Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension
Abstract
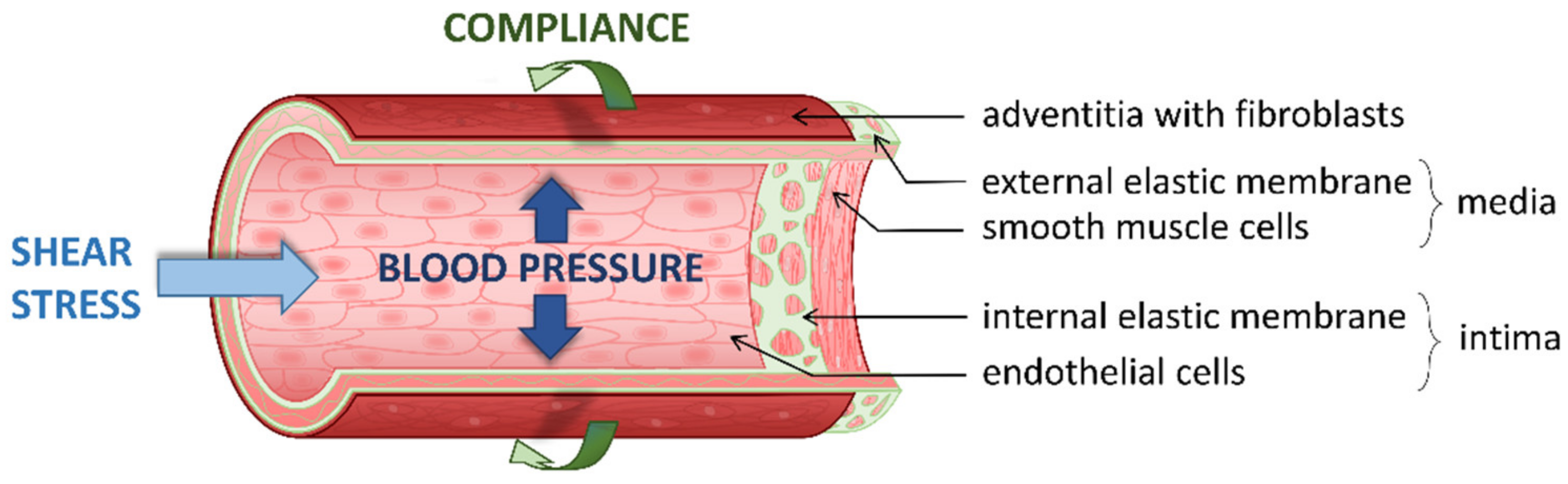
1. Introduction
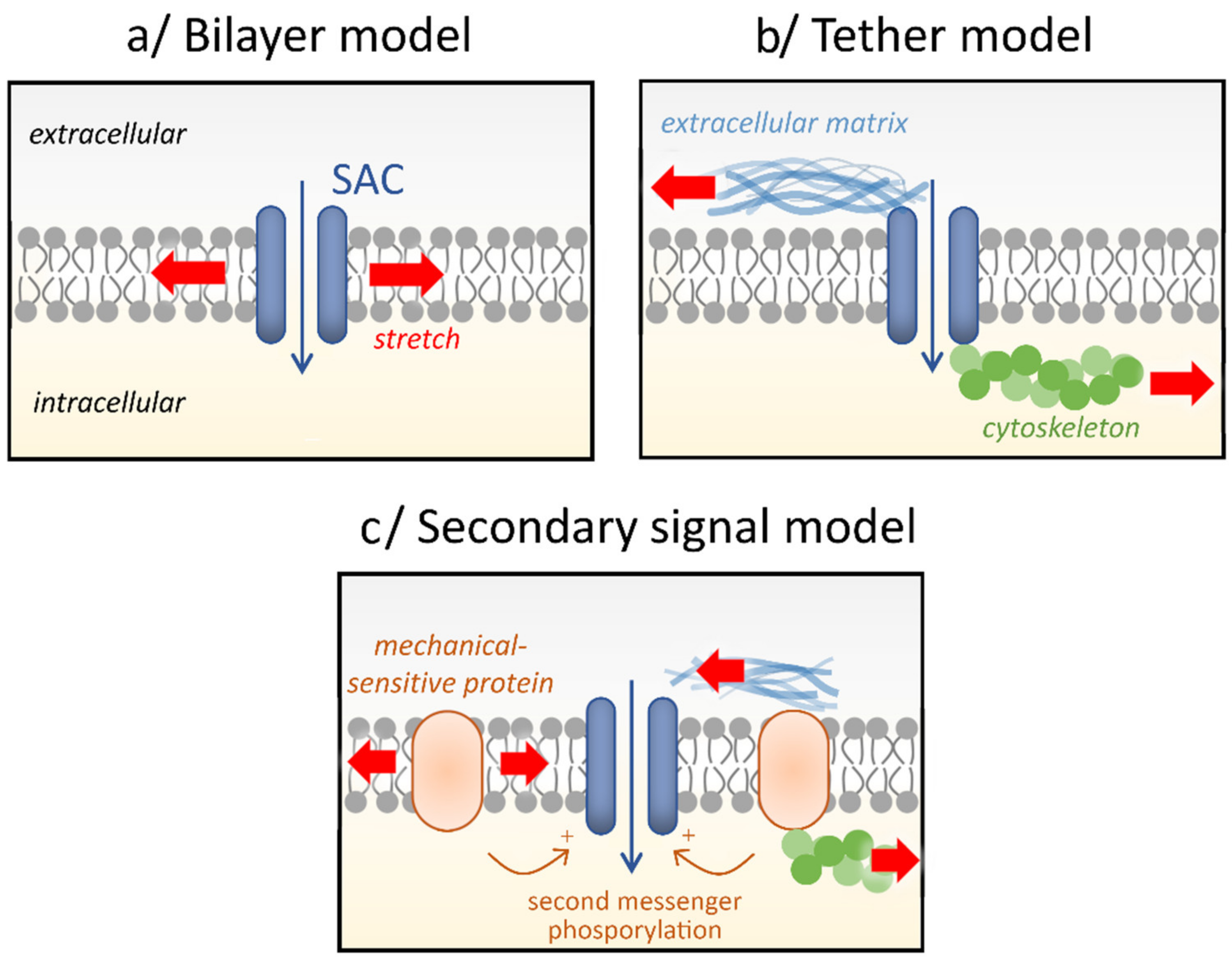
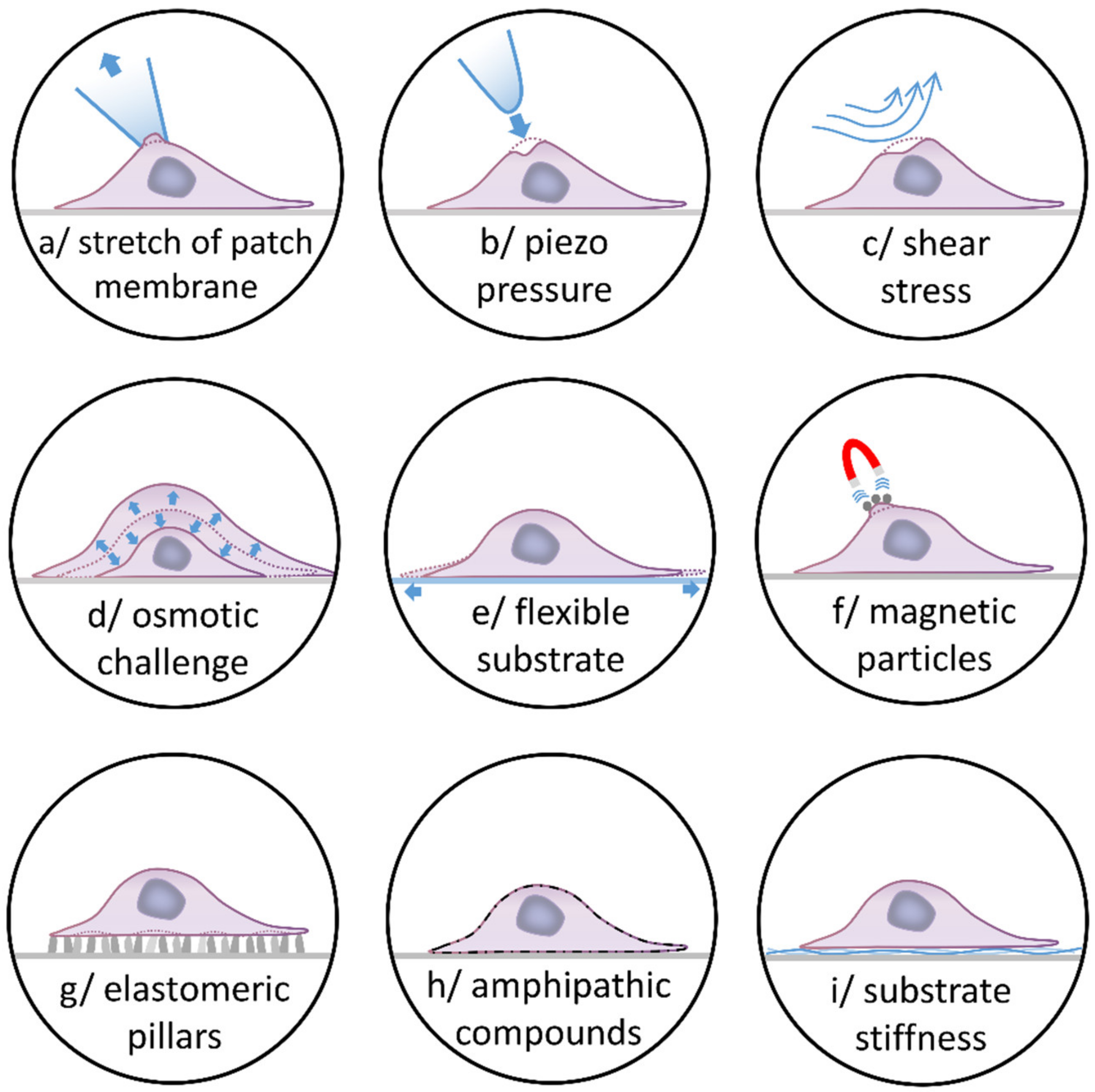
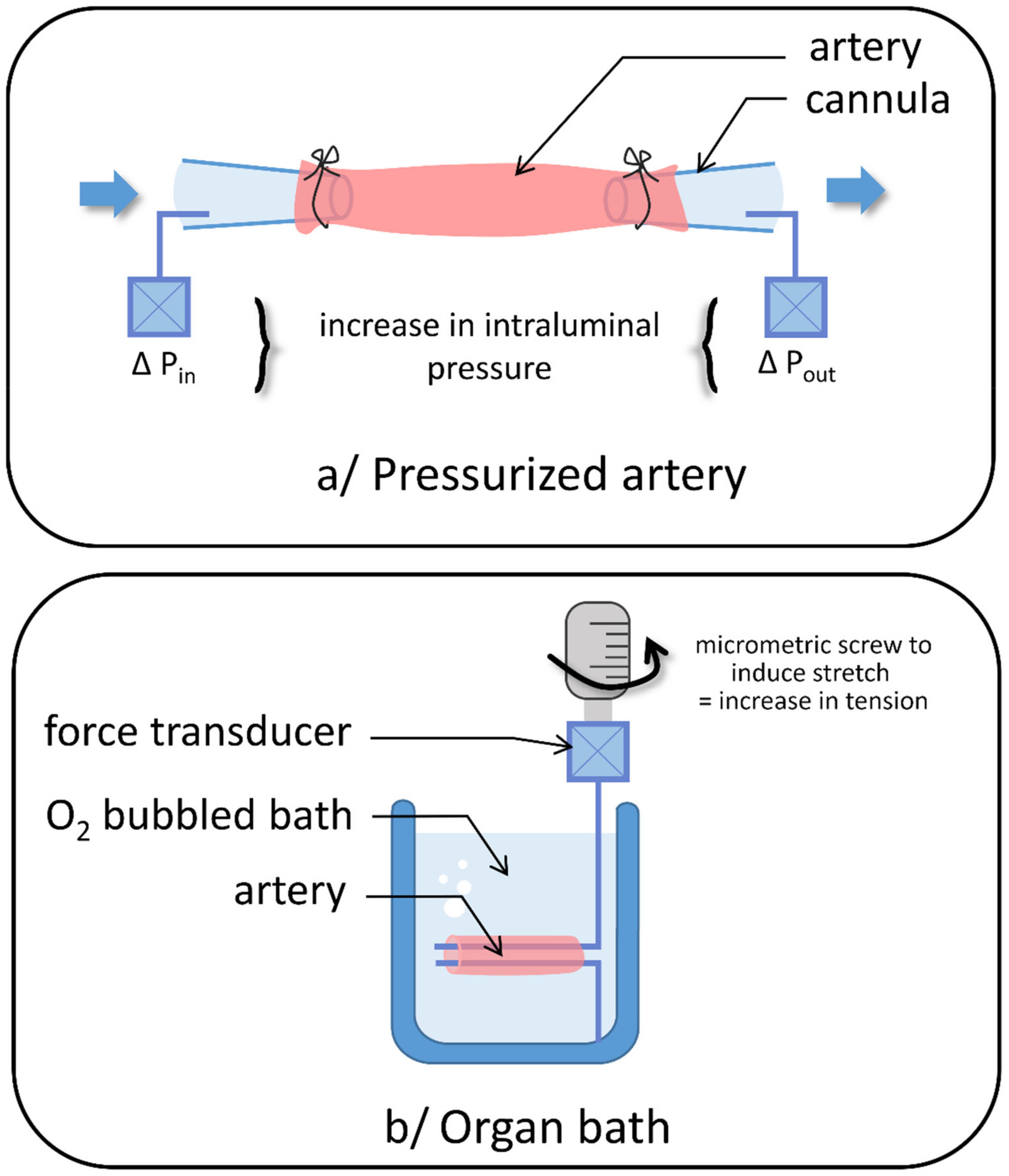
2. Stretch-Activated Channels
3. TRP Channels
3.1. TRPC
3.1.1. TRPC1
3.1.2. TRPC3
3.1.3. TRPC4
3.1.4. TRPC5
3.1.5. TRPC6
3.2. TRPV
3.2.1. TRPV1
3.2.2. TRPV2
3.2.3. TRPV4
3.3. TRPM
3.3.1. TRPM3
3.3.2. TRPM4
3.3.3. TRPM7
3.4. TRPA1
3.5. TRPP
4. Piezo Channels
5. Other Mechanosensitive Channels
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yamashiro, Y.; Yanagisawa, H. The Molecular Mechanism of Mechanotransduction in Vascular Homeostasis and Disease. Clin. Sci. 2020, 134, 2399–2418. [Google Scholar] [CrossRef]
- Bayliss, W.M. On the Local Reactions of the Arterial Wall to Changes of Internal Pressure. J. Physiol. 1902, 28, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Haeger, C.M.; Dieffenbach, P.B.; Sicard, D.; Chrobak, I.; Coronata, A.M.; Suarez Velandia, M.M.; Vitali, S.; Colas, R.A.; Norris, P.C.; et al. Distal Vessel Stiffening Is an Early and Pivotal Mechanobiological Regulator of Vascular Remodeling and Pulmonary Hypertension. JCI Insight 2016, 1, e86987. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Galie, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An Insider View on the World Symposium on Pulmonary Hypertension. Lancet Respir. Med. 2019, 7, 484–485. [Google Scholar] [CrossRef]
- Broughton, B.R.; Walker, B.R.; Resta, T.C. Chronic Hypoxia Induces Rho kinase-Dependent Myogenic Tone in Small Pulmonary Arteries. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L797–L806. [Google Scholar] [CrossRef] [PubMed]
- De La Roque, E.D.; Thiaudiere, E.; Ducret, T.; Marthan, R.; Franconi, J.M.; Guibert, C.; Parzy, E. Effect of Chronic Hypoxia on Pulmonary Artery Blood Velocity in Rats as Assessed by Electrocardiography-Triggered Three-Dimensional Time-Resolved MR Angiography. NMR Biomed. 2011, 24, 225–230. [Google Scholar] [CrossRef]
- Wu, D.; Birukov, K. Endothelial Cell Mechano-Metabolomic Coupling to Disease States in the Lung Microvasculature. Front. Bioeng. Biotechnol. 2019, 7, 172. [Google Scholar] [CrossRef]
- Song, S.; Yamamura, A.; Yamamura, H.; Ayon, R.J.; Smith, K.A.; Tang, H.; Makino, A.; Yuan, J.X. Flow Shear Stress Enhances Intracellular Ca2+ Signaling in Pulmonary Artery Smooth Muscle Cells from Patients with Pulmonary Arterial Hypertension. Am. J. Physiol. Cell Physiol. 2014, 307, C373–C383. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular Mechanotransduction: Putting All the Pieces Together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Corey, D.P.; Hudspeth, A.J. Response Latency of Vertebrate Hair Cells. Biophys. J. 1979, 26, 499–506. [Google Scholar] [CrossRef]
- Guharay, F.; Sachs, F. Stretch-Activated Single Ion Channel Currents in Tissue-Cultured Embryonic Chick Skeletal Muscle. J. Physiol. 1984, 352, 685–701. [Google Scholar] [CrossRef]
- Folgering, J.H.; Sharif-Naeini, R.; Dedman, A.; Patel, A.; Delmas, P.; Honore, E. Molecular Basis of the Mammalian Pressure-Sensitive Ion Channels: Focus on Vascular Mechanotransduction. Prog. Biophys. Mol. Biol. 2008, 97, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Beech, D.J.; Kalli, A.C. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Douguet, D.; Patel, A.; Xu, A.; Vanhoutte, P.M.; Honore, E. Piezo Ion Channels in Cardiovascular Mechanobiology. Trends Pharmacol. Sci. 2019, 40, 956–970. [Google Scholar] [CrossRef]
- Bialecki, R.A.; Kulik, T.J.; Colucci, W.S. Stretching Increases Calcium Influx and Efflux in Cultured Pulmonary Arterial Smooth Muscle Cells. Am. J. Physiol. 1992, 263, L602–L606. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, Y.; Lee, Y.H.; Earm, Y.E.; Ho, W.K. Mechanosensitive Cation Channels in Arterial Smooth Muscle Cells Are Activated by Diacylglycerol and Inhibited by Phospholipase C Inhibitor. Circ. Res. 2003, 93, 557–564. [Google Scholar] [CrossRef]
- Park, K.S.; Lee, H.A.; Earm, K.H.; Ko, J.H.; Earm, Y.E.; Kim, S.J. Differential Distribution of Mechanosensitive Nonselective Cation Channels in Systemic and Pulmonary Arterial Myocytes of Rabbits. J. Vasc. Res. 2006, 43, 347–354. [Google Scholar] [CrossRef]
- Ducret, T.; El Arrouchi, J.; Courtois, A.; Quignard, J.F.; Marthan, R.; Savineau, J.P. Stretch-Activated Channels in Pulmonary Arterial Smooth Muscle Cells from Normoxic and Chronically Hypoxic Rats. Cell Calcium 2010, 48, 251–259. [Google Scholar] [CrossRef]
- Gilbert, G.; Ducret, T.; Marthan, R.; Savineau, J.P.; Quignard, J.F. Stretch-Induced Ca2+ Signalling in Vascular Smooth Muscle Cells Depends on Ca2+ Store Segregation. Cardiovasc. Res. 2014, 103, 313–323. [Google Scholar] [CrossRef]
- Parpaite, T.; Cardouat, G.; Mauroux, M.; Gillibert-Duplantier, J.; Robillard, P.; Quignard, J.F.; Marthan, R.; Savineau, J.P.; Ducret, T. Effect of Hypoxia on TRPV1 and TRPV4 Channels in Rat Pulmonary Arterial Smooth Muscle Cells. Pflugers Arch. 2016, 468, 111–130. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoo, H.Y.; Jang, J.H.; Lin, H.Y.; Seo, E.Y.; Zhang, Y.H.; Kim, S.J. Wall Stretch and Thromboxane A2 Activate NO Synthase (eNOS) in Pulmonary Arterial Smooth Muscle Cells via H2O2 and Akt-Dependent Phosphorylation. Pflugers Arch. 2016, 468, 705–716. [Google Scholar] [CrossRef]
- Yang, X.R.; Lin, A.H.; Hughes, J.M.; Flavahan, N.A.; Cao, Y.N.; Liedtke, W.; Sham, J.S. Upregulation of Osmo-Mechanosensitive TRPV4 Channel Facilitates Chronic Hypoxia-Induced Myogenic Tone and Pulmonary Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L555–L568. [Google Scholar] [CrossRef]
- Lee, H.A.; Baek, E.B.; Park, K.S.; Jung, H.J.; Kim, J.I.; Kim, S.J.; Earm, Y.E. Mechanosensitive Nonselective Cation Channel Facilitation by Endothelin-1 Is Regulated by Protein Kinase C in Arterial Myocytes. Cardiovasc. Res. 2007, 76, 224–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gilbert, G.; Ducret, T.; Savineau, J.P.; Marthan, R.; Quignard, J.F. Caveolae Are Involved in Mechanotransduction during Pulmonary Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L1078–L1087. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, A.; Gilbert, G.; Pele, T.; Deweirdt, J.; Henrion, D.; Baudrimont, I.; Campagnac, M.; Marthan, R.; Guibert, C.; Ducret, T.; et al. Stretch-Activated Piezo1 Channel in Endothelial Cells Relaxes Mouse Intrapulmonary Arteries. Am. J. Respir. Cell Mol. Biol. 2019, 60, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Winston, F.K.; Thibault, L.E.; Macarak, E.J. An Analysis of the Time-Dependent Changes in Intracellular Calcium Concentration in Endothelial Cells in Culture Induced by Mechanical Stimulation. J. Biomech. Eng. 1993, 115, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Ghatak, C.; Yasmann, A.; Nishizawa, K.; Sachs, F.; Ladokhin, A.S.; Sukharev, S.I.; Suchyna, T.M. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels. Biophys. J. 2017, 112, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in Structure and Physiology of Mechanically Activated Ion Channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef]
- Delmas, P.; Hao, J.; Rodat-Despoix, L. Molecular Mechanisms of Mechanotransduction in Mammalian Sensory Neurons. Nat. Rev. Neurosci. 2011, 12, 139–153. [Google Scholar] [CrossRef]
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179. [Google Scholar] [CrossRef] [PubMed]
- Deweirdt, J.; Ducret, T.; Quignard, J.F.; Freund-Michel, V.; Lacomme, S.; Gontier, E.; Muller, B.; Marthan, R.; Guibert, C.; Baudrimont, I. Effects of FW2 Nanoparticles Toxicity in a New In Vitro Pulmonary Vascular Cells Model Mimicking Endothelial Dysfunction. Cardiovasc. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Cao, S.; Stowe, J.C.; Valdez-Jasso, D. Substrate Stiffness and Stretch Regulate Profibrotic Mechanosignaling in Pulmonary Arterial Adventitial Fibroblasts. Cells 2021, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Staruschenko, A.; Jeske, N.A.; Akopian, A.N. Contribution of TRPV1-TRPA1 Interaction to the Single Channel Properties of the TRPA1 Channel. J. Biol. Chem. 2010, 285, 15167–15177. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Jian, Z.; Kawarabayashi, Y. Mechanosensitive TRP Channels in Cardiovascular Pathophysiology. Pharmacol. Ther. 2009, 123, 371–385. [Google Scholar] [CrossRef]
- Inoue, R.; Jensen, L.J.; Shi, J.; Morita, H.; Nishida, M.; Honda, A.; Ito, Y. Transient Receptor Potential Channels in Cardiovascular Function and Disease. Circ. Res. 2006, 99, 119–131. [Google Scholar] [CrossRef]
- Watanabe, H.; Murakami, M.; Ohba, T.; Takahashi, Y.; Ito, H. TRP Channel and Cardiovascular Disease. Pharmacol. Ther. 2008, 118, 337–351. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Nabissi, M. TRP Channels: New Potential Therapeutic Approaches in CNS Neuropathies. CNS Neurol. Disord. Drug Targets 2013, 12, 274–293. [Google Scholar] [CrossRef]
- Walker, R.L.; Koh, S.D.; Sergeant, G.P.; Sanders, K.M.; Horowitz, B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am. J. Physiol. Cell Physiol. 2002, 283, C1637–C1645. [Google Scholar] [CrossRef]
- Freichel, M.; Tsvilovskyy, V.; Camacho-Londono, J.E. TRPC4- and TRPC4-Containing Channels. Handb. Exp. Pharmacol. 2014, 222, 85–128. [Google Scholar] [CrossRef]
- Vazquez, G.; Wedel, B.J.; Aziz, O.; Trebak, M.; Putney, J.W., Jr. The Mammalian TRPC Cation Channels. Biochim. Biophys. Acta 2004, 1742, 21–36. [Google Scholar] [CrossRef]
- Beech, D.J. Integration of Transient Receptor Potential Canonical Channels with Lipids. Acta Physiol. 2012, 204, 227–237. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Freichel, M.; Vogel, S.M.; Paria, B.C.; Mehta, D.; Flockerzi, V.; Malik, A.B. Impairment of Store-Operated Ca2+ Entry in TRPC4−/− Mice Interferes with Increase in Lung Microvascular Permeability. Circ. Res. 2002, 91, 70–76. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Ahmmed, G.U.; Vogel, S.M.; Malik, A.B. Ca2+ Signaling, TRP Channels, and Endothelial Permeability. Microcirculation 2006, 13, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, A.; Almalouf, P.; Toba, M.; O’Neill, K.; Qian, X.; Francis, M.; Taylor, M.S.; Alexeyev, M.; McMurtry, I.F.; Oka, M.; et al. TRPC4 Inactivation Confers a Survival Benefit in Severe Pulmonary Arterial Hypertension. Am. J. Pathol. 2013, 183, 1779–1788. [Google Scholar] [CrossRef]
- Francis, M.; Xu, N.; Zhou, C.; Stevens, T. Transient Receptor Potential Channel 4 Encodes a Vascular Permeability Defect and High-Frequency Ca2+ Transients in Severe Pulmonary Arterial Hypertension. Am. J. Pathol. 2016, 186, 1701–1709. [Google Scholar] [CrossRef]
- McDaniel, S.S.; Platoshyn, O.; Wang, J.; Yu, Y.; Sweeney, M.; Krick, S.; Rubin, L.J.; Yuan, J.X. Capacitative Ca2+ Entry in Agonist-Induced Pulmonary Vasoconstriction. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L870–L880. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shimoda, L.A.; Sylvester, J.T. Capacitative Calcium Entry and TRPC Channel Proteins Are Expressed in Rat Distal Pulmonary Arterial Smooth Muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L848–L858. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fantozzi, I.; Remillard, C.V.; Landsberg, J.W.; Kunichika, N.; Platoshyn, O.; Tigno, D.D.; Thistlethwaite, P.A.; Rubin, L.J.; Yuan, J.X. Enhanced Expression of Transient Receptor Potential Channels in Idiopathic Pulmonary Arterial Hypertension. Proc. Natl. Acad. Sci. USA 2004, 101, 13861–13866. [Google Scholar] [CrossRef]
- Grimm, C.; Kraft, R.; Sauerbruch, S.; Schultz, G.; Harteneck, C. Molecular and Functional Characterization of the Melastatin-Related Cation Channel TRPM3. J. Biol. Chem. 2003, 278, 21493–21501. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient Receptor Potential Cation Channels in Disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef]
- Launay, P.; Fleig, A.; Perraud, A.L.; Scharenberg, A.M.; Penner, R.; Kinet, J.P. TRPM4 Is a Ca2+-Activated Nonselective Cation Channel Mediating Cell Membrane Depolarization. Cell 2002, 109, 397–407. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Janssens, A.; Owsianik, G.; Wang, C.; Zhu, M.X.; Voets, T. The selectivity filter of the cation channel TRPM4. J. Biol. Chem. 2005, 280, 22899–22906. [Google Scholar] [CrossRef]
- Earley, S.; Waldron, B.J.; Brayden, J.E. Critical Role for Transient Receptor Potential Channel TRPM4 in Myogenic Constriction of Cerebral Arteries. Circ. Res. 2004, 95, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 Provides an Ion Channel Mechanism for Cellular Entry of Trace Metal Ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a Bifunctional Protein with Kinase and Ion Channel Activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Shimizu, T.; Okada, Y. TRPM7 Is a Stretch- and Swelling-Activated Cation Channel Involved in Volume Regulation in Human Epithelial Cells. Am. J. Physiol. Cell Physiol. 2007, 292, C460–C467. [Google Scholar] [CrossRef]
- Hiraishi, K.; Kurahara, L.H.; Feng, J.; Yamamura, A.; Cui, Y.; Yahiro, E.; Yokomise, H.; Go, T.; Ishikawa, K.; Yokota, N.; et al. Substantial Involvement of TRPM7 Inhibition in the Therapeutic Effect of Ophiocordyceps sinensis on Pulmonary Hypertension. Transl. Res. 2021, 233, 127–143. [Google Scholar] [CrossRef]
- Earley, S.; Brayden, J.E. Transient Receptor Potential Channels in the Vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef]
- Du, J.; Fu, J.; Xia, X.M.; Shen, B. The Functions of TRPP2 in the Vascular System. Acta Pharmacol. Sin. 2016, 37, 13–18. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, R.; Zhou, M.; Li, J.; Yao, X.; Du, J.; Chen, J.; Shen, B. TRPP2 and STIM1 form a Microdomain to Regulate Store-Operated Ca2+ Entry and Blood Vessel Tone. Cell Commun. Signal. 2020, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.; Bai, S.; Yin, S.; Jiang, S.; Ye, L.; Hou, W.; Yao, Y.; Wang, H.; Shen, Y.; Shen, B.; et al. Role of TRPP2 in Mouse Airway Smooth Muscle Tension and Respiration. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L466–L474. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ayon, R.J.; Yamamura, A.; Yamamura, H.; Dash, S.; Babicheva, A.; Tang, H.; Sun, X.; Cordery, A.G.; Khalpey, Z.; et al. Capsaicin-Induced Ca2+ Signaling Is Enhanced via Upregulated TRPV1 Channels in Pulmonary Artery Smooth Muscle Cells from Patients with Idiopathic PAH. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L309–L325. [Google Scholar] [CrossRef]
- Dahan, D.; Ducret, T.; Quignard, J.F.; Marthan, R.; Savineau, J.P.; Esteve, E. Implication of the Ryanodine Receptor in TRPV4-Induced Calcium Response in Pulmonary Arterial Smooth Muscle Cells from Normoxic and Chronically Hypoxic Rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L824–L833. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a Nonselective Cation Channel That Confers Sensitivity to Extracellular Osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef]
- Firth, A.L.; Remillard, C.V.; Yuan, J.X. TRP Channels in Hypertension. Biochim. Biophys. Acta 2007, 1772, 895–906. [Google Scholar] [CrossRef]
- Lin, M.J.; Leung, G.P.; Zhang, W.M.; Yang, X.R.; Yip, K.P.; Tse, C.M.; Sham, J.S. Chronic Hypoxia-Induced Upregulation of Store-Operated and Receptor-Operated Ca2+ Channels in Pulmonary Arterial Smooth Muscle Cells: A Novel Mechanism of Hypoxic Pulmonary Hypertension. Circ. Res. 2004, 95, 496–505. [Google Scholar] [CrossRef]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a Human Homolog of a Drosophila Store-Operated Channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef]
- Thakore, P.; Earley, S. Transient Receptor Potential Channels and Endothelial Cell Calcium Signaling. Compr. Physiol. 2019, 9, 1249–1277. [Google Scholar] [CrossRef] [PubMed]
- Malczyk, M.; Veith, C.; Fuchs, B.; Hofmann, K.; Storch, U.; Schermuly, R.T.; Witzenrath, M.; Ahlbrecht, K.; Fecher-Trost, C.; Flockerzi, V.; et al. Classical Transient Receptor Potential Channel 1 in hypoxia-Induced Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2013, 188, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Kini, V.; Chavez, A.; Mehta, D. A New Role for PTEN in Regulating Transient Receptor Potential Canonical Channel 6-Mediated Ca2+ Entry, Endothelial Permeability, and Angiogenesis. J. Biol. Chem. 2010, 285, 33082–33091. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Zhang, M.F.; Yang, N.; Liu, Q.; Wang, R.X.; Cao, Y.N.; Yang, X.R.; Sham, J.S.; Lin, M.J. Enhanced Store-Operated Ca2+ Entry and TRPC Channel Expression in Pulmonary Arteries of Monocrotaline-Induced Pulmonary Hypertensive Rats. Am. J. Physiol. Cell Physiol. 2012, 302, C77–C87. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Chen, Y.; Yang, K.; Wang, S.; Lu, W.; Wang, J. Upregulation of Canonical Transient Receptor Potential Channel in the Pulmonary Arterial Smooth Muscle of a Chronic Thromboembolic Pulmonary Hypertension Rat Model. Hypertens. Res. 2015, 38, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ran, P.; Zhang, D.; Peng, G.; Li, B.; Zhong, N.; Wang, J. Sildenafil Inhibits Chronically Hypoxic Upregulation of Canonical Transient Receptor Potential Expression in Rat Pulmonary Arterial Smooth Muscle. Am. J. Physiol. Cell Physiol. 2010, 298, C114–C123. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, X.R.; Fu, Z.; Paudel, O.; Abramowitz, J.; Birnbaumer, L.; Sham, J.S. Classical Transient Receptor Potential 1 and 6 Contribute to Hypoxic Pulmonary Hypertension through Differential Regulation of Pulmonary Vascular Functions. Hypertension 2014, 63, 173–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yang, K.; Tian, L.; Fu, X.; Wang, Y.; Sun, Y.; Jiang, Q.; Lu, W.; Wang, J. BMP4 Increases the Expression of TRPC and Basal [Ca2+]i via the p38MAPK and ERK1/2 Pathways Independent of BMPRII in PASMCs. PLoS ONE 2014, 9, e112695. [Google Scholar] [CrossRef]
- Li, X.; Lu, W.; Fu, X.; Zhang, Y.; Yang, K.; Zhong, N.; Ran, P.; Wang, J. BMP4 Increases Canonical Transient Receptor Potential Protein Expression by Activating p38 MAPK and ERK1/2 Signaling Pathways in Pulmonary Arterial Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2013, 49, 212–220. [Google Scholar] [CrossRef]
- Sun, C.K.; Zhen, Y.Y.; Lu, H.I.; Sung, P.H.; Chang, L.T.; Tsai, T.H.; Sheu, J.J.; Chen, Y.L.; Chua, S.; Chang, H.W.; et al. Reducing TRPC1 Expression through Liposome-Mediated siRNA Delivery Markedly Attenuates Hypoxia-Induced Pulmonary Arterial Hypertension in a Murine Model. Stem Cells Int. 2014, 2014, 316214. [Google Scholar] [CrossRef]
- Wang, J.; Yang, K.; Xu, L.; Zhang, Y.; Lai, N.; Jiang, H.; Zhang, Y.; Zhong, N.; Ran, P.; Lu, W. Sildenafil Inhibits Hypoxia-Induced Transient Receptor Potential Canonical Protein Expression in Pulmonary Arterial Smooth Muscle via cGMP-PKG-PPARgamma Axis. Am. J. Respir. Cell Mol. Biol. 2013, 49, 231–240. [Google Scholar] [CrossRef]
- Gonzalez-Cobos, J.C.; Trebak, M. TRPC Channels in Smooth Muscle Cells. Front. Biosci. (Landmark Ed.) 2010, 15, 1023–1039. [Google Scholar] [CrossRef] [PubMed]
- Rodat, L.; Savineau, J.P.; Marthan, R.; Guibert, C. Effect of Chronic Hypoxia on Voltage-Independent Calcium Influx Activated by 5-HT in Rat Intrapulmonary Arteries. Pflugers Arch. 2007, 454, 41–51. [Google Scholar] [CrossRef]
- Zhang, S.; Patel, H.H.; Murray, F.; Remillard, C.V.; Schach, C.; Thistlethwaite, P.A.; Insel, P.A.; Yuan, J.X. Pulmonary Artery Smooth Muscle Cells from Normal Subjects and IPAH Patients Show Divergent cAMP-Mediated Effects on TRPC Expression and Capacitative Ca2+ Entry. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1202–L1210. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, D.; Ma, S.; He, H.; Luo, Z.; Feng, X.; Cao, T.; Ma, L.; Yan, Z.; Liu, D.; et al. Increased rhythmicity in hypertensive arterial smooth muscle is linked to transient receptor potential canonical channels. J. Cell. Mol. Med. 2010, 14, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Negri, S.; Faris, P.; Berra-Romani, R.; Guerra, G.; Moccia, F. Endothelial Transient Receptor Potential Channels and Vascular Remodeling: Extracellular Ca2+ Entry for Angiogenesis, Arteriogenesis and Vasculogenesis. Front. Physiol. 2019, 10, 1618. [Google Scholar] [CrossRef]
- Singh, I.; Knezevic, N.; Ahmmed, G.U.; Kini, V.; Malik, A.B.; Mehta, D. Galphaq-TRPC6-Mediated Ca2+ Entry Induces RhoA Activation and Resultant Endothelial Cell Shape Change in Response to Thrombin. J. Biol. Chem. 2007, 282, 7833–7843. [Google Scholar] [CrossRef] [PubMed]
- Malczyk, M.; Erb, A.; Veith, C.; Ghofrani, H.A.; Schermuly, R.T.; Gudermann, T.; Dietrich, A.; Weissmann, N.; Sydykov, A. The Role of Transient Receptor Potential Channel 6 Channels in the Pulmonary Vasculature. Front. Immunol. 2017, 8, 707. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.C.; Gurney, A.M. Store-Operated Channels Mediate Ca2+ Influx and Contraction in Rat Pulmonary Artery. Circ. Res. 2001, 89, 923–929. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Yang, K.; Wang, Y.; Tian, L.; Zhang, J.; Wang, E.W.; Sun, D.; Lu, W.; Wang, J. Chronic Hypoxia Increases TRPC6 Expression and Basal Intracellular Ca2+ Concentration in Rat Distal Pulmonary venous Smooth Muscle. PLoS ONE 2014, 9, e112007. [Google Scholar] [CrossRef]
- Kunichika, N.; Landsberg, J.W.; Yu, Y.; Kunichika, H.; Thistlethwaite, P.A.; Rubin, L.J.; Yuan, J.X. Bosentan Inhibits Transient Receptor Potential Channel Expression in Pulmonary Vascular Myocytes. Am. J. Respir. Crit. Care Med. 2004, 170, 1101–1107. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Q.; Wan, L.; Yang, K.; Zhang, Y.; Chen, Y.; Wang, E.; Lai, N.; Zhao, L.; Jiang, H.; et al. Sodium Tanshinone IIA Sulfonate Inhibits Canonical Transient Receptor Potential Expression in Pulmonary Arterial Smooth Muscle from Pulmonary hypertensive Rats. Am. J. Respir. Cell Mol. Biol. 2013, 48, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Keller, S.H.; Remillard, C.V.; Safrina, O.; Nicholson, A.; Zhang, S.L.; Jiang, W.; Vangala, N.; Landsberg, J.W.; Wang, J.Y.; et al. A Functional Single-Nucleotide Polymorphism in the TRPC6 Gene Promoter Associated with Idiopathic Pulmonary Arterial Hypertension. Circulation 2009, 119, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.A.; Wan, J.; Song, S.; Smith, K.A.; Gu, Y.; Tauseef, M.; Tang, H.; Makino, A.; Mehta, D.; Yuan, J.X. Upregulated Expression of STIM2, TRPC6, and Orai2 Contributes to the Transition of Pulmonary Arterial Smooth Muscle Cells from a Contractile to Proliferative Phenotype. Am. J. Physiol. Cell Physiol. 2015, 308, C581–C593. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Voiriot, G.; Tang, H.; Fraidenburg, D.R.; Song, S.; Yamamura, H.; Yamamura, A.; Guo, Q.; Wan, J.; Pohl, N.M.; et al. Notch Activation of Ca2+ Signaling in the Development of Hypoxic Pulmonary Vasoconstriction and Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2015, 53, 355–367. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, J.; Zhu, S.; Lan, T.; Yang, J.; Li, L. Chrysin Alleviates Chronic Hypoxia-Induced Pulmonary Hypertension by Reducing Intracellular Calcium Concentration in Pulmonary Arterial Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2019, 74, 426–435. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, J.; Chen, X.; Zhang, S.; Zhu, L.; Peng, Y.; Guo, Z. Chrysin Alleviates Monocrotaline-Induced Pulmonary Hypertension in Rats through Regulation of Intracellular Calcium Homeostasis in Pulmonary Arterial Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2020, 75, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, E.; Akin, A.T.; Tufan, E.; Basaran, K.E.; Taheri, S.; Ozdamar, S.; Yakan, B. The Effect of Chloroquine on the TRPC1, TRPC6, and CaSR in the Pulmonary Artery Smooth Muscle Cells in Hypoxia-Induced Experimental Pulmonary Artery Hypertension. J. Biochem. Mol. Toxicol. 2021, 35, e22636. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Peng, G.; Liu, N.; Tian, H.; Pan, D.; Liu, L.; Yang, X.; Li, C.; Li, W.; et al. Topotecan Prevents Hypoxia-Induced Pulmonary Arterial Hypertension and Inhibits Hypoxia-Inducible Factor-1alpha and TRPC Channels. Int. J. Biochem. Cell Biol. 2018, 104, 161–170. [Google Scholar] [CrossRef]
- Ciura, S.; Bourque, C.W. Transient Receptor Potential Vanilloid 1 Is Required for Intrinsic Osmoreception in Organum Vasculosum Lamina Terminalis Neurons and for Normal Thirst Responses to Systemic Hyperosmolality. J. Neurosci. 2006, 26, 9069–9075. [Google Scholar] [CrossRef]
- Naeini, R.S.; Witty, M.F.; Seguela, P.; Bourque, C.W. An N-Terminal Variant of Trpv1 Channel Is Required for Osmosensory Transduction. Nat. Neurosci. 2006, 9, 93–98. [Google Scholar] [CrossRef]
- Muraki, K.; Iwata, Y.; Katanosaka, Y.; Ito, T.; Ohya, S.; Shigekawa, M.; Imaizumi, Y. TRPV2 Is a Component of Osmotically Sensitive Cation Channels in Murine Aortic Myocytes. Circ. Res. 2003, 93, 829–838. [Google Scholar] [CrossRef]
- Liedtke, W.; Choe, Y.; Marti-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid Receptor-Related Osmotically Activated Channel (VR-OAC), a Candidate Vertebrate Osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Wissenbach, U.; Bodding, M.; Droogmans, G. Differential Activation of the Volume-Sensitive Cation Channel TRP12 (OTRPC4) and Volume-Regulated Anion Currents in HEK-293 Cells. Pflugers Arch. 2001, 443, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Naeini, R.; Dedman, A.; Folgering, J.H.; Duprat, F.; Patel, A.; Nilius, B.; Honore, E. TRP Channels and Mechanosensory Transduction: Insights into the Arterial Myogenic Response. Pflugers Arch. 2008, 456, 529–540. [Google Scholar] [CrossRef]
- Soya, M.; Sato, M.; Sobhan, U.; Tsumura, M.; Ichinohe, T.; Tazaki, M.; Shibukawa, Y. Plasma membrane stretch activates transient receptor potential vanilloid and ankyrin channels in Merkel cells from hamster buccal mucosa. Cell Calcium 2014, 55, 208–218. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozlowska, H.; Kloza, M.; Sadowska, O.; Kozlowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory Effects of Cannabidiol in Human Pulmonary and Rat Small Mesenteric Arteries: Modification by Hypertension and the Potential Pharmacological Opportunities. J. Hypertens. 2020, 38, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Dahan, D.; Cardouat, G.; Gillibert-Duplantier, J.; Marthan, R.; Savineau, J.P.; Ducret, T. Involvement of TRPV1 and TRPV4 Channels in Migration of Rat Pulmonary Arterial Smooth Muscle Cells. Pflugers Arch. 2012, 464, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Wang, J.; Wang, C.; Liu, J.; Shi, L.P.; Xu, M.; Wang, C. Functional Expression of Transient Receptor Potential Vanilloid-Related Channels in Chronically Hypoxic Human Pulmonary Arterial Smooth Muscle Cells. J. Membr. Biol. 2008, 223, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Lin, M.J.; McIntosh, L.S.; Sham, J.S. Functional Expression of Transient Receptor Potential Melastatin- and Vanilloid-Related Channels in Pulmonary Arterial and Aortic Smooth Muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L1267–L1276. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Lu, W.; Li, X.; Chen, Y.; Zhong, N.; Ran, P.; Wang, J. Expression of Store-Operated Ca2+ Entry and Transient Receptor Potential Canonical and Vanilloid-Related Proteins in Rat Distal Pulmonary Venous Smooth Muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L621–L630. [Google Scholar] [CrossRef] [PubMed]
- Dubes, V.; Parpaite, T.; Ducret, T.; Quignard, J.F.; Mornet, S.; Reinhardt, N.; Baudrimont, I.; Dubois, M.; Freund-Michel, V.; Marthan, R.; et al. Calcium Signalling Induced by In Vitro Exposure to Silicium Dioxide Nanoparticles in Rat Pulmonary Artery Smooth Muscle Cells. Toxicology 2017, 375, 37–47. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Park, S.J.; Seo, E.Y.; Park, K.S.; Han, J.A.; Kim, K.S.; Shin, D.H.; Earm, Y.E.; Zhang, Y.H.; Kim, S.J. Role of Thromboxane A2-Activated Nonselective Cation Channels in Hypoxic Pulmonary Vasoconstriction of Rat. Am. J. Physiol. Cell Physiol. 2012, 302, C307–C317. [Google Scholar] [CrossRef]
- Marziano, C.; Hong, K.; Cope, E.L.; Kotlikoff, M.I.; Isakson, B.E.; Sonkusare, S.K. Nitric Oxide-Dependent Feedback Loop Regulates Transient Receptor Potential Vanilloid 4 (TRPV4) Channel Cooperativity and Endothelial Function in Small Pulmonary Arteries. J. Am. Heart Assoc. 2017, 6, e007157. [Google Scholar] [CrossRef]
- Ottolini, M.; Daneva, Z.; Chen, Y.L.; Cope, E.L.; Kasetti, R.B.; Zode, G.S.; Sonkusare, S.K. Mechanisms Underlying Selective Coupling of Endothelial Ca2+ Signals with eNOS vs. IK/SK Channels in Systemic and Pulmonary Arteries. J. Physiol. 2020, 598, 3577–3596. [Google Scholar] [CrossRef]
- Ducret, T.; Guibert, C.; Marthan, R.; Savineau, J.P. Serotonin-Induced Activation of TRPV4-like Current in Rat Intrapulmonary Arterial Smooth Muscle Cells. Cell Calcium 2008, 43, 315–323. [Google Scholar] [CrossRef]
- Xia, Y.; Fu, Z.; Hu, J.; Huang, C.; Paudel, O.; Cai, S.; Liedtke, W.; Sham, J.S. TRPV4 Channel Contributes to Serotonin-Induced Pulmonary Vasoconstriction and the Enhanced Vascular Reactivity in Chronic Hypoxic Pulmonary Hypertension. Am. J. Physiol. Cell Physiol. 2013, 305, C704–C715. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, C. A Novel Mechanism of Sildenafil Improving the Excessive Proliferation and H2S Production in Pulmonary Arterial Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2019, 74, 355–363. [Google Scholar] [CrossRef]
- Cussac, L.A.; Cardouat, G.; Tiruchellvam Pillai, N.; Campagnac, M.; Robillard, P.; Montillaud, A.; Guibert, C.; Gailly, P.; Marthan, R.; Quignard, J.F.; et al. TRPV4 Channel Mediates Adventitial Fibroblast Activation and Adventitial Remodeling in Pulmonary Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L135–L146. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.V.; Singh, T.U.; Parida, S.; Reddy, C.E.N.; Thangamalai, R.; Kandasamy, K.; Singh, V.; Mishra, S.K. TRPV4 Channel Activation Leads to Endothelium-Dependent Relaxation Mediated by Nitric Oxide and Endothelium-Derived Hyperpolarizing Factor in Rat Pulmonary Artery. Pharmacol. Res. 2013, 78, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Yun, X.; Kliment, C.; Huetsch, J.; Damarla, M.; Shimoda, L.A. Reactive Oxygen Species Induced Ca2+ Influx via TRPV4 and Microvascular Endothelial Dysfunction in the SU5416/Hypoxia Model of Pulmonary Arterial Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L893–L907. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xia, L.; Jin, Z.; Jin, R.; Paudel, O.; Sham, J.S.K. Cytochrome P450 Epoxygenase-Dependent Activation of TRPV4 Channel Participates in Enhanced Serotonin-Induced Pulmonary Vasoconstriction in Chronic Hypoxic Pulmonary Hypertension. Anal. Cell. Pathol. 2020, 2020, 8927381. [Google Scholar] [CrossRef]
- Goldenberg, N.M.; Wang, L.; Ranke, H.; Liedtke, W.; Tabuchi, A.; Kuebler, W.M. TRPV4 Is Required for Hypoxic Pulmonary Vasoconstriction. Anesthesiology 2015, 122, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Honda, A.; Inoue, R.; Ito, Y.; Abe, K.; Nelson, M.T.; Brayden, J.E. Membrane Stretch-Induced Activation of a TRPM4-like Nonselective Cation Channel in Cerebral Artery Myocytes. J. Pharmacol. Sci. 2007, 103, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Shimizu, T.; Okada, Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell. Physiol. Biochem. 2007, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Earley, S. TRPM4 Channels in Smooth Muscle Function. Pflugers Arch. 2013, 465, 1223–1231. [Google Scholar] [CrossRef]
- Mathar, I.; Vennekens, R.; Meissner, M.; Kees, F.; Van der Mieren, G.; Londono, J.E.C.; Uhl, S.; Voets, T.; Hummel, B.; van den Bergh, A.; et al. Increased Catecholamine Secretion Contributes to Hypertension in TRPM4-Deficient Mice. J. Clin. Investig. 2010, 120, 3267–3279. [Google Scholar] [CrossRef]
- Touyz, R.M. Transient Receptor Potential Melastatin 6 and 7 Channels, Magnesium Transport, and Vascular Biology: Implications in Hypertension. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1103–H1118. [Google Scholar] [CrossRef]
- Xing, J.; Wang, M.; Hong, J.; Gao, Y.; Liu, Y.; Gu, H.; Dong, J.; Li, L. TRPM7 channel inhibition exacerbates pulmonary arterial hypertension through MEK/ERK pathway. Aging 2019, 11, 4050–4065. [Google Scholar] [CrossRef]
- Corey, D.P.; Garcia-Anoveros, J.; Holt, J.R.; Kwan, K.Y.; Lin, S.Y.; Vollrath, M.A.; Amalfitano, A.; Cheung, E.L.; Derfler, B.H.; Duggan, A.; et al. TRPA1 Is a Candidate for the Mechanosensitive Transduction Channel of Vertebrate Hair Cells. Nature 2004, 432, 723–730. [Google Scholar] [CrossRef]
- Sharif-Naeini, R.; Folgering, J.H.; Bichet, D.; Duprat, F.; Lauritzen, I.; Arhatte, M.; Jodar, M.; Dedman, A.; Chatelain, F.C.; Schulte, U.; et al. Polycystin-1 and -2 Dosage Regulates Pressure Sensing. Cell 2009, 139, 587–596. [Google Scholar] [CrossRef]
- Narayanan, D.; Bulley, S.; Leo, M.D.; Burris, S.K.; Gabrick, K.S.; Boop, F.A.; Jaggar, J.H. Smooth Muscle Cell Transient Receptor Potential Polycystin-2 (TRPP2) Channels Contribute to the Myogenic Response in Cerebral Arteries. J. Physiol. 2013, 591, 5031–5046. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.Q.; et al. Structure and Mechanogating Mechanism of the Piezo1 Channel. Nature 2018, 554, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Bae, C.; Gottlieb, P.A.; Sachs, F. Ionic Selectivity and Permeation Properties of Human PIEZO1 Channels. PLoS ONE 2015, 10, e0125503. [Google Scholar] [CrossRef]
- Morley, L.C.; Shi, J.; Gaunt, H.J.; Hyman, A.J.; Webster, P.J.; Williams, C.; Forbes, K.; Walker, J.J.; Simpson, N.A.B.; Beech, D.J. Piezo1 Channels Are Mechanosensors in Human Fetoplacental Endothelial Cells. Mol. Hum. Reprod. 2018, 24, 510–520. [Google Scholar] [CrossRef]
- Shin, K.C.; Park, H.J.; Kim, J.G.; Lee, I.H.; Cho, H.; Park, C.; Sung, T.S.; Koh, S.D.; Park, S.W.; Bae, Y.M. The Piezo2 Ion Channel Is Mechanically Activated by Low-Threshold Positive Pressure. Sci. Rep. 2019, 9, 6446. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Kala, S.; Zhu, J.; Xian, Q.; Qiu, W.; Li, G.; Zhu, T.; Meng, L.; Zhang, R.; et al. The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. iScience 2019, 21, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Li, L.; Zhang, X.; Liu, J.; Hao, J.; Zhu, J.; Wu, H.; Chen, W.; Zhang, Q. Roles of TRPV4 and Piezo Channels in Stretch-Evoked Ca2+ Response in Chondrocytes. Exp. Biol. Med. 2020, 245, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical Activation of the Mechanotransduction Channel Piezo1. eLife 2015, 4, e07369. [Google Scholar] [CrossRef]
- Lacroix, J.J.; Botello-Smith, W.M.; Luo, Y. Probing the Gating Mechanism of the Mechanosensitive Channel Piezo1 with the Small Molecule Yoda1. Nat. Commun. 2018, 9, 2029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A Lever-like Transduction Pathway for Long-Distance Chemical- and Mechano-Gating of the Mechanosensitive Piezo1 Channel. Nat. Commun. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The Mechanosensitive Ion Channel Piezo1 Is Inhibited by the Peptide GsMTx4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef]
- Alcaino, C.; Knutson, K.; Gottlieb, P.A.; Farrugia, G.; Beyder, A. Mechanosensitive ion Channel Piezo2 Is Inhibited by D-GsMTx4. Channels 2017, 11, 245–253. [Google Scholar] [CrossRef]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo Proteins Are Pore-Forming Subunits of Mechanically Activated Channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 Analogue (Dooku1) Which Antagonizes Yoda1-Evoked Activation of Piezo1 and Aortic Relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Cheng, W.; Deng, B.; He, Y.; Zhang, L.; Ning, Y.; Li, J. Tubeimoside I Antagonizes Yoda1-Evoked Piezo1 Channel Activation. Front. Pharmacol. 2020, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Capuano, V.; Olschewski, A.; Sabourin, J.; Nagaraj, C.; Girerd, B.; Weatherald, J.; Humbert, M.; Antigny, F. Ion Channels in Pulmonary Hypertension: A Therapeutic Interest? Int. J. Mol. Sci. 2018, 19, 3162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, Y.; Lu, D.; Huang, T.; Yan, K.; Yang, W.; Gao, J. Mechanosensitive Piezo1 Channel Activation Promotes Ventilator-Induced Lung Injury via Disruption of Endothelial Junctions in ARDS Rats. Biochem. Biophys. Res. Commun. 2021, 556, 79–86. [Google Scholar] [CrossRef]
- Liao, J.; Lu, W.; Chen, Y.; Duan, X.; Zhang, C.; Luo, X.; Lin, Z.; Chen, J.; Liu, S.; Yan, H.; et al. Upregulation of Piezo1 (Piezo Type Mechanosensitive Ion Channel Component 1) Enhances the Intracellular Free Calcium in Pulmonary Arterial Smooth Muscle Cells from Idiopathic Pulmonary Arterial Hypertension Patients. Hypertension 2021, 77, 1974–1989. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Hough, R.F. Piezo1 in the Lung: At Last! Am. J. Respir. Cell Mol. Biol. 2019, 60, 609–610. [Google Scholar] [CrossRef]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 Integration of Vascular Architecture with Physiological Force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, V.; Mathur, J.; Mao, R.; Bayrak-Toydemir, P.; Procter, M.; Cahalan, S.M.; Kim, H.J.; Bandell, M.; Longo, N.; Day, R.W.; et al. Impaired PIEZO1 Function in Patients with a Novel Autosomal Recessive Congenital Lymphatic Dysplasia. Nat. Commun. 2015, 6, 8329. [Google Scholar] [CrossRef] [PubMed]
- Retailleau, K.; Duprat, F.; Arhatte, M.; Ranade, S.S.; Peyronnet, R.; Martins, J.R.; Jodar, M.; Moro, C.; Offermanns, S.; Feng, Y.; et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015, 13, 1161–1171. [Google Scholar] [CrossRef]
- Allison, S.J. Hypertension: Mechanosensation by PIEZO1 in Blood Pressure Control. Nat. Rev. Nephrol. 2017, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial Cation Channel PIEZO1 Controls Blood Pressure by Mediating Flow-Induced ATP Release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef] [PubMed]
- Iring, A.; Jin, Y.J.; Albarran-Juarez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Kunne, C.; et al. Shear Stress-Induced Endothelial Adrenomedullin Signaling Regulates Vascular Tone and Blood Pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef] [PubMed]
- John, L.; Ko, N.L.; Gokin, A.; Gokina, N.; Mandala, M.; Osol, G. The Piezo1 Cation Channel Mediates Uterine Artery Shear Stress Mechanotransduction and Vasodilation during Rat Pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1019–H1026. [Google Scholar] [CrossRef] [PubMed]
- Rode, B.; Shi, J.; Endesh, N.; Drinkhill, M.J.; Webster, P.J.; Lotteau, S.J.; Bailey, M.A.; Yuldasheva, N.Y.; Ludlow, M.J.; Cubbon, R.M.; et al. Piezo1 Channels Sense Whole Body Physical Activity to Reset Cardiovascular Homeostasis and Enhance Performance. Nat. Commun. 2017, 8, 350. [Google Scholar] [CrossRef]
- Dora, K.A. Endothelial-Smooth Muscle Cell Interactions in the Regulation of Vascular Tone in Skeletal Muscle. Microcirculation 2016, 23, 626–630. [Google Scholar] [CrossRef]
- Genet, N.; Billaud, M.; Rossignol, R.; Dubois, M.; Gillibert-Duplantier, J.; Isakson, B.E.; Marthan, R.; Savineau, J.P.; Guibert, C. Signaling Pathways Linked to Serotonin-Induced Superoxide Anion Production: A Physiological Role for Mitochondria in Pulmonary Arteries. Front. Physiol. 2017, 8, 76. [Google Scholar] [CrossRef]
- Billaud, M.; Marthan, R.; Savineau, J.P.; Guibert, C. Vascular Smooth Muscle Modulates Endothelial Control of Vasoreactivity via Reactive Oxygen Species Production through Myoendothelial Communications. PLoS ONE 2009, 4, e6432. [Google Scholar] [CrossRef] [PubMed]
- McHugh, B.J.; Buttery, R.; Lad, Y.; Banks, S.; Haslett, C.; Sethi, T. Integrin Activation by Fam38A uses a Novel Mechanism of R-Ras Targeting to the Endoplasmic Reticulum. J. Cell Sci. 2010, 123, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of Cyclical Force by PIEZO1 Is Essential for Innate Immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef]
- Sun, Y.; Leng, P.; Song, M.; Li, D.; Guo, P.; Xu, X.; Gao, H.; Li, Z.; Li, C.; Zhang, H. Piezo1 Activates the NLRP3 Inflammasome in Nucleus Pulposus Cell-Mediated by Ca2+/NF-kappaB Pathway. Int. Immunopharmacol. 2020, 85, 106681. [Google Scholar] [CrossRef]
- Albarran-Juarez, J.; Iring, A.; Wang, S.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and Gq/G11 Promote Endothelial Inflammation Depending on Flow Pattern and Integrin Activation. J. Exp. Med. 2018, 215, 2655–2672. [Google Scholar] [CrossRef]
- Fulton, D.J.R.; Li, X.; Bordan, Z.; Haigh, S.; Bentley, A.; Chen, F.; Barman, S.A. Reactive Oxygen and Nitrogen Species in the Development of Pulmonary Hypertension. Antioxidants 2017, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, M.; Liu, G.; Zhang, X.; Zhi, L.; Zhao, J.; Wang, G. The Function of Piezo1 in Colon Cancer Metastasis and Its Potential Regulatory Mechanism. J. Cancer Res. Clin. Oncol. 2020, 146, 1139–1152. [Google Scholar] [CrossRef]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.K.; Hu, M.; Draghici, D.; et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 2018, 100, 799–815.e7. [Google Scholar] [CrossRef]
- Richter, K.; Kiefer, K.P.; Grzesik, B.A.; Clauss, W.G.; Fronius, M. Hydrostatic Pressure Activates ATP-Sensitive K+ Channels in Lung Epithelium by ATP Release through Pannexin and Connexin Hemichannels. FASEB J. 2014, 28, 45–55. [Google Scholar] [CrossRef]
- Xin, F.; Cheng, Y.; Ren, J.; Zhang, S.; Liu, P.; Zhao, H.; Huang, H.; Wang, W. The Extracellular Loop of the Auxiliary beta1-Subunit Is Involved in the Regulation of BKCa Channel Mechanosensitivity. Am. J. Physiol. Cell Physiol. 2018, 315, C485–C493. [Google Scholar] [CrossRef]
- Wiedmann, F.; Rinne, S.; Donner, B.; Decher, N.; Katus, H.A.; Schmidt, C. Mechanosensitive TREK-1 Two-Pore-Domain Potassium (K2P) Channels in the Cardiovascular System. Prog. Biophys. Mol. Biol. 2021, 159, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Z.M.; Wu, X.; Zhang, L.; Cao, Y.; Zhou, P. Distinct Molecular Mechanisms Underlying Potassium Efflux for NLRP3 Inflammasome Activation. Front. Immunol. 2020, 11, 609441. [Google Scholar] [CrossRef] [PubMed]
- Mondejar-Parreno, G.; Cogolludo, A.; Perez-Vizcaino, F. Potassium (K+) Channels in the Pulmonary Vasculature: Implications in Pulmonary Hypertension Physiological, Pathophysiological and Pharmacological Regulation. Pharmacol. Ther. 2021, 225, 107835. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Qi, Z.; Naruse, K.; Sokabe, M. Characterization of a Functionally Expressed Stretch-Activated BKca Channel Cloned from Chick Ventricular Myocytes. J. Membr. Biol. 2003, 196, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Kirber, M.T.; Ordway, R.W.; Clapp, L.H.; Walsh, J.V., Jr.; Singer, J.J. Both Membrane Stretch and Fatty Acids Directly Activate Large Conductance Ca2+-Activated K+ Channels in Vascular Smooth Muscle Cells. FEBS Lett. 1992, 297, 24–28. [Google Scholar] [CrossRef]
- Ferraz, A.P.; Seara, F.A.C.; Baptista, E.F.; Barenco, T.S.; Sottani, T.B.B.; Souza, N.S.C.; Domingos, A.E.; Barbosa, R.A.Q.; Takiya, C.M.; Couto, M.T.; et al. BKCa Channel Activation Attenuates the Pathophysiological Progression of Monocrotaline-Induced Pulmonary Arterial Hypertension in Wistar Rats. Cardiovasc. Drugs Ther. 2020, 35, 719–732. [Google Scholar] [CrossRef]
- Huang, H.; Liang, L.; Liu, P.; Wei, H.; Sachs, F.; Niu, W.; Wang, W. Mechanical Effects on KATP Channel Gating in Rat Ventricular Myocytes. PLoS ONE 2013, 8, e63337. [Google Scholar] [CrossRef][Green Version]
- Clapp, L.H.; Gurney, A.M. ATP-Sensitive K+ Channels Regulate Resting Potential of Pulmonary Arterial Smooth Muscle Cells. Am. J. Physiol. 1992, 262, H916–H920. [Google Scholar] [CrossRef]
- Cui, Y.; Tran, S.; Tinker, A.; Clapp, L.H. The Molecular Composition of K(ATP) Channels in Human Pulmonary Artery Smooth Muscle Cells and Their Modulation by Growth. Am. J. Respir. Cell Mol. Biol. 2002, 26, 135–143. [Google Scholar] [CrossRef]
- Koh, S.D.; Monaghan, K.; Sergeant, G.P.; Ro, S.; Walker, R.L.; Sanders, K.M.; Horowitz, B. TREK-1 Regulation by Nitric Oxide and cGMP-Dependent Protein Kinase. An Essential Role in Smooth Muscle Inhibitory Neurotransmission. J. Biol. Chem. 2001, 276, 44338–44346. [Google Scholar] [CrossRef]
- Gardener, M.J.; Johnson, I.T.; Burnham, M.P.; Edwards, G.; Heagerty, A.M.; Weston, A.H. Functional Evidence of a Role for Two-Pore Domain Potassium Channels in Rat Mesenteric and Pulmonary Arteries. Br. J. Pharmacol. 2004, 142, 192–202. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H.; Ding, J.; Wang, H.; Hu, G. Anti-Proliferating Effect of Iptakalim, a Novel KATP Channel Opener, in Cultured Rabbit Pulmonary Arterial Smooth Muscle Cells. Eur. J. Pharmacol. 2005, 511, 81–87. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Cui, T.; Cai, Q.; Wang, H.; Kong, H.; Xie, W. Iptakalim Ameliorates Hypoxia-Impaired Human Endothelial Colony-Forming Cells Proliferation, Migration, and Angiogenesis via Akt/eNOS Pathways. Pulm. Circ. 2019, 9, 2045894019875417. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Long, C.; Cui, W.; Wang, H. Iptakalim Ameliorates Monocrotaline-Induced Pulmonary Arterial Hypertension in Rats. J. Cardiovasc. Pharmacol. Ther. 2013, 18, 60–69. [Google Scholar] [CrossRef]
- Hayabuchi, Y. The Action of Smooth Muscle Cell Potassium Channels in the Pathology of Pulmonary Arterial Hypertension. Pediatr. Cardiol. 2017, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]

| Channel | Cell Type (Species) | Conductance (pS) | Permeability | Activator | Inhibitor | PA Phenotype in KO Mice | References |
|---|---|---|---|---|---|---|---|
| TRPA1 | - | 9–16 53.1–62.8 | - | osmolarity mustard oil | HC030031 | not described in PA | [35] |
| TRPC1 | human, mouse, and rat PA (PAEC and PASMC) | 16 | PCa/PNa < 10 | stretch store depletion | 2-APB Gd3+/La3+ | reduced hyperreactivity, remodeling, and vasomotor tone | [36,37,38,39] |
| TRPC3 | rat PA human PASMC | 66 | PCa/PNa = 1.6 | stretch store depletion DAG and analogs | 2-APB Gd3+/La3+ | not described in PA | [36,37,38,39] |
| TRPC4 | human, mouse, and rat PA (PAEC and PASMC) | 17.5–41 | PCa/PNa = 1.1–7 | store depletion, arachidonic acid calmidazolium | 2-APB SKF96365 Gd3+/La3+ niflumic acid DIDS | reduced vascular permeability (and remodeling in KO rats) | [40,41,42,43,44,45,46,47] |
| TRPC5 | human, mouse, and rat PA human PASMC | 64 | PCa/PNa = 9 | stretch hypotonocity roziglitazone | 2-APB La3+ | not described in PA | [36,38,39,48,49,50] |
| TRPC6 | human, mouse, and rat PA (PAEC and PASMC) | 28–37 | PCa/PNa = 4–5 | stretch hypotonicity store depletion DAG and analogs | 2-APB SKF96365 Cd2+, La3+, Gd3+ | reduced hyperreactivity, remodeling and vasomotor tone | [36,37,38,39] |
| TRPM3 | rat PA | 65–133 | PCa/PNa = 1.5–2 | hypotonicity | not described in PA | [51,52] | |
| TRPM4 | rat PA | 24–25 | Na+//K+ > Cs+ > Li+ | stretch | not described in PA | [52,53,54,55] | |
| TRPM7 | rat PA human and rat PASMC | 105 22–37 | Zn2+ ≈ Ni2+ >> Ba2+ > Co2+ > Mg2+ ≥ Mn2+ ≥ Sr2+ ≥ Cd2+ ≥ Ca2+ PNa/PCa = 3 | stretch osmolarity | not viable → conditional deletion: reduced remodeling and vasomotor tone | [52,56,57,58,59] | |
| TRPP1 | - | 135–175 | - | PKD1 stretch | - | not described in PA | [36,60] |
| TRPP2 | - | 177 | PCa/PNa = 1–5 | shear stress hypotonicity triptolide | Gd3+/La3+ | not described in PA (conditional in smooth muscle cells) | [36,37,61,62,63] |
| TRPV1 | human and rat PA (PAEC and PASMC) | 35–80 (unitary) 143–144 (whole cell) | PCa/PNa = 10 (capsaicin-activated) | hypotonicity capsaicin 2-APB cannabidiol resiniferatoxin | capsazepine AMG9810 A784168 5’-Iodoresiniferatoxin | not described in PA | [36,52,64] |
| TRPV2 | human and rat PASMC | - | PCa/PNa = 1–3 | hypotonicity stretch 2-APB | tranilast | not described in PA | [36,52] |
| TRPV4 | human, mouse, and rat PA (PAAF PAEC, PASMC) | 30–90 (unitary) 452 ± 63 (whole cell) | PCa/PNa = 6–10 | hypotonicity shear stress 4-αPDD GSK1016790A | HC607047 RN-1734 GSK2193874 | reduced hyperreactivity, remodeling and vasomotor tone | [23,36,52,65,66] |
| Channel | Cell Type (Species) | Conductance (pS) | Permeability | Activator | Inhibitor | PA Phenotype in KO Mice | References |
|---|---|---|---|---|---|---|---|
| Piezo1 | human, mouse, and rat PA human and mouse PAEC human PASMC | 22–30 | Ca2+ > Na+, K+, Mg2+ PCa/PCs = 2; PNa/PCs = 1.1; PK/PCs = 1.1; PMg/PCs = 0.5 | negative and positive pressures, shear stress, ultrasound waves, Yoda1, Jedi1/2 | GsMTx-4 ruthenium red Gd3+ dooku1 tubeimoside I | not viable → endothelium specific conditional deletion: no effect | [26,132,134,135,137,138,139,140,141,142,144,145,146,147,148,149,150] |
| Piezo2 | human PA | 27–28 | - | negative and positive pressures | GsMTx-4 ruthenium red Gd3+ | not described in PA | [132,136,138,143,147] |
| Channel | Cell Type (Species) | Conductance (pS) | Permeability | Activator | Inhibitor | PA Phenotype in KO Mice | References |
|---|---|---|---|---|---|---|---|
| BKCa | rabbit and rat PASMC | 273 | K+ | negative pressure, calcium | - | not described in PA | [170,174,175,176] |
| KATP | human and rabbit PASMC | 42–55 (pressure), 28 (Levcromakalim) | K+ | negative and positive pressures, levcromakalim, iptakalim | intracellular ATP glibenclamide | not described in PA | [177,178,179] |
| TREK-1 | mouse PA rat PASMC | 90 | K+ | negative pressure | - | not described in PA | [180,181] |
| TREK-2 | mouse and rat PA | - | K+ | stretch | - | not described in PA | [180,181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbeau, S.; Gilbert, G.; Cardouat, G.; Baudrimont, I.; Freund-Michel, V.; Guibert, C.; Marthan, R.; Vacher, P.; Quignard, J.-F.; Ducret, T. Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension. Biomolecules 2021, 11, 1389. https://doi.org/10.3390/biom11091389
Barbeau S, Gilbert G, Cardouat G, Baudrimont I, Freund-Michel V, Guibert C, Marthan R, Vacher P, Quignard J-F, Ducret T. Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension. Biomolecules. 2021; 11(9):1389. https://doi.org/10.3390/biom11091389
Chicago/Turabian StyleBarbeau, Solène, Guillaume Gilbert, Guillaume Cardouat, Isabelle Baudrimont, Véronique Freund-Michel, Christelle Guibert, Roger Marthan, Pierre Vacher, Jean-François Quignard, and Thomas Ducret. 2021. "Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension" Biomolecules 11, no. 9: 1389. https://doi.org/10.3390/biom11091389
APA StyleBarbeau, S., Gilbert, G., Cardouat, G., Baudrimont, I., Freund-Michel, V., Guibert, C., Marthan, R., Vacher, P., Quignard, J.-F., & Ducret, T. (2021). Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension. Biomolecules, 11(9), 1389. https://doi.org/10.3390/biom11091389






