Abstract
Sponges are aquatic, spineless organisms that belong to the phylum Porifera. They come in three primary classes: Hexactinellidae, Demospongiae, and Calcarea. The Demospongiae class is the most dominant, making up over 90% of sponge species. One of the most widely studied genera within the Demospongiae class is Xestospongia, which is found across Southeast Asian waters. This genus is of particular interest due to the production of numerous primary and secondary metabolites with a wide range of biological potentials. In the current review, the antioxidant, anticancer, anti-inflammatory, antibacterial, antiviral, antiparasitic, and cytotoxic properties of metabolites from several varieties of Southeast Asian Xestospongia spp. were discussed. A total of 40 metabolites of various natures, including alkaloids, fatty acids, steroids, and quinones, were highlighted in X. bergquistia, X. testudinaria, X. muta, X. exigua, X. ashmorica and X. vansoesti. The review aimed to display the bioactivity of Xestospongia metabolites and their potential for use in the pharmaceutical sector. Further research is needed to fully understand their bioactivities.
1. Introduction
Sponges, aquatic animals of the phylum Porifera, have existed for millions of years as the simplest multicellular organisms. They are filter feeders and are known for their unique species diversity and morphological complexity [1,2]. Sponge species number over 8000 and are found in temperate, tropical, and polar regions, inhabiting a wide range of freshwater and marine habitats [3]. They are an important source of metabolites. More than 5300 distinct metabolites produced by sponges and the accompanying microbes are known and more than 200 novel sponge metabolites are reported each year [3]. Alkaloids, fatty acids, sterols, terpenoids, polyketones, macrolides, quinines, glucosides, and peptides are a few examples of novel metabolites that have been identified from marine sponges [4,5,6,7,8,9].
There are three primary classes of sponges: Hexactinellidae, Demospongiae, and Calcarea. The Demospongiae class is the most dominant, comprising over 90% of sponge species. The genus Xestospongia (Petrosiidae) is widely studied due to its various primary and secondary metabolites with various biological potentials. They are also known as “giant barrel sponges” and have a large, erect, barrel-shaped appearance with variations in height, diameter, and surface complexity among distinct species [10]. However, they can be distinguished by their unique morphological characteristics, which include a thick cortex, a large central osculum, and a porous spongin skeleton [11,12,13]. Their external morphology also varied from smooth to highly digitated or lamellate surfaces (Figure 1) [10,11,14,15].

Figure 1.
X. testudinaria morphotypes found in Sabah, Malaysia, waters include (a) digitate (outer body surface cover with digitate or spiky projections), (b) Smooth (lack of surface projections, (c,d) Lamellate (pronounced and smooth flanges extending from the base to the apex of its exterior).
Xestospongia spp. are classified as follows within the phylum Porifera. Kingdom: Animalia (animals), Phylum: Porifera (sponges), Class: Demospongiae (demosponges), Order: Haplosclerida, Family: Xestospongiidae, Genus: Xestospongia. There are over 30 Xestospongia spp., including X. bergquistia (Fromont, 1991) [16], X. testudinaria (Lamarck, 1815) [17], X. muta (Schmidt, 1870) [18], X. exigua (Kirkpatrick, 1900) [19], X. ashmorica (Hooper, 1984) [20] and X. vansoesti [21] etc.
Xestospongia spp. can be found in a variety of habitats in the Southeast Asia region. They are typically found in shallow tropical coral reefs but can also be found in deeper waters. They are often found attached to sand, rocks, corals, or other benthic substrates [22,23,24,25,26]. In terms of ecology, Xestospongia spp. play a key role in the coral reef ecosystem. They are filter feeders, which means they filter water through their bodies to obtain food particles. The huge size of these sponges is particularly crucial because body size is mechanistically connected to pumping and nutrition cycling. This process also helps to remove excess nutrients and sediment from the water, which can help maintain the overall health condition of the coral reef ecosystem [27,28]. Xestospongia spp. also provide important habitats for other organisms, such as algae, bacteria, fish, crustaceans, and other invertebrates, which use the sponges as a place to hide, breed, and forage for food (Figure 2). Some Xestospongia spp. also form symbiotic relationships with other prokaryotes [23,29,30,31].

Figure 2.
Numerous symbiotic macroscopic creatures reside inside or on the surface of a sponge. (a) Crinoid feather star, (b) Red lionfish, and (c,d) Synaptid sea cucumber.
However, Xestospongia spp. are facing potential threats to their survival. These include pollution, rise in temperature due to climate changes and associated diseases such as generalised necrosis, cyclic spotted bleaching, sponge orange band, tissue hardening condition and tissue wasting disease [32,33,34,35]. These threats can have negative impacts on the overall health of coral reef ecosystems and therefore on Xestospongia spp. as well [36,37].
Xestospongia species are found in tropical and subtropical waters throughout Southeast Asia (Figure 3). They are noticed in several places in Indonesia, including Pecaron Bay, Pasir Putih, Situbondo, East Java, Sabang Island, North Sulawesi, and Bandung. They can frequently be seen in deep waters, lagoons, and coral reefs [38,39,40,41]. Xestospongia spp. are observed in the Philippines in several places, including the Manila Channel off Mindoro Island [42]. They are located in Malaysia in several places, including Sepanggar and Gaya Islands, Sabah, Mentigi Island, Johor, Bidong Island, Terengganu, and Langkawi [43,44,45]. In Thailand, they are found in Chon Buri and Rayong Provinces [46] while in Vietnam, they are found in Ha Long Bay and Khanh Hoa [47,48].
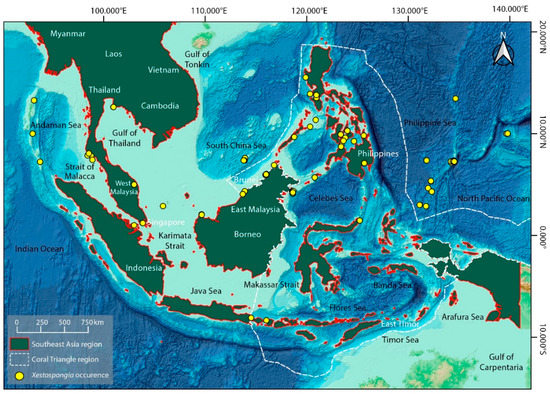
Figure 3.
The location of Xestospongia spp. in Southeast Asia is indicated on the map. Malaysia, Indonesia, Thailand, Myanmar, Vietnam, Cambodia, Brunei, Singapore, Timor-Leste, and the Philippines are the nations that makeup Southeast Asia. Data for Xestospongia spp. (Yellow dots) are taken from the Ocean Biodiversity Information System (OBIS) (https://obis.org/; accessed on 25 December 2022) and recreated as a distribution map from the GEBCO World Map 2014. (www.gebco.net; accessed on 25 December 2022).
It is also worth noting that the diversity of Xestospongia spp. in Southeast Asia is not well understood, and more research is needed to fully understand the diversity, distribution, and abundance of these species in the region.
This review comprehensively addresses the secondary metabolites of marine sponges of the genus Xestospongia in Southeast Asian waters with potential bioactive properties, including antioxidant, anticancer, antiplasmodial, antibiotic, antibacterial, antifungal, cardiotonic, cytotoxic, antimalarial, and antihelminthic properties. The review collects and compares information from peer-reviewed articles from 1974 to 2022 on secondary metabolites isolated from Xestospongia spp. The information was retrieved from several internet databases (PubMed, Web of Science, Scopus, and registries including dissertations and proceedings). The database was searched for marine sponges, Xestospongia, Indo-Pacific, Southeast Asia, the South China Sea, bioactivity, morphotypes, haplotypes, secondary metabolites and marine natural products.
2. Secondary Metabolites in Xestospongia spp. and Their Bioactivity
In Southeast Asian waters, Xestospongia spp. has been found to produce a variety of secondary metabolites of various nature including alkaloids, steroids, fatty acids, quinone etc. These compounds have been shown to have antioxidant, anti-inflammatory, antiparasitic, antitumor, and antimicrobial properties [4,5,6,49,50,51,52,53,54,55,56]. However, more research is needed to fully understand these metabolites’ bioactivity and potential uses. Some of the important Xestospongia spp. have been discussed below.
2.1. Xestospongia bergquistia (Fromont, 1991)
X. bergquistia is an abundant member of the coral reef community found in the Philippines, Indian Ocean, Indonesia, and Malaysia [15,57]. Recent studies have isolated three unique pentacyclic polyhydroxylated steroids, known as xestobergsterol A (1), B (2), and C (3), from the methanol/toluene extract of this species. These compounds are notable for being the first steroids to have five carbocyclic rings. Compounds 1 and 2 have been found to have anti-inflammatory activity, with both displaying potent inhibition of histamine release from rats’ mast cells induced by anti-IgE (Immunoglobulin E) in a dose-dependent manner [4,5,6]. The anti-inflammatory activity of the compound 1 was found to be approximately 5200 times stronger than that of disodium cromoglycate, a commonly used antiallergy medication. In addition to their anti-inflammatory properties, compounds 1 and 3 were also found to exhibit cytotoxic activity against L-1210 murine leukaemia cells. Specifically, they displayed IC50 values of 4.0 μg/mL and 4.1 μg/mL, respectively. However, compound 2 was found to have negligible cytotoxic effects [49,50]. The chemical structures of compounds 1-3 are shown in Figure 4.
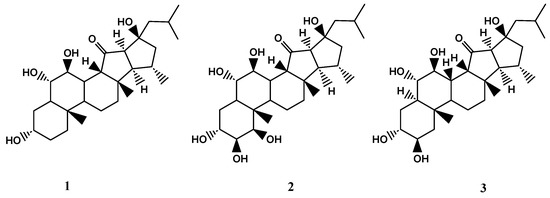
Figure 4.
The chemical structures of xestobergsteroles A-C (1-3).
Overall, these findings suggest that compounds 1-3 isolated from X. bergquistia have potential therapeutic applications in the fields of anti-inflammatory and cancer treatment. However, further research is needed to fully understand the properties and potential uses of these compounds.
2.2. Xestospongia muta (Schmidt, 1870)
X. muta is a species of sponge found on Sabang Island, Indonesia, and other parts of the world. Studies have shown that the tissues of this sponge contain symbionts of the Synechococcus group, which are a type of microorganism that lives in symbiotic relationships with other organisms [37,38].
A manzamine alkaloid, named manzamine C (4) (Figure 5), was isolated from X. muta, and displayed inhibition activity against human pancreatic cell carcinoma (PANC-1) under glucose starvation conditions with IC50 values of 10 μM, whereas no growth inhibition was observed up to 100 μM under the general culture conditions. Additionally, the compound 4 also exhibited strong antileishmanial and antimalarial activity against drug-sensitive and drug-resistant strains of Plasmodium [58,59]. These findings suggest that compound 4 has the potential as a treatment for these diseases, and further research is needed to explore this possibility.
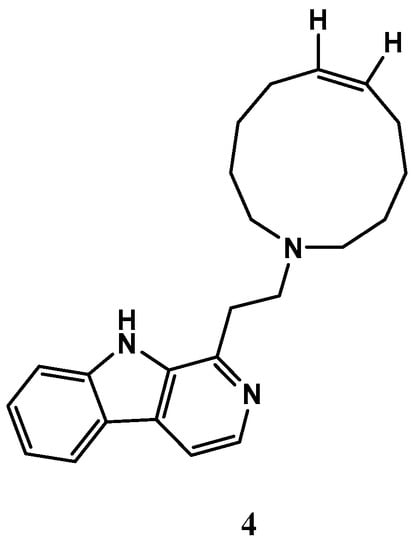
Figure 5.
The chemical structure of manzamine C (4).
In addition to the pure secondary metabolites the fractions of the solvent extract of X. muta also displayed protective properties. The X. muta collected in coastal Terengganu (Malaysia) was found to have cardiovascular protective properties. The study showed that various fractions of this species contain various fatty acids with cardiovascular protective activity, with Fraction-7 being the most notable. Fraction 7 was obtained from the methanol extract of X. muta, and gas chromatography and mass spectrometry (GCMS) analysis of this fraction indicated the presence of 58 compounds. In vitro research in HepG2 cells displayed that fraction-7 of X. muta boosted the expression of Scavenger receptor class B type I (SR-BI) mRNA by 129%. SR-BI is the primary receptor for high-density lipoprotein (HDL) cholesterol, which is crucial for preventing atherosclerosis [44].
2.3. Xestospongia exigua (Kirkpatrick, 1900)
X. exigua is abundant in tropical Southeast Asia, the Western South Pacific, Papua New Guinea, and Australia [53,60,61]. In natural product chemistry, X. exigua has attracted a lot of attention due to the diversity of secondary metabolites that have been isolated from it. Since the 1980s, over 24 different bioactive metabolites have been identified from this species, and these metabolites have been found to display a wide range of bioactivities, including vasodilation, cytotoxicity, and antibacterial effects [53,61,62,63,64,65].
The metabolites identified in X. exigua exhibited diverse chemical structures, including alkaloids, quinones, and sterols [61,62,63]. The compounds (+)-xestospongin (A–D) (5-8) and (+)-araguspongines K (9) and L (10) exhibited vasodilation activity [62,63]. Similarly, xestosin A (11), a new bis-quinolizidine alkaloid, was isolated from X. exigua collected in Papua New Guinea [60]. Exiguamine A. (12) displayed inhibition of indoleamine-2,3- dioxygenase [64]. Other compounds isolated from X. exigua such as motuporamines A- I (13-21) displayed cytotoxicity against human cancer cell lines [65]. According to the literature, Motuporamines G, H and I (19-21) shared identical chemical structures and varied in chemical shifts. Halenaquinone (22) displayed antibacterial properties [52]. Exiguaquinol (23) showed inhibition of Helicobacter pylori glutamate racemase (MurI) with an IC50 value of 4.4 μM. MurI catalyses the conversion of L and D-glutamate, supplying D-glutamate for integration into the elongating peptidoglycan chain that constitutes the cell walls [51]. Clionasterol (24) and 5α,8α-Epidioxy-24αethylcholest-6-en-βb-ol (25) were reported to inhibit the human complement system [53]. The compound 25 also exhibited anti-inflammatory properties. At the mRNA and protein levels, 25 reduced the liposaccharide-induced production of inflammation mediators involved in the NF-κB pathway, including tumour necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and cyclooxygenase-2 (COX-2) [66]. The chemical structures of compounds reported from X. exigua are displayed in Figure 6.
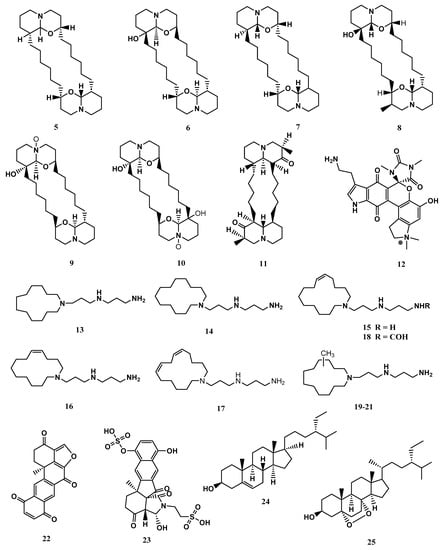
Figure 6.
The chemical structures of compounds isolated from Xestospongia exigua (5-25).
Similarly, the crude extract of X. exigua also displayed protective activity. The cytotoxic and antioxidant activities of the crude extract of X. exigua collected from Pecaron Bay, Pasir Putih, Situbondo, East Java, Indonesia, were determined. The cytotoxic assay was carried out using MTT methods for cancerous cells, including HT-29, T47D, and Casky, while the antioxidant assay was conducted using the DPPH (2,2-diphenyl-1-picrylhydrazyl) method. The ethanol extract of X. exigua (1 mg/mL) showed cytotoxic activity with IC50 values of 124 (HT-29), 98 (T47D), and 68 μg/mL (Casky), while antioxidant activity was 89 μg/mL [40].
The above information displayed that the metabolites detected in X. exigua have a diverse range of chemical structures, including alkaloids, quinones, and sterols, with protective activity such as indoleamine-2,3-dioxygenase inhibition, vasodilation, and cytotoxicity against human cancer cell lines. Some metabolites demonstrated anti-inflammatory and antibacterial properties. Crude extract of X. exigua also showed cytotoxic and antioxidant properties. These results demonstrated the potential of X. exigua as a source of bioactive metabolites for further research.
2.4. Xestospongia testudinaria (Lamarck, 1815)
X. testudinaria is a well-known sponge species that is common across the Indo-Pacific and dominates coral reef sponge ecosystems in Indonesia. The function of the so-called “giant barrel sponge” in the reef ecology as well as its different bioactive substances have been extensively researched. Important metabolites include nonanedioic acid (azelaic acid) (26), which displayed bactericidal activity in a variety of Gram-positive and Gram-negative bacteria such as Propionibacterium acnes, Staphylococcus epidermidis, S. aureus, Pseudomonas aeruginosa, Escherichia coli, Corynebacterium diphtheriae and Proteus mirabilis [67]. It also displayed potent anti-inflammatory and antioxidant properties. Compound 26 treatment dramatically decreased the number of inflammatory papules and pustules in individuals. It promoted the transcription of genes involved in the production of proinflammatory cytokines, including IL-1β, IL-6 or TNFα [67]. Tetradecanoic acid (27), at concentrations of 1.0, 2.5, and 5.0 ppm, displayed larvicidal activity against Aedes aegypti and Culex quinquefasciatus mosquitoes’ larvae with LC50 values of 14.08 and 25.10 ppm, respectively [68]. Trans phytol (28), at a concentration of 10 μg/mL showed aromatase inhibition activity with an IC50 value of 1 μM. The compound 28 reduced aromatase mRNA and protein expression levels in human ovarian granulosa-like KGN cells [69]. In vertebrates, the sole enzyme that catalyzes the biosynthesis of estrogens is aromatase. Overexposure to estrogens causes endometrial, ovarian and breast cancer, so lowering estrogen levels by inhibiting aromatase becomes a possibility in the prevention and treatment of estrogen-mediated cancer [69,70]. In another study, 28 displayed antioxidant properties, at a concentration of 7.2 μg/mL displayed 59.89 and 62.79% scavenging capacity of DPPH• and ABTS•+ (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid), respectively. The compound 28 (administered via the intraperitoneal (i.p.) route at doses of 25, 50 and 75 mg/kg), reduced lipid peroxidation (LP) and nitrite (NO2−) levels and elevated reduced glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) activities in the Swiss mouse hippocampus [71]. Pentadecanoic acid (29) displayed anticancer effects against human breast carcinoma MCF-7/stem-like cells (SC). Furthermore,29 reduced interleukin-6 (IL-6)-induced JAK2/STAT3 signalling. In populations of CD44+/CD24 stem-like cells derived from human breast cancer cells, JAK2/STAT3 signalling is essential for maintenance [72,73]. Thus, targeting JAK2/STAT3 signalling is seen as a viable therapeutic approach. Additionally, 29 caused a cell cycle arrest at the sub-G1 phase and aided caspase-dependent death in MCF-7/SC cells [72,73]. Palmitic acid (30) displayed anticancer properties against the human prostate cancer cell lines PC3 and DU145 as well as the subcutaneous xenograft model. Both in vitro and in vivo, 30 reduce the development of prostate cancer cells. The exposure of 30 induced G1 phase arrest, linked with the downregulation of cyclin D1 and p-Rb and upregulation of p27 [54]. The compound 30 displayed anticancer properties against murine colorectal carcinoma (CT-26) and (MC-38) cell lines [55]. It also demonstrated antiviral activity against viremia of carp virus (SVCV) infection using Zebrafish (Danio rerio) model. The findings showed that a low concentration of 30 modulates the infection and reduced Zebrafish mortality [56]. At concentrations ranging from 0.1 to 1.0 mg/mL, 9,12-octadecadienoic acid (linoleic acid) (31) demonstrated antibacterial activity against five Gram-positive bacteria, including Bacillus cereus, B. pumilus, B. subtilis, Micrococcus kristinae, and S. aureus, but was inactive against Gram-negative species (Enterobacter cloacae, E. coli, Klebsiella pneumoniae, P. aeruginosa and Serratia marcescens) [74]. Heneicosane (32) at a concentration of 10 μg/mL displayed excellent antimicrobial potential against S. pneumoniae (zone of inhibition = ZOI = 31 ± 0.64 mm) and Aspergillus fumigatus (ZOI = 29 ± 0.86 mm) respectively [75]. 1-iodohexadecane (hexadecyl iodide or cetyl iodide) (33) reduced 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis (AD) (a chronic inflammatory dermal) in mice. The treatment with bioactive alkane at a concentration of 100μg/mL for 21 days improved AD-like skin lesions, suppressed epidermal thickness and elevated filaggrin [76,77]. The above-mentioned long-chain fatty acids from X. testudinaria are displayed in Figure 7.
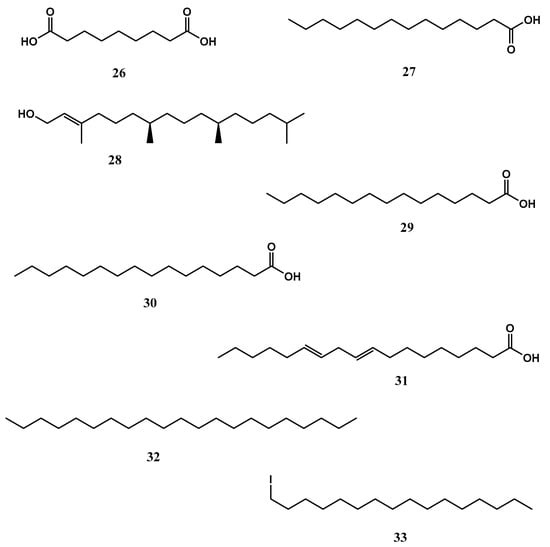
Figure 7.
The chemical structures of long-chained fatty acids from Xestospongia testudinaria (26-33).
The above studies indicated that potential secondary metabolites were found in X. testudinaria with different biological activities, including anti-inflammatory, antioxidant, larvicidal, aromatase inhibition, anticancer, antiviral, and antimicrobial.
In another study, the organic (methanol—dichloromethane) extracts of X. testudinaria from Langkawi, Malaysia, displayed antimicrobial activity against S. aureus, B. cereus, and E. coli with ZOI of 11.5, 12 and 9 mm, while no antimicrobial activity was noticed in the aqueous extract of the same species against the same bacteria [45]. Similarly, the symbiotic bacteria extract isolated from X. testudinaria was found to possess antibacterial activity against different types of bacteria, including S. aureus, P. aeruginosa, E. coli, and Salmonella typhi, as conducted by the disc diffusion dilution method. The n-hexane, ethyl acetate, and n-butanol fractions of the extract demonstrated antibacterial activities. Additionally, phytochemical screening of the extract revealed the existence of important metabolites, such as alkaloids, steroids, and triterpenoids [78]. The chloroform fraction of methanol extract of X. tesdudinaria displayed anticancer properties against the HeLa cell line using the MTT method. The fraction displayed anticancer activity with IC50 values of 2.273 ppm. The GC-MS analysis indicated the presence of 21 metabolites [77]. Another study reported the antioxidant, anti-inflammatory, and immunomodulatory properties of the methanolic extract of X. testudinaria. against carrageenan-induced rat hind paw edema. The methanolic extract of X. testudinaria at a concentration of 100 mg/kg significantly decreased percentage increase in paw weight after carrageenan injection. The histopathological observation indicated that the extract administration reduced inflammatory cell infiltrate and capillary congestion. The extract boosted reduced glutathione, glutathione peroxidase, and catalase activities while decreasing malondialdehyde (MDA) and nitric oxide (NO) levels. Inflammatory cytokines such as tumour necrosis factor (TNF), interleukin-1 (IL-1), and IL-6 were also lowered [79]. The extract of X. testudinaria also possessed poisonous effects on brine shrimp (Artemia salina), with LC50 values ranging from 0.56 to 6.99 μM [80,81]. In addition to this, the extract of X. testudinaria from Bandung, Indonesia, mixed with the marine sponge Melophlus sarasinorum to form a scaffold for bone tissue, promoting the growth and division of bone cells. The study displayed promising results for bone tissue engineering [41].
Thus, in addition to the protective nature of the pure secondary metabolites of X. testudinaria, the crude extract and fractions of the sponge also displayed a wide range of protective properties, including antimicrobial, anticancer, antioxidant, anti-inflammatory, and immunomodulatory properties. Phytochemical screening of these extracts and fractions further revealed the presence of important metabolites. These results demonstrated the potential of X. testudinaria as a source of biologically active compounds with a range of therapeutic applications.
2.5. Xestospongia ashmorica (Hooper, 1984)
X. ashmorica is a marine sponge found in the Manila Channel off Mindoro Island in the Philippines. In 1996, researchers isolated three alkaloids from this sponge, named manzamine A (34), manzamine E (35), and manzamine F (36). These alkaloids were found to have cytotoxic properties against L5178 mouse lymphoma cells at a concentration range of 0.3 to 20 μg/mL. The median effective dose (ED50) for compounds 34-36 were 1.8, 6.6 and 2.3 g/mL respectively, showing that all the compounds were active against the selected cell line [42]. The structures of the compounds 34-36 from X. ashmorica are illustrated in Figure 8.
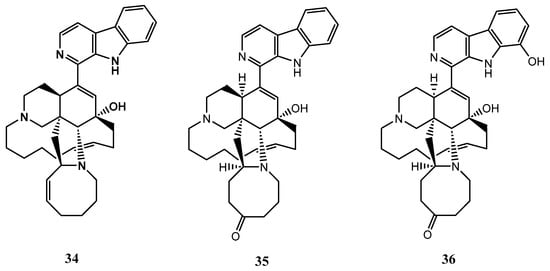
Figure 8.
The chemical structures of manzamines A, E and F (34-36).
2.6. Xestospongia vansoesti (Bakus & Nishiyama, 2000)
The compounds salsolinol (37), norsalsolinol (38), cis-4-hydroxysalsolinol (39), and trans-4-hydroxysalsolinol (40) were isolated from the marine sponge X. vansoesti found in North Sulawesi, Indonesia. These compounds are all tetrahydroisoquinoline alkaloids. Compound (37) showed cytotoxicity against various cancer cell lines such as murine leukaemia (L1210) with IC50 values of 8 mg/mL, human amnion (FL) with IC50 values of 13 mg/mL, human oral epidermoid carcinoma (KB) with IC50 values of 20 mg/mL and human lung adenocarcinoma (A549) with IC50 values of 27 mg/mL, respectively. Additionally, both compounds 37 and 38 inhibited the activity of the proteasome with IC50 values of 50 and 32 mg/mL, respectively, and were also cytotoxic to human cancer cell lines (HeLa) with IC50 values of 17 and 7 mg/mL However, compounds 39 and 40 were found to have no protosome inhibitory effect and no cytotoxicity against HeLa [39]. The chemical structures of compounds 37-40 are exhibited in Figure 9.
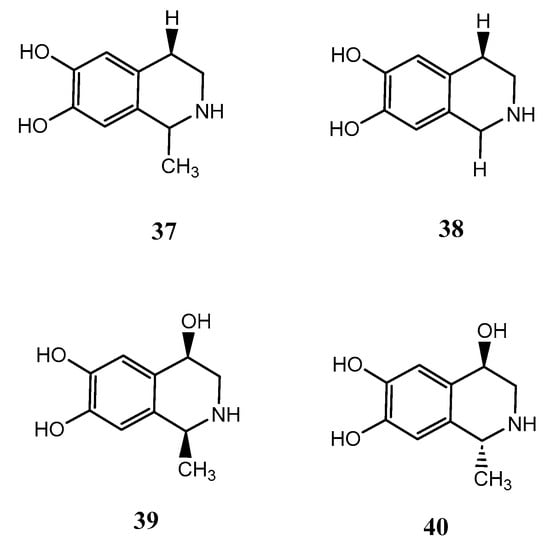
Figure 9.
The chemical structures of salsalinol derivatives from Xestospongia vansoesti (37-40).
3. Summary of Xestospongia Metabolites
Table 1 below summarizes the metabolites detected in the extracts of various species of Xestospongia, including their bioactivity, class, molecular formula (MF), and molecular weight (MW).

Table 1.
Summary of bioactive metabolites isolated from Xestospongia spp.
Xestospongia spp., found in Southeast Asian waters, are a rich source of secondary metabolites with potential for application in various industries, such as pharmaceuticals, biotechnology, and agriculture. Among the species, X. exigua displayed a high number of metabolites with bioactivity, followed by X. testudinaria, X. bergquistia, X. ashmorica, X. vansoesti, and X. muta. Despite the potential application, there is still limited knowledge about Xestospongia spp. and the metabolites they produce. To address this knowledge gap, Southeast Asian nations must collaborate on research. The primary objectives of the research should be the identification, classification, and biosynthetic and biotechnological potential of natural compounds from Xestospongia spp.
The efficiency of the bioactive secondary metabolites discovered in Xestospongia spp. must also be determined by clinical study. Additionally, the generation of metabolites by the host sponge and its microbial population must also be investigated individually to confirm the source of these metabolites, which will not only assist in establishing the origin of the metabolites but also shed light on the sponge’s host microbial community’s biochemistry.
Further, to aid future identification and metabolomics studies, a mass spectrometry database for Xestospongia spp. related metabolites should be established. This will provide a valuable resource for researchers studying these species, allowing for rapid identification and characterization of new natural products.
Overall, further research on Xestospongia spp. is necessary to fully understand the potential of these species and their secondary metabolites.
4. Conclusions
The genus Xestospongia within the Demospongiae class of sponges from Southeast Asian waters is a rich source of biologically active compounds. This review highlights the various structural diversity of the metabolites, including alkaloids (xestospongin, araguspongine, exiguamine, motuporamine, manzamine, salsolinol and norsalsolinol), fatty acids (nonanedioic acid, phytol, pentadecanoic acid, palmitic acid, and 9,12-octadecadienoic acid), steroids (xestobergsterol, clionasterol and 5α,8α-epidioxy-24αethylcholest-6-en-βb-ol), alkane (heneicosane and iodohexadecane), and quinones (halenaquinone and exiguaquinol), that have been isolated from Xestospongia spp. and found to exhibit a wide range of bioactivity. These compounds displayed antioxidant properties, which means they can protect cells from damage caused by free radicals. They also demonstrated anticancer properties, which could make them useful in the development of new cancer therapies. Additionally, the compounds displayed anti-inflammatory properties, which could be beneficial in the treatment of chronic diseases such as rheumatoid arthritis and asthma. They also indicated anti-bacterial, antiviral, antiparasitic, and cytotoxic properties.
Author Contributions
Conceptualization, M.D.S., F.A.K., W.S.C. and N.B.M.; methodology, M.D.S.; validation, M.D.S. and K.P.; resources, M.D.S., F.A.K., W.S.C., N.B.M., K.H.O. and K.P.; data curation, M.D.S., F.A.K., W.S.C. and N.B.M.; writing—original draft preparation, M.D.S., F.A.K., W.S.C. and N.B.M.; writing—review and editing, M.D.S., F.A.K., W.S.C., N.B.M., K.H.O. and K.P.; supervision, M.D.S.; project administration, M.D.S.; funding acquisition, M.D.S. and W.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research work has been supported by the Ministry of Higher Education, the Fundamental Research Grant Scheme (FRGS) 2022, FRGS/1/2022/WAB05/UMS/02/1, FRG0568-1/2022; Universiti Malaysia Sabah Project: SLB2232.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hooper, J.N.A.; Van Soest, R.W.M. Systema Porifera. A guide to the classification of sponges. In Systema Porifera; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–7. [Google Scholar]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; De Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global diversity of sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed]
- Laport, M.S.; Santos, O.C.S.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Johnson, T.W. First total synthesis of xestobergsterol A and active structural analogues of the xestobergsterols. Tetrahedron 2001, 57, 1449–1481. [Google Scholar] [CrossRef]
- Kobayashi, J.; Shinonaga, H.; Shigemori, H.; Umeyama, A.; Shoji, N.; Arihara, S. Xestobergsterol C, a new pentacyclic steroid from the okinawan marine sponge ircinia sp. and absolute stereochemistry of xestobergsterol A. J. Nat. Prod. 1995, 58, 312–318. [Google Scholar] [CrossRef]
- Shoji, N.; Umeyama, A.; Shin, K.; Takeda, K.; Arihara, S.; Kobayashi, J.; Takei, M. Two Unique Pentacyclic Steroids with Cis C/D Ring Junction from Xestospongia bergquistia Fromont, Powerful Inhibitors of Histamine Release. J. Org. Chem. 1992, 57, 2996–2997. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, F.; Al-Kareef, A.M.Q.; Wang, H. Anticancer agents from marine sponges. J. Asian Nat. Prod. Res. 2015, 17, 64–88. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Perkins, M.V.; Zhang, W.; Franco, C.M.M. New marine natural products from sponges (Porifera) of the order Dictyoceratida (2001 to 2012); a promising source for drug discovery, exploration and future prospects. Biotechnol. Adv. 2016, 34, 473–491. [Google Scholar] [CrossRef]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef]
- Swierts, T.; Peijnenburg, K.T.C.A.; de Leeuw, C.; Cleary, D.F.R.; Hörnlein, C.; Setiawan, E.; Wörheide, G.; Erpenbeck, D.; de Voogd, N.J. Lock, stock and two different barrels: Comparing the genetic composition of morphotypes of the Indo-Pacific sponge Xestospongia testudinaria. PLoS ONE 2013, 8, e74396. [Google Scholar] [CrossRef]
- López-Legentil, S.; Pawlik, J.R. Genetic structure of the Caribbean giant barrel sponge Xestospongia muta using the I3-M11 partition of COI. Coral Reefs 2009, 28, 157–165. [Google Scholar] [CrossRef]
- Zea, S.; Rutzler, K. A new species of Xestospongia (Porifera: Demospongea) from the Colombian Caribbean. Caldasia 1983, 13, 817–831. [Google Scholar]
- De Carvalho, S.M.; Lopes, D.A.; Cosme, B.; Hajdu, E. Seven new species of sponges (Porifera) from deep-sea coral mounds at Campos Basin (SW Atlantic). Helgol. Mar. Res. 2016, 70, 1–33. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, T.; Yang, X.W.; Huang, R.; Yang, B.; Tang, L.; Liu, Y. Chemical and biological aspects of marine sponges of the genus Xestospongia. Chem. Biodivers. 2010, 7, 2201–2227. [Google Scholar] [CrossRef]
- Setiawan, E.; de Voogd, N.J.; Swierts, T.; Hooper, J.N.A.; Wörheide, G.; Erpenbeck, D. MtDNA diversity of the Indonesian giant barrel sponge Xestospongia testudinaria (Porifera: Haplosclerida)—Implications from partial cytochrome oxidase 1 sequences. J. Mar. Biol. Assoc. U. K. 2016, 96, 323–332. [Google Scholar] [CrossRef]
- Fromont, J. Descriptions of species of the Petrosida (Porifera: Demospongiae) occurring in the tropical waters of the Great Barrier Reef. Beagle Rec. Museums Art Gall. North. Territ. 1991, 8, 73–95. [Google Scholar] [CrossRef]
- De Monte Lamarck, J.B.P. Suite des polypiers empâtés. Mémoirs du Muséum d’Histoire naturelle. Paris 1815, 1, 69–80. [Google Scholar]
- Schmidt, E.O. Grundzüge Einer Spongien-Fauna des Atlantischen Gebietes; Hansebooks: Norderstedt, Germany, 1870. [Google Scholar]
- Kirkpatrick, R. On the Sponges of Christmas Island. Proc. Zoolological Soc. London 1900, 68, 127–141. [Google Scholar]
- Hooper, J.N.A. A New Genus and Two New Species of Haplosclerid Sponges (Porifera: Demospongiae) from the Timor Sea, Northwest Australia; Stillwell and Co.: Melbourne, Australia, 1984. [Google Scholar]
- Bakus, G.J.; Nishiyama, G.K. Three species of toxic sponges from Cebu, Philippines (Porifera: Demospongiae). Proc. Biol. Soc. Washingt. 2000, 113, 1162–1172. [Google Scholar]
- Darumas, U.; Chavanich, S.; Suwanborirux, K. Distribution Patterns of the Renieramycin-Producing Sponge, Xestospongia sp., and Its Association with Other Reef Organisms in the Gulf of Thailand. Zool. Stud. 2007, 46, 695–704. [Google Scholar]
- Swierts, T.; Cleary, D.F.R.; de Voogd, N.J. Prokaryotic communities of Indo-Pacific giant barrel sponges are more strongly influenced by geography than host phylogeny. FEMS Microbiol. Ecol. 2018, 94, fiy194. [Google Scholar] [CrossRef]
- Chong, W.S.; Zaki, N.H.M.; Hossain, M.S.; Muslim, A.M.; Pour, A.B. Introducing Theil-Sen estimator for sun glint correction of UAV data for coral mapping. Geocarto Int. 2021, 37, 4527–4556. [Google Scholar] [CrossRef]
- Safuan, C.D.M.; Ismail, K.; Khalil, I.; Ali, A.; Chong, W.S.; Chan, A.A.; Ismail, M.N.; Repin, I.M.; Bachok, Z. Quantification of coral reef benthos for coral health assessment in Labuan Marine Park, Malaysia. J. Sustain. Sci. Manag. 2018, 13, 101–112. [Google Scholar]
- Zaki, N.H.M.; Chong, W.S.; Muslim, A.M.; Reba, M.N.M.; Hossain, M.S. Assessing optimal UAV-data pre-processing workflows for quality ortho-image generation to support coral reef mapping. Geocarto Int. 2022, 37, 1–25. [Google Scholar] [CrossRef]
- McGrath, E. Demography and Impacts of Habitat Degradation on the Giant Barrel Sponge Xestospongia spp. in the Indo-Pacific. Doctoral Thesis, Victoria University of Wellington, Wellington, New Zealand, 2018. [Google Scholar]
- Schönberg, C.H.L. No taxonomy needed: Sponge functional morphologies inform about environmental conditions. Ecol. Indic. 2021, 129, 107806. [Google Scholar] [CrossRef]
- Marty, M.J.; Vicente, J.; Oyler, B.L.; Place, A.; Hill, R.T. Sponge symbioses between Xestospongia deweerdtae and Plakortis spp. are not motivated by shared chemical defense against predators. PLoS ONE 2017, 12, e0174816. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, J.H.; Lee, H.K. Microbial Symbiosis in Marine Sponges. J. Microbiol. 2001, 39, 254–264. [Google Scholar]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-Associated Microorganisms: Evolution, Ecology, and Biotechnological Potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef]
- Wulff, J.L. Rapid diversity and abundance decline in a Caribbean coral reef sponge community. Biol. Conserv. 2006, 127, 167–176. [Google Scholar] [CrossRef]
- Wulff, J.L. Disease prevalence and population density over time in three common Caribbean coral reef sponge species. J. Mar. Biol. Assoc. U. K. 2007, 87, 1715–1720. [Google Scholar] [CrossRef]
- Cowart, J.D.; Henkel, T.P.; McMurray, S.E.; Pawlik, J.R. Sponge orange band (SOB): A pathogenic-like condition of the giant barrel sponge, Xestospongia muta. Coral Reefs 2006, 25, 513. [Google Scholar] [CrossRef]
- Angermeier, H.; Kamke, J.; Abdelmohsen, U.R.; Krohne, G.; Pawlik, J.R.; Lindquist, N.L.; Hentschel, U. The pathology of sponge orange band disease affecting the Caribbean barrel sponge Xestospongia muta. FEMS Microbiol. Ecol. 2011, 75, 218–230. [Google Scholar] [CrossRef]
- Garcia-Hernandez, J.E.; Tuohy, E.; Toledo-Rodríguez, D.A.; Sherman, C.; Schizas, N.V.; Weil, E. Detrimental conditions affecting Xestospongia muta across shallow and mesophotic coral reefs off the southwest coast of Puerto Rico. Dis. Aquat. Organ. 2021, 147, 47–61. [Google Scholar] [CrossRef]
- López-Legentil, S.; Song, B.; Mcmurray, S.E.; Pawlik, J.R. Bleaching and stress in coral reef ecosystems: hsp70 expression by the giant barrel sponge Xestospongia muta. Mol. Ecol. 2008, 17, 1840–1849. [Google Scholar] [CrossRef]
- Keumala, S.; Illahi, G.F.; Sakinah, R.; Razi, N.M.; Khairunnisa, K.; Kurnianda, V. Bioactivity of Indonesian’s Marine Sponge Xestospongia muta as Antidormant Mycobacterium smegmatis. Med. Chem. 2018, 8, 9. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Ueoka, R.; Yamanokuchi, R.; Horiuchi, N.; Ikeda, T.; Rotinsulu, H.; Mangindaan, R.E.P.; Ukai, K.; Kobayashi, H.; Namikoshi, M.; et al. Isolation of salsolinol, a tetrahydroisoquinoline alkaloid, from the marine sponge Xestospongia cf. vansoesti as a proteasome inhibitor. Chem. Pharm. Bull. 2011, 59, 287–290. [Google Scholar] [CrossRef]
- Abdillah, S.; Nurhayati, A.P.D.; Nurhatika, S.; Setiawan, E.; Heffen, W.L. Cytotoxic and antioxidant activities of marine sponge diversity at Pecaron Bay Pasir Putih Situbondo East Java, Indonesia. J. Pharm. Res. 2013, 6, 685–689. [Google Scholar] [CrossRef]
- Rahmanisa, S.; Prajatelistia, E.; Wibowo, I.; Barlian, A. 3D Biosilica Scaffolds from Melophlus sarasinorum and Xestospongia testudinaria Indonesian Sponges are Biocompatible for Cell Growth and Differentiation of Human Wharton’s Jelly Mesenchymal Stem Cell in Bone Tissue Engineering. Indones. Biomed. J. 2022, 14, 382–392. [Google Scholar] [CrossRef]
- Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; Müller, W.E.G.; Van Soest, R.W.M. Four new bioactive manzamine-type alkaloids from the Philippine marine sponge Xestospongia ashmorica. J. Nat. Prod. 1996, 59, 1056–1060. [Google Scholar] [CrossRef]
- Quah, Y.; Mohd Ismail, N.I.; Ooi, J.L.S.; Affendi, Y.A.; Abd Manan, F.; Wong, F.C.; Chai, T.T. Identification of Novel Cytotoxic Peptide KENPVLSLVNGMF from Marine Sponge Xestospongia testudinaria, with Characterization of Stability in Human Serum. Int. J. Pept. Res. Ther. 2018, 24, 189–199. [Google Scholar] [CrossRef]
- Azemi, N.A.; Azemi, A.K.; Abu-Bakar, L.; Sevakumaran, V.; Muhammad, T.S.T.; Ismail, N. Xestospongia muta Fraction-7 and Linoleic Acid: Effects on SR-BI Gene Expression and HDL Cholesterol Uptake. Mar. Drugs 2022, 20, 762. [Google Scholar] [CrossRef]
- Qaralleh, H.; Idid, S.; Saad, S.; Susanti, D.; Taher, M.; Khleifat, K. Antifungal and Antibacterial Activities of Four Malaysian Sponge Species (Petrosiidae). J. Mycol. Med. 2010, 20, 315–320. [Google Scholar] [CrossRef]
- Putchakarn, S. Species diversity of marine sponges along Chanthaburi and Trat Provinces, the eastern coast of the Gulf of Thailand. Publ. Seto Mar. Biol. Lab. 2011, 41, 17–23. [Google Scholar] [CrossRef]
- Azzini, F.; Calcinai, B.; Cerrano, C.; Bavestrello, G.; Pansini, M. Sponges of the marine karst lakes and of the coast of the islands of Ha Long Bay (North Vietnam). Porifera Res. Biodevers. Innovat. Sustain. 2007, 157–164. [Google Scholar]
- Nguyen, X.C.; Longeon, A.; Pham, V.C.; Urvois, F.; Bressy, C.; Van Trinh, T.T.; Nguyen, H.N.; Phan, V.K.; Chau, V.M.; Briand, J.-F.; et al. Antifouling 26,27-cyclosterols from the Vietnamese marine sponge Xestospongia testudinaria. J. Nat. Prod. 2013, 76, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A. Steroids from sponges: Recent reports. Steroids 1999, 64, 687–714. [Google Scholar] [CrossRef]
- Terracciano, S.; Aquino, M.; Rodriquez, M.; Chiara Monti, M.; Casapullo, A.; Riccio, R.; Gomez-Paloma, L. Chemistry and Biology of Anti-Inflammatory Marine Natural Products: Molecules Interfering with Cyclooxygenase, NF-kB and Other Unidentified Targets. Curr. Med. Chem. 2006, 13, 1947–1969. [Google Scholar] [CrossRef]
- Leone, P.D.A.; Carroll, A.R.; Towerzey, L.; King, G.; McArdle, B.M.; Kern, G.; Fisher, S.; Hooper, J.N.A.; Quinn, R.J. Exiguaquinol: A novel pentacyclic hydroquinone from Neopetrosia exigua that inhibits Helicobacter pylori MurI. Org. Lett. 2008, 10, 2585–2588. [Google Scholar] [CrossRef]
- Roll, D.M.; Scheuer, P.J.; Matsumoto, G.K.; Clardy, J. Halenaquinone, a Pentacyclic Polyketide from a Marine Sponge. J. Am. Chem. Soc. 1983, 105, 6177–6178. [Google Scholar] [CrossRef]
- Cerqueira, F.; Watanadilok, R.; Sonchaeng, P.; Kijjoa, A.; Pinto, M.; Van Ufford, H.Q.; Kroes, B.; Beukelman, C.; Nascimento, M.S.J. Clionasterol: A potent inhibitor of complement component C1. Planta Med. 2003, 69, 174–176. [Google Scholar] [CrossRef]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic acid inhibits prostate cancer cell proliferation and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef]
- De Araujo Junior, R.F.; Eich, C.; Jorquera, C.; Schomann, T.; Baldazzi, F.; Chan, A.B.; Cruz, L.J. Ceramide and palmitic acid inhibit macrophage-mediated epithelial–mesenchymal transition in colorectal cancer. Mol. Cell. Biochem. 2020, 468, 153–168. [Google Scholar] [CrossRef]
- Librán-Pérez, M.; Pereiro, P.; Figueras, A.; Novoa, B. Antiviral activity of palmitic acid via autophagic flux inhibition in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 95, 595–605. [Google Scholar] [CrossRef]
- Comendador, J.E.B. A barrel of sponges: A Xestospongia testudinaria species complex in the Verde Island Passage, Philippines. Master’s Thesis, San Francisco State University, San Francisco, CA, USA, 2020. [Google Scholar]
- Ashok, P.; Ganguly, S.; Murugesan, S. Manzamine alkaloids: Isolation, cytotoxicity, antimalarial activity and SAR studies. Drug Discov. Today 2014, 19, 1781–1791. [Google Scholar] [CrossRef]
- Ashok, P.; Lathiya, H.; Murugesan, S. Manzamine alkaloids as antileishmanial agents: A review. Eur. J. Med. Chem. 2015, 97, 928–936. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kaneko, M.; Okamura, H.; Nakatani, M.; van Soest, R.W.M.; Shiro, M. A new quinolizidine alkaloid from the Papua New Guinean sponge Xestospongia exigua. J. Nat. Prod. 2000, 63, 1310–1311. [Google Scholar] [CrossRef]
- Majali, I.; Qaralleh, H.N.; Idid, S.Z.; Saad, S.; Susanti, D.; Althunibat, O.Y. Potential Antimicrobial Activity of Marine Sponge Neopetrosia exigua. J. Basic Appl. Res. 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Nakagawa, M.; Endo, M.; Tanaka, N.; Gen-Pei, L. Structures of xestospongin A, B, C and D, novel vasodilativecompounds from marine sponge, xestospongia exigua. Tetrahedron Lett. 1984, 25, 3227–3230. [Google Scholar] [CrossRef]
- Orabi, K.Y.; El Sayed, K.A.; Hamann, M.T.; Chuck Dunbar, D.; Al-Said, M.S.; Higa, T.; Kelly, M. Araguspongines K and L, new bioactive bis-1-oxaquinolizidine N-oxide alkaloids from red sea specimens of Xestospongia exigua. J. Nat. Prod. 2002, 65, 1782–1785. [Google Scholar] [CrossRef]
- Brastianos, H.C.; Vottero, E.; Patrick, B.O.; Van Soest, R.; Matainaho, T.; Mauk, A.G.; Andersen, R.J. Exiguamine A, an indoleamine-2,3-dioxygenase (IDO) inhibitor isolated from the marine sponge Neopetrosia exigua. J. Am. Chem. Soc. 2006, 128, 16046–16047. [Google Scholar] [CrossRef]
- Williams, D.E.; Lassota, P.; Andersen, R.J. Motuporamines A−C, Cytotoxic Alkaloids Isolated from the Marine Sponge Xestospongia exigua (Kirkpatrick). J. Org. Chem. 1998, 63, 4838–4841. [Google Scholar] [CrossRef]
- Pereira, R.B.; Pereira, D.M.; Jiménez, C.; Rodríguez, J.; Nieto, R.M.; Videira, R.A.; Silva, O.; Andrade, P.B.; Valentaõ, P. Anti-inflammatory effects of 5α,8α-epidioxycholest-6-en-3β-ol, a steroidal endoperoxide isolated from aplysia depilans, based on bioguided fractionation and NMR analysis. Mar. Drugs 2019, 17, 330. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.A.; Hegel, J.K.E. Azelaic acid: Properties and mode of action. Skin Pharmacol. Physiol. 2013, 27, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Jebanesan, A.; Govindarajan, M.; Rajasekar, P. Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera:Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yuan, Y.; Lu, D.; Du, B.; Xiong, L.; Shi, J.; Yang, L.; Liu, W.; Yuan, X.; Zhang, G.; et al. Two natural products, trans-phytol and (22E)-ergosta-6,9,22-triene-3β,5α,8α-triol, inhibit the biosynthesis of estrogen in human ovarian granulosa cells by aromatase (CYP19). Toxicol. Appl. Pharmacol. 2014, 279, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: From bench to treatment. Pharmacol. Rev. 2005, 57, 359–383. [Google Scholar] [CrossRef]
- Costa, J.; Islam, M.; Santos, P.; Ferreira, P.; Oliveira, G.; Alencar, M.; Paz, M.; Ferreira, É.; Feitosa, C.; Citó, A.; et al. Evaluation of Antioxidant Activity of Phytol Using Non- and Pre-Clinical Models. Curr. Pharm. Biotechnol. 2016, 17, 1278–1284. [Google Scholar] [CrossRef]
- Marotta, L.L.C.; Almendro, V.; Marusyk, A.; Shipitsin, M.; Schemme, J.; Walker, S.R.; Bloushtain-Qimron, N.; Kim, J.J.; Choudhury, S.A.; Maruyama, R.; et al. The JAK2/STAT3 signaling pathway is required for growth of CD44 +CD24- stem cell-like breast cancer cells in human tumors. J. Clin. Investig. 2011, 121, 2723–2735. [Google Scholar] [CrossRef]
- To, N.B.; Nguyen, Y.T.K.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic acid, an odd-chain fatty acid, suppresses the stemness of MCF-7/SC human breast cancer stem-like cells through JAK2/STAT3 signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.D.; Meyer, J.J.M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Vanitha, V.; Vijayakumar, S.; Nilavukkarasi, M.; Punitha, V.N.; Vidhya, E.; Praseetha, P.K. Heneicosane—A novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind. Crops Prod. 2020, 154, 112748. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Il Hwang, D.; Kim, N.Y.; Kim, B.; Lee, H.M. 1-Iodohexadecane Alleviates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in Mice: Possible Involvements of the Skin Barrier and Mast Cell SNARE Proteins. Molecules 2022, 27, 1560. [Google Scholar] [CrossRef]
- Swantara, M.D.; Rita, W.S.; Suartha, N.; Agustina, K.K. Anticancer activities of toxic isolate of Xestospongia testudinaria sponge. Vet. World 2019, 12, 1434–1440. [Google Scholar] [CrossRef]
- Cita, Y.P.; Suhermanto, A.; Radjasa, O.K.; Sudharmono, P. Antibacterial activity of marine bacteria isolated from sponge Xestospongia testudinaria from Sorong, Papua. Asian Pac. J. Trop. Biomed. 2017, 7, 450–454. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Shaala, L.A.; Abbas, A.T.; Abdel-dayem, U.A.; Azhar, E.I.; Ali, S.S.; van Soest, R.W.M.; Youssef, D.T.A. Evaluation of the Anti-Inflammatory, Antioxidant and Immunomodulatory Effects of the Organic Extract of the Red Sea Marine Sponge Xestospongia testudinaria against Carrageenan Induced Rat Paw Inflammation. PLoS ONE 2015, 10, e0138917. [Google Scholar] [CrossRef]
- Swantara, I.M.D.; Rita, W.S. Toxicity of sponge extract Xestospongia testudinaria. Int. J. Eng. Sci. Invent. 2018, 7, 55–58. [Google Scholar]
- Zhou, C.; Yuan, K.; Tang, X.; Hu, N.; Peng, W. Molecular genetic evidence for polyandry in Ascaris suum. Parasitol. Res. 2011, 108, 703–708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).