Abstract
7DW8-5 is a potent glycolipid adjuvant that improves malaria vaccine efficacy in mice by inducing IFN-γ and increasing protective CD8+ T cell responses. The addition of 7DW8-5 was previously shown to improve the efficacy of a CD8+ T cell-mediated heterologous ‘prime-and-trap’ malaria vaccine against Plasmodium yoelii sporozoite challenge in inbred female mice. Here, we report significant differential sex-specific responses to 7DW8-5 in inbred and outbred mice. Male mice express significantly less IFN-γ and IL-4 compared to females following intravenous 7DW8-5 administration. Additionally, unlike in female mice, 7DW8-5 did not improve the vaccine efficacy against sporozoite challenge in prime-and-trap vaccinated male mice. Our findings highlight the importance of including both female and male sexes in experimental adjuvant studies.
1. Introduction
Adjuvants are compounds that are added to vaccines to enhance immune responses. Currently very few adjuvants have been licensed for clinical vaccination and the majority of these have a bias toward enhancing humoral and helper T cell immune responses (reviewed in [1]). There is a critical lack of approved adjuvants that enhance cytotoxic CD8+ T cell responses to vaccination [1]. Importantly, many pathogens, including the Plasmodium parasites that cause malaria, are best controlled by the host through mechanisms that include cytotoxic CD8+ cell responses [2]. Thus, more adjuvants, and especially those that improve cytotoxic CD8+ T cell responses, will likely be needed to improve the efficacy of vaccines targeting these complex pathogens.
Glycolipid adjuvants that stimulate iNKT cells are of increasing interest due to their ability to activate not only humoral immunity, but also cytotoxic CD8+ T cells (reviewed in [3]). α-Galactosylceramide (α-GalCer) is a glycolipid adjuvant that binds CD1d on antigen presenting cells (APCs), activates iNKT cells, and induces a cascade of immune cell activation that improves vaccine-induced immune responses [4,5,6]. α-GalCer has been studied for many years and clinical trials with this compound demonstrated safety and potent enhancement of immune responses in humans (reviewed in [7]). However, several clinical trials with α-GalCer have shown suboptimal results, which inspired the development of synthetic α-GalCer analogs. There are now hundreds of α-GalCer analogs that have been developed, which have different toxicological profiles and/or immunostimulatory effects [8].
7DW8-5 is a synthetic analog of α-GalCer that has significant translational potential. 7DW8-5 has been tested as an adjuvant and therapy for a variety of infectious diseases and cancer (reviewed in [9]). 7DW8-5 binds CD1d with higher affinity than α-GalCer and has shown higher efficacy at a 100-fold lower dose compared to α-GalCer in rodent and in vitro human cell models [10]. Importantly, 7DW8-5 increases the efficacy of live-attenuated malaria sporozoite (spz) vaccines by increasing CD8+ T cell responses [11,12]. However, the majority of pre-clinical rodent studies involving 7DW8-5 were completed in female inbred mice. Since substantial sex-specific differences in immune responses to adjuvants including α-GalCer have been noted [13,14], we sought to directly compare the impact of biological sex on 7DW8-5 adjuvant effects.
2. Materials & Methods
Mice: Male and female inbred BALB/cJ or Swiss outbred J:ARC(S) mice were purchased at 4–6 weeks of age from Jackson Laboratories (Barr Harbor, ME, USA). All animals were housed at the University of Washington Institutional Animal Care and Use Committee (IACUC)-approved animal facility and were used under an approved IACUC protocol (4317-01 to S.C.M.).
7DW8-5: 7DW8-5 was gifted from M. Tsuji and prepared as described [12]. All mice received 2 μg 7DW8-5 administered via intravenous (IV) or intradermal (ID) route. IV injections were administered retro-orbitally in 100 µL with an Exel International Insulin Syringes with a 29 G permanently attached needle (product #26018). ID injections were administered on the lower back near the base of the tail in two 10 µL injections with a BD Veo Insulin Syringe with Ultra-Fine needle 6 mm × 31 G 3/10 mL/cc (product #324909).
ELISA: Plasma was isolated from mice and IFN-γ and IL-4 cytokines were measured with commercially available ELISA kits (BioLegend, San Diego, CA, USA) as previously described [12]. All plasma was frozen at −80 °C in single-use aliquots.
Plasmodium yoelii DNA prime-and-RAS trap immunization and challenge: Prime-and-trap vaccines were prepared as previously described with minor modifications described below [12]. Briefly, a DNA insert encoding the full-length Plasmodium yoelii (Py) circumsporozoite protein (CSP) without the major repeat region (248aa: MKKCTILVVASLLLVDSLLPGYGQNKSVQAQRNLNELCYNEENDNKLYHVLNSKNGKIYNRNIVNRLLGDALNGKPEEKKDDPPKDGNKDDLPKEEKKDDLPKEEKKDDPPKDPKKDDPPKEAQNKLNQPVVADENVDQ|PRPQPDGNNNNNNNNGNNNEDSYVPSAEQILEFVKQISSQLTEEWSQCSVTCGSGVRVRKRKNVNKQPENLTLEDIDTEICKMDKCSSIFNIVSNSLGFVILLVLVFFN) was cloned into the pUb.3 vector, loaded onto gold beads, and administered to mice via gene gun (ggCSP). One month later, 2 × 104 total cryopreserved Py radiation attenuated sporozoites (cryo-RAS, Sanaria Inc., Rockville, MD, USA) with or without 7DW8-5 were administered ID into the rear left footpad in two 2.5 µL injections with a NanoFil syringe (World Precision Instruments, LLC, Sarasota, FL, USA). Cryo-RAS and 7DW8-5 were mixed immediately before injection. Animals were challenged IV via the retro-orbital route with 1 × 103 freshly dissected wild-type infectious Py spz (obtained from Seattle Children’s Research Institute, Seattle, WA, USA) and monitored for the presence of blood stage parasites by Giemsa stain thin blood smear microscopy for 14 days.
Statistics: ELISA data was analyzed with non-parametric Mann–Whitney tests. All error bars represent the standard deviation (SD) of the mean with individual mouse samples shown, if applicable. Protection data was analyzed with Fisher Exact test. p values above 0.05 were considered not significant (ns). GraphPad Prism 9.1.2 Software (San Diego, CA, USA) was used for all calculations.
3. Results
As a first step towards evaluating the impact of biological sex on 7DW8-5 adjuvant effects, we reviewed prior literature on 7DW8-5 and searched for information on research subject sex. A total of 17 papers were identified by searching “7DW8-5” on PubMed®. Two papers were excluded -- one contained no animal experiments [15] and one was a review article with limited primary research data [16]. Of the 15 remaining papers, 13 used 7DW8-5 in rodent models, one used 7DW8-5 in non-human primates (NHP), and one used 7DW8-5 with human ex vivo samples (Table 1). The single study in NHPs used only male animals, while only 9 of the 13 papers in rodent models listed the sex of the animals, and all of these used female mice (Table 1). We also noted that these studies of 7DW8-5 in rodent models were also exclusively performed in inbred mouse models. Thus, we concluded there was a need for further evaluation of the impact of biological sex on 7DW8-5 adjuvant effects in pre-clinical research models.

Table 1.
Research subject sex in published research articles with 7DW8-5.
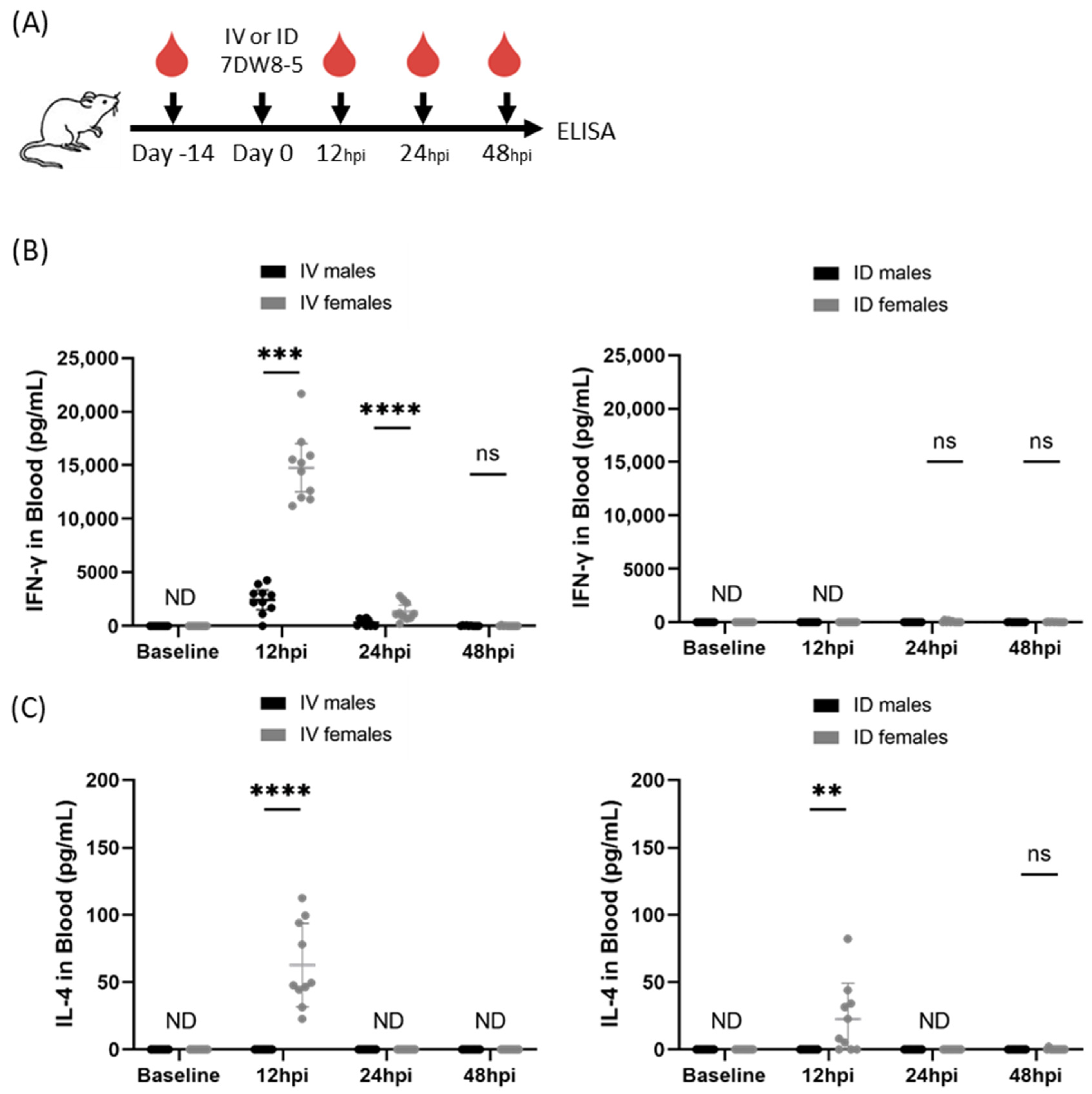
We previously reported that IV administration of 2 μg 7DW8-5 induced potent transient levels of IFN-γ cytokines in female inbred BALB/cJ mouse blood with strong responses peaking at 12 h and declining by 24 h [12]. To assess the impact of biological sex on this phenomenon, we repeated the same experiment in male BALB/cJ mice and found an ~6-fold reduction in IFN-γ induced by IV 7DW8-5 administration in male mice compared to female mice at the 12 h timepoint (Figure 1). 7DW8-5 is known to preferentially induce Th1 cytokines (e.g., IFN-γ), but we have also shown that detectable levels of Th2 cytokines (e.g., IL-4) are also induced by IV 7DW8-5 administration with similar expression kinetics [12]. Since 7DW8-5 is also known to induce IL-4 production, we also assessed IL-4 levels in the same mice. We found that there was no detectable IL-4 induced by IV 7DW8-5 administration in male mice, but we did detect IL-4 in female mice at the 12 h timepoint (Figure 1). Thus, male and female BALB/cJ mice show striking sex-specific differences in the levels of these plasma cytokines in response to IV 7DW8-5 administration.
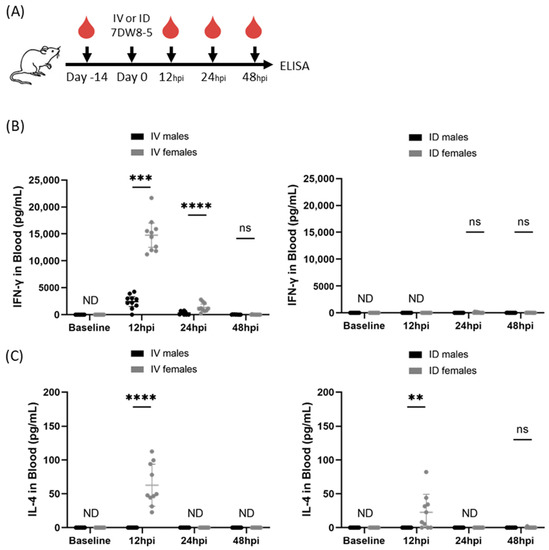
Figure 1.
7DW8-5 induces higher levels of systemic IFN-γ and IL-4 in female vs. male BALB/cJ mice. (A) Experimental design of blood plasma ELISA studies. IFN-γ (B) or IL-4 (C) cytokine levels induced by IV-7DW8-5 (left) or ID-7DW8-5 (right) in mouse blood plasma. Female IV data adapted from Watson et al. for comparison [12]. Error bars represent the SD of the mean of N = 10 BALB/cJ mice across two independent experiments. ELISA data analyzed with Mann–Whitney Tests, **** p < 0.0001, *** p < 0.001, ** p < 0.01, ns p > 0.05. ND = Not detected. ID = two 10 μL injections for all.
Non-systemic administration routes are also of interest. This is because non-IV administration of 7DW8-5 induced little to no systemic cytokine responses in female mice [11], which may be expected to translate to a better safety profile for clinical use. Therefore, we next evaluated the impact of sex on ID 7DW8-5 administration in mice. We administered 2 µg 7DW8-5 ID to male and female BALB/cJ mice and assessed IFN-γ and IL-4 by ELISA as above. As expected, the ID route induced significantly lower levels of these cytokines, but female mice still had higher levels than males at the 12 h timepoint (Figure 1). Thus, male and female BALB/cJ mice also show sex-specific differences in the levels of these plasma cytokines in response to 7DW8-5 non-systemic ID administration.
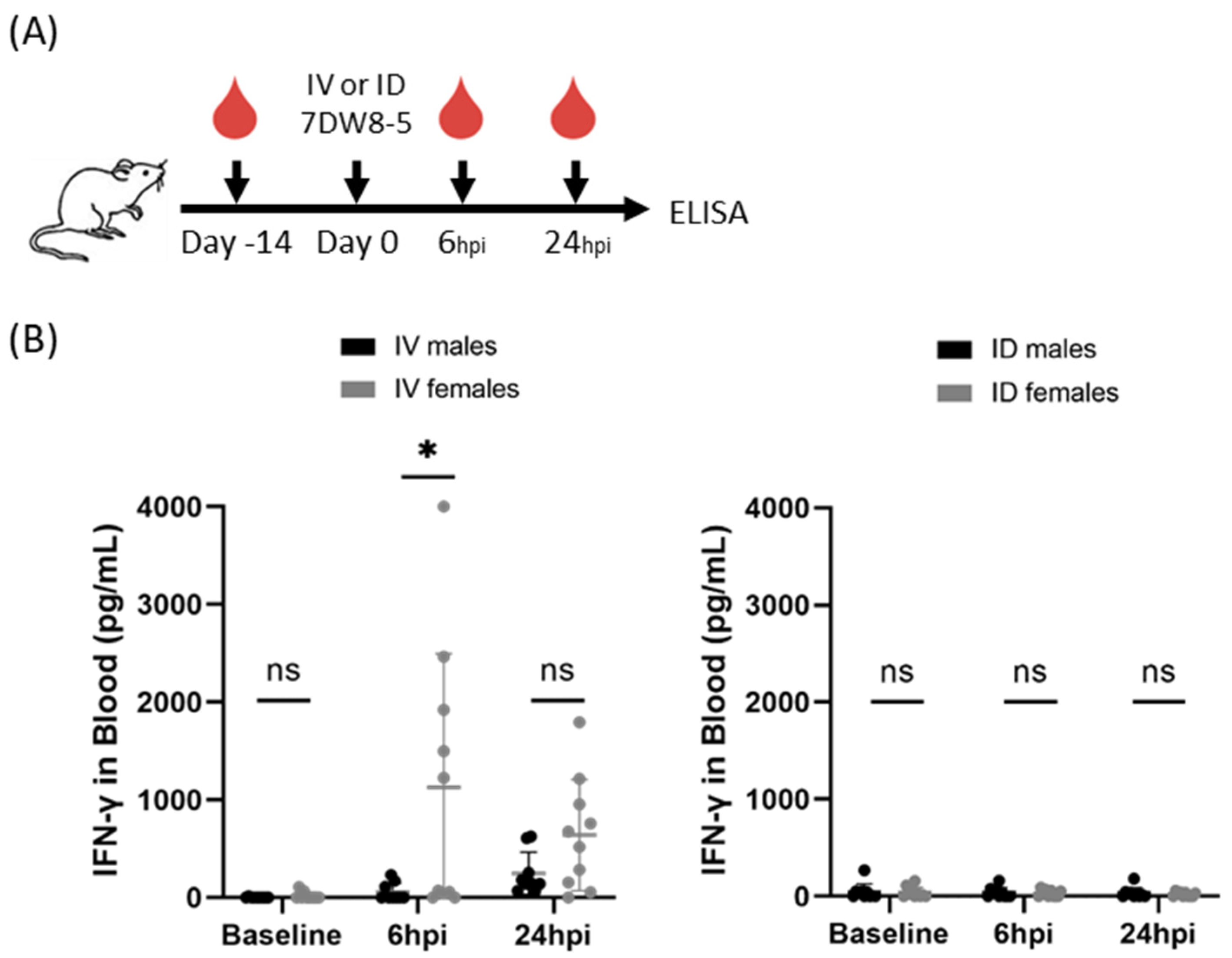
As it was possible that these findings were a phenomenon of the BALB/cJ mouse model, we further wanted to investigate if this sex-specific phenotype was apparent in another mouse model. To assess whether these findings were generalizable to outbred mouse strains, we next compared IFN-γ induction following systemic IV or local ID administration of 7DW8-5 in Swiss outbred mice. As seen in the inbred BALB/cJ mice, we found higher levels of this cytokine in females compared to male mice following IV 7DW8-5 administration at 6 h post-injection (Figure 2). As expected, only low levels of IFN-γ were induced by ID 7DW8-5 administration in these mice. Thus, male and female genetically diverse Swiss outbred mice also showed sex-specific differences in the levels of plasma IFN-γ in response to 7DW8-5.
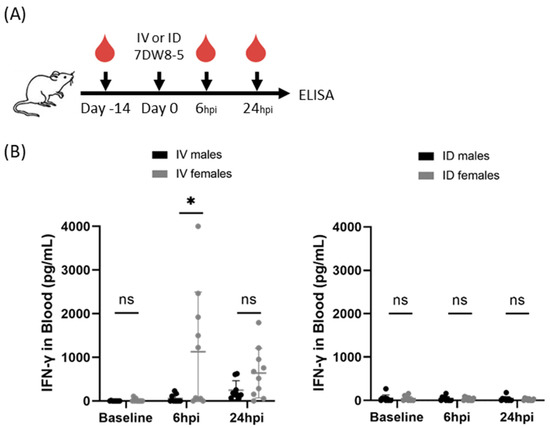
Figure 2.
7DW8-5 induces higher levels of systemic IFN-γ in female vs. male Swiss outbred mice. (A) Experimental design of blood plasma ELISA studies. (B) IFN-γ cytokine levels induced by IV-7DW8-5 (left) or ID-7DW8-5 (right) in mouse blood plasma. Error bars represent the SD of the mean of N = 9–10 Swiss outbred mice across two independent experiments. ELISA data analyzed with Mann–Whitney Tests, * p < 0.05, ns p > 0.05. ID = two 10 μL injections for all.
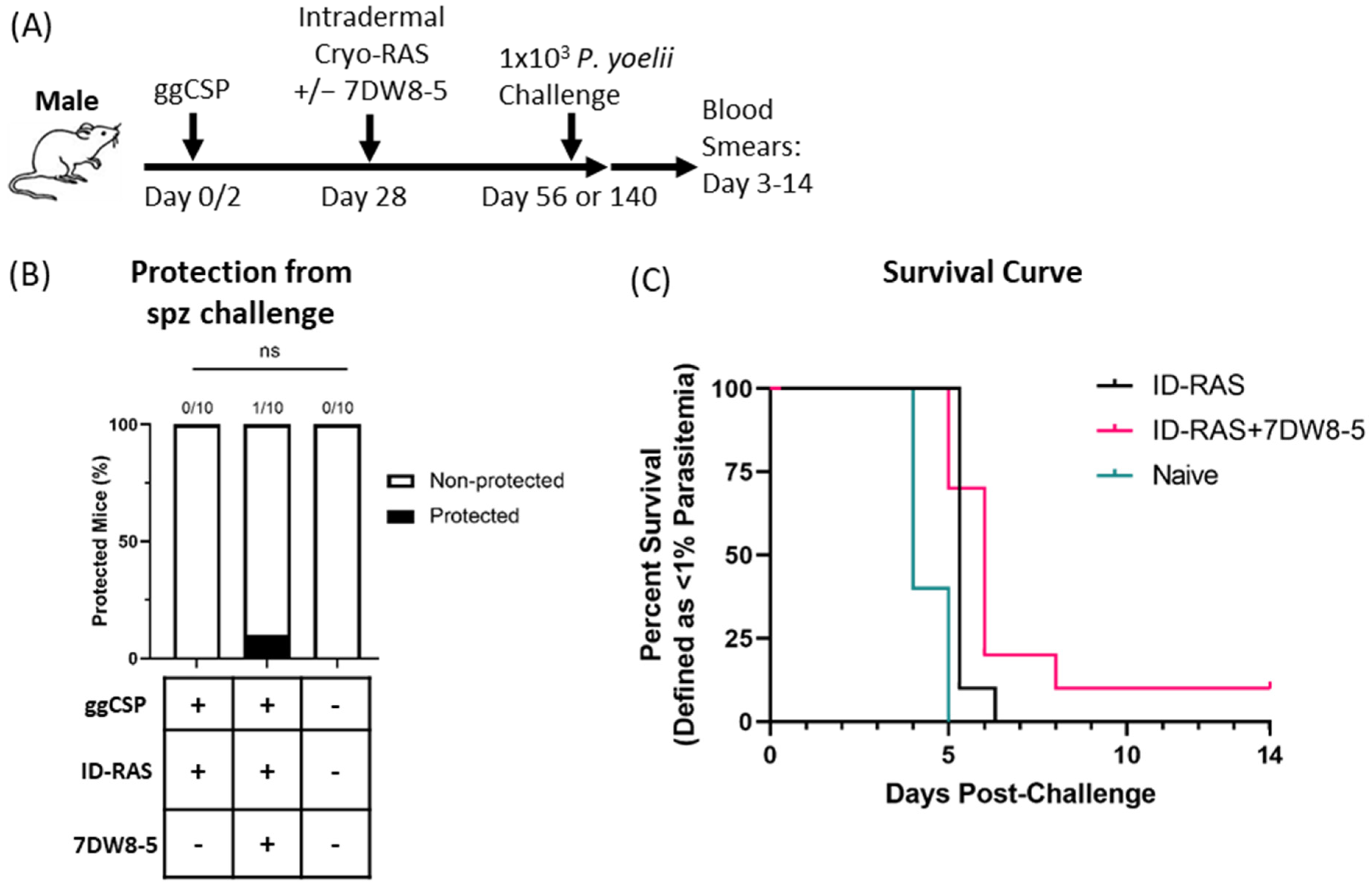
Lastly, we wanted to determine if 7DW8-5 would be an effective adjuvant in male BALB/cJ mice. Previous literature has demonstrated that live RAS malaria vaccines are improved by the co-administration of 7DW8-5 in female mice [11]. Additionally, our group also demonstrated that 7DW8-5 improved a heterologous two step DNA-prime-and-RAS trap malaria vaccine strategy in female BALB/cJ mice [12]. Moreover, recent data from our group found that 7DW8-5 co-administration with ID-RAS was required to achieve 100% protection in female BALB/cJ mice (In preparation and [29]). Here, we evaluated the efficacy of the same DNA-prime and 7DW8-5-adjuvanted ID-RAS trap vaccine in male BALB/cJ mice. Strikingly, we found that 7DW8-5 did not significantly improve vaccine efficacy, with poor protection seen following DNA-prime and ID-RAS trap groups both with and without 7DW8-5 (Figure 3). Thus, the adjuvant effects of 7DW8-5 are drastically different in male and female mice when used in this malaria vaccine strategy.
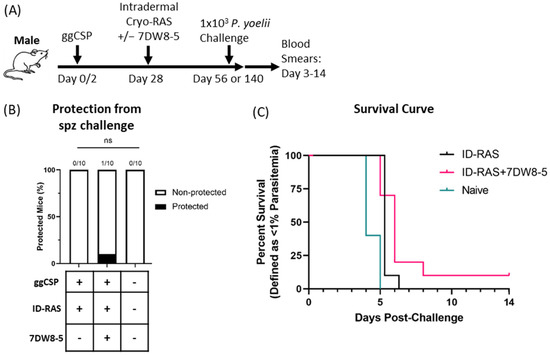
Figure 3.
Prime-and-trap vaccination is not protective in male BALB/cJ mice. (A) Experimental design of prime-and-trap protection studies. (B) Results of protection studies after challenge with 1 × 103 WT purified Py spz administered four weeks after trapping with 2 × 104 ID administered cryo-RAS +/− 7DW8-5. Protection data was from N = 10 male BALB/cJ mice across two independent experiments and analyzed with Fisher Exact Test, ns p > 0.05. ID = two 2.5 μL injections for all. (C) Survival curve of mice from (B).
4. Discussion
Novel adjuvants are critically needed for the clinic. Glycolipids are potent adjuvants that have been shown to have potent immunostimulatory effects, including the ability to induce cytotoxic CD8+ T cell responses [3]. As such, they are of increasing interest for use in vaccines targeting complex pathogens like Plasmodium. 7DW8-5 is of particular interest due its ability to activate iNKT cells and induce Th1-like responses, including the activation of CD8+ T cells, and to do so at lower doses than α-GalCer [10]. Previous reports in rodent models only examined adjuvant effects of 7DW8-5 in female inbred mice. However, since significant biological sex-based differences have been noted for α-GalCer [13,14], here we sought to investigate if 7DW8-5 similarly induced different adjuvant effects in male and female mice. We found that IFN-γ was induced by IV 7DW8-5 administration in both inbred and outbred mice, but the cytokine levels were significantly lower in males compared to females. Additionally, we found that unlike in females, 7DW8-5 did not improve the efficacy of a DNA prime-and-RAS trap malaria vaccine in male BALB/cJ mice. Taken together, we demonstrate that 7DW8-5 adjuvant effects can be drastically different in male and female mice.
Our findings are supported by other studies that found significant sex-specific differences in the iNKT immune responses of inbred C57BL/6 mice [13,14]. Although both reports concluded that IFN-γ cytokine levels induced by glycolipids were higher in female mice than male mice, these prior studies proposed different mechanisms and contributions from sex hormones. Gourdy et al. suggested that estrogens but not testosterone were responsible for the differential immune responses [13], while Lotter et al. suggested that testosterone moderated cytokine production of iNKT cells in mice [14]. Based on this data, we hypothesize that in our model, high testosterone in male mice inhibits the induction of IFN-γ following 7DW8-5 administration. Future efforts in the laboratory will seek to investigate the role of estrogens and androgens in mouse models of prime-and-trap malaria vaccination. A limitation of this study is that we did not account for weight differences between male and female mice. On average, six-week-old BALB/cJ male mice weigh more than age matched female mice (male: 23.7 g SD 1.4 vs. female: 19.1 g SD 1.2) [30]. Thus, female mice are receiving a higher dose (mg/kg) of adjuvant. It is possible that a weight normalized adjuvant dose would minimize the differences in cytokine induction that we observed. We also noted that the experiments in Swiss outbred mice resulted in greater variation of the data, which was not unexpected since outbred mice are more genetically diverse and may better represent how diverse human populations may respond to adjuvants. As a future pre-clinical step, our understanding of 7DW8-5 will also greatly benefit from studies using NHP models of malaria cryo-RAS vaccination and challenge.
In 2016, the NIH introduced a policy mandating inclusion of sex as a biological variable in all vertebrate animals and human studies [31,32,33]. Our findings here indeed highlight the importance of including both biological sexes in experimental adjuvant studies, documenting sex-specific data in publications, and considering how biological sex may impact pre-clinical and clinical outcomes. Strengthened efforts to investigate sex as a biological variable in pre-clinical research studies may be especially important for translating research findings from rodent models to larger NHP models and ultimately to humans.
Author Contributions
F.N.W., M.J.S. and S.C.M. conceived and supervised the study. F.N.W., C.J.D., A.C.K. and E.C. performed the experiments. S.C., B.K.L.S., S.L.H. and M.T. provided key resources, analysis, and discussion. All the authors discussed the results, wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NIH grant 1R01AI141857 to S.C.M. and NIH Diversity Supplement funding 04S1 and NIH Fellowship 1F31AI169754-01 to F.N.W.
Institutional Review Board Statement
All animals were housed at the University of Washington Institutional Animal Care and Use Committee (IACUC)-approved animal facility and were used under an approved IACUC protocol (4317-01 to S.C.M.).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable, all data is contained within the article.
Acknowledgments
We thank Moriya Tsuji for gifting the 7DW8-5 adjuvant. We thank Tess Seltzer, Veronica Primavera, Cecilia Kalthoff, and Alexis Kaushansky (Seattle Children’s Research Institute) for assistance and support of Plasmodium yoelii–infected mosquito production and Sanaria, Inc. for the cryopreserved irradiated P. yoelii sporozoites. We also thank the veterinary staff of the UW Department of Comparative Medicine.
Conflicts of Interest
S.C.M. filed a patent application on selected aspects of the prime-and-trap concept through the University of Washington. S.C.M. has equity in a startup company (Sound Vaccines, Inc.) that is negotiating with the University of Washington for rights to this intellectual property. The relationship between the authors and Sound Vaccines, Inc., has been reviewed by the University of Washington and complies with all University and State of Washington policies on such activities. S.C., B.K.L.S. and S.L.H. are paid employees of Sanaria Inc. M.T., S.C., and S.L.H. are inventors on two patents related to 7DW8-5 and Plasmodium SPZ, both of which are assigned in part to Sanaria Inc.—(1) Title: Pharmaceutical Compositions Comprising Attenuated Sporozoites and Glycolipid Adjuvants. Inventors: Chakravarty, Hoffman, Tsuji. Date of Filing: 28 October 2013, Date of Issue: 8 March 2016. US Patent Issue Number: 9,278,125; (2) Title: Pharmaceutical Compositions Comprising Attenuated Sporozoites and Glycolipid Adjuvants (methods of use). Inventors: Chakravarty, Hoffman, Tsuji. Date of Filing: 18 Feb 2016, Date of Issue: 9 May 2017. US Patent Issue Number: 9,642,909. MT is also an inventor of four patients related to 7DW8-5, all of which are assigned to Rockefeller University. (1) Glycolipids and analogues thereof as antigens for NK T cells. Date of Issue: 19 May 19 2009. US Patent Issue Number: 7,534,434; (2) Glycolipids and analogues thereof as antigens for NK T cells. Date of Issue: 12 April 2011. US Patent Issue Number: 7,923,013; (3) Glycolipids and analogues thereof as antigens for NK T cells. Date of Issue: 24 April 2012. US Patent Issue Number: 8,163,290 B2; (4) Glycolipids and analogues thereof as antigens for NK T cells. Date of Issue: 19 November 2013. US Patent Issue Number: 8,586,051. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.R.; Sedegah, M.; Beaudoin, R.L.; Miller, L.H.; Good, M.F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. USA 1988, 85, 573–576. [Google Scholar] [CrossRef]
- Fujii, S.; Motohashi, S.; Shimizu, K.; Nakayama, T.; Yoshiga, Y.; Taniguchi, M. Adjuvant activity mediated by iNKT cells. Semin. Immunol. 2010, 22, 97–102. [Google Scholar] [CrossRef]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Motoki, K.; Taniguchi, M. CD1d-restricted and TCR-mediated activation of v-alpha-14 NKT cells by glycosylceramides. Science 1997, 278, 1626–1629. [Google Scholar] [CrossRef]
- Kitamura, H.; Iwakabe, K.; Yahata, T.; Nishimura, S.; Ohta, A.; Ohmi, Y.; Nishimura, T. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 1999, 189, 1121–1128. [Google Scholar] [CrossRef]
- Fujii, S.; Shimizu, K.; Smith, C.; Bonifaz, L.; Steinman, R.M. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 2003, 198, 267–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Springfield, R.; Chen, S.; Li, X.; Feng, X.; Moshirian, R.; Yuan, W. alpha-GalCer and iNKT Cell-Based Cancer Immunotherapy: Realizing the Therapeutic Potentials. Front. Immunol. 2019, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-T.; Huang, J.-R.; Yu, A.L. Tailored design of NKT-stimulatory glycolipids for polarization of immune responses. J. Biomed. Sci. 2017, 24, 22. [Google Scholar] [CrossRef]
- Coelho-Dos-Reis, J.G.; Li, X.; Tsuji, M. Development of a novel mechanism-based glycolipid adjuvant for vaccination. F1000Research 2018, 7, 676. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fujio, M.; Imamura, M.; Wu, D.; Vasan, S.; Wong, C.H.; Ho, D.D.; Tsuji, M. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc. Natl. Acad. Sci. USA 2010, 107, 13010–13015. [Google Scholar] [CrossRef]
- Li, X.; Kawamura, A.; Andrews, C.D.; Miller, J.L.; Wu, D.; Tsao, T.; Tsuji, M. Colocalization of a CD1d-Binding Glycolipid with a Radiation-Attenuated Sporozoite Vaccine in Lymph Node-Resident Dendritic Cells for a Robust Adjuvant Effect. J. Immunol. 2015, 195, 2710–2721. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.; Shears, M.; Matsubara, J.; Kalata, A.; Seilie, A.; Talavera, I.C.; Murphy, S.C. Cryopreserved Sporozoites with and without the Glycolipid Adjuvant 7DW8-5 Protect in Prime-and-Trap Malaria Vaccination. Am. J. Trop. Med. Hyg. 2022, 106, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Gourdy, P.; Araujo, L.M.; Zhu, R.; Garmy-Susini, B.; Diem, S.; Laurell, H.; Herbelin, A. Relevance of sexual dimorphism to regulatory T cells: Estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood 2005, 105, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Lotter, H.; Helk, E.; Bernin, H.; Jacobs, T.; Prehn, C.; Adamski, J.; Tannich, E. Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFN-gamma secretion in natural killer T cells. PLoS ONE 2013, 8, e55694. [Google Scholar] [CrossRef]
- Panza, L.; Compostella, F.; Imperio, D. A versatile synthesis of alpha-GalCer and its analogues exploiting a cyclic carbonate as phytosphingosine 3,4-diol protecting group. Carbohydr. Res. 2019, 472, 50–57. [Google Scholar] [CrossRef]
- Padte, N.N.; Li, X.; Tsuji, M.; Vasan, S. Clinical development of a novel CD1d-binding NKT cell ligand as a vaccine adjuvant. Clin. Immunol. 2011, 140, 142–151. [Google Scholar] [CrossRef]
- Feng, H.; Sun, R.; Song, G.; Zhu, S.; Nie, Z.; Lin, L.; Shu, J. A Glycolipid alpha-GalCer Derivative, 7DW8-5 as a Novel Mucosal Adjuvant for the Split Inactivated Influenza Vaccine. Viruses 2022, 14, 1174. [Google Scholar] [CrossRef]
- Feng, H.; Nakajima, N.; Wu, L.; Yamashita, M.; Lopes, T.J.S.; Tsuji, M.; Kawaoka, Y. A Glycolipid Adjuvant, 7DW8-5, Enhances the Protective Immune Response to the Current Split Influenza Vaccine in Mice. Front. Microbiol. 2019, 10, 2157. [Google Scholar] [CrossRef]
- Lang, G.A.; Ballard-Norman, K.; Amadou Amani, S.; Shadid, T.M.; Ballard, J.D.; Lang, M.L. Use of a Clostridioides difficile Murine Immunization and Challenge Model to Evaluate Single and Combination Vaccine Adjuvants Consisting of Alum and NKT Cell-Activating Ligands. Front. Immunol. 2021, 12, 818734. [Google Scholar] [CrossRef]
- Seki, T.; Liu, J.; Brutkiewicz, R.R.; Tsuji, M. A Potent CD1d-binding Glycolipid for iNKT-Cell-based Therapy Against Human Breast Cancer. Anticancer Res. 2019, 39, 549–555. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Kawamura, A.; Funakoshi, R.; Porcelli, S.A.; Tsuji, M. Co-localization of a CD1d-binding glycolipid with an adenovirus-based malaria vaccine for a potent adjuvant effect. Vaccine 2017, 35, 3171–3177. [Google Scholar] [CrossRef]
- Venkataswamy, M.M.; Ng, T.W.; Kharkwal, S.S.; Carreno, L.J.; Johnson, A.J.; Kunnath-Velayudhan, S.; Porcelli, S.A. Improving Mycobacterium bovis Bacillus Calmette-Guerin as a vaccine delivery vector for viral antigens by incorporation of glycolipid activators of NKT cells. PLoS ONE 2014, 9, e108383. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hegazy, W.A.; Guo, L.; Gao, X.; Courtney, A.N.; Kurbanov, S.; Liu, D.; Tian, G.; Manuel, E.R.; Diamond, D.J.; et al. Effective cancer vaccine platform based on attenuated salmonella and a type III secretion system. Cancer Res. 2014, 74, 6260–6270. [Google Scholar] [CrossRef]
- Lee, C.; Hong, S.N.; Kim, Y.H. A glycolipid adjuvant, 7DW8-5, provides a protective effect against colonic inflammation in mice by the recruitment of CD1d-restricted natural killer T cells. Intest. Res. 2020, 18, 402–411. [Google Scholar] [CrossRef]
- Coelho-Dos-Reis, J.G.; Huang, J.; Tsao, T.; Pereira, F.V.; Funakoshi, R.; Nakajima, H.; Sugimiya, H.; Tsuji, M. Co-administration of alpha-GalCer analog and TLR4 agonist induces robust CD8+ T-cell responses to PyCS protein and WT-1 antigen and activates memory-like effector NKT cells. Clin. Immunol. 2016, 168, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, J.; Kaneko, I.; Zhang, M.; Iwanaga, S.; Yuda, M.; Tsuji, M. A potent adjuvant effect of a CD1d-binding NKT cell ligand in human immune system mice. Expert Rev. Vaccines 2017, 16, 73–80. [Google Scholar] [CrossRef]
- Padte, N.N.; Boente-Carrera, M.; Andrews, C.D.; McManus, J.; Grasperge, B.F.; Gettie, A.; Tsuji, M. A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS ONE 2013, 8, e78407. [Google Scholar] [CrossRef] [PubMed]
- Ghnewa, Y.G.; O’Reilly, V.P.; Vandenberghe, E.; Browne, P.V.; McElligott, A.M.; Doherty, D.G. Retinoic acid induction of CD1d expression primes chronic lymphocytic leukemia B cells for killing by CD8+ invariant natural killer T cells. Clin. Immunol. 2017, 183, 91–98. [Google Scholar] [CrossRef]
- Watson, F.; Kalata, A.; Shears, M.; Chakravarty, S.; Sim, B.; Hoffman, S. (Eds.) Intradermally delivered glycolipid-adjuvanted radiation-attenuated sporozoites are protective in Prime-and-Trap malaria vaccination. Presented at the American Society of Tropical Medicine and Hygiene Annual Meeting, Seattle, WA, USA, 30 October–3 November 2022. [Google Scholar]
- The Jackson Laboratory. Body Weight Information for BALB/CJ (000651). Available online: https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000651 (accessed on 10 November 2022).
- National Institute of Health. NOT-OD-15-102: Consideration of Sex as a Biological Variable in NIH-Funded Research. 2015. Available online: https://orwh.od.nih.gov/sites/orwh/files/docs/NOT-OD-15-102_Guidance.pdf (accessed on 8 November 2022).
- Miller, L.R.; Marks, C.; Becker, J.B.; Hurn, P.D.; Chen, W.J.; Woodruff, T.; Clayton, J.A. Considering sex as a biological variable in preclinical research. FASEB J. 2017, 31, 29–34. [Google Scholar] [CrossRef]
- Arnegard, M.E.; Whitten, L.A.; Hunter, C.; Clayton, J.A. Sex as a Biological Variable: A 5-Year Progress Report and Call to Action. J. Womens Health 2020, 29, 858–864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).