Essential Oils: Chemistry and Pharmacological Activities
Abstract
1. Introduction
2. Materials and Methods
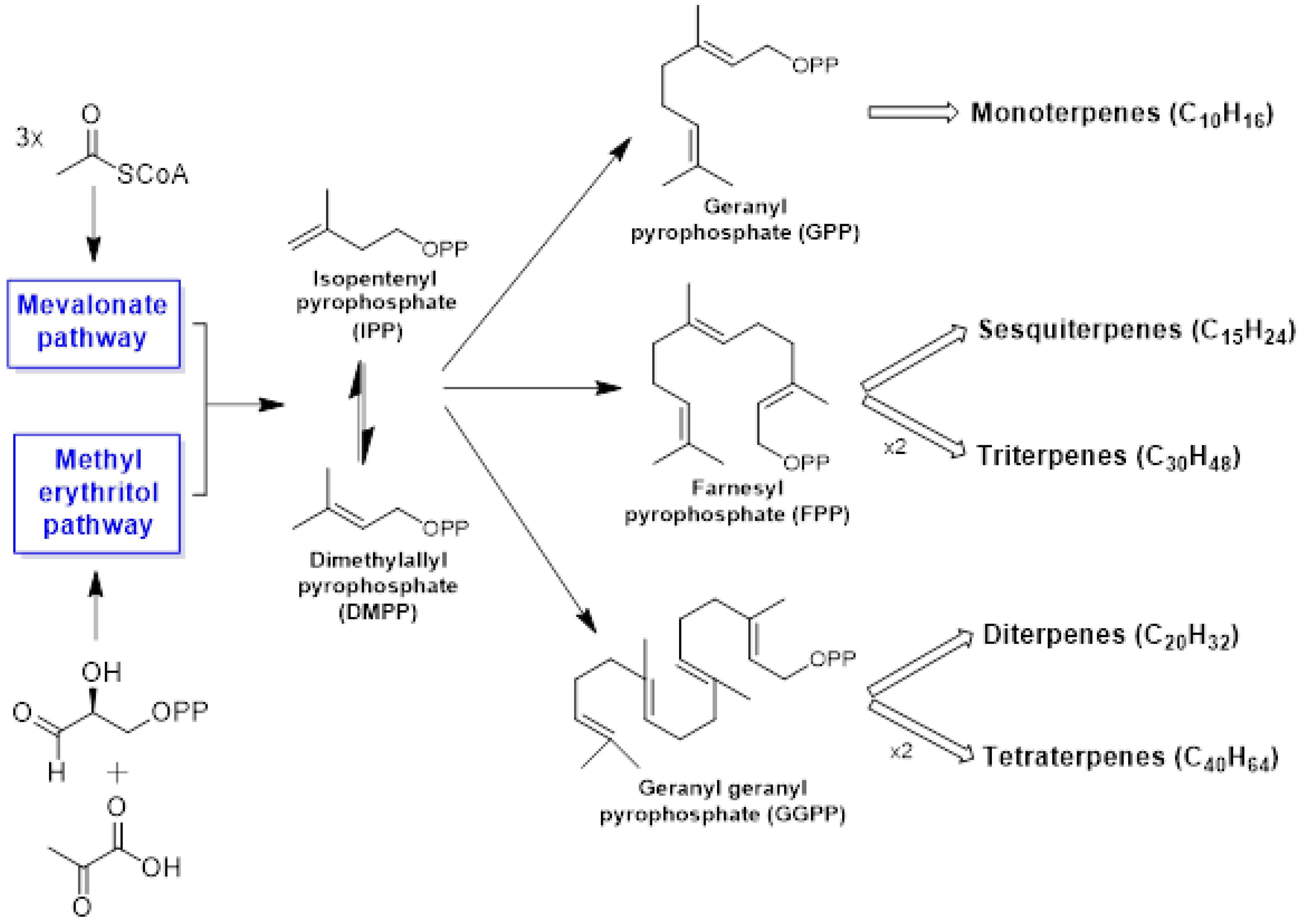
3. Chemistry of Essential Oils
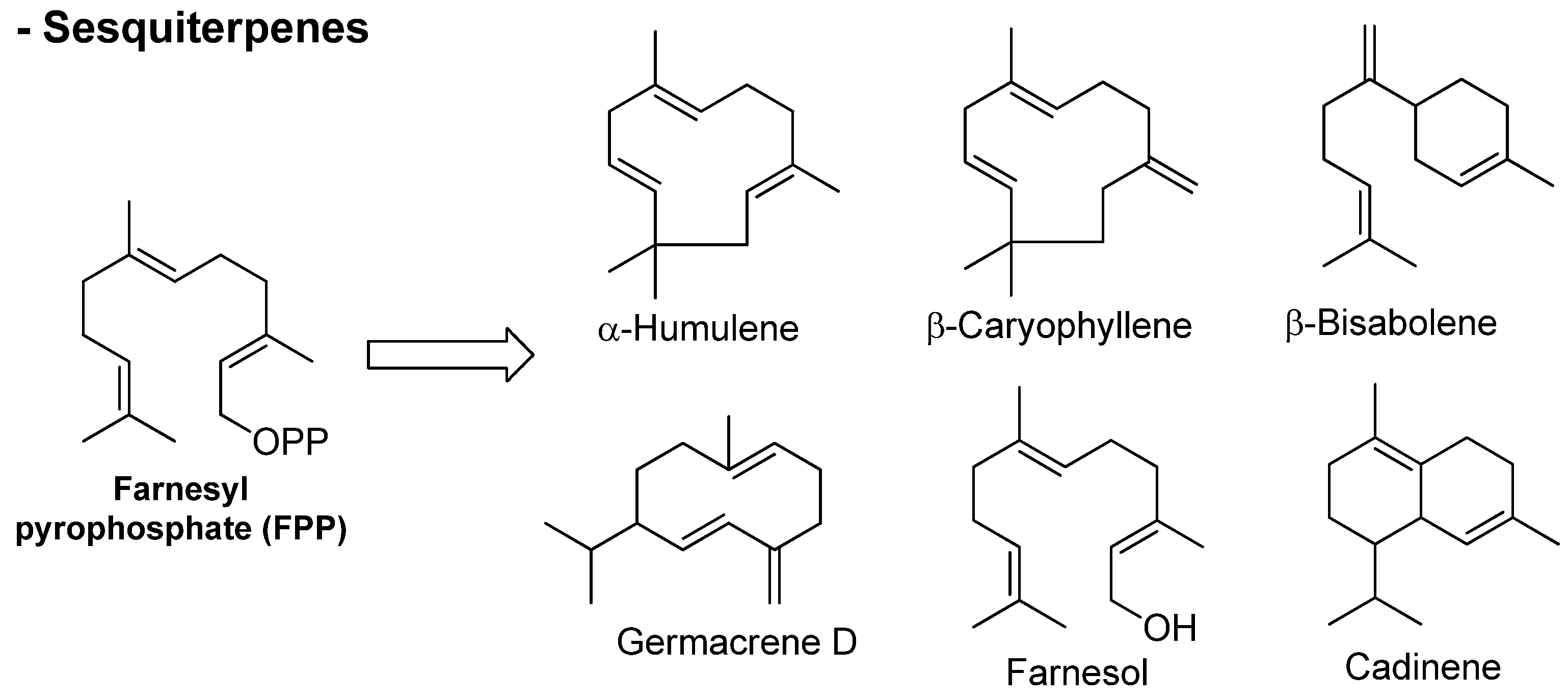
3.1. Terpenes
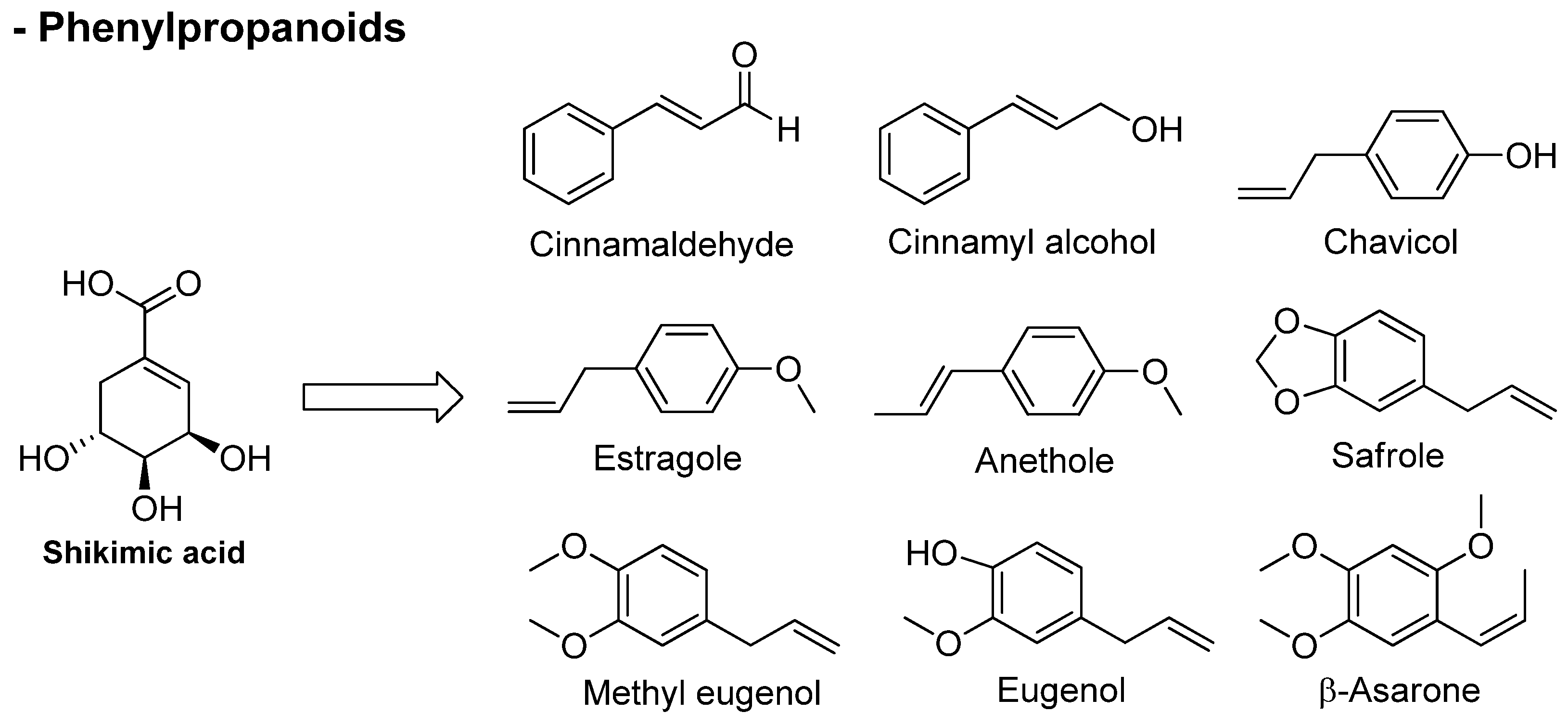
3.2. Phenylpropanoids
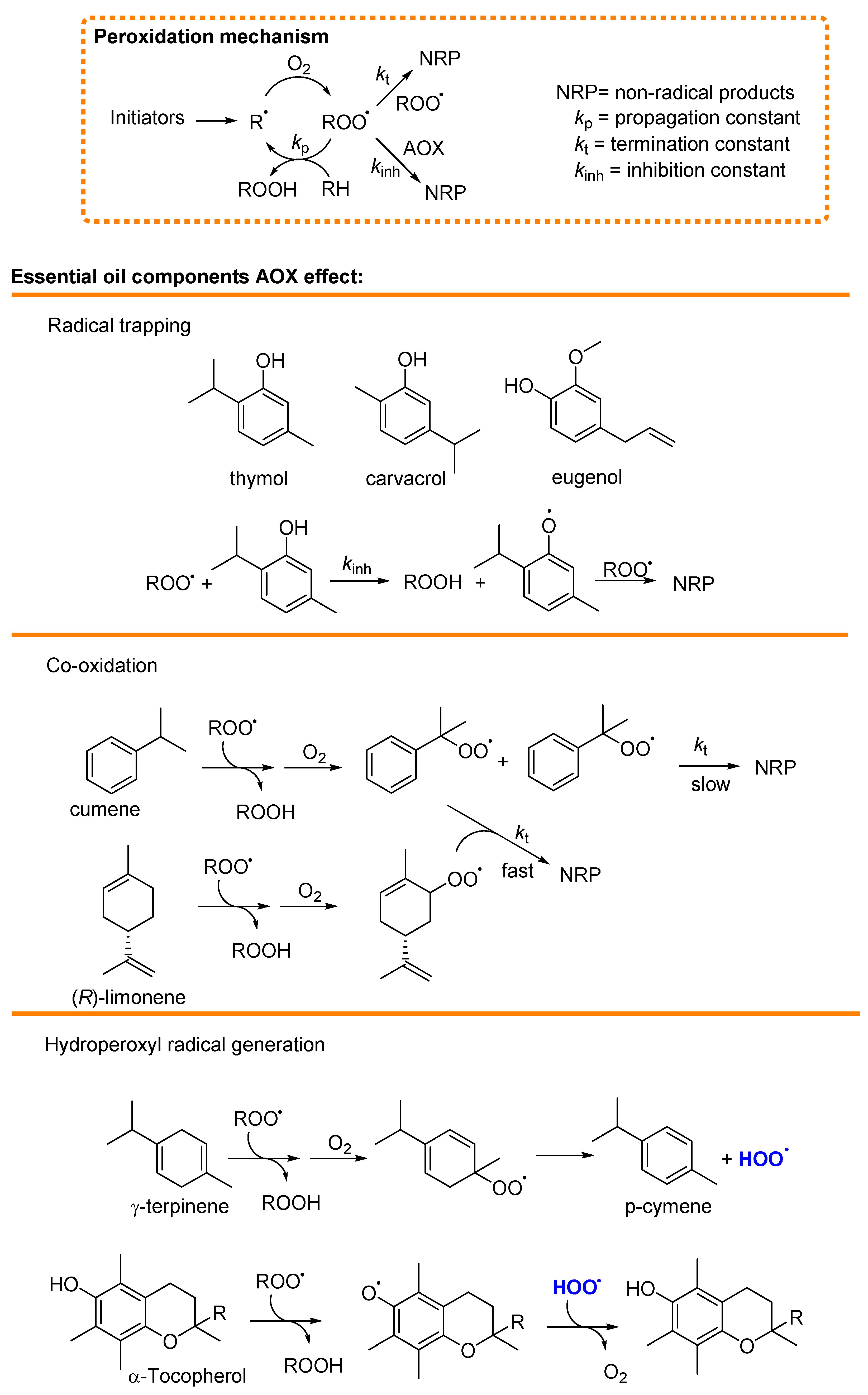
4. Antioxidants
4.1. Mechanism of Antioxidant Activity of Main Essential Oils and Their Constituents
4.1.1. Radical Trapping
4.1.2. Termination Enhancers
4.1.3. Hydroperoxyl Radical (HOO•) Generation
4.2. Methods Suggested to Explore the Radical Trapping Activity
5. Antiviral Activity
6. Antimicrobial Activity
6.1. Antibacterial Activity
6.2. Antifungal Activity
7. Antitumor Activity
8. Anti-Inflammatory Activity
8.1. Inflammatory Response
8.2. Anti-Inflammatory Mechanisms
8.2.1. Inhibition of the Release of Mediators by Inflammatory Cells
8.2.2. Interaction with Sensory Receptors
8.2.3. Suppression of the NLRP3 Inflammasome
8.2.4. Effects on NF-κB Signaling Pathway
8.2.5. Regulation of the Intestinal Microbiota and Barrier Function
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guenther, E. The Essential Oils: Individual Essential Oils of the Plant Families Gramineae, Lauraceae, Burseraceae, Myrtaceae, Umbelliferae and Geraniaceae, 4th ed.; Van Nostrand: Paris, France, 1950; pp. 1–752. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Association Française de Normalisation (AFNOR). Huiles Essentielles, Tome 2, Monographies Relatives Aux Huiles Essentielles, 6th ed.; AFNOR, Association Française de Normalisation: Paris, France, 2000. [Google Scholar]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar] [CrossRef]
- Sell, C. Chemistry of essential oils. In Handbook of Essential Oils. Science, Technology, and Applications; Bașer, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 121–150. [Google Scholar]
- Schmidt, E. Production of essential oils. In Handbook of Essential Oils. Science, Technology, and Applications; Bașer, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 83–119. [Google Scholar]
- De Cássia da Silveira e Sá, R.; Lima, T.C.; Da Nóbrega, F.R.; De Brito, A.E.M.; De Sousa, D.P. Analgesic-like Activity of Essential Oil Constituents: An Update. Int. J. Mol. Sci. 2017, 18, 2392. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Silva, R.H.N.; Silva, E.F.d.; Gavioli, E.C. Essential Oils and Their Constituents: An Alternative Source for Novel Antidepressants. Molecules 2017, 22, 1290. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Hocayen, P.D.A.S.; Andrade, L.N.; Andreatini, R. A Systematic Review of the Anxiolytic-like Effects of Essential Oils in Animal Models. Molecules 2015, 20, 18620–18660. [Google Scholar] [CrossRef]
- Pandey, V.K.; Tripathi, A.; Srivastava, S.; Dar, A.H.; Singh, R.; Farooqui, A.; Pandey, S. Exploiting the bioactive properties of essential oils and their potential applications in food industry. Food Sci. Biotechnol. 2023, 32, 885–902. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The Effects of Essential Oils on the Nervous System: A Scoping Review. Molecules 2023, 28, 3771. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef]
- Machado, T.Q.; da Fonseca, A.C.C.; Duarte, A.B.S.; Robbs, B.K.; de Sousa, D.P. A Narrative Review of the Antitumor Activity of Monoterpenes from Essential Oils: An Update. Biomed. Res. Int. 2022, 2022, 6317201. [Google Scholar] [CrossRef]
- Kong, A.S.-Y.; Maran, S.; Yap, P.S.-X.; Lim, S.-H.E.; Yang, S.-K.; Cheng, W.-H.; Tan, Y.-H.; Lai, K.-S. Anti- and Pro-Oxidant Properties of Essential Oils against Antimicrobial Resistance. Antioxidants 2022, 11, 1819. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.C.; Silva, T.K.M.; Silva, F.L.; Barbosa Filho, J.M.; Marques, M.O.M.; La Corte, R.; Cavalcanti, S.C.H.; Sousa, D.P. Larvicidal activity of Mentha x villosa Hudson essential oil, rotundifolone and derivatives. Chemosphere 2013, 104, 37–43. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Boumaiza, A.; Nada, H.G.; Rajabi, M.; Mousa, S.A. Eucalyptus globulus essential oil as a natural food preservative: Antioxidant, antibacterial and antifungal properties in vitro and in a real food matrix (orangina fruit juice). Appl. Sci. 2020, 10, 5581. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Falah, F.; Arab, F.L.; Vasiee, M.; Yazdi, F.T. Chemical Composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid. Based Complement. Altern. Med. 2020, 2020, 5190603. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Dewick, P. The biosynthesis of C5-C25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef]
- Tian, H.; Zada, B.; Singh, B.H.; Wang, C.; Kim, S.-W. Synthetic Biology Approaches for the Production of Isoprenoids in Escherichia coli. In Current Developments in Biotechnology and Bioengineering, 1st ed.; Singh, S.P., Pandey, A., Du, G., Kumar, S., Eds.; Elsevier: New York, NY, USA, 2020; pp. 311–329. [Google Scholar]
- Leite, A.C.; Lopes, A.A.; Massuo, J.K.; Bolzani, V.d.S.; Furlan, M. Biosynthetic origin of the isoprene units in chromenes of Piper aduncum (Piperaceae). J. Braz. Chem. Soc. 2007, 18, 1500–1503. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Rohmer, M.; Schwender, J. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiol. Plantarum. 2006, 101, 643–652. [Google Scholar] [CrossRef]
- Chatzivasileiou, A.O.; Ward, V.; Edgar, S.M.; Stephanopoulos, G. Two-step pathway for isoprenoid synthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 506–511. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Physiology: Rockville, MD, USA, 2000; Volume 24, pp. 1250–1318. [Google Scholar]
- Simões, C.M.O.; Schenkel, E.P.; Gosmann, G.; Mello, J.C.P.; Mentz, L.A.; Petrovik, P.R. Farmacognosia: Da Planta ao Medicamento; Editora UFRGS: Porto Alegre, Brazil, 2010. [Google Scholar]
- Jaeger, R.; Cuny, E. Terpenoids with Special Pharmacological Significance: A Review. Nat. Prod. Commun. 2016, 11, 1373–1390. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.S.; Croteau, R.B. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant. Sci. 2002, 7, 366–373. [Google Scholar] [CrossRef]
- Avila, C. Terpenoids in Marine Heterobranch Molluscs. Mar. Drugs 2020, 18, 162. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Feijó, E.V.R.S.; Oliveira, R.A.; Costa, L.C.B. Light affects Varronia curassavica essential oil yield by increasing trichomes frequency. Rev. Bras. Farmacogn. 2014, 24, 516–523. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Butnariu, M.; Sarac, I. Essential Oils from Plants. J. Biotechnol. Biomed. Sci. 2018, 1, 35–43. [Google Scholar] [CrossRef]
- Ampadu, G.A.A.; Mensah, J.O.; Darko, G.; Borquaye, L.S. Essential Oils from the Fruits and Leaves of Spondias mombin Linn.: Chemical Composition, Biological Activity, and Molecular Docking Study. Evid. Based Complement. Altern. Med. 2022, 2022, 7211015. [Google Scholar]
- Vigan, M. Essential oils: Renewal of interest and toxicity. Eur. J. Dermatol. 2010, 20, 685–692. [Google Scholar] [PubMed]
- Chami, F.; Chami, N.; Bennis, S.; Trouillas, J.; Remmal, A. Evaluation of carvacrol and eugenol as prophylaxis and treatment of vaginal candidiasis in an immunosuppressed rat model. J. Antimicrob. Chemother. 2004, 54, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef]
- Saranraj, P.; Devi, V.D. Essential oils and its antibacterial properties—A review. Life Sci. Arch. 2017, 3, 994–1011. [Google Scholar]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Methods to Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef]
- Guo, Y.; Pizzol, R.; Gabbanini, S.; Baschieri, A.; Amorati, R.; Valgimigli, L. Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils. Molecules 2021, 26, 5237. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Valgimigli, L. Measuring Antioxidant Activity in Bioorganic Samples by the Differential Oxygen Uptake Apparatus: Recent Advances. J. Chem. 2017, 2017, 6369358. [Google Scholar] [CrossRef]
- Baschieri, A.; Ajvazi, M.D.; Tonfack, J.L.F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Cedrowski, J.; Litwinienko, G.; Baschieri, A.; Amorati, R. Hydroperoxyl Radicals (HOOC): Vitamin E Regeneration and H-Bond Effects on the Hydrogen Atom Transfer. Chem. Eur. J. 2016, 22, 16441–16445. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOOC to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Ingold, K.U. Mechanism of Inhibition of Lipid Peroxidation by γ-Terpinene, an Unusual and Potentially Useful Hydrocarbon Antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-X.; Liu, Z.-Q. Unusual Antioxidant Behavior of α- and γ-Terpinene in Protecting Methyl Linoleate, DNA, and Erythrocyte. J. Agric. Food Chem. 2009, 57, 3943–3948. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem. 2021, 345, 128468. [Google Scholar] [CrossRef]
- Mollica, F.; Gelabert, I.; Amorati, R. Synergic Antioxidant Effects of the Essential Oil Component γ-Terpinene on High-Temperature Oil Oxidation. ACS Food Sci. Technol. 2022, 2, 180–186. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Guo, L.; Jiang, H.; Ji, Q. In Vitro Evaluation of Antioxidant and Antimicrobial Activities of Melaleuca alternifolia Essential Oil. BioMed Res. Int. 2018, 2018, 2396109. [Google Scholar] [CrossRef]
- Kallel, I.; Hadrich, B.; Gargouri, B.; Chaabane, A.; Lassoued, S.; Gdoura, R.; Bayoudh, A.; Messaoud, E.B. Optimization of Cinnamon (Cinnamomum zeylanicum Blume) Essential Oil Extraction: Evaluation of Antioxidant and Antiproliferative Effects. Evid. Based Complement. Alternat. Med. 2019, 2019, 6498347. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Damien Dorman, H.J. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef]
- Allahverdiyev, A.; Duran, N.; Ozguven, M.; Koltas, S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine 2004, 11, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.A.; Stashenko, E.; Ocazionez, R.E. Comparative study on in vitro activities of citral, limonene and essential oils from Lippia citriodora and L. alba on yellow fever vírus. Nat. Prod. Commun. 2013, 8, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Picard, M.; Bénard, S.; Desvignes, C.; Desprès, P.; Diotel, N.; El Kalamouni, C. Ayapana triplinervis Essential Oil and Its Main Component Thymohydroquinone Dimethyl Ether Inhibit Zika Virus at Doses Devoid of Toxicity in Zebrafish. Molecules 2019, 24, 3447. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2020, 152, 104620. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Forsch. Komplementmed. 2009, 16, 79–90. [Google Scholar] [CrossRef]
- Lu, M.; Han, Z.; Xu, Y.; Yao, L. In Vitro and In Vivo anti-tobacco mosaic virus activities of essential oils and individual compounds. J. Microbiol. Biotechnol. 2013, 23, 771–778. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schön, K.; Reichling, J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie 2001, 56, 343–347. [Google Scholar]
- Reichling, J.; Koch, C.; Stahl-Biskup, E.; Sojka, C.; Schnitzler, P. Virucidal activity of a beta-triketone-rich essential oil of Leptospermum scoparium (manuka oil) against HSV-1 and HSV-2 in cell culture. Planta Med. 2005, 71, 1123–1127. [Google Scholar] [CrossRef]
- Schnitzler, P.; Koch, C.; Reichling, J. Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrob. Agents. Chemother. 2007, 51, 1859–1862. [Google Scholar] [CrossRef]
- Garozzo, A.; Timpanaro, R.; Bisignano, B.; Furneri, P.M.; Bisignano, G.; Castro, A. In vitro antiviral activity of Melaleuca alternifolia essential oil. Lett. Appl. Microbiol. 2009, 49, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.L.; Roehe, P.M.; Fuentefria, A.M.; Henriques, A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Biol. 2015, 53, 682–688. [Google Scholar] [CrossRef] [PubMed]
- García, C.C.; Talarico, L.; Almeida, N.; Colombres, S.; Duschatzky, C.; Damonte, E.B. Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother. Res. 2003, 17, 1073–1075. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. A Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef]
- Pourghanbari, G.; Nili, H.; Moattari, A.; Mohammadi, A.; Iraji, A. Antiviral activity of the oseltamivir and Melissa officinalis L. essential oil against avian influenza A virus (H9N2). Virus Dis. 2016, 27, 170–178. [Google Scholar] [CrossRef]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014, 116, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J. Chemical Constituents of Essential Oils Possessing Anti-Influenza A/WS/33 Virus Activity. Osong. Public Health Res. Perspect. 2018, 9, 348–353. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Liao, Q.; Qian, Z.; Liu, R.; An, L.; Chen, X. Germacrone inhibits early stages of influenza virus infection. Antivir. Res. 2013, 100, 578–588. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Zu, S.; Sun, X.; Liu, C.; Liu, D.; Zhang, X.; Tian, J.; Qu, L. In vitro antiviral effect of germacrone on feline calicivirus. Arch. Virol. 2016, 161, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Paulpandi, M.; Kannan, S.; Thangam, R.; Kaveri, K.; Gunasekaran, P.; Rejeeth, C. In vitro anti-viral effect of β-santalol against influenza viral replication. Phytomedicine 2012, 19, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Vimalanathan, S.; Hudson, J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent. Oil. Nat. Prod. 2014, 2, 47–53. [Google Scholar]
- Feriotto, G.; Marchetti, N.; Costa, V.; Beninati, S.; Tagliati, F.; Mischiati, C. Chemical Composition of Essential Oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and Their Effects on the HIV-1 Tat Protein Function. Chem. Biodivers. 2018, 15, e1700436. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Ćavar Zeljković, S.; Schadich, E.; Džubák, P.; Hajdúch, M.; Tarkowski, P. Antiviral Activity of Selected Lamiaceae Essential Oils and Their Monoterpenes Against SARS-Cov-2. Front Pharmacol. 2022, 13, 893634. [Google Scholar] [CrossRef]
- Mori, K.; Obossou, E.K.; Suwa, S.; Miura, S.; Oh, S.; Jinbo, N.; Ishibashi, Y.; Shikamoto, Y.; Hosono, T.; Toda, T. Human Immunodeficiency virus type 1 (hiv-1) reverse transcriptase inhibitory effect of Cymbopogon nardus essential oil. Int. J. Adv. Res. 2016, 2, 7–13. [Google Scholar]
- Civitelli, L.; Panella, S.; Marcocci, M.E.; De Petris, A.; Garzoli, S.; Pepi, F.; Vavala, E.; Ragno, R.; Nencioni, L.; Palamara, A.T.; et al. In vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomedicine 2014, 21, 857–865. [Google Scholar] [CrossRef]
- Lai, W.L.; Chuang, H.S.; Lee, M.H.; Wei, C.L.; Lin, C.-F.; Tsai, Y.C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014, 6, 149–155. [Google Scholar] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.A.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell Mol. Biol. 2017, 63, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Benali, T.; Lemhadri, A.; Harboul, K.; Chtibi, H.; Khabbach, A.; Jadouali, S.M.; Quesada-Romero, L.; Louahlia, S.; Hammani, K.; Ghaleb, A.; et al. Chemical Profiling and Biological Properties of Essential Oils of Lavandula stoechas L. Collected from Three Moroccan Sites: In Vitro and In Silico Investigations. Plants 2023, 12, 1413. [Google Scholar] [CrossRef] [PubMed]
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral activity of the Lippia graveolens (Mexican oregano) essential oil and its main compound carvacrol against human and animal viroses. Braz. J. Microbiol. 2011, 42, 1616–1624. [Google Scholar] [CrossRef]
- Garber, A.; Barnard, L.; Pickrell, C. Review of Whole Plant Extracts with Activity Against Herpes Simplex Viruses In Vitro and In Vivo. J. Evid. Based Integr. Med. 2021, 26, 2515690X20978394. [Google Scholar] [CrossRef] [PubMed]
- Flechas, M.C.; Ocazionez, R.E.; Stashenko, E.E. Evaluation of in vitro antiviral activity of essential oil compounds against dengue virus. Pharmacogn. J. 2018, 10, 55–59. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds—A Scientific Approach. Planta Med. 2022, 88, 587–603. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Contreras-Castillo, C.J.; Da Gloria, E.M. In vitro mechanism of antibacterial action of a citrus essential oil on an enterotoxigenic Escherichia coli and Lactobacillus rhamnosus. J. Appl. Microbiol. 2020, 129, 541–553. [Google Scholar] [CrossRef]
- Li, Z.-H.; Cai, M.; Liu, Y.-S.; Sun, P.-L.; Luo, S.-L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Piasecki, B.; Balázs, V.L.; Kieltyka-Dadasiewicz, A.; Szabó, P.; Kocsis, B.; Horváth, G.; Ludwiczuk, A. Microbiological Studies on the Influence of Essential Oils from Several Origanum Species on Respiratory Pathogens. Molecules 2023, 28, 3044. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Turgis, M.; Han, J.; Caillet, S.; Lacroix, M. Antimicrobial activity of mustard essential oil against Escherichia coli O157: H7 and Salmonella typhi. Food Control 2009, 20, 1073–1079. [Google Scholar] [CrossRef]
- Bouhdid, S.; Abrini, J.; Zhiri, A.; Espuny, M.J.; Manresa, A. Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J. Appl. Microbiol. 2009, 106, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendide, K.; Remmal, A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on Escherichia coli and Bacillus subtilis. J. Essent. Oil Res. 2003, 15, 356–362. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Burt, S.A.; van der Zee, R.; Koets, A.P.; de Graaff, A.M.; van Knapen, F.; Gaastra, W.; Haagsman, H.P.; Veldhuizen, E.J.A. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157: H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef]
- Frazzon, A.P.G.; Correa, M.S.; Lauer Júnior, C.M.; Frazzon, J. Modulation of gene expression by essential oils in bacteria. Adv. Biotech. Micro. 2018, 8, 1–3. [Google Scholar]
- Zani, F.; Massimo, G.; Benvenuti, S.; Bianchi, A.; Albasini, A.; Melegari, M.; Vampa, G.; Bellotti, A.; Mazza, P. Studies on the genotoxic properties of essential oils with Bacillus subtilis rec-assay and Salmonella/microsome reversion assay. Planta Medica 1991, 57, 237–241. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Zahin, M.; Hasan, S.; Husain, F.M.; Ahmad, I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2009, 49, 354–360. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Freires, I.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; de Alencar, S.M.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.T.; Rosalen, P.L. Coriandrum sativum L. (coriander) essential oil: Antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef]
- Pozniakovsky, A.I.; Knorre, D.A.; Markova, O.V.; Hyman, A.A.; Skulachev, V.P.; Severin, F.F. Role of mitochondria in the pheromone-and amiodarone-induced programmed death of yeast. J. Cell. Biol. 2005, 168, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Shahwar, M.K.; El-Ghorab, A.H.; Anjum, F.M.; Butt, M.S.; Hussain, S.; Nadeem, M. Characterization of Coriander (Coriandrum sativum L.) Seeds and Leaves: Volatile and Non Volatile Extracts. Int. J. Food Prop. 2012, 15, 736–747. [Google Scholar] [CrossRef]
- Danielli, L.J.; Pippi, B.; Duarte, J.A.; Maciel, A.J.; Lopes, W.; Machado, M.M.; Oliveira, L.F.S.; Vainstein, M.H.; Teixeira, M.L.; Bordignon, S.A.L. Antifungal mechanism of action of Schinus lentiscifolius Marchand essential oil and its synergistic effect in vitro with terbinafine and ciclopirox against dermatophytes. J. Pharm. Pharmacol. 2018, 70, 1216–1227. [Google Scholar] [CrossRef]
- de Macêdo Andrade, A.C.; Rosalen, P.L.; Freires, I.A.; Scotti, L.; Scotti, M.T.; Aquino, S.G.; de Castro, R.D. Antifungal activity, mode of action, docking prediction and anti-biofilm effects of (+)-β-pinene enantiomers against Candida spp. Curr. Top. Med. Chem. 2018, 18, 2481–2490. [Google Scholar] [CrossRef]
- Medeiros, C.I.S.; de Sousa, M.N.A.; Filho, G.G.A.; Freitas, F.O.R.; Uchoa, D.P.L.; Nobre, M.S.C.; Bezerra, A.L.D.; Rolim, L.A.D.M.M.; Morais, A.M.B.; Nogueira, T.B.S.S.; et al. Antifungal activity of linalool against fluconazole-resistant clinical strains of vulvovaginal Candida albicans and its predictive mechanism of action. Braz. J. Med. Biol. Res. 2022, 55, e11831. [Google Scholar] [CrossRef]
- Rivera-Yañez, R.C.; Terrazas, L.I.; Jimenez-Estrada, M.; Campos, J.E.; Flores-Ortiz, C.M.; Hernandez, L.B.; Cruz-Sanchez, T.; Garrido-Fariña, G.I.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene-An In Vitro Study. Molecules 2017, 22, 2095. [Google Scholar] [CrossRef]
- Sousa, J.P.; Queiroz, E.O.; Guerra, F.Q.S.; Mendes, J.M.; Pedrosa, Z.V.; Abrahão Filho, A.O.; Pereira, F.O.; Trajano, V.N.; Souza, F.S.; Lima, E.O. Morphological alterations and time-kill studies of the essential oil from the leaves of Coriandrum sativum L. on Candida albicans. Bol. Latinoam. Caribe Plant Med. Aromats. 2016, 15, 398–406. [Google Scholar]
- Agassi, S.F.T.; Yeh, T.M.; Chang, C.D.; Hsu, J.L.; Shih, W.L. Potentiation of Differentiation and Apoptosis in a Human Promyelocytic Leukemia Cell Line by Garlic Essential Oil and Its Organosulfur Compounds. Anticancer Res. 2020, 40, 6345–6354. [Google Scholar] [CrossRef] [PubMed]
- Ahani, N.; Sangtarash, M.H.; Alipour Eskandani, M.; Houshmand, M. Zataria multiflora Boiss. Essential Oil Induce Apoptosis in Two Human Colon Cancer Cell Lines (HCT116 & SW48). Iran. J. Public Health 2020, 49, 753–762. [Google Scholar] [PubMed]
- Seal, S.; Chatterjee, P.; Bhattacharya, S.; Pal, D.; Dasgupta, S.; Kundu, R.; Mukherjee, S.; Bhattacharya, S.; Bhuyan, M.; Bhattacharyya, P.R.; et al. Vapor of volatile oils from Litsea cubeba seed induces apoptosis and causes cell cycle arrest in lung cancer cells. PLoS ONE 2012, 7, e47014. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, M.; Kumar, A.; Suravajhala, R. Cedrus deodara (Bark) Essential Oil Induces Apoptosis in Human Colon Cancer Cells by Inhibiting Nuclear Factor kappa B. Curr. Top. Med. Chem. 2020, 20, 1981–1992. [Google Scholar] [CrossRef] [PubMed]
- Poma, P.; Labbozzetta, M.; Ramarosandratana, A.V.; Rosselli, S.; Tutone, M.; Sajeva, M.; Notarbartolo, M. In Vitro Modulation of P-Glycoprotein Activity by Euphorbia intisy Essential Oil on Acute Myeloid Leukemia Cell Line HL-60R. Pharmaceuticals 2021, 14, 111. [Google Scholar] [CrossRef]
- Cha, J.D.; Moon, S.E.; Kim, H.Y.; Cha, I.H.; Lee, K.Y. Essential oil of Artemisia capillaris induces apoptosis in KB cells via mitochondrial stress and caspase activation mediated by MAPK-stimulated signaling pathway. J. Food Sci. 2009, 74, 75–81. [Google Scholar] [CrossRef]
- Ren, Z.; Elson, C.E.; Gould, M.N. Inhibition of type I and type II geranylgeranyl-protein transferases by the monoterpene perillyl alcohol in NIH3T3 cells. Biochem. Pharmacol. 1997, 54, 113–120. [Google Scholar] [CrossRef]
- Stayrook, K.R.; Mckinzie, J.H.; Barbhaiya, L.H.; Crowell, P.L. Effects of the antitumor agent perillyl alcohol on H-Ras vs. K-Ras farnesylation and signal transduction in pancreatic cells. Anticancer Res. 1998, 18, 823–828. [Google Scholar]
- Roepke, M.; Diestel, A.; Bajbouj, K.; Walluscheck, D.; Schonfeld, P.; Roessner, A.; Schneider-Stock, R.; Gali-Muhtasib, H. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol. Ther. 2007, 6, 160–169. [Google Scholar] [CrossRef]
- Alam, A.; Jawaid, T.; Alsanad, S.M.; Kamal, M.; Balaha, M.F. Composition, Antibacterial Efficacy, and Anticancer Activity of Essential Oil Extracted from Psidium guajava (L.) Leaves. Plants 2023, 12, 246. [Google Scholar] [CrossRef]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; Sousa, D.P. Antitumor Activity of Monoterpenes Found in Essential Oils. Sci. World J. 2014, 2014, 953451. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Xi, G.; Zhang, M.; Chen, Y.; Lei, B.; Dong, X.; Yang, Y. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013, 29, 349–354. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco. Targets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Zahra, H.A.; Ammar, R.B.; Mohamed, M.E.; Ibrahim, H.-I.M. Beta-Caryophyllene Enhances the Anti-Tumor Activity of Cisplatin in Lung Cancer Cell Lines through Regulating Cell Cycle and Apoptosis Signaling Molecules. Molecules 2022, 27, 8354. [Google Scholar] [CrossRef] [PubMed]
- Rezaieseresht, H.; Shobeiri, S.S.; Kaskani, A. Chenopodium botrys Essential Oil as A Source of Sesquiterpenes to Induce Apoptosis and G1 Cell Cycle Arrest in Cervical Cancer Cells. Iran. J. Pharm. Res. 2020, 19, 341–351. [Google Scholar]
- Beeby, E.; Magalhães, M.; Lemos, M.F.L.; Pires, I.M.; Cabral, C. Cytotoxic effects of Ridolfia segetum (L.) Moris phytoproducts in cancer cells. J. Ethnopharmacol. 2021, 267, 113515. [Google Scholar] [CrossRef]
- Ilhan, S. Essential Oils from Vitex agnus castus L. Leaves Induces Caspase-Dependent Apoptosis of Human Multidrug-Resistant Lung Carcinoma Cells through Intrinsic and Extrinsic Pathways. Nutr. Cancer 2021, 73, 694–702. [Google Scholar] [CrossRef]
- Anunciação, T.A.D.; Costa, R.G.A.; Lima, E.J.S.P.; Silva, V.R.; Santos, L.S.; Soares, M.B.P.; Dias, R.B.; Rocha, C.A.G.; Costa, E.V.; Silva, F.M.A.D.; et al. In vitro and in vivo inhibition of HCT116 cells by essential oils from bark and leaves of Virola surinamensis (Rol. ex Rottb.) Warb. (Myristicaceae). J. Ethnopharmacol. 2020, 262, 113166. [Google Scholar] [CrossRef]
- Lima, E.J.S.P.; Fontes, S.S.; Nogueira, M.L.; Silva, V.R.; Santos, L.S.; D’Elia, G.M.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Vannier-Santos, M.A.; et al. Essential oil from leaves of Conobea scoparioides (Cham. & Schltdl.) Benth. (Plantaginaceae) causes cell death in HepG2 cells and inhibits tumor development in a xenograft model. Biomed. Pharmacother. 2020, 129, 110402. [Google Scholar] [PubMed]
- Nogueira, M.L.; Lima, E.J.S.P.; Adrião, A.A.X.; Fontes, S.S.; Silva, V.R.; Santos, L.S.; Soares, M.B.P.; Dias, R.B.; Rocha, C.A.G.; Costa, E.V.; et al. Cyperus articulatus L. (Cyperaceae) Rhizome Essential Oil Causes Cell Cycle Arrest in the G2/M Phase and Cell Death in HepG2 Cells and Inhibits the Development of Tumors in a Xenograft Model. Molecules 2020, 25, 2687. [Google Scholar] [CrossRef] [PubMed]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Van Cuong, P.; Dang, N.H.; Dang, H.D.; Nguyen Quang, T.; Dat, N.T. Essential Oils of Lemongrass (Cymbopogon citratus Stapf) Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. BioMed Res. Int. 2020, 11, 5924856. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.L.; Sun, H.; Liu, C.; Li, J.; Liang, C.X.; Zhang, R.R.; Ge, F.R.; Liu, W. Croton tiglium essential oil compounds have anti-proliferative and pro-apoptotic effects in A549 lung cancer cell lines. PLoS ONE 2020, 15, e0231437. [Google Scholar] [CrossRef] [PubMed]
- Azadi, M.; Jamali, T.; Kianmehr, Z.; Kavoosi, G.; Ardestani, S.K. In-vitro (2D and 3D cultures) and in-vivo cytotoxic properties of Zataria multiflora essential oil (ZEO) emulsion in breast and cervical cancer cells along with the investigation of immunomodulatory potential. J. Ethnopharmacol. 2020, 257, 112865. [Google Scholar] [CrossRef]
- Athamneh, K.; Alneyadi, A.; Alsamri, H.; Alrashedi, A.; Palakott, A.; El-Tarabily, K.A.; Eid, A.H.; Al Dhaheri, Y.; Iratni, R. Origanum majorana Essential Oil Triggers p38 MAPK-Mediated Protective Autophagy, Apoptosis, and Caspase-Dependent Cleavage of P70S6K in Colorectal Cancer Cells. Biomolecules 2020, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xin, C.; Qiu, J.; Wang, Z. Essential Oil from Pinus Koraiensis Pinecones Inhibits Gastric Cancer Cells via the HIPPO/YAP Signaling Pathway. Molecules 2019, 24, 3851. [Google Scholar] [CrossRef]
- Lima, E.J.S.P.; Alves, R.G.; D’Elia, G.M.A.; Anunciação, T.A.D.; Silva, V.R.; Santos, L.S.; Soares, M.B.P.; Cardozo, N.M.D.; Costa, E.V.; Silva, F.M.A.D.; et al. Antitumor Effect of the Essential Oil from the Leaves of Croton matourensis Aubl. (Euphorbiaceae). Molecules 2018, 23, 2974. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-proliferative activity of Origanum vulgare inhibited lipogenesis and induced mitochondrial mediated apoptosis in human stomach cancer cell lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef]
- Oliveira, F.P.; Rodrigues, A.C.B.d.C.; de Lima, E.J.S.P.; Silva, V.R.; Santos, L.d.S.; Anunciação, T.A.d.; Nogueira, M.L.; Soares, M.B.P.; Dias, R.B.; Gurgel Rocha, C.A.; et al. Essential Oil from Bark of Aniba parviflora (Meisn.) Mez (Lauraceae) Reduces HepG2 Cell Proliferation and Inhibits Tumor Development in a Xenograft Model. Chem. Biodivers. 2021, 18, e2000938. [Google Scholar] [CrossRef]
- Costa, R.G.A.; Anunciação, T.A.D.; Araujo, M.S.; Souza, C.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Silva, F.M.A.D.; Koolen, H.H.F.; et al. In vitro and in vivo growth inhibition of human acute promyelocytic leukemia HL-60 cells by Guatteria megalophylla Diels (Annonaceae) leaf essential oil. Biomed. Pharmacother. 2020, 122, 109713. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Kariagina, A.; Doseff, A.I. Anti-Inflammatory Mechanisms of Dietary Flavones: Tapping into Nature to Control Chronic Inflammation in Obesity and Cancer. Int. J. Mol. Sci. 2022, 23, 15753. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Kim, D.H.; Jung, H.J.; Arulkumar, R.; Chung, H.Y.; Yu, B.P. Chronic Inflammation as an Underlying Mechanism of Ageing and Ageing-Related Diseases. Subcell. Biochem. 2023, 103, 31–44. [Google Scholar]
- Freire, M.O.; Van Dyke, T.E. Natural resolution of inflammation. Periodontology 2013, 63, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef] [PubMed]
- de Lavor, E.M.; Fernandes, A.W.C.; Teles, R.B.A.; Leal, A.E.B.P.; Oliveira Júnior, R.G.; Silva, M.G.E.; Oliveira, A.P.; Silva, J.C.; Araújo, M.T.M.F.; Coutinho, H.D.M.; et al. Essential Oils and Their Major Compounds in the Treatment of Chronic Inflammation: A Review of Antioxidant Potential in Preclinical Studies and Molecular Mechanisms. Oxid. Med. Cell Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Altern. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef]
- Mnif, W.; Dhifi, W.; Jelali, N.; Baaziz, H.; Hadded, A.; Hamdi, N. Characterization of Leaves Essential oil of Pelargonium graveolens Originating from Tunisia: Chemical Composition, Antioxidant and Biological Activities. J. Essent. Oil-Bear. Plants 2011, 14, 761–769. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Poznańska-Kurowska, K.; Kaszuba, A.; Kowalczyk, E. The antibacterial activity of geranium oil against Gram-negative bacteria isolated from difficult-to-heal wounds. Burns 2013, 40, 1046–1051. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid. Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef]
- Huang, L.; Pi, J.; Wu, J.; Zhou, H.; Cai, J.; Li, T.; Liu, L. A rapid and sensitive assay based on particle analysis for cell degranulation detection in basophils and mast cells. Pharmacol. Res. 2016, 111, 374–383. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-alpha signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sato, H.; Yorita, M.; Nakayama, H.; Miyazato, H.; Sugimoto, K.; Jippo, T. Inhibitory effects of geranium essential oil and its major component, citronellol, on degranulation and cytokine production by mast cells. Biosci. Biotechnol. Biochem. 2016, 80, 1172–1178. [Google Scholar] [CrossRef]
- Iscan, G.; Kirimer, N.; Demirci, F.; Demirci, B.; Noma, Y.; Başer, K.H.C. Biotransformation of (−)-(R)-alpha-phellandrene: Antimicrobial activity of its major metabolite. Chem. Biodivers. 2012, 9, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Onyenekwe, P.C.; Stahl, M.; Adejo, G. Post-irradiation changes of the volatile oil constituents of Monodora myristica (Gaertn) Dunal. Nat. Prod. Res. 2012, 26, 2030–2034. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Abbasi, N. Hypolipidemic activity of Anethum graveolens in rats. Phytother. Res. 2008, 22, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.L.G.; Cunha, F.V.M.; Sousa-Neto, B.P.S.; Oliveira, L.S.A.; Lopes, M.E.; Rezende, D.C.; Sousa, I.J.O.; Nogueira, K.M.; Souza, L.K.M.; Medeiros, J.V.R.; et al. α-Phellandrene attenuates tissular damage, oxidative stress, and TNF-α levels on acute model ifosfamide-induced hemorrhagic cystitis in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.M.C.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef]
- Piccinelli, A.C.; Santos, J.A.; Konkiewitz, E.C.; Oesterreich, S.A.; Formagio, A.S.N.; Croda, J.; Ziff, E.B.; Kassuya, C.A.L. Antihyperalgesic and antidepressive actions of (R)-(+)-limonene, α-phellandrene, and essential oil from Schinus terebinthifolius fruits in a neuropathic pain model. Nutr. Neurosci. 2015, 18, 217–224. [Google Scholar] [CrossRef]
- Siqueira, H.D.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; da Silva, F.V.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Cândido, A.G.F.; Wong, D.V.T.; et al. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Costa, R.; Dugo, P.; Navarra, M.; Raymo, V.; Dugoa, G.; Mondello, L. Study on the chemical composition variability of some processed bergamot (Citrus bergamia) essential oils. Flavour Fragr. J. 2010, 25, 4–12. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Jiang, Y.; Zhou, T.; Fan, G.; Wu, Y. Separation and determination of coumarins in Fructus cnidii extracts by pressurized capillary electrochromatography using a packed column with a monolithic outlet frit. J. Pharm. Biomed. Anal. 2009, 50, 695–702. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, A.; Kaur, J.; Bhatti, M.S.; Singh, P.; Bhatti, R. Bergapten inhibits chemically induced nociceptive behavior and inflammation in mice by decreasing the expression of spinal PARP, iNOS, COX-2 and inflammatory cytokines. Inflammopharmacology 2019, 27, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, D.B.; Obiri, D.D.; Osafo, N.; Antwi, A.O.; Essel, L.B.; Duduyemi, B.M.; Ekor, M. Allergic Airway-Induced Hypersensitivity Is Attenuated by Bergapten in Murine Models of Inflammation. Adv. Pharmacol. Sci. 2019, 2019, 6097349. [Google Scholar] [CrossRef]
- Ham, J.R.; Choi, R.-Y.; Lee, H.-I.; Lee, M.-K. Methoxsalen and Bergapten Prevent Diabetes-Induced Osteoporosis by the Suppression of Osteoclastogenic Gene Expression in Mice. Int. J. Mol. Sci. 2019, 20, 1298. [Google Scholar] [CrossRef]
- Adakudugu, E.A.; Ameyaw, E.O.; Obese, E.; Biney, R.P.; Henneh, I.T.; Aidoo, D.B.; Oge, E.N.; Attah, I.Y.; Obiri, D.D. Protective effect of bergapten in acetic acid-induced colitis in rats. Heliyon 2020, 6, e04710. [Google Scholar] [CrossRef]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef] [PubMed]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.C.; Rios, E.R.V.; Rocha, N.F.M.; Citó, M.C.O.; Fernandes, M.L.; Sousa, D.P.; Vasconcelos, S.M.M.; Sousa, F.C.F. Antinociceptive activity of carvacrol (5-isopropyl-2-methylphenol) in mice. J. Pharm. Pharmacol. 2012, 64, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.C.; Venâncio, E.T.; Sousa, D.P.; Fonteles, M.M.F.; Vasconcelos, S.M.M.; Viana, G.S.B.; Sousa, F.C.F. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: Involvement with GABAergic transmission. Fundam. Clin. Pharmacol. 2010, 24, 437–443. [Google Scholar] [CrossRef]
- Zhao, W.; Deng, C.; Han, Q.; Xu, H.; Chen, Y. Carvacrol may alleviate vascular inflammation in diabetic db/db mice. Int. J. Mol. Med. 2020, 46, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, E.M.; Souza, L.K.; Araújo, T.S.; Nogueira, K.M.; Sousa, F.B.; Araújo, A.R.; Martins, C.S.; Pacífico, D.M.; de C Brito, G.A.; Souza, E.P.; et al. Carvacrol reduces irinotecan-induced intestinal mucositis through inhibition of inflammation and oxidative damage via TRPA1 receptor activation. Chem. Biol. Interact. 2016, 260, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, E.M.; Sousa, N.A.; de Araújo, S.; Júnior, J.L.P.; Araújo, A.R.; Iles, B.; Pacífico, D.M.; Brito, G.A.C.; Souza, E.P.; Sousa, D.P.; et al. Carvacryl acetate, a novel semisynthetic monoterpene ester, binds to the TRPA1 receptor and is effective in attenuating irinotecan-induced intestinal mucositis in mice. J. Pharm. Pharmacol. 2017, 69, 1773–1785. [Google Scholar] [CrossRef]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflug. Arch. 2012, 464, 425–458. [Google Scholar] [CrossRef]
- Earley, S.; Gonzales, A.L.; Crnich, R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ. Res. 2009, 104, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory Properties of the Monoterpene 1.8-cineole: Current Evidence for Co-medication in Inflammatory Airway Diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.O.B.P.B.; Rodrigues, L.B.; Cesário, F.R.A.S.; Oliveira, M.R.C.; Tintino, C.D.M.; Castro, F.F.E.; Alcântara, I.S.; Fernandes, M.N.M.; Albuquerque, T.R.; Silva, M.S.A.; et al. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1,8-cineole (eucalyptol). Biomed. Pharmacother. 2017, 96, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Seol, G.H.; Kim, K.Y. Eucalyptol and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 389–398. [Google Scholar]
- Caceres, A.I.; Liu, B.; Jabba, S.V.; Achanta, S.; Morris, J.B.; Jordt, S.E. Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017, 174, 867–879. [Google Scholar] [CrossRef]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef]
- Ramachandran, R.; Hyun, E.; Zhao, L.; Lapointe, T.K.; Chapman, K.; Hirota, C.L.; Ghosh, S.; McKemy, D.D.; Vergnolle, N.; Beck, P.L.; et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 7476–7481. [Google Scholar]
- Wang, X.-P.; Yu, X.; Yan, X.-J.; Lei, F.; Chai, Y.-S.; Jiang, J.-F.; Yuan, Z.-Y.; Xing, D.-M.; Du, L.-J. TRPM8 in the negative regulation of TNFα expression during cold stress. Sci. Rep. 2017, 7, 45155. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Hsu, J.-S.; Li, C.-C.; Chen, K.-M.; Liu, C.-T. Protective effect of leaf essential oil from Cinnamomum osmophloeum Kanehira on endotoxin-induced intestinal injury in mice associated with suppressed local expression of molecules in the signaling pathways of TLR4 and NLRP3. PLoS ONE 2015, 10, e0120700. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Huang, H. Protective effect of linalool against lipopolysaccharide/D-galactosamine-induced liver injury in mice. Int. Immunopharmacol. 2014, 23, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013, 180, 47–54. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Kim, J.H.; Jung, H.S.; Sohn, Y.; Choi, Y.J.; Hwang, M.K.; Kim, S.H.; Kim, J.; Yang, W.M. Schizonepeta tenuifolia inhibits the development of atopic dermatitis in mice. Phytother. Res. 2013, 27, 1131–1135. [Google Scholar] [CrossRef]
- Brankovic, S.V.; Kitic, D.V.; Radenkovic, M.M.; Veljkovic, S.M.; Golubovic, T.D. Calcium blocking activity as a mechanism of the spasmolytic effect of the essential oil of Calamintha glandulosa Silic on the isolated rat ileum. Gen Physiol. Biophys. 2009, 28, 174–178. [Google Scholar] [PubMed]
- de Sousa, D.P.; Nóbrega, F.F.F.; de Lima, M.R.V.; de Almeida, R.N. Pharmacological activity of (R)-(+)-pulegone, a chemical constituent of essential oils. Z Naturforsch. C J Biosci. 2011, 2011, 353–359. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Lee, H.; Jo, S.Y.; Yang, W.M. (R)-(+)-pulegone suppresses allergic and inflammation responses on 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice model. J. Dermatol. Sci. 2018, 91, 292–300. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, J.; Lv, H.; Wen, T.; Shi, B.; Liu, X.; Zeng, N. Pulegone inhibits inflammation via suppression of NLRP3 inflammasome and reducing cytokine production in mice. Immunopharmacol. Immunotoxicol. 2019, 41, 420–427. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Q.; Lv, H.; Wang, F.; Liu, R.; Zeng, N. Effect of pulegone on the NLPR3 inflammasome during inflammatory activation of THP-1 cells. Exp. Ther. Med. 2020, 19, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-Y.; Lin, J.-J.; Jiang, J.-G.; Wang, T.-X.; Zhu, W. Potential roles of dietary flavonoids from Citrus aurantium L. var. amara Engl. in atherosclerosis development. Food Funct. 2020, 11, 561–571. [Google Scholar] [PubMed]
- Shen, C.Y.; Yang, L.; Jiang, J.G.; Zheng, C.Y.; Zhu, W. Immune enhancement effects and extraction optimization of polysaccharides from Citrus aurantium L. var. amara Engl. Food Funct. 2017, 8, 796–807. [Google Scholar] [CrossRef]
- Jiang, M.-H.; Yang, L.; Zhu, L.; Piao, J.-H.; Jiang, J.-G. Comparative GC/MS analysis of essential oils extracted by 3 methods from the bud of Citrus aurantium L. var. amara Engl. J. Food Sci. 2011, 76, 1219–1225. [Google Scholar]
- Liu, T.; Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.M.; Stashenko, E.; Duque, J.E. Insecticidal and Repellent Activity of Several Plant-Derived Essential Oils Against Aedes aegypti. J. Am. Mosq. Control Assoc. 2017, 1, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tolba, H.; Moghrani, H.; Benelmouffok, A.; Kellou, D.; Maachi, R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition, antifungal activity. J. Mycol. Med. 2015, 25, 128–133. [Google Scholar] [CrossRef]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.L.; Matos, F.J.A. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Ho, C.-L.; Li, L.-H.; Weng, Y.-C.; Hua, K.-F.; Ju, T.-C. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement Med. Ther. 2020, 20, 200. [Google Scholar] [CrossRef]
- Bastaki, S.M.; Adeghate, E.; Amir, N.; Ojha, S.; Oz, M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. Am. J. Transl. Res. 2018, 10, 4210–4222. [Google Scholar]
- Liu, Z.; Shen, C.; Tao, Y.; Wang, S.; Wei, Z.; Cao, Y.; Wu, H.; Fan, F.; Lin, C.; Shan, Y.; et al. Chemopreventive efficacy of menthol on carcinogen-induced cutaneous carcinoma through inhibition of inflammation and oxidative stress in mice. Food Chem. Toxicol. 2015, 82, 12–18. [Google Scholar] [CrossRef]
- Shahid, M.; Lee, M.Y.; Yeon, A.; Cho, E.; Sairam, V.; Valdiviez, L.; You, S.; Kim, J. Menthol, a unique urinary volatile compound, is associated with chronic inflammation in interstitial cystitis. Sci. Rep. 2018, 8, 10859. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, G.; Zhang, F.; Feng, C.; Liang, M.; Jia, P.; Zhao, Z.; Guo, H.; Zhao, Y. Effects of Artemisia annua L. Essential Oil on Osteoclast Differentiation and Function Induced by RANKL. Evid. Based Complement. Altern. Med. 2022, 2022, 1322957. [Google Scholar]
- Silveira e Sá, R.C.; Andrade, L.N.; Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef] [PubMed]
- Parente, M.S.R.; Custódio, F.R.; Cardoso, N.A.; Lima, M.J.A.; Melo, T.S.d.; Linhares, M.I.; Siqueira, R.M.P.; Nascimento, A.Á.d.; Catunda Júnior, F.E.A.; Melo, C.T.V.d. Antidepressant-Like Effect of Lippia sidoides CHAM (Verbenaceae) Essential Oil and Its Major Compound Thymol in Mice. Sci. Pharm. 2018, 86, 27. [Google Scholar] [CrossRef]
- Barbosa, R.; Cruz-Mendes, Y.; Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Ribeiro, N.M.; Morais, L.P.; Leal-Cardoso, J.H. Effects of Lippia sidoides essential oil, thymol, p-cymene, myrcene and caryophyllene on rat sciatic nerve excitability. Braz. J. Med. Biol. Res. 2017, 50, e6351. [Google Scholar] [CrossRef]
- Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.M.L.; Rondon, F.C.; Lobo, C.H.; Moura, A.A.A.N.; Sales, A.D.; Rodrigues, A.P.R.; Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agents. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef] [PubMed]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.-C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol Improves Barrier Function and Attenuates Inflammatory Responses in Porcine Intestinal Epithelial Cells during Lipopolysaccharide (LPS)-Induced Inflammation. J. Agric. Food Chem. 2019, 67, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Mendes SS, Bomfim RR, Jesus HC; et al. Evaluation of the analgesic and anti-inflammatory effects of the essential oil of Lippia gracilis leaves. J. Ethnopharmacol. 2010, 129, 391–397. [CrossRef] [PubMed]
- Pandur, E.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas aeruginosa LPS-Activated THP-1 Macrophages. Antioxidants 2022, 11, 1330. [Google Scholar] [CrossRef] [PubMed]



| Essential Oil | Main Components a | Assay | Activity | Ref. |
|---|---|---|---|---|
| Thyme (T. vulgaris L.) | Carvacrol, p-cymene | O2 uptake during inhibited autoxidation | Yes, similar to BHT | [46] |
| Oregano (O. vulgare L.) | Carvacrol, p-cymene | O2 uptake during inhibited autoxidation | Yes, similar to BHT | [46] |
| Savory (Satureja hortensis L.) | Carvacrol, p-cymene, γ-terpinene | O2 uptake during inhibited autoxidation | Yes, similar to BHT | [46] |
| Clove buds (E. caryophyllus Spreng) | Eugenol | O2 uptake during inhibited autoxidation | Yes, similar to BHT | [46] |
| Cinnamon (C. zeylanicum Blume) | Eugenol | O2 uptake during inhibited autoxidation | Yes, similar to BHT | [46] |
| Melaleuca alternifolia (M. alternifolia Cheel.) | Terpinen-4-ol, γ-terpinene, α-terpinene | TBARS | Yes, similar to α-tocopherol | [55] |
| Cinnamomum zeylanicum bark | Cinnamaldehyde, eugenol | β-carotene bleaching | Yes, no comparison available | [18,56] |
| Marine fennel (Crithmum maritimum L.) | γ-Terpinene, limonene, | Conjugated dienes, TBARS | Yes, similar to α-tocopherol and BHT | [57] |
| Common fennel (Foeniculum vulgare Mill.) | Estragole, α-pinene, thymol methyl ether | Conjugated dienes, TBARS | Yes, similar to α-tocopherol and BHT | [57] |
| Constituent | Source | Target Virus | Mechanism | IC50 | SI | Reference |
|---|---|---|---|---|---|---|
| Germacrone | Curcuma longa L. | Influenza | Inhibits multiple steps in the viral life cycle | 6.03 μM | >41 | [77] |
| Eugenol | Cinnamomum zeylanicum Blume. | Influenza | Not determined | <3.1 μL/mL | Not determined | [80] |
| β-Santalol | Santalum album L. | Influenza | Inhibits viral replication | 10–100 μg/mL | Not determined | [79] |
| Carvacrol | Thymus vulgaris L. | Influenza | Not determined | 2.6 μg/mL | <0.15 | [76,86] |
| HSV-1 | Inhibits viral attachment to host cells | 7 μM | 43 | |||
| Human rotavirus | Inhibits viral replication | 27.9 μg/mL | 33 | |||
| β-Citronellol | Cymbopogon nardus (L.) Rendle. | HIV-1 | Inhibits reverse transcriptase | 2.6 mg/mL | Not determined | [84] |
| β-Caryophyllene | Cinnamomum zeylanicum Blume. and Syzygium aromaticum (L.) Merrill & Perry | HSV-1 | Inhibits viral attachment to host cells | 0.25 μg/mL | 140 | [87,92,93] |
| Dengue-2 virus | Inhibits multiple steps in viral life cycle | 22.5 μM | 71.1 | |||
| Limonene | Citrus bergamia Risso & Poit. | HSV-1 | Inhibits viral attachment to host cells | 5.9 μg/mL | 10.2 | [76,88] |
| β-Pinene | Pinus pinaster Aiton | HSV-1 | Inhibits viral attachment to host cells | 3.5 μg/mL | 24.3 | [88,94] |
| Thymol | Thymus vulgaris L. | HSV-1 | Inhibits viral attachment to host cells | 7 μM | 43 | [82,86] |
| Farnesol | Matricaria chamomilla L. | HSV-1 | Inhibits viral attachment to host cells | 3.5 μg/mL | 11.4 | [61,87] |
| p-Cymene | Thymus vulgaris L. | HSV-1 | Inhibits viral attachment to host cells | >0.1% | Not determined | [89] |
| Antimicrobial Activity | Mechanism of Action | References |
|---|---|---|
| Antibacterial | Plasmatic membrane and wall cell alteration. | [97] |
| Change in electrical conductivity of plasma membrane. | [99] | |
| Decrease in ATP production. | [100] | |
| Change in protein synthesis. | [104] | |
| Inhibition of biofilm formation. | [108] | |
| Gene modulation. | [106] | |
| Antifungal | Action on wall cell and plasmatic membrane. | [109] |
| Inhibition of ergosterol synthesis. | [110,112] | |
| Decreasing intracellular ATPase activity and increasing production of Ros. | [110] | |
| Changes on fungal micromorphology. | [117] |
| EO Bearing Plants | Part of the Plant Used | Main Chemical Constituents | Mechanisms Reported | References |
|---|---|---|---|---|
| Euphorbia intisy Drake | stem | heptacosane and phytol | suppression of P-gp protein/inhibition of NF-κB | [122] |
| Chenopodium botrys L. | aerial parts | α-eudesmol, elemol acetate, elemol, and α-chenopodiol-6-acetate | induction of apoptosis/augmentation of expression of p21 and p53 | [132] |
| Ridolfia segetum (L.) Moris | - | α-phellandrene, terpinolene, ß-phellandrene, and dillapiol | induction of apoptosis/p21 stabilization | [133] |
| Allium sativum L. | bulb | diallyl disulfide and diallyl trisulfide | augmentation of ROS, induction of apoptosis, and differentiation | [118] |
| Vitex agnus-castus L. | leaves | 1,8-cineole, eucalyptol, oleic acid, and caryophyllene | induction of apoptosis through triggering both extrinsic and intrinsic pathways | [134] |
| Virola surinamensis (Rol. ex Rottb.) Warb. | bark and leaves | aristolene, α-gurjunene, valencene, germacrene d, δ-guaiene, β-elemene, α-farnesene, bicyclogermacrene, and α-cubebene | induction of apoptotic cell death | [135] |
| Cedrus deodara (Roxb. ex D. Don) G. Don | bark | 9-octadecenoic acid, copaene, and 9(E),11(E)- conjugated linoleic acid | induction of apoptosis/inhibition of NF-κB | [121] |
| Conobea scoparioides (Cham. & Schltdl.) Benth. | leaves | thymol methyl ether, thymol, and α-phellandrene | induction of apoptotic cell death | [136] |
| Zataria multiflora Boiss. | - | - | augmentation of ROS and induction of apoptosis | [119] |
| Cyperus articulatus L. | rhizome | muskatone, cyclocolorenone, α-pinene, pogostol, α-copaene, and caryophyllene oxide | induction of apoptotic cell death | [137] |
| Cymbopogon citratus (DC.) Stapf | leaves and culms | myrcene, neral and geranial | induction of apoptotic cell death | [138] |
| Croton tiglium L. | fruits | 17-octadecynoic acid, tetradecanoic acid, 17-octadecynoic acid methyl ester, n-hexadecanoic acid, n-decanoic acid, linoleic acid ethyl ester, and iso-propyl 9-octadecenoate | induction of apoptotic cell death and inhibition of migration | [139] |
| Zataria multiflora Boiss. | - | carvacrol, γ-terpinene, carvacrol methyl ether, p-cymene, and thymol | immunomodulation | [140] |
| Origanum majorana L. | - | - | p38 MAPK-mediated protective autophagy and apoptosis | [141] |
| Pinus koraiensis Siebold & Zucc. | pinecones | α-pinene, limonene, and β-pinene | induction of apoptosis via the HIPPO/YAP signaling pathway | [142] |
| Croton matourensis Aubl. | leaves | β-caryophyllene, thunbergol, cembrene, p-cymene, and β-elemene | induction of apoptotic cell death | [143] |
| Origanum vulgare L. | - | thymol, ρ-cymene, γ-terpinene, and carvacrol | inhibition of lipogenesis and induction of apoptosis | [144] |
| Aniba parviflora (Meisn.) Mez | bark | linalool, α-humulene, δ-cadinene, α-copaene, and germacrene b | induction of apoptotic cell death | [145] |
| Guatteria megalophylla Diels | leaves | spathulenol, γ-muurolene, bicyclogermacrene, β-elemene, and δ-elemene | induction of apoptotic cell death | [146] |
| Litsea cubeba (Lour.) Pers. | seed | citronellal, neo-isopulegol, isopulegol, and citronellol | induction of apoptotic cell death by suppression of AKT/mTOR pathway | [120] |
| Artemisia capillaris Thunb. | - | - | induction of apoptotic cell death by activation of MAPK | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. https://doi.org/10.3390/biom13071144
de Sousa DP, Damasceno ROS, Amorati R, Elshabrawy HA, de Castro RD, Bezerra DP, Nunes VRV, Gomes RC, Lima TC. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules. 2023; 13(7):1144. https://doi.org/10.3390/biom13071144
Chicago/Turabian Stylede Sousa, Damião P., Renan Oliveira S. Damasceno, Riccardo Amorati, Hatem A. Elshabrawy, Ricardo D. de Castro, Daniel P. Bezerra, Vitória Regina V. Nunes, Rebeca C. Gomes, and Tamires C. Lima. 2023. "Essential Oils: Chemistry and Pharmacological Activities" Biomolecules 13, no. 7: 1144. https://doi.org/10.3390/biom13071144
APA Stylede Sousa, D. P., Damasceno, R. O. S., Amorati, R., Elshabrawy, H. A., de Castro, R. D., Bezerra, D. P., Nunes, V. R. V., Gomes, R. C., & Lima, T. C. (2023). Essential Oils: Chemistry and Pharmacological Activities. Biomolecules, 13(7), 1144. https://doi.org/10.3390/biom13071144











