Pyroptosis in Periprosthetic Osteolysis
Abstract
:1. Introduction
2. Pathogenesis of Periprosthetic Osteolysis
3. Pyroptosis in PPO
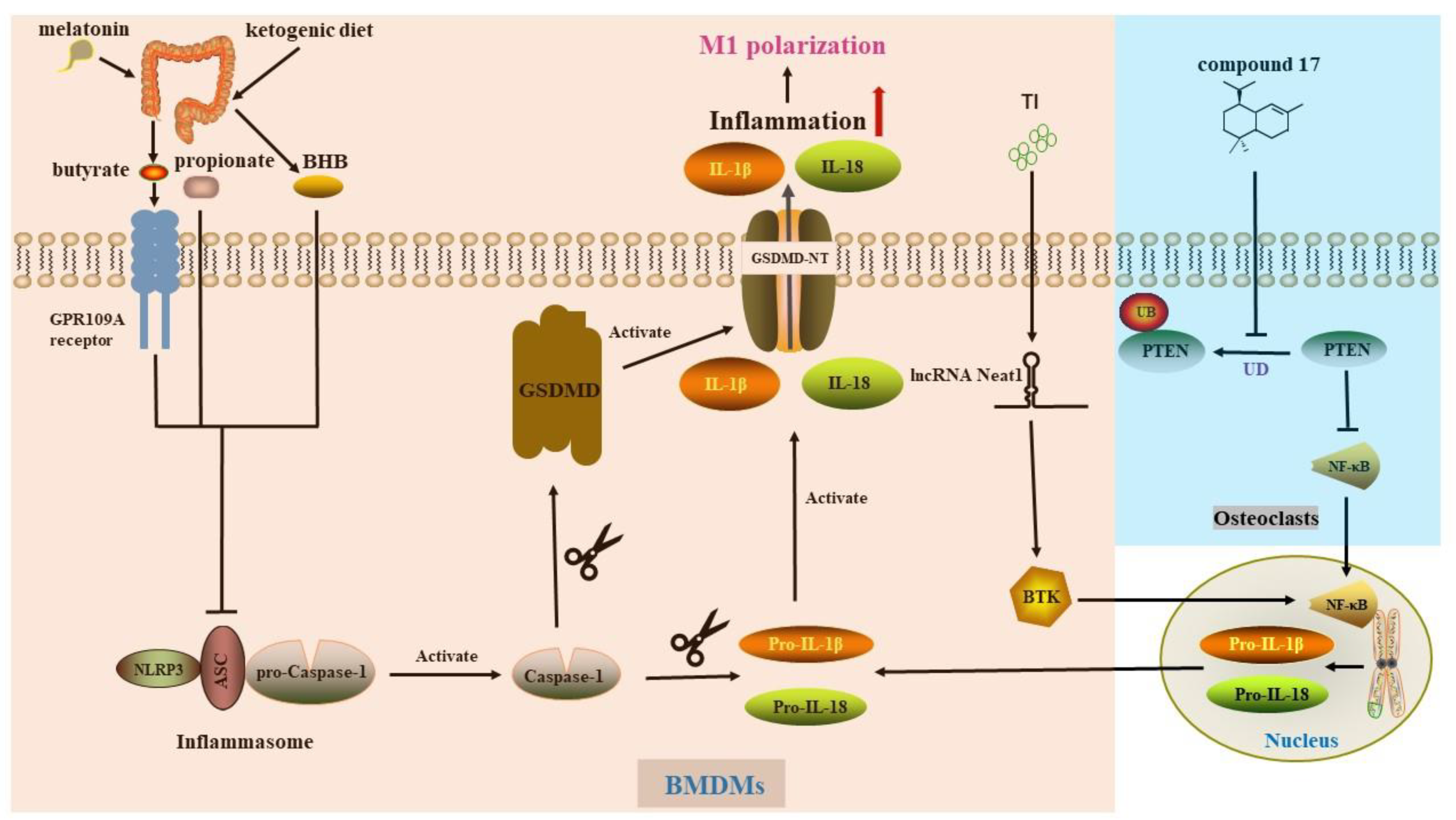
4. Pyroptosis in Macrophage
5. Pyroptosis in Osteocyte
6. Pyroptosis in Osteoblast
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Gallo, J.; Goodman, S.; Konttinen, Y.; Wimmer, M.; Holinka, M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, P.; Lichstein, P.; Shen, C.; Tokarski, A.; Parvizi, J. Why are total knee arthroplasties failing today--has anything changed after 10 years? J. Arthroplast. 2014, 29, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Camuzard, O.; Breuil, V.; Carle, G.; Pierrefite-Carle, V. Autophagy Involvement in Aseptic Loosening of Arthroplasty Components. J. Bone Jt. Surg. 2019, 101, 466–472. [Google Scholar] [CrossRef]
- Vanhegan, I.; Malik, A.; Jayakumar, P.; Ul Islam, S.; Haddad, F. A financial analysis of revision hip arthroplasty: The economic burden in relation to the national tariff. J. Bone Jt. Surg. 2012, 94, 619–623. [Google Scholar] [CrossRef]
- Hunt, L.; Whitehouse, M.; Beswick, A.; Porter, M.; Howard, P.; Blom, A. Implications of Introducing New Technology: Comparative Survivorship Modeling of Metal-on-Metal Hip Replacements and Contemporary Alternatives in the National Joint Registry. J. Bone Jt. Surg. 2018, 100, 189–196. [Google Scholar] [CrossRef]
- Jagga, S.; Sharma, A.; Lee, Y.; Nam, J.; Lee, S. Sclerostin-Mediated Impaired Osteogenesis by Fibroblast-Like Synoviocytes in the Particle-Induced Osteolysis Model. Front. Mol. Biosci. 2021, 8, 666295. [Google Scholar] [CrossRef]
- Desai, M.; Bancroft, L. The case. Diagnosis: Periprosthetic osteolysis. Orthopedics 2008, 31, 518–615. [Google Scholar] [CrossRef]
- Delanois, R.; Mistry, J.; Gwam, C.; Mohamed, N.; Choksi, U.; Mont, M. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J. Arthroplast. 2017, 32, 2663–2668. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Wang, W.; Ge, G.; Xu, N.; Zheng, D.; Jiang, S.; Zhao, G.; Xu, Y.; Wang, Y.; et al. Harmine Alleviates Titanium Particle-Induced Inflammatory Bone Destruction by Immunomodulatory Effect on the Macrophage Polarization and Subsequent Osteogenic Differentiation. Front. Immunol. 2021, 12, 657687. [Google Scholar] [CrossRef]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets. Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9, S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negishi-Koga, T.; Shinohara, M.; Komatsu, N.; Bito, H.; Kodama, T.; Friedel, R.; Takayanagi, H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 2011, 17, 1473–1480. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Yan, M.; Mao, H.; Pan, C.; Gan, M.; Fan, J.; Wang, G. Inhibition of osteolysis after local administration of osthole in a TCP particles-induced osteolysis model. Int. Orthop. 2016, 40, 1545–1552. [Google Scholar] [CrossRef]
- Shi, J.; Gu, Y.; Wang, Y.; Bai, J.; Xiong, L.; Tao, Y.; Xue, Y.; Xu, Y.; Yang, H.; Ye, H.; et al. Inhibitory effect of acetyl-11-keto-β-boswellic acid on titanium particle-induced bone loss by abrogating osteoclast formation and downregulating the ERK signaling pathway. Int. Immunopharmacol. 2021, 94, 107459. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Z.; Gan, J.; Liu, N.; Zhou, G.; Shi, T.; Wang, Z.; Wang, R.; Bao, N.; Guo, T.; et al. The fibroblast expression of RANKL in CoCrMo-particle-induced osteolysis is mediated by ER stress and XBP1s. Acta Biomater. 2015, 24, 352–360. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Ma, Y.; Liu, G.; Shi, H.; Chen, J.; Dong, L.; Zhao, J.; Zhang, J. Particle-induced osteolysis mediated by endoplasmic reticulum stress in prosthesis loosening. Biomaterials 2013, 34, 2611–2623. [Google Scholar] [CrossRef]
- Kręcisz, B.; Kieć-Świerczyńska, M.; Chomiczewska-Skóra, D. Allergy to orthopedic metal implants—A prospective study. Int. J. Occup. Med. Environ. Health 2012, 25, 463–469. [Google Scholar] [CrossRef]
- Sabokbar, A.; Pandey, R.; Athanasou, N. The effect of particle size and electrical charge on macrophage-osteoclast differentiation and bone resorption. J. Mater. Sci. Mater. Med. 2003, 14, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 2007, 28, 5044–5048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, D.; Ribeiro-da-Silva, M.; Mateus, A.; Alves, C.; Machado, G.; Machado-Santos, J.; Paramos-de-Carvalho, D.; Alencastre, I.; Henrique, R.; Costa, G.; et al. Immune response and innervation signatures in aseptic hip implant loosening. J. Transl. Med. 2016, 14, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Arra, M.; Mbalaviele, G.; Swarnkar, G.; Abu-Amer, Y. Inflammatory Responses Reprogram T Through Impairment of Neuropilin-1. Sci. Rep. 2019, 9, 10429. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, R.; Xie, C.; Wei, X.; Zhang, M.; Zhang, X.; Flick, L.; Schwarz, E.; O’Keefe, R. PGE2 signaling through the EP4 receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J. Bone Miner. Res. 2009, 24, 1753–1762. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Ruaro, B.; Casabella, A.; Paolino, S.; Pizzorni, C.; Ghio, M.; Seriolo, C.; Molfetta, L.; Odetti, P.; Smith, V.; Cutolo, M. Dickkopf-1 (Dkk-1) serum levels in systemic sclerosis and rheumatoid arthritis patients: Correlation with the Trabecular Bone Score (TBS). Clin. Rheumatol. 2018, 37, 3057–3062. [Google Scholar] [CrossRef]
- Holt, G.; Murnaghan, C.; Reilly, J.; Meek, R. The biology of aseptic osteolysis. Clin. Orthop. Relat. Res. 2007, 460, 240–252. [Google Scholar] [CrossRef]
- Goodman, S.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, T.; Anoushiravani, A.; Sayeed, Z.; Chambers, M.; El-Othmani, M.; Saleh, K. Osteolysis Complicating Total Knee Arthroplasty. JBJS Rev. 2016, 4, e1. [Google Scholar] [CrossRef]
- Friedlander, A. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 1986, 261, 7123–7126. [Google Scholar] [CrossRef] [PubMed]
- Cookson, B.; Brennan, M. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.; Abrams, J.; Adam, D.; Agostinis, P.; Alnemri, E.; Altucci, L.; Amelio, I.; Andrews, D.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, I.; Ijaz, S.; Mokhtari, T.; Gholaminejhad, M.; Mahdavipour, M.; Jameie, B.; Akbari, M.; Hassanzadeh, G. Subventricular zone-derived extracellular vesicles promote functional recovery in rat model of spinal cord injury by inhibition of NLRP3 inflammasome complex formation. Metab. Brain Dis. 2020, 35, 809–818. [Google Scholar] [CrossRef]
- McKenzie, B.; Mamik, M.; Saito, L.; Boghozian, R.; Monaco, M.; Major, E.; Lu, J.; Branton, W.; Power, C. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, E6065–E6074. [Google Scholar] [CrossRef] [Green Version]
- Pecorelli, A.; Cordone, V.; Messano, N.; Zhang, C.; Falone, S.; Amicarelli, F.; Hayek, J.; Valacchi, G. Altered inflammasome machinery as a key player in the perpetuation of Rett syndrome oxinflammation. Redox Biol. 2020, 28, 101334. [Google Scholar] [CrossRef]
- Humphries, F.; Bergin, R.; Jackson, R.; Delagic, N.; Wang, B.; Yang, S.; Dubois, A.; Ingram, R.; Moynagh, P. The E3 ubiquitin ligase Pellino2 mediates priming of the NLRP3 inflammasome. Nat. Commun. 2018, 9, 1560. [Google Scholar] [CrossRef] [Green Version]
- Petit, A.; Zukor, D.; Antoniou, J.; Ralston, W.; Huk, O. Expression of caspase-8 and caspase-3 proteins in interface membranes from aseptically loose total hip arthroplasty. J. Mater. Sci. Mater. Med. 2002, 13, 1001–1005. [Google Scholar] [CrossRef]
- Zheng, Z.; Deng, W.; Bai, Y.; Miao, R.; Mei, S.; Zhang, Z.; Pan, Y.; Wang, Y.; Min, R.; Deng, F.; et al. YersiniaThe Lysosomal Rag-Ragulator Complex Licenses RIPK1 and Caspase-8-mediated Pyroptosis by. Science 2021, 372, eabg0269. [Google Scholar] [CrossRef]
- Van Hauwermeiren, F.; Van Opdenbosch, N.; Van Gorp, H.; de Vasconcelos, N.; van Loo, G.; Vandenabeele, P.; Kanneganti, T.; Lamkanfi, M. Bacillus anthracis induces NLRP3 inflammasome activation and caspase-8-mediated apoptosis of macrophages to promote lethal anthrax. Proc. Natl. Acad. Sci. USA 2022, 119, e2116415119. [Google Scholar] [CrossRef] [PubMed]
- LaRock, D.; Johnson, A.; Wilde, S.; Sands, J.; Monteiro, M.; LaRock, C. Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature 2022, 605, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhou, F.; Zhang, L. Novel pyroptosis-independent functions of gasdermins. Signal Transduct. Target. Ther. 2022, 7, 127. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrín, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, C.; Rathkey, J.; Yang, J.; Dubyak, G.; Abbott, D.; Xiao, T. Structures of the Gasdermin D C-Terminal Domains Reveal Mechanisms of Autoinhibition. Structure 2018, 26, 778–784.e773. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, S.; Miao, E. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Dong, N.; Wu, X.; Hong, T.; Shen, X.; Guo, X.; Wang, H.; Yu, L.; Zhao, H.; Fang, Q. Elevated Serum Ninjurin-1 Is Associated with a High Risk of Large Artery Atherosclerotic Acute Ischemic Stroke. Transl. Stroke Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Kristjansson, R.; Oddsson, A.; Helgason, H.; Sveinbjornsson, G.; Arnadottir, G.; Jensson, B.; Jonasdottir, A.; Jonasdottir, A.; Bragi Walters, G.; Sulem, G.; et al. Common and rare variants associating with serum levels of creatine kinase and lactate dehydrogenase. Nat. Commun. 2016, 7, 10572. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shao, F. NINJ1, rupturing swollen membranes for cataclysmic cell lysis. Mol. Cell 2021, 81, 1370–1371. [Google Scholar] [CrossRef]
- Kayagaki, N.; Kornfeld, O.; Lee, B.; Stowe, I.; O’Rourke, K.; Li, Q.; Sandoval, W.; Yan, D.; Kang, J.; Xu, M.; et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 2021, 591, 131–136. [Google Scholar] [CrossRef]
- Nandakumar, R.; Tschismarov, R.; Meissner, F.; Prabakaran, T.; Krissanaprasit, A.; Farahani, E.; Zhang, B.; Assil, S.; Martin, A.; Bertrams, W.; et al. Intracellular bacteria engage a STING-TBK1-MVB12b pathway to enable paracrine cGAS-STING signalling. Nat. Microbiol. 2019, 4, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, G.; Wu, Y.; Xiong, Y. Inflammasome Complexes: Crucial mediators in osteoimmunology and bone diseases. Int. Immunopharmacol. 2022, 110, 109072. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zheng, J.; Fan, Y.; Wu, J. TI: NLRP3 Inflammasome-Dependent Pyroptosis in CNS Trauma: A Potential Therapeutic Target. Front. Cell Dev. Biol. 2022, 10, 821225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Miller-Little, W.; Li, X. Inflammasome-independent functions of AIM2. J. Exp. Med. 2021, 218, e20210273. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Rocha, F.; Delitto, A.; de Souza, J.; González-Maldonado, L.; Wallet, S.; Rossa Junior, C. Relevance of Caspase-1 and Nlrp3 Inflammasome on Inflammatory Bone Resorption in A Murine Model of Periodontitis. Sci. Rep. 2020, 10, 7823. [Google Scholar] [CrossRef]
- Zhong, Y.; Kinio, A.; Saleh, M. Functions of NOD-Like Receptors in Human Diseases. Front. Immunol. 2013, 4, 333. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, M.; Chen, Z.; Yu, Y.; Shi, H.; Yu, Y.; Wang, Y.; Chen, R.; Ge, J. Mitochondrial calpain-1 activates NLRP3 inflammasome by cleaving ATP5A1 and inducing mitochondrial ROS in CVB3-induced myocarditis. Basic Res. Cardiol. 2022, 117, 40. [Google Scholar] [CrossRef]
- Neel, D.; Basu, H.; Gunner, G.; Chiu, I. Catching a killer: Mechanisms of programmed cell death and immune activation in Amyotrophic Lateral Sclerosis. Immunol. Rev. 2022, 311, 130–150. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Ning, B.; Su, X.; Yang, B.; Dong, H.; Yin, B.; Pang, Z.; Shen, S. Listeria monocytogenesIntravenous Delivery of Living Elicits Gasdmermin-Dependent Tumor Pyroptosis and Motivates Anti-Tumor Immune Response. ACS Nano 2022, 16, 4102–4115. [Google Scholar] [CrossRef] [PubMed]
- Jämsen, E.; Pajarinen, J.; Kouri, V.; Rahikkala, A.; Goodman, S.; Manninen, M.; Nordström, D.; Eklund, K.; Nurmi, K. Tumor necrosis factor primes and metal particles activate the NLRP3 inflammasome in human primary macrophages. Acta Biomater. 2020, 108, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, F.; Zhang, C.; Zhang, Q.; Su, X.; Zhu, X.; Liu, A.; Shi, W.; Lin, W.; Jin, Z.; et al. Melatonin alleviates titanium nanoparticles induced osteolysis via activation of butyrate/GPR109A signaling pathway. J. Nanobiotechnology 2021, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.; Abdallah, D.; Shebl, A.; El-Abhar, H. The interrupted cross-talk of inflammatory and oxidative stress trajectories signifies the effect of artesunate against hepatic ischemia/reperfusion-induced inflammasomopathy. Toxicol. Appl. Pharmacol. 2020, 409, 115309. [Google Scholar] [CrossRef]
- Zhao, L.; Xing, R.; Wang, P.; Zhang, N.; Yin, S.; Li, X.; Zhang, L. NLRP1 and NLRP3 inflammasomes mediate LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol. Med. Rep. 2018, 17, 5463–5469. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Fan, Q.; Luo, X.; Cheng, W.; Wang, Y.; Li, Z.; Chen, X.; Wu, D. Silencing of Cathepsin B suppresses the proliferation and invasion of endometrial cancer. Oncol. Rep. 2013, 30, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Tong, B.; Wan, B.; Wei, Z.; Wang, T.; Zhao, P.; Dou, Y.; Lv, Z.; Xia, Y.; Dai, Y. Role of cathepsin B in regulating migration and invasion of fibroblast-like synoviocytes into inflamed tissue from patients with rheumatoid arthritis. Clin. Exp. Immunol. 2014, 177, 586–597. [Google Scholar] [CrossRef]
- Bruchard, M.; Mignot, G.; Derangère, V.; Chalmin, F.; Chevriaux, A.; Végran, F.; Boireau, W.; Simon, B.; Ryffel, B.; Connat, J.; et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 2013, 19, 57–64. [Google Scholar] [CrossRef]
- Chevriaux, A.; Pilot, T.; Derangère, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rébé, C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Biol. 2020, 8, 167. [Google Scholar] [CrossRef]
- Ma, L.; Han, Z.; Yin, H.; Tian, J.; Zhang, J.; Li, N.; Ding, C.; Zhang, L. Characterization of Cathepsin B in Mediating Silica Nanoparticle-Induced Macrophage Pyroptosis via an NLRP3-Dependent Manner. J. Inflamm. Res. 2022, 15, 4537–4545. [Google Scholar] [CrossRef]
- Son, H.; Lee, J.; Lee, H.; Kim, N.; Jo, Y.; Lee, G.; Hong, S.; Kwon, M.; Kim, N.; Kim, H.; et al. viaBenzydamine inhibits osteoclast differentiation and bone resorption down-regulation of interleukin-1 expression. Acta Pharm. Sinica. B 2020, 10, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.; Lee, F.; Konttinen, Y.T.; Wilkinson, J.; Smith, R. What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J. Am. Acad. Orthop. Surg. 2008, 16 (Suppl. S1), S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Funes, S.; Rios, M.; Escobar-Vera, J.; Kalergis, A. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, W.; Simonet, W.; Lacey, D. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, K.; Hock, J.; Wang, C.; Yu, X. CLCN7Enhanced but hypofunctional osteoclastogenesis in an autosomal dominant osteopetrosis type II case carrying a c.1856C>T mutation in. Bone Res. 2016, 4, 16035. [Google Scholar] [CrossRef] [Green Version]
- Shippy, D.; Wilhelm, C.; Viharkumar, P.; Raife, T.; Ulland, T. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflammation 2020, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, S.; Cho, W.; Lee, S.; Kim, S.; Kim, J.; Choi, E.; Kim, J.; Yu, J.; Lee, B.; et al. ViaShort Term Isocaloric Ketogenic Diet Modulates NLRP3 Inflammasome B-hydroxybutyrate and Fibroblast Growth Factor 21. Front. Immunol. 2022, 13, 843520. [Google Scholar] [CrossRef]
- Wallisch, J.; Simon, D.; Bayır, H.; Bell, M.; Kochanek, P.; Clark, R. Cerebrospinal Fluid NLRP3 is Increased After Severe Traumatic Brain Injury in Infants and Children. Neurocritical Care 2017, 27, 44–50. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.; Rajendiran, T.; Núñez, G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [Green Version]
- Haridas, B.; Kossoff, E. Dietary Treatments for Epilepsy. Neurol. Clin. 2022, 40, 785–797. [Google Scholar] [CrossRef]
- Spigoni, V.; Cinquegrani, G.; Iannozzi, N.; Frigeri, G.; Maggiolo, G.; Maggi, M.; Parello, V.; Dei Cas, A. Activation of G protein-coupled receptors by ketone bodies: Clinical implication of the ketogenic diet in metabolic disorders. Front. Endocrinol. 2022, 13, 972890. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, L.; Gentileschi, P.; Sbraccia, P.; Guglielmi, V. Ketogenic Diet for Preoperative Weight Reduction in Bariatric Surgery: A Narrative Review. Nutrients 2022, 14, 3610. [Google Scholar] [CrossRef]
- Dal Bello, S.; Valdemarin, F.; Martinuzzi, D.; Filippi, F.; Gigli, G.; Valente, M. Ketogenic Diet in the Treatment of Gliomas and Glioblastomas. Nutrients 2022, 14, 3851. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Letchumanan, V.; Tan, L.; Hong, K.; Wong, S.; Ab Mutalib, N.; Lee, L.; Law, J. Ketogenic Diet: A Dietary Intervention via Gut Microbiome Modulation for the Treatment of Neurological and Nutritional Disorders (a Narrative Review). Nutrients 2022, 14, 3566. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Teng, Y.; Zhang, C.; Pan, Y.; Zhang, Q.; Zhu, X.; Liu, N.; Su, X.; Lin, J. The ketone body β-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J. Nanobiotechnology 2022, 20, 120. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Teng, Y.; Pan, Y.; Liu, N.; Liu, P.; Zhu, X.; Su, X.; Lin, J. Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles. Mil. Med. Res. 2022, 9, 46. [Google Scholar] [CrossRef]
- Ell, B.; Mercatali, L.; Ibrahim, T.; Campbell, N.; Schwarzenbach, H.; Pantel, K.; Amadori, D.; Kang, Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 2013, 24, 542–556. [Google Scholar] [CrossRef] [Green Version]
- Ikegame, M.; Hattori, A.; Tabata, M.; Kitamura, K.; Tabuchi, Y.; Furusawa, Y.; Maruyama, Y.; Yamamoto, T.; Sekiguchi, T.; Matsuoka, R.; et al. Melatonin is a potential drug for the prevention of bone loss during space flight. J. Pineal Res. 2019, 67, e12594. [Google Scholar] [CrossRef]
- Puchner, A.; Saferding, V.; Bonelli, M.; Mikami, Y.; Hofmann, M.; Brunner, J.; Caldera, M.; Goncalves-Alves, E.; Binder, N.; Fischer, A.; et al. Non-classical monocytes as mediators of tissue destruction in arthritis. Ann. Rheum. Dis. 2018, 77, 1490–1497. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, C.; Wang, G.; Sun, Y.; Deng, Z.; Chen, L.; Chen, K.; Tickner, J.; Kenny, J.; Song, D.; et al. Loureirin B suppresses RANKL-induced osteoclastogenesis and ovariectomized osteoporosis via attenuating NFATc1 and ROS activities. Theranostics 2019, 9, 4648–4662. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Hu, J.; Tang, Y.; Tian, Z.; Hu, W.; Zeng, F.; Tan, J.; Dai, Q.; Hou, Z.; et al. Epothilone B prevents lipopolysaccharide-induced inflammatory osteolysis through suppressing osteoclastogenesis via STAT3 signaling pathway. Aging 2020, 12, 11698–11716. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.; Hammer, N.; Campbell, J.; Benson, M.; Perrien, D.; Mrak, L.; Smeltzer, M.; Torres, V.; Skaar, E. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host Microbe 2013, 13, 759–772. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Xie, Z.; Hu, B.; Zhang, B.; Ma, Y.; Pan, X.; Huang, H.; Wang, J.; Zhao, X.; Jie, Z.; et al. The Nrf2 activator RTA-408 attenuates osteoclastogenesis by inhibiting STING dependent NF-κb signaling. Redox Biol. 2020, 28, 101309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, L.; He, H.; Zhu, S.; Zhang, W.; Liu, X.; Zhao, X.; Gao, C.; Mei, M.; Bao, S.; et al. NF-kappa B mediated up-regulation of CCCTC-binding factor in pediatric acute lymphoblastic leukemia. Mol. Cancer 2014, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Yang, J.; Feng, X.; Wang, H.; Ye, S.; Yang, P.; Tan, W.; Wei, G.; Zhou, Y. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol. Cancer 2013, 12, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Yu, Q.; Xin, L.; Guo, L. Circular RNA circC3P1 restrains kidney cancer cell activity by regulating miR-21/PTEN axis and inactivating PI3K/AKT and NF- kB pathways. J. Cell. Physiol. 2020, 235, 4001–4010. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Huang, N.; Zheng, Y.; Cai, L.; Ke, Q.; Wu, S. MicroRNA-455-3p regulates proliferation and osteoclast differentiation of RAW264.7 cells by targeting PTEN. BMC Musculoskelet. Disord. 2022, 23, 340. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Yang, T.; Su, S.; Hu, Y.; Zhong, D. Pseudogene PTENP1 sponges miR-214 to regulate the expression of PTEN to modulate osteoclast differentiation and attenuate osteoporosis. Cytotherapy 2020, 22, 412–423. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, W.; Wang, C.; Zhang, W.; Zhou, C.; Jiang, G.; Hong, J.; Yan, S.; Yan, W. Catalpol suppresses osteoclastogenesis and attenuates osteoclast-derived bone resorption by modulating PTEN activity. Biochem. Pharmacol. 2020, 171, 113715. [Google Scholar] [CrossRef]
- Sugatani, T.; Alvarez, U.; Hruska, K. PTEN regulates RANKL- and osteopontin-stimulated signal transduction during osteoclast differentiation and cell motility. J. Biol. Chem. 2003, 278, 5001–5008. [Google Scholar] [CrossRef]
- Shalev, M.; Elson, A. The roles of protein tyrosine phosphatases in bone-resorbing osteoclasts. Biochim. Biophys. Acta. Mol. Cell Res. 2019, 1866, 114–123. [Google Scholar] [CrossRef]
- Scheffner, M.; Staub, O. HECT E3s and human disease. BMC Biochem. 2007, 8, S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Hu, W.; Wang, Y.; Li, Y.; Li, X.; Li, H.; Tang, Y.; Zhang, L.; Dong, Y.; Yang, X.; et al. A selected small molecule prevents inflammatory osteolysis through restraining osteoclastogenesis by modulating PTEN activity. Clin. Transl. Med. 2020, 10, e240. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shichita, T.; Okada, M.; Komine, R.; Noguchi, Y.; Yoshimura, A.; Morita, R. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 2015, 6, 7360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Wen, Z.; Li, S.; Chen, Z.; Li, C.; Ouyang, Z.; Lin, C.; Kuang, M.; Xue, C.; Ding, Y. LncRNA Neat1 promotes the macrophage inflammatory response and acts as a therapeutic target in titanium particle-induced osteolysis. Acta Biomater. 2022, 142, 345–360. [Google Scholar] [CrossRef]
- Gao, X.; Ge, J.; Li, W.; Zhou, W.; Xu, L.; Geng, D. miR-34a carried by adipocyte exosomes inhibits the polarization of M1 macrophages in mouse osteolysis model. J. Biomed. Mater. Res. Part A 2021, 109, 994–1003. [Google Scholar] [CrossRef]
- Sapir-Koren, R.; Livshits, G. Osteocyte control of bone remodeling: Is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles? Osteoporos. Int. 2014, 25, 2685–2700. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, M.; Niu, W.; Mao, H.; Yang, P.; Xu, B.; Sun, Y. Tricalcium phosphate particles promote pyroptotic death of calvaria osteocytes through the ROS/NLRP3/Caspase-1 signaling axis in amouse osteolysis model. Int. Immunopharmacol. 2022, 107, 108699. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Guo, J.; Yang, X.; Sun, X.; Fu, Z.; Qin, A.; Wu, Y.; Zhao, J. BNTA alleviates inflammatory osteolysis by the SOD mediated anti-oxidation and anti-inflammation effect on inhibiting osteoclastogenesis. Front. Pharmacol. 2022, 13, 939929. [Google Scholar] [CrossRef]
- Xing, F.; Geng, L.; Guan, H.; Liu, D.; Li, Y.; Zeng, L.; Chen, Y.; Tian, R.; Li, Z.; Cao, R.; et al. Astragalin mitigates inflammatory osteolysis by negatively modulating osteoclastogenesis via ROS and MAPK signaling pathway. Int. Immunopharmacol. 2022, 112, 109278. [Google Scholar] [CrossRef]
- Wei, L.; Chen, W.; Huang, L.; Wang, H.; Su, Y.; Liang, J.; Lian, H.; Xu, J.; Zhao, J.; Liu, Q. Alpinetin ameliorates bone loss in LPS-induced inflammation osteolysis via ROS mediated P38/PI3K signaling pathway. Pharmacol. Res. 2022, 184, 106400. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Huang, X.; Yue, J.; Xiang, H.; Shaheen, S.; Jiang, Z.; Tao, Y.; Tu, J.; Liu, Z.; Yao, Y.; et al. SIRT3-mediated deacetylation of NLRC4 promotes inflammasome activation. Theranostics 2021, 11, 3981–3995. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Bai, J.; Li, N.; Li, M.; Sun, H.; Zhang, W.; Ge, G.; Liang, X.; Tao, H.; Xue, Y.; et al. Protective effects of sirtuin 3 on titanium particle-induced osteogenic inhibition by regulating the NLRP3 inflammasome via the GSK-3β/β-catenin signalling pathway. Bioact. Mater. 2021, 6, 3343–3357. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Takatori, Y.; Kyomoto, M.; Ishihara, K.; Kawaguchi, H.; Hashimoto, M.; Tanaka, T.; Oshima, H.; Tanaka, S. Wear resistance of the biocompatible phospholipid polymer-grafted highly cross-linked polyethylene liner against larger femoral head. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, A.; Pajarinen, J.; Lin, T.; Jiang, X.; Gibon, E.; Córdova, L.; Loi, F.; Lu, L.; Jämsen, E.; Egashira, K.; et al. Mutant CCL2 protein coating mitigates wear particle-induced bone loss in a murine continuous polyethylene infusion model. Biomaterials 2017, 117, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohmann, C.; Dean, D.; Bonewald, L.; Schwartz, Z.; Boyan, B. Nitric oxide and prostaglandin E2 production in response to ultra-high molecular weight polyethylene particles depends on osteoblast maturation state. J. Bone Jt. Surg. 2002, 84, 411–419. [Google Scholar] [CrossRef]
- Vendittoli, P.; Shahin, M.; Rivière, C.; Barry, J.; Lavoie, P.; Duval, N. Ceramic-on-ceramic total hip arthroplasty is superior to metal-on-conventional polyethylene at 20-year follow-up: A randomised clinical trial. Orthop. Traumatol. Surg. Res. OTSR 2021, 107, 102744. [Google Scholar] [CrossRef]
- Perry, M.; Mortuza, F.; Ponsford, F.; Elson, C.; Atkins, R. Analysis of cell types and mediator production from tissues around loosening joint implants. Br. J. Rheumatol. 1995, 34, 1127–1134. [Google Scholar] [CrossRef]
- Sharma, A.; Jagga, S.; Chakraborty, C.; Lee, S. Fibroblast-Like-Synoviocytes Mediate Secretion of Pro-Inflammatory Cytokines via ERK and JNK MAPKs in Ti-Particle-Induced Osteolysis. Materials 2020, 13, 3628. [Google Scholar] [CrossRef]
- Nam, J.; Sharma, A.; Jagga, S.; Lee, D.; Sharma, G.; Nguyen, L.; Lee, Y.; Chang, J.; Chakraborty, C.; Lee, S. Suppression of osteogenic activity by regulation of WNT and BMP signaling during titanium particle induced osteolysis. J. Biomed. Mater. Res. Part A 2017, 105, 912–926. [Google Scholar] [CrossRef]
- Lee, S.; Sharma, A.; Choi, B.; Jung, J.; Chang, J.; Park, S.; Salvati, E.; Purdue, E.; Song, D.; Nam, J. The effect of TNFα secreted from macrophages activated by titanium particles on osteogenic activity regulated by WNT/BMP signaling in osteoprogenitor cells. Biomaterials 2012, 33, 4251–4263. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hao, J.; Wu, J.; Li, Y.; Cai, X.; Zheng, Y. Prussian Blue Nanozyme as a Pyroptosis Inhibitor Alleviates Neurodegeneration. Adv. Mater. 2022, 34, e2106723. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, S.; Kersse, K.; Festjens, N.; Lamkanfi, M.; Vandenabeele, P. Inflammatory caspases: Targets for novel therapies. Curr. Pharm. Des. 2007, 13, 367–385. [Google Scholar] [CrossRef]
- Linton, S. Caspase inhibitors: A pharmaceutical industry perspective. Curr. Top. Med. Chem. 2005, 5, 1697–1717. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, J.; Zhang, P.; Li, H.; Shen, H.; Li, X.; Chen, G. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J. Neurosci. Res. 2019, 97, 645–660. [Google Scholar] [CrossRef]
- Coll, R.; Robertson, A.; Chae, J.; Higgins, S.; Muñoz-Planillo, R.; Inserra, M.; Vetter, I.; Dungan, L.; Monks, B.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, C.; Swartzwelter, B.; Gamboni, F.; Neff, C.; Richter, K.; Azam, T.; Carta, S.; Tengesdal, I.; Nemkov, T.; D’Alessandro, A.; et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA 2018, 115, E1530–E1539. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, Z.; Wang, C.; Yang, R.; Rathkey, J.; Pinkard, O.; Shi, W.; Chen, Y.; Dubyak, G.; Abbott, D.; et al. Mechanism of gasdermin D recognition by inflammatory caspases and their inhibition by a gasdermin D-derived peptide inhibitor. Proc. Natl. Acad. Sci. USA 2018, 115, 6792–6797. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Reilly, B.; Jha, A.; Murao, A.; Lee, Y.; Brenner, M.; Aziz, M.; Wang, P. Active Release of eCIRP via Gasdermin D Channels to Induce Inflammation in Sepsis. J. Immunol. 2022, 208, 2184–2195. [Google Scholar] [CrossRef]
- Gurung, P.; Anand, P.; Malireddi, R.; Vande Walle, L.; Van Opdenbosch, N.; Dillon, C.; Weinlich, R.; Green, D.; Lamkanfi, M.; Kanneganti, T. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 2014, 192, 1835–1846. [Google Scholar] [CrossRef] [PubMed]

| Therapeutic Target/Agents | Animal | Cell Type | Animal Model | In Vitro Cell Model | Caspase | Gasdermins | Inflammasome | Ref. |
|---|---|---|---|---|---|---|---|---|
| BHB | C57BL/6J mice (male) | BMDMs | Calvarial osteolysis induced by CoCrMo alloy particles (average 1.69 μm) | Stimulated by LPS and CoCrMo alloy particles | Caspase-1 | GSDMD | NLRP3 | [63] |
| Melatonin | C57BL/6J mice (male) | BMDMs | Calvarial osteolysis induced by Ti particles (average 500 nm) | Stimulated by LPS and Ti particles | Caspase-1 | Not detected | NLRP3 | [52] |
| propionate, butyrate | C57BL/6J mice (male) | BMDMs | Calvarial osteolysis induced by CoCrMo alloy particles | Stimulated by LPS and CoCrMo alloy particles | Caspase-1 | GSDMD | NLRP3 | [64] |
| compound 17 small molecule | C57BL/6J mice (female) | Osteoclasts induced from BMDMs | Calvarial osteolysis induced by LPS | Osteoclast stimulated by RANKL or LPS | Caspase-1 | Not detected | NLRP3 | [78] |
| Neat1 downregulation (si-Neat1) | C57BL/6J mice (male) | BMDMs | Calvarial osteolysis induced by Ti particles | Stimulated by LPS and Ti particles | Caspase-1 | Not detected | NLRP3 | [80] |
| miR-34a carried by EXOs | C57BL/6J mice (male) | RAW 264.7 | Calvarial osteolysis induced by Ti particles | Stimulated by Ti particles | Not detected | Not detected | NLRP3 | [81] |
| NAC VX765 * MCC950 ** | ICR mice (male) | Osteocytes | Calvarial osteolysis induced by TCP particles (average 1.997 μm) | Not established | Caspase-1 | GSDMD | NLRP3 | [82] |
| SIRT3 | SD rat (male) | MSCs and Osteoblast | Femoral osteolysis induced intramedullary implantation of Ti rod and particles | MSCs stimulated by Ti particles | Caspase-1 | GSDMD | NLRP3 | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Yin, Z.; Lai, P.; Liu, X.; Ma, J. Pyroptosis in Periprosthetic Osteolysis. Biomolecules 2022, 12, 1733. https://doi.org/10.3390/biom12121733
Yin J, Yin Z, Lai P, Liu X, Ma J. Pyroptosis in Periprosthetic Osteolysis. Biomolecules. 2022; 12(12):1733. https://doi.org/10.3390/biom12121733
Chicago/Turabian StyleYin, Jian, Zhaoyang Yin, Peng Lai, Xinhui Liu, and Jinzhong Ma. 2022. "Pyroptosis in Periprosthetic Osteolysis" Biomolecules 12, no. 12: 1733. https://doi.org/10.3390/biom12121733
APA StyleYin, J., Yin, Z., Lai, P., Liu, X., & Ma, J. (2022). Pyroptosis in Periprosthetic Osteolysis. Biomolecules, 12(12), 1733. https://doi.org/10.3390/biom12121733





