Emerging Roles of the Unique Molecular Chaperone Cosmc in the Regulation of Health and Disease
Abstract
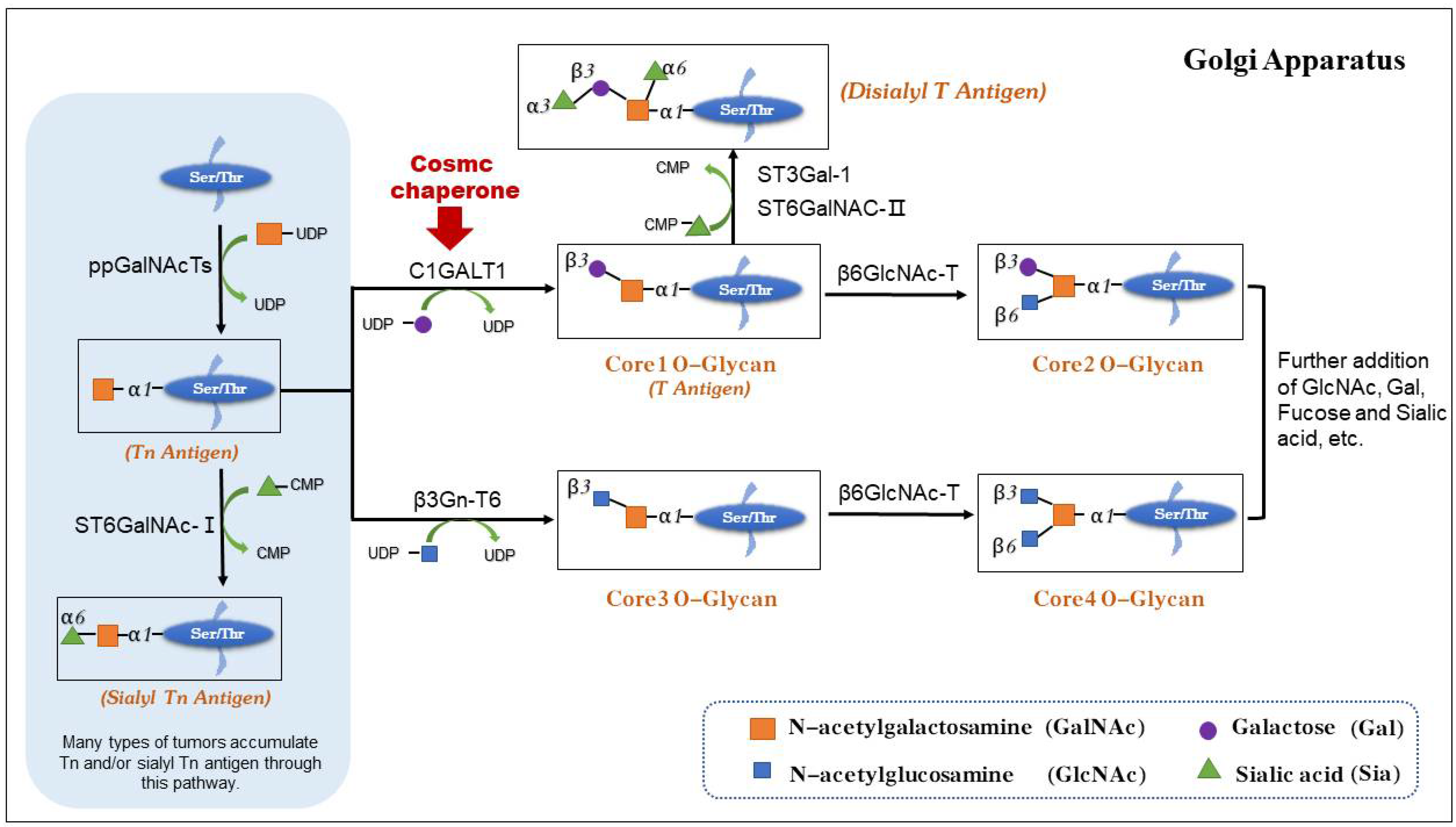
:1. Introduction
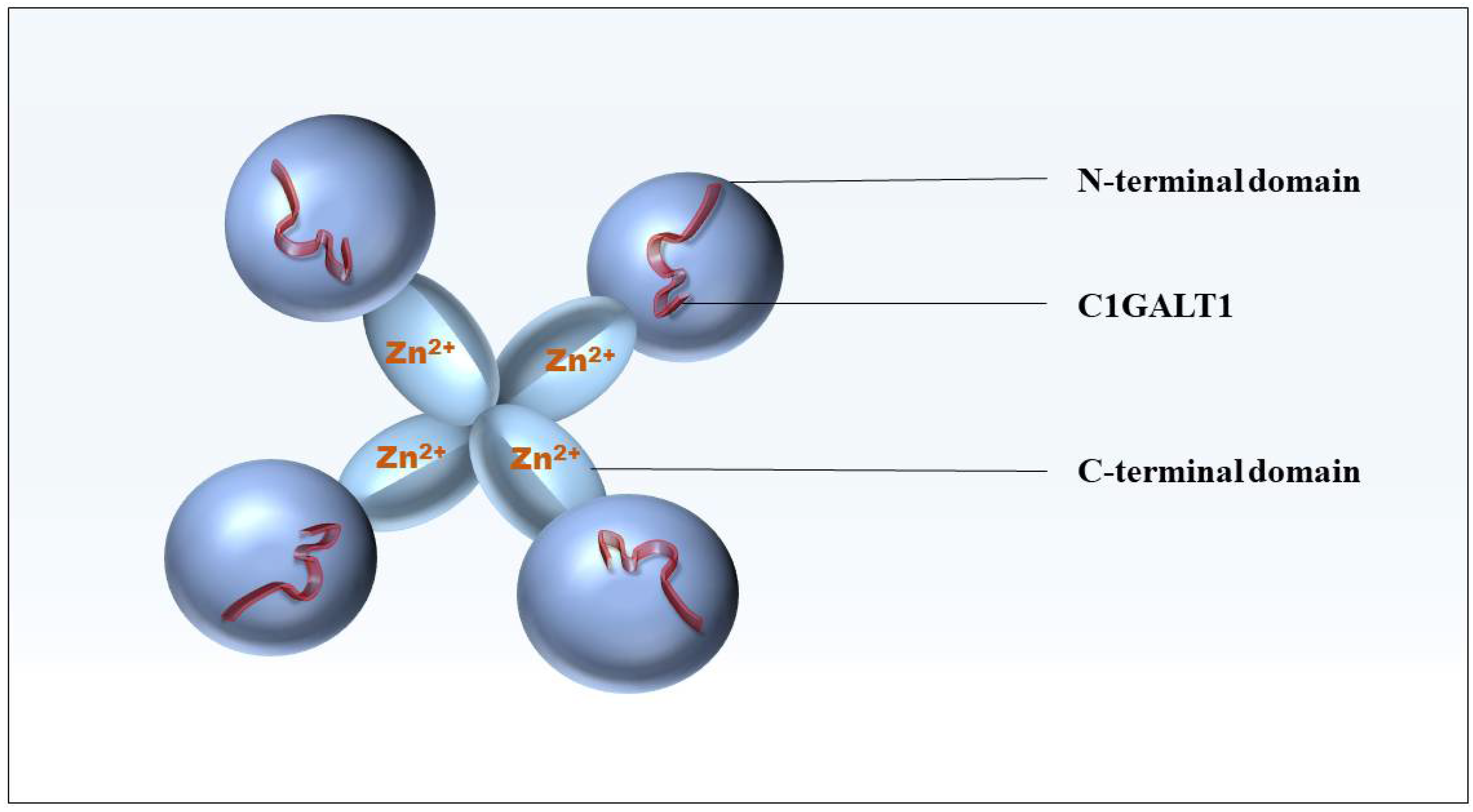
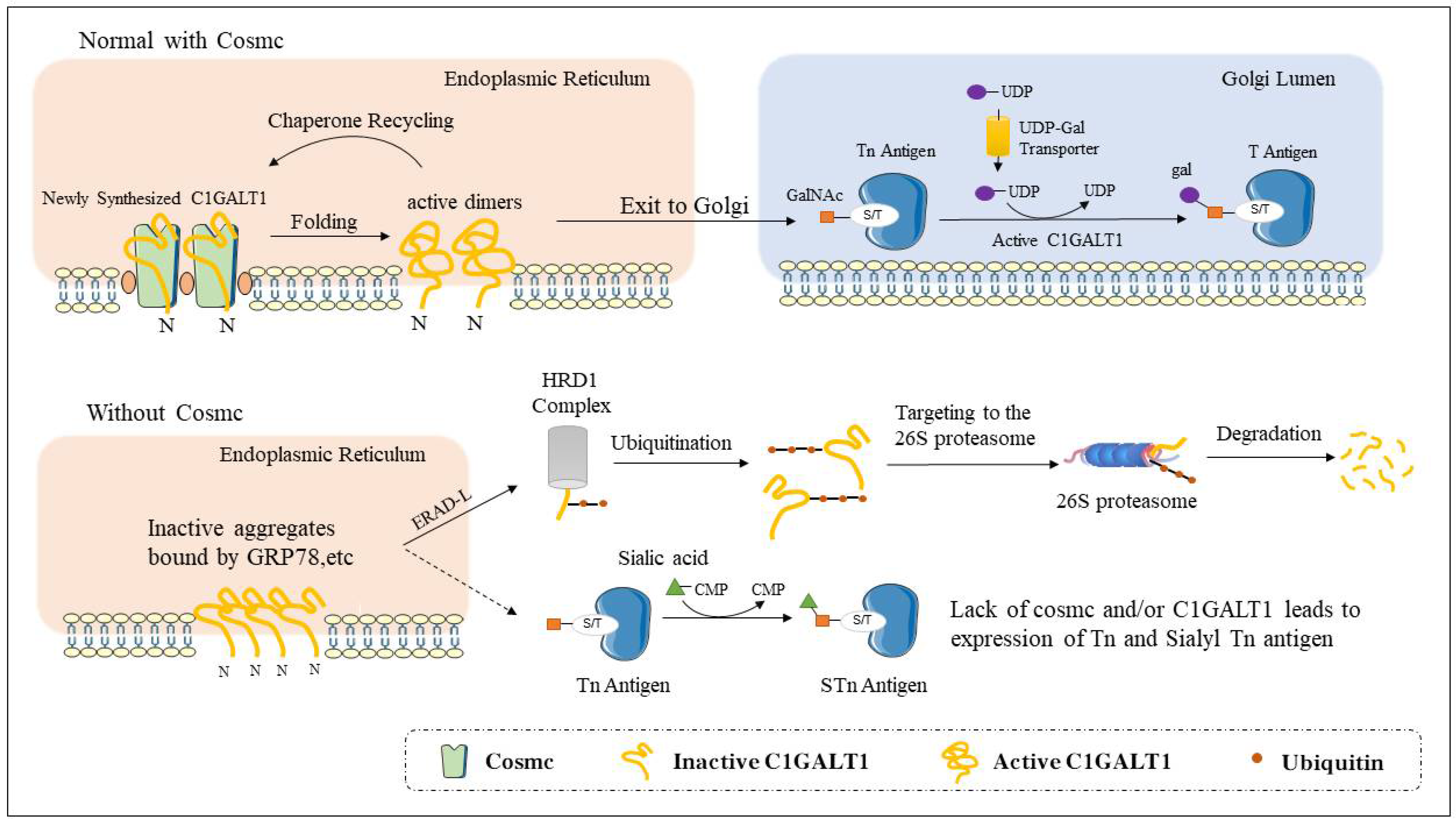
2. The Structure and Function of Cosmc
3. Roles of Cosmc in Normal Development
3.1. Cosmc Affects Platelet Production
3.2. Cosmc Affects Kidney Development
3.3. Cosmc Affects the Function of Immune Cells
3.3.1. Cosmc and Extended O-glycosylation Are Key Factors Controlling B-Cell Homing and Maintaining B-Cell Immune Tolerance
3.3.2. Cosmc and Extended O-glycosylation Are Key Factors in Maintaining Peripheral T Cells
3.3.3. Cosmc and Extended O-glycosylation Mediate Phagocytosis of Apoptotic Cells by Macrophages
4. Roles of Cosmc in Non-neoplastic Diseases
4.1. Immune Diseases
4.1.1. Immunoglobulin A Nephropathy (IgAN)
4.1.2. Tn Syndrome
4.2. Inflammatory Diseases
4.2.1. Lung Inflammation
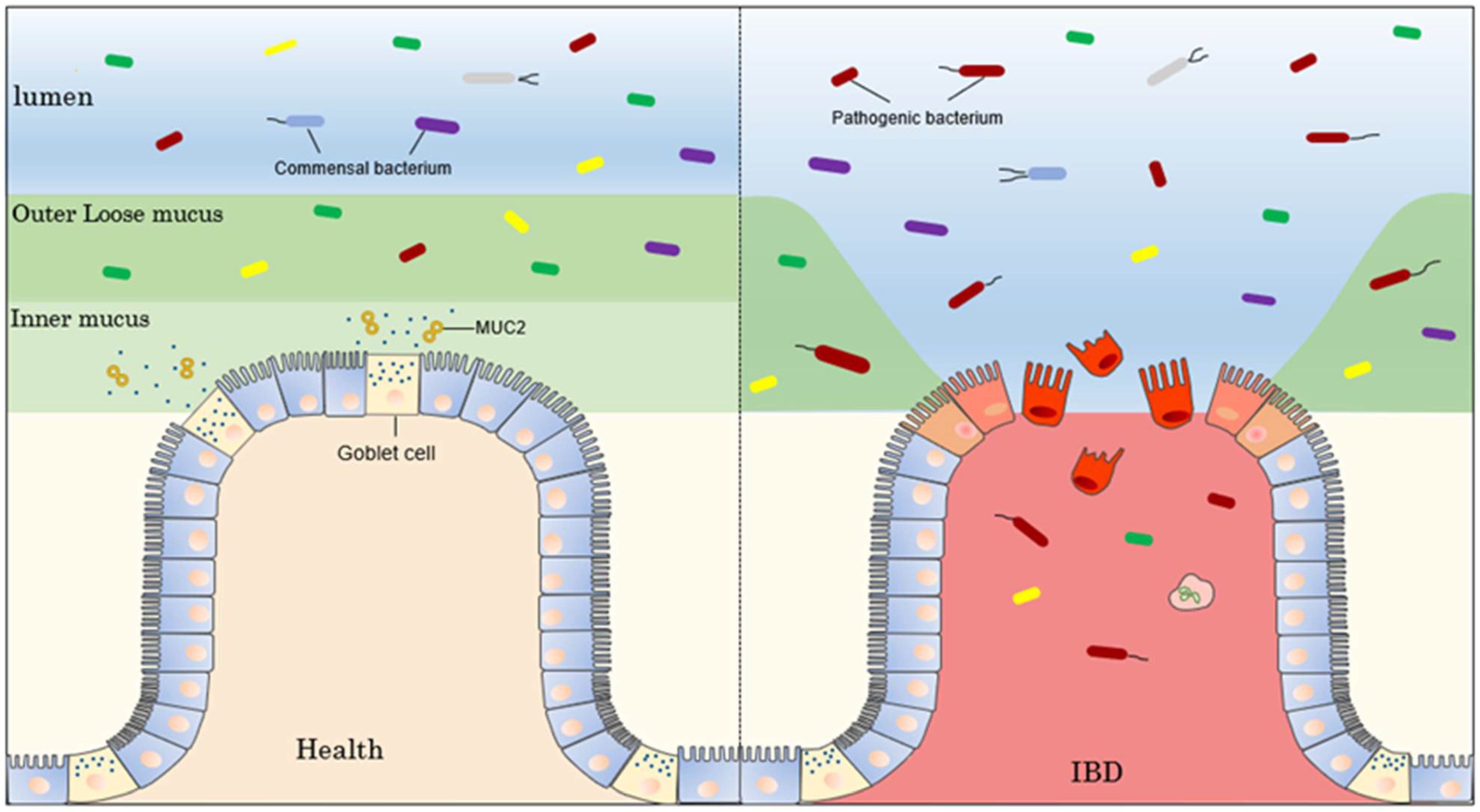
4.2.2. Bowel Inflammation
4.3. Neurodegenerative Diseases
4.4. Viral Diseases
5. Cosmc Function in Tumorigenesis
5.1. Regulation of Cell Proliferation
5.2. Regulation of Cell Apoptosis
5.3. Regulation of Cell Migration
5.4. Regulation of Immune Surveillance
5.5. Regulation of Angiogenesis
5.6. Cosmc as Novel Prognosis Biomarker
6. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eichler, J. Protein glycosylation. Curr. Biol. 2019, 29, R229–R231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodoratou, E.; Thaçi, K.; Agakov, F.; Timofeeva, M.N.; Štambuk, J.; Pučić-Baković, M.; Vučković, F.; Orchard, P.; Agakova, A.; Din, F.V.N.; et al. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci. Rep. 2016, 6, 28098. [Google Scholar] [CrossRef] [Green Version]
- Stotter, B.R.; Talbot, B.E.; Capen, D.E.; Artelt, N.; Zeng, J.; Matsumoto, Y.; Endlich, N.; Cummings, R.D.; Schlondorff, J.S. Cosmc-dependent mucin-type O-linked glycosylation is essential for podocyte function. Am. J. Physiol. Renal. Physiol. 2020, 318, F518–F530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Hagen, K.G.T. A Mucin-type O-Glycosyltransferase Modulates Cell Adhesion during Drosophila Development. J. Biol. Chem. 2008, 283, 34076–34086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, A.; Duarte, H.O.; Reis, C.A. The role of O-glycosylation in human disease. Mol. Asp. Med. 2021, 79, 100964. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA 2016, 88, 275–286. [Google Scholar] [CrossRef]
- Hurtado-Guerrero, R. Recent structural and mechanistic insights into protein O-GalNAc glycosylation. Biochem. Soc. Trans. 2016, 44, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Ashikov, A.; Liu, H.; Griffith, C.L.; Bakker, H.; Doering, T.L. Cryptococcus neoformans UGT1 encodes a UDP-Galactose/UDP-GalNAc transporter. Glycobiology 2017, 27, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Levery, S.B.; Steentoft, C.; Halim, A.; Narimatsu, Y.; Clausen, H.; Vakhrushev, S.Y. Advances in mass spectrometry driven O-glycoproteomics. Biochim. Biophys. Acta 2015, 1850, 33–42. [Google Scholar] [CrossRef]
- Narimatsu, Y.; Ikehara, Y.; Iwasaki, H.; Nonomura, C.; Sato, T.; Nakanishi, H.; Narimatsu, H. Immunocytochemical analysis for intracellular dynamics of C1GalT associated with molecular chaperone, Cosmc. Biochem. Biophys. Res. Commun. 2008, 366, 199–205. [Google Scholar] [CrossRef]
- Lin, M.-C.; Chien, P.-H.; Wu, H.-Y.; Chen, S.-T.; Juan, H.-F.; Lou, P.-J.; Huang, M.-C. C1GALT1 predicts poor prognosis and is a potential therapeutic target in head and neck cancer. Oncogene 2018, 37, 5780–5793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, T.; Wang, Y.; Aryal, R.P.; Lehoux, S.D.; Ding, X.; Kudelka, M.R.; Cutler, C.; Zeng, J.; Wang, J.; Sun, X.; et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteom. Clin. Appl. 2013, 7, 618–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, T.; Brewer, K.; D’Souza, A.; Cummings, R.D.; Canfield, W.M. Cloning and Expression of Human Core 1 β1,3-Galactosyltransferase. J. Biol. Chem. 2002, 277, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, T.; Otto, V.I.; Cummings, R.D. The Tn Antigen Structural Simplicity and Biological Complexity. Angew. Chem. Int. Ed. Engl. 2011, 50, 1770–1791. [Google Scholar] [CrossRef]
- Narimatsu, Y.; Kubota, T.; Furukawa, S.; Shimojima, M.; Iwasaki, H.; Tozawa, Y.; Tachibana, K.; Narimatsu, H. Co-translational function of Cosmc, core 1 synthase specific molecular chaperone, revealed by a cell-free translation system. FEBS Lett. 2011, 585, 1276–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Jobe, S.M.; Ding, X.; Choo, H.; Archer, D.R.; Mi, R.; Ju, T.; Cummings, R.D. Platelet biogenesis and functions require correct protein O-glycosylation. Proc. Natl. Acad. Sci. USA 2012, 109, 16143–16148. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Eljalby, M.; Aryal, R.P.; Lehoux, S.; Stavenhagen, K.; Kudelka, M.R.; Wang, Y.; Wang, J.; Ju, T.; Von Andrian, U.H.; et al. Cosmc controls B cell homing. Nat. Commun. 2020, 11, 3990. [Google Scholar] [CrossRef]
- Mi, R.; Song, L.; Wang, Y.; Ding, X.; Zeng, J.; Lehoux, S.; Aryal, R.P.; Wang, J.; Crew, V.K.; van Die, I.; et al. Epigenetic Silencing of the Chaperone Cosmc in Human Leukocytes Expressing Tn Antigen. J. Biol. Chem. 2012, 287, 41523–41533. [Google Scholar] [CrossRef] [Green Version]
- Springer, G.F. T and Tn, General Carcinoma Autoantigens. Science 1984, 224, 1198–1206. [Google Scholar] [CrossRef]
- Pang, X.; Li, H.; Guan, F.; Li, X. Multiple Roles of Glycans in Hematological Malignancies. Front. Oncol. 2018, 8, 364. [Google Scholar] [CrossRef]
- Thomas, D.; Sagar, S.; Caffrey, T.; Grandgenett, P.M.; Radhakrishnan, P. Truncated O-glycans promote epithelial-to-mesenchymal transition and stemness properties of pancreatic cancer cells. J. Cell. Mol. Med. 2019, 23, 6885–6896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, T.; Cummings, R.D. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature 2005, 437, 1252. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Cummings, R.D. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc. Natl. Acad. Sci. USA 2002, 99, 16613–16618. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Mi, R.; Wang, Y.; Li, Y.; Lin, L.; Yao, B.; Song, L.; van Die, I.; Chapman, A.B.; Cummings, R.D.; et al. Promoters of Human Cosmc and T-synthase Genes Are Similar in Structure, Yet Different in Epigenetic Regulation. J. Biol. Chem. 2015, 290, 19018–19033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanes, M.S.; Moremen, K.W.; Cummings, R.D. Biochemical characterization of functional domains of the chaperone Cosmc. PLoS ONE 2017, 12, e0180242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Ihara, Y.; Leach, M.R.; Cohen-Doyle, M.F.; Williams, D.B. Calreticulin functions in vitro as a molecular chaperone for both glycosylated and non-glycosylated proteins. EMBO J. 1999, 18, 6718–6729. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Ju, T.; Cummings, R.D. The Transmembrane Domain of the Molecular Chaperone Cosmc Directs Its Localization to the Endoplasmic Reticulum. J. Biol. Chem. 2011, 286, 11529–11542. [Google Scholar] [CrossRef] [Green Version]
- Kudo, T.; Iwai, T.; Kubota, T.; Iwasaki, H.; Takayma, Y.; Hiruma, T.; Inaba, N.; Zhang, Y.; Gotoh, M.; Togayachi, A.; et al. Molecular Cloning and Characterization of a Novel UDP-Gal:GalNAcα Peptide β1,3-Galactosyltransferase (C1Gal-T2), an Enzyme Synthesizing a Core 1 Structure of O-Glycan. J. Biol. Chem. 2002, 277, 47724–47731. [Google Scholar] [CrossRef] [Green Version]
- Aryal, R.P.; Ju, T.; Cummings, R.D. Identification of a Novel Protein Binding Motif within the T-synthase for the Molecular Chaperone Cosmc. J. Biol. Chem. 2014, 289, 11630–11641. [Google Scholar] [CrossRef] [Green Version]
- Aryal, R.P.; Ju, T.; Cummings, R.D. The Endoplasmic Reticulum Chaperone Cosmc Directly Promotes in Vitro Folding of T-synthase. J. Biol. Chem. 2010, 285, 2456–2462. [Google Scholar] [CrossRef]
- Ju, T.; Aryal, R.P.; Kudelka, M.R.; Wang, Y.; Cummings, R.D. The Cosmc connection to the Tn antigen in cancer. Cancer Biomark. 2014, 14, 63–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, T.; Aryal, R.P.; Stowell, C.J.; Cummings, R.D. Regulation of protein O-glycosylation by the endoplasmic reticulum–localized molecular chaperone Cosmc. J. Cell Biol. 2008, 182, 531–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, M.; Lee, A.S. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007, 581, 3641–3651. [Google Scholar] [CrossRef] [Green Version]
- Ahner, A.; Brodsky, J.L. Checkpoints in ER-associated degradation: Excuse me, which way to the proteasome? Trends Cell Biol. 2004, 14, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ju, T.; Ding, X.; Xia, B.; Wang, W.; Xia, L.; He, M.; Cummings, R.D. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc. Natl. Acad. Sci. USA 2010, 107, 9228–9233. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, P.; Stanley, A.M.; Rapoport, T.A. Retrotranslocation of a Misfolded Luminal ER Protein by the Ubiquitin-Ligase Hrd1p. Cell 2010, 143, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Aryal, R.P.; Stavenhagen, K.; Luo, C.; Liu, R.; Wang, X.; Chen, J.; Li, H.; Matsumoto, Y.; Wang, Y.; et al. Cosmc deficiency causes spontaneous autoimmunity by breaking B cell tolerance. Sci. Adv. 2021, 7, eabg9118. [Google Scholar] [CrossRef]
- E Cutler, C.; Jones, M.B.; A Cutler, A.; Mener, A.; Arthur, C.M.; Stowell, S.R.; Cummings, R.D. Cosmc is required for T cell persistence in the periphery. Glycobiology 2019, 29, 776–788. [Google Scholar] [CrossRef] [Green Version]
- Wakui, H.; Fuseya, S.; Suzuki, R.; Shimbo, M.; Okada, R.; Hamada, M.; Kuno, A.; Hagiwara, K.; Sato, T.; Narimatsu, H.; et al. Incomplete clearance of apoptotic cells by core 1-derived O-glycan-deficient resident peritoneal macrophages. Biochem. Biophys. Res. Commun. 2018, 495, 2017–2023. [Google Scholar] [CrossRef]
- Andrews, R.; Gardiner, E.; Shen, Y.; Whisstock, J.; Berndt, M. Glycoprotein Ib–IX–V. Int. J. Biochem. Cell Biol. 2003, 35, 1170–1174. [Google Scholar] [CrossRef]
- Weeterings, C.; de Groot, P.G.; Adelmeijer, J.; Lisman, T. The glycoprotein Ib-IX-V complex contributes to tissue factor–independent thrombin generation by recombinant factor VIIa on the activated platelet surface. Blood 2008, 112, 3227–3233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, W.S.; Viney, E.M.; Zhang, J.-G.; Metcalf, D.; Kauppi, M.; Hyland, C.D.; Carpinelli, M.R.; Stevenson, W.; Croker, B.A.; Hilton, A.A.; et al. Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proc. Natl. Acad. Sci. USA 2006, 103, 16442–16447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiser, J.; Altintas, M.M. Podocytes. F1000Research 2016, 5, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlomchik, M.J.; Craft, J.E.; Mamula, M.J. From T to B and back again: Positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 2001, 1, 147–153. [Google Scholar] [CrossRef]
- Harris, D.P.; Haynes, L.; Sayles, P.C.; Duso, D.K.; Eaton, S.M.; Lepak, N.M.; Johnson, L.L.; Swain, S.L.; Lund, F.E. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 2000, 1, 475–482. [Google Scholar] [CrossRef]
- Hauser, M.A.; Kindinger, I.; Laufer, J.M.; Späte, A.-K.; Bucher, D.; Vanes, S.L.; Krueger, W.A.; Wittmann, V.; Legler, D.F. Distinct CCR7 glycosylation pattern shapes receptor signaling and endocytosis to modulate chemotactic responses. J. Leukoc. Biol. 2016, 99, 993–1007. [Google Scholar] [CrossRef] [Green Version]
- Le, Y.; Zhou, Y.; Iribarren, P.; Wang, J. Chemokines and chemokine receptors: Their manifold roles in homeostasis and disease. Cell. Mol. Immunol. 2004, 1, 95–104. [Google Scholar]
- Rawlings, D.J.; Metzler, G.; Wray-Dutra, M.; Jackson, S.W. Altered B cell signalling in autoimmunity. Nat. Rev. Immunol. 2017, 17, 421–436. [Google Scholar] [CrossRef] [Green Version]
- Niiro, H.; Clark, E.A. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2002, 2, 945–956. [Google Scholar] [CrossRef]
- Ward, S.G. T lymphocytes on the move: Chemokines, PI 3-kinase and beyond. Trends Immunol. 2006, 27, 80–87. [Google Scholar] [CrossRef]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.-Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Zhang, J.; Zhou, N.; Liu, X.; Shen, Y. DNA Methylation in Cosmc Promoter Region and Aberrantly Glycosylated IgA1 Associated with Pediatric IgA Nephropathy. PLoS ONE 2015, 10, e0112305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Zhou, Q.; Yang, L.-C.; Li, Z.; Su, B.-H.; Luo, H.; Fan, J.-M. Peripheral B lymphocyte beta1,3-galactosyltransferase and chaperone expression in immunoglobulin A nephropathy. J. Intern. Med. 2005, 258, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhou, X.; Kolosov, V.P.; Perelman, J.M. The Cosmc-mediated effects of neutrophil elastase on T antigen expression in BEAS-2B cells. Respir. Physiol. Neurobiol. 2020, 281, 103496. [Google Scholar] [CrossRef]
- Kudelka, M.R.; Hinrichs, B.H.; Darby, T.; Moreno, C.S.; Nishio, H.; Cutler, C.E.; Wang, J.; Wu, H.; Zeng, J.; Wang, Y.; et al. Cosmc is an X-linked inflammatory bowel disease risk gene that spatially regulates gut microbiota and contributes to sex-specific risk. Proc. Natl. Acad. Sci. USA 2016, 113, 14787–14792. [Google Scholar] [CrossRef] [Green Version]
- Gollamudi, S.; Lekhraj, R.; Lalezari, S.; Lalezari, P. COSMC mutations reduce T-synthase activity in advanced Alzheimer’s disease. Alzheimer’s Dement. 2020, 6, e12040. [Google Scholar] [CrossRef]
- Mazurov, D.; Ilinskaya, A.; Heidecker, G.; Filatov, A. Role of O-Glycosylation and Expression of CD43 and CD45 on the Surfaces of Effector T Cells in Human T Cell Leukemia Virus Type 1 Cell-to-Cell Infection. J. Virol. 2012, 86, 2447–2458. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.N.; Tang, S.C.W.; Schena, F.P.; Novak, J.; Tomino, Y.; Fogo, A.B.; Glassock, R.J. IgA nephropathy. Nat. Rev. Dis. Prim. 2016, 2, 16001. [Google Scholar] [CrossRef]
- Schena, F.P.; Nistor, I. Epidemiology of IgA Nephropathy: A Global Perspective. Semin. Nephrol. 2018, 38, 435–442. [Google Scholar] [CrossRef]
- Zhai, Y.-L.; Zhu, L.; Shi, S.-F.; Liu, L.-J.; Lv, J.-C.; Zhang, H. Increased APRIL Expression Induces IgA1 Aberrant Glycosylation in IgA Nephropathy. Medicine 2016, 95, e3099. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Dwek, R.A.; Edge, C.J.; Rademacher, T.W. O-Linked oligosaccharides from human serum immunoglobulin A1. Biochem. Soc. Trans. 1989, 17, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Hiki, Y.; Iwase, H.; Horii, A.; Tanaka, A.; Nishikido, J.; Hotta, K.; Kobayashi, Y. Evidence for involvement of IgA1 hinge glycopeptide in the IgA1-IgA1 interaction in IgA nephropathy. J. Am. Soc. Nephrol. 1997, 8, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Tomana, M.; Novak, J.; Julian, B.A.; Matousovic, K.; Konecny, K.; Mestecky, J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J. Clin. Investig. 1999, 104, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppo, R.; Amore, A. Aberrant glycosylation in IgA nephropathy (IgAN). Kidney Int. 2004, 65, 1544–1547. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; Gharavi, A.G.; et al. The Pathophysiology of IgA Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Hiki, Y.; Kokubo, T.; Iwase, H.; Masaki, Y.; Sano, T.; Tanaka, A.; Toma, K.; Hotta, K.; Kobayashi, Y. Underglycosylation of IgA1 Hinge Plays a Certain Role for Its Glomerular Deposition in IgA Nephropathy. J. Am. Soc. Nephrol. 1999, 10, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kobayashi, N.; Ikeda, T.; Suzuki, Y.; Tsuge, T.; Horikoshi, S.; Emancipator, S.N.; Tomino, Y. Down-regulation of core 1 β1,3-galactosyltransferase and Cosmc by Th2 cytokine alters O-glycosylation of IgA1. Nephrol. Dial. Transplant. 2010, 25, 3890–3897. [Google Scholar] [CrossRef] [Green Version]
- Vainchenker, W.; Vinci, G.; Testa, U.; Henri, A.; Tabilio, A.; Fache, M.P.; Rochant, H.; Cartron, J.P. Presence of the Tn antigen on hematopoietic progenitors from patients with the Tn syndrome. J. Clin. Investig. 1985, 75, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Berger, E.G. Tn-syndrome. Biochim. Biophys. Acta 1999, 1455, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Yago, T.; Fu, J.; McDaniel, J.M.; Miner, J.J.; McEver, R.P.; Xia, L. Core 1-derived O-glycans are essential E-selectin ligands on neutrophils. Proc. Natl. Acad. Sci. USA 2010, 107, 9204–9209. [Google Scholar] [CrossRef] [PubMed]
- Tjew, S.L.; Brown, K.L.; Kannagi, R.; Johnson, P. Expression of N-acetylglucosamine 6-O-sulfotransferases (GlcNAc6STs)-1 and -4 in human monocytes: GlcNAc6ST-1 is implicated in the generation of the 6-sulfo N-acetyllactosamine/Lewis x epitope on CD44 and is induced by TNF-α. Glycobiology 2005, 15, 7C–13C. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-M.; Nowack, D.D.; Omenn, G.S.; Haab, B.B. Mucin Glycosylation Is Altered by Pro-Inflammatory Signaling in Pancreatic-Cancer Cells. J. Proteome Res. 2009, 8, 1876–1886. [Google Scholar] [CrossRef]
- Livraghi-Butrico, A.; Grubb, B.R.; Wilkinson, K.J.; Volmer, A.S.; A Burns, K.; Evans, C.M.; O’Neal, W.K.; Boucher, R.C. Erratum: Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2017, 10, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsuzzaman; Uddin, S.; Shah, M.A.; Mathew, B. Natural inhibitors on airway mucin: Molecular insight into the therapeutic potential targeting MUC5AC expression and production. Life Sci. 2019, 231, 116485. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.A. Role of Neutrophil Elastase in Hypersecretion During COPD Exacerbations, and Proposed Therapies. Chest 2000, 117, 386S–389S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Perez-Munoz, M.E.; Bergstrom, K.; Peng, V.; Schmaltz, R.; Jimenez-Cardona, R.; Marsteller, N.; McGee, S.; Clavel, T.; Ley, R.; Fu, J.; et al. Discordance between changes in the gut microbiota and pathogenicity in a mouse model of spontaneous colitis. Gut Microbes 2014, 5, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [Green Version]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, M.A.B.; Asker, N.; Hansson, G.C. O-Glycosylated MUC2 Monomer and Dimer from LS 174T Cells Are Water-soluble, whereas Larger MUC2 Species Formed Early during Biosynthesis Are Insoluble and Contain Nonreducible Intermolecular Bonds. J. Biol. Chem. 1998, 273, 18864–18870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Wei, B.; Wen, T.; Johansson, M.E.; Liu, X.; Bradford, E.; Thomsson, K.A.; McGee, S.; Mansour, L.; Tong, M.; et al. Loss of intestinal core 1–derived O-glycans causes spontaneous colitis in mice. J. Clin. Investig. 2011, 121, 1657–1666. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Gao, F.; Slavney, A.; Ma, L.; Waldman, Y.Y.; Sams, A.J.; Billing-Ross, P.; Madar, A.; Spritz, R.; Keinan, A. Accounting for eXentricities: Analysis of the X Chromosome in GWAS Reveals X-Linked Genes Implicated in Autoimmune Diseases. PLoS ONE 2014, 9, e113684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2017, 53, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Cruchaga, C.; Del-Aguila, J.L.; Saef, B.; Black, K.; Fernandez, M.V.; Budde, J.; Ibanez, L.; Deming, Y.; Kapoor, M.; Tosto, G.; et al. Polygenic risk score of sporadic late-onset Alzheimer’s disease reveals a shared architecture with the familial and early-onset forms. Alzheimer’s Dement. 2018, 14, 205–214. [Google Scholar] [CrossRef]
- Tao, P.-F.; Huang, H.-C. Regulation of AβPP Glycosylation Modification and Roles of Glycosylation on AβPP Cleavage in Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 2115–2124. [Google Scholar] [CrossRef]
- Lalezari, P.; Lekhraj, R.; Kimiabakhsh, B.; Carrasco, E.; Casper, D.J.A.S. Dementia. P2–006: Tn antigen in the brain: A newly recognized glycoprotein in Alzheimer’s disease. Alzheimer’s Dement. 2013, 9, P347–P348. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Shmueli, M.D.; Raz, C.; Yanku, M.; Zilberzwige, S.; Gazit, E.; Segal, D. Interplay between protein glycosylation pathways in Alzheimer’s disease. Sci. Adv. 2017, 3, e1601576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cifuentes-Munoz, N.; El Najjar, F.; Dutch, R.E. Viral cell-to-cell spread: Conventional and non-conventional ways. Adv. Virus Res. 2020, 108, 85–125. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.Q.; Silva, M.T.T. The HTLV-1 neurological complex. Lancet Neurol. 2006, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, D.; Cui, J.; Li, J.; Jiang, H. Demethylation of the Cosmc Promoter Alleviates the Progression of Breast Cancer Through Downregulation of the Tn and Sialyl-Tn Antigens. Cancer Manag. Res. 2020, 12, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Kilickap, S.; Kaya, Y.; Yucel, B.; Tuncer, E.; Babacan, N.A.; Elagoz, S. Higher Ki67 expression is associates with unfavorable prognostic factors and shorter survival in breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 1381–1385. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, P.; Dabelsteen, S.; Madsen, F.B.; Francavilla, C.; Kopp, K.L.; Steentoft, C.; Vakhrushev, S.Y.; Olsen, J.V.; Hansen, L.; Bennett, E.P.; et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. USA 2014, 111, E4066–E4075. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, B.T.; Picksak, A.-S.; Kwiatkowski, M.; Grupp, K.; Jücker, M.; Bachmann, K.; Mercanoglu, B.; Izbicki, J.R.; Kahlert, C.; Bockhorn, M.; et al. Truncated O-GalNAc glycans impact on fundamental signaling pathways in pancreatic cancer. Glycobiology. 2021, cwab088. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Xu, F.; Dong, X.; Cheng, Y.; Hu, Y.; Gao, T.; Liu, J.; Yang, L.; Jia, X.; et al. Aberrant O-glycosylation contributes to tumorigenesis in human colorectal cancer. J. Cell. Mol. Med. 2018, 22, 4875–4885. [Google Scholar] [CrossRef] [Green Version]
- Coelho, R.; Marcos-Silva, L.; Mendes, N.; Pereira, D.; Brito, C.; Jacob, F.; Steentoft, C.; Mandel, U.; Clausen, H.; David, L.; et al. Mucins and Truncated O-Glycans Unveil Phenotypic Discrepancies between Serous Ovarian Cancer Cell Lines and Primary Tumours. Int. J. Mol. Sci. 2018, 19, 2045. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Huang, M.-J.; Liao, Y.-Y.; Chen, C.-H.; Huang, M.-C. C1GALT1 Seems to Promote In Vitro Disease Progression in Ovarian Cancer. Int. J. Gynecol. Cancer 2017, 27, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Chen, C.-H.; Chen, Y.-H.; Huang, M.-J.; Huang, J.; Hung, J.-S.; Chen, M.-T.; Huang, M.-C. COSMC Is Overexpressed in Proliferating Infantile Hemangioma and Enhances Endothelial Cell Growth via VEGFR2. PLoS ONE 2013, 8, e56211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinnin, M.; Medici, D.; Park, L.; Limaye, N.; Liu, Y.; Boscolo, E.; Bischoff, J.; Vikkula, M.; Boye, E.; Olsen, B.R. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat. Med. 2008, 14, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Szegezdi, E.; Keane, M.; de Jong, S.; Samali, A. TRAIL receptor signalling and modulation: Are we on the right TRAIL? Cancer Treat. Rev. 2009, 35, 280–288. [Google Scholar] [CrossRef]
- Wagner, K.W.; A Punnoose, E.; Januario, T.; A Lawrence, D.; Pitti, R.M.; Lancaster, K.; Lee, D.; Von Goetz, M.; Yee, S.F.; Totpal, K.; et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 2007, 13, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wen, T.; Yan, R.; Kim, S.; Stowell, S.R.; Wang, W.; Wang, Y.; An, G.; Cummings, R.D.; Ju, T. O-glycans on death receptors in cells modulate their sensitivity to TRAIL-induced apoptosis through affecting on their stability and oligomerization. FASEB J. 2020, 34, 11786–11801. [Google Scholar] [CrossRef]
- Ding, R.; Hu, X.; Hu, W.; Du, Z.; Huang, P.; Wang, M.; Sheng, J.; Ma, Y.; Wang, A.; Luan, X.; et al. Cosmc transfection decreases malignant behavior of Tn+ cells and enhances sensitivity to apoptosis when induced by Apo2L/TRAIL via alteration of O-glycan structure. Aging 2021, 13, 23393–23406. [Google Scholar] [CrossRef]
- Hofmann, B.T.; Schlüter, L.; Lange, P.; Mercanoglu, B.; Ewald, F.; Fölster, A.; Picksak, A.-S.; Harder, S.; El Gammal, A.T.; Grupp, K.; et al. COSMC knockdown mediated aberrant O-glycosylation promotes oncogenic properties in pancreatic cancer. Mol. Cancer 2015, 14, 109. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Garg, M. Mechanistic regulation of epithelial-to-mesenchymal transition through RAS signaling pathway and therapeutic implications in human cancer. J. Cell Commun. Signal. 2018, 12, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Liu, J.; Dong, X.; Hu, X.; Jiang, Y.; Li, L.; Du, T.; Yang, L.; Wen, T.; An, G.; et al. Tn antigen promotes human colorectal cancer metastasis via H-Ras mediated epithelial-mesenchymal transition activation. J. Cell. Mol. Med. 2019, 23, 2083–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiguchi, K.; Shirakihara, T.; Nakano, A.; Imamura, T.; Miyazono, K.; Saitoh, M. Role of Ras Signaling in the Induction of Snail by Transforming Growth Factor-β. J. Biol. Chem. 2009, 284, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Yoh, K.E.; Regunath, K.; Guzman, A.; Lee, S.-M.; Pfister, N.T.; Akanni, O.; Kaufman, L.J.; Prives, C.; Prywes, R. Repression of p63 and induction of EMT by mutant Ras in mammary epithelial cells. Proc. Natl. Acad. Sci. USA 2016, 113, E6107–E6116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. 2016, 11, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [Green Version]
- Bidlingmaier, S.; Zhu, X.; Liu, B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J. Mol. Med. 2008, 86, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Cui, C.; Ge, Y.; Chen, H.; Li, Q.; Yang, Z.; Wu, G.; Sun, S.; Chen, K.; Gu, J.; et al. α2,3-Sialylation regulates the stability of stem cell marker CD133. J. Biochem. 2010, 148, 273–280. [Google Scholar] [CrossRef]
- Mereiter, S.; Martins, M.; Gomes, C.; Balmaña, M.; Macedo, J.A.; Polom, K.; Roviello, F.; Magalhães, A.; Reis, C.A. O-glycan truncation enhances cancer-related functions of CD 44 in gastric cancer. FEBS Lett. 2019, 593, 1675–1689. [Google Scholar] [CrossRef]
- Costello, R.; A Gastaut, J.; Olive, D. Tumor escape from immune surveillance. Arch. Immunol. Ther. Exp. 1999, 47, 83–88. [Google Scholar]
- Rodríguez, E.; Schetters, S.T.T.; Van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- McGreal, E.P.; Miller, J.L.; Gordon, S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr. Opin. Immunol. 2005, 17, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Higashi, N.; Fujioka, K.; Denda-Nagai, K.; Hashimoto, S.-I.; Nagai, S.; Sato, T.; Fujita, Y.; Morikawa, A.; Tsuiji, M.; Miyata-Takeuchi, M.; et al. The Macrophage C-type Lectin Specific for Galactose/N-Acetylgalactosamine Is an Endocytic Receptor Expressed on Monocyte-derived Immature Dendritic Cells. J. Biol. Chem. 2002, 277, 20686–20693. [Google Scholar] [CrossRef] [Green Version]
- Zizzari, I.G.; Napoletano, C.; Battisti, F.; Rahimi, H.; Caponnetto, S.; Pierelli, L.; Nuti, M.; Rughetti, A. MGL Receptor and Immunity: When the Ligand Can Make the Difference. J. Immunol. Res. 2015, 2015, 4506951. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, S.J.; Gringhuis, S.I.; Geijtenbeek, T.B.H.; Van Kooyk, Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat. Immunol. 2006, 7, 1200–1208. [Google Scholar] [CrossRef]
- van Vliet, S.J.; van Liempt, E.; Geijtenbeek, T.B.; van Kooyk, Y. Differential regulation of C-type lectin expression on tolerogenic dendritic cell subsets. Immunobiology 2006, 211, 577–585. [Google Scholar] [CrossRef] [PubMed]
- da Costa, V.; van Vliet, S.J.; Carasi, P.; Frigerio, S.; García, P.A.; Croci, D.O.; Festari, M.F.; Costa, M.; Landeira, M.; Rodríguez-Zraquia, S.A.; et al. The Tn antigen promotes lung tumor growth by fostering immunosuppression and angiogenesis via interaction with Macrophage Galactose-type lectin 2 (MGL2). Cancer Lett. 2021, 518, 72–81. [Google Scholar] [CrossRef]
- Jentoft, N. Why are proteins O-glycosylated? Trends Biochem. Sci. 1990, 15, 291–294. [Google Scholar] [CrossRef]
- Madsen, C.B.; Lavrsen, K.; Steentoft, C.; Vester-Christensen, M.B.; Clausen, H.; Wandall, H.H.; Pedersen, A.E. Glycan Elongation Beyond the Mucin Associated Tn Antigen Protects Tumor Cells from Immune-Mediated Killing. PLoS ONE 2013, 8, e72413. [Google Scholar] [CrossRef]
- Gubbels, J.A.A.; Felder, M.; Horibata, S.; Belisle, J.A.; Kapur, A.; Holden, H.; Petrie, S.; Migneault, M.; Rancourt, C.; Connor, J.P.; et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer 2010, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hida, K.; Maishi, N.; Torii, C.; Hida, Y. Tumor angiogenesis—Characteristics of tumor endothelial cells. Int. J. Clin. Oncol. 2016, 21, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-Q.; Du, W.-L.; Cai, M.-H.; Yao, J.-Y.; Zhao, Y.-Y.; Mou, X.-Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K.B.; Costello, C.E.; Rahimi, N. Glycosylation in the Tumor Microenvironment: Implications for Tumor Angiogenesis and Metastasis. Cells 2019, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Tong, Y.; Li, Z.; Yan, H.; Ye, F.; Wang, Y.; XC, X. C1GALT1C1/COSMC is a novel prognostic biomarker for hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 310–320. [Google Scholar] [CrossRef]
| Location | Funtion | Citation |
|---|---|---|
| Platelet | Mediates platelet production by affecting the expression and function of platelet glycoproteins. | [16] |
| Kidney | Maintains the normal function of podocyte cells in the kidney. | [3] |
| B cell | Mediate the homing of B cells by affecting chemokines and can maintain the immune tolerance of B cells. | [17,37] |
| T cell | Mediates the homing of T cells and maintains the presence of peripheral T cells. | [38] |
| Macrophage cell | Affect the phagocytosis of apoptotic cells by macrophages. | [39] |
| Diseases | Effects | Citation |
|---|---|---|
| Immunoglobulin A Nephropathy | Downregulation of Cosmc expression causes abnormal glycosylation of IgA1, which is involved in the pathogenesis of IGAN. | [53,54] |
| Tn syndrome | Loss of Cosmc function causes abnormal expression of Tn antigens, resulting in Tn syndrome. | [16,18] |
| Inflammatory pneumonia | Cosmc regulates the glycosylation of airway mucin 5AC via T antigen and plays an important role in the stimulation of T antigen overexpression by the inflammatory factor neutrophil elastase. | [55] |
| Inflammatory bowel disease | Cosmc spatially regulates the intestinal microbiota in a region-specific manner, and its functional deficiency causes a decrease in intestinal mucosal MUC2 protein, causing IBD with a sex-specific profile. | [56] |
| Alzheimer’s disease | Cosmc mutations cause abnormal glycosylation in late-onset AD and affect the progression of AD. | [57] |
| HTLV-1 infection | Cosmc enhances HTLV-1 virus infection between cells by affecting the glycosylation of CD43 and CD45. | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, T.; Qiao, M.; Xie, J.; Li, Z.; Xie, H. Emerging Roles of the Unique Molecular Chaperone Cosmc in the Regulation of Health and Disease. Biomolecules 2022, 12, 1732. https://doi.org/10.3390/biom12121732
Xiang T, Qiao M, Xie J, Li Z, Xie H. Emerging Roles of the Unique Molecular Chaperone Cosmc in the Regulation of Health and Disease. Biomolecules. 2022; 12(12):1732. https://doi.org/10.3390/biom12121732
Chicago/Turabian StyleXiang, Ting, Muchuan Qiao, Jiangbo Xie, Zheng Li, and Hailong Xie. 2022. "Emerging Roles of the Unique Molecular Chaperone Cosmc in the Regulation of Health and Disease" Biomolecules 12, no. 12: 1732. https://doi.org/10.3390/biom12121732
APA StyleXiang, T., Qiao, M., Xie, J., Li, Z., & Xie, H. (2022). Emerging Roles of the Unique Molecular Chaperone Cosmc in the Regulation of Health and Disease. Biomolecules, 12(12), 1732. https://doi.org/10.3390/biom12121732






