The Immunogenicity of a VLP-based Malaria Vaccine Targeting CSP in Pregnant and Neonatal Mice
Abstract
1. Introduction
2. Materials and Methods
3. Results
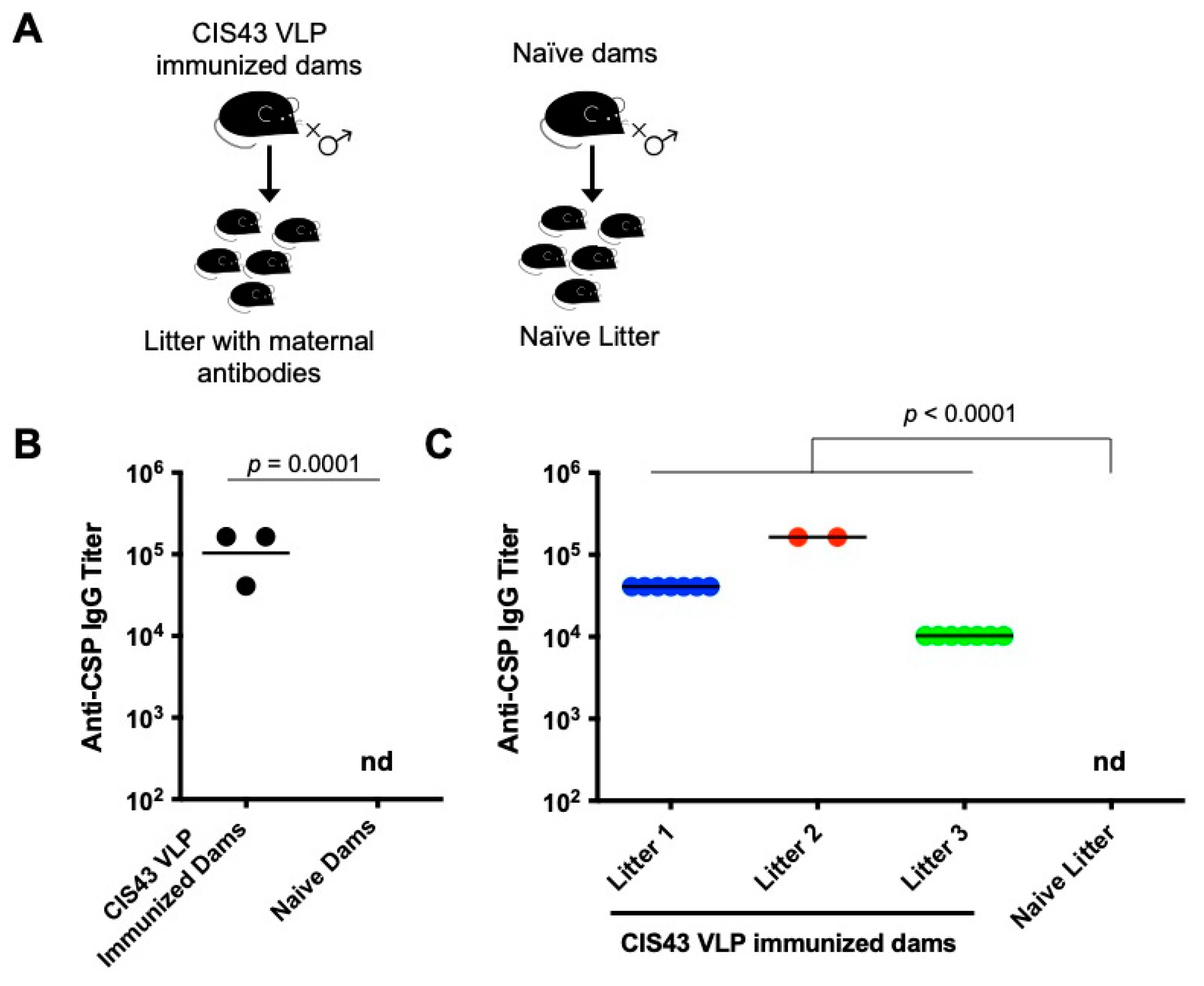
3.1. CIS43 VLP Immunization of Female Mice Elicits Anti-CSP IgG That is Transferred to Their Pups
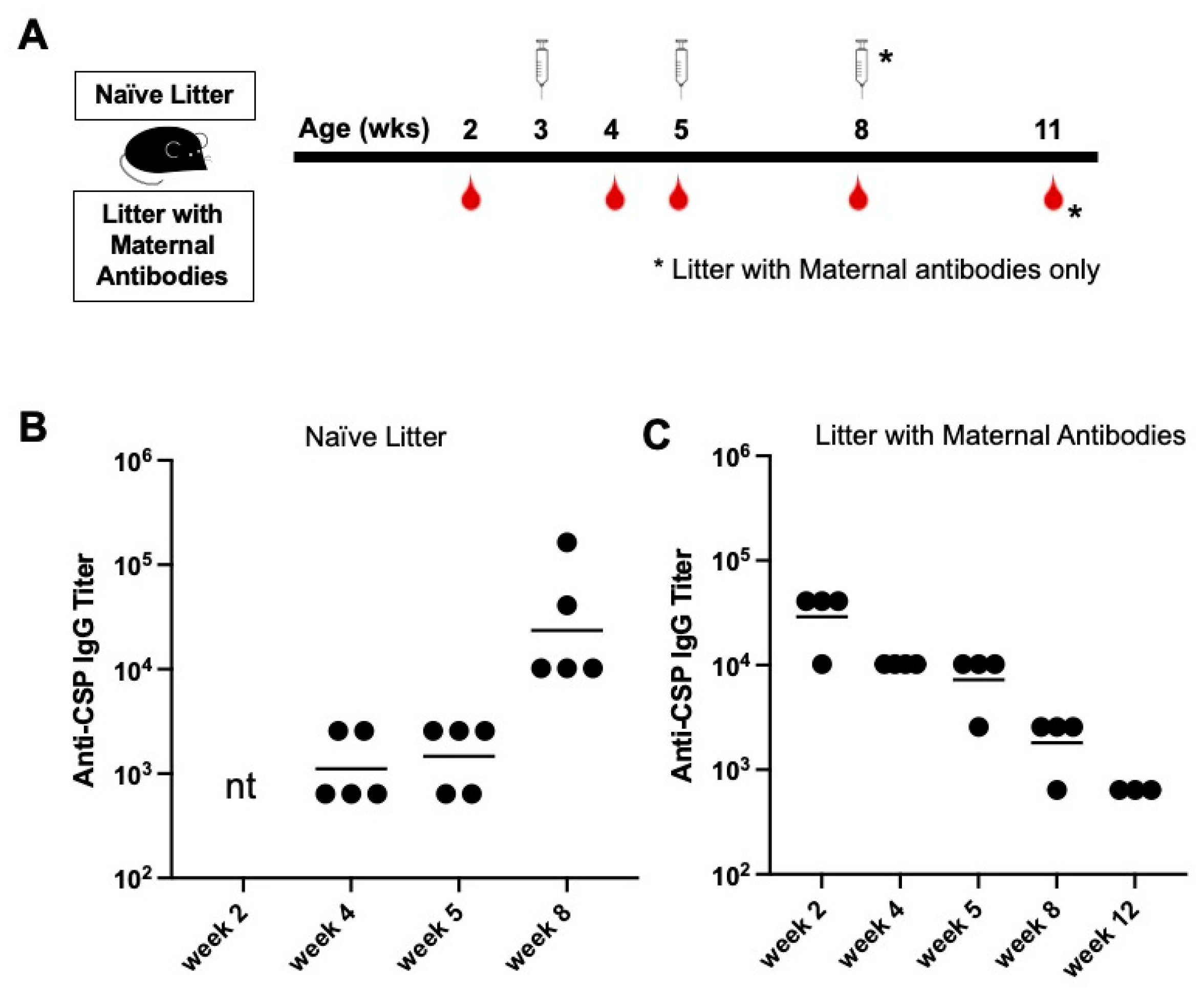
3.2. Young Mice with Anti-CSP Maternal Antibodies Fail to Respond to Immunization with CIS43 VLPs
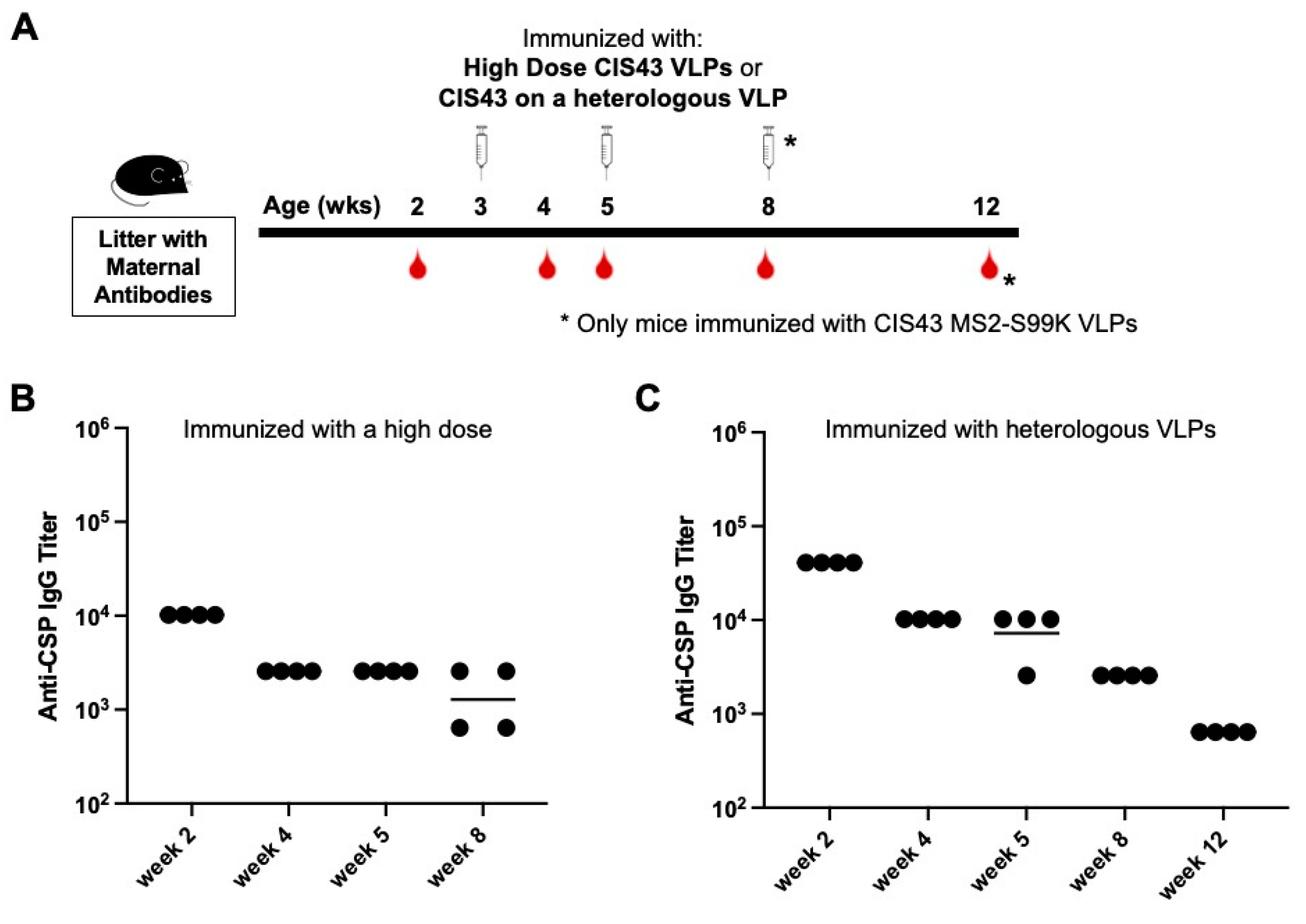
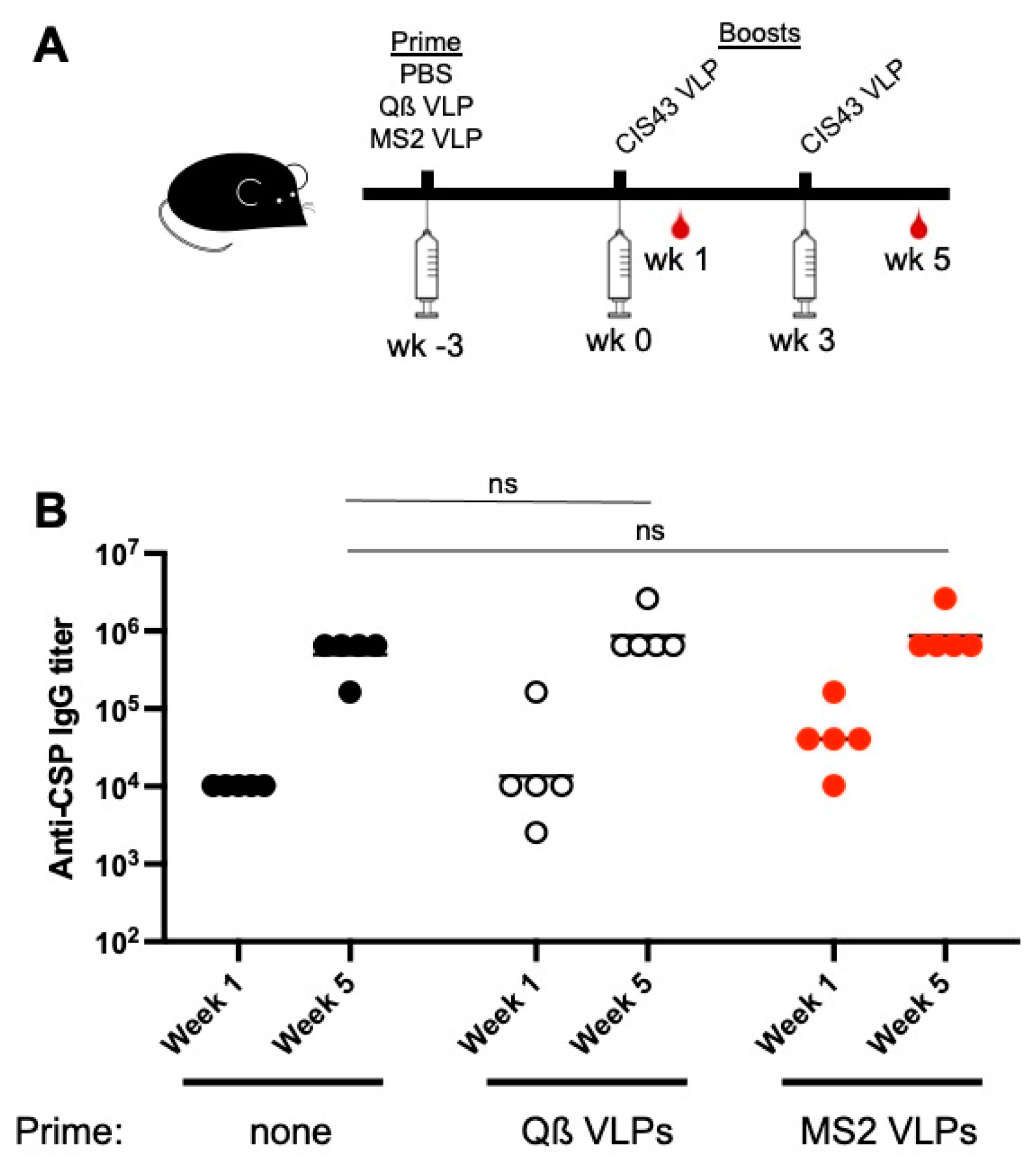
3.3. Neither Increased Dose nor Changing the VLP Platform Overcome Maternal Antibody-mediated Inhibition
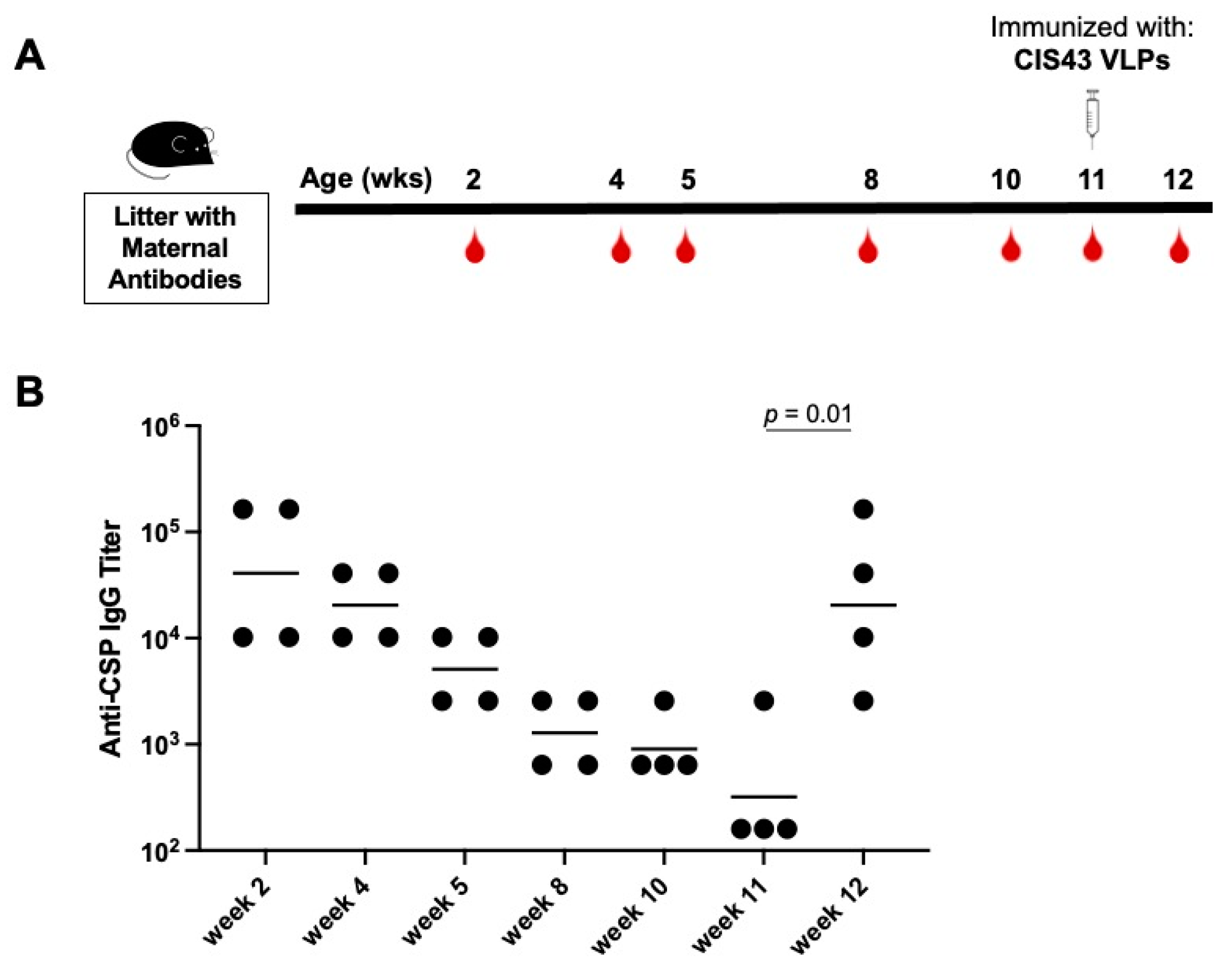
3.4. Delaying de novo Immunization Overcomes Vaccine Inhibition Mediated by Anti-CSP Maternal Antibodies
3.5. Pre-Existing Antibodies against the VLP Platform Do Not Inhibit Anti-CSP Responses in Adult Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, I.; Esposito, D.B.; Gracey, K.D.; Shapiro, E.D.; Vazquez, M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin. Infect. Dis. 2010, 51, 1355–1361. [Google Scholar] [CrossRef]
- Manske, J.M. Efficacy and effectiveness of maternal influenza vaccination during pregnancy: A review of the evidence. Matern. Child Health J. 2014, 18, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef]
- Patel, C.D.; Backes, I.M.; Taylor, S.A.; Jiang, Y.; Marchant, A.; Pesola, J.M.; Coen, D.M.; Knipe, D.M.; Ackerman, M.E.; Leib, D.A. Maternal immunization confers protection against neonatal herpes simplex mortality and behavioral morbidity. Sci. Transl. Med. 2019, 11, eaau6039. [Google Scholar] [CrossRef] [PubMed]
- Van Rie, A.; Wendelboe, A.M.; Englund, J.A. Role of maternal pertussis antibodies in infants. Pediatr. Infect. Dis. J. 2005, 24, S62–S65. [Google Scholar] [CrossRef]
- Healy, C.M.; Rench, M.A.; Baker, C.J. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin. Infect. Dis. 2013, 56, 539–544. [Google Scholar] [CrossRef]
- Villafana, T.; Falloon, J.; Griffin, M.P.; Zhu, Q.; Esser, M.T. Passive and active immunization against respiratory syncytial virus for the young and old. Expert Rev. Vaccines 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Edwards, K.M. Maternal antibodies and infant immune responses to vaccines. Vaccine 2015, 33, 6469–6472. [Google Scholar] [CrossRef]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.A.; Barrios, C.; Martinez, X.; Brandt, C.; Berney, M.; Cordova, M.; Kovarik, J.; Lambert, P.H. Influence of maternal antibodies on vaccine responses: Inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur. J. Immunol. 1998, 28, 4138–4148. [Google Scholar] [CrossRef]
- Siegrist, C.A.; Lambert, P.H. Maternal immunity and infant responses to immunization: Factors influencing infant responses. Dev. Biol. Stand. 1998, 95, 133–139. [Google Scholar] [PubMed]
- Jones, C.; Pollock, L.; Barnett, S.M.; Battersby, A.; Kampmann, B. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine 2014, 32, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Gans, H.; DeHovitz, R.; Forghani, B.; Beeler, J.; Maldonado, Y.; Arvin, A.M. Measles and mumps vaccination as a model to investigate the developing immune system: Passive and active immunity during the first year of life. Vaccine 2003, 21, 3398–3405. [Google Scholar] [CrossRef] [PubMed]
- Njie-Jobe, J.; Nyamweya, S.; Miles, D.J.; van der Sande, M.; Zaman, S.; Touray, E.; Hossin, S.; Adetifa, J.; Palmero, M.; Burl, S.; et al. Immunological impact of an additional early measles vaccine in Gambian children: Responses to a boost at 3 years. Vaccine 2012, 30, 2543–2550. [Google Scholar] [CrossRef]
- Whittle, H.; Hanlon, P.; O’Neill, K.; Hanlon, L.; Marsh, V.; Jupp, E.; Aaby, P. Trial of high-dose Edmonston-Zagreb measles vaccine in the Gambia: Antibody response and side-effects. Lancet 1988, 2, 811–814. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2021; WHO: Geneva, Switzerland, 2021; p. 322. [Google Scholar]
- Apinjoh, T.O.; Anchang-Kimbi, J.K.; Mugri, R.N.; Njua-Yafi, C.; Tata, R.B.; Chi, H.F.; Tangoh, D.A.; Loh, B.T.; Achidi, E.A. Determinants of infant susceptibility to malaria during the first year of life in South Western cameroon. Open Forum Infect. Dis. 2015, 2, ofv012. [Google Scholar] [CrossRef]
- Franks, S.; Koram, K.A.; Wagner, G.E.; Tetteh, K.; McGuinness, D.; Wheeler, J.G.; Nkrumah, F.; Ranford-Cartwright, L.; Riley, E.M. Frequent and persistent, asymptomatic Plasmodium falciparum infections in African infants, characterized by multilocus genotyping. J. Infect. Dis. 2001, 183, 796–804. [Google Scholar] [CrossRef]
- Riley, E.M.; Wagner, G.E.; Akanmori, B.D.; Koram, K.A. Do maternally acquired antibodies protect infants from malaria infection? Parasite Immunol. 2001, 23, 51–59. [Google Scholar] [CrossRef]
- Snow, R.W.; Nahlen, B.; Palmer, A.; Donnelly, C.A.; Gupta, S.; Marsh, K. Risk of severe malaria among African infants: Direct evidence of clinical protection during early infancy. J. Infect. Dis. 1998, 177, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Falade, C.; Mokuolu, O.; Okafor, H.; Orogade, A.; Falade, A.; Adedoyin, O.; Oguonu, T.; Aisha, M.; Hamer, D.H.; Callahan, M.V. Epidemiology of congenital malaria in Nigeria: A multi-centre study. Trop. Med. Int. Health 2007, 12, 1279–1287. [Google Scholar] [CrossRef]
- Mwaniki, M.K.; Talbert, A.W.; Mturi, F.N.; Berkley, J.A.; Kager, P.; Marsh, K.; Newton, C.R. Congenital and neonatal malaria in a rural Kenyan district hospital: An eight-year analysis. Malar. J. 2010, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, K.R.; Dent, A.E. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 2016, 143, 129–138. [Google Scholar] [CrossRef]
- Riley, E.M.; Wagner, G.E.; Ofori, M.F.; Wheeler, J.G.; Akanmori, B.D.; Tetteh, K.; McGuinness, D.; Bennett, S.; Nkrumah, F.K.; Anders, R.F.; et al. Lack of association between maternal antibody and protection of African infants from malaria infection. Infect. Immun. 2000, 68, 5856–5863. [Google Scholar] [CrossRef] [PubMed]
- Jelinkova, L.; Flores-Garcia, Y.; Shapiro, S.; Roberts, B.T.; Petrovsky, N.; Zavala, F.; Chackerian, B. A vaccine targeting the L9 epitope of the malaria circumsporozoite protein confers protection from blood-stage infection in a mouse challenge model. NPJ Vaccines 2022, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Jelinkova, L.; Jhun, H.; Eaton, A.; Petrovsky, N.; Zavala, F.; Chackerian, B. An epitope-based malaria vaccine targeting the junctional region of circumsporozoite protein. NPJ Vaccines 2021, 6, 13. [Google Scholar] [CrossRef]
- Kisalu, N.K.; Idris, A.H.; Weidle, C.; Flores-Garcia, Y.; Flynn, B.J.; Sack, B.K.; Murphy, S.; Schon, A.; Freire, E.; Francica, J.R.; et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med. 2018, 24, 408–416. [Google Scholar] [CrossRef]
- Wang, L.T.; Pereira, L.S.; Flores-Garcia, Y.; O’Connor, J.; Flynn, B.J.; Schon, A.; Hurlburt, N.K.; Dillon, M.; Yang, A.S.P.; Fabra-Garcia, A.; et al. A Potent Anti-Malarial Human Monoclonal Antibody Targets Circumsporozoite Protein Minor Repeats and Neutralizes Sporozoites in the Liver. Immunity 2020, 53, 733–744. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Berkowitz, N.M.; Idris, A.H.; Coates, E.E.; Holman, L.A.; Mendoza, F.; Gordon, I.J.; Plummer, S.H.; Trofymenko, O.; Hu, Z.; et al. A Monoclonal Antibody for Malaria Prevention. N. Engl. J. Med. 2021, 385, 803–814. [Google Scholar] [CrossRef]
- Wu, R.L.; Idris, A.H.; Berkowitz, N.M.; Happe, M.; Gaudinski, M.R.; Buettner, C.; Strom, L.; Awan, S.F.; Holman, L.A.; Mendoza, F.; et al. Low-Dose Subcutaneous or Intravenous Monoclonal Antibody to Prevent Malaria. N. Engl. J. Med. 2022, 387, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kayentao, K.; Ongoiba, A.; Preston, A.C.; Healy, S.A.; Doumbo, S.; Doumtabe, D.; Traore, A.; Traore, H.; Djiguiba, A.; Li, S.; et al. Safety and Efficacy of a Monoclonal Antibody against Malaria in Mali. N. Engl. J. Med. 2022, 387, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Aida, Y.; Pabst, M.J. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 1990, 132, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Noe, A.R.; Espinosa, D.; Li, X.; Coelho-Dos-Reis, J.G.; Funakoshi, R.; Giardina, S.; Jin, H.; Retallack, D.M.; Haverstock, R.; Allen, J.R.; et al. A full-length Plasmodium falciparum recombinant circumsporozoite protein expressed by Pseudomonas fluorescens platform as a malaria vaccine candidate. PLoS ONE 2014, 9, e107764. [Google Scholar] [CrossRef]
- Otero, C.E.; Langel, S.N.; Blasi, M.; Permar, S.R. Maternal antibody interference contributes to reduced rotavirus vaccine efficacy in developing countries. PLoS Pathog. 2020, 16, e1009010. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.; Ennis, F.A.; Saltzman, E.J.; Krugman, S. Persistence of maternal antibody in infants beyond 12 months: Mechanism of measles vaccine failure. J. Pediatr. 1977, 91, 715–718. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Yuan, L.; Azevedo, M.S.; Jeong, K.I.; Gonzalez, A.M.; Iosef, C.; Lovgren-Bengtsson, K.; Morein, B.; Lewis, P.; Saif, L.J. High titers of circulating maternal antibodies suppress effector and memory B-cell responses induced by an attenuated rotavirus priming and rotavirus-like particle-immunostimulating complex boosting vaccine regimen. Clin. Vaccine Immunol. 2006, 13, 475–485. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, X.E.; Teter, C.; Zhong, H.; Pang, Z.; Ding, L.; Li, F.; Zhou, Y.; Zhang, L. Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine 2014, 32, 6091–6097. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Zhang, L.; Liu, Z.; Li, J.; Ji, Z.; Xu, R.; Zhao, N.; Li, F.; Chen, X.; et al. Maternal immunization promotes the immune response of neonates towards hepatitis B vaccine. J. Viral. Hepat. 2013, 20, 875–881. [Google Scholar] [CrossRef]
- Siegrist, C.A. Mechanisms by which maternal antibodies influence infant vaccine responses: Review of hypotheses and definition of main determinants. Vaccine 2003, 21, 3406–3412. [Google Scholar] [CrossRef]
- Borras, E.; Urbiztondo, L.; Costa, J.; Batalla, J.; Torner, N.; Plasencia, A.; Salleras, L.; Dominguez, A.; Working Group for the Study of Measles Immunity in Children. Measles antibodies and response to vaccination in children aged less than 14 months: Implications for age of vaccination. Epidemiol. Infect. 2012, 140, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelínková, L.; Roberts, B.; Ajayi, D.T.; Peabody, D.S.; Chackerian, B. The Immunogenicity of a VLP-based Malaria Vaccine Targeting CSP in Pregnant and Neonatal Mice. Biomolecules 2023, 13, 202. https://doi.org/10.3390/biom13020202
Jelínková L, Roberts B, Ajayi DT, Peabody DS, Chackerian B. The Immunogenicity of a VLP-based Malaria Vaccine Targeting CSP in Pregnant and Neonatal Mice. Biomolecules. 2023; 13(2):202. https://doi.org/10.3390/biom13020202
Chicago/Turabian StyleJelínková, Lucie, Bryce Roberts, Diane T. Ajayi, David S. Peabody, and Bryce Chackerian. 2023. "The Immunogenicity of a VLP-based Malaria Vaccine Targeting CSP in Pregnant and Neonatal Mice" Biomolecules 13, no. 2: 202. https://doi.org/10.3390/biom13020202
APA StyleJelínková, L., Roberts, B., Ajayi, D. T., Peabody, D. S., & Chackerian, B. (2023). The Immunogenicity of a VLP-based Malaria Vaccine Targeting CSP in Pregnant and Neonatal Mice. Biomolecules, 13(2), 202. https://doi.org/10.3390/biom13020202





