Therapeutic Potential of Leaves from Fridericia chica (Bonpl.) L. G. Lohmann: Botanical Aspects, Phytochemical and Biological, Anti-Inflammatory, Antioxidant and Healing Action
Abstract
1. Introduction
2. Revision Strategy
3. Ethnobotany
4. Chemical Composition
5. Pharmacological Properties
5.1. Healing Activity
5.2. Anti-Inflammatory Activity
5.3. Antioxidant Activity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takemura, O.S.; Iinuma, M.; Tosa, H.; Miguel, O.G.; Moreira, E.A.; Nozawa, Y. A flavone from leaves of Arrabidaea chica f. cuprea. Phytochemistry 1995, 38, 1299–1300. [Google Scholar] [CrossRef]
- Pauletti, P.M.; Bolzani, V.S.; Young, M.C.M. Chemical constituents of Arrabidaea samydoides (Bignonia-ceae). Química Nova 2003, 26, 641–643. [Google Scholar] [CrossRef]
- Lorenzi, H.; Matos, F.J. Plantas Medicinais No Brasil: Nativas e Exóticas; Instituto Plantarum de Estudos da Flora: São Paulo, Brazil, 2002; p. 512. [Google Scholar]
- Chapman, E.; Perkin, A.G.; Robinson, R. The colouring matters of carajura. J. Chem. Soc. 1927, 49, 3015. [Google Scholar] [CrossRef]
- Cronquist, A. An Integrated System of Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1981; pp. 248–250. [Google Scholar]
- Lohmann, L.G. Bignoniaceae in Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Behrens, M.D.; Tellis, C.J.M. Arrabidaea chica (Humb. & Bonpl.) B. Verlot (Bignoniaceae). Rev. Fitos 2012, 7, 236–244. [Google Scholar]
- Joly, A.B. Botânica: Introdução à Taxonomia Vegetal; Editora Nacional: São Paulo, Brazil, 1993; p. 776. [Google Scholar]
- Puhl, M.C.M.N.; Milaneze-Gutierre, M.A.; Nakamura, C.V.; Cortez, D.A.G. Morpho-anatomy of leaves and young stems of Arrabidaea chica (Humb. & Bonpl.) B. Verl. (Bignoniaceae). Lat. Am. J. Pharm. 2007, 26, 224–229. [Google Scholar]
- Corrêa, M.P.; Pena, L.A. Dicionário das Plantas Úteis do Brasil e das Exóticas Cultivadas; Ministério da Agricultura, Instituto Brasileiro de Desenvolvimento Florestal: Rio de Janeiro, Brazil, 1984; pp. 1934–1974. [Google Scholar]
- Ferreira, F.A.G.; Carvalho, C.M.; Costa, J.C.; Ferreira, J.M.R.; Silva, F. Comprovação do potencial medici-nal de Arrabidaea chica (Bignoniaceae). Sci. Prima 2013, 1, 1–6. [Google Scholar]
- Borrás, M.R.L. Plantas da Amazônia: Medicinais ou Mágicas—Plantas Comercializadas no Mercado Municipal Adolpho Lisboa; Editora Valer, Governo do Estado do Amazonas: Manaus, Brazil, 2003; p. 322. [Google Scholar]
- Moreira, L.S.; Silva, S.A. Ação Inibitória do Crajiru Arrabdaea chica (Humb&Bonpl.) B.Verlt Sobre Staphylococcus sp. como Microoganismo Oportunista no Tratamento da Acne Vulgar. Revista Olhar Científico 2016, 2, 210. [Google Scholar]
- Jorge, M.P.; Madjarof, C.; Gois Ruiz, A.L.; Fernandes, A.T.; Ferreira Rodrigues, R.A.; de Oliveira Sousa, I.M.; Foglio, M.A.; de Carvalho, J.E. Evaluation of wound healing properties of Arrabidaea chica Verlot extract. J. Ethnopharmacol. 2008, 118, 361–366. [Google Scholar] [CrossRef]
- Oliveira, D.P.C.; Borrás, M.R.L.; Ferreira, L.C.L.; López-Lozano, J.L. Anti-inflammatory activity of the aqueous extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. on the self-induced inflammatory process from venoms amazonians snakes. Rev. Bras. de Farmacogn. 2009, 19, 643–649. [Google Scholar]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef]
- Zorn, B.; García-Piñeres, A.J.; Castro, V.; Murillo, R.; Mora, G.; Merfort, I. 3-Desoxyanthocyanidins from Arrabidaea chica. Phytochemistry 2001, 56, 831–835. [Google Scholar] [CrossRef]
- Devia, B.; Llabres, G.; Wouters, J.; Dupont, L.; Escribano-Bailon, M.T.; de Pascual-Teresa, S.; Angenot, L.; Tits, M. New 3-deoxyanthocyanidins from leaves of Arrabidaea chica. Phytochem. Anal. PCA 2002, 13, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.C.; Lopes, A.; Sousa, E.; Camelo, D.S.; Lima, F.; Rocha, C.; Silva, G.; Garcia, J.; Cartágenes, M. Effects of Extract of Arrabidaea chica Verlot on an Experimental Model of Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4717. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, W.L.R.; Pinto, L.N.; Quignard, E.; Vieira, J.M.S.; Silva-Júnior, O.C.; Albuquerque, S. Arrabidaea chica (HBK) Verlot: Phytochemical approach, antifungal and trypanocidal activities. Braz. J. Pharmacogn. 2008, 18, 544–548. [Google Scholar] [CrossRef]
- Siraichi, J.T.; Felipe, D.F.; Brambilla, L.Z.; Gatto, M.J.; Terra, V.A.; Cecchini, A.L.; Cortez, L.E.; Ro-drigues-Filho, E.; Cortez, D.A. Antioxidant capacity of the leaf extract obtained from Arrabidaea chica cultivat-ed in Southern Brazil. PLoS ONE 2013, 8, e72733. [Google Scholar]
- de Siqueira, F.C.; Leitão, D.D.S.T.C.; Mercadante, A.Z.; Chiste, R.C.; Lopes, A.S. Profile of phenolic compounds and carotenoids of Arrabidaea chica leaves and the in vitro singlet oxygen quenching capacity of their hydrophilic extract. Food Res. Int. 2019, 126, 108597. [Google Scholar] [CrossRef]
- Vasconcelos, C.C.; Lopes, A.; de Jesus Garcia Ataide, E.; Carvalho, K.; de Brito, M.; Rodrigues, M.S.; de Mo-rais, S.V.; Silva, G.; da Rocha, C.Q.; Garcia, J.; et al. Arrabidaea chica Verlot fractions re-duce MIA-induced osteoarthritis progression in rat knees. Inflammopharmacology 2021, 29, 735–752. [Google Scholar] [CrossRef]
- Miranda, N.; Gerola, A.P.; Novello, C.R.; Ueda-Nakamura, T.; de Oliveira Silva, S.; Dias-Filho, B.P.; Hioka, N.; de Mello, J.; Nakamura, C.V. Pheophorbide a, a compound isolated from the leaves of Arrabidaea chica, induces photodynamic inactivation of Trypanosoma cruzi. Photodiagnosis Photodyn. Ther. 2017, 19, 256–265. [Google Scholar] [CrossRef]
- Huang, X.F.; Zhang, J.L.; Huang, D.P.; Huang, A.S.; Huang, H.T.; Liu, Q.; Liu, X.H.; Liao, H.L. A net-work pharmacology strategy to investigate the anti-inflammatory mechanism of luteolin combined with in vitro transcriptomics and proteomics. Int. Immunopharmacol. 2020, 86, 106727. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Gan, F.F.; Shelar, S.B.; Ng, K.Y.; Chew, E.H. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 59, 272–280. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, Y.J.; Bai, L.; Liu, Y.X.; Fu, X.Q.; Zhu, P.L.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Wang, Y.P.; et al. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine Int. J. Phytother. Phytopharm. 2020, 68, 153173. [Google Scholar] [CrossRef]
- Nickavar, B.; Rezaee, J.; Nickavar, A. Effect-Directed Analysis for the Antioxidant Compound in Salvia verticillata. Iran. J. Pharm. Res. IJPR 2016, 15, 241–246. [Google Scholar]
- Huang, W.C.; Liou, C.J. Dietary acacetin reduces airway hyperresponsiveness and eosinophil infiltration by modulating eotaxin-1 and th2 cytokines in a mouse model of asthma. Evid. Based Compl. Alter. Med. eCAM 2012, 2012, 910520. [Google Scholar] [CrossRef]
- More, G.; Lall, N.; Hussein, A.; Tshikalange, T.E. Antimicrobial Constituents of Artemisia afra Jacq. ex Willd. against Periodontal Pathogens. Evid. Based Compl. Alter. Med. eCAM 2012, 2012, 252758. [Google Scholar]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Syed Mohamad, S.; Khatib, A.; Latip, J. Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. BioMed Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef]
- Pandith, H.; Zhang, X.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W.; Baek, S.J. Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-κB pathway. J. Ethnopharmacol. 2013, 147, 434–441. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, N.; Li, S.; Chen, M.; Pu, P. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed. Pharmacother. 2019, 117, 109042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, X.; Ouyang, X.; Chen, D. Dual Effect of Glucuronidation of a Pyrogallol-Type Phytophenol Antiox-idant: A Comparison between Scutellarein and Scutellarin. Molecules 2018, 23, 3225. [Google Scholar] [CrossRef]
- Ana Silvia, G.R.; Gabriela, T.T.; Maribel, H.R.; Nayeli, M.B.; José Luis, T.E.; Alejandro, Z.; Manasés, G.C. Effect of Terpenoids and Flavonoids Isolated from Baccharis conferta Kunth on TPA-Induced Ear Edema in Mice. Molecules 2020, 25, 1379. [Google Scholar] [CrossRef]
- Yu, C.I.; Cheng, C.I.; Kang, Y.F.; Chang, P.C.; Lin, I.P.; Kuo, Y.H.; Jhou, A.J.; Lin, M.Y.; Chen, C.Y.; Lee, C.H. Hispidulin Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia and Attenu-ates the Activation of Akt, NF-κB, and STAT3 Pathway. Neurotox. Res. 2020, 38, 163–174. [Google Scholar] [CrossRef]
- Dabaghi-Barbosa, P.; Mariante Rocha, A.; Franco da Cruz Lima, A.; Heleno de Oliveira, B.; Benigna Martinelli de Oliveira, M.; Gunilla Skare Carnieri, E.; Cadena, S.M.; Eliane Merlin Rocha, M. Hispidulin: Antioxidant properties and effect on mitochondrial energy metabolism. Free. Radic. Res. 2005, 39, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apig-enin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharmacal Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structur-ally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar] [CrossRef]
- Fidelis, Q.C.; Faraone, I.; Russo, D.; Aragão Catunda-Jr, F.E.; Vignola, L.; de Carvalho, M.G.; de Tommasi, N.; Milella, L. Chemical and Biological insights of Ouratea hexasperma (A. St.-Hil.) Baill.: A source of bioactive compounds with multifunctional properties. Nat. Prod. Res. 2019, 33, 1500–1503. [Google Scholar] [CrossRef]
- Süntar, I.; Küpeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef]
- Yao, H.; Yuan, Z.; Wei, G.; Chen, C.; Duan, J.; Li, Y.; Wang, Y.; Zhang, C.; Liu, Y. Thevetiaflavone from Wikstroemia indica ameliorates PC12 cells injury induced by OGD/R via improving ROS mediated mitochondrial dysfunction. Mol. Med. Rep. 2017, 16, 9197–9202. [Google Scholar] [CrossRef][Green Version]
- Han, H.S.; Shin, J.S.; Lee, S.B.; Park, J.C.; Lee, K.T. Cirsimarin, a flavone glucoside from the aerial part of Cirsium japonicum var. ussuriense (Regel) Kitam. ex Ohwi, suppresses the JAK/STAT and IRF-3 signaling pathway in LPS-stimulated RAW 264.7 macrophages. Chem. Interact. 2018, 293, 38–47. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, Y.; Xie, F.; Zhuang, X.; Wang, X.; Yu, X. Development and Validation of a UPLC-MS/MS Method for the Quantitative Determination and Pharmacokinetic Analysis of Cirsimarin in Rat Plasma. BioMed Res. Int. 2021, 2021, 9953664. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; Oliveira, L.; Fujiike, A.; Tuttis, K.; Ribeiro, D.L.; Camara, M.; Rocha, C.; Cólus, I. Flavone cirsimarin impairs cell proliferation, migration, and invasion in MCF-7 cells grown in 2D and 3D models. Toxicology 2022, 83, 105416. [Google Scholar] [CrossRef]
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Ji, L.; Zhao, X.; Si, C.L.; Jiang, Y.; Wang, G. Apigenin-7-O-β-D-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signaling path-ways in RAW 264.7 macrophages and protects mice against endotoxin shock. Food Funct. 2016, 7, 1002–1013. [Google Scholar] [CrossRef]
- Li, S.; Xu, T.; Liu, S.; Liu, Z.; Pi, Z.; Song, F.; Jin, Y. Exploring the potential pharmacodynamic material basis and pharmacologic mechanism of the Fufang-Xialian-Capsule in chronic atrophic gastritis by network pharma-cology approach based on the components absorbed into the blood. R. Soc. Open Sci. 2018, 5, 171806. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Hu, Z.; Bian, Y.; Su, W.; Li, X.; Li, S.; Wu, J.; Shi, L.; Song, Y.; Zheng, G.; et al. Scutellarin Attenuates the IL-1β-Induced Inflammation in Mouse Chondrocytes and Prevents Osteoarthritic Progression. Front. Pharmacol. 2020, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.X.; Huang, D.Y.; Li, H.X.; Zhang, L.N.; Lv, Y.H.; Cui, H.D.; Zheng, J.H. Scutellarin promotes in vitro angiogenesis in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2010, 400, 151–156. [Google Scholar] [CrossRef]
- Kadioglu, O.; Nass, J.; Saeed, M.E.; Schuler, B.; Efferth, T. Kaempferol Is an Anti-Inflammatory Compound with Activity towards NF-κB Pathway Proteins. Anticancer. Res. 2015, 35, 2645–2650. [Google Scholar] [PubMed]
- Park, M.Y.; Ji, G.E.; Sung, M.K. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig. Dis. Sci. 2012, 57, 355–363. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Anti-inflammatory action of isorhamnetin. Inflammation 2014, 37, 1200–1201. [Google Scholar] [CrossRef]
- Bakır, T.; Sönmezoğlu, I.; Imer, F.; Apak, R. Antioxidant/prooxidant effects of α-tocopherol, quercetin and iso-rhamnetin on linoleic acid peroxidation induced by Cu(II) and H2O2. Int. J. Food Sci. Nutr. 2014, 65, 226–234. [Google Scholar] [CrossRef]
- Marzouk, M.S.; Moharram, F.A.; Haggag, E.G.; Ibrahim, M.T.; Badary, O.A. Antioxidant flavonol glycosides from Schinus molle. Phytother. Res. 2006, 20, 200–205. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Sa, Y.J.; Kim, M.J. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J. Agric. Food Chem. 2011, 59, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, T.; Niu, J.G.; He, Z.Q.; Liu, Y.; Wang, F. Amentoflavone protects hippocampal neurons: Anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen. Res. 2015, 10, 1125–1133. [Google Scholar] [PubMed]
- Bajpai, V.K.; Park, I.; Lee, J.; Shukla, S.; Nile, S.H.; Chun, H.S.; Khan, I.; Oh, S.Y.; Lee, H.; Huh, Y.S.; et al. Anti-oxidant and antimicrobial efficacy of a biflavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chem. 2019, 271, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; da Silva, M.S.; Cárdeno, A.; Aparicio-Soto, M.; Salvador, M.J.; Frankland Sawaya, A.C.; Souza-Brito, A.R.; de la Lastra, C.A. Abarema cochliacarpos reduces LPS-induced inflammatory response in murine peritoneal macrophages regulating ROS-MAPK signal pathway. J. Ethnopharmacol. 2013, 149, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.B.; Sousa, C.; Costa, A.; Andrade, P.B.; Valentão, P. Glutathione and the antioxidant potential of binary mixtures with flavonoids: Synergisms and antagonisms. Molecules 2013, 18, 8858–8872. [Google Scholar] [CrossRef]
- Vasconcelos, P.C.D.P.; Seito, L.N.; Di Stasi, L.C.; Akiko Hiruma-Lima, C.; Pellizzon, C.H. Epicatechin used in the treatment of intestinal inflammatory disease: An analysis by experimental models. Evid. Based Compl. Altern. Med. 2012, 2012, 508902. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, D.; Kumari, A.; Yadav, S.K. Encapsulation of catechin and epicatechin on BSA NPS im-proved their stability and antioxidant potential. EXCLI J. 2014, 13, 331–346. [Google Scholar]
- Islam, M.T.; Ayatollahi, S.A.; Zihad, S.; Sifat, N.; Khan, M.R.; Paul, A.; Salehi, B.; Islam, T.; Mubarak, M.S.; Martins, N.; et al. Phytol anti-inflammatory activity: Pre-clinical assessment and possible mechanism of action elucidation. Cell. Mol. Biol. 2020, 66, 264–269. [Google Scholar] [CrossRef]
- Santos, C.C.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.; de Oliveira, G.A.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and Antioxidant Activities of Phytol In Vivo and In Vitro Models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Amarowicz, R. Squalene: A natural antioxidant? Eur. J. Lipid Sci. Technol. 2009, 111, 411–412. [Google Scholar] [CrossRef]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2010, 54, 551–558. [Google Scholar] [CrossRef]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory Activity of β-Carotene, Lycopene and Tri-n-butylborane, a Scavenger of Reactive Oxygen Species. In Vivo 2018, 32, 255–264. [Google Scholar] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, M.; Orhan, C.; Muz, O.E.; Sahin, N.; Juturu, V.; Sahin, K. Lutein and zeaxanthin isomers modulates lipid metabolism and the inflammatory state of retina in obesity-induced high-fat diet rodent model. BMC Ophthalmol. 2017, 17, 129. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef]
- Chung, R.; Leanderson, P.; Lundberg, A.K.; Jonasson, L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 2017, 262, 87–93. [Google Scholar] [CrossRef]
- Wisniewska-Becker, A.; Nawrocki, G.; Duda, M.; Subczynski, W.K. Structural aspects of the antioxidant activi-ty of lutein in a model of photoreceptor membranes. Acta Biochim. Pol. 2012, 59, 119–124. [Google Scholar] [CrossRef]
- Freitas de Lima, F.; Lescano, C.H.; Arrigo, J.; Cardoso, C.; Coutinho, J.P.; Moslaves, I.; Ximenes, T.; Kadri, M.; Weber, S.S.; Perdomo, R.T.; et al. Anti-inflammatory, anti-proliferative and cytoprotective potential of the Attalea phalerata Mart. ex Spreng. pulp oil. PLoS ONE 2018, 13, e0195678. [Google Scholar] [CrossRef]
- Araki, M.; Kaku, N.; Harada, M.; Ando, Y.; Yamaguchi, R.; Shindo, K. Production of Auroxanthins from Violaxanthin and 9-cis-Violaxanthin by Acidic Treatment and the Antioxidant Activities of Violaxanthin, 9-cis-Violaxanthin, and Auroxanthins. J. Agric. Food Chem. 2016, 64, 9352–9355. [Google Scholar] [CrossRef]
- Serbinova, E.A.; Packer, L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol. 1994, 234, 354–366. [Google Scholar] [PubMed]
- Shuid, A.N.; Mohamad, S.; Muhammad, N.; Fadzilah, F.M.; Mokhtar, S.A.; Mohamed, N.; Soelaiman, I.N. Effects of α-tocopherol on the early phase of osteoporotic fracture healing. J. Orthop.Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 55, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Liapina, E.A.; Machneva, T.V.; Larkina, E.A.; Tkachevskaia, E.P.; Osipov, A.N.; Mironov, A.F. Effect of the photosensitizers pheophorbid a and protoporphyrin IX on skin wound healing by the action of low-intensity laser irradiation. Biofizika 2010, 55, 350–355. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Corrêa, M.F.P.; Melo, G.O.; Costa, S.S. Natural products from plant origin potentially usefull in the asthma therapy. Braz. J. Pharmacogn. 2008, 18, 785–797. [Google Scholar] [CrossRef]
- Brasil. Relação Nacional de Medicamentos Essenciais: Rename 2013. Brasília, DF: Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Assistência Farmacêutica e Insumos Estra-tégicos, 8th ed. 2013. Available online: https://www.prefeitura.sp.gov.br/cidade/secretarias/upload/rename/livro-rename-2013-atualizado.pdf (accessed on 15 May 2022).
- Marmitt, D.J.; Rempel, C.; Goettert, M.I.; Silva, A.C. Medicinal Plants RENISUS with Potential Anti-inflammatory: Systematic Review in Three Scientific Databases. Rev. Fitos 2015, 9, 73–159. [Google Scholar]
- Lopes, G.F.G.; Pantoja, S.C.S. Levantamento das espécies de plantas medicinais utilizadas pela população de Santa Cruz–Rio de Janeiro–RJ. Rev. Eletrônica Novo Enfoque 2013, 16, 62–80. [Google Scholar]
- Feijó, A.M.; Heiden, G. Medicinal plants used by elderly people with Diabetes mellitus in the treatment of the disease symptoms. Rev. Bras. Plantas Med. 2012, 14, 50–56. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Azevedo, M.M.; Chaves, F.C.; Alviano, C.S.; Alviano, D.S.; Vermelho, A.B. Arrabidaea chica hexanic extract induces mitochondrion damage and peptidase inhibition on Leishmania spp. BioMed. Res. Int. 2014, 985171. [Google Scholar]
- Cortez de Sá, J.; Almeida-Souza, F.; Mondêgo-Oliveira, R.; Oliveira, I.; Lamarck, L.; Magalhães, I.; Ataídes-Lima, A.F.; Ferreira, H.; Abreu-Silva, A.L. Leishmanicidal, cytotoxicity and wound healing potential of Arrabidaea chica Verlot. BMC Compl. Altern. Med. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mafioleti, L.; da Silva Junior, I.F.; Colodel, E.M.; Flach, A.; Martins, D.T. Evaluation of the toxicity and anti-microbial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. J. Ethnopharmacol. 2013, 150, 576–582. [Google Scholar] [PubMed]
- Torres, C.A.; Zampini, I.C.; Nuñez, M.B.; Isla, M.I.; Castro, M.P.; Gonzalez, A.M. In vitro antimicrobial activity of 20 selected climber species from the Bignoniaceae family. Nat. Prod. Res. 2013, 27, 2144–2148. [Google Scholar] [CrossRef]
- Hofling, J.F.; Anibal, P.C.; Obando-Pereda, G.A.; Peixoto, I.A.T.; Furletti, V.F.; Foglio, M.A.; Gonçalves, R.B. Antimicrobial potential of some plant extracts against Candida species. Braz. J. Biol. 2010, 70, 1065–1068. [Google Scholar] [CrossRef]
- Kohn, L.K.; Foglio, M.A.; Rodrigues, R.A.; Sousa, I.M.O.; Martini, M.C.; Padilla, M.A.; Lima-Neto, D.F.; Arns, C.W. In-Vitro Antiviral Activities of Extracts of Plants of The Brazilian Cerrado against the Avian Metapneumovirus (aMPV). Rev. Bras. De Ciência Avícola 2015, 17, 275–280. [Google Scholar] [CrossRef]
- Brandão, G.C.; Kroon, E.G.; Santos, J.R.; Stehmann, J.R.; Lombardi, J.A.; Oliveira, A.B. Antiviral activities of plants occurring in the state of Minas Gerais, Brazil: Part 2. Screening Bignoniaceae species. Rev. Bras. Farmacogn. 2010, 20, 742–750. [Google Scholar] [CrossRef]
- Ribeiro, A.F.C.; Telles, T.C.; Ferraz, V.P.; Souza-Fagundes, E.M.; Cassali, G.D.; Carvalho, A.T.; Melo, M.M. Effect of Arrabidaea chica extracts on the Ehrlich solid tumor development. Rev. Bras. de Farmacogn. 2012, 22, 364–373. [Google Scholar] [CrossRef]
- Rocha, K.B.F.; Oliveira, C.N.; Azevedo, I.M.; Macedo, R.; Medeiros, A.C. Effect of Arrabidaea chica extract against chemically induced breast cancer in animal model. Acta Cirúrgica Bras. 2019, 34, e201901001. [Google Scholar] [CrossRef]
- Taffarello, D.; Jorge, M.P.; Sousa, I.M.O.; Duarte, M.C.T.; Figueira, G.M.; Queiroz, N.C.A.; Rodrigues, R.A.F.; Carvalho, J.E.; Goes, A.L.T.R.; Foglio, M.A. Activity of Arrabidaea chica (Humb. & Bonpl.) Verlot ex-tracts obtained by biotechnological processes on fibroblast and human tumor cells. Química Nova 2013, 36, 431–436. [Google Scholar]
- Evangelista, S.S.; Sampaio, F.C.; Parente, R.C.; Bandeira, M.F.C.L. Phytotherapics in Odontology: Ethnobo-tanical study in Manaus. Rev. Bras. de Plantas Med. 2013, 15, 513–519. [Google Scholar] [CrossRef]
- Elisabetsky, E.; Wannmacher, L. The status of ethnopharmacology in Brazil. J. Ethnopharmacol. 1993, 38, 137–143. [Google Scholar] [CrossRef]
- Odonne, G.; Valadeau, C.; Alban-Castillo, J.; Stien, D.; Sauvain, M.; Bourdy, G. Medical ethnobotany of the Chayahuita of the Paranapura basin (Peruvian Amazon). J. Ethnopharmacol. 2013, 146, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Stojanović-Radić, Z.; Fokou, P.V.T.; Sharifi-Rad, M.; Mahady, G.B.; Iriti, M. Me-dicinal plants used in the treatment of tuberculosis-Ethnobotanical and ethnopharmacological approaches. Biotechnol. Adv. 2020, 44, 107629. [Google Scholar] [CrossRef]
- Moraes, L.L.C.; Fritas, J.L.; Matos-Filho, J.R.; Silva, R.B.L.; Borges, C.H.A.; Santos, A.C. A Ethno-knowledge of medicinal plants in a community in the eastern Amazon. Rev. Ciên. Agrár. 2019, 42, 565–573. [Google Scholar]
- Cartagenes, M.d.S.S.; Lima, N.F.M.C.; França, L.G.; Pessoa, D.L.R.; Amaral, F.M.M.; Abreu, I.C.; Silva, S.N.; Borges, M.O.R.; Medeiros, I.A. Avaliação da atividade anti-hipertensiva do extrato de Arrabi-daea chica Verlot em ratos espontaneamente hipertensos. Rev. Ciên. Saúde 2015, 16, 1–11. [Google Scholar]
- Coelho-Ferreira, M. Medicinal knowledge and plant utilization in an Amazonian coastal community of Marudá, Pará State (Brazil). J. Ethnopharmacol. 2009, 126, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Muehlmann, L.A.; Longo, J.P.F.; Silva, R.C.; Graebner, I.B.; Degterev, I.A.; Lucci, C.M.; Azevedo, R.B.; Garcia, M.P. Photodynamic Therapy Based on Arrabidaea chica (Crajiru) Extract Nanoemulsion: In vitro Activity against Monolayers and Spheroids of Human Mammary Adenocarcinoma MCF-7 Cells. J. Nanomed. Nanotechnol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Souza, H.Q.; Hidalgo, A.F.; Chaves, F.C.M. Preparo do corante de crajirú (Arrabidaea chica (Bonpl.) B. Verl.) e sua aplicação em Histologia. J. Braz. Assoc. Hortic. Sci. 2007, 25, S36. [Google Scholar]
- Lima de Medeiros, B.J.; Dos Santos Costa, K.; Alves Ribeiro, J.F.; Carrera Silva, J.O.; Ramos Barbosa, W.L.; Tavares Carvalho, J.C. Liver protective activity of a hydroethanolic extract of Arrabidaea chica (Humb. and Bonpl.) B. Verl. (pariri). Pharmacogn. Res. 2011, 3, 79–84. [Google Scholar]
- Do Amaral, R.R.; Santos, A.A.; Saravia, A.; Botas, G.; Cruz, R.A.; Fernandes, C.P.; Boylan, F. Biological activities of Arrabidaea chica (Bonpl.) B. Verl. leaves. Lat. Am. J. Pharm. 2012, 31, 451–455. [Google Scholar]
- Gonzalez, A.C.O.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. de Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Pristo, I. Cicatrização de feridas: Fases e fatores de influência. Acta Vet. Bras. 2012, 6, 267–271. [Google Scholar]
- Martelli, A.; Andrade, T.A.M.; dos Santos, G.M.T. Perspectivas na utilização de fitoterápicos na cicatriza-ção tecidual: Revisão sistemática. Arch. Health Investig. 2018, 7, 344–350. [Google Scholar] [CrossRef]
- Servat-Medina, L.; González-Gómez, A.; Reyes-Ortega, F.; Sousa, I.M.; Queiroz, N.; Zago, P.M.; Jorge, M.P.; Monteiro, K.M.; de Carvalho, J.E.; San Román, J.; et al. Chitosan-tripolyphosphate nanoparticles as Arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int. J. Nanomed. 2015, 10, 3897–3909. [Google Scholar] [CrossRef]
- Aro, A.A.; Freitas, K.M.; Foglio, M.A.; Carvalho, J.E.; Dolder, H.; Gomes, L.; Vidal, B.C.; Pimentel, E.R. Effect of the Arrabidaea chica extract on collagen fiber organization during healing of partially transected ten-don. Life Sci. 2013, 92, 799–807. [Google Scholar] [CrossRef]
- Aro, A.A.; Simões, G.F.; Esquisatto, M.A.; Foglio, M.A.; Carvalho, J.E.; Oliveira, A.L.; Gomes, L.; Pimen-tel, E.R. Arrabidaea chica extract improves gait recovery and changes collagen content during healing of the Achilles tendon. Injury 2013, 44, 884–892. [Google Scholar] [CrossRef]
- Pires, A.; Westin, C.B.; Hernandez-Montelongo, J.; Sousa, I.; Foglio, M.A.; Moraes, A.M. Flexible, dense and porous chitosan and alginate membranes containing the standardized extract of Arrabidaea chica Verlot for the treatment of skin lesions. Mater. Sci. Engineering. C Mater. Biol. Appl. 2020, 112, 110869. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kuprash, D.V.; Nedospasov, S.A. Molecular and Cellular Mechanisms of Inflammation. Biochemistry 2016, 81, 1237–1239. [Google Scholar] [CrossRef]
- Groh, L.; Keating, S.T.; Joosten, L.; Netea, M.G.; Riksen, N.P. Monocyte and macrophage immunometabo-lism in atherosclerosis. Semin. Immunopathol. 2018, 40, 203–214. [Google Scholar] [CrossRef]
- Kiripolsky, J.; McCabe, L.G.; Kramer, J.M. Innate immunity in Sjögren’s syndrome. Clin. Immunol. 2017, 182, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Scrivo, R.; Vasile, M.; Bartosiewicz, I.; Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Chronic Inflammatory Diseases, Anti-Inflammatory Agents and Their Delivery Nanosystems. Pharmaceutics 2021, 13, 64. [Google Scholar] [CrossRef]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef]
- Brasil. Relação Nacional de Medicamentos Essenciais: RENAME 2014. Brasília: Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Assistência Farmacêutica e Insumos Estratégi-cos. 9th edição. 2015. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/relacao_nacional_medicamentos_essenciais_rename_2014.pdf (accessed on 1 July 2022).
- Chi, Y.S.; Jong, H.G.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of naturally occurring prenyl-ated flavonoids on enzymes metabolizing arachidonic acid: Cyclooxygenases and lipoxygenases. Biochem. Pharmacol. 2001, 62, 1185–1191. [Google Scholar] [CrossRef]
- Jang, D.S.; Cuendet, M.; Hawthorne, M.E.; Kardono, L.B.; Kawanishi, K.; Fong, H.H.; Mehta, R.G.; Pez-zuto, J.M.; Kinghorn, A.D. Prenylated flavonoids of the leaves of Macaranga conifera with inhibitory activi-ty against cyclooxygenase-2. Phytochemistry 2002, 61, 867–872. [Google Scholar] [CrossRef]
- Michel, A.F.; Melo, M.M.; Campos, P.P.; Oliveira, M.S.; Oliveira, F.A.; Cassali, G.D.; Ferraz, V.P.; Cota, B.B.; Andrade, S.P.; Souza-Fagundes, E.M. Evaluation of anti-inflammatory, antiangiogenic and antiprolifera-tive activities of Arrabidaea chica crude extracts. J. Ethnopharmacol. 2015, 165, 29–38. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.F.D.; Silva, W.D.; Colleto, G.M.D.D. Controle de qualidade dos venenos animais e dos corres-pondentes antivenenos. I Padronização dos métodos de ensaio das atividades bioquímicas e farmacológicas dos venenos de algumas espécies do gênero Bothrops e Crotalus usando amostras secas a temperaturas ambiente ou lioflizadas. Memórias Inst. Butantan 1991, 53, 149–159. [Google Scholar]
- Koutoulogenis, G.S.; Kokotou, M.G.; Hayashi, D.; Mouchlis, V.D.; Dennis, E.A.; Kokotos, G. 2-Oxoester Phospholipase A2 Inhibitors with Enhanced Metabolic Stability. Biomolecules 2020, 10, 491. [Google Scholar] [CrossRef]
- Takenaka, I.K.T.M.; Amorim, J.M.; de Barros, P.A.V.; Brandao, G.C.; Contarini, S.M.L.; de Sales Souza e Melo, É.; Fernandes, S.O.A. Chemical characterization and anti-inflammatory assessment of the hydroethanol-ic extract of Fridericia chica. Rev. Bras. de Farmacogn. 2020, 30, 559–567. [Google Scholar] [CrossRef]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Torres, C.A.; Pérez Zamora, C.M.; Nuñez, M.B.; Gonzalez, A.M. In vitro antioxidant, antilipoxygenase and antimicrobial activities of extracts from seven climbing plants belonging to the Bignoniaceae. J. Integr. Med. 2018, 16, 255–262. [Google Scholar] [CrossRef]
- Lima, J.; de Oliveira, R.G.; Silva, V.C.; de Sousa, P.T., Jr.; Violante, I.; Macho, A.; Martins, D. Anti-inflammatory activity of 4’,6,7-trihydroxy-5-methoxyflavone from Fridericia chica (Bonpl.) L.G.Lohmann. Nat. Prod. Res. 2020, 34, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Introductory Remarks. In Oxidative stress; Elsevier: Amsterdam, The Netherlands, 1985; pp. 1–8. [Google Scholar]
- Sies, H. Biological redox systems and oxidative stress. Cell. Mol. Life Sci. CMLS 2007, 64, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Carvalho, A.V.; Cavalcante, M.A.; Santana, C.L.; Alves, R.M. Physical and chemical characteristics of ma-trices of yellow mobin fruits in the state of Pará. Aliment. Nutr. Araraquara 2011, 22, 45–53. [Google Scholar]
- Aprioku, J.S. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J. Reprod. Infertil. 2013, 14, 158–172. [Google Scholar] [PubMed]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35 Pt 5, 1147–1150. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I. Reactive oxygen species, lipid peroxidation and antioxidative defense mechanism. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 44–57. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive oxygen and nitrogen species: Impact on endothelial dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging--matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Katoch, N.; Kaur, P.; Kashyap, P.; Gupta, S.; Dahiya, R.S. Role of oxidative stress in cardiovascular diseases. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 870–881. [Google Scholar]
- Fibach, E.; Rachmilewitz, E. The role of oxidative stress in hemolytic anemia. Curr. Mol. Med. 2008, 8, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: Comparison of aging and cancer. Aging Dis. 2013, 5, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford Scholarship Online: Oxford, UK, 2015. [Google Scholar]
- Augustyniak, A.; Bartosz, G.; Cipak, A.; Duburs, G.; Horáková, L.; Luczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free. Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Processing 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. PTR 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.; Lyu, X.; Lee, J.J.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I. Phenolic Compounds as Nutraceuticals or Functional Food Ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondelle, Y. Antioxidant activities and polyphenolic con-tents of fifteen selected plant species from the Amazonian region. Food Chem. 2007, 101, 1012–1018. [Google Scholar] [CrossRef]
- Paula, J.T.; Paviani, L.C.; Foglio, M.A.; Sousa, I.M.O.; Duarte, G.H.B.; Jorge, M.P.; Eberlin, M.N.; Ca-bral, F.A. Extraction of anthocyanins and luteolin from Arrabidaea chica by sequential extraction in fixed bed using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids 2014, 86, 100–107. [Google Scholar] [CrossRef]
- Dos Santos, V.C.; Longo, T.B.; Garcia, A.L.; Richter, M.F.; Guecheva, T.N.; Henriques, J.A.; Ferraz, A.; Picada, J.N. Evaluation of the mutagenicity and genotoxicity of Arrabidaea chica Verlot (Bignoneaceae), an Amazon plant with medicinal properties. J. Toxicol. Environ. Health Part A 2013, 76, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, T.F.; Prado, L.; Santos, F.S.; de Souza, A.P.; Guecheva, T.N.; Henriques, J.A.; Ferraz, A.; Corrêa, D.S.; Dihl, R.R.; Picada, J.N. Evaluation of Safety of Arrabidaea chica Verlot (Bignoniaceae), a Plant with Healing Properties. J. Toxicol. Environ. Health Part A 2015, 78, 1170–1180. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; De la Parra-Guerra, A.; Caballero-Gallardo, K.; Sierra-Marquez, L.; Fuentes-Lopez, K.; Franco-Marmolejo, J.; Jannasch, A.S.; Sepulveda, M.S.; Stashenko, E. The aqueous extract of Fridericia chica grown in northern Colombia ameliorates toxicity induced by Tergitol on Caenorhabditis elegans. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2021, 244, 109026. [Google Scholar]
- Martins, F.J.; Caneschi, C.A.; Vieira, J.L.; Barbosa, W.; Raposo, N.R. Antioxidant activity and potential pho-toprotective from amazon native flora extracts. J. Photochem. Photobiol. B Biol. 2016, 161, 34–39. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Volpato, H.; Lazarin-Bidóia, D.; Desoti, V.C.; de Souza, R.O.; Fonseca, M.; Ueda-Nakamura, T.; Nakamura, C.V.; Silva, S.O. The extended production of UV-induced reactive oxygen species in L929 fi-broblasts is attenuated by posttreatment with Arrabidaea chica through scavenging mechanisms. J. Photochem. Photobiol. B Biol. 2018, 178, 175–181. [Google Scholar] [CrossRef]
- Teixeira, T.S.; Vale, R.C.; Almeida, R.R.; Ferreira, T.P.S.; Guimarães, L.G.L. Antioxidant Potential and its Correlation with the Contents of Phenolic Compounds and Flavonoids of Methanolic Extracts from Different Medicinal Plants. Rev. Virtual Química 2017, 9, 1546–1559. [Google Scholar] [CrossRef]
- Brasil. Programa Nacional de Plantas Medicinais e Fitoterápicos. Brasília: Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Assistência Farmacêutica e Insumos Estratégicos. 2009. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/programa_nacional_plantas_medicinais_fitoterapicos.pdf (accessed on 3 June 2022).
- Panizza, S.T. Como Prescrever ou Recomendar Plantas Medicinais; Conbrafito: São Luís, Brazil, 2010; p. 15. [Google Scholar]
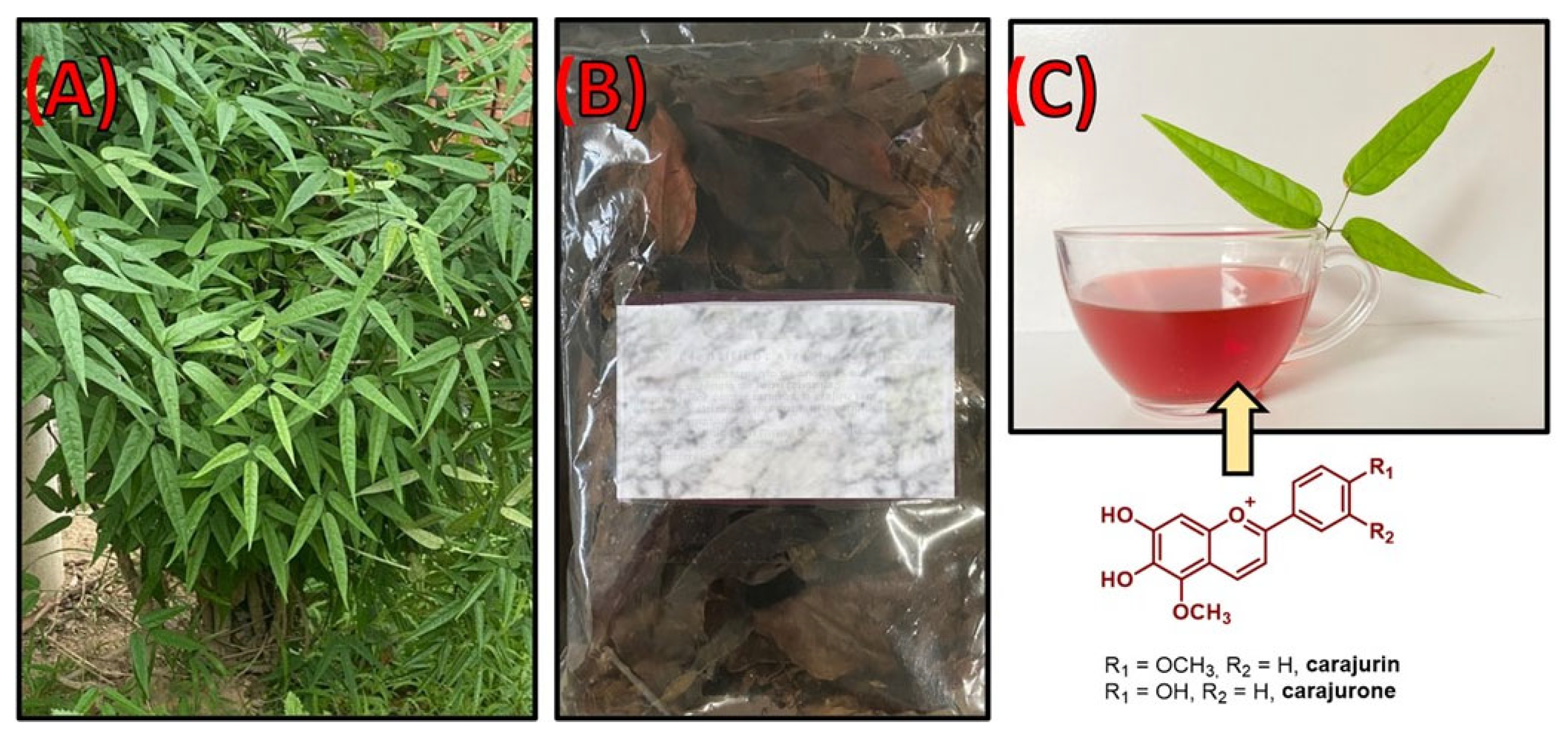

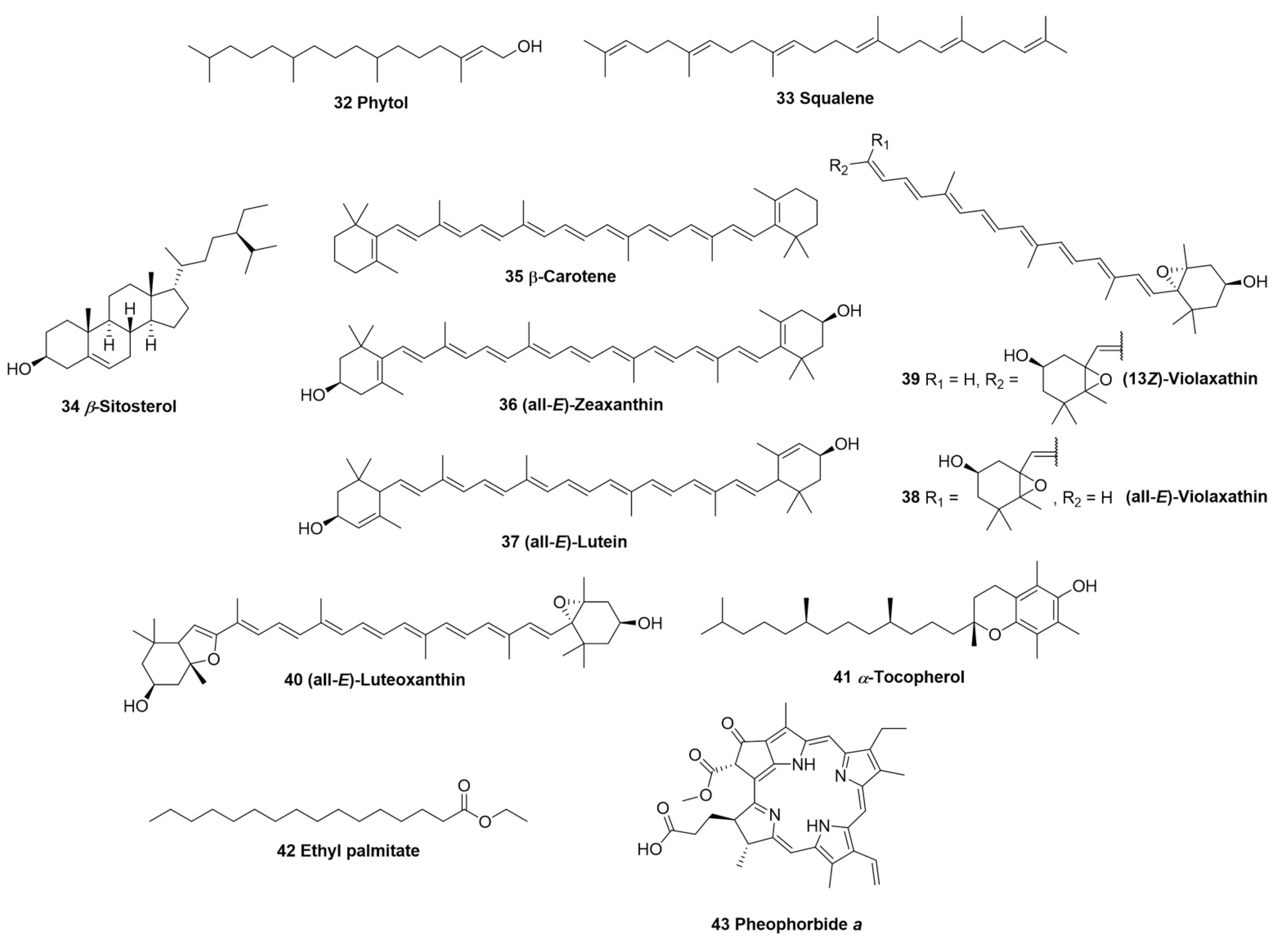
| Compound No. | Name | Source | Isolation/Detection | Biological Activity * |
|---|---|---|---|---|
| Anthocyanins | ||||
| 1 | Carajurin | leaves, MeOH | Isolation [17] | Anti-inflammatory [17] |
| 2 | Carajurone | leaves, MeOH | Isolation [17] | N.A. |
| 3 | 3’-Hydroxy-carajurone | leaves, MeOH | Isolation [17] | N.A. |
| 4 | 3’-Hydroxy-carajurin | leaves, MeOH | Isolation [17] | N.A. |
| 5 | 6,7,3’,4’-Tetrahydroxy-5-methoxyflavylium | leaves, DCM fraction | Isolation [18] | N.A. |
| 6 | 6,7,4’-Trihydroxy-5-methoxyflavylium | leaves, DCM fraction | Isolation [18] | N.A. |
| Flavones | ||||
| 7 | Carajuflavone | leaves, AcOEt fraction | Isolation [1] | N.A. |
| 8 | Luteolin | leaves, AcOEt fraction | Isolation [1] | Anti-inflammatory [25] Antioxidant [26] |
| 9 | Chrysoeriol | leaves, 70% EtOH | HPLC-MS detection [19] | Anti-inflammatory [27] Antioxidant [28] |
| 10 | 4′-Hydroxy-3,7-dimethoxyflavone | leaves, EtOH | Isolation [20] | N.A. |
| 11 | 5,7-dimethoxy-4′-hydroxyflavone | leaves, 70% EtOH | HPLC-MS detection [19] | N.A. |
| 12 | Acacetin | leaves, MeOH | Isolation [17] | Anti-inflammatory [29] Antioxidant [30] |
| 13 | Isoscutellarein | leaves, 90% EtOH | HPLC-MS detection [21] | Antioxidant [31] |
| 14 | Scutellarein | leaves, 90% EtOH | HPLC-MS detection, isolation [21] | Anti-inflammatory [32,33] Antioxidant [34] |
| 15 | 6-Hydroxyluteolin | leaves, 90% EtOH | HPLC-MS detection [21] | N.A. |
| 16 | Hispidulin | leaves, 90% EtOH | HPLC-MS detection [21] | Anti-inflammatory [35,36] Antioxidant [37] |
| 17 | Apigenin | leaves, 90% EtOH | HPLC-MS detection, isolation [21] | Anti-inflammatory [38,39] Antioxidant [40] Healing [41] |
| 18 | Thevetiaflavone | leaves, AcOEt fraction | Isolation [1] | Antioxidant [42] |
| 19 | Cirsimarin | leaves, 70% EtOH | HPLC-MS detection [19] | Anti-inflammatory [43] Antioxidant [44] Healing [45] |
| 20 | Apigenin 7-glucuronide | leaves, 80% MeOH | HPLC-MS detection [22] | Anti-inflammatory [46] Antioxidant [47] |
| 21 | Scutellarin | leaves, 80% MeOH | HPLC-MS detection [22] | Anti-inflammatory [48] Antioxidant [34] Healing [49] |
| 22 | Chrysoeriol-O-glucoside | leaves, 70% EtOH | HPLC-MS detection [19] | N.A. |
| Flavonols | ||||
| 23 | Quercetin-O-gallate | leaves, 70% EtOH | HPLC-MS detection [19] | N.A. |
| 24 | Kaempferol | leaves, 70% EtOH | HPLC-MS detection [19] | Anti-inflammatory [50,51] Antioxidant [31] Healing [52] |
| 25 | Isorhamnetin | leaves, 70% EtOH | HPLC-MS detection [19] | Anti-inflammatory [53] Antioxidant [54] |
| 26 | Hyperin 6”-gallate | leaves, 70% EtOH | HPLC-MS detection [19] | Antioxidant [55] |
| 27 | Quercetin-O-glucoside | leaves, 70% EtOH | HPLC-MS detection [19] | Antioxidant [56] |
| 28 | Isorhamnetin-3-O-glucoside | leaves, 70% EtOH | HPLC-MS detection [19] | Antioxidant [57] |
| Flavone dimer | ||||
| 29 | Amentoflavone | leaves, 70% EtOH | HPLC-MS detection [19] | Anti-inflammatory [58] Antioxidant [59] |
| Flavan-3-ols | ||||
| 30 | Catechin | leaves, 70% EtOH | HPLC-MS detection [19] | Anti-inflammatory [60] Anti-oxidant [61] |
| 31 | Epicatechin | leaves, 70% EtOH | HPLC-MS detection [19] | Anti-inflammatory [62] Antioxidant [63] |
| Terpenes | ||||
| 32 | Phytol | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Anti-inflammatory [64] Antioxidant [65] |
| 33 | Squalene | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Antioxidant [66] Anti-inflammatory [67] |
| 34 | β-Sitosterol | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Anti-inflammatory [68] |
| 35 | β-Carotene | leaves, acetone | HPLC-MS detection [22] | Anti-inflammatory [69] Antioxidant [69] Healing [70] |
| 36 | (all-E)-Zeaxanthin | leaves, acetone | HPLC-MS detection [22] | Anti-inflammatory [71] Antioxidant [72] |
| 37 | (all-E)-Lutein | leaves, acetone | HPLC-MS detection [22] | Anti-inflammatory [73] Antioxidant [74] |
| 38 | (all-E)-Violaxanthin | leaves, acetone | HPLC-MS detection [22] | Anti-inflammatory [75] Antioxidant [75] |
| 39 | (13Z)-Violaxanthin | leaves, acetone | HPLC-MS detection [22] | Antioxidant [76] |
| 40 | (all-E)-Luteoxanthin | leaves, acetone | HPLC-MS detection [22] | N.A. |
| Tocochromanol | ||||
| 41 | α-Tocopherol | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Antioxidant [77] Healing [78] |
| Fatty acid | ||||
| 42 | Ethyl palmitate | leaves, 70% EtOH, hexane fraction | GC-MS detection [23] | Anti-inflammatory [79] |
| Alkaloid | ||||
| 43 | Pheophorbide a | leaves, 90% EtOH | Isolation [24] | Anti-inflammatory [80] Antioxidant [81] Healing [82] |
| Authors | Type of Study | Action | Etiological Agent | Plant Material/Part | Treatment |
|---|---|---|---|---|---|
| [20] | In vitro study | Antifungal and antiprotozoal | Trichophyton mentagrophytes (fungus) and Trypanosoma cruzi | Ethanolic extract of leaves | 4 mg/mL (trypanocide); 3.125 mg/mL (fungicide) |
| [89] | In vitro study | Antiprotozoal | Leishmania amazonensis and Leishmania infantum | Hexanic leaf extract | 37.2 µg/mL (L. amazonensis); 18.6 µg/mL (L. infantum) |
| [90] | In vitro and in vivo study | Antiprotozoal and healing | Leishmania amazonensi; Swiss Webster mouse | Ethanolic extract of leaves and fractions | Leishmanicidal effect: 60–155.9 μg/mL; healing effect: 10 mg/g |
| [24] | In vitro study | Antiprotozoal | Trypanosoma cruzi | Hydroethanolic extract of leaves and fractions | 24.8 μg/mL–213 μg/mL (hydroethanolic extract); 2.3 μg/mL–10 μg/mL (feoforbide-a) |
| [91] | In vivo Study | Antimicrobial | Helicobacter pylori 43,504 and Enterococcus faecalis 29,212 | Hydroethanolic extract of leaves | 12.5 μg/mL (H. pylori); 100 μg/mL (E. faecalis) |
| [92] | In vitro study | Antimicrobial | Staphylococcus sp. | Hydroalcoholic extract | 250 μg gallic acid equivalent (GAE)/mL–MIC; 1000 μg GAE/mL–MBC |
| [93] | In vitro study | Antimicrobial | Candida sp. | Dichloromethane extract of leaves | 0.007–0.03 mg/mL |
| [94] | In vitro study | Antiviral | aMPV cepa SHS/669/03 | Ethanolic extract of leaves | 2.5 μg/mL |
| [95] | In vitro study | Antiviral | Human Herpes Virus type 1 (HHV-1); murine Encephalomyocarditis virus (EMCV); Vaccinia Virus strain Western Reserve (VACV-WR) | Ethanolic extract of leaves | EC50: 245.7 μg/mL (HHV-1); 86.3 μg/mL (VACV-WR) |
| [96] | In vivo study | Antitumor | Solid Ehrlich tumor | Ethanolic extract and aqueous extract of leaves | 30 mg/kg body weight (10 days of oral treatment) |
| [97] | In vivo study | Antitumor | 7,12-dimethyl injection-induced breast cancer-1,2-benzanthracene (DMBA) | Hydroalcoholic extract of leaves | Oral administration for 16 weeks: extract at a dose of 300 mg/kg; 7,12-dimethyl-1,2-benzanthracene (DMBA) associated with vincristine 250 µg /mL |
| [98] | In vitro study | Antitumor and fibroblast proliferation | Human tumor cell lines: MCF-7 (breast), NCI-ADR/RES (ovary with multiple drug resistance phenotype), UACC-62 (melanoma), NCI–h460 (lung), PC-3 (prostate), HT29 (colon), OVCAR-03 (ovary), 786-0 (kidney) and K562 (leukemia). Fibroblasts: obtained from 3T3 mice | Crude leaf extracts (without and with enzyme treatment) | 0.25; 2.5 and 25 μg/mL without enzymatic treatment (fibroblast proliferation); 7.4 and 8.7 μg/mL with enzymatic treatment (cytostatic effect for UACC-62–melanoma lineage) |
| [99] | Survey of use by health professionals | Anti-inflammatory | Oral diseases | Leaves | Tea (no dose determined) |
| [19] | In vivo study | Analgesic and anti-inflammatory | Osteoarthritis induced by sodium monoiodoacetate | Hydroalcoholic extract of leaves | Oral administration s. i.d. for 25 days: 50 mg/kg, 150 mg/kg, 450 mg/kg |
| [100] | Survey of studies with herbal medicines | Anti-acne | Does not apply | Not mentioned | Not informed |
| [101] | Survey of traditional use | Treatment of skin irritation and healing | Measles and smallpox | Leaves | Infusion and bath (measles and smallpox); leaves macerated and applied to the affected area (lesions) |
| [102] | Review | Treatment of tuberculosis-related symptoms | Mycobacterium tuberculosis | Not specified | Not specified |
| [103] | Survey of traditional use | Anemia and weakness | Does not apply | Leaves | Not specified |
| [104] | In vivo study | Antihypertensive | Does not apply | Hydroalcoholic extract of leaves | Oral administration of the extract at doses of 100 mg/kg; 250 mg/ kg and 500 mg/kg |
| [105] | Survey of traditional use | (1) Anemia, weakness, restoration facial color in malaria patients; (2) ovarian cysts, cystitis, hepatitis, liver, diarrhea; (3) flu, cough, anemia; (4) aids getting pregnant, ulcers, (5) vaginal itching | Does not apply | Leaves | (1) maceration or tea; (2) tea or infusion; (3) syrup; (4) bottled; (5) bath. No dose determined |
| [21] | Ex vivo study | Photoprotection | Does not apply | Various parts | Topical application of nonionic cream with 2.5% ethylacetate fraction and 2.5% hexane fraction |
| [106] | In vitro study | Photosensitization | MCF-7 cells of human breast adenocarcinoma | Extract nanoemulsion produced from aerial parts | CC50: 1.3 μg ACE/mL |
| [107] | In vitro study | Anti-hepatoxic | Does not apply | Leaves | 0.25–1.25 mg/mL |
| [108] | In vivo study | Anti-hepatoxic | Carbon tetrachloride | Ethanolic extract of leaves | 300, 500 and 600 mg/kg |
| [109] | In vitro study | Antioxidant | Free Radical DPPH (1,1-diphenyl-2-picrylidazyl | Ethanolic extract of leaves and fractions | 5, 10, 25, 50, 125 and 250 µg/mLin ethanol |
| [14] | In vitro study | Antioxidant | Free Radical DPPH (1,1-diphenyl-2-picrylidazyl | Methanolic extract of leaves | 0.25; 2.5; 25 and 250 μg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batalha, A.D.d.S.J.; Souza, D.C.d.M.; Ubiera, R.D.; Chaves, F.C.M.; Monteiro, W.M.; da Silva, F.M.A.; Koolen, H.H.F.; Boechat, A.L.; Sartim, M.A. Therapeutic Potential of Leaves from Fridericia chica (Bonpl.) L. G. Lohmann: Botanical Aspects, Phytochemical and Biological, Anti-Inflammatory, Antioxidant and Healing Action. Biomolecules 2022, 12, 1208. https://doi.org/10.3390/biom12091208
Batalha ADdSJ, Souza DCdM, Ubiera RD, Chaves FCM, Monteiro WM, da Silva FMA, Koolen HHF, Boechat AL, Sartim MA. Therapeutic Potential of Leaves from Fridericia chica (Bonpl.) L. G. Lohmann: Botanical Aspects, Phytochemical and Biological, Anti-Inflammatory, Antioxidant and Healing Action. Biomolecules. 2022; 12(9):1208. https://doi.org/10.3390/biom12091208
Chicago/Turabian StyleBatalha, Adriane Dâmares de Sousa Jorge, Damy Caroline de Melo Souza, Rosmery Duran Ubiera, Francisco Celio Maia Chaves, Wuelton Marcelo Monteiro, Felipe Moura Araújo da Silva, Hector Henrique Ferreira Koolen, Antônio Luiz Boechat, and Marco Aurélio Sartim. 2022. "Therapeutic Potential of Leaves from Fridericia chica (Bonpl.) L. G. Lohmann: Botanical Aspects, Phytochemical and Biological, Anti-Inflammatory, Antioxidant and Healing Action" Biomolecules 12, no. 9: 1208. https://doi.org/10.3390/biom12091208
APA StyleBatalha, A. D. d. S. J., Souza, D. C. d. M., Ubiera, R. D., Chaves, F. C. M., Monteiro, W. M., da Silva, F. M. A., Koolen, H. H. F., Boechat, A. L., & Sartim, M. A. (2022). Therapeutic Potential of Leaves from Fridericia chica (Bonpl.) L. G. Lohmann: Botanical Aspects, Phytochemical and Biological, Anti-Inflammatory, Antioxidant and Healing Action. Biomolecules, 12(9), 1208. https://doi.org/10.3390/biom12091208








