Gabapentin Disrupts Binding of Perlecan to the α2δ1 Voltage Sensitive Calcium Channel Subunit and Impairs Skeletal Mechanosensation
Abstract
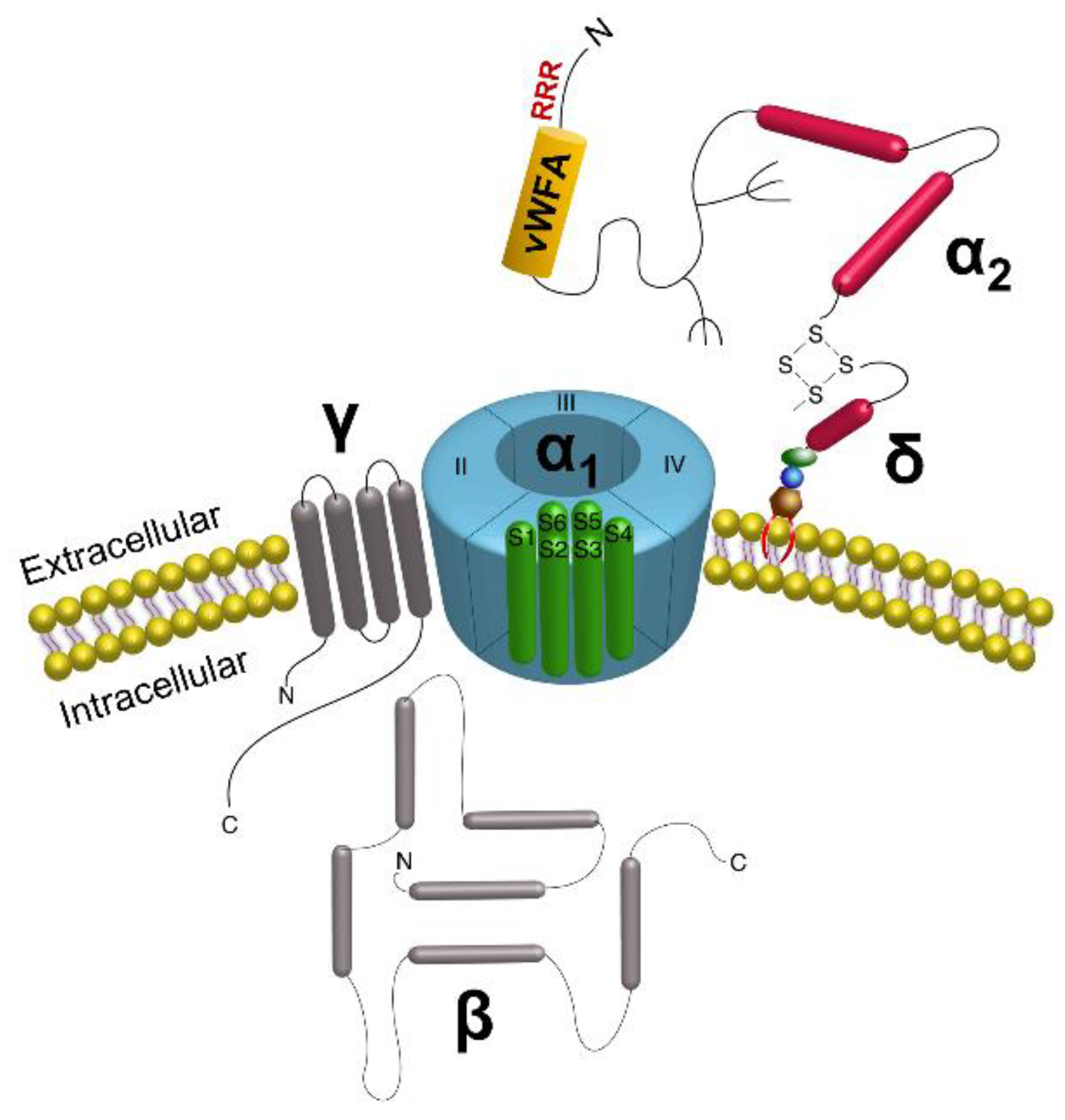
:1. Introduction
2. Materials and Methods
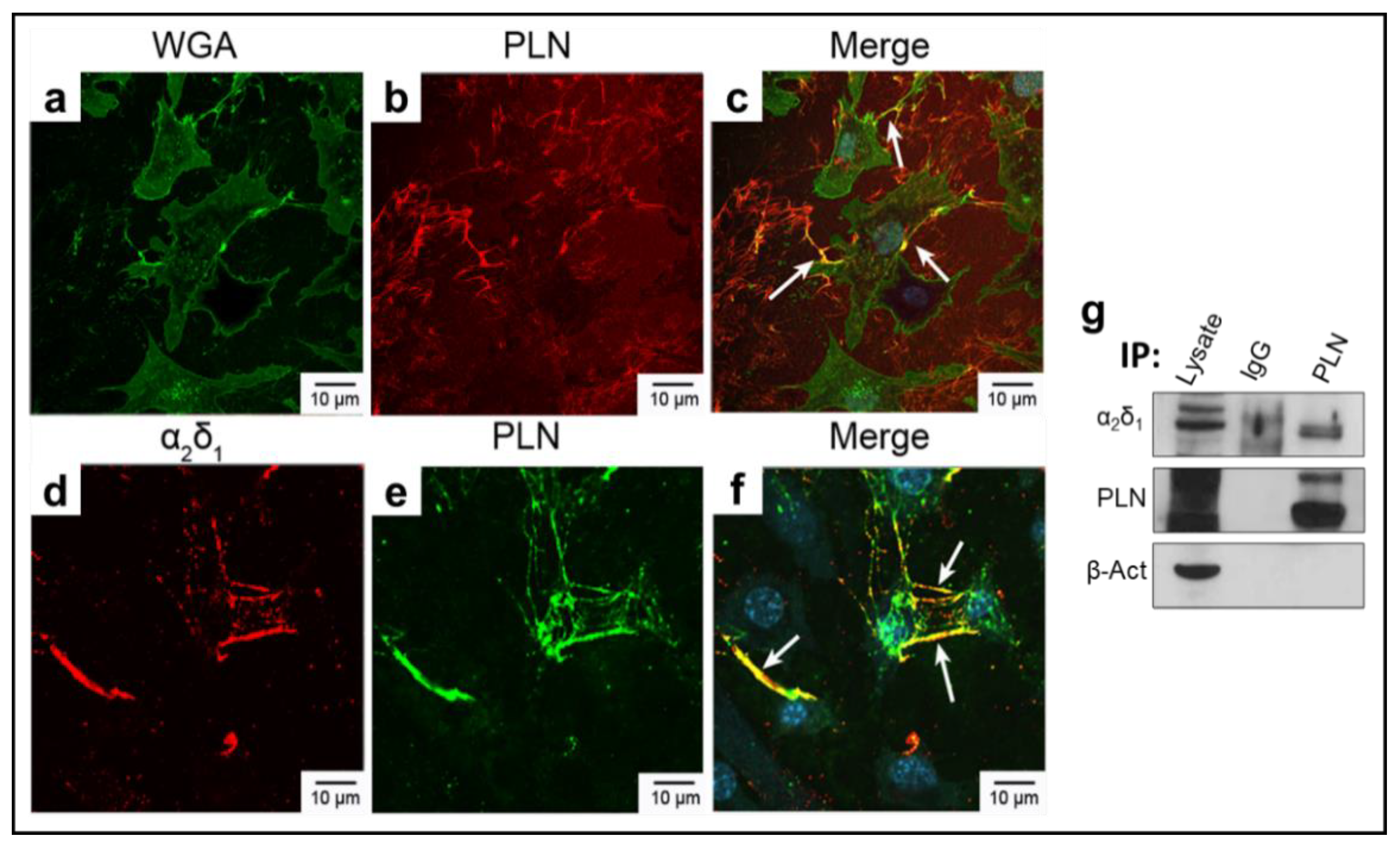
2.1. Cell Culture and Immunofluorescence
2.2. Co-Immunoprecipitation and Western Blotting
2.3. Recombinant α2δ1 Polypeptides
2.4. Recombinant Perlecan Proteins
2.4.1. Full-Length Perlecan
2.4.2. Perlecan Domains I, III and IV
2.4.3. Perlecan Domain V
2.5. Localized Surface Plasmon Resonance (LSPR) Experiments
2.5.1. Synthesis of Gold Triangular Nanoprisms (Au TNPs)
2.5.2. α2-Functionalized Au TNPs
2.5.3. Protein Binding Curves and Spectroscopy Characterization
2.5.4. Data Processing
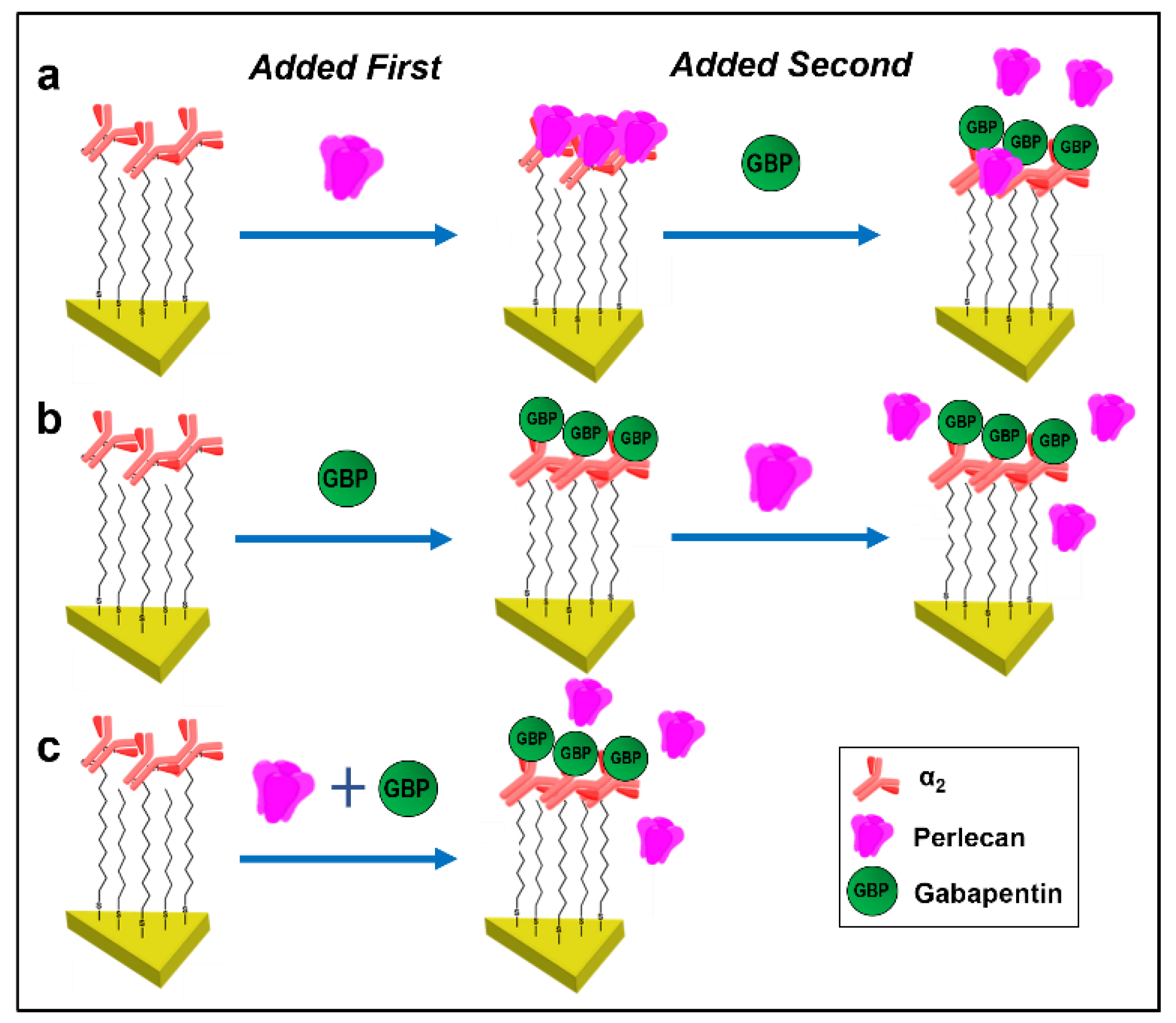
2.6. Drug Binding Experiments
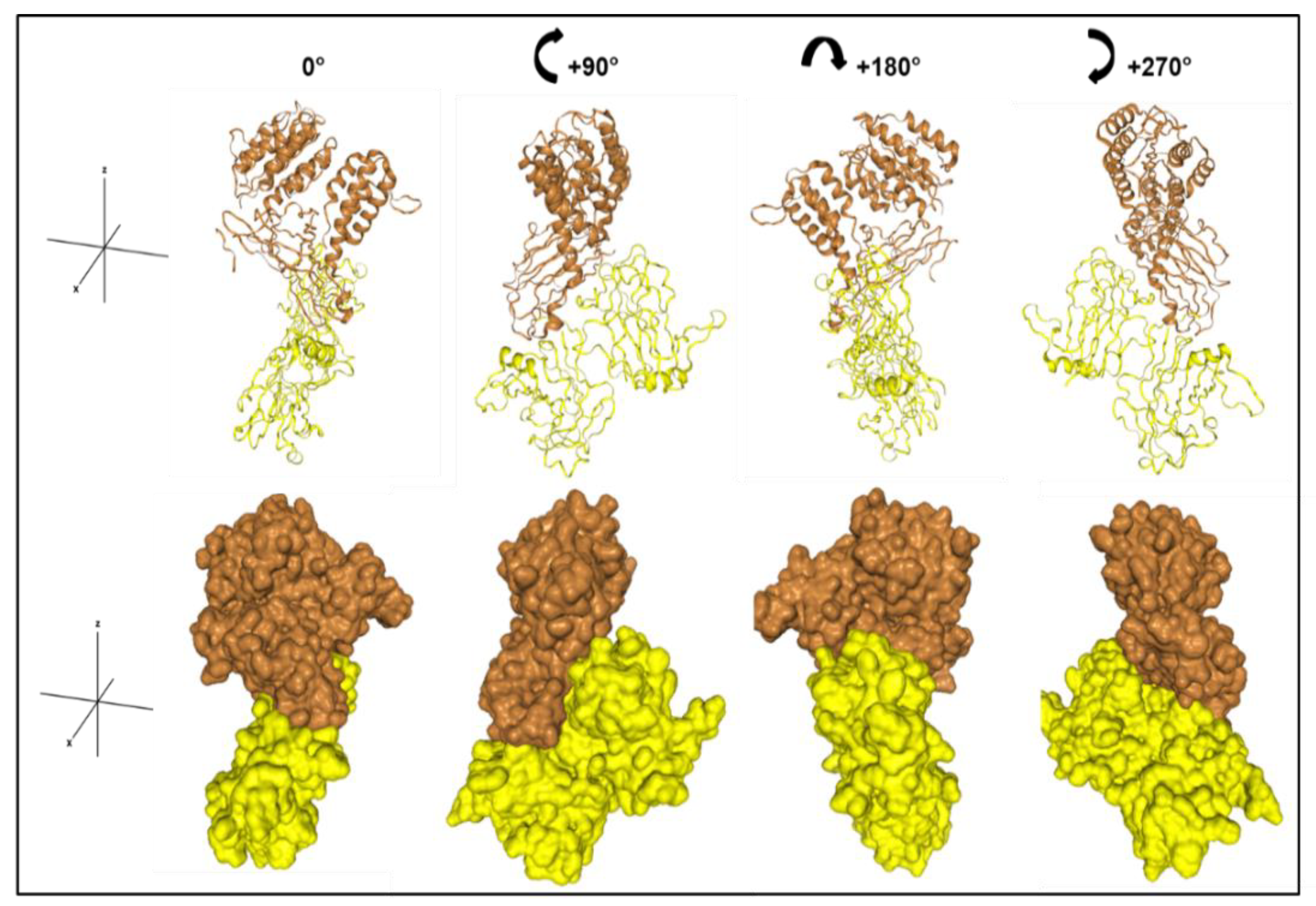
2.7. Docking Models
2.8. Animal Experiments and In Vivo Ulnar Loading
2.9. Dynamic Histomorphometry
3. Results
3.1. α2δ1 and Perlecan (PLN) Co-Localize in Murine Osteocyte-like Cells
3.2. α2δ1 and PLN Bind with High Affinity, Which Is Mediated by PLN Domain III-2
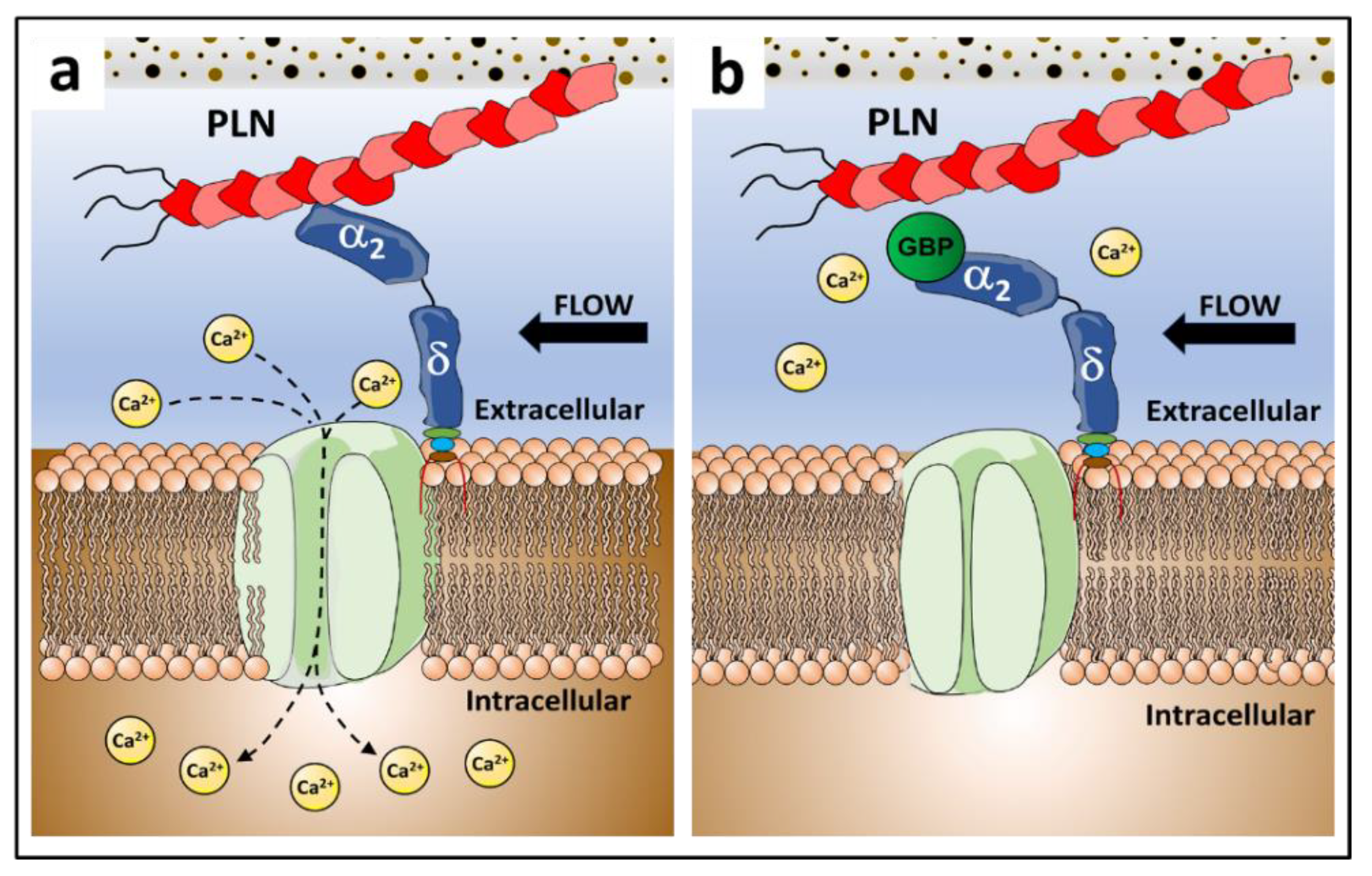
3.3. Gabapentin Interferes with PLN::α2δ1 Binding
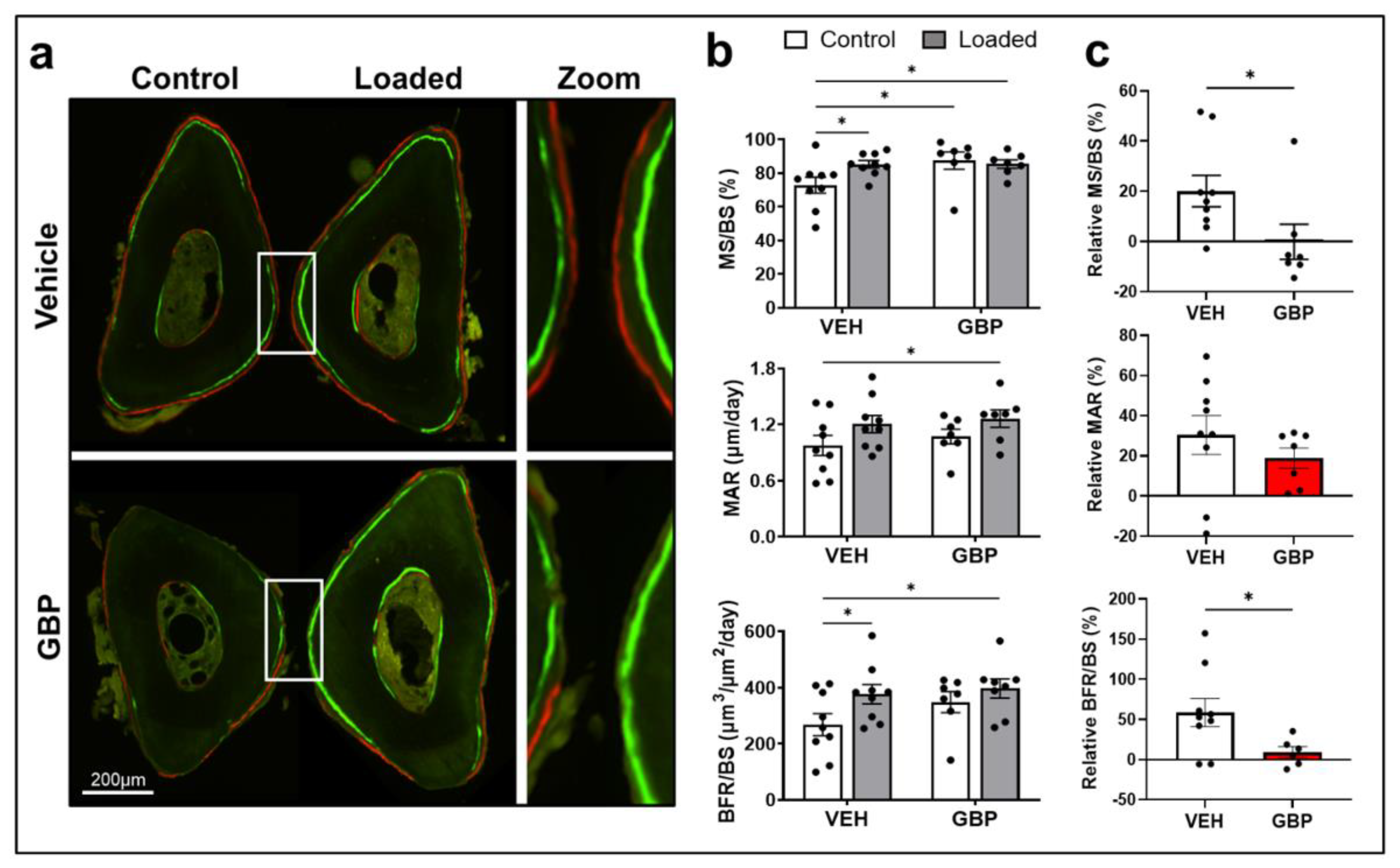
3.4. Gabapentin Impairs Bone Mechanosensation In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Lanyon, L. Regulation of bone formation by applied dynamic loads. J. Bone Jt. Surg. 1984, 66, 397–402. [Google Scholar] [CrossRef]
- Fritton, S.P.; McLeod, K.J.; Rubin, C.T. Quantifying the strain history of bone: Spatial uniformity and self-similarity of low-magnitude strains. J. Biomech. 2000, 33, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; Semeins, C.M.; Veldhuijzen, J.P.; Burger, E.H. Effect of mechanical stimulation on the production of soluble bone factors in cultured fetal mouse calvariae. Cell Tissue Res. 1993, 271, 513–517. [Google Scholar] [CrossRef]
- You, J.; Yellowley, C.E.; Donahue, H.J.; Zhang, Y.; Chen, Q.; Jacobs, C.R. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J. Biomech. Eng. Trans. ASME 2000, 122, 387–393. [Google Scholar] [CrossRef]
- Smalt, R.; Mitchell, F.T.; Howard, R.L.; Chambers, T.J. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am. J. Physiol. 1997, 273, E751–E758. [Google Scholar]
- You, L.D.; Weinbaum, S.; Cowin, S.C.; Schaffler, M.B. Ultrastructure of the osteocyte process and its pericellular matrix. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. Off. Publ. Am. Assoc. Anat. 2004, 278, 505–513. [Google Scholar] [CrossRef]
- Han, Y.; Cowin, S.C.; Schaffler, M.B.; Weinbaum, S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc. Natl. Acad. Sci. USA 2004, 101, 16689–16694. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.R.; Modla, S.; Grindel, B.J.; Czymmek, K.J.; Kirn-Safran, C.B.; Wang, L.; Duncan, R.L.; Farach-Carson, M.C. Perlecan/Hspg2 deficiency alters the pericellular space of the lacunocanalicular system surrounding osteocytic processes in cortical bone. J. Bone Miner. Res. 2011, 26, 618–629. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Lai, X.; Price, C.; Thompson, W.R.; Li, W.; Quabili, T.R.; Tseng, W.J.; Liu, X.S.; Zhang, H.; Pan, J.; et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J. Bone Miner. Res. 2014, 29, 878–891. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.T.; Allen, F.D.; Pollack, S.R.; Brighton, C.T. Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J. Biomech. 1996, 29, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Duncan, R.L.; Burr, D.B.; Gattone, V.H.; Turner, C.H. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology 2003, 144, 1226–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Duncan, R.L.; Burr, D.B.; Turner, C.H. L-type calcium channels mediate mechanically induced bone formation in vivo. J. Bone Miner. Res. 2002, 17, 1795–1800. [Google Scholar] [CrossRef]
- Pei, S.; Parthasarathy, S.; Parajuli, A.; Martinez, J.; Lv, M.; Jiang, S.; Wu, D.; Wei, S.; Lu, X.L.; Farach-Carson, M.C. Perlecan/Hspg2 deficiency impairs bone’s calcium signaling and associated transcriptome in response to mechanical loading. Bone 2020, 131, 115078. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C. Calcium channel auxiliary alpha2delta and beta subunits: Trafficking and one step beyond. Nat. Rev. Neurosci. 2012, 13, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Shirokov, R.; Ferreira, G.; Yi, J.; Ríos, E. Inactivation of gating currents of L-type calcium channels: Specific role of the α2δ subunit. J. Gen. Physiol. 1998, 111, 807–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shistik, E.; Ivanina, T.; Puri, T.; Hosey, M.; Dascal, N. Ca2+ current enhancement by alpha 2/delta and beta subunits in Xenopus oocytes: Contribution of changes in channel gating and alpha 1 protein level. J. Physiol. 1995, 489, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, R.; Mehrke, G.; Gingrich, K.; Hofmann, F.; Kass, R. Influence of L-type Ca channel alpha 2/delta-subunit on ionic and gating current in transiently transfected HEK 293 cells. Am. J. Physiol. Heart Circ. Physiol. 1996, 270, H1521–H1528. [Google Scholar] [CrossRef]
- Wright, C.S.; Robling, A.G.; Farach-Carson, M.C.; Thompson, W.R. Skeletal Functions of Voltage Sensitive Calcium Channels. Curr. Osteoporos. Rep. 2021, 19, 206–221. [Google Scholar] [CrossRef]
- Gee, N.S.; Brown, J.P.; Dissanayake, V.U.; Offord, J.; Thurlow, R.; Woodruff, G.N. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J. Biol. Chem. 1996, 271, 5768–5776. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Offord, J.; Oxender, D.L.; Su, T.Z. Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. Biochem. J. 1999, 342 Pt 2, 313–320. [Google Scholar] [CrossRef]
- Jette, N.; Lix, L.M.; Metge, C.J.; Prior, H.J.; McChesney, J.; Leslie, W.D. Association of antiepileptic drugs with nontraumatic fractures: A population-based analysis. Arch. Neurol. 2011, 68, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Ensrud, K.E.; Walczak, T.S.; Blackwell, T.L.; Ensrud, E.R.; Barrett-Connor, E.; Orwoll, E.S.; Osteoporotic Fractures in Men Study Research, G. Antiepileptic drug use and rates of hip bone loss in older men: A prospective study. Neurology 2008, 71, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Sofu, H.; Kockara, N.; Aydin, B.K.; Suleyman, B.; Tayfur, M.; Malkoc, I. Should orthopedic surgeons consider the effects of gabapentin administration on bone healing while treating a long bone fracture: Experimental study in a rat model. SICOT J. 2016, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Bonewald, L.F. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Metab. 1999, 17, 61–65. [Google Scholar] [CrossRef]
- Kato, Y.; Windle, J.J.; Koop, B.A.; Mundy, G.R.; Bonewald, L.F. Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 1997, 12, 2014–2023. [Google Scholar] [CrossRef]
- Shao, Y.; Alicknavitch, M.; Farach-Carson, M.C. Expression of voltage sensitive calcium channel (VSCC) L-type Cav1.2 (α1C) and T-type Cav3.2 (α1H) subunits during mouse bone development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 234, 54–62. [Google Scholar]
- Thompson, W.R.; Majid, A.S.; Czymmek, K.J.; Ruff, A.L.; García, J.; Duncan, R.L.; Farach-Carson, M.C. Association of the α2δ1 subunit with Cav3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. J. Bone Miner. Res. 2011, 26, 2125–2139. [Google Scholar] [CrossRef] [Green Version]
- García, K.; Nabhani, T.; García, J. The calcium channel α2/δ1 subunit is involved in extracellular signalling. J. Physiol. 2008, 586, 727–738. [Google Scholar] [CrossRef]
- Thompson, W.R.; Guilluy, C.; Xie, Z.; Sen, B.; Brobst, K.E.; Yen, S.S.; Uzer, G.; Styner, M.; Case, N.; Burridge, K.; et al. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells 2013, 31, 2528–2537. [Google Scholar] [CrossRef] [Green Version]
- Noonan, D.M.; Fulle, A.; Valente, P.; Cai, S.; Horigan, E.; Sasaki, M.; Yamada, Y.; Hassell, J.R. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J. Biol. Chem. 1991, 266, 22939–22947. [Google Scholar] [CrossRef]
- Grindel, B.; Martinez, J.; Pennington, C.; Muldoon, M.; Stave, J.; Chung, L.; Farach-Carson, M. Matrilysin/matrix metalloproteinase-7 (MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol. 2014, 36, 64–76. [Google Scholar] [CrossRef]
- Tellman, T.V.; Cruz, L.A.; Grindel, B.J.; Farach-Carson, M.C. Cleavage of the Perlecan-Semaphorin 3A-Plexin A1-Neuropilin-1 (PSPN) Complex by Matrix Metalloproteinase 7/Matrilysin Triggers Prostate Cancer Cell Dyscohesion and Migration. Int. J. Mol. Sci. 2021, 22, 3218. [Google Scholar] [CrossRef]
- Fuki, I.V.; Iozzo, R.V.; Williams, K.J. Perlecan heparan sulfate proteoglycan: A novel receptor that mediates a distinct pathway for ligand catabolism. J. Biol. Chem. 2000, 275, 25742–25750. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, F.H.B.; Lewis, M.S.; Truong, A.; Carson, D.D.; Farach-Carson, M.C. SULF1 suppresses Wnt3A-driven growth of bone metastatic prostate cancer in perlecan-modified 3D cancer-stroma-macrophage triculture models. PLoS ONE 2020, 15, e0230354. [Google Scholar]
- Yang, W.; Gomes , R., Jr.; Alicknavitch, M.; Farach-Carson, M.; Carson, D. Perlecan domain I promotes fibroblast growth factor 2 delivery in collagen I fibril scaffolds. Tissue Eng. 2005, 11, 76–89. [Google Scholar] [CrossRef]
- Couchman, J.R.; Ljubimov, A.V.; Sthanam, M.; Horchar, T.; Hassell, J.R. Antibody mapping and tissue localization of globular and cysteine-rich regions of perlecan domain III. J. Histochem. Cytochem. 1995, 43, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Clarke, D.; Al Ahmad, A.; Kahle, M.; Parham, C.; Auckland, L.; Shaw, C.; Fidanboylu, M.; Orr, A.W.; Ogunshola, O.; et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J. Clin. Investig. 2011, 121, 3005–3023. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape-and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef]
- Sardar, R.; Shumaker-Parry, J.S. Spectroscopic and microscopic investigation of gold nanoparticle formation: Ligand and temperature effects on rate and particle size. J. Am. Chem. Soc. 2011, 133, 8179–8190. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Joshi, G.K.; McClory, P.J.; Dolai, S.; Sardar, R. Improved localized surface plasmon resonance biosensing sensitivity based on chemically-synthesized gold nanoprisms as plasmonic transducers. J. Mater. Chem. 2012, 22, 923–931. [Google Scholar] [CrossRef]
- Joshi, G.K.; McClory, P.J.; Muhoberac, B.B.; Kumbhar, A.; Smith, K.A.; Sardar, R. Designing Efficient Localized Surface Plasmon Resonance-Based Sensing Platforms: Optimization of Sensor Response by Controlling the Edge Length of Gold Nanoprisms. J. Phys. Chem. C 2012, 116, 20990–21000. [Google Scholar] [CrossRef]
- Haes, A.J.; Hall, W.P.; Chang, L.; Klein, W.L.; Van Duyne, R.P. A localized surface plasmon resonance biosensor: A first steps toward an assay for Alzheimer’s disease. Nano Lett. 2004, 4, 1029–1034. [Google Scholar] [CrossRef]
- Beeram, S.R.; Zamborini, F.P. Selective Attachment of Antibodies to the Edges of Gold Nanostructures for Enhanced Localized Surface Plasmon Resonance Biosensing. J. Am. Chem. Soc. 2009, 131, 11689–11691. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.; Sangha, A.; Sardar, R. Achieving biosensing at attomolar concentrations of cardiac troponin T in human biofluids by developing a label-free nanoplasmonic analytical assay. Analyst 2017, 142, 2442–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masterson, A.N.; Liyanage, T.; Berman, C.; Kaimakliotis, H.; Johnson, M.; Sardar, R. A novel liquid biopsy-based approach for highly specific cancer diagnostics: Mitigating false responses in assaying patient plasma-derived circulating microRNAs through combined SERS and plasmon-enhanced fluorescence analyses. Analyst 2020, 145, 4173–4180. [Google Scholar] [CrossRef]
- Hati, S.; Langlais, S.R.; Masterson, A.N.; Liyanage, T.; Muhoberac, B.B.; Kaimakliotis, H.; Johnson, M.; Sardar, R. Photoswitchable Machine-Engineered Plasmonic Nanosystem with High Optical Response for Ultrasensitive Detection of microRNAs and Proteins Adaptively. Anal. Chem. 2021, 93, 13935–13944. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.-Y. HDOCK: A web server for protein–protein and protein–DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, T.; Gat, Y.; Fass, D. Laminin L4 domain structure resembles adhesion modules in ephrin receptor and other transmembrane glycoproteins. FEBS J. 2015, 282, 2746–2757. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coderre, T.J.; Kumar, N.; Lefebvre, C.D.; Yu, J.S. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J. Neurochem. 2005, 94, 1131–1139. [Google Scholar] [CrossRef]
- Kuraishi, Y.; Iida, Y.; Zhang, H.-W.; Uehara, S.; Nojima, H.; Murata, J.; Saiki, I.; Takahata, H.; Ouchi, H. Suppression by gabapentin of pain-related mechano-responses in mice given orthotopic tumor inoculation. Biol. Pharm. Bull. 2003, 26, 550–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.N. The Hill equation revisited: Uses and misuses. FASEB J. 1997, 11, 835–841. [Google Scholar] [CrossRef]
- Wallner, B.; Elofsson, A. Can correct protein models be identified? Protein Sci. 2003, 12, 1073–1086. [Google Scholar] [CrossRef] [Green Version]
- Siew, N.; Elofsson, A.; Rychlewski, L.; Fischer, D. MaxSub: An automated measure for the assessment of protein structure prediction quality. Bioinformatics 2000, 16, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.R.; Rubin, C.T.; Rubin, J. Mechanical regulation of signaling pathways in bone. Gene 2012, 503, 179–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compton, J.T.; Lee, F.Y. A review of osteocyte function and the emerging importance of sclerostin. J. Bone Jt. Surg. 2014, 96, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loiselle, A.E.; Paul, E.M.; Lewis, G.S.; Donahue, H.J. Osteoblast and osteocyte-specific loss of Connexin43 results in delayed bone formation and healing during murine fracture healing. J. Orthop. Res. 2013, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Cherian, P.P.; Siller-Jackson, A.J.; Gu, S.; Wang, X.; Bonewald, L.F.; Sprague, E.; Jiang, J.X. Mechanical Strain Opens Connexin 43 Hemichannels in Osteocytes: A Novel Mechanism for the Release of Prostaglandin. Mol. Biol. Cell 2005, 16, 3100–3106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzer, G.; Pongkitwitoon, S.; Ian, C.; Thompson, W.R.; Rubin, J.; Chan, M.E.; Judex, S. Gap Junctional Communication in Osteocytes Is Amplified by Low Intensity Vibrations In Vitro. PLoS ONE 2014, 9, e90840. [Google Scholar] [CrossRef] [PubMed]
- Cabahug-Zuckerman, P.; Stout Jr, R.F.; Majeska, R.J.; Thi, M.M.; Spray, D.C.; Weinbaum, S.; Schaffler, M.B. Potential role for a specialized β3 integrin-based structure on osteocyte processes in bone mechanosensation. J. Orthop. Res. 2018, 36, 642–652. [Google Scholar]
- Lee, D.Y.; Li, Y.S.J.; Chang, S.F.; Zhou, J.; Ho, H.M.; Chiu, J.J.; Chien, S. Oscillatory Flow-induced Proliferation of Osteoblast-like Cells Is Mediated by αvδ3 and δ1 Integrins through Synergistic Interactions of Focal Adhesion Kinase and Shc with Phosphatidylinositol 3-Kinase and the Akt/mTOR/p70S6K Pathway. J. Biol. Chem. 2010, 285, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Sen, B.; Xie, Z.; Case, N.; Thompson, W.R.; Uzer, G.; Styner, M.; Rubin, J. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J. Bone Miner. Res. 2014, 29, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.R.; Yen, S.S.; Uzer, G.; Xie, Z.; Sen, B.; Styner, M.; Burridge, K.; Rubin, J. LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage. Bone 2018, 107, 172–180. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kameo, Y.; Kamioka, H.; Adachi, T. High-resolution image-based simulation reveals membrane strain concentration on osteocyte processes caused by tethering elements. Biomech. Model. Mechanobiol. 2021, 20, 2353–2360. [Google Scholar] [CrossRef]
- Brown, G.N.; Leong, P.L.; Guo, X.E. T-Type voltage-sensitive calcium channels mediate mechanically-induced intracellular calcium oscillations in osteocytes by regulating endoplasmic reticulum calcium dynamics. Bone 2016, 88, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genetos, D.C.; Geist, D.J.; Liu, D.; Donahue, H.J.; Duncan, R.L. Fluid Shear-Induced ATP Secretion Mediates Prostaglandin Release in MC3T3-E1 Osteoblasts. J. Bone Miner. Res. 2005, 20, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C. Voltage-gated calcium channels and their auxiliary subunits: Physiology and pathophysiology and pharmacology. J. Physiol. 2016, 594, 5369–5390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tétreault, M.-P.; Bourdin, B.; Briot, J.; Segura, E.; Lesage, S.; Fiset, C.; Parent, L. Identification of glycosylation sites essential for surface expression of the CaVα2δ1 subunit and modulation of the cardiac Cav1.2 channel activity. J. Biol. Chem. 2016, 291, 4826–4843. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Schaffler, M.B.; Weinbaum, S.; Spray, D.C. Matrix-dependent adhesion mediates network responses to physiological stimulation of the osteocyte cell process. Proc. Natl. Acad. Sci. USA 2013, 110, 12096–12101. [Google Scholar] [CrossRef] [Green Version]
- Wilchek, M.; Bayer, E.A. Introduction to avidin-biotin technology. Methods Enzym. 1990, 184, 5–13. [Google Scholar] [CrossRef]
- Mongiat, M.; Taylor, K.; Otto, J.; Aho, S.; Uitto, J.; Whitelock, J.M.; Iozzo, R.V. The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7. J. Biol. Chem. 2000, 275, 7095–7100. [Google Scholar] [CrossRef] [Green Version]
- Göhring, W.; Sasaki, T.; Heldin, C.H.; Timpl, R. Mapping of the binding of platelet-derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope. Eur. J. Biochem. 1998, 255, 60–66. [Google Scholar] [CrossRef]
- Smith, S.M.-L.; West, L.A.; Hassell, J.R. The core protein of growth plate perlecan binds FGF-18 and alters its mitogenic effect on chondrocytes. Arch. Biochem. Biophys. 2007, 468, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.M.; Bateman, J.F.; Hansen, U.; Wilson, R.; Bruckner, P.; Owens, R.T.; Sasaki, T.; Timpl, R.; Fitzgerald, J. WARP is a novel multimeric component of the chondrocyte pericellular matrix that interacts with perlecan. J. Biol. Chem. 2006, 281, 7341–7349. [Google Scholar] [CrossRef] [Green Version]
- Dolphin, A.C. The α2δ subunits of voltage-gated calcium channels. Biochim. Et Biophys. Acta Biomembr. 2013, 1828, 1541–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensrud, K.E.; Walczak, T.S.; Blackwell, T.; Ensrud, E.R.; Bowman, P.J.; Stone, K.L. Antiepileptic drug use increases rates of bone loss in older women: A prospective study. Neurology 2004, 62, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.A.; Hynes, R.O. Distribution and evolution of von Willebrand/integrin A domains: Widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 2002, 13, 3369–3387. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, C.; Allen, N.J.; Susman, M.W.; O’Rourke, N.A.; Park, C.Y.; Ozkan, E.; Chakraborty, C.; Mulinyawe, S.B.; Annis, D.S.; Huberman, A.D.; et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009, 139, 380–392. [Google Scholar] [CrossRef]
- Kanda, J.; Izumo, N.; Kobayashi, Y.; Onodera, K.; Shimakura, T.; Yamamoto, N.; Takahashi, H.E.; Wakabayashi, H. Effects of the Antiepileptic Drugs Phenytoin, Gabapentin, and Levetiracetam on Bone Strength, Bone Mass, and Bone Turnover in Rats. Biol. Pharm. Bull. 2017, 40, 1934–1940. [Google Scholar] [CrossRef] [Green Version]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Guilak, F.; Hayes, A.J.; Melrose, J. Perlecan in pericellular mechanosensory cell-matrix communication, extracellular matrix stabilisation and mechanoregulation of load-bearing connective tissues. Int. J. Mol. Sci. 2021, 22, 2716. [Google Scholar] [CrossRef]
| Perlecan Domain/Subdomain | KD (M) 1 | R-Squared 2 |
|---|---|---|
| Undigested Full Length | 3.6 × 10−9 | 0.993 |
| Digested Full Length | 2.6 × 10−7 | 0.996 |
| Domain I | 7.7 × 10−6 | 0.990 |
| Domain III-2 | 8.0 × 10−11 | 0.999 |
| Domain III-2 (w/cystine) | 7.7 × 10−6 | 0.993 |
| Domain IV-I | 1.4 × 10−7 | 0.994 |
| Domain IV-2 | 4.3 × 10−4 | 0.983 |
| Domain IV-3 | 2.8 × 10−4 | 0.995 |
| Domain V | 5.1 × 10−3 | 0.979 |
| Exp | Sensors | ΔλLSPR (nm) | Added First to Sensors | ΔλLSPR (nm) | Added Second to Sensors | ΔλLSPR (nm) |
|---|---|---|---|---|---|---|
| 1 | α2 | +39 ± 3.9 | Full length PLN | +14.4 ± 1.5 | GBP | −4.1 ± 0.8 |
| 2 | α2 | +39 ± 3.9 | GBP | +5.8 ± 0.6 | Full length PLN | 0.1 ± 0.1 |
| 3 | α2 | +39 ± 3.9 | Full length PLN + GBP | +2.7 ± 0.6 | - | - |
| 4 | α2 | +39 ± 3.9 | PLN Dm III-2 | +12.7 ± 1.1 | GBP | −4.3 ± 0.9 |
| 5 | α2 | +39 ± 3.9 | GBP | +5.4 ± 0.7 | PLN Dm III-2 | +0.4 ± 0.5 |
| 6 | α2 | +39 ± 3.9 | PLN Dm III-2 + GBP | +4.9 ± 0.9 | - | - |
| VEH 1 Treated Mice | GBP 1 Treated Mice | 2-Way ANOVA 3 | |||||
|---|---|---|---|---|---|---|---|
| Bone Parameters 2 | Non-Loaded Ulna (Ctrl) | Loaded Ulna | Non-Loaded Ulna (Ctrl) | Loaded Ulna | L | T | L×T |
| MS/BS (%) | 72.81 ± 4.7 (9) a | 85.23 ± 2.2 (9) b | 87.43 ± 5.12 (7) b | 85.38 ± 2.51 (7) b | 0.192 | 0.067 | 0.072 |
| MAR (μm/day) | 0.976 ± 0.11 (9) a | 1.21 ± 0.09 (9) ab | 1.072 ± 0.08 (7) ab | 1.26 ± 0.08 (7) b | 0.039 | 0.431 | 0.431 |
| BFR/BS (μm3/μm2/day) | 268.5 ± 39.13 (9) a | 377 ± 34.25 (9) b | 348.9 ± 37.24 (7) ab | 398.1 ± 34 (7) b | 0.041 | 0.181 | 0.417 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes Fernandez, P.C.; Wright, C.S.; Masterson, A.N.; Yi, X.; Tellman, T.V.; Bonteanu, A.; Rust, K.; Noonan, M.L.; White, K.E.; Lewis, K.J.; et al. Gabapentin Disrupts Binding of Perlecan to the α2δ1 Voltage Sensitive Calcium Channel Subunit and Impairs Skeletal Mechanosensation. Biomolecules 2022, 12, 1857. https://doi.org/10.3390/biom12121857
Reyes Fernandez PC, Wright CS, Masterson AN, Yi X, Tellman TV, Bonteanu A, Rust K, Noonan ML, White KE, Lewis KJ, et al. Gabapentin Disrupts Binding of Perlecan to the α2δ1 Voltage Sensitive Calcium Channel Subunit and Impairs Skeletal Mechanosensation. Biomolecules. 2022; 12(12):1857. https://doi.org/10.3390/biom12121857
Chicago/Turabian StyleReyes Fernandez, Perla C., Christian S. Wright, Adrianna N. Masterson, Xin Yi, Tristen V. Tellman, Andrei Bonteanu, Katie Rust, Megan L. Noonan, Kenneth E. White, Karl J. Lewis, and et al. 2022. "Gabapentin Disrupts Binding of Perlecan to the α2δ1 Voltage Sensitive Calcium Channel Subunit and Impairs Skeletal Mechanosensation" Biomolecules 12, no. 12: 1857. https://doi.org/10.3390/biom12121857
APA StyleReyes Fernandez, P. C., Wright, C. S., Masterson, A. N., Yi, X., Tellman, T. V., Bonteanu, A., Rust, K., Noonan, M. L., White, K. E., Lewis, K. J., Sankar, U., Hum, J. M., Bix, G., Wu, D., Robling, A. G., Sardar, R., Farach-Carson, M. C., & Thompson, W. R. (2022). Gabapentin Disrupts Binding of Perlecan to the α2δ1 Voltage Sensitive Calcium Channel Subunit and Impairs Skeletal Mechanosensation. Biomolecules, 12(12), 1857. https://doi.org/10.3390/biom12121857











