Abstract
Extensive control efforts have significantly reduced malaria cases and deaths over the past two decades, but in recent years, coupled with the COVID-19 pandemic, success has stalled. The WHO has urged the implementation of a number of interventions, including vaccines. The modestly effective RTS,S/AS01 pre-erythrocytic vaccine has been recommended by the WHO for use in sub-Saharan Africa against Plasmodium falciparum in children residing in moderate to high malaria transmission regions. A second pre-erythrocytic vaccine, R21/Matrix-M, was also recommended by the WHO on 3 October 2023. However, the paucity and limitations of pre-erythrocytic vaccines highlight the need for asexual blood-stage malaria vaccines that prevent disease caused by blood-stage parasites. Few asexual blood-stage vaccine candidates have reached phase 2 clinical development, and the challenges in terms of their efficacy include antigen polymorphisms and low immunogenicity in humans. This review summarizes the history and progress of asexual blood-stage malaria vaccine development, highlighting the need for novel candidate vaccine antigens/molecules.
1. Introduction
Malaria, a disease of global health priority, is caused by infection, with the Plasmodium parasite transmitted by anopheline mosquitoes. Global malaria deaths decreased steadily from 864,000 in 2000 to 576,000 in 2019; however, a new increase in deaths to an estimated 608,000 in 2022 is thought to be caused by disruptions to malaria control efforts during the COVID-19 pandemic [1]. These recent events emphasize the need to develop highly effective malaria vaccines to control and eventually eradicate the disease.
Malaria vaccines can be classified into three groups, each of which focuses on the specific developmental stages of the parasite: the pre-erythrocytic (sporozoite and liver stages), asexual blood (merozoite, trophozoite, schizont, and parasite-infected erythrocyte), and sexual stages within the bloodstream and the mosquito (gametocyte, gamete, and zygote/ookinete) (Figure 1).
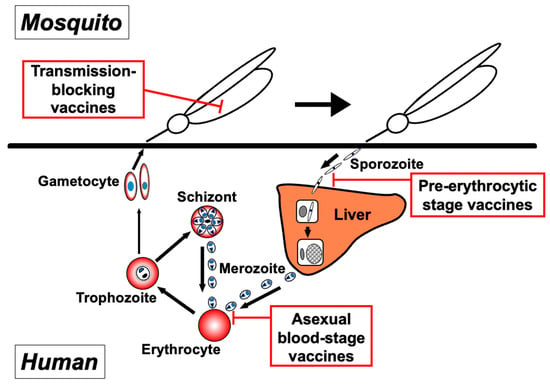
Figure 1.
Targets of three approaches to malaria vaccines within the parasite lifecycle. Malaria vaccine development can be categorized into three groups, with each targeting distinct parasite developmental stages: pre-erythrocytic-stage vaccines, asexual blood-stage vaccines, and transmission-blocking vaccines.
The Malaria Vaccine Technology Roadmap has been updated for the development of new malaria vaccines by 2030. The renewed roadmap calls for developing vaccines with at least 75% protective efficacy against clinical malaria, targeting both Plasmodium falciparum and Plasmodium vivax, and vaccines that reduce the transmission of the parasite [2,3]. One leading malaria vaccine, RTS,S/AS01, is a pre-erythrocytic vaccine that uses the circumsporozoite protein (CSP) of P. falciparum, which is expressed during the sporozoite stage. The RTS,S antigen consists of the C-terminal region and central repeats of CSP and is expressed with fused (20%) and free (80%) hepatitis B surface antigen (HBsAg), which self-assembles into virus-like particles [4,5] and is formulated with the AS01 adjuvant. Phase 3 trials showed moderate efficacy against clinical malaria [6] with short duration [7]. Since 2019, large pilot implementation programs have been conducted in Ghana, Kenya, and Malawi [8]. A significant reduction in severe malaria cases by approximately 30% in the first 2 years [9] was noted. On 6 October 2021, the World Health Organization (WHO) recommended the broad use of this vaccine among children over 5 months old living in moderate to high falciparum malaria transmission regions [10].
Currently, the second-generation malaria vaccine candidates in clinical development include the most advanced R21/Matrix-M [11,12]. R21 is also a pre-erythrocytic malaria vaccine antigen and shares similarities with RTS,S, including the fusion of HBsAg to the C-terminal domain and central repeats of CSP. However, unlike RTS,S, R21 consists solely of the fusion protein with HBsAg, resulting in greater CSP coverage within the virus-like particles [13,14]. After preclinical studies of R21 formulated with multiple adjuvants, Matrix-M (R21/Matrix-M) was selected for clinical development because of its higher immunogenicity. In a phase 2b trial involving 5- to 17-month-old Burkinabe children, R21/Matrix-M had an efficacy of 77% against clinical malaria [13]. Based on these trial results, the vaccine recently obtained regulatory clearance in Ghana, Nigeria, and Burkina Faso (https://www.gavi.org/vaccineswork/five-things-you-need-know-about-new-r21-malaria-vaccine; https://allafrica.com/stories/202304190024.html; https://www.ox.ac.uk/news/2023-07-24-oxford-r21matrix-m-malaria-vaccine-receives-regulatory-clearance-use-burkina-faso; accessed on 1 October 2023) and was recommended by the WHO on 3 October 2023 [1].
RTS,S and R21 are both classified as pre-erythrocytic vaccines and this distinction is important; in a study conducted by White et al. [7], the level of anti-CSP antibodies provided by the RTS,S/AS01 vaccine helped inform its efficacy profile and estimate the duration of protection. In low-transmission areas, efficacy against clinical malaria declines because of a reduction in anti-CSP antibody titers. In high-transmission areas, efficacy against clinical malaria decreases much faster in vaccinated individuals because of RTS,S blocking sporozoite invasion, which results in lowered blood-stage immunity. Hence, White et al. [7] demonstrated that pre-erythrocytic anti-infection vaccines for malaria have limitations and highlighted the significance of developing vaccines that target asexual blood stages, where the associated manifestations of the disease are predominantly attributed. Moreover, it is theoretically possible to enhance the protective immunity provided by blood-stage vaccines through natural infection with the parasite [15].
The value of blood-stage vaccines cannot be overemphasized. In addition to the renewed Malaria Vaccine Technology Roadmap [2], a recent WHO publication, “Malaria vaccines: preferred product characteristics and clinical development considerations” [16], stated two priorities. Strategic Goal 1 called for “malaria vaccines that prevent human blood-stage infection of the individual level”, and Goal 2 described “malaria vaccines that reduce morbidity and mortality in individuals of risk in malaria-endemic areas”. Another recent publication, the “WHO Guidelines for Malaria” [17], also emphasized the need for asexual blood-stage vaccine development for malaria eradication. In this review, we summarize the history and progress of the development of asexual blood-stage malaria vaccines and stress the importance of discovering novel blood-stage vaccine candidate molecules to help address these roadmap goals [15].
2. Complex Processes of the Asexual Blood-Stage Malaria Lifecycle and the Molecules Involved in These Steps
The clinical manifestations of malaria are caused by the highly regulated amplification cycles of the merozoite invasion of erythrocytes, the development of the trophozoite and schizont stages, and the subsequent egress of ~32 mature daughter merozoites. The egress and invasion processes result in critical parasite proteins being exposed to host immune responses [18,19,20] (Figure 2). This protein repertoire is considered to represent asexual blood-stage malaria vaccine candidates. The catalog comprises invasion-related proteins found on the merozoite surface or within merozoite secretory organelles, such as micronemes, rhoptries, and dense granules. Merozoite surface proteins (MSPs) can be anchored by glycosylphosphatidylinositol (GPI), integrated into parasite membranes, or associated with membrane proteins. During schizogony, the secretory organellar proteins of the merozoite are initially retained in the micronemes, rhoptries, or dense granules. Following this, some microneme and rhoptry proteins are relocated to the merozoite surface after egress [21]. Once released into the bloodstream, the merozoite invasion cascade, which includes pre-invasion, internalization, and echinocytosis, is completed within a few minutes (as summarized in [22]).
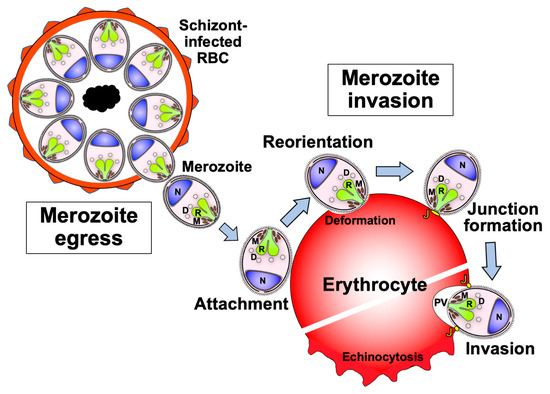
Figure 2.
Schematic representation of the sequential events of the merozoite from egress to erythrocyte invasion. Soon after the merozoite egresses from the schizont, it attaches to the surface of a new erythrocyte. Then, the merozoite reorients its apical end toward the erythrocyte. After reorientation, the merozoite forms a tight junction between its surface and the erythrocyte surface. Finally, the merozoite starts to invade the erythrocyte via a moving junction. Once invasion begins, the infected erythrocyte plasma membrane deforms and exhibits echinocytosis until the completion of invasion. In addition to the merozoite surface proteins, the secretory organelles of the merozoite are known to contain the necessary molecules for its invasion. M: microneme organelle; R: rhoptry organelle; D: dense granule organelle; J: junction between the merozoite surface and erythrocyte plasma membrane; PV: parasitophorous vacuole that surrounds the invading merozoite; N: nucleus.
The pre-invasion step, which involves merozoite attachment and reorientation, is the first contact between merozoites and erythrocytes and is mediated by molecular interactions that are not fully understood. The dynamic interplay between the merozoite and erythrocyte during the pre-invasion step results in erythrocyte deformation driven by a parasite actin-myosin motor [22,23]. Then, the merozoite reorients the apical end toward the erythrocyte membrane through erythrocyte-binding antigens (EBAs) and reticulocyte-binding-like homologs (Rhs) [23]. The merozoite anchors its apical via the interaction of Rh5 with an erythrocyte surface protein, basigin, resulting in irreversible merozoite attachment to the erythrocyte. A tight junction is formed between the parasite apical membrane antigen (AMA) 1, and the rhoptry neck protein (RON) complex, RON2 and RON4. The merozoite invades the erythrocyte using the parasite actin-myosin motor, creating a parasitophorous vacuole (PV) membrane that surrounds the parasite [24]. After the invasion, membrane fusion occurs at the posterior end of the merozoite to enclose it within the PV and erythrocyte plasma membranes. Echinocytosis occurs once the parasite has started invasion into the erythrocyte, which leads to erythrocyte shrinkage and the formation of spiky protrusions on the surface [22] (Figure 2). Over the next 48 h, the parasite develops into the schizont stage of up to 32 merozoites (for P. falciparum) before egressing to invade new erythrocytes.
EBAs and Rhs bind specific erythrocyte receptors, including glycophorins A, B, and C, and complement receptor 1 (CR1). In P. falciparum, there are redundant functions in the individual members of these protein families that need to be considered in the process of selecting vaccine candidates [25]. For example, the 175-kDa EBA (EBA175) binds to glycophorin A on the erythrocyte surface, whereas Rh4 binds to CR1 [26]. After parasite reorientation, Rh5 forms a complex with Rh5-interacting protein (Ripr) [27] and cysteine-rich protective antigen (CyRPA) [28]. Rh5 binds to basigin on the erythrocyte surface, which is crucial for erythrocyte invasion by the merozoite [29]. Recently, Plasmodium thrombospondin-related apical merozoite protein (PTRAMP) and cysteine-rich small secreted protein (CSS) in P. falciparum were identified as the interacting proteins of the Rh5 complex. The PTRAMP/CSS/Ripr/CyRPA/Rh5 complex plays a significant role in anchoring the contact between merozoite and erythrocyte membranes and is, therefore, essential for merozoite invasion [30,31].
3. Discovery of Asexual Blood-Stage Malaria Vaccine Candidate Molecules
The discoveries of asexual blood-stage malaria vaccine candidates before the whole genome sequence era are summarized in a review paper by Good and Miller [32]. Since this information is valuable for considering the future direction of malaria vaccine research, we extracted the essence of their descriptions in the following paragraphs.
Historically, the immunization of Rhesus monkeys with Plasmodium knowlesi parasite antigens emulsified in Freund’s complete adjuvant (FCA) conferred protection [33,34]. Immune serum from infected monkeys could passively transfer immunity [35]. With the establishment of a continuous in vitro culture for P. falciparum [36], monkeys could be immunized with this human parasite [37,38]. In addition, the injection of immunoglobulin purified from adult human serum with naturally acquired immunity to malaria was successful in treating infected non-immune children in Gambia [39] and Thailand [40]. However, because of the limitations of whole parasites as vaccine antigens and a suitable human-compatible adjuvant, researchers have turned to cloning the genes of malaria antigens [32].
Malaria blood-stage antigens were initially cloned in 1983 from P. falciparum and P. knowlesi malaria parasites using λgt11 expression libraries [41,42]. For P. falciparum, the leading subunit vaccine antigens, including MSP1 [43], MSP2 [44], MSP3 [45], and AMA1 [46], were identified by screening the P. falciparum expression libraries using either antibodies from malaria-immune Papua New Guinean adults or protective mouse monoclonal antibodies. Similarly, serine repeat antigen 5 (SERA5) [47] and glutamate-rich protein (GLURP) [48,49] were independently identified by screening P. falciparum expression libraries with malaria-immune human antibodies from African countries.
A “functional approach” based on the erythrocyte-binding phenotype of the parasite proteins (ligands) was also initiated. Two pioneering works were reported in 1990 on the discovery of parasite ligands, P. knowlesi and P. vivax Duffy binding proteins (DBP) [50,51], and P. falciparum 175 kDa erythrocyte binding antigen (EBA175) [52].
The discovery of the DBP gene involved the identification of soluble parasite proteins found in both P. knowlesi and P. vivax culture supernatants that specifically bind to Duffy-positive human erythrocytes. The 135 kDa P. knowlesi Duffy antigen-binding protein was purified using Duffy-positive human erythrocytes. Briefly, anti-135 kDa antibodies were affinity purified from the immune serum of a rhesus monkey using strips of the nitrocellulose membrane from the 135 kDa region of the gel. A λgt11 expression library derived from P. knowlesi asexual blood-stage cDNA was screened with these specific antibodies, leading to the cloning of the PkDBP gene [50]. By using this information, a gene encoding the DBP of P. vivax (PvDBP) was also cloned [51].
The 175 kDa P. falciparum antigen, EBA175, is a soluble parasite protein that accumulates in the supernatants of schizont cultures [53]. In order to identify the gene for EBA175, EBA175 antigens were affinity purified from the culture supernatants of P. falciparum using intact erythrocytes. Monospecific antibodies against EBA175 were then isolated from the sera of Aotus monkeys immunized against P. falciparum parasites using affinity purification against EBA175 absorbed to a nitrocellulose membrane [54]. Affinity-purified monospecific antibodies against EBA175 were used to screen a P. falciparum λgt11 expression library, and an eba175 gene was cloned [52]. This gene was classified as a member of the erythrocyte binding-like (EBL) protein family, along with PkDBP and PvDBP, based on its primary structure and amino acid homology, all of which have conserved 5′ and 3′ cysteine-rich regions [55]. Finally, several EBA175 paralogs have been identified from the P. falciparum genome [56,57,58].
The discovery of P. vivax reticulocyte-binding proteins 1 and 2 (PvRBP1 and PvRBP2) was made using an approach similar to that used for identifying EBLs [59]. The orthologous Py235 gene family of these proteins was identified in the rodent malaria parasite Plasmodium yoelii [60,61]. In the case of P. falciparum, a search of the then incomplete genome data led to the identification of an ortholog of PvRBP1, which was named normocyte binding protein 1 (PfNBP1) [62]. This protein was subsequently categorized as a member of the reticulocyte binding-like (RBL) family of invasion-related proteins [63]. A similar bioinformatic approach was employed to identify and characterize four RBL family members in P. falciparum (PfRh), namely PfRh1/PfNBP1, PfRh2a, PfRh2b, and PfRh4 [62,64,65,66].
A number of these blood-stage antigens discovered in the pre-genome era have continued to progress to vaccine clinical trials.
After the availability of parasite genome databases, an additional PfRh member, Rh5, was identified [67,68,69]. Rh5 forms a complex with CyRPA [28] and Ripr [27], as described above, and is abbreviated as the RCR protein complex. Each RCR complex member is highly conserved, essential for invasion, and antibody-susceptible [70,71]. Despite its poor natural immunogenicity, Rh5 remains a blood-stage vaccine antigen candidate because of its indispensable role and highly conserved sequences in field isolates [20,72,73,74].
Apart from the merozoite proteins, a search of the malaria parasite genome was conducted using an α-helical coiled-coil structure, which led to the identification of P27A protein as a fragment of trophozoite exported protein 1 (TEX1), with the potential to serve as a target for blood-stage vaccines [75]. Similarly, other nonmerozoite proteins, P. falciparum schizont egress antigen-1 (PfSEA-1) and P. falciparum glutamic acid-rich protein (PfGARP), have been identified as blood-stage vaccine candidates from the library screenings of proteins that are only reactive to the sera from malaria-protected children [76,77]. These studies indicated that not only merozoite-stage proteins but also trophozoite antigens could be developed as blood-stage vaccines [78]. The clinical development of a number of antigens is described in the following section.
4. Current Status of Leading Asexual Blood-Stage Malaria Vaccine Candidates
The road to asexual blood-stage vaccine development has been arduous, with most clinical trials showing limited efficacy, primarily due to extensive polymorphisms across parasite isolates, the involvement of functionally redundant merozoite invasion pathways, and, in some cases, safety concerns [79]. Only a few vaccine candidates are currently undergoing clinical trials for further evaluation, and their clinical development outcomes are summarized in this section and Table 1. The status of protein structural analyses is incorporated into Table 1 because the latest approaches to improving vaccine antigen design are based on structural information.

Table 1.
Clinical development of the asexual blood-stage malaria vaccine candidates of P. falciparum.
4.1. AMA1
AMA1 from P. falciparum has shown promise as a potential blood-stage vaccine candidate, despite being highly polymorphic. Anti-AMA1 antibodies have strongly inhibited merozoite invasion in vitro, and the knockdown of the ama1 gene led to a loss of merozoite invasion [100,101]. Several AMA1 vaccine clinical trials have been conducted up to phase 2b; however, the results have been mixed. One phase 2b trial based on AMA1-C1 (a combination of 3D7- and FVO-types of AMA1 adjuvanted with Alhydrogel) in Malian children showed no protection against clinical malaria [102] or strain-specific protection [103]. Another trial using AMA1-3D7 formulated with an AS02A adjuvant (FMP2.1/AS02A) showed no reduction in malaria episodes, and only children infected with the vaccine strain sequence had a significant decrease in clinical episodes [80,81].
In order to address concerns about antigen polymorphisms, three AMA1 ‘Diversity Covering’ (DiCo) proteins, which account for major AMA1 polymorphisms, have been synthesized [104]. In rabbits and monkeys, the three AMA1 DiCo protein mixtures successfully induced cross-reactive antibodies that had in vitro parasite growth inhibition assay (GIA) activity against several P. falciparum strains [104,105]. The broadened antibody response is attributed to increased levels of cross-reactive antibodies [106]. However, in Burkina Faso clinical trials, the levels of anti-AMA antibodies against the natural AMA1 variants were only slightly increased despite the significant antibody increase observed for anti-PfAMA1 DiCo antibodies. Moreover, the presence of antibodies against other malaria antigens could have countered the GIA activity of anti-AMA1 [88], resulting in limited efficacy. The utility of a single antigen, AMA1, as a vaccine formulation remains a topic of debate [89,90].
Administering AMA1 with RON2 has the potential to improve the AMA1-based vaccines [107], based on RON2 mediating the binding and tight junction formation of AMA1 protein to establish the attachment of the parasite to red blood cells. In a P. falciparum Aotus model, an AMA1 + RON2 combination vaccine induced higher levels of antibodies against AMA1 than AMA1 alone [108]. The binary complex vaccine increased neutralizing antibodies targeting the AMA1-RON2 interaction, and this was significantly correlated with vaccine efficacy [108]. In order to prepare for future clinical trials, the identity and integrity of the candidate antigens were characterized, with the results indicating that the complex was stable for 72 h at 4 °C [109]. Recently, growth-inhibitory epitopes outside the RON2 binding site have been reported using the AMA1-RON2 fusion protein as an immunogen [110]. However, further progress in the development of the AMA1-RON2 complex vaccine has not been reported.
4.2. MSP1
Antibodies that target proteins on the surface of merozoites can prevent invasion or inhibit parasite growth in vitro [111,112]. One well-studied antigen, MSP1, is a high-molecular-weight protein that mediates initial attachment by binding to heparin-like proteoglycans or Band 3 [113,114]. Before merozoite egresses, MSP1 is cleaved into several smaller fragments. In the Aotus monkey, protective immunity is achieved against MSP119 [115]. Several vaccine formulations of MSP1 have been tested in malaria-naïve subjects using controlled human malaria infections (CHMI). A phase 2b trial using a single MSP1 antigen-based vaccine (MSP142-3D7/AS02) on healthy Kenyan children aged 12–47 months showed no significant clinical efficacy [83]. Similarly, a phase 2a trial in UK adults was conducted using a combination blood-stage vaccine (MSP1, AMA1, a combination of MSP1 + AMA1, or MSP1 + thrombospondin-related adhesive protein (TRAP)) primed with replication-deficient chimpanzee adenovirus serotype 63 (ChAd63) vectored vaccine and boosted with attenuated orthopoxviral-modified vaccinia virus Ankara (MVA) vectored vaccine. The vaccine was safe but did not show blood-stage efficacy after sporozoite challenge [102]. More recently, a first-in-human phase 1 study demonstrated that the full-length MSP1 vaccine was safe and immunogenic. The MSP1-specific antibodies in the vaccinees activated complement, opsonized merozoites, and triggered respiratory bursts in neutrophils. However, vaccine-induced human antibodies did not show in vitro GIA activity [116,117].
4.3. MSP2 (Combination B Vaccine)
MSP2 is a roughly 25 kDa protein that is expressed on the merozoite surface. It is divided into two distinct types, 3D7 and FC27, which are defined based on their central variable regions. The regions surrounding this variable part are highly conserved and categorized into N- and C-terminal regions [118,119]. The MSP2 protein is intrinsically disordered and lacks a known 3D structure, with the exception of a single disulfide bond in the C-terminal region. The N-terminal region tends to form an α-helical structure [120].
A malaria vaccine called Combination B comprises the blood-stage proteins of P. falciparum, including K1 strain-type MSP1 (blocks 3 and 4), 3D7-type full-length MSP2, and FC27-type C-terminal ring-infected erythrocyte surface antigen (RESA) (70% of the region). This vaccine formulation was developed with the Montanide ISA720 adjuvant and was tested in Papua New Guinea [85]. A phase 2b study showed that the Combination B vaccine group had lower infection rates for the homologous 3D7-MSP2 parasite, indicating allele-specific protection in naturally exposed populations. In a phase 1 trial of a vaccine containing both 3D7 and FC27 types of MSP2, the induced antibodies showed ADCI activity but required reformulation due to reactogenicity [121]. MSP2 chimeras were also produced, which consisted of the variable region of both the 3D7 and FC27 types and the conserved regions. The immunization of animals with recombinant proteins resulted in a significant antibody response to both types [122].
In further attempts to overcome strain-specificity, researchers have explored targeting epitopes that are located exclusively within conserved regions. Specifically, the C-terminal conserved region of MSP2 was recognized by mouse monoclonal antibodies (mAb). Although these five mAbs bind to overlapping epitopes [123], they exhibit different binding characteristics, and notably, mAbs 4D11 and 9G8 strongly recognized MSP2. Recently, a novel strategy to design a vaccine antigen based on its structure was employed for MSP2, using the crystal structure of the 4D11 variable fragment complexed with its minimal target epitope. This approach involved molecular dynamics simulations and surface plasmon resonance techniques to design multiple constrained peptides that mimic the 4D11-bound epitope structure. These peptides were used to immunize mice, which resulted in the generation of antibodies in all groups. The specificity of antibody responses revealed that a single point mutation can refocus the antibody response to a more favorable epitope [124]. These promising advancements in MSP2-based vaccine design hold great potential to strategize more effective malaria vaccine antigens.
4.4. MSP3 and GMZ2
MSP3 is a 48 kDa protein expressed on the merozoite surface and forms a protein complex with MSP1 [114]. The C-terminal region of MSP3 is highly conserved [125], and a long synthetic peptide (MSP3-LSP) derived from this region has been developed as a vaccine antigen for clinical trials [126,127]. In a phase 1b study, Burkinabe children who received the MSP3-LSP vaccine formulated with aluminum hydroxide adjuvant showed significantly fewer clinical malaria episodes compared with the control group, although this trial was initially not designed to measure vaccine efficacy [128].
GMZ2 is an MSP3-based vaccine made of a recombinant protein fusion of MSP3 with a part of the 145 kDa glutamate-rich protein (GLURP) [129,130,131]. Although the function of GLURP remains largely unknown, the GMZ2 vaccine elicited functional antibodies in a phase 1 study [86]. The efficacy of GMZ2 has been evaluated through a multicenter phase 2b trial in children from sub-Saharan countries [86]. The vaccine was immunogenic and well-tolerated, and the vaccine efficacy (VE) was 14% in children who received three doses of the vaccine, as per protocol analysis, adjusted for age and site.
In further attempts to improve efficacy, a recent study conducted on 50 Gabonese adults evaluated the efficacy of two formulations: GMZ2/Alhydrogel and GMZ2/CAF01. The study, using CHMI by intravenously injecting live P. falciparum sporozoites (PfSPZ Challenge) to assess vaccine efficacy, showed that the use of the novel adjuvant CAF01 did not significantly affect vaccine efficacy [132]. Despite being immunogenic, MSP3-based vaccines face significant challenges in achieving improved protective efficacy [133].
4.5. EBA175
During the invasion process by P. falciparum, the 175 kDa merozoite surface receptor EBA175 binds to the sialic acid residues of erythrocyte glycophorin A [53,134]. The binding activity is mainly limited to Region II of EBA175 (RII); therefore, this domain is a potential candidate for a malaria vaccine [55,134]. A phase 1a clinical trial was conducted in malaria-naïve US adults using a vaccine antigen called EBA175 RII-NG, which was expressed without glycosylation in Pichia pastoris and was formulated with aluminum phosphate adjuvant. The vaccine was safe, immunogenic, and induced human antibodies showing GIA activity [135]. The vaccine was further assessed for safety and immunogenicity in semi-immune Ghanaian adults in a phase 1b trial. The study showed that the EBA175 RII-NG vaccine was well tolerated, safe, and immunogenic in semi-immune Ghanaian adults [87]. Although recommended for further vaccine development, it is critical to note that different field parasite isolates express varying levels of EBA and Rh proteins, including EBA175, EBA140, EBA181, Rh1, and Rh2, owing to redundant merozoite invasion pathways [136]. This implies that EBA175 alone is inadequate as a vaccine antigen against P. falciparum, and a multivalent vaccine that combines other blood-stage proteins should be developed. This has not yet been attempted.
4.6. SERA5 (BK-SE36 Vaccine)
The serine repeat antigen 5 (SERA5) of P. falciparum is abundantly expressed in schizonts and is secreted into the parasitophorous vacuole to play a vital role in the survival of the parasite. Upon merozoite egress, the SERA5 protein undergoes sequential proteolytic processing to generate five distinct fragments, namely, P47, P73, P50, P6, and P18. This cleavage process involves the initial processing of the 120 kDa precursor protein into P47 and P73 fragments, which are then processed into smaller fragments [137,138,139,140,141]. Epidemiological studies have shown that antibody levels specific to the N-terminal P47 region of SERA5 are negatively correlated with malaria symptoms [142]. Worldwide, the SERA5 sequences of P. falciparum are highly conserved and show no evidence of positive selection [143]. African strain variations did not appear to affect the immune response to BK-SE36, with sequence analyses performed on strains collected from clinical trials and follow-up studies in Africa [144]. Immune-target epitopes in the P47 region that are recognized in humans have been identified in N-terminal repetitive sequences [145]. In Uganda, a phase 1b trial of the SE36 vaccine antigen, composed of the P47 region, excluding the serine repeats and formulated with aluminum hydroxide (BK-SE36), demonstrated that the vaccine was safe and immunogenic [146]. A follow-up study provided promising efficacy signals (against clinical malaria) [146] and boosting in vaccinated volunteers because of natural infection [147]. The vaccine was also safe and immunogenic in Burkinabe children, with a higher antibody response in the younger age group [148]. Recently, a novel adjuvant, CpG-ODN(K3), was shown to increase the immune response, particularly in malaria-immune individuals or those with a history of repeated malaria infection. The first-in-human BK-SE36/CpG-ODN(K3) trial in Japan demonstrated that the vaccine was safe and immunogenic [149]. Recently, a phase 1b trial of the BK-SE36/CpG-ODN(K3) vaccine in healthy malaria-exposed Burkinabe adults and children reported that the vaccine was well-tolerated and immunogenic [89]. These findings provide a foundation for additional proof-of-concept investigations aimed at demonstrating the effectiveness of the vaccine.
4.7. Rh5
Rh5 is a 45 kDa component of the Rh5/CyRPA/Ripr (RCR) merozoite protein complex [70,138]. Rh5 induces strain-transcending antibodies in animals [72]. Although the levels of these antibodies are low in malaria-exposed individuals [72,74], human anti-Rh5 IgGs have shown growth inhibitory activity in vitro [74,150]. Full-length Rh5 (RH5_FL) vaccination in animals has also induced high growth-inhibitory antibodies against P. falciparum lab strains and field-isolated parasites [72,151,152,153]. In a study conducted in Aotus monkeys, immunization with 3D7-type Rh5, using ChAd63 for priming and MVA for boosting, protected the animals against challenge infection with heterologous FVO [112].
The first clinical trial of the Rh5 vaccine, based on 3D7-type Rh5, was conducted in healthy adults. The vaccine was administered with the viral vectors ChAd63 and MVA at 8-week intervals and was well tolerated. The Rh5 vaccine elicited antibodies that can effectively inhibit the growth of various parasite strains. The antibodies were capable of identifying both linear and conformational epitopes within Rh5. This is the first evidence where significant Rh5-specific immunity has been demonstrated in humans [154]. In order to assess safety, immunogenicity, and efficacy, a phase 1/2a clinical trial of the RH5.1 (recombinant Rh5 protein) formulated with AS01B adjuvant was conducted in healthy UK adults [91]. The RH5.1/AS01B study demonstrated a significant reduction in the parasite multiplication rate (PMR) following blood-stage CHMI. Thus, this clinical trial was a milestone in the field of asexual blood-stage malaria vaccines. In a subset of vaccine recipients who received an additional booster dose, a stronger inhibition in PMR was observed, which confirmed the findings. Although the vaccine was effective, it only caused a slight delay of 1 to 2 days in the time taken to diagnose the disease [71]. In order to achieve greater protection, research has focused on improved immunogens and formulations that target Rh5 or its invasion complex; for example, a computational approach to design RH5 vaccine antigen variants [155] and structure-guided vaccine antigen development [31,156]. The following clinical trials are currently underway, including a multistage P. falciparum malaria vaccine using Matrix-M with RH5.2 VLP and/or R21 (accessed at ClinicalTrials.gov: NCT05790889, NCT05357560, NCT04318002, and NCT05385471).
4.8. Ripr
Ripr is another potential antigen that targets the RCR complex. Strain-transcending GIA activities in P. falciparum were observed with specific antibodies raised against recombinant Ripr [70]. Rabbit anti-Ripr antibodies showed superior GIA activity to anti-Rh5 antibodies, as reported by Healer et al. [157]. In our own study, we produced a region of Ripr (K279-D995) using the wheat germ cell-free system (WGCFS) [158], and anti-Ripr 3D7-type antibodies significantly inhibited parasite growth against both 3D7 and heterologous FVO strains [159]. Ripr poses a significant challenge as a vaccine target due to its large size of 126 kDa and high cysteine content of 87 residues. In order to address this, we produced 11 truncated fragments of Ripr and generated fragment-specific polyclonal antibodies in animals. Using these antibodies in GIA, we found that a specific Ripr fragment, PfRipr5, spanning C720-D934, was the most potent antigen. It induces the strongest growth-inhibitory antibodies at a level comparable to that of anti-full-length Ripr antibodies [160]. Our findings demonstrate that PfRipr5 is a promising antigen for a novel asexual blood-stage vaccine against P. falciparum.
The PfRipr5 recombinant protein was successfully produced on a large scale using the insect High Five cells/baculovirus system for further testing cGMP-compliant methods. The purified PfRipr5 was thermally stable and highly pure and demonstrated a high binding capacity to anti-PfRipr5 functional mAb [161]. Purified PfRipr5 was tested in combination with licensed adjuvants, such as Alhydrogel, GLA-SE, and CAF01, and it showed acceptable compatibility and produced comparable levels of anti-PfRipr5 antibodies in rabbits. The highest GIA activity was observed when PfRipr5 was formulated with the CAF01 adjuvant [93]. Further preclinical and clinical studies are planned to develop a PfRipr5-based vaccine. In addition, the fine structure of Ripr in the RCR complex was recently reported [31]. It may be useful for the future Ripr-based vaccine design.
4.9. CyRPA
CyRPA is another essential merozoite antigen and is a member of the RCR complex. Anti-CyRPA antibodies have strain-transcending GIA activities and synergistic effects with anti-Rh5 [28]. Naturally acquired antibodies against CyRPA prevent parasite invasion of erythrocytes and reduce malaria episodes [162,163]. The virosome-based CyRPA vaccine has been shown to elicit antibodies that are protective both in vitro and in vivo [164]. Somanathan et al. [165] successfully developed a large-scale production process for CyRPA with the refolding of tag-free CyRPA antigen in E. coli. Alhydrogel-formulated tag-free CyRPA induced a high antibody response exhibiting strain-transcending GIA activity. Another approach for establishing scalable, cost-effective, robust, and high-yield CyRPA production processes was successfully achieved using High Five insect cells [166]. When formulated in lipid-based virosome nanoparticles, rabbit anti-CyRPA antibodies showed potent GIA activities [166]. Recently, the recombinant CyRPA antigen, produced in mammalian HEK293 cells and formulated with GLA-SE adjuvant, was also immunogenic in animals and induced anti-CyRPA antibodies with potent GIA activities [95]. Further development efforts are required.
4.10. P27A
A genome-wide approach has been used to identify new vaccine-candidate antigens that are potential targets for growth-inhibitory antibodies [167]. One of the targets identified through this approach was a protein fragment known as P27A (a part of TEX1) [75,168]. The P27A fragment is intrinsically unstructured [167]. Human antibodies that target P27A are capable of inhibiting parasite invasion in vitro, and the immune response to P27A has been linked to protection against malaria [167,168]. Clinical trials were conducted to evaluate the safety and efficacy of a P27A vaccine, with adjuvants Alhydrogel or GLA-SE, in both malaria-naïve individuals from Switzerland and healthy adults from Tanzania [98]. The vaccine was safe, induced high levels of antibodies (especially when used with GLA-SE), and exhibited effective growth-inhibitory activities [98].
4.11. PfSEA-1
PfSEA-1 is a 244 kDa protein expressed in schizont-infected erythrocytes that plays a crucial role in blood-stage parasite growth. In malaria-endemic areas, the development of antibodies against PfSEA-1A (aa 801–1023) was observed [76]. In vitro studies have shown that these antibodies against PfSEA-1A inhibit parasite egress from schizont-infected erythrocytes. Studies conducted on Tanzanian children and Kenyan adults have revealed that individuals with antibodies to recombinant PfSEA-1A experience a significant reduction in parasitemia and the incidence of severe malaria [76]. Recently, maternally derived antibodies against PfSEA-1A in cord blood reduced severe malaria in infants. These observations were also replicated using a rodent malaria model [99]. The results suggest that the vaccination of pregnant women with PfSEA-1 may provide their offspring with a survival advantage [99]. Despite these promising findings, further vaccine development efforts on PfSEA-1 have not been reported.
4.12. PfGARP
Two separate groups discovered PfGARP. Almukadi et al. [169] used human erythrocytes as bait to screen the phage cDNA libraries of P. falciparum. They found a clone of PfGARP (aa 356–552; PfGARP-L) and a smaller PfGARP fragment (aa 392–437; PfGARP-S). PfGARP is a novel erythrocyte-binding protein that uses band 3 as its erythrocyte receptor. Raj and colleagues [77] performed differential biopanning using a T7 bacteriophage cDNA library of blood-stage P. falciparum 3D7. They identified that PfGARP is a protective antigen recognized by antibodies in children with immunity to P. falciparum. PfGARP is an 80 kDa protein that contains a Plasmodium export element (PEXEL) motif, enabling its translocation into infected erythrocytes and expression on the infected erythrocyte surface. Non-human primates vaccinated with PfGARP-based mRNA or protein vaccines showed partial protection against P. falciparum challenge. Longitudinal studies have shown that Tanzanian children and Kenyan adults who lack anti-PfGARP antibodies are at a greater risk of contracting severe malaria [77]. Sequence analysis confirmed that PfGARP is highly conserved, suggesting that a PfGARP-based vaccine may elicit strain-transcending immunity [170]. Similar to PfSEA-1, further vaccine development efforts have not been reported.
4.13. Others
In this review article, we did not describe two types of asexual blood-stage vaccines of P. falciparum that are under clinical development, namely, placental malaria vaccines [171,172,173], including the structural design of a VAR2CSA vaccine antigen [174] and whole blood-stage parasite vaccines [32,175,176,177,178,179].
5. Conclusions
Recently, decades of research on malaria vaccines have resulted in significant advances, culminating in the recommendation of the RTS,S/AS01 and R21 vaccines by the WHO. The progress suggests that a promising strategy to interrupt the parasite lifecycle at multiple stages could involve using a combination of pre-erythrocytic-stage vaccines (such as RTS,S/AS01 or R21) that are partially effective, along with asexual blood-stage vaccines. This combination can theoretically prevent parasite infection and the development of a blood-stage infection in the human host. Currently, the most advanced asexual blood-stage malaria vaccines are AMA1-based FMP2.1/AS02A [80] and MSP3-based GMZ2 [86]. They were tested in phase 2b trials; however, the vaccines had no significant clinical efficacy.
The high polymorphism levels in P. falciparum vaccine antigens are a major reason why the vaccine trials of leading asexual blood-stage targets were unsuccessful [80,81,83,85]. These polymorphisms often result in strain-specific immunity that hampers vaccine efficacy in clinical trials. Various studies that attempted to address the effects of allele-specific protective efficacy by combining several diversity-covering variants were met with a number of challenges [104]. Therefore, emerging evidence suggests that conserved antigens across multiple strains could be a more straightforward approach to attain high protective efficacy in the field [112]. Until recently, only one vaccine candidate has been considered to be such a conserved blood-stage vaccine candidate, namely, P. falciparum reticulocyte-binding homolog 5, Rh5, and it showed promising vaccine efficacy in a phase 1/2a trials [91]. As described above, a number of novel blood-stage vaccine candidates have been reported after complete sequence information for multiple parasite genomes has become available; however, unfortunately, to date, no additional asexual blood-stage vaccines have proceeded to phase 2b clinical trials. So far, the most advanced vaccine candidates are Rh5 and BK-SE36 vaccines, but proof-of-concept trials have not yet been reported. Thus, further genome-wide discovery efforts for a novel, highly conserved asexual blood-stage vaccine candidates are urgently needed.
The history of malaria vaccine development summarized in this review dissects the approaches to antigen discovery, protein expression technologies, the use of adjuvants, alternative delivery platforms, and the key lessons learned in the iterative phases of vaccine development. CHMI is now a popular tool in the early clinical phase that can significantly accelerate the vaccine developmental timelines. Numerous vaccine clinical trial sites have been established in malaria-endemic countries. These dramatic advances emphasize to the research community that a pipeline is available for the successful development of second-generation malaria vaccines. In order to achieve success in developing a vaccine for asexual blood-stage malaria, it is crucial to prioritize genome-wide identification of novel vaccine antigens.
Author Contributions
Conceptualization, E.T. and T.T.; data curation, T.T.; writing—original draft preparation, E.T. and T.T.; writing—review and editing, H.O., M.M., D.I., H.N., T.Y. and I.H.; Visualization, D.I. and M.M.; funding acquisition, E.T. and T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant numbers JP20H03481, JP21H02724, JP21KK0138, and JP23KK0140. T.Y. is supported by an EU Advanced Research Fellowship.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Thomas J. Templeton for his critical reading of this manuscript and Nirianne Marie Q. Palacpac for her valuable comments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- WHO. World Malaria Report 2023; WHO Press: Geneva, Switzerland, 2023. [Google Scholar]
- Malaria Vaccine Funders Group. Malaria Vaccine Technology Roadmap; WHO Press: Geneva, Switzerland, 2013. [Google Scholar]
- Moorthy, V.S.; Newman, R.D.; Okwo-Bele, J.M. Malaria vaccine technology roadmap. Lancet 2013, 382, 1700–1701. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M.; McGovern, T.W.; Krzych, U.; Cohen, J.C.; Schneider, I.; LaChance, R.; Heppner, D.G.; Yuan, G.; Hollingdale, M.; Slaoui, M.; et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J. Infect. Dis. 1995, 171, 1576–1585. [Google Scholar] [CrossRef]
- Cohen, J.; Nussenzweig, V.; Nussenzweig, R.; Vekemans, J.; Leach, A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum. Vaccines 2010, 6, 90–96. [Google Scholar] [CrossRef] [PubMed]
- RTS, S.C.T.P. Efficacy and safety of RTS, S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- White, M.T.; Verity, R.; Griffin, J.T.; Asante, K.P.; Owusu-Agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Lusingu, J.; Ansong, D.; et al. Immunogenicity of the RTS, S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef]
- Adepoju, P. RTS,S malaria vaccine pilots in three African countries. Lancet 2019, 393, 1685. [Google Scholar] [CrossRef]
- Vogel, G. WHO gives first malaria vaccine the green light. Science 2021, 374, 245–246. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2021; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Datoo, M.S.; Natama, M.H.; Some, A.; Traore, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, H.M.; Some, A.; Bellamy, D.; Traore, O.; Rouamba, T.; Tahita, M.C.; Ido, N.F.A.; Yameogo, P.; Valia, D.; et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: A phase 1/2b randomised controlled trial. Lancet Infect. Dis. 2022, 22, 1728–1736. [Google Scholar] [CrossRef]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef]
- Regules, J.A.; Cummings, J.F.; Ockenhouse, C.F. The RTS,S vaccine candidate for malaria. Expert Rev. Vaccines 2011, 10, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Takashima, E. Antibody titre as a surrogate of protection of the first malaria subunit vaccine, RTS, S/AS01. Lancet Infect. Dis. 2015, 15, 1371–1372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- WHO. Malaria Vaccines: Preferred Product Characteristics and Clinical Development Considerations; WHO Press: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. WHO Guidelines for Malaria; WHO Press: Geneva, Switzerland, 2022. [Google Scholar]
- Aikawa, M.; Miller, L.H.; Johnson, J.; Rabbege, J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J. Cell Biol. 1978, 77, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Arumugam, T.U.; Reiling, L.; Healer, J.; Hodder, A.N.; Fowkes, F.J.; Cross, N.; Langer, C.; Takeo, S.; Uboldi, A.D.; et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J. Immunol. 2013, 191, 795–809. [Google Scholar] [CrossRef]
- Osier, F.H.; Mackinnon, M.J.; Crosnier, C.; Fegan, G.; Kamuyu, G.; Wanaguru, M.; Ogada, E.; McDade, B.; Rayner, J.C.; Wright, G.J.; et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci. Transl. Med. 2014, 6, 247ra102. [Google Scholar] [CrossRef]
- Beeson, J.G.; Drew, D.R.; Boyle, M.J.; Feng, G.; Fowkes, F.J.; Richards, J.S. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 2016, 40, 343–372. [Google Scholar] [CrossRef]
- Weiss, G.E.; Gilson, P.R.; Taechalertpaisarn, T.; Tham, W.H.; de Jong, N.W.; Harvey, K.L.; Fowkes, F.J.; Barlow, P.N.; Rayner, J.C.; Wright, G.J.; et al. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 2015, 11, e1004670. [Google Scholar] [CrossRef] [PubMed]
- Yahata, K.; Hart, M.N.; Davies, H.; Asada, M.; Wassmer, S.C.; Templeton, T.J.; Treeck, M.; Moon, R.W.; Kaneko, O. Gliding motility of Plasmodium merozoites. Proc. Natl. Acad. Sci. USA 2021, 118, e2114442118. [Google Scholar] [CrossRef]
- Riglar, D.T.; Richard, D.; Wilson, D.W.; Boyle, M.J.; Dekiwadia, C.; Turnbull, L.; Angrisano, F.; Marapana, D.S.; Rogers, K.L.; Whitchurch, C.B.; et al. Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Tham, W.H.; Healer, J.; Cowman, A.F. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Tham, W.H.; Kennedy, A.T. Malaria: A master lock for deadly parasites. Nature 2015, 522, 158–159. [Google Scholar] [CrossRef]
- Chen, L.; Lopaticki, S.; Riglar, D.T.; Dekiwadia, C.; Uboldi, A.D.; Tham, W.H.; O’Neill, M.T.; Richard, D.; Baum, J.; Ralph, S.A.; et al. An EGF-like protein forms a complex with PfRh5 and is required for invasion of human erythrocytes by Plasmodium falciparum. PLoS Pathog. 2011, 7, e1002199. [Google Scholar] [CrossRef]
- Reddy, K.S.; Amlabu, E.; Pandey, A.K.; Mitra, P.; Chauhan, V.S.; Gaur, D. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion. Proc. Natl. Acad. Sci. USA 2015, 112, 1179–1184. [Google Scholar] [CrossRef]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T.; et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Scally, S.W.; Triglia, T.; Evelyn, C.; Seager, B.A.; Pasternak, M.; Lim, P.S.; Healer, J.; Geoghegan, N.D.; Adair, A.; Tham, W.H.; et al. PCRCR complex is essential for invasion of human erythrocytes by Plasmodium falciparum. Nat. Microbiol. 2022, 7, 2039–2053. [Google Scholar] [CrossRef]
- Farrell, B.; Alam, N.; Hart, M.N.; Jamwal, A.; Ragotte, R.J.; Walters-Morgan, H.; Draper, S.J.; Knuepfer, E.; Higgins, M.K. The PfRCR complex bridges malaria parasite and erythrocyte during invasion. Nature 2023. [Google Scholar] [CrossRef]
- Good, M.F.; Miller, L.H. Interpreting challenge data from early phase malaria blood stage vaccine trials. Expert Rev. Vaccines 2018, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Freund, J.; Thomson, K.J.; Sommer, H.E.; Walter, A.W.; Pisani, T.M. Immunization of monkeys against malaria by means of killed parasites with adjuvants. Am. J. Trop. Med. Hyg. 1948, 28, 1–22. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Good, M.F. Whole parasite blood stage malaria vaccines: A convergence of evidence. Hum. Vaccines 2010, 6, 114–123. [Google Scholar] [CrossRef]
- Coggeshall, L.T.; Kumm, H.W. Demonstration of Passive Immunity in Experimental Monkey Malaria. J. Exp. Med. 1937, 66, 177–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Mitchell, G.H.; Richards, W.H.; Butcher, G.A.; Cohen, S. Merozoite vaccination of douroucouli monkeys against falciparum malaria. Lancet 1977, 1, 1335–1338. [Google Scholar] [CrossRef]
- Siddiqui, W.A. An effective immunization of experimental monkeys against a human malaria parasite, Plasmodium falciparum. Science 1977, 197, 388–389. [Google Scholar] [CrossRef]
- Cohen, S.; Mc, G.I.; Carrington, S. Gamma-globulin and acquired immunity to human malaria. Nature 1961, 192, 733–737. [Google Scholar] [CrossRef]
- Bouharoun-Tayoun, H.; Attanath, P.; Sabchareon, A.; Chongsuphajaisiddhi, T.; Druilhe, P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 1990, 172, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Ozaki, L.S.; Gwadz, R.W.; Cochrane, A.H.; Nussenzweig, V.; Nussenzweig, R.S.; Godson, G.N. Cloning and expression in E. coli of the malarial sporozoite surface antigen gene from Plasmodium knowlesi. Nature 1983, 302, 536–538. [Google Scholar] [CrossRef]
- Kemp, D.J.; Coppel, R.L.; Cowman, A.F.; Saint, R.B.; Brown, G.V.; Anders, R.F. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: Detection with antibodies from immune humans. Proc. Natl. Acad. Sci. USA 1983, 80, 3787–3791. [Google Scholar] [CrossRef]
- Holder, A.A.; Lockyer, M.J.; Odink, K.G.; Sandhu, J.S.; Riveros-Moreno, V.; Nicholls, S.C.; Hillman, Y.; Davey, L.S.; Tizard, M.L.; Schwarz, R.T.; et al. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature 1985, 317, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Smythe, J.A.; Coppel, R.L.; Brown, G.V.; Ramasamy, R.; Kemp, D.J.; Anders, R.F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1988, 85, 5195–5199. [Google Scholar] [CrossRef]
- McColl, D.J.; Silva, A.; Foley, M.; Kun, J.F.; Favaloro, J.M.; Thompson, J.K.; Marshall, V.M.; Coppel, R.L.; Kemp, D.J.; Anders, R.F. Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 1994, 68, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.G.; Marshall, V.M.; Smythe, J.A.; Crewther, P.E.; Lew, A.; Silva, A.; Anders, R.F.; Kemp, D.J. Integral membrane protein located in the apical complex of Plasmodium falciparum. Mol. Cell Biol. 1989, 9, 3151–3154. [Google Scholar] [CrossRef] [PubMed]
- Horii, T.; Bzik, D.J.; Inselburg, J. Characterization of antigen-expressing Plasmodium falciparum cDNA clones that are reactive with parasite inhibitory antibodies. Mol. Biochem. Parasitol. 1988, 30, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Borre, M.B.; Dziegiel, M.; Hogh, B.; Petersen, E.; Rieneck, K.; Riley, E.; Meis, J.F.; Aikawa, M.; Nakamura, K.; Harada, M.; et al. Primary structure and localization of a conserved immunogenic Plasmodium falciparum glutamate rich protein (GLURP) expressed in both the preerythrocytic and erythrocytic stages of the vertebrate life cycle. Mol. Biochem. Parasitol. 1991, 49, 119–131. [Google Scholar] [CrossRef]
- Dziegiel, M.; Borre, M.B.; Jepsen, S.; Hogh, B.; Petersen, E.; Vuust, J. Recombinant Plasmodium falciparum glutamate rich protein; purification and use in enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 1991, 44, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Hudson, D.E.; Torii, M.; Ward, G.E.; Wellems, T.E.; Aikawa, M.; Miller, L.H. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell 1990, 63, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.D.; Kaslow, D.C.; Adams, J.H.; Miller, L.H. Cloning of the Plasmodium vivax Duffy receptor. Mol. Biochem. Parasitol. 1991, 44, 125–132. [Google Scholar] [CrossRef]
- Sim, B.K.; Orlandi, P.A.; Haynes, J.D.; Klotz, F.W.; Carter, J.M.; Camus, D.; Zegans, M.E.; Chulay, J.D. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J. Cell Biol. 1990, 111, 1877–1884. [Google Scholar] [CrossRef]
- Camus, D.; Hadley, T.J. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 1985, 230, 553–556. [Google Scholar] [CrossRef]
- Orlandi, P.A.; Sim, B.K.; Chulay, J.D.; Haynes, J.D. Characterization of the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 1990, 40, 285–294. [Google Scholar] [CrossRef]
- Adams, J.H.; Sim, B.K.; Dolan, S.A.; Fang, X.; Kaslow, D.C.; Miller, L.H. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 1992, 89, 7085–7089. [Google Scholar] [CrossRef]
- Mayer, D.C.; Kaneko, O.; Hudson-Taylor, D.E.; Reid, M.E.; Miller, L.H. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc. Natl. Acad. Sci. USA 2001, 98, 5222–5227. [Google Scholar] [CrossRef] [PubMed]
- Narum, D.L.; Fuhrmann, S.R.; Luu, T.; Sim, B.K. A novel Plasmodium falciparum erythrocyte binding protein-2 (EBP2/BAEBL) involved in erythrocyte receptor binding. Mol. Biochem. Parasitol. 2002, 119, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.K.; Triglia, T.; Reed, M.B.; Cowman, A.F. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol. Microbiol. 2001, 41, 47–58. [Google Scholar] [CrossRef]
- Galinski, M.R.; Medina, C.C.; Ingravallo, P.; Barnwell, J.W. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell 1992, 69, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.K.; Sinha, K.A.; Brown, K.N.; Holder, A.A. A gene coding for a high-molecular mass rhoptry protein of Plasmodium yoelii. Mol. Biochem. Parasitol. 1994, 65, 171–177. [Google Scholar] [CrossRef]
- Ogun, S.A.; Holder, A.A. A high molecular mass Plasmodium yoelii rhoptry protein binds to erythrocytes. Mol. Biochem. Parasitol. 1996, 76, 321–324. [Google Scholar] [CrossRef]
- Rayner, J.C.; Vargas-Serrato, E.; Huber, C.S.; Galinski, M.R.; Barnwell, J.W. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J. Exp. Med. 2001, 194, 1571–1581. [Google Scholar] [CrossRef][Green Version]
- Miller, L.H.; Baruch, D.I.; Marsh, K.; Doumbo, O.K. The pathogenic basis of malaria. Nature 2002, 415, 673–679. [Google Scholar] [CrossRef]
- Rayner, J.C.; Galinski, M.R.; Ingravallo, P.; Barnwell, J.W. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc. Natl. Acad. Sci. USA 2000, 97, 9648–9653. [Google Scholar] [CrossRef]
- Triglia, T.; Thompson, J.; Caruana, S.R.; Delorenzi, M.; Speed, T.; Cowman, A.F. Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect. Immun. 2001, 69, 1084–1092. [Google Scholar] [CrossRef][Green Version]
- Kaneko, O.; Mu, J.; Tsuboi, T.; Su, X.; Torii, M. Gene structure and expression of a Plasmodium falciparum 220-kDa protein homologous to the Plasmodium vivax reticulocyte binding proteins. Mol. Biochem. Parasitol. 2002, 121, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Cowman, A.F.; Crabb, B.S. Invasion of red blood cells by malaria parasites. Cell 2006, 124, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Hayton, K.; Gaur, D.; Liu, A.; Takahashi, J.; Henschen, B.; Singh, S.; Lambert, L.; Furuya, T.; Bouttenot, R.; Doll, M.; et al. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe 2008, 4, 40–51. [Google Scholar] [CrossRef]
- Rodriguez, M.; Lustigman, S.; Montero, E.; Oksov, Y.; Lobo, C.A. PfRH5: A novel reticulocyte-binding family homolog of plasmodium falciparum that binds to the erythrocyte, and an investigation of its receptor. PLoS ONE 2008, 3, e3300. [Google Scholar] [CrossRef]
- Ragotte, R.J.; Higgins, M.K.; Draper, S.J. The RH5-CyRPA-Ripr Complex as a Malaria Vaccine Target. Trends Parasitol. 2020, 36, 545–559. [Google Scholar] [CrossRef]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef]
- Douglas, A.D.; Williams, A.R.; Illingworth, J.J.; Kamuyu, G.; Biswas, S.; Goodman, A.L.; Wyllie, D.H.; Crosnier, C.; Miura, K.; Wright, G.J.; et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat. Commun. 2011, 2, 601. [Google Scholar] [CrossRef]
- Payne, R.O.; Silk, S.E.; Elias, S.C.; Milne, K.H.; Rawlinson, T.A.; Llewellyn, D.; Shakri, A.R.; Jin, J.; Labbe, G.M.; Edwards, N.J.; et al. Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies. JCI Insight 2017, 2, e93683. [Google Scholar] [CrossRef]
- Tran, T.M.; Ongoiba, A.; Coursen, J.; Crosnier, C.; Diouf, A.; Huang, C.Y.; Li, S.; Doumbo, S.; Doumtabe, D.; Kone, Y.; et al. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J. Infect. Dis. 2014, 209, 789–798. [Google Scholar] [CrossRef]
- Villard, V.; Agak, G.W.; Frank, G.; Jafarshad, A.; Servis, C.; Nebie, I.; Sirima, S.B.; Felger, I.; Arevalo-Herrera, M.; Herrera, S.; et al. Rapid identification of malaria vaccine candidates based on alpha-helical coiled coil protein motif. PLoS ONE 2007, 2, e645. [Google Scholar] [CrossRef]
- Raj, D.K.; Nixon, C.P.; Nixon, C.E.; Dvorin, J.D.; DiPetrillo, C.G.; Pond-Tor, S.; Wu, H.W.; Jolly, G.; Pischel, L.; Lu, A.; et al. Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 2014, 344, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.K.; Das Mohapatra, A.; Jnawali, A.; Zuromski, J.; Jha, A.; Cham-Kpu, G.; Sherman, B.; Rudlaff, R.M.; Nixon, C.E.; Hilton, N.; et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature 2020, 582, 104–108. [Google Scholar] [CrossRef]
- Duffy, P.E. Current approaches to malaria vaccines. Curr. Opin. Microbiol. 2022, 70, 102227. [Google Scholar] [CrossRef] [PubMed]
- Ntege, E.H.; Takashima, E.; Morita, M.; Nagaoka, H.; Ishino, T.; Tsuboi, T. Blood-stage malaria vaccines: Post-genome strategies for the identification of novel vaccine candidates. Expert Rev. Vaccines 2017, 16, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Thera, M.A.; Doumbo, O.K.; Coulibaly, D.; Laurens, M.B.; Ouattara, A.; Kone, A.K.; Guindo, A.B.; Traore, K.; Traore, I.; Kouriba, B.; et al. A field trial to assess a blood-stage malaria vaccine. N. Engl. J. Med. 2011, 365, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Laurens, M.B.; Thera, M.A.; Coulibaly, D.; Ouattara, A.; Kone, A.K.; Guindo, A.B.; Traore, K.; Traore, I.; Kouriba, B.; Diallo, D.A.; et al. Extended safety, immunogenicity and efficacy of a blood-stage malaria vaccine in malian children: 24-month follow-up of a randomized, double-blinded phase 2 trial. PLoS ONE 2013, 8, e79323. [Google Scholar] [CrossRef]
- Bai, T.; Becker, M.; Gupta, A.; Strike, P.; Murphy, V.J.; Anders, R.F.; Batchelor, A.H. Structure of AMA1 from Plasmodium falciparum reveals a clustering of polymorphisms that surround a conserved hydrophobic pocket. Proc. Natl. Acad. Sci. USA 2005, 102, 12736–12741. [Google Scholar] [CrossRef]
- Ogutu, B.R.; Apollo, O.J.; McKinney, D.; Okoth, W.; Siangla, J.; Dubovsky, F.; Tucker, K.; Waitumbi, J.N.; Diggs, C.; Wittes, J.; et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE 2009, 4, e4708. [Google Scholar] [CrossRef]
- Morgan, W.D.; Birdsall, B.; Frenkiel, T.A.; Gradwell, M.G.; Burghaus, P.A.; Syed, S.E.; Uthaipibull, C.; Holder, A.A.; Feeney, J. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J. Mol. Biol. 1999, 289, 113–122. [Google Scholar] [CrossRef]
- Genton, B.; Betuela, I.; Felger, I.; Al-Yaman, F.; Anders, R.F.; Saul, A.; Rare, L.; Baisor, M.; Lorry, K.; Brown, G.V.; et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 2002, 185, 820–827. [Google Scholar] [CrossRef]
- Sirima, S.B.; Mordmuller, B.; Milligan, P.; Ngoa, U.A.; Kironde, F.; Atuguba, F.; Tiono, A.B.; Issifou, S.; Kaddumukasa, M.; Bangre, O.; et al. A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children. Vaccine 2016, 34, 4536–4542. [Google Scholar] [CrossRef] [PubMed]
- Koram, K.A.; Adu, B.; Ocran, J.; Karikari, Y.S.; Adu-Amankwah, S.; Ntiri, M.; Abuaku, B.; Dodoo, D.; Gyan, B.; Kronmann, K.C.; et al. Safety and Immunogenicity of EBA-175 RII-NG Malaria Vaccine Administered Intramuscularly in Semi-Immune Adults: A Phase 1, Double-Blinded Placebo Controlled Dosage Escalation Study. PLoS ONE 2016, 11, e0163066. [Google Scholar] [CrossRef] [PubMed]
- Tolia, N.H.; Enemark, E.J.; Sim, B.K.; Joshua-Tor, L. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 2005, 122, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, A.; Bougouma, E.C.; Palacpac, N.M.Q.; Houard, S.; Nebie, I.; Sawadogo, J.; Berges, G.D.; Soulama, I.; Diarra, A.; Hien, D.; et al. Safety and immunogenicity of BK-SE36/CpG malaria vaccine in healthy Burkinabe adults and children: A phase 1b randomised, controlled, double-blinded, age de-escalation trial. Front. Immunol. 2023, 14, 1267372. [Google Scholar] [CrossRef]
- Hodder, A.N.; Malby, R.L.; Clarke, O.B.; Fairlie, W.D.; Colman, P.M.; Crabb, B.S.; Smith, B.J. Structural insights into the protease-like antigen Plasmodium falciparum SERA5 and its noncanonical active-site serine. J. Mol. Biol. 2009, 392, 154–165. [Google Scholar] [CrossRef]
- Minassian, A.M.; Silk, S.E.; Barrett, J.R.; Nielsen, C.M.; Miura, K.; Diouf, A.; Loos, C.; Fallon, J.K.; Michell, A.R.; White, M.T.; et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med 2021, 2, 701–719.e719. [Google Scholar] [CrossRef]
- Wright, K.E.; Hjerrild, K.A.; Bartlett, J.; Douglas, A.D.; Jin, J.; Brown, R.E.; Illingworth, J.J.; Ashfield, R.; Clemmensen, S.B.; de Jongh, W.A.; et al. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature 2014, 515, 427–430. [Google Scholar] [CrossRef]
- Takashima, E.; Nagaoka, H.; Correia, R.; Alves, P.M.; Roldao, A.; Christensen, D.; Guderian, J.A.; Fukushima, A.; Viebig, N.K.; Depraetere, H.; et al. A novel asexual blood-stage malaria vaccine candidate: PfRipr5 formulated with human-use adjuvants induces potent growth inhibitory antibodies. Front. Immunol. 2022, 13, 1002430. [Google Scholar] [CrossRef]
- Wong, W.; Huang, R.; Menant, S.; Hong, C.; Sandow, J.J.; Birkinshaw, R.W.; Healer, J.; Hodder, A.N.; Kanjee, U.; Tonkin, C.J.; et al. Structure of Plasmodium falciparum Rh5-CyRPA-Ripr invasion complex. Nature 2019, 565, 118–121. [Google Scholar] [CrossRef]
- Tamborrini, M.; Schafer, A.; Hauser, J.; Zou, L.; Paris, D.H.; Pluschke, G. The malaria blood stage antigen PfCyRPA formulated with the TLR-4 agonist adjuvant GLA-SE elicits parasite growth inhibitory antibodies in experimental animals. Malar. J. 2023, 22, 210. [Google Scholar] [CrossRef]
- Favuzza, P.; Guffart, E.; Tamborrini, M.; Scherer, B.; Dreyer, A.M.; Rufer, A.C.; Erny, J.; Hoernschemeyer, J.; Thoma, R.; Schmid, G.; et al. Structure of the malaria vaccine candidate antigen CyRPA and its complex with a parasite invasion inhibitory antibody. eLife 2017, 6, e20383. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Wong, W.; Thompson, J.K.; Healer, J.; Goddard-Borger, E.D.; Lawrence, M.C.; Cowman, A.F. Structural basis for inhibition of erythrocyte invasion by antibodies to Plasmodium falciparum protein CyRPA. eLife 2017, 6, e21347. [Google Scholar] [CrossRef] [PubMed]
- Steiner-Monard, V.; Kamaka, K.; Karoui, O.; Roethlisberger, S.; Audran, R.; Daubenberger, C.; Fayet-Mello, A.; Erdmann-Voisin, A.; Felger, I.; Geiger, K.; et al. The Candidate Blood-stage Malaria Vaccine P27A Induces a Robust Humoral Response in a Fast Track to the Field Phase 1 Trial in Exposed and Nonexposed Volunteers. Clin. Infect. Dis. 2019, 68, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, J.D.; Raj, D.K.; Michelow, I.C.; Park, S.; Nixon, C.E.; McDonald, E.A.; Nixon, C.P.; Pond-Tor, S.; Jha, A.; Taliano, R.J.; et al. Maternally-derived Antibodies to Schizont Egress Antigen-1 and Protection of Infants From Severe Malaria. Clin. Infect. Dis. 2019, 68, 1718–1724. [Google Scholar] [CrossRef]
- Lamarque, M.H.; Roques, M.; Kong-Hap, M.; Tonkin, M.L.; Rugarabamu, G.; Marq, J.B.; Penarete-Vargas, D.M.; Boulanger, M.J.; Soldati-Favre, D.; Lebrun, M. Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat. Commun. 2014, 5, 4098. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.; Azevedo, M.F.; Gilson, P.R.; Weiss, G.E.; O’Neill, M.T.; Wilson, D.W.; Crabb, B.S.; Cowman, A.F. Conditional expression of apical membrane antigen 1 in Plasmodium falciparum shows it is required for erythrocyte invasion by merozoites. Cell Microbiol. 2014, 16, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Sagara, I.; Dicko, A.; Ellis, R.D.; Fay, M.P.; Diawara, S.I.; Assadou, M.H.; Sissoko, M.S.; Kone, M.; Diallo, A.I.; Saye, R.; et al. A randomized controlled phase 2 trial of the blood stage AMA1-C1/Alhydrogel malaria vaccine in children in Mali. Vaccine 2009, 27, 3090–3098. [Google Scholar] [CrossRef]
- Ouattara, A.; Mu, J.; Takala-Harrison, S.; Saye, R.; Sagara, I.; Dicko, A.; Niangaly, A.; Duan, J.; Ellis, R.D.; Miller, L.H.; et al. Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine. Malar. J. 2010, 9, 175. [Google Scholar] [CrossRef]
- Remarque, E.J.; Faber, B.W.; Kocken, C.H.; Thomas, A.W. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect. Immun. 2008, 76, 2660–2670. [Google Scholar] [CrossRef][Green Version]
- Kusi, K.A.; Remarque, E.J.; Riasat, V.; Walraven, V.; Thomas, A.W.; Faber, B.W.; Kocken, C.H. Safety and immunogenicity of multi-antigen AMA1-based vaccines formulated with CoVaccine HT and Montanide ISA 51 in rhesus macaques. Malar. J. 2011, 10, 182. [Google Scholar] [CrossRef]
- Dutta, S.; Dlugosz, L.S.; Drew, D.R.; Ge, X.; Ababacar, D.; Rovira, Y.I.; Moch, J.K.; Shi, M.; Long, C.A.; Foley, M.; et al. Overcoming antigenic diversity by enhancing the immunogenicity of conserved epitopes on the malaria vaccine candidate apical membrane antigen-1. PLoS Pathog. 2013, 9, e1003840. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Ekanem, E.; Diouf, A.; Tonkin, M.L.; Miura, K.; Boulanger, M.J.; Long, C.A.; Narum, D.L.; Miller, L.H. Immunization with a functional protein complex required for erythrocyte invasion protects against lethal malaria. Proc. Natl. Acad. Sci. USA 2014, 111, 10311–10316. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Baldeviano, G.C.; Miura, K.; Diouf, A.; Ventocilla, J.A.; Leiva, K.P.; Lugo-Roman, L.; Lucas, C.; Orr-Gonzalez, S.; Zhu, D.; et al. A malaria vaccine protects Aotus monkeys against virulent Plasmodium falciparum infection. NPJ Vaccines 2017, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Dai, W.; Srinivasan, P.; McClellan, H.; Braden, D.; Allee-Munoz, A.; Hurtado, P.A.G.; Miller, L.H.; Duffy, P.E. Characterization of AMA1-RON2L complex with native gel electrophoresis and capillary isoelectric focusing. Electrophoresis 2022, 43, 509–515. [Google Scholar] [CrossRef]
- Patel, P.N.; Dickey, T.H.; Diouf, A.; Salinas, N.D.; McAleese, H.; Ouahes, T.; Long, C.A.; Miura, K.; Lambert, L.E.; Tolia, N.H. Structure-based design of a strain transcending AMA1-RON2L malaria vaccine. Nat. Commun. 2023, 14, 5345. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.J.; Reiling, L.; Feng, G.; Langer, C.; Osier, F.H.; Aspeling-Jones, H.; Cheng, Y.S.; Stubbs, J.; Tetteh, K.K.; Conway, D.J.; et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015, 42, 580–590. [Google Scholar] [CrossRef]
- Douglas, A.D.; Baldeviano, G.C.; Lucas, C.M.; Lugo-Roman, L.A.; Crosnier, C.; Bartholdson, S.J.; Diouf, A.; Miura, K.; Lambert, L.E.; Ventocilla, J.A.; et al. A PfRH5-based vaccine is efficacious against Heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe 2015, 17, 130–139. [Google Scholar] [CrossRef]
- Das, S.; Hertrich, N.; Perrin, A.J.; Withers-Martinez, C.; Collins, C.R.; Jones, M.L.; Watermeyer, J.M.; Fobes, E.T.; Martin, S.R.; Saibil, H.R.; et al. Processing of Plasmodium falciparum Merozoite Surface Protein MSP1 Activates a Spectrin-Binding Function Enabling Parasite Egress from RBCs. Cell Host Microbe 2015, 18, 433–444. [Google Scholar] [CrossRef]
- Lin, C.S.; Uboldi, A.D.; Epp, C.; Bujard, H.; Tsuboi, T.; Czabotar, P.E.; Cowman, A.F. Multiple Plasmodium falciparum Merozoite Surface Protein 1 Complexes Mediate Merozoite Binding to Human Erythrocytes. J. Biol. Chem. 2016, 291, 7703–7715. [Google Scholar] [CrossRef]
- Kumar, S.; Yadava, A.; Keister, D.B.; Tian, J.H.; Ohl, M.; Perdue-Greenfield, K.A.; Miller, L.H.; Kaslow, D.C. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol. Med. 1995, 1, 325–332. [Google Scholar] [CrossRef]
- Blank, A.; Furle, K.; Jaschke, A.; Mikus, G.; Lehmann, M.; Husing, J.; Heiss, K.; Giese, T.; Carter, D.; Bohnlein, E.; et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. NPJ Vaccines 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, M.; Furle, K.; Hibbert, J.; Ulmer, A.; Ali, A.; Giese, T.; Blank, A.; Haefeli, W.E.; Bohnlein, E.; Lanzer, M.; et al. Multifunctional IgG/IgM antibodies and cellular cytotoxicity are elicited by the full-length MSP1 SumayaVac-1 malaria vaccine. NPJ Vaccines 2023, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Smythe, J.A.; Coppel, R.L.; Day, K.P.; Martin, R.K.; Oduola, A.M.; Kemp, D.J.; Anders, R.F. Structural diversity in the Plasmodium falciparum merozoite surface antigen 2. Proc. Natl. Acad. Sci. USA 1991, 88, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Fenton, B.; Clark, J.T.; Khan, C.M.; Robinson, J.V.; Walliker, D.; Ridley, R.; Scaife, J.G.; McBride, J.S. Structural and antigenic polymorphism of the 35- to 48-kilodalton merozoite surface antigen (MSA-2) of the malaria parasite Plasmodium falciparum. Mol. Cell Biol. 1991, 11, 963–971. [Google Scholar] [CrossRef]
- Zhang, X.; Perugini, M.A.; Yao, S.; Adda, C.G.; Murphy, V.J.; Low, A.; Anders, R.F.; Norton, R.S. Solution conformation, backbone dynamics and lipid interactions of the intrinsically unstructured malaria surface protein MSP2. J. Mol. Biol. 2008, 379, 105–121. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Marjason, J.; Elliott, S.; Fahey, P.; Bang, G.; Malkin, E.; Tierney, E.; Aked-Hurditch, H.; Adda, C.; Cross, N.; et al. A phase 1 trial of MSP2-C1, a blood-stage malaria vaccine containing 2 isoforms of MSP2 formulated with Montanide(R) ISA 720. PLoS ONE 2011, 6, e24413. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Andrew, D.; MacRaild, C.A.; Morales, R.A.; Beeson, J.G.; Anders, R.F.; Richards, J.S.; Norton, R.S. Strain-transcending immune response generated by chimeras of the malaria vaccine candidate merozoite surface protein 2. Sci. Rep. 2016, 6, 20613. [Google Scholar] [CrossRef]
- Adda, C.G.; MacRaild, C.A.; Reiling, L.; Wycherley, K.; Boyle, M.J.; Kienzle, V.; Masendycz, P.; Foley, M.; Beeson, J.G.; Norton, R.S.; et al. Antigenic characterization of an intrinsically unstructured protein, Plasmodium falciparum merozoite surface protein 2. Infect. Immun. 2012, 80, 4177–4185. [Google Scholar] [CrossRef]
- Seow, J.; Das, S.C.; Morales, R.A.V.; Ataide, R.; Krishnarjuna, B.; Silk, M.; Chalmers, D.K.; Richards, J.; Anders, R.F.; MacRaild, C.A.; et al. Guiding the Immune Response to a Conserved Epitope in MSP2, an Intrinsically Disordered Malaria Vaccine Candidate. Vaccines 2021, 9, 855. [Google Scholar] [CrossRef]
- Huber, W.; Felger, I.; Matile, H.; Lipps, H.J.; Steiger, S.; Beck, H.P. Limited sequence polymorphism in the Plasmodium falciparum merozoite surface protein 3. Mol. Biochem. Parasitol. 1997, 87, 231–234. [Google Scholar] [CrossRef]
- Singh, S.; Soe, S.; Mejia, J.P.; Roussilhon, C.; Theisen, M.; Corradin, G.; Druilhe, P. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 2004, 190, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Druilhe, P.; Spertini, F.; Soesoe, D.; Corradin, G.; Mejia, P.; Singh, S.; Audran, R.; Bouzidi, A.; Oeuvray, C.; Roussilhon, C. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med. 2005, 2, e344. [Google Scholar] [CrossRef]
- Sirima, S.B.; Cousens, S.; Druilhe, P. Protection against malaria by MSP3 candidate vaccine. N. Engl. J. Med. 2011, 365, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, M.P.; Jogdand, P.S.; Singh, S.K.; Esen, M.; Christiansen, M.; Issifou, S.; Hounkpatin, A.B.; Ateba-Ngoa, U.; Kremsner, P.G.; Dziegiel, M.H.; et al. The malaria vaccine candidate GMZ2 elicits functional antibodies in individuals from malaria endemic and non-endemic areas. J. Infect. Dis. 2013, 208, 479–488. [Google Scholar] [CrossRef]
- Mordmuller, B.; Szywon, K.; Greutelaers, B.; Esen, M.; Mewono, L.; Treut, C.; Murbeth, R.E.; Chilengi, R.; Noor, R.; Kilama, W.L.; et al. Safety and immunogenicity of the malaria vaccine candidate GMZ2 in malaria-exposed, adult individuals from Lambarene, Gabon. Vaccine 2010, 28, 6698–6703. [Google Scholar] [CrossRef]
- Belard, S.; Issifou, S.; Hounkpatin, A.B.; Schaumburg, F.; Ngoa, U.A.; Esen, M.; Fendel, R.; de Salazar, P.M.; Murbeth, R.E.; Milligan, P.; et al. A randomized controlled phase Ib trial of the malaria vaccine candidate GMZ2 in African children. PLoS ONE 2011, 6, e22525. [Google Scholar] [CrossRef] [PubMed]
- Nouatin, O.; Ibanez, J.; Fendel, R.; Ngoa, U.A.; Lorenz, F.R.; Dejon-Agobe, J.C.; Edoa, J.R.; Flugge, J.; Bruckner, S.; Esen, M.; et al. Cellular and antibody response in GMZ2-vaccinated Gabonese volunteers in a controlled human malaria infection trial. Malar. J. 2022, 21, 191. [Google Scholar] [CrossRef]
- Alves, K.C.S.; Guimaraes, J.M.; Almeida, M.E.M.; Mariuba, L.A.M. Plasmodium falciparum merozoite surface protein 3 as a vaccine candidate: A brief review. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e23. [Google Scholar] [CrossRef]
- Sim, B.K.; Chitnis, C.E.; Wasniowska, K.; Hadley, T.J.; Miller, L.H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 1994, 264, 1941–1944. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Patel, S.M.; Atmar, R.L.; Lanford, T.A.; Dube, T.; Thompson, D.; Sim, B.K.; Long, C.; Keitel, W.A. Safety and immunogenicity of a recombinant nonglycosylated erythrocyte binding antigen 175 Region II malaria vaccine in healthy adults living in an area where malaria is not endemic. Clin. Vaccine Immunol. 2010, 17, 1552–1559. [Google Scholar] [CrossRef]
- Bei, A.K.; Membi, C.D.; Rayner, J.C.; Mubi, M.; Ngasala, B.; Sultan, A.A.; Premji, Z.; Duraisingh, M.T. Variant merozoite protein expression is associated with erythrocyte invasion phenotypes in Plasmodium falciparum isolates from Tanzania. Mol. Biochem. Parasitol. 2007, 153, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mitamura, T.; Fox, B.A.; Bzik, D.J.; Horii, T. Differential localization of processed fragments of Plasmodium falciparum serine repeat antigen and further processing of its N-terminal 47 kDa fragment. Parasitol. Int. 2002, 51, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Li, J.; Itagaki, S.; Okech, B.A.; Egwang, T.G.; Matsuoka, H.; Palacpac, N.M.; Mitamura, T.; Horii, T. Serine repeat antigen (SERA5) is predominantly expressed among the SERA multigene family of Plasmodium falciparum, and the acquired antibody titers correlate with serum inhibition of the parasite growth. J. Biol. Chem. 2002, 277, 47533–47540. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.K.; Good, R.T.; Drew, D.R.; Delorenzi, M.; Sanders, P.R.; Hodder, A.N.; Speed, T.P.; Cowman, A.F.; de Koning-Ward, T.F.; Crabb, B.S. A subset of Plasmodium falciparum SERA genes are expressed and appear to play an important role in the erythrocytic cycle. J. Biol. Chem. 2002, 277, 47524–47532. [Google Scholar] [CrossRef]
- McCoubrie, J.E.; Miller, S.K.; Sargeant, T.; Good, R.T.; Hodder, A.N.; Speed, T.P.; de Koning-Ward, T.F.; Crabb, B.S. Evidence for a common role for the serine-type Plasmodium falciparum serine repeat antigen proteases: Implications for vaccine and drug design. Infect. Immun. 2007, 75, 5565–5574. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, R.; Kavishwar, M.; Withers-Martinez, C.; Hackett, F.; Collins, C.R.; Howell, S.A.; Yeoh, S.; Knuepfer, E.; Atid, A.J.; Holder, A.A.; et al. Plasmodium falciparum SERA5 plays a non-enzymatic role in the malarial asexual blood-stage lifecycle. Mol. Microbiol. 2015, 96, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Palacpac, N.M.; Arisue, N.; Tougan, T.; Ishii, K.J.; Horii, T. Plasmodium falciparum serine repeat antigen 5 (SE36) as a malaria vaccine candidate. Vaccine 2011, 29, 5837–5845. [Google Scholar] [CrossRef]
- Tanabe, K.; Arisue, N.; Palacpac, N.M.; Yagi, M.; Tougan, T.; Honma, H.; Ferreira, M.U.; Farnert, A.; Bjorkman, A.; Kaneko, A.; et al. Geographic differentiation of polymorphism in the Plasmodium falciparum malaria vaccine candidate gene SERA5. Vaccine 2012, 30, 1583–1593. [Google Scholar] [CrossRef]
- Arisue, N.; Palacpac, N.M.Q.; Ntege, E.H.; Yeka, A.; Balikagala, B.; Kanoi, B.N.; Bougouma, E.C.; Tiono, A.B.; Nebie, I.; Diarra, A.; et al. African-specific polymorphisms in Plasmodium falciparum serine repeat antigen 5 in Uganda and Burkina Faso clinical samples do not interfere with antibody response to BK-SE36 vaccination. Front. Cell Infect. Microbiol. 2022, 12, 1058081. [Google Scholar] [CrossRef]
- Yagi, M.; Bang, G.; Tougan, T.; Palacpac, N.M.; Arisue, N.; Aoshi, T.; Matsumoto, Y.; Ishii, K.J.; Egwang, T.G.; Druilhe, P.; et al. Protective epitopes of the Plasmodium falciparum SERA5 malaria vaccine reside in intrinsically unstructured N-terminal repetitive sequences. PLoS ONE 2014, 9, e98460. [Google Scholar] [CrossRef]
- Palacpac, N.M.; Ntege, E.; Yeka, A.; Balikagala, B.; Suzuki, N.; Shirai, H.; Yagi, M.; Ito, K.; Fukushima, W.; Hirota, Y.; et al. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLoS ONE 2013, 8, e64073. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Palacpac, N.M.; Ito, K.; Oishi, Y.; Itagaki, S.; Balikagala, B.; Ntege, E.H.; Yeka, A.; Kanoi, B.N.; Katuro, O.; et al. Antibody titres and boosting after natural malaria infection in BK-SE36 vaccine responders during a follow-up study in Uganda. Sci. Rep. 2016, 6, 34363. [Google Scholar] [CrossRef]
- Bougouma, E.C.; Palacpac, N.M.Q.; Tiono, A.B.; Nebie, I.; Ouedraogo, A.; Houard, S.; Yagi, M.; Coulibaly, S.A.; Diarra, A.; Tougan, T.; et al. Safety and immunogenicity of BK-SE36 in a blinded, randomized, controlled, age de-escalating phase Ib clinical trial in Burkinabe children. Front. Immunol. 2022, 13, 978591. [Google Scholar] [CrossRef] [PubMed]
- Ezoe, S.; Palacpac, N.M.Q.; Tetsutani, K.; Yamamoto, K.; Okada, K.; Taira, M.; Nishida, S.; Hirata, H.; Ogata, A.; Yamada, T.; et al. First-in-human randomised trial and follow-up study of Plasmodium falciparum blood-stage malaria vaccine BK-SE36 with CpG-ODN(K3). Vaccine 2020, 38, 7246–7257. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Ahouidi, A.D.; Bei, A.K.; Dieye, T.N.; Mboup, S.; Harrison, S.C.; Duraisingh, M.T. Plasmodium falciparum merozoite surface antigen, PfRH5, elicits detectable levels of invasion-inhibiting antibodies in humans. J. Infect. Dis. 2013, 208, 1679–1687. [Google Scholar] [CrossRef]
- Williams, A.R.; Douglas, A.D.; Miura, K.; Illingworth, J.J.; Choudhary, P.; Murungi, L.M.; Furze, J.M.; Diouf, A.; Miotto, O.; Crosnier, C.; et al. Enhancing blockade of Plasmodium falciparum erythrocyte invasion: Assessing combinations of antibodies against PfRH5 and other merozoite antigens. PLoS Pathog. 2012, 8, e1002991. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, L.Y.; Bartholdson, S.J.; Crosnier, C.; Campos, M.G.; Wanaguru, M.; Nguon, C.; Kwiatkowski, D.P.; Wright, G.J.; Rayner, J.C. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine 2013, 31, 373–379. [Google Scholar] [CrossRef]
- Reddy, K.S.; Pandey, A.K.; Singh, H.; Sahar, T.; Emmanuel, A.; Chitnis, C.E.; Chauhan, V.S.; Gaur, D. Bacterially expressed full-length recombinant Plasmodium falciparum RH5 protein binds erythrocytes and elicits potent strain-transcending parasite-neutralizing antibodies. Infect. Immun. 2014, 82, 152–164. [Google Scholar] [CrossRef]
- Payne, R.O.; Silk, S.E.; Elias, S.C.; Miura, K.; Diouf, A.; Galaway, F.; de Graaf, H.; Brendish, N.J.; Poulton, I.D.; Griffiths, O.J.; et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight 2017, 2, e96381. [Google Scholar] [CrossRef]
- Campeotto, I.; Goldenzweig, A.; Davey, J.; Barfod, L.; Marshall, J.M.; Silk, S.E.; Wright, K.E.; Draper, S.J.; Higgins, M.K.; Fleishman, S.J. One-step design of a stable variant of the malaria invasion protein RH5 for use as a vaccine immunogen. Proc. Natl. Acad. Sci. USA 2017, 114, 998–1002. [Google Scholar] [CrossRef]
- Alanine, D.G.W.; Quinkert, D.; Kumarasingha, R.; Mehmood, S.; Donnellan, F.R.; Minkah, N.K.; Dadonaite, B.; Diouf, A.; Galaway, F.; Silk, S.E.; et al. Human Antibodies that Slow Erythrocyte Invasion Potentiate Malaria-Neutralizing Antibodies. Cell 2019, 178, 216–228.e221. [Google Scholar] [CrossRef] [PubMed]
- Healer, J.; Wong, W.; Thompson, J.K.; He, W.; Birkinshaw, R.W.; Miura, K.; Long, C.A.; Soroka, V.; Sogaard, T.M.M.; Jorgensen, T.; et al. Neutralising antibodies block the function of Rh5/Ripr/CyRPA complex during invasion of Plasmodium falciparum into human erythrocytes. Cell Microbiol. 2019, 21, e13030. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Takeo, S.; Iriko, H.; Jin, L.; Tsuchimochi, M.; Matsuda, S.; Han, E.T.; Otsuki, H.; Kaneko, O.; Sattabongkot, J.; et al. Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates. Infect. Immun. 2008, 76, 1702–1708. [Google Scholar] [CrossRef]
- Ntege, E.H.; Arisue, N.; Ito, D.; Hasegawa, T.; Palacpac, N.M.Q.; Egwang, T.G.; Horii, T.; Takashima, E.; Tsuboi, T. Identification of Plasmodium falciparum reticulocyte binding protein homologue 5-interacting protein, PfRipr, as a highly conserved blood-stage malaria vaccine candidate. Vaccine 2016, 34, 5612–5622. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Kanoi, B.N.; Ntege, E.H.; Aoki, M.; Fukushima, A.; Tsuboi, T.; Takashima, E. Antibodies against a short region of PfRipr inhibit Plasmodium falciparum merozoite invasion and PfRipr interaction with Rh5 and SEMA7A. Sci. Rep. 2020, 10, 6573. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Fernandes, B.; Castro, R.; Nagaoka, H.; Takashima, E.; Tsuboi, T.; Fukushima, A.; Viebig, N.K.; Depraetere, H.; Alves, P.M.; et al. Asexual Blood-Stage Malaria Vaccine Candidate PfRipr5: Enhanced Production in Insect Cells. Front. Bioeng. Biotechnol. 2022, 10, 908509. [Google Scholar] [CrossRef]
- Valmaseda, A.; Macete, E.; Nhabomba, A.; Guinovart, C.; Aide, P.; Bardaji, A.; Bassat, Q.; Nhampossa, T.; Maculuve, S.; Casellas, A.; et al. Identifying Immune Correlates of Protection against Plasmodium falciparum Through a Novel Approach to Account for Heterogeneity in Malaria Exposure. Clin. Infect. Dis. 2018, 66, 586–593. [Google Scholar] [CrossRef]
- Mian, S.Y.; Somanathan, A.; Chaddha, K.; Pandey, A.K.; Singh, H.; Krishna, S.; Chaturvedi, N.; Uchoi, S.; Shukla, M.M.; Bharti, P.K.; et al. Plasmodium falciparum Cysteine-Rich Protective Antigen (CyRPA) Elicits Detectable Levels of Invasion-Inhibitory Antibodies during Natural Infection in Humans. Infect. Immun. 2022, 90, e0037721. [Google Scholar] [CrossRef]
- Tamborrini, M.; Hauser, J.; Schafer, A.; Amacker, M.; Favuzza, P.; Kyungtak, K.; Fleury, S.; Pluschke, G. Vaccination with virosomally formulated recombinant CyRPA elicits protective antibodies against Plasmodium falciparum parasites in preclinical in vitro and in vivo models. NPJ Vaccines 2020, 5, 9. [Google Scholar] [CrossRef]
- Somanathan, A.; Mian, S.Y.; Chaddha, K.; Uchoi, S.; Bharti, P.K.; Tandon, R.; Gaur, D.; Chauhan, V.S. Process development and preclinical evaluation of a major Plasmodium falciparum blood stage vaccine candidate, Cysteine-Rich Protective Antigen (CyRPA). Front. Immunol. 2022, 13, 1005332. [Google Scholar] [CrossRef]
- Fernandes, B.; Sousa, M.; Castro, R.; Schafer, A.; Hauser, J.; Schulze, K.; Amacker, M.; Tamborrini, M.; Pluschke, G.; Alves, P.M.; et al. Scalable Process for High-Yield Production of PfCyRPA Using Insect Cells for Inclusion in a Malaria Virosome-Based Vaccine Candidate. Front. Bioeng. Biotechnol. 2022, 10, 879078. [Google Scholar] [CrossRef] [PubMed]
- Olugbile, S.; Kulangara, C.; Bang, G.; Bertholet, S.; Suzarte, E.; Villard, V.; Frank, G.; Audran, R.; Razaname, A.; Nebie, I.; et al. Vaccine potentials of an intrinsically unstructured fragment derived from the blood stage-associated Plasmodium falciparum protein PFF0165c. Infect. Immun. 2009, 77, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Kulangara, C.; Luedin, S.; Dietz, O.; Rusch, S.; Frank, G.; Mueller, D.; Moser, M.; Kajava, A.V.; Corradin, G.; Beck, H.P.; et al. Cell biological characterization of the malaria vaccine candidate trophozoite exported protein 1. PLoS ONE 2012, 7, e46112. [Google Scholar] [CrossRef]
- Almukadi, H.; Schwake, C.; Kaiser, M.M.; Mayer, D.C.G.; Schiemer, J.; Baldwin, M.R.; Hegde, S.; Lu, Y.; Hanada, T.; Chishti, A.H. Human erythrocyte band 3 is a host receptor for Plasmodium falciparum glutamic acid-rich protein. Blood 2019, 133, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Rojrung, R.; Kuamsab, N.; Putaporntip, C.; Jongwutiwes, S. Analysis of sequence diversity in Plasmodium falciparum glutamic acid-rich protein (PfGARP), an asexual blood stage vaccine candidate. Sci. Rep. 2023, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Doritchamou, J.Y.A.; Renn, J.P.; Hviid, L.; Duffy, P.E. A conformational epitope in placental malaria vaccine antigen VAR2CSA: What does it teach us? PLoS Pathog. 2023, 19, e1011370. [Google Scholar] [CrossRef]
- Doritchamou, J.Y.A.; Suurbaar, J.; Tuikue Ndam, N. Progress and new horizons toward a VAR2CSA-based placental malaria vaccine. Expert Rev. Vaccines 2021, 20, 215–226. [Google Scholar] [CrossRef]
- Tomlinson, A.; Semblat, J.P.; Gamain, B.; Chene, A. VAR2CSA-Mediated Host Defense Evasion of Plasmodium falciparum Infected Erythrocytes in Placental Malaria. Front. Immunol. 2020, 11, 624126. [Google Scholar] [CrossRef]
- Wang, K.; Dagil, R.; Lavstsen, T.; Misra, S.K.; Spliid, C.B.; Wang, Y.; Gustavsson, T.; Sandoval, D.R.; Vidal-Calvo, E.E.; Choudhary, S.; et al. Cryo-EM reveals the architecture of placental malaria VAR2CSA and provides molecular insight into chondroitin sulfate binding. Nat. Commun. 2021, 12, 2956. [Google Scholar] [CrossRef]
- Raja, A.I.; Stanisic, D.I.; Good, M.F. Chemical Attenuation in the Development of a Whole-Organism Malaria Vaccine. Infect. Immun. 2017, 85, 10-1128. [Google Scholar] [CrossRef]
- Stanisic, D.I.; Fink, J.; Mayer, J.; Coghill, S.; Gore, L.; Liu, X.Q.; El-Deeb, I.; Rodriguez, I.B.; Powell, J.; Willemsen, N.M.; et al. Vaccination with chemically attenuated Plasmodium falciparum asexual blood-stage parasites induces parasite-specific cellular immune responses in malaria-naive volunteers: A pilot study. BMC Med. 2018, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Low, L.M.; Ssemaganda, A.; Liu, X.Q.; Ho, M.F.; Ozberk, V.; Fink, J.; Sundac, L.; Alcorn, K.; Morrison, A.; O’Callaghan, K.; et al. Controlled Infection Immunization Using Delayed Death Drug Treatment Elicits Protective Immune Responses to Blood-Stage Malaria Parasites. Infect. Immun. 2019, 87, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Ho, M.F.; Nevagi, R.; Cooper, E.; Walton, M.; Islam, M.T.; Hussein, W.M.; Skwarczynski, M.; Toth, I.; Good, M.F. Development and Evaluation of a Cryopreserved Whole-Parasite Vaccine in a Rodent Model of Blood-Stage Malaria. mBio 2021, 12, e0265721. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Good, M.F. Malaria Vaccines: Progress to Date. BioDrugs 2023, 37, 737–756. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).