaccines 2017, 16, 769–779. [Google Scholar] [CrossRef]
- Kanoi, B.N.; Nagaoka, H.; Morita, M.; Tsuboi, T.; Takashima, E. Leveraging the wheat germ cell-free protein synthesis system to accelerate malaria vaccine development. Parasitol. Int. 2021, 80, 102224. [Google Scholar] [CrossRef]
- Tsuboi, T.; Takeo, S.; Iriko, H.; Jin, L.; Tsuchimochi, M.; Matsuda, S.; Han, E.-T.; Otsuki, H.; Kaneko, O.; Sattabongkot, J.; et al. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect. Immun. 2008, 76, 1702–1708. [Google Scholar] [CrossRef]
- Rui, E.; Fernandez-Becerra, C.; Takeo, S.; Sanz, S.; Lacerda, M.V.; Tsuboi, T.; del Portillo, H.A. Plasmodium vivax: Comparison of immunogenicity among proteins expressed in the cell-free systems of Escherichia coli and wheat germ by suspension array assays. Malar. J. 2011, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Takashima, E.; Kanoi, B.N.; Nagaoka, H.; Morita, M.; Hassan, I.; Palacpac, N.M.; Egwang, T.G.; Horii, T.; Gitaka, J.; Tsuboi, T. Meta-Analysis of Human Antibodies Against Plasmodium falciparum Variable Surface and Merozoite Stage Antigens. Front. Immunol. 2022, 13, 887219. [Google Scholar] [CrossRef] [PubMed]
- Landier, J.; Parker, D.M.; Thu, A.M.; Carrara, V.I.; Lwin, K.M.; Bonnington, C.A.; Pukrittayakamee, S.; Delmas, G.; Nosten, F.H. The role of early detection and treatment in malaria elimination. Malar. J. 2016, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Kumpitak, C.; Ponlawat, A.; Maneechai, N.; Phunkitchar, V.; Rachapaew, N.; Zollner, G.; Sattabongkot, J. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J. Med. Entomol. 2004, 41, 201–208. [Google Scholar] [CrossRef]
- Sakamoto, H.; Takeo, S.; Takashima, E.; Miura, K.; Kanoi, B.N.; Kaneko, T.; Han, E.-T.; Tachibana, M.; Matsuoka, K.; Sattabongkot, J.; et al. Identification of target proteins of clinical immunity to Plasmodium falciparum in a region of low malaria transmission. Parasitol. Int. 2018, 67, 203–208. [Google Scholar] [CrossRef]
- Kanoi, B.N.; Nagaoka, H.; White, M.T.; Morita, M.; Palacpac, N.M.; Ntege, E.H.; Balikagala, B.; Yeka, A.; Egwang, T.G.; Horii, T.; et al. Global repertoire of human antibodies against Plasmodium falciparum RIFINs, SURFINs, and STEVORs in a malaria exposed population. Front. Immunol. 2020, 11, 893. [Google Scholar] [CrossRef]
- Kanoi, B.N.; Nagaoka, H.; Morita, M.; White, M.T.; Palacpac, N.M.; Ntege, E.H.; Balikagala, B.; Yeka, A.; Egwang, T.G.; Horii, T.; et al. Comprehensive analysis of antibody responses to Plasmodium falciparum erythrocyte membrane protein 1 domains. Vaccine 2018, 36, 6826–6833. [Google Scholar] [CrossRef]
- Morita, M.; Takashima, E.; Ito, D.; Miura, K.; Thongkukiatkul, A.; Diouf, A.; Fairhurst, R.M.; Diakite, M.; Long, C.A.; Torii, M.; et al. Immunoscreening of Plasmodium falciparum proteins expressed in a wheat germ cell-free system reveals a novel malaria vaccine candidate. Sci. Rep. 2017, 7, 46086. [Google Scholar] [CrossRef]
- Longley, R.J.; White, M.T.; Takashima, E.; Morita, M.; Kanoi, B.N.; Suen, C.S.L.W.; Betuela, I.; Kuehn, A.; Sripoorote, P.; Franca, C.T.; et al. Naturally acquired antibody responses to more than 300 Plasmodium vivax proteins in three geographic regions. PLoS Negl. Trop. Dis. 2017, 11, e0005888. [Google Scholar] [CrossRef]
- Doolan, D.L.; Dobaño, C.; Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef]
- Travassos, M.A.; Niangaly, A.; Bailey, J.A.; Ouattara, A.; Coulibaly, D.; Laurens, M.B.; Pablo, J.; Jasinskas, A.; Nakajima-Sasaki, R.; Berry, A.A.; et al. Seroreactivity to Plasmodium falciparum erythrocyte membrane protein 1 intracellular domain in malaria-exposed children and adults. J. Infect. Dis. 2013, 208, 1514–1519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crompton, P.D.; Kayala, M.A.; Traore, B.; Kayentao, K.; Ongoiba, A.; Weiss, G.E.; Molina, D.M.; Burk, C.R.; Waisberg, M.; Jasinskas, A.; et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. USA 2010, 107, 6958–6963. [Google Scholar] [CrossRef] [PubMed]
- Camponovo, F.; Campo, J.J.; Le, T.Q.; Oberai, A.; Hung, C.; Pablo, J.V.; Teng, A.A.; Liang, X.; Sim, B.K.L.; Jongo, S.; et al. Proteome-wide analysis of a malaria vaccine study reveals personalized humoral immune profiles in Tanzanian adults. Elife 2020, 9, e53080. [Google Scholar] [CrossRef] [PubMed]
- Kamuyu, G.; Tuju, J.; Kimathi, R.; Mwai, K.; Mburu, J.; Kibinge, N.; Kwan, M.C.; Hawkings, S.; Yaa, R.; Chepsat, E.; et al. KILchip v1. 0: A novel Plasmodium falciparum merozoite protein microarray to facilitate malaria vaccine candidate prioritization. Front. Immunol. 2018, 9, 2866. [Google Scholar] [CrossRef]
- Yman, V.; Tuju, J.; White, M.T.; Kamuyu, G.; Mwai, K.; Kibinge, N.; Asghar, M.; Sundling, C.; Sondén, K.; Murungi, L.; et al. Distinct kinetics of antibodies to 111 Plasmodium falciparum proteins identifies markers of recent malaria exposure. Nat. Commun. 2022, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Doolan, D.L.; Mu, Y.; Unal, B.; Sundaresh, S.; Hirst, S.; Valdez, C.; Randall, A.; Molina, D.; Liang, X.; Freilich, D.A.; et al. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 2008, 8, 4680–4694. [Google Scholar] [CrossRef] [PubMed]
- Harbers, M. Wheat germ systems for cell-free protein expression. FEBS Lett. 2014, 588, 2762–2773. [Google Scholar] [CrossRef]
- Eglen, R.M.; Reisine, T.; Roby, P.; Rouleau, N.; Illy, C.; Bossé, R.; Bielefeld, M. The use of AlphaScreen technology in HTS: Current status. Curr. Chem. Genom. 2008, 1, 2–10. [Google Scholar] [CrossRef]
- Matsuoka, K.; Komori, H.; Nose, M.; Endo, Y.; Sawasaki, T. Simple screening method for autoantigen proteins using the N-terminal biotinylated protein library produced by wheat cell-free synthesis. J. Proteome Res. 2010, 9, 4264–4273. [Google Scholar] [CrossRef]
- Sierecki, E.; Giles, N.; Polinkovsky, M.; Moustaqil, M.; Alexandrov, K.; Gambin, Y. A cell-free approach to accelerate the study of protein–protein interactions in vitro. Interface Focus 2013, 3, 20130018. [Google Scholar] [CrossRef]
- Nagayoshi, Y.; Nakamura, M.; Matsuoka, K.; Ohtsuka, T.; Mori, Y.; Kono, H.; Aso, T.; Ideno, N.; Takahata, S.; Ryo, A. Profiling of autoantibodies in sera of pancreatic cancer patients. Ann. Surg. Oncol. 2014, 21, 459–465. [Google Scholar] [CrossRef]
- Raghavan, M.; Kalantar, K.L.; Duarte, E.; Teyssier, N.; Takahashi, S.; Kung, A.F.; Rajan, J.V.; Rek, J.; Tetteh, K.K.; Drakeley, C.; et al. Antibodies to repeat-containing antigens in Plasmodium falciparum are exposure-dependent and short-lived in children in natural malaria infections. Elife 2023, 12, e81401. [Google Scholar] [CrossRef]
- Vatti, A.; Monsalve, D.M.; Pacheco, Y.; Chang, C.; Anaya, J.-M.; Gershwin, M.E. Original antigenic sin: A comprehensive review. J. Autoimmun. 2017, 83, 12–21. [Google Scholar] [CrossRef]
- Badaut, C.; Visitdesotrakul, P.; Chabry, A.; Bigey, P.; Tornyigah, B.; Roman, J.; Maroufou, J.A.; Amoussou, A.; Ayivi, B.S.; Sagbo, G.; et al. IgG acquisition against PfEMP1 PF11_0521 domain cassette DC13, DBLβ3_D4 domain, and peptides located within these constructs in children with cerebral malaria. Sci. Rep. 2021, 11, 3680. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, M.; Weedall, G.D. Malaria in the ‘Omics Era’. Genes 2021, 12, 843. [Google Scholar] [CrossRef]
- Oleinikov, A.V.; Amos, E.; Frye, I.T.; Rossnagle, E.; Mutabingwa, T.K.; Fried, M.; Duffy, P.E. High throughput functional assays of the variant antigen PfEMP1 reveal a single domain in the 3D7 Plasmodium falciparum genome that binds ICAM1 with high affinity and is targeted by naturally acquired neutralizing antibodies. PLoS Pathog. 2009, 5, e1000386. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Lavstsen, T.; Berger, S.S.; Wang, C.W.; Petersen, J.E.; Avril, M.; Brazier, A.J.; Freeth, J.; Jespersen, J.S.; Nielsen, M.A.; et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013, 498, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, F.; Adams, Y.; Bengtsson, A.; Olsen, R.W.; Turner, L.; Ndam, N.T.; Ecklu-Mensah, G.; Moussiliou, A.; Ofori, M.F.; Gamain, B.; et al. Structure-guided identification of a family of dual receptor-binding PfEMP1 that is associated with cerebral malaria. Cell Host Microbe 2017, 21, 403–414. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, K.; Piccoli, L.; Foglierini, M.; Tan, J.; Jin, W.; Gorman, J.; Tsybovsky, Y.; Zhang, B.; Traore, B.; et al. Structural basis of malaria RIFIN binding by LILRB1-containing antibodies. Nature 2021, 592, 639–643. [Google Scholar] [CrossRef]
- Saito, F.; Hirayasu, K.; Satoh, T.; Wang, C.W.; Lusingu, J.; Arimori, T.; Shida, K.; Palacpac, N.M.Q.; Itagaki, S.; Iwanaga, S.; et al. Immune evasion of Plasmodium falciparum by RIFIN via inhibitory receptors. Nature 2017, 552, 101–105. [Google Scholar] [CrossRef]

| Characteristics | Asy (n = 17) | Sym (n = 21) |
|---|---|---|
| Age in years at sampling, median (range) | 30 (11–50) | 23 (17–50) |
| Gender (male %) | 58 | 90 |
| Median parasitemia (range) % | 0.003 (0.0006–0.2) | 0.6 (0.0021–2.9) |
| Gene ID and Domain | Protein ID | OR | Lower CI | Upper CI | p Value | |
|---|---|---|---|---|---|---|
| 1 | PF3D7_1219300_CIDRα3.4 | DC239 | 0.002882631 | 0.000123653 | 0.067200484 | 0.000272011 |
| 2 | PF3D7_1150400_DBLβ3 | DC217 | 0.037865179 | 0.006190304 | 0.231615732 | 0.000395639 |
| 3 | PF3D7_1240300_CIDRα4 | DC243 | 0.002350607 | 0.0000779 | 0.070905936 | 0.000496605 |
| 4 | PF3D7_0412900_CIDRα3.1 | DC47 | 0.009541722 | 0.000692251 | 0.131519501 | 0.000509778 |
| 5 | PF3D7_0223500_CIDRα3.4 | DC15 | 0.008724198 | 0.000563243 | 0.135131077 | 0.000694702 |
| 6 | PF3D7_0421300_DBLα0.24 | DC66 | 0.002910712 | 0.0000985 | 0.085977897 | 0.000723691 |
| 7 | PF3D7_0937800_DBLδ1 | DC195 | 0.004367111 | 0.000178979 | 0.106558101 | 0.000856835 |
| 8 | PF3D7_1035500 (MSP6) | WE18 | 0.011459908 | 0.000822541 | 0.159663158 | 0.000883835 |
| 9 | PF3D7_0425800_CIDRβ5 | DC76 | 0.016955413 | 0.001510087 | 0.19037717 | 0.000952049 |
| 10 | PF3D7_1366400 (RHOP148) | WE76.2 | 0.006899884 | 0.00035859 | 0.132765465 | 0.000972586 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, I.; Kanoi, B.N.; Nagaoka, H.; Sattabongkot, J.; Udomsangpetch, R.; Tsuboi, T.; Takashima, E. High-Throughput Antibody Profiling Identifies Targets of Protective Immunity against P. falciparum Malaria in Thailand. Biomolecules 2023, 13, 1267. https://doi.org/10.3390/biom13081267
Hassan I, Kanoi BN, Nagaoka H, Sattabongkot J, Udomsangpetch R, Tsuboi T, Takashima E. High-Throughput Antibody Profiling Identifies Targets of Protective Immunity against P. falciparum Malaria in Thailand. Biomolecules. 2023; 13(8):1267. https://doi.org/10.3390/biom13081267
Chicago/Turabian StyleHassan, Ifra, Bernard N. Kanoi, Hikaru Nagaoka, Jetsumon Sattabongkot, Rachanee Udomsangpetch, Takafumi Tsuboi, and Eizo Takashima. 2023. "High-Throughput Antibody Profiling Identifies Targets of Protective Immunity against P. falciparum Malaria in Thailand" Biomolecules 13, no. 8: 1267. https://doi.org/10.3390/biom13081267
APA StyleHassan, I., Kanoi, B. N., Nagaoka, H., Sattabongkot, J., Udomsangpetch, R., Tsuboi, T., & Takashima, E. (2023). High-Throughput Antibody Profiling Identifies Targets of Protective Immunity against P. falciparum Malaria in Thailand. Biomolecules, 13(8), 1267. https://doi.org/10.3390/biom13081267






