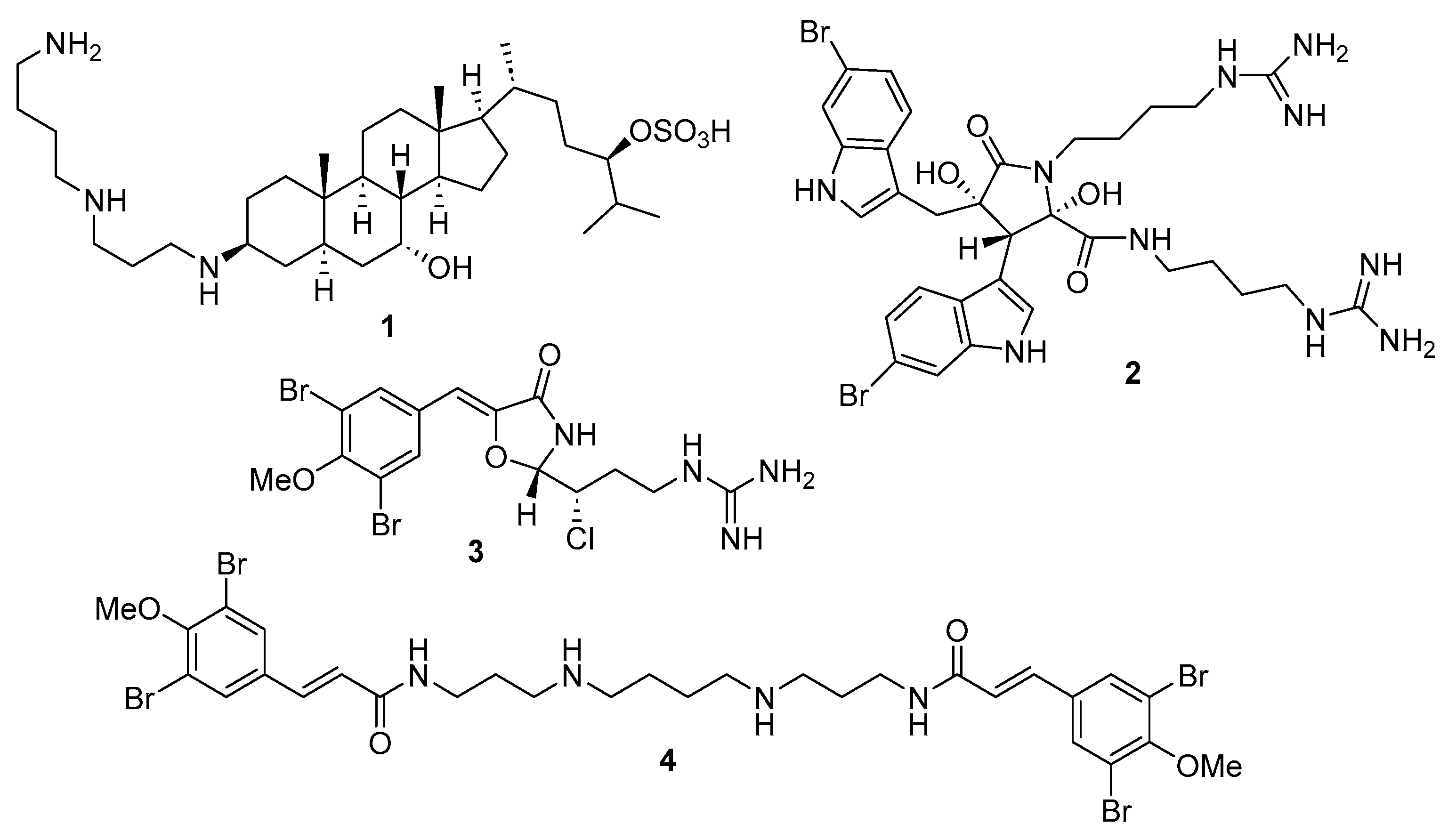
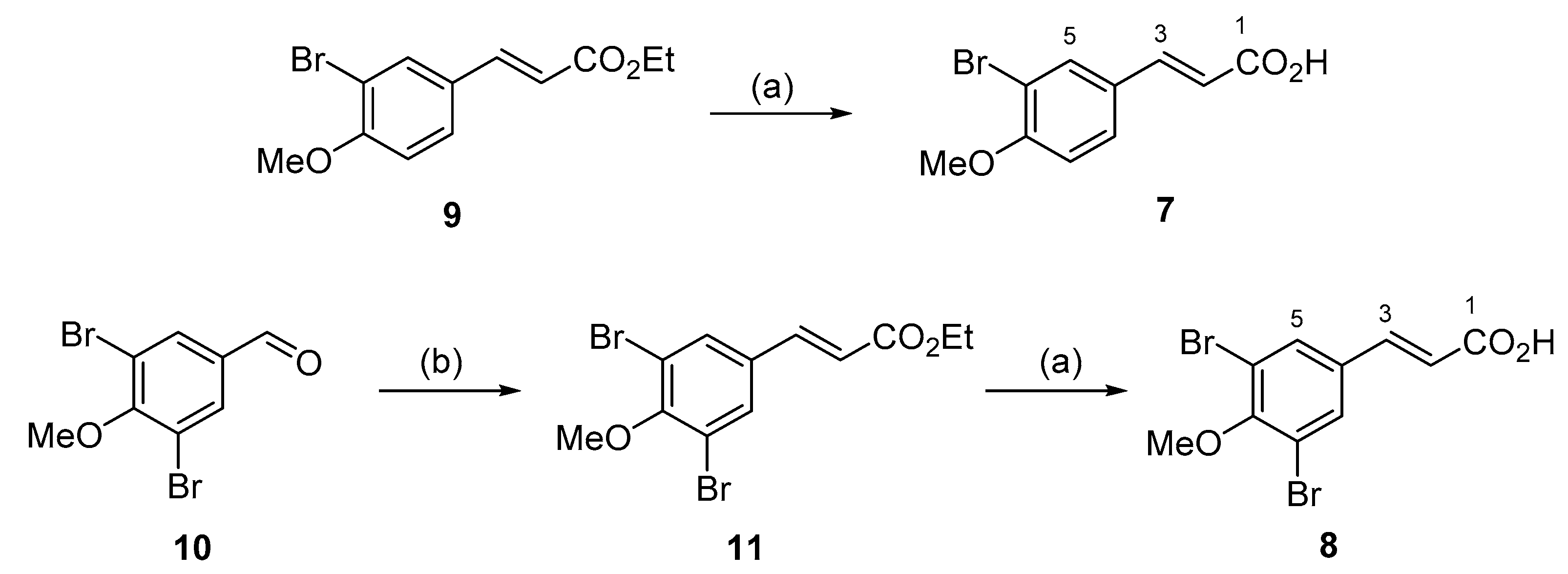
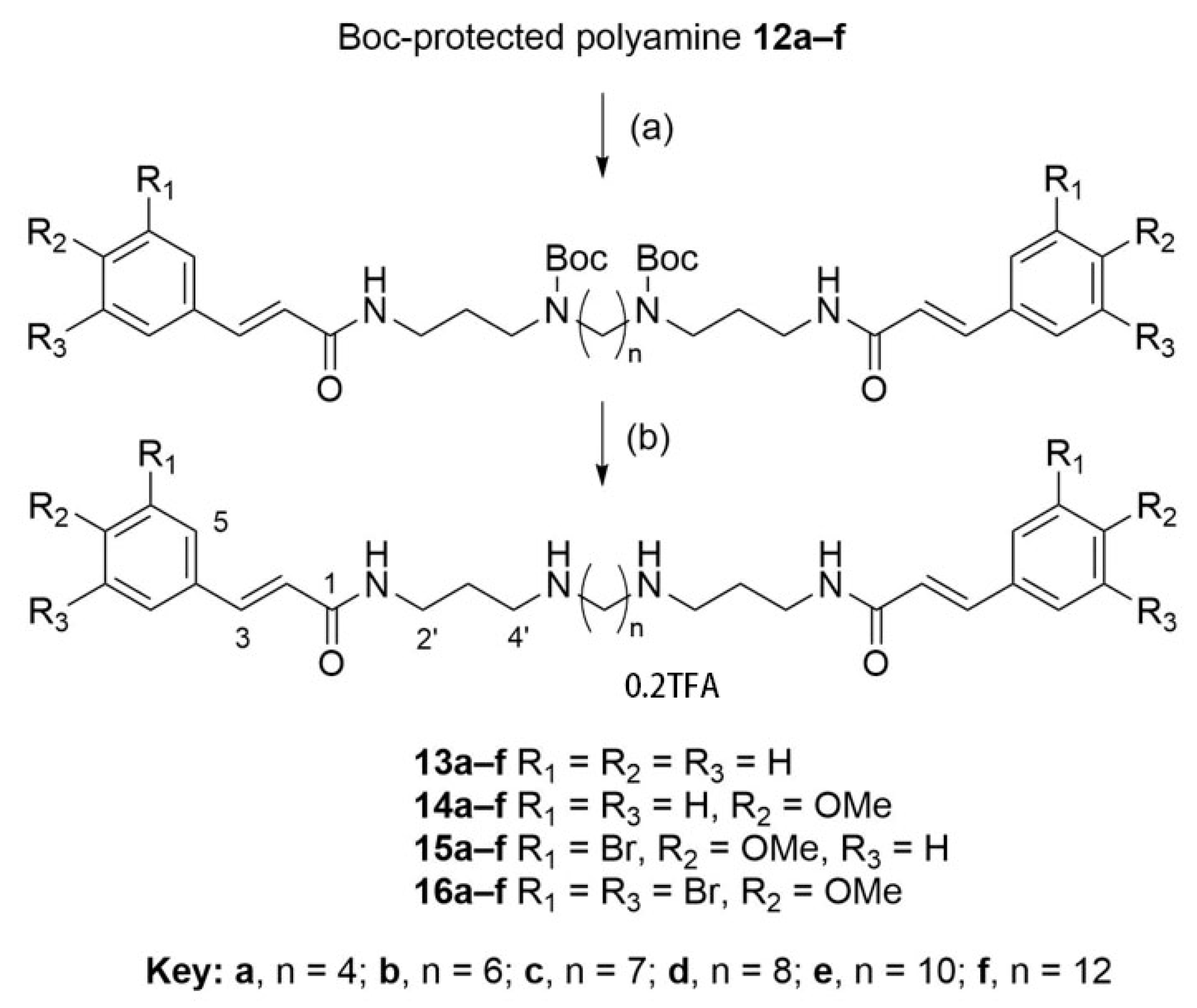
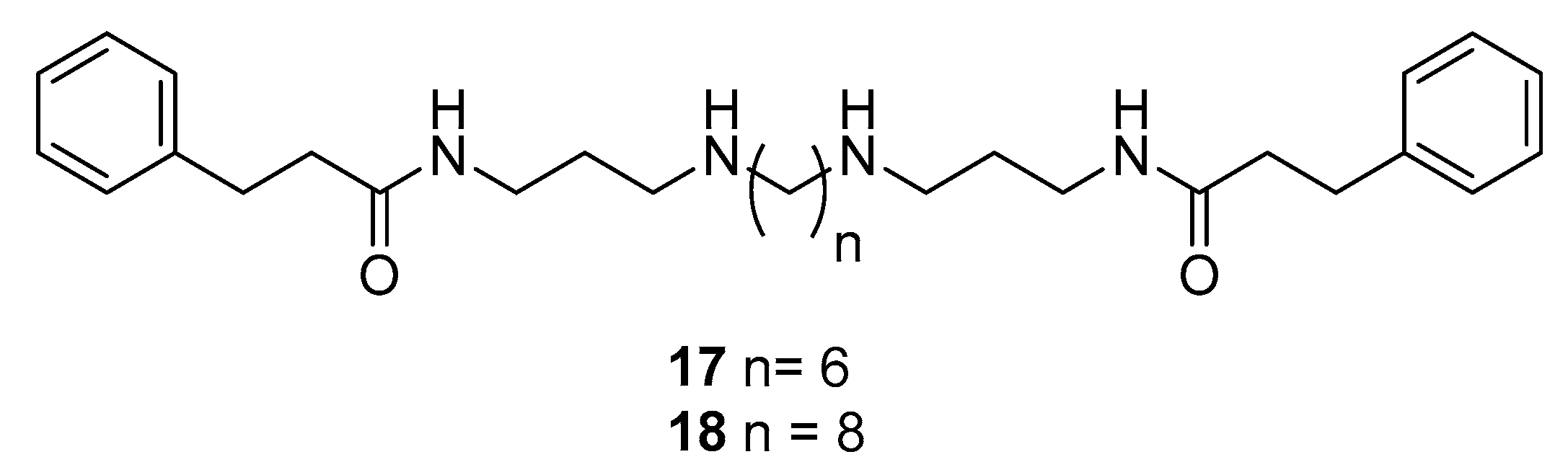
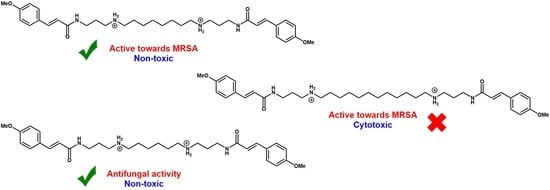
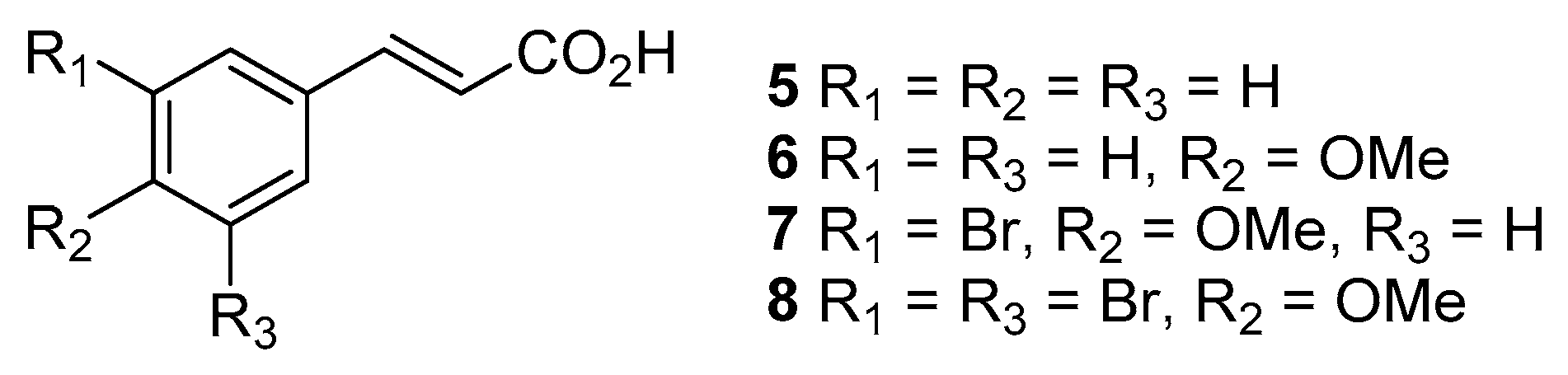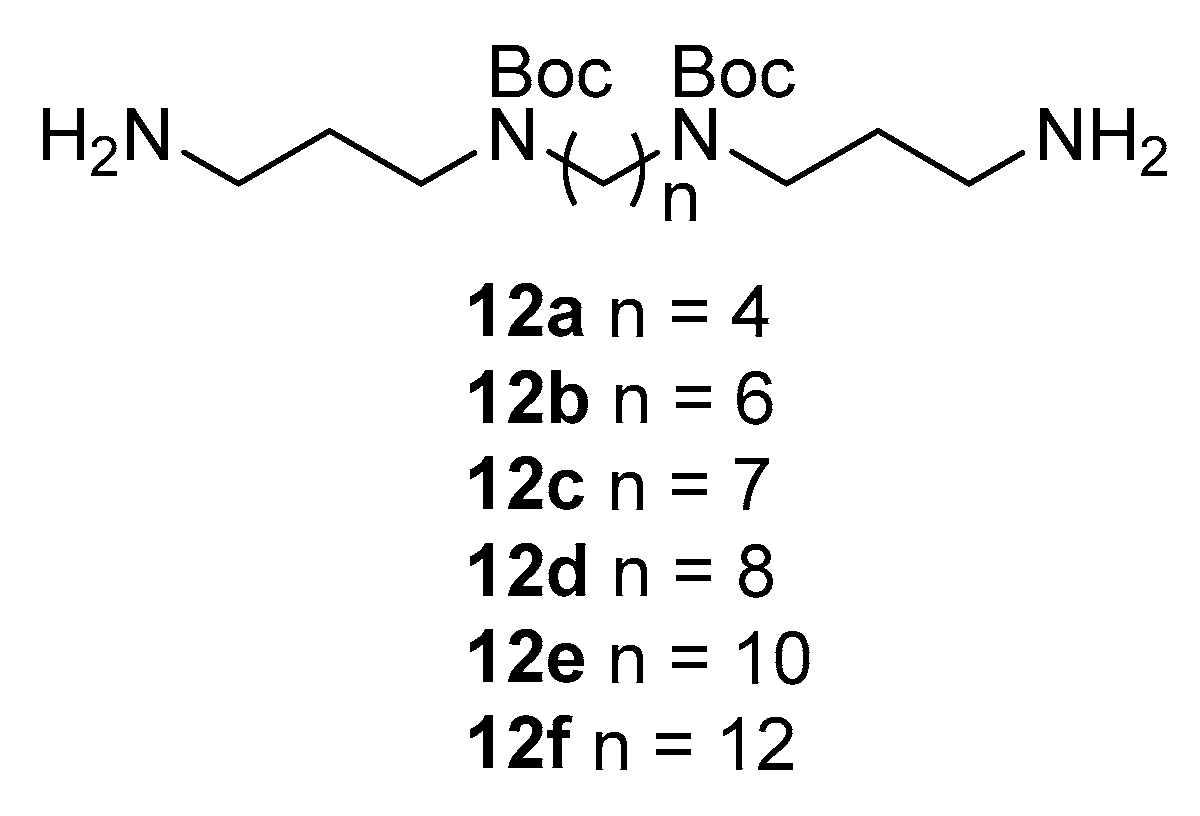2.2. Synthesis of Compounds
2.2.1. (E)-3-(3-Bromo-4-methoxyphenyl)acrylic Acid (7)
Ethyl (
E)-3-(3-bromo-4-methoxyphenyl)acrylate (
9) [
22] (451 mg, 1.58 mmol) was dissolved in EtOH (5 mL), and 1N NaOH (10 mL) was added and then heated at reflux under N
2 for 4 h. EtOH was removed under reduced pressure and the aqueous layer was washed with EtOAc (2 × 10 mL). The aqueous layer was then acidified with 10% HCl (10 mL) and extracted with EtOAc (2 × 10 mL). The combined organic layers were dried with anhydrous MgSO
4 and the solvent removed under reduced pressure to afford (
E)-3-(3-bromo-4-methoxyphenyl)acrylic acid (
7) as a white solid (355 mg, 87%).
1H NMR (DMSO-
d6, 400 MHz) δ 12.29 (1H, br s, COOH), 7.96 (1H, d,
J = 2.1 Hz), 7.70 (1H, dd,
J = 8.6, 2.1 Hz), 7.51 (1H, d,
J = 16.0 Hz), 7.14 (1H, d,
J = 8.6 Hz), 6.46 (1H, d,
J = 16.0 Hz), 3.89 (3H, s);
13C NMR (DMSO-
d6, 100 MHz) 167.6, 156.7, 142.3, 132.5, 129.3, 128.4, 118.1, 112.7, 111.2, 56.5. The
1H and
13C NMR data agreed with literature values [
27].
2.2.2. (E)-3-(3,5-Dibromo-4-methoxyphenyl)acrylic Acid (8)
To a solution of NaH (60%) (202 mg, 5.0 mmol) in tetrahydrofuran (THF) (5 mL) was added triethyl phosphonoacetate (0.94 mL, 4.7 mmol) at 0 °C and stirred for 45 min. A solution of 3,5-dibromo-4-methoxybenzaldehyde (
10) [
21] (948 mg, 3.2 mmol) in THF (5 mL) was added dropwise and the reaction stirred at rt under N
2 for 24 h. The crude product mixture was poured onto ice/H
2O and extracted with EtOAc (2 × 10 mL), the organic layer was washed with brine (20 mL) and was then dried (anhydrous MgSO
4) and the solvent was removed under reduced pressure. The product was purified by silica gel column chromatography (1% EtOAc/hexane) to afford ethyl (
E)-3-(3,5-dibromo-4-methoxyphenyl)acrylate (
11) as a white solid (788 mg, 68%).
1H NMR (CDCl
3, 400 MHz) δ 7.66 (2H, s), 7.50 (1H, d,
J = 15.8 Hz), 6.36 (1H, d,
J = 15.8 Hz), 4.26 (2H, q,
J = 7.3 Hz), 3.91 (3H, s), 1.33 (3H, t,
J = 7.3 Hz);
13C NMR (CDCl
3, 100 MHz) δ 166.4, 155.6, 141.1, 133.3, 132.1, 120.3, 118.9, 60.9, 14.4. The
1H and
13C NMR data agreed with those reported in the literature [
19]. The product (788 mg, 2.2 mmol) was dissolved in EtOH (5 mL), 1N NaOH (10 mL) added and then heated at reflux under N
2 for 4 h. EtOH was removed under reduced pressure and the aqueous layer was washed with EtOAc (2 × 10 mL). The aqueous layer was then acidified with 10% HCl (10 mL) and extracted with EtOAc (2 × 10 mL). The combined organic layers were dried with anhydrous MgSO
4 and the solvent was removed under reduced pressure to afford (
E)-3-(3,5-dibromo-4-methoxyphenyl)acrylic acid (
8) as a white solid (632 mg, 86%).
1H NMR (DMSO-
d6, 400 MHz) δ 12.48 (1H, br s), 8.05 (2H, s), 7.50 (1H, d,
J = 16.1 Hz), 6.62 (1H, d,
J = 15.6 Hz), 3.82 (3H, s);
13C NMR (DMSO-
d6, 100 MHz) δ 167.2, 154.4, 140.4, 133.7, 132.3, 121.5, 118.0, 60.5. The
1H and
13C NMR data agreed with those reported in the literature [
19].
2.2.3. N1,N4-Bis(3-cinnamamidopropyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (13a)
Using general procedure A, reaction of cinnamic acid (5) (81 mg, 0.55 mmol), EDC·HCl (124 mg, 0.65 mmol), HOBt (87 mg, 0.64 mmol), DIPEA (0.26 mL, 1.49 mmol) and di-tert-butyl butane 1,4-diylbis((3-aminopropyl)carbamate) (12a) (100 mg, 0.25 mmol) afforded di-tert-butyl butane-1,4-diylbis((3-cinnamamidopropyl) carbamate) as a colorless oil (112 mg, 68%). Using general procedure C, a sub-sample of this product (83 mg, 0.13 mmol) was deprotected to afford the di-TFA salt 13a as a colorless oil (76 mg, 85%). Rf = 0.46 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3308, 2944, 2833, 1673, 1450, 1116, 1022 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.59–7.54 (6H, m, H-3, 2H-5), 7.41–7.37 (6H, m, 2H-6, H-7), 6.62 (2H, d, J = 15.8 Hz, H-2), 3.44 (4H, t, J = 6.4 Hz, H2-2′), 3.08–3.04 (8H, m, H2-4′, H2-6′), 1.96 (4H, tt, J = 6.5, 6.5 Hz, H2-3′), 1.89–1.81 (4H, m, H2-7′); 13C NMR (CD3OD, 100 MHz) δ 169.7 (C-1), 142.4 (C-3), 136.0 (C-4), 131.1 (C-7), 130.0 (C-6), 128.9 (C-5), 121.2 (C-2), 48.1 (C-6′), 46.3 (C-4′), 37.0 (C-2′), 27.8 (C-3′), 24.3 (C-7′); (+)-HRESIMS [M+H]+ m/z 463.3076 (calcd for C28H39N4O2, 463.3068).
2.2.4. N1,N6-Bis(3-cinnamamidopropyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (13b)
Using general procedure A, reaction of cinnamic acid (5) (37.8 mg, 0.255 mmol), EDC·HCl (57.9 mg, 0.302 mmol), HOBt (40.8 mg, 0.302 mmol), DIPEA (0.12 mL, 0.69 mmol) and di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (12b) (50 mg, 0.12 mmol) afforded di-tert-butyl hexane-1,6-diylbis((3-cinnamamidopropyl)carbamate) as a colorless oil (53 mg, 64%). Using general procedure C, a sub-sample of this product (21 mg, 0.030 mmol) was deprotected to afford the di-TFA salt 13b as an orange gum (9 mg, 41%). Rf = 0.47 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3747, 3293, 3057, 2866, 1674, 1619, 1553, 1448, 1202, 1133, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.60–7.54 (6H, m, H-3, 2H-5), 7.42–7.36 (6H, m, 2H-6, H-7), 6.62 (2H, d, J = 16.0 Hz, H-2), 3.43 (4H, t, J = 6.4 Hz, H2-2′), 3.07–3.00 (8H, m, H2-4′, H2-6′), 1.95 (4H, tt, J = 6.5, 6.5 Hz, H2-3′), 1.80–1.71 (4H, m, H2-7′), 1.52–1.47 (4H, m, H2-8′); 13C NMR (CD3OD, 100 MHz) δ 169.6 (C-1), 142.4 (C-3), 136.1 (C-4), 131.1 (C-7), 130.0 (C-6), 128.9 (C-5), 121.2 (C-2), 48.4 (C-6′), 46.3 (C-4′), 37.0 (C-2′), 27.8 (C-3′), 27.1 (C-7′/C-8′), 27.0 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 491.3374 (calcd for C30H43N4O2, 491.3381).
2.2.5. N1,N7-Bis(3-cinnamamidopropyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (13c)
Using general procedure A, reaction of cinnamic acid (5) (36.7 mg, 0.248 mmol), EDC·HCl (56.1 mg, 0.293 mmol), HOBt (39.6 mg, 0.293 mmol), DIPEA (0.118 mL, 0.677 mmol) and di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (12c) (50 mg, 0.112 mmol) afforded di-tert-butyl heptane-1,7-diylbis((3-cinnamamidopropyl)carbamate) as a colorless oil (36 mg, 45%). Using general procedure C, this product (36 mg, 0.051 mmol) was deprotected to afford the di-TFA salt 13c as a colorless gum (21 mg, 56%). Rf = 0.47 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3285, 3032, 2939, 1659, 1620, 1556, 1450, 1343, 1286, 1200, 1180, 1131, 980, 834, 800, 767, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.60–7.54 (6H, m, H-3, 2H-5), 7.42–7.36 (6H, m, 2H-6, H-7), 6.62 (2H, d, J = 15.9 Hz, H-2), 3.43 (4H, t, J = 6.4 Hz, H2-2′), 3.06–2.97 (8H, m, H2-4′, H2-6′), 1.99–1.90 (4H, m, H2-3′), 1.77–1.68 (4H, m, H2-7′), 1.49–1.41 (6H, m, H2-8′, H2-9′); 13C NMR (CD3OD, 100 MHz) δ 169.6 (C-1), 142.4 (C-3), 136.1 (C-4), 131.0 (C-7), 130.0 (C-6), 128.9 (C-5), 121.2 (C-2), 48.9 (C-6′), 46.3 (C-4′), 37.1 (C-2′), 29.6 (C-9′), 27.7 (C-3′), 27.24 (C-7′/C-8′), 27.15 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 505.3520 (calcd for C31H45N4O2, 505.3537).
2.2.6. N1,N8-Bis(3-cinnamamidopropyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (13d)
Using general procedure A, reaction of cinnamic acid (5) (35.5 mg, 0.24 mmol), EDC·HCl (54.3 mg, 0.283 mmol), HOBt (38.3 mg, 0.283 mmol), DIPEA (0.114 mL, 0.654 mmol) and di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (12d) (50 mg, 0.11 mmol) afforded di-tert-butyl octane-1,8-diylbis((3-cinnamamidopropyl)carbamate) as a colorless oil (37 mg, 47%). Using general procedure C, a sub-sample of this product (19 mg, 0.026 mmol) was deprotected to afford the di-TFA salt 13d as a colorless gum (8 mg, 41%). Rf = 0.47 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3284, 2935, 2858, 1674, 1621, 1555, 1451, 1343, 1202, 1133, 980, 845, 800, 768, 721 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.60–7.54 (6H, m, H-3, 2H-5), 7.43–7.36 (6H, m, 2H-6, H-7), 6.62 (2H, d, J = 15.9 Hz, H-2), 3.43 (4H, t, J = 6.4 Hz, H2-2′), 3.07–2.97 (8H, m, H2-4′, H2-6′), 1.94 (4H, tt, J = 6.5, 6.5 Hz, H2-3′), 1.76–1.67 (4H, m, H2-7′), 1.47–1.39 (4H, m, H2-8′, H2-9′); 13C NMR (CD3OD, 100 MHz) δ 169.6 (C-1), 142.4 (C-3), 136.1 (C-4), 131.1 (C-7), 130.0 (C-6), 128.9 (C-5), 121.2 (C-2), 48.8 (C-6′), 46.3 (C-4′), 37.0 (C-2′), 29.9 (C-9′), 27.8 (C-3′), 27.4 (C-7′/C-8′), 27.3 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 519.3698 (calcd for C32H47N4O2, 519.3694).
2.2.7. N1,N10-Bis(3-cinnamamidopropyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (13e)
Using general procedure A, reaction of cinnamic acid (5) (67 mg, 0.45 mmol), EDC·HCl (103 mg, 0.54 mmol), HOBt (72 mg, 0.53 mmol), DIPEA (0.22 mL, 1.26 mmol) and di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (12e) (100 mg, 0.21 mmol) afforded di-tert-butyl decane-1,10-diylbis((3-cinnamamidopropyl)carbamate) as a colorless oil (60 mg, 38%). Using general procedure C, a sub-sample of this product (42 mg, 0.056 mmol) was deprotected to afford the di-TFA salt 13e as a colorless oil (40 mg, 92%). Rf = 0.25 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3311, 2944, 2832, 1683, 1448, 1115, 1022 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.60–7.54 (6H, m, H-3, 2H-5), 7.43–7.36 (6H, m, 2H-6, H-7), 6.62 (2H, d, J = 15.9 Hz, H-2), 3.43 (4H, t, J = 6.5 Hz, H2-2′), 3.06–2.96 (8H, m, H2-4′, H2-6′), 1.94 (4H, tt, J = 6.5, 6.5 Hz, H2-3′), 1.75–1.66 (4H, m, H2-7′), 1.46–1.34 (12H, m, H2-8′, H2-9′, H2-10′); 13C NMR (CD3OD, 100 MHz) δ 169.6 (C-1), 142.4 (C-3), 136.1 (C-4), 131.1 (C-7), 130.0 (C-6), 128.9 (C-5), 121.2 (C-2), 48.8 (C-6′), 46.3 (C-4′), 37.0 (C-2′), 30.4 (C-9′/C-10′), 30.2 (C-9′/C-10′), 27.8 (C-3′), 27.5 (C-7′/C-8′), 27.4 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 547.4022 (calcd for C34H51N4O2, 547.4007).
2.2.8. N1,N12-Bis(3-cinnamamidopropyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (13f)
Using general procedure A, reaction of cinnamic acid (5) (31.6 mg, 0.213 mmol), EDC·HCl (48.4 mg, 0.253 mmol), HOBt (34.1 mg, 0.252 mmol), DIPEA (0.101 mL, 0.582 mmol) and di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (12f) (5.0 mg, 0.0097 mmol) afforded di-tert-butyl dodecane-1,12-diylbis((3-cinnamamidopropyl)carbamate as a colorless oil (7.0 mg, 93%). Using general procedure C, this product (7.0 mg, 0.0090 mmol) was deprotected to afford the di-TFA salt 13f as a colorless gum (4.0 mg, 55%). Rf = 0.16 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3276, 3076, 2926, 2859, 1675, 1621, 1546, 1460, 1202, 1133 cm−1; 1H NMR (CD3OD, 400 MHz) δ 7.61–7.54 (6H, m, H-3, 2H-5), 7.43–7.37 (6H, m, 2H-6, H-7), 6.62 (2H, d, J = 15.9 Hz, H-2), 3.43 (4H, t, J = 6.5 Hz, H2-2′), 3.07–2.96 (8H, m, H2-4′, H2-5′), 1.94 (4H, tt, J = 6.5, 6.5 Hz, H2-3′), 1.76–1.65 (4H, m, H2-7′), 1.48–1.27 (16H, m, H2-8′, H2-9′, H2-10′, H2-11′); 13C NMR (CD3OD, 100 MHz) δ 169.7 (C-1), 142.5 (C-3), 136.1 (C-4), 131.1 (C-7), 130.0 (C-6), 128.9 (C-5), 121.1 (C-2), 48.8 (C-6′), 46.3 (C-4′), 37.0 (C-2′), 30.7 (C-9′/C-10′/C-11′), 30.5 (C-9′/C-10′/C-11′), 30.2 (C-9′/C-10′/C-11′), 27.8 (C-3′), 27.5 (C-7′/C-8′), 27.3 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 575.4299 (calcd for C36H55N4O2, 575.4320).
2.2.9. N1,N4-Bis(3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (14a)
Using general procedure B, reaction of 4-methoxycinnamic acid (6) (55 mg, 0.309 mmol), EDC·HCl (67 mg, 0.350 mmol), DMAP (76 mg, 0.622 mmol) and di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (12a) (50 mg, 0.124 mmol) afforded di-tert-butyl butane-1,4-diylbis ((3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (53 mg, 59%). Using general procedure C, a sub-sample of this product (27 mg, 0.037 mmol) was deprotected to afford the di-TFA salt 14a as a yellow oil (13 mg, 46%). Rf = 0.34 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3247, 3031, 2839, 1673, 1604, 1514, 1200, 1138, 1027, 830, 799, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.59 (4H, br s, NH2-5′), 8.26 (2H, t, J = 5.9 Hz, NH-1′), 7.51 (4H, d, J = 8.9 Hz, 2H-5), 7.38 (2H, d, J = 15.7 Hz, H-3), 6.97 (4H, d, J = 8.9 Hz, 2H-6), 6.48 (2H, d, J = 15.7 Hz, H-2), 3.78 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.96–2.90 (8H, m, H2-4′, H2-6′), 1.79 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.66–1.61 (4H, m, H2-7′); 13C NMR (DMSO-d6, 100 MHz) δ 165.8 (C-1), 160.4 (C-7), 138.6 (C-3), 129.1 (C-5), 127.3 (C-4), 119.3 (C-2), 114.4 (C-6), 55.3 (OMe), 46.1 (C-6′), 44.7 (C-4′), 35.8 (C-2′), 26.2 (C-3′), 22.7 (C-7′); (+)-HRESIMS [M+H]+ m/z 523.3287 (calcd for C30H43N4O4, 523.3279).
2.2.10. N1,N6-Bis(3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (14b)
Following general procedure B, reaction of 4-methoxycinnamic acid (6) (52 mg, 0.292 mmol), EDC·HCl (62 mg, 0.323 mmol), DMAP (71 mg, 0.581 mmol) and di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (12b) (50 mg, 0.116 mmol) afforded di-tert-butyl hexane-1,6-diylbis((3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (29 mg, 33%). Using general procedure C, a sub-sample of this product was deprotected to afford the di-TFA salt 14b as a yellow oil (10 mg, 64%). Rf = 0.34 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3285, 2840, 1674, 1603, 1514, 1202, 1175, 1133, 832, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.45 (4H, br s, NH2-5′), 8.24 (2H, t, J = 5.5 Hz, NH-1′), 7.51 (4H, d, J = 8.9 Hz, 2H-5), 7.38 (2H, d, J = 16.1 Hz, H-3), 6.97 (4H, d, J = 9.3 Hz, 2H-6), 6.47 (2H, d, J = 15.7 Hz, H-2), 3.79 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.94–2.86 (8H, m, H2-4′, H2-6′), 1.83–1.73 (4H, m, H2-3′), 1.60–1.54 (4H, m, H2-7′), 1.34–1.30 (4H, m, H2-8′); 13C NMR (DMSO-d6, 100 MHz) δ 165.8 (C-1), 160.4 (C-7), 138.6 (C-3), 129.1 (C-5), 127.3 (C-4), 119.3 (C-2), 114.4 (C-6), 55.3 (OMe), 46.6 (C-6′), 44.7 (C-4′), 35.8 (C-2′), 26.2 (C-3′), 25.5 (C-7′/C-8′), 25.4 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 551.3593 (calcd for C32H47N4O4, 551.3592).
2.2.11. N1,N7-Bis(3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (14c)
Using general procedure B, reaction of 4-methoxycinnamic acid (6) (53 mg, 0.300 mmol), EDC·HCl (65 mg, 0.340 mmol), DMAP (73 mg, 0.600 mmol) and di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (12c) (50 mg, 0.112 mmol) afforded di-tert-butyl heptane-1,7-diylbis((3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (45 mg, 52%). Using general procedure C, a sub-sample of this product (23 mg, 0.030 mmol) was deprotected to afford the di-TFA salt 14c as a yellow oil (19 mg, 80%). Rf = 0.34 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3280, 2938, 1657, 1602, 1513, 1200, 1174, 1131, 1028, 830, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.61 (4H, br s, NH2-5′), 8.29 (2H, t, J = 5.7 Hz, NH-1′), 7.51 (4H, d, J = 8.8 Hz, 2H-5), 7.38 (2H, d, J = 15.8 Hz, H-3), 6.97 (4H, d, J = 8.8 Hz, 2H-6), 6.49 (2H, d, J = 15.8 Hz, H-2), 3.78 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.97–2.83 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.62–1.53 (4H, m, H2-7′), 1.33–1.26 (6H, m, H2-8′, H2-9′); 13C NMR (DMSO-d6, 100 MHz) δ 165.8 (C-1), 160.4 (C-7), 138.6 (C-3), 129.1 (C-5), 127.4 (C-4), 119.4 (C-2), 114.4 (C-6), 55.3 (OMe), 46.7 (C-6′), 44.7 (C-4′), 35.8 (C-2′), 28.0 (C-9′), 26.1 (C-3′), 25.7 (C-7′/C-8′), 25.4 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 565.3748 (calcd for C33H49N4O4, 565.3748).
2.2.12. N1,N8-Bis(3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (14d)
Using general procedure B, reaction of 4-methoxycinnamic acid (6) (52 mg, 0.292 mmol), EDC·HCl (62 mg, 0.323 mmol), DMAP (71 mg, 0.581 mmol) and di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (12d) (50 mg, 0.109 mmol) afforded di-tert-butyl octane-1,8-diylbis((3-((E)-3-(4-methoxyphenyl)acrylamido)propyl) carbamate) as a white oil (49 mg, 58%). Using general procedure C, a sub-sample of this product (25 mg, 0.032 mmol) was deprotected to afford the di-TFA salt 14d as a yellow oil (17 mg, 66%). Rf = 0.26 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3282, 2937, 2840, 1658, 1602, 1513, 1423, 1174, 1130, 1028, 830, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.51 (4H, br s, NH2-5′), 8.25 (2H, t, J = 5.8 Hz, NH-1′), 7.51 (4H, d, J = 8.5 Hz, 2H-5), 7.38 (2H, d, J = 15.6 Hz, H-3), 6.97 (4H, d, J = 8.5 Hz, 2H-6), 6.48 (2H, d, J = 15.6 Hz, H-2), 3.78 (6H, s, OMe), 3.25 (4H, tt, J = 6.4, 6.4 Hz, H2-2′), 2.96–2.83 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.60–1.52 (4H, m, H2-7′), 1.32–1.24 (8H, m, H2-8′, H2-9′); 13C NMR (DMSO-d6, 100 MHz) δ 165.8 (C-1), 160.4 (C-7), 138.6 (C-3), 129.1 (C-5), 127.3 (C-4), 119.4 (C-2), 114.4 (C-6), 55.3 (OMe), 46.8 (C-6′), 44.7 (C-4′), 35.8 (C-2′), 28.3 (C-9′), 26.2 (C-3′), 25.8 (C-7′/C-8′), 25.5 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 579.3895 (calcd for C34H51N4O4, 579.3905).
2.2.13. N1,N10-Bis(3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (14e)
Using general procedure B, reaction of 4-methoxycinnamic acid (6) (46 mg, 0.258 mmol), EDC·HCl (55 mg, 0.287 mmol), DMAP (63 mg, 0.516 mmol) and di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (12e) (50 mg, 0.103 mmol) afforded di-tert-butyl decane-1,10-diylbis((3-((E)-3-(4-methoxyphenyl)acrylamido)propyl) carbamate) as a white oil (30 mg, 36%). Using general procedure C, this product (30 mg, 0.037 mmol) was deprotected to afford the di-TFA salt 14e as a yellow oil (18 mg, 58%). Rf = 0.19 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3386, 2932, 2855, 1672, 1602, 1513, 1424, 1201, 1133, 1030, 832, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.46 (4H, br s, NH2-5′), 8.24 (2H, t, J = 5.8 Hz, NH-1′), 7.51 (4H, d, J = 8.8 Hz, 2H-5), 7.39 (2H, d, J = 15.8 Hz, H-3), 6.97 (4H, d, J = 8.8 Hz, 2H-6), 6.48 (2H, d, J = 15.8 Hz, H-2), 3.78 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.96–2.84 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.60–1.52 (4H, m, H2-7′), 1.32–1.23 (12H, m, H2-8′, H2-9′, H2-10′); 13C NMR (DMSO-d6, 100 MHz) δ 165.8 (C-1), 160.4 (C-7), 138.6 (C-3), 129.1 (C-5), 127.3 (C-4), 119.3 (C-2), 114.4 (C-6), 55.2 (OMe), 46.8 (C-6′), 44.7 (C-4′), 35.8 (C-2′), 28.7 (C-9′), 28.5 (C-10′), 26.2 (C-3′), 25.9 (C-7′/C-8′), 25.5 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 607.4220 (calcd for C36H55N4O4, 607.4218).
2.2.14. N1,N12-Bis(3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (14f)
Using general procedure B, reaction of 4-methoxycinnamic acid (6) (46 mg, 0.258 mmol), EDC·HCl (55 mg, 0.287 mmol), DMAP (63 mg, 0.516 mmol) and di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (12f) (50 mg, 0.097 mmol) afforded di-tert-butyl dodecane-1,12-diylbis((3-((E)-3-(4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (37 mg, 46%). Using general procedure C, a sub-sample of this product (19 mg, 0.023 mmol) was deprotected to afford the di-TFA salt 14f as a yellow oil (12 mg, 61%). Rf = 0.09 (MeOH:10% HCl, 7:3); IR (ATR) νmax 2930, 1673, 1603, 1513, 1202, 1175, 1133, 831, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.48 (4H, br s, NH2-5′), 8.26 (2H, t, J = 5.7 Hz, NH-1′), 7.51 (4H, d, J = 8.7 Hz, 2H-5), 7.38 (2H, d, J = 15.7 Hz, H-3), 6.97 (4H, d, J = 8.7 Hz, 2H-6), 6.48 (2H, d, J = 15.7 Hz, H-2), 3.78 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.95–2.84 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.60–1.52 (4H, m, H2-7′), 1.31–1.22 (16H, m, H2-8′, H2-9′, H2-10′, H2-11′); 13C NMR (DMSO-d6, 100 MHz) δ 165.8 (C-1), 160.4 (C-7), 138.6 (C-3), 129.1 (C-5), 127.3 (C-4), 119.3 (C-2), 114.4 (C-6), 55.3 (OMe), 46.8 (C-6′), 44.7 (C-4′), 35.8 (C-2′), 28.9 (C-9′/C-10′/C-11′), 28.8 (C-9′/C-10′/C-11′), 28.5 (C-9′/C-10′/C-11′), 26.2 (C-3′), 25.9 (C-7′/C-8′), 25.5 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 635.4532 (calcd for C38H59N4O4, 635.4531).
2.2.15. N1,N4-Bis(3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (15a)
Using general procedure B, reaction of 3-bromo-4-methoxycinnamic acid (7) (50 mg, 0.194 mmol), EDC·HCl (42 mg, 0.219 mmol), DMAP (48 mg, 0.39 mmol) and di-tert-butyl butane 1,4-diylbis((3-aminopropyl)carbamate) (12a) (50 mg, 0.124 mmol) afforded di-tert-butyl butane-1,4-diylbis((3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido) propyl)carbamate) as a white oil (50 mg, 36%). Using general procedure C, a sub-sample of this product (25 mg, 0.028 mmol) was deprotected to afford the di-TFA salt 15a as a yellow oil (7 mg, 27%). Rf = 0.29 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3284, 2843, 1673, 1497, 1261, 1201, 1131, 1052, 800, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.57 (4H, br s, NH2-5′), 8.25 (2H, t, J = 5.7 Hz, NH-1′), 7.80 (2H, d, J = 2.1 Hz, H-5), 7.57 (2H, dd, J = 8.6, 2.1 Hz, H-9), 7.36 (2H, d, J = 16.0 Hz, H-3), 7.15 (2H, d, J = 8.6 Hz, H-8), 6.54 (2H, d, J = 16.0 Hz, H-2), 3.88 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.97–2.90 (8H, m, H2-4′, H2-6′), 1.79 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.65–1.61 (4H, m, H2-7′); 13C NMR (DMSO-d6, 100 MHz) δ 165.4 (C-1), 156.3 (C-7), 137.2 (C-3), 131.7 (C-5), 128.9 (C-4), 128.5 (C-9), 120.9 (C-2), 112.9 (C-8), 111.1 (C-6), 56.4 (OMe), 46.1 (C-6′), 44.7 (C-4′), 35.9 (C-2′), 26.1 (C-3′), 22.7 (C-7′); (+)-HRESIMS [M+H]+ m/z 679.1476 (calcd for C30H4179Br2N4O4, 679.1489).
2.2.16. N1,N6-Bis(3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (15b)
Using general procedure B, reaction of 3-bromo-4-methoxycinnamic acid (7) (50 mg, 0.194 mmol), EDC·HCl (42 mg, 0.219 mmol), DMAP (47 mg, 0.385 mmol) and di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (12b) (34 mg, 0.079 mmol) afforded di-tert-butyl hexane-1,6-diylbis((3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido) propyl) carbamate as a white oil (42 mg, 58%). Using general procedure C, a sub-sample of this product (21 mg, 0.023 mmol) was deprotected to afford the di-TFA salt 15b as a yellow oil (10 mg, 46%). Rf = 0.29 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3285, 2945, 2845, 1659, 1596, 1497, 1261, 1200, 1133, 1052, 801, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.51 (4H, br s, NH2-5′), 8.25 (2H, t, J = 5.6 Hz, NH-1′), 7.80 (2H, d, J = 2.1 Hz, H-5), 7.57 (2H, dd, J = 8.5, 2.1 Hz, H-9), 7.36 (2H, d, J = 15.9 Hz, H-3), 7.15 (2H, d, J = 8.5 Hz, H-8), 6.54 (2H, d, J = 15.7 Hz, H-2), 3.88 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.95–2.86 (8H, m, H2-4′, H2-6′), 1.79 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.60–1.54 (4H, m H2-7′), 1.34–1.29 (4H, m, H2-8′); 13C NMR (DMSO-d6, 100 MHz) δ 165.4 (C-1), 156.2 (C-7), 137.1 (C-3), 131.7 (C-5), 128.9 (C-4), 128.5 (C-9), 120.9 (C-2), 112.9 (C-8), 111.1 (C-6), 56.4 (OMe), 46.6 (C-6′), 44.7 (C-4′), 35.9 (C-2′), 26.1 (C-3′), 25.5 (C-7′/C-8′), 25.4 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 707.1782 (calcd for C32H4579Br2N4O4, 707.1802).
2.2.17. N1,N7-Bis(3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (15c)
Using general procedure B, reaction of 3-bromo-4-methoxycinnamic acid (7) (77 mg, 0.300 mmol), EDC·HCl (65 mg, 0.339 mmol), DMAP (73 mg, 0.600 mmol) and di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (12c) (50 mg, 0.11 mmol) afforded di-tert-butyl heptane-1,7-diylbis((3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido) propyl)carbamate) as a white oil (61 mg, 59%). Using general procedure C, a sub-sample of this product (31 mg, 0.034 mmol) was deprotected to afford the di-TFA salt 15c as a yellow oil (22 mg, 69%). Rf = 0.20 (MeOH:10% HCl, 7:3); IR (ATR) νmax 2971, 1738, 1676, 1498, 1366, 1204, 1134, 1053, 800, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.53 (4H, br s, NH2-5′), 8.25 (2H, t, J = 5.9 Hz, NH-1′), 7.80 (2H, d, J = 2.2 Hz, H-5), 7.57 (2H, dd, J = 8.5, 2.0 Hz, H-9), 7.36 (2H, d, J = 15.7 Hz, H-3), 7.15 (2H, d, J = 8.7 Hz, H-8), 6.55 (2H, d, J = 15.9 Hz, H-2), 3.88 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.95–2.86 (8H, m, H2-4′, H2-6′), 1.79 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.60–1.53 (4H, m, H2-7′), 1.32–1.27 (6H, m, H2-8′, H2-9′); 13C NMR (DMSO-d6, 100 MHz) δ 165.4 (C-1), 156.2 (C-7), 137.1 (C-3), 131.7 (C-5), 129.0 (C-4), 128.5 (C-9), 120.9 (C-2), 112.9 (C-8), 111.1 (C-6), 56.4 (OMe), 46.7 (C-6′), 44.7 (C-4′), 35.9 (C-2′), 28.0 (C-8′/C-9′), 26.1 (C-3′), 25.7 (C-8′/C-9′), 25.4 (C-7′); (+)-HRESIMS [M+H]+ m/z 721.1961 (calcd for C33H4779Br2N4O4, 721.1959).
2.2.18. N1,N8-Bis(3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (15d)
Using general procedure B, reaction of 3-bromo-4-methoxycinnamic acid (7) (75 mg, 0.29 mmol), EDC·HCl (62 mg, 0.323 mmol), DMAP (71 mg, 0.58 mmol) and di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (12d) (50 mg, 0.109 mmol) afforded di-tert-butyl octane-1,8-diylbis((3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido) propyl)carbamate) as a white oil (79 mg, 77%). Using general procedure C, a sub-sample of this product (40 mg, 0.043 mmol) was deprotected to afford the di-TFA salt 15d as a yellow oil (29 mg, 70%). Rf = 0.23 (MeOH:10% HCl, 7:3); IR (ATR) νmax 3395, 1678, 1498, 1202, 1133, 1025, 998, 827, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.60 (4H, br s, NH2-5′), 8.28 (2H, t, J = 5.8 Hz, NH-1′), 7.80 (2H, d, J = 2.3 Hz, H-5), 7.57 (2H, dd, J = 8.8, 2.1 Hz, H-9), 7.35 (2H, d, J = 15.7 Hz, H-3), 7.15 (2H, d, J = 8.9 Hz, H-8), 6.55 (2H, d, J = 15.7 Hz, H-2), 3.88 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.95–2.85 (8H, m, H2-4′, H2-6′), 1.79 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.60–1.53 (4H, m, H2-7′), 1.32–1.23 (8H, m, H2-8′, H2-9′); 13C NMR (DMSO-d6, 100 MHz) δ 165.4 (C-1), 156.2 (C-7), 137.1 (C-3), 131.8 (C-5), 129.0 (C-4), 128.5 (C-9), 121.0 (C-2), 112.9 (C-8), 111.1 (C-6), 56.4 (OMe), 46.8 (C-6′), 44.7 (C-4′), 35.9 (C-2′), 28.3 (C-8′/C-9′), 26.1 (C-3′), 25.8 (C-8′/C-9′), 25.4 (C-7′); (+)-HRESIMS [M+H]+ m/z 735.2115 (calcd for C34H4979Br2N4O4, 735.2115).
2.2.19. N1,N10-Bis(3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (15e)
Using general procedure B, reaction of 3-bromo-4-methoxycinnamic acid (7) (70 mg, 0.273 mmol), EDC·HCl (59 mg, 0.308 mmol), DMAP (67 mg, 0.548 mmol) and di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (12e) (50 mg, 0.103 mmol) afforded di-tert-butyl decane-1,10-diylbis((3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido) propyl)carbamate) as a white oil (87 mg, 88%). Using general procedure C, a sub-sample of this product (25 mg, 0.026 mmol) was deprotected to afford the di-TFA salt 15e as a yellow oil (10 mg, 39%). Rf = 0.11 (MeOH:10% HCl, 7:3); IR (ATR) νmax 2931, 2854, 1672, 1596, 1498, 1261, 1201, 1053, 800, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.37 (4H, br s, NH2-5′), 8.21 (2H, t, J = 5.7 Hz, NH-1′), 7.80 (2H, d, J = 2.3 Hz, H-5), 7.57 (2H, dd, J = 8.2, 2.1 Hz, H-9), 7.36 (2H, d, J = 15.8 Hz, H-3), 7.16 (2H, d, J = 8.2 Hz, H-8), 6.53 (2H, d, J = 15.8 Hz, H-2), 3.88 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.94–2.86 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.58–1.52 (4H, m, H2-7′), 1.32–1.23 (12H, m, H2-8′, H2-9′, H2-10′); 13C NMR (DMSO-d6, 100 MHz) δ 165.5 (C-1), 156.3 (C-7), 137.3 (C-3), 131.8 (C-5), 129.0 (C-4), 128.6 (C-9), 120.9 (C-2), 112.9 (C-8), 111.2 (C-6), 56.5 (OMe), 46.8 (C-6′), 44.7 (C-4′), 35.9 (C-2′), 28.8 (C-9′/C-10′), 28.6 (C-9′/C-10′), 26.2 (C-3′), 25.9 (C-8′), 25.5 (C-7′); (+)-HRESIMS [M+H]+ m/z 763.2427 (calcd for C36H5379Br2N4O4, 763.2428).
2.2.20. N1,N12-Bis(3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (15f)
Using general procedure B, reaction of 3-bromo-4-methoxycinnamic acid (7) (66 mg, 0.257 mmol), EDC·HCl (55 mg, 0.287 mmol), DMAP (63 mg, 0.516 mmol) and di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (12f) (50 mg, 0.097 mmol) afforded di-tert-butyl dodecane-1,12-diylbis ((3-((E)-3-(3-bromo-4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (41 mg, 43%). Using general procedure C, a sub-sample of this product (21 mg, 0.021 mmol) was deprotected to afford the di-TFA salt 15f as a yellow oil (6 mg, 28%). Rf = 0.09 (MeOH:10% HCl, 7:3); IR (ATR) νmax 2930, 1676, 1498, 1262, 1202, 1134, 1053, 800, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.44 (4H, br s, NH2-5′), 8.24 (2H, t, J = 5.4 Hz, NH-1′), 7.80 (2H, d, J = 2.3 Hz, H-5), 7.57 (2H, dd, J = 8.6, 2.1 Hz, H-9), 7.36 (2H, d, J = 15.8 Hz, H-3), 7.15 (2H, d, J = 8.5 Hz, H-8), 6.54 (2H, d, J = 15.8 Hz, H-2), 3.88 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.91–2.84 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.59–1.52 (4H, m, H2-7′), 1.30–1.22 (16H, m, H2-8′, H2-9′, H2-10′, H2-11′); 13C NMR (DMSO-d6, 100 MHz) δ 165.5 (C-1), 156.3 (C-7), 137.2 (C-3), 131.7 (C-5), 129.0 (C-4), 128.5 (C-9), 120.9 (C-2), 112.9 (C-8), 111.1 (C-6), 56.4 (OMe), 46.8 (C-6′), 44.7 (C-4′), 35.9 (C-2′), 29.0 (C-9′/C-10′/C-11′), 28.9 (C-9′/C-10′/C-11′), 28.5 (C-9′/C-10′/C-11′), 26.1 (C-3′), 25.9 (C-8′), 25.5 (C-7′); (+)-HRESIMS [M+H]+ m/z 791.2710 (calcd for C38H5779Br2N4O4, 791.2741).
2.2.21. N1,N4-Bis(3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (16a)
Using general procedure B, reaction of 3,5-dibromo-4-methoxycinnamic acid (
8) (109 mg, 0.324 mmol), EDC·HCl (67 mg, 0.350 mmol), DMAP (76 mg, 0.622 mmol) and di-
tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (
12a) (50 mg, 0.124 mmol) afforded di-
tert-butyl butane-1,4-diylbis((3-((
E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (61 mg, 47%). Using general procedure C, a sub-sample of this product (31 mg, 0.030 mmol) was deprotected to afford the di-TFA salt
16a as a yellow oil (19 mg, 60%). R
f = 0.08 (MeOH:10% HCl, 7:3); IR (ATR) ν
max 1738, 1366, 1217, 733 cm
−1;
1H NMR (DMSO-
d6, 400 MHz) δ 8.61 (4H, br s, NH
2-5′), 8.30 (2H, t,
J = 5.8 Hz, NH-1′), 7.88 (4H, s, 2H-5), 7.35 (2H, d,
J = 15.9 Hz, H-3), 6.66 (2H, d,
J = 15.9 Hz, H-2), 3.81 (6H, s, OMe), 3.25 (4H, dt,
J = 6.4, 6.4 Hz, H
2-2′), 2.96–2.90 (8H, m, H
2-4′, H
2-6′), 1.79 (4H, tt,
J = 7.5, 7.5 Hz, H
2-3′), 1.65–1.61 (4H, m, H
2-7′);
13C NMR (DMSO-
d6, 100 MHz) δ 164.9 (C-1), 154.0 (C-7), 135.5 (C-3), 134.3 (C-4), 131.6 (C-5), 124.1 (C-2), 118.0 (C-6), 60.6 (OMe), 46.1 (C-6′), 44.7 (C-4′), 36.0 (C-2′), 26.0 (C-3′), 22.7 (C-7′); (+)-HRESIMS [M+H]
+ m/
z 834.9688 (calcd for C
30H
3979Br
4N
4O
4, 834.9699). The
1H and
13C NMR data agreed with those reported in the literature [
19].
2.2.22. N1,N6-Bis(3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (16b)
Following general procedure B, reaction of 3,5-dibromo-4-methoxycinnamic acid (
8) (102 mg, 0.304 mmol), EDC·HCl (62 mg, 0.323 mmol), DMAP (71 mg, 0.581 mmol) and di-
tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (
12b) (50 mg, 0.116 mmol) afforded di-
tert-butylhexane-1,6-diylbis((3-((
E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (72 mg, 58%). Using general procedure C, a sub-sample of this product (36 mg, 0.034 mmol) was deprotected to afford the di-TFA salt
16b as a yellow oil (23 mg, 62%). R
f = 0.08 (MeOH:10% HCl, 7:3); IR (ATR) ν
max 2971, 1738, 1366, 1217, 733 cm
−1;
1H NMR (DMSO-
d6, 400 MHz) δ 8.50 (4H, br s, NH
2-5′), 8.29 (2H, t,
J = 5.8 Hz, NH-1′), 7.88 (4H, s, 2H-5), 7.35 (2H, d,
J = 15.6 Hz, H-3), 6.66 (2H, d,
J = 15.6 Hz, H-2), 3.82 (6H, s, OMe), 3.25 (4H, dt,
J = 6.4, 6.4 Hz, H
2-2′), 2.95–1.85 (8H, m, H
2-4′, H
2-6′), 1.79 (4H, tt,
J = 7.5, 7.5 Hz, H
2-3′), 1.60–1.53 (4H, m, H
2-7′), 1.34–1.29 (4H, m, H
2-8′);
13C NMR (DMSO-
d6, 100 MHz) δ 164.9 (C-1), 153.9 (C-7), 135.4 (C-3), 134.3 (C-4), 131.6 (C-5), 124.2 (C-2), 118.0 (C-6), 60.6 (OMe), 46.7 (C-6′), 44.7 (C-4′), 36.0 (C-2′), 26.0 (C-3′), 25.5 (C-7′/C-8′), 25.4 (C-7′/C-8′); (+)-HRESIMS [M+H]
+ m/
z 862.9996 (calcd for C
32H
4379Br
4N
4O
4, 863.0012). The
1H and
13C NMR data agreed with those reported in the literature [
19]. The literature data reported for this compound contains an extra, spurious,
13C NMR signal at δ
C 115.2.
2.2.23. N1,N7-Bis(3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (16c)
Following general procedure B, reaction of 3,5-dibromo-4-methoxycinnamic acid (8) (98 mg, 0.292 mmol), EDC·HCl (60 mg, 0.313 mmol), DMAP (69 mg, 0.565 mmol) and di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (12c) (50 mg, 0.112 mmol) afforded di-tert-butylheptane-1,7-diylbis((3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (71 mg, 58%). Using general procedure C, a sub-sample of this product (36 mg, 0.033 mmol) was deprotected to afford the di-TFA salt 16c as a yellow oil (22 mg, 60%). Rf = 0.08 (MeOH:10% HCl, 7:3); IR (ATR) νmax 2938, 1665, 1473, 1202, 1133, 984, 800, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.45 (4H, br s, NH2-5′), 8.27 (2H, t, J = 5.9 Hz, NH-1′), 7.89 (4H, s, 2H-5), 7.35 (2H, d, J = 16.0 Hz, H-3), 6.66 (2H, d, J = 16.0 Hz, H-2), 3.82 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.95–2.85 (8H, m, H2-4′, H2-6′), 1.79 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.59–1.53 (4H, m, H2-7′), 1.31–1.27 (6H, m, H2-8′, H2-9′); 13C NMR (DMSO-d6, 100 MHz) δ 164.9 (C-1), 154.0 (C-7), 135.5 (C-3), 134.3 (C-4), 131.6 (C-5), 124.1 (C-2), 118.0 (C-6), 60.6 (OMe), 46.7 (C-6′), 44.7 (C-4′), 36.0 (C-2′), 28.0 (C-9′), 26.0 (C-3′), 25.8 (C-7′/C-8′), 25.4 (C-7′/C-8′); (+)-HRESIMS [M+H]+ m/z 877.0168 (calcd for C33H4579Br4N4O4, 877.0169).
2.2.24. N1,N8-Bis(3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (16d)
Following general procedure B, reaction of 3,5-dibromo-4-methoxycinnamic acid (8) (95 mg, 0.283 mmol), EDC·HCl (59 mg, 0.308 mmol), DMAP (66 mg, 0.540 mmol) and di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (12d) (50 mg, 0.109 mmol) afforded di-tert-butyloctane-1,8-diylbis((3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido) propyl)carbamate) as a white oil (57 mg, 48%). Using general procedure C, a sub-sample of this product (28.5 mg, 0.026 mmol) was deprotected to afford the di-TFA salt 16d as a yellow oil (22 mg, 75%). Rf = 0.08 (MeOH:10% HCl, 7:3); IR (ATR) νmax 1740, 1366, 1217 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.46 (4H, br s, NH2-5′), 8.27 (2H, t, J = 5.8 Hz, NH-1′), 7.88 (4H, s, 2H-5), 7.36 (2H, d, J = 16.0 Hz, H-3), 6.66 (2H, d, J = 16.0 Hz, H-2), 3.81 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.94–2.85 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.59–1.52 (4H, m, H2-7′), 1.31–1.25 (8H, m, H2-8′, H2-9′); 13C NMR (DMSO-d6, 100 MHz) δ 164.9 (C-1), 154.0 (C-7), 135.5 (C-3), 134.3 (C-4), 131.6 (C-5), 124.2 (C-2), 118.0 (C-6), 60.6 (OMe), 46.8 (C-6′), 44.7 (C-4′), 36.0 (C-2′), 28.3 (C-8′/C-9′), 26.0 (C-3′), 25.8 (C-8′/C-9′), 25.5 (C-7′); (+)-HRESIMS [M+H]+ m/z 891.0316 (calcd for C34H4779Br4N4O4, 891.0325).
2.2.25. N1,N10-Bis(3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (16e)
Using general procedure B, reaction of 3,5-dibromo-4-methoxycinnamic acid (8) (90 mg, 0.268 mmol), EDC·HCl (55 mg, 0.287 mmol), DMAP (63 mg, 0.516 mmol) and di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (12e) (50 mg, 0.103 mmol) afforded di-tert-butyldecane-1,10-diylbis((3-((E)-3-(3,5-dibromo-4-methox-phenyl)acrylamido)propyl)carbamate) as a white oil (30 mg, 26%). Using general procedure C, a sub-sample of this product (22 mg, 0.020 mmol) was deprotected to afford the di-TFA salt 16e as a yellow oil (18 mg, 80%). Rf = 0.08 (MeOH:10% HCl, 7:3); IR (ATR) νmax 2933, 1674, 1473, 1203, 1135, 800, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.46 (4H, br s, NH2-5′), 8.28 (2H, t, J = 5.8 Hz, NH-1′), 7.88 (4H, s, 2H-5), 7.35 (2H, d, J = 15.7 Hz, H-3), 6.66 (2H, d, J = 15.7 Hz, H-2), 3.82 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.94–2.85 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.59–1.52 (4H, m, H2-7′), 1.30–1.23 (12H, m, H2-8′, H2-9′, H2-10′); 13C NMR (DMSO-d6, 100 MHz) δ 164.9 (C-1), 153.9 (C-7), 135.4 (C-3), 134.3 (C-4), 131.6 (C-5), 124.1 (C-2), 118.0 (C-6), 60.5 (OMe), 46.8 (C-6′), 44.6 (C-4′), 35.9 (C-2′), 28.7 (C-9′/C-10′), 28.5 (C-9′/C-10′), 26.0 (C-3′), 25.9 (C-8′), 25.5 (C-7′); (+)-HRESIMS [M+H]+ m/z 919.0649 (calcd for C36H5179Br4N4O4, 919.0638).
2.2.26. N1,N12-Bis(3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (16f)
Using general procedure B, reaction of 3,5-dibromo-4-methoxycinnamic acid (8) (85 mg, 0.253 mmol), EDC·HCl (52 mg, 0.271 mmol), DMAP (59 mg, 0.483 mmol) and di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (12f) (50 mg, 0.097 mmol) afforded di-tert-butyldodecane-1,12-diylbis((3-((E)-3-(3,5-dibromo-4-methoxyphenyl)acrylamido)propyl)carbamate) as a white oil (44 mg, 39%). Using general procedure C, a sub-sample of this product (22 mg, 0.019 mmol) was deprotected to afford the di-TFA salt 16f as a yellow oil (16 mg, 71%). Rf = 0.08 (MeOH:10% HCl, 7:3); IR (ATR) νmax 2931, 1675, 1366, 1203, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.42 (4H, br s, NH2-5′), 8.27 (2H, t, J = 5.8 Hz, NH-1′), 7.88 (4H, s, 2H-5), 7.35 (2H, d, J = 15.6 Hz, H-3), 6.66 (2H, d, J = 15.6 Hz, H-2), 3.82 (6H, s, OMe), 3.25 (4H, dt, J = 6.4, 6.4 Hz, H2-2′), 2.94–2.84 (8H, m, H2-4′, H2-6′), 1.78 (4H, tt, J = 7.5, 7.5 Hz, H2-3′), 1.58–1.51 (4H, m, H2-7′), 1.30–1.22 (16H, m, H2-8′, H2-9′, H2-10′, H2-11′); 13C NMR (DMSO-d6, 100 MHz) δ 164.9 (C-1), 153.9 (C-7), 135.5 (C-3), 134.3 (C-4), 131.6 (C-5), 124.1 (C-2), 118.0 (C-6), 60.5 (OMe), 46.8 (C-6′), 44.6 (C-4′), 35.9 (C-2′), 29.0 (C-9′/C-10′/C-11′), 28.9 (C-9′/C-10′/C-11′), 28.5 (C-9′/C-10′/C-11′), 26.0 (C-3′), 25.9 (C-8′), 25.5 (C-7′); (+)-HRESIMS [M+H]+ m/z 947.0951 (calcd for C38H5579Br4N4O4, 947.0951).