Revisiting the Large-Conductance Calcium-Activated Potassium (BKCa) Channels in the Pulmonary Circulation
Abstract
:1. Introduction
2. The Pulmonary Circulation, a System with Many Unique Features
3. Structure and Gating of the Large-Conductance Calcium (Ca2+)-Activated Potassium (BKCa) Channels
4. The Role of the α-Subunit in the Pulmonary Circulation
5. β-Subunit or the Modulatory Subunit of the BKCa Channel
6. γ-Subunit or the Auxiliary Subunit of the BKCa Channel
7. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olschewski, A.; Papp, R.; Nagaraj, C.; Olschewski, H. Ion channels and transporters as therapeutic targets in the pulmonary circulation. Pharm. Ther. 2014, 144, 349–368. [Google Scholar] [CrossRef] [PubMed]
- Mondejar-Parreno, G.; Cogolludo, A.; Perez-Vizcaino, F. Potassium (K+) channels in the pulmonary vasculature: Implications in pulmonary hypertension Physiological, pathophysiological and pharmacological regulation. Pharm. Ther. 2021, 225, 107835. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, A.; Weir, E.K. Redox regulation of ion channels in the pulmonary circulation. Antioxid. Redox Signal. 2015, 22, 465–485. [Google Scholar] [CrossRef] [Green Version]
- Morrell, N.W.; Aldred, M.A.; Chung, W.K.; Elliott, C.G.; Nichols, W.C.; Soubrier, F.; Trembath, R.C.; Loyd, J.E. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801899. [Google Scholar] [CrossRef] [Green Version]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmuller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, G.; Berghold, A.; Scheidl, S.; Olschewski, H. Pulmonary arterial pressure during rest and exercise in healthy subjects: A systematic review. Eur. Respir. J. 2009, 34, 888–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, J.A.; Tribble, C.G.; Chan, B.B.; Flanagan, T.L.; Kron, I.L. Reduced-size porcine lung transplantation: Long-term studies of pulmonary vascular resistance. Ann. Thorac. Surg. 1992, 53, 583–588. [Google Scholar] [CrossRef]
- Suresh, K.; Shimoda, L.A. Lung Circulation. Compr. Physiol. 2016, 6, 897–943. [Google Scholar] [CrossRef] [PubMed]
- Euler, U.V.; Liljestrand, G. Observations on the Pulmonary Arterial Blood Pressure in the Cat. Acta Physiol. Scand. 1946, 12, 301–320. [Google Scholar] [CrossRef]
- Weissmann, N.; Grimminger, F.; Olschewski, A.; Seeger, W. Hypoxic pulmonary vasoconstriction: A multifactorial response? Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L314–L317. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef]
- Weissmann, N.; Grimminger, F.; Walmrath, D.; Seeger, W. Hypoxic vasoconstriction in buffer-perfused rabbit lungs. Respir. Physiol. 1995, 100, 159–169. [Google Scholar] [CrossRef]
- Hughes, J.M. Hypoxic pulmonary vasoconstriction: Clinical implications. Eur. Respir. J. 2016, 47, 31–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.S.; Micco, A.J.; Czartolomna, J.; Latham, L.; Voelkel, N.F. Rapid onset of hypoxic vasoconstriction in isolated lungs. J. Appl. Physiol. 1992, 72, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Tajima, F.; Osada, H.; Nakamura, A.; Yagura, S.; Kawai, T.; Suzuki, M.; Torikata, C. Pulmonary, vascular responses in rats exposed to chronic hypobaric hypoxia at two different altitude levels. Pathol. Res. Pr. 1996, 192, 1057–1067. [Google Scholar] [CrossRef]
- Olschewski, A.; Hong, Z.; Nelson, D.P.; Weir, E.K. Graded response of K+ current, membrane potential, and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L1143–L1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brimioulle, S.; Lejeune, P.; Vachiery, J.L.; Leeman, M.; Melot, C.; Naeije, R. Effects of acidosis and alkalosis on hypoxic pulmonary vasoconstriction in dogs. Am. J. Physiol. 1990, 258, H347–H353. [Google Scholar] [CrossRef]
- Olschewski, A.; Li, Y.; Tang, B.; Hanze, J.; Eul, B.; Bohle, R.M.; Wilhelm, J.; Morty, R.E.; Brau, M.E.; Weir, E.K.; et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ. Res. 2006, 98, 1072–1080. [Google Scholar] [CrossRef] [Green Version]
- Remme, C.A.; Wilde, A.A. A new, sympathetic look at KATP channels in the heart. Cardiovasc. Res. 1999, 43, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Hartzell, H.C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog. Biophys. Mol. Biol. 1988, 52, 165–247. [Google Scholar] [CrossRef]
- Archer, S.; Michelakis, E. The mechanism(s) of hypoxic pulmonary vasoconstriction: Potassium channels, redox O(2) sensors, and controversies. News Physiol. Sci. 2002, 17, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelakis, E.D.; Thebaud, B.; Weir, E.K.; Archer, S.L. Hypoxic pulmonary vasoconstriction: Redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J. Mol. Cell Cardiol. 2004, 37, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Cornfield, D.N.; Reeve, H.L.; Tolarova, S.; Weir, E.K.; Archer, S. Oxygen causes fetal pulmonary vasodilation through activation of a calcium-dependent potassium channel. Proc. Natl. Acad. Sci. USA 1996, 93, 8089–8094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, V.A.; Rhodes, M.T.; Reeve, H.L.; Cornfield, D.N. Oxygen-induced fetal pulmonary vasodilation is mediated by intracellular calcium activation of K(Ca) channels. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L1379–L1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, W.F. Potassium channels in the peripheral microcirculation. Microcirculation 2005, 12, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Kapela, A.; Behringer, E.J.; Segal, S.S.; Tsoukias, N.M. Biophysical properties of microvascular endothelium: Requirements for initiating and conducting electrical signals. Microcirculation 2018, 25, e12429. [Google Scholar] [CrossRef] [PubMed]
- Siemen, D.; Loupatatzis, C.; Borecky, J.; Gulbins, E.; Lang, F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem. Biophys. Res. Commun. 1999, 257, 549–554. [Google Scholar] [CrossRef]
- Heinen, A.; Aldakkak, M.; Stowe, D.F.; Rhodes, S.S.; Riess, M.L.; Varadarajan, S.G.; Camara, A.K. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1400–H1407. [Google Scholar] [CrossRef] [Green Version]
- Aon, M.A.; Cortassa, S.; Wei, A.C.; Grunnet, M.; O’Rourke, B. Energetic performance is improved by specific activation of K+ fluxes through K(Ca) channels in heart mitochondria. Biochim. Biophys. Acta 2010, 1797, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, Y.; Shimada, H.; Taniguchi, J. Ca2+-activated K+-channels in the nuclear envelope isolated from single pancreatic acinar cells. Pflug. Arch. 1995, 430, 148–150. [Google Scholar] [CrossRef]
- Gobeil, F., Jr.; Dumont, I.; Marrache, A.M.; Vazquez-Tello, A.; Bernier, S.G.; Abran, D.; Hou, X.; Beauchamp, M.H.; Quiniou, C.; Bouayad, A.; et al. Regulation of eNOS expression in brain endothelial cells by perinuclear EP(3) receptors. Circ. Res. 2002, 90, 682–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, M.M.; Eghbali, M.; Alioua, A.; Song, M.; Knaus, H.G.; Stefani, E.; Toro, L. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc. Natl. Acad. Sci. USA 2004, 101, 10072–10077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Alioua, A.; Kumar, Y.; Eghbali, M.; Stefani, E.; Toro, L. MaxiK channel partners: Physiological impact. J. Physiol. 2006, 570, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, B.H.; Olesen, S.P.; Ronn, L.C.; Grunnet, M. BK channel activators and their therapeutic perspectives. Front. Physiol. 2014, 5, 389. [Google Scholar] [CrossRef] [Green Version]
- Knaus, H.G.; Folander, K.; Garcia-Calvo, M.; Garcia, M.L.; Kaczorowski, G.J.; Smith, M.; Swanson, R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J. Biol. Chem. 1994, 269, 17274–17278. [Google Scholar] [CrossRef]
- McManus, O.B.; Helms, L.M.; Pallanck, L.; Ganetzky, B.; Swanson, R.; Leonard, R.J. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 1995, 14, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Valverde, M.A.; Rojas, P.; Amigo, J.; Cosmelli, D.; Orio, P.; Bahamonde, M.I.; Mann, G.E.; Vergara, C.; Latorre, R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science 1999, 285, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Meera, P.; Toro, L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane beta-subunit homolog. Proc. Natl. Acad. Sci. USA 1999, 96, 4137–4142. [Google Scholar] [CrossRef]
- Meera, P.; Wallner, M.; Toro, L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl. Acad. Sci. USA 2000, 97, 5562–5567. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Aldrich, R.W. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 2010, 466, 513–516. [Google Scholar] [CrossRef]
- Yan, J.; Aldrich, R.W. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 7917–7922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magleby, K.L. Gating mechanism of BK (Slo1) channels: So near, yet so far. J. Gen. Physiol. 2003, 121, 81–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol. Rev. 2017, 97, 39–87. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Suzuki, Y.; Yamamura, H.; Takeshima, H.; Imaizumi, Y. A junctophilin-caveolin interaction enables efficient coupling between ryanodine receptors and BKCa channels in the Ca2+ microdomain of vascular smooth muscle. J. Biol. Chem. 2019, 294, 13093–13105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golowasch, J.; Kirkwood, A.; Miller, C. Allosteric effects of Mg2+ on the gating of Ca2+-activated K+ channels from mammalian skeletal muscle. J. Exp. Biol. 1986, 124, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Meera, P.; Wallner, M.; Jiang, Z.; Toro, L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (KV,Ca beta) of maxi K channels. FEBS Lett. 1996, 382, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Connolly, M.; Nagaraj, C.; Tang, B.; Balint, Z.; Popper, H.; Smolle-Juettner, F.M.; Lindenmann, J.; Kwapiszewska, G.; Aaronson, P.I.; et al. Peroxisome proliferator-activated receptor-beta/delta, the acute signaling factor in prostacyclin-induced pulmonary vasodilation. Am. J. Respir. Cell Mol. Biol. 2012, 46, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Adelman, J.P.; Shen, K.Z.; Kavanaugh, M.P.; Warren, R.A.; Wu, Y.N.; Lagrutta, A.; Bond, C.T.; North, R.A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 1992, 9, 209–216. [Google Scholar] [CrossRef]
- Shen, K.Z.; Lagrutta, A.; Davies, N.W.; Standen, N.B.; Adelman, J.P.; North, R.A. Tetraethylammonium block of Slowpoke calcium-activated potassium channels expressed in Xenopus oocytes: Evidence for tetrameric channel formation. Pflug. Arch. 1994, 426, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.A.; Zhu, S.; White, R.E. Hypoxia modulates cyclic AMP activation of BkCa channels in rat pulmonary arterial smooth muscle. Lung 2005, 183, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, C.; Tang, B.; Balint, Z.; Wygrecka, M.; Hrzenjak, A.; Kwapiszewska, G.; Stacher, E.; Lindenmann, J.; Weir, E.K.; Olschewski, H.; et al. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur. Respir. J. 2013, 41, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCullough, D.J.; Vang, A.; Choudhary, G. NS1619-induced vasodilation is enhanced and differentially mediated in chronically hypoxic lungs. Lung 2014, 192, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Revermann, M.; Neofitidou, S.; Kirschning, T.; Schloss, M.; Brandes, R.P.; Hofstetter, C. Inhalation of the BK(Ca)-opener NS1619 attenuates right ventricular pressure and improves oxygenation in the rat monocrotaline model of pulmonary hypertension. PLoS ONE 2014, 9, e86636. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, B.H.; Nardi, A.; Calloe, K.; Madsen, L.S.; Olesen, S.P.; Grunnet, M. The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol. Pharm. 2007, 72, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, H.; Rose, F.; Schermuly, R.; Ghofrani, H.A.; Enke, B.; Olschewski, A.; Seeger, W. Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharm. Ther. 2004, 102, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, R.; Liu, P.; Gu, Y. Docosahexaenoic acid attenuates hypoxic pulmonary vasoconstriction by activating the large conductance Ca2+-activated K+ currents in pulmonary artery smooth muscle cells. Pulm Pharm. Ther. 2014, 28, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, C.; Tang, B.; Nagy, B.M.; Papp, R.; Jain, P.P.; Marsh, L.M.; Meredith, A.L.; Ghanim, B.; Klepetko, W.; Kwapiszewska, G.; et al. Docosahexaenoic acid causes rapid pulmonary arterial relaxation via KCa channel-mediated hyperpolarisation in pulmonary hypertension. Eur. Respir. J. 2016, 48, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Aursnes, M.; Hansen, T.V.; Tungen, J.E.; Galpin, J.D.; Leisle, L.; Ahern, C.A.; Xu, R.; Heinemann, S.H.; Hoshi, T. Atomic determinants of BK channel activation by polyunsaturated fatty acids. Proc. Natl. Acad. Sci. USA 2016, 113, 13905–13910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gai, X.Y.; Wei, Y.H.; Zhang, W.; Wuren, T.N.; Wang, Y.P.; Li, Z.Q.; Liu, S.; Ma, L.; Lu, D.X.; Zhou, Y.; et al. Echinacoside induces rat pulmonary artery vasorelaxation by opening the NO-cGMP-PKG-BKCa channels and reducing intracellular Ca2+ levels. Acta Pharm. Sin. 2015, 36, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Dumas-de-La-Roque, E.; Begueret, H.; Marthan, R.; Fayon, M.; Dos Santos, P.; Savineau, J.P.; Baulieu, E.E. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc. Natl. Acad. Sci. USA 2003, 100, 9488–9493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, W.; Hoidal, J.R.; Farrukh, I.S. Role of a novel KCa opener in regulating K+ channels of hypoxic human pulmonary vascular cells. Am. J. Respir. Cell Mol. Biol. 1999, 20, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.D.; Xu, R.; Reynolds, M.F.; Garcia, M.L.; Heinemann, S.H.; Hoshi, T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature 2003, 425, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Vang, A.; Mazer, J.; Casserly, B.; Choudhary, G. Activation of endothelial BKCa channels causes pulmonary vasodilation. Vasc. Pharm. 2010, 53, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.P.; Seara, F.A.C.; Baptista, E.F.; Barenco, T.S.; Sottani, T.B.B.; Souza, N.S.C.; Domingos, A.E.; Barbosa, R.A.Q.; Takiya, C.M.; Couto, M.T.; et al. BKCa Channel Activation Attenuates the Pathophysiological Progression of Monocrotaline-Induced Pulmonary Arterial Hypertension in Wistar Rats. Cardiovasc. Drugs Ther. 2021, 35, 719–732. [Google Scholar] [CrossRef]
- Milara, J.; Ballester, B.; Morell, A.; Ortiz, J.L.; Escriva, J.; Fernandez, E.; Perez-Vizcaino, F.; Cogolludo, A.; Pastor, E.; Artigues, E.; et al. JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: An experimental study. Thorax 2018, 73, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Cornfield, D.N.; Resnik, E.R.; Herron, J.M.; Abman, S.H. Chronic intrauterine pulmonary hypertension decreases calcium-sensitive potassium channel mRNA expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L857–L862. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.K.; Cheng, Y.J.; Chung, H.H.; Wu, J.R.; Chen, I.J.; Wu, B.N. KMUP-1 ameliorates monocrotaline-induced pulmonary arterial hypertension through the modulation of Ca2+ sensitization and K+-channel. Life Sci. 2010, 86, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Cogolludo, A.; Mondejar-Parreno, G. Transcriptomic profile of cationic channels in human pulmonary arterial hypertension. Sci. Rep. 2021, 11, 15829. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.P.; Morera, F.J.; Carvacho, I.; Latorre, R. A marriage of convenience: Beta-subunits and voltage-dependent K+ channels. J. Biol. Chem. 2007, 282, 24485–24489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orio, P.; Rojas, P.; Ferreira, G.; Latorre, R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol. Sci. 2002, 17, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkov, G.V.; Bonev, A.D.; Heppner, T.J.; Brenner, R.; Aldrich, R.W.; Nelson, M.T. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J. Physiol. 2001, 537, 443–452. [Google Scholar] [CrossRef]
- Nimigean, C.M.; Magleby, K.L. The beta subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J. Gen. Physiol. 1999, 113, 425–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, L.; Cox, D.H. Gating and ionic currents reveal how the BKCa channel’s Ca2+ sensitivity is enhanced by its beta1 subunit. J. Gen. Physiol. 2005, 126, 393–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, R.; Perez, G.J.; Bonev, A.D.; Eckman, D.M.; Kosek, J.C.; Wiler, S.W.; Patterson, A.J.; Nelson, M.T.; Aldrich, R.W. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 2000, 407, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, J.M.; Tomas, M.; Vazquez, E.; Orio, P.; Latorre, R.; Senti, M.; Marrugat, J.; Valverde, M.A. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J. Clin. Investig. 2004, 113, 1032–1039. [Google Scholar] [CrossRef]
- Seibold, M.A.; Wang, B.; Eng, C.; Kumar, G.; Beckman, K.B.; Sen, S.; Choudhry, S.; Meade, K.; Lenoir, M.; Watson, H.G.; et al. An african-specific functional polymorphism in KCNMB1 shows sex-specific association with asthma severity. Hum. Mol. Genet. 2008, 17, 2681–2690. [Google Scholar] [CrossRef] [Green Version]
- Barnes, E.A.; Lee, L.; Barnes, S.L.; Brenner, R.; Alvira, C.M.; Cornfield, D.N. beta1-Subunit of the calcium-sensitive potassium channel modulates the pulmonary vascular smooth muscle cell response to hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L265–L275. [Google Scholar] [CrossRef]
- Resnik, E.; Herron, J.; Fu, R.; Ivy, D.D.; Cornfield, D.N. Oxygen tension modulates the expression of pulmonary vascular BKCa channel alpha- and beta-subunits. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L761–L768. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.T.; Kim, Y.M.; Adams, E.; Lyu, S.C.; Alvira, C.M.; Cornfield, D.N. Hypoxia-inducible factor-1alpha regulates KCNMB1 expression in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L352–L359. [Google Scholar] [CrossRef] [Green Version]
- Babicheva, A.; Ayon, R.J.; Zhao, T.; Ek Vitorin, J.F.; Pohl, N.M.; Yamamura, A.; Yamamura, H.; Quinton, B.A.; Ba, M.; Wu, L.; et al. MicroRNA-mediated downregulation of K+ channels in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L10–L26. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, N.; Lou, T.; Jin, Y.; Wu, Y.; Ye, Z.; Pan, J. microRNA-183 down-regulates the expression of BKCabeta1 protein that is related to the severity of chronic obstructive pulmonary disease. Hippokratia 2014, 18, 328–332. [Google Scholar]
- Kovacs, G.; Agusti, A.; Barbera, J.A.; Celli, B.; Criner, G.; Humbert, M.; Sin, D.D.; Voelkel, N.; Olschewski, H. Pulmonary Vascular Involvement in Chronic Obstructive Pulmonary Disease. Is There a Pulmonary Vascular Phenotype? Am. J. Respir. Crit. Care Med. 2018, 198, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, A.M.; Grabauskas, G.; Huang, S.K. The Role of KCNMB1 and BK Channels in Myofibroblast Differentiation and Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 62, 191–203. [Google Scholar] [CrossRef]
- Evanson, K.W.; Bannister, J.P.; Leo, M.D.; Jaggar, J.H. LRRC26 is a functional BK channel auxiliary gamma subunit in arterial smooth muscle cells. Circ. Res. 2014, 115, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Manzanares, D.; Srinivasan, M.; Salathe, S.T.; Ivonnet, P.; Baumlin, N.; Dennis, J.S.; Conner, G.E.; Salathe, M. IFN-gamma-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L453–L462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtson, C.D.; Kim, M.D.; Anabtawi, A.; He, J.; Dennis, J.S.; Miller, S.; Yoshida, M.; Baumlin, N.; Salathe, M. Hyperglycaemia in cystic fibrosis adversely affects BK channel function critical for mucus clearance. Eur. Respir. J. 2021, 57, 2000509. [Google Scholar] [CrossRef]
- Manzanares, D.; Krick, S.; Baumlin, N.; Dennis, J.S.; Tyrrell, J.; Tarran, R.; Salathe, M. Airway Surface Dehydration by Transforming Growth Factor beta (TGF-beta) in Cystic Fibrosis Is Due to Decreased Function of a Voltage-dependent Potassium Channel and Can Be Rescued by the Drug Pirfenidone. J. Biol. Chem. 2015, 290, 25710–25716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.D.; Baumlin, N.; Yoshida, M.; Polineni, D.; Salathe, S.F.; David, J.K.; Peloquin, C.A.; Wanner, A.; Dennis, J.S.; Sailland, J.; et al. Losartan Rescues Inflammation-related Mucociliary Dysfunction in Relevant Models of Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.; Olesen, S.P. BK channel modulators: A comprehensive overview. Curr. Med. Chem 2008, 15, 1126–1146. [Google Scholar] [CrossRef]
- Malerba, M.; DAmato, M.; Radaeli, A.; Giacovelli, G.; Rovati, L.; Arshad, S.H.; Holgate, S.T. Efficacy of Andolast in Mild to Moderate Asthma: A Randomized, Controlled, Double-Blind Multicenter Study (The Andast Trial). Curr. Pharm. Des. 2015, 21, 3835–3843. [Google Scholar] [CrossRef]
- Michelakis, E.D.; McMurtry, M.S.; Sonnenberg, B.; Archer, S.L. The NO—K+ channel axis in pulmonary arterial hypertension. Activation by experimental oral therapies. Adv. Exp. Med. Biol. 2003, 543, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Inholte, C.; Ghofrani, H.A.; Gall, H.; Weissmann, N.; Weidenbach, A.; Seeger, W.; Grimminger, F. Lung vasodilatory response to inhaled iloprost in experimental pulmonary hypertension: Amplification by different type phosphodiesterase inhibitors. Respir. Res. 2005, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, V.V.; Benza, R.L.; Rubin, L.J.; Channick, R.N.; Voswinckel, R.; Tapson, V.F.; Robbins, I.M.; Olschewski, H.; Rubenfire, M.; Seeger, W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. J. Am. Coll Cardiol. 2010, 55, 1915–1922. [Google Scholar] [CrossRef] [Green Version]
- Olschewski, H.; Simonneau, G.; Galie, N.; Higenbottam, T.; Naeije, R.; Rubin, L.J.; Nikkho, S.; Speich, R.; Hoeper, M.M.; Behr, J.; et al. Inhaled iloprost for severe pulmonary hypertension. N. Engl. J. Med. 2002, 347, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Clapp, L.H.; Turcato, S.; Hall, S.; Baloch, M. Evidence that Ca2+-activated K+ channels play a major role in mediating the vascular effects of iloprost and cicaprost. Eur. J. Pharm. 1998, 356, 215–224. [Google Scholar] [CrossRef]
- Olschewski, H.; Olschewski, A.; Rose, F.; Schermuly, R.; Schutte, H.; Weissmann, N.; Seeger, W.; Grimminger, F. Physiologic basis for the treatment of pulmonary hypertension. J. Lab. Clin. Med. 2001, 138, 287–297. [Google Scholar] [CrossRef] [PubMed]
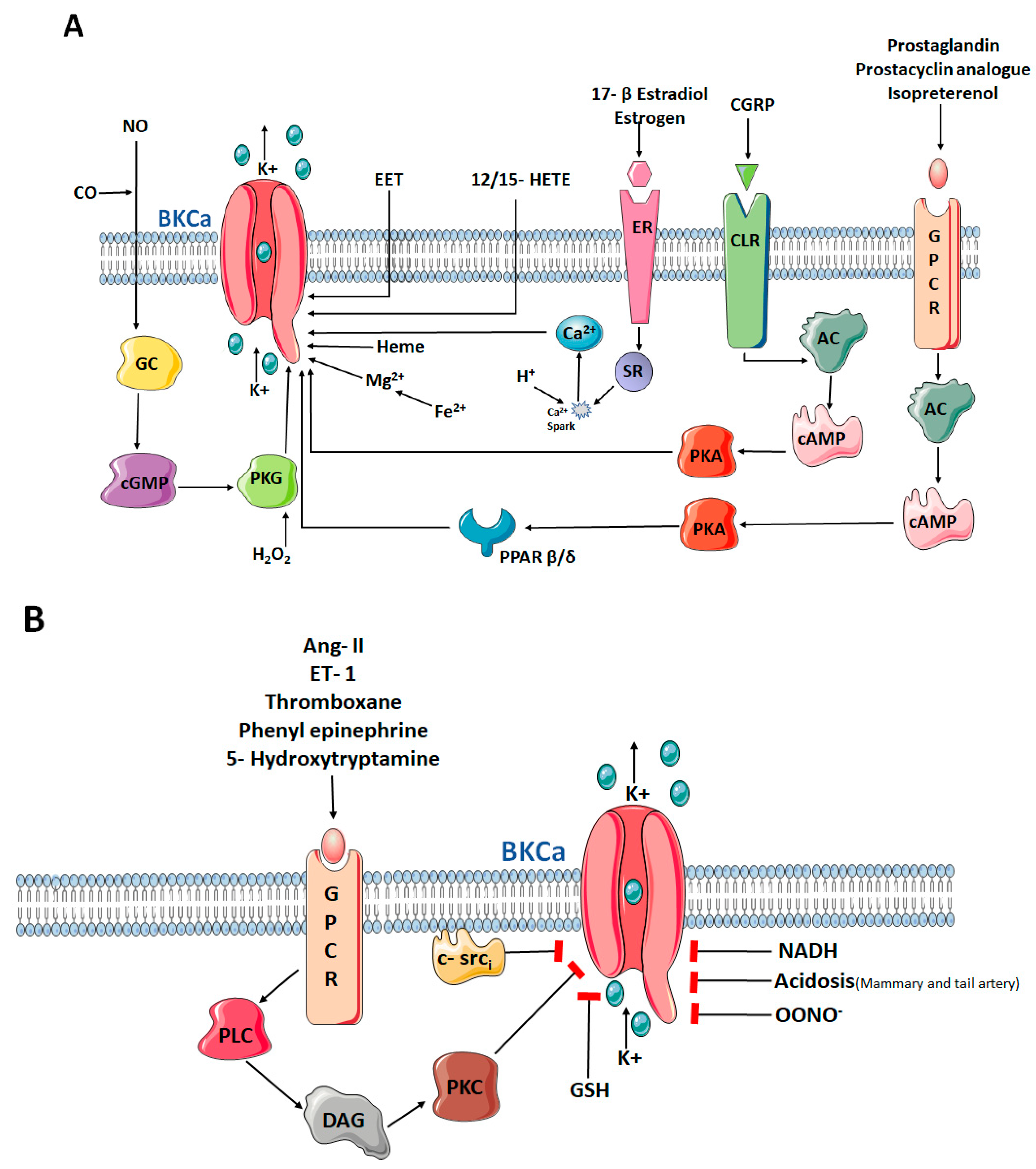
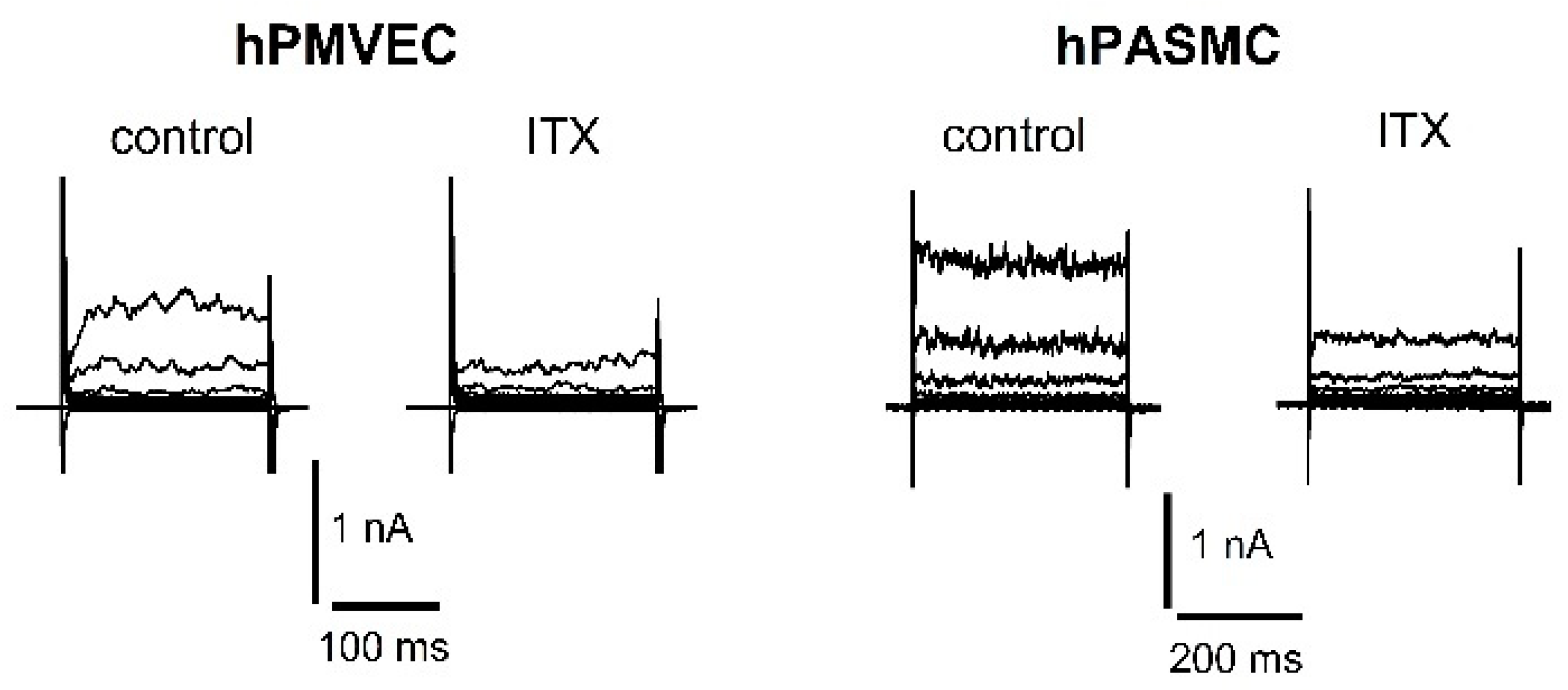
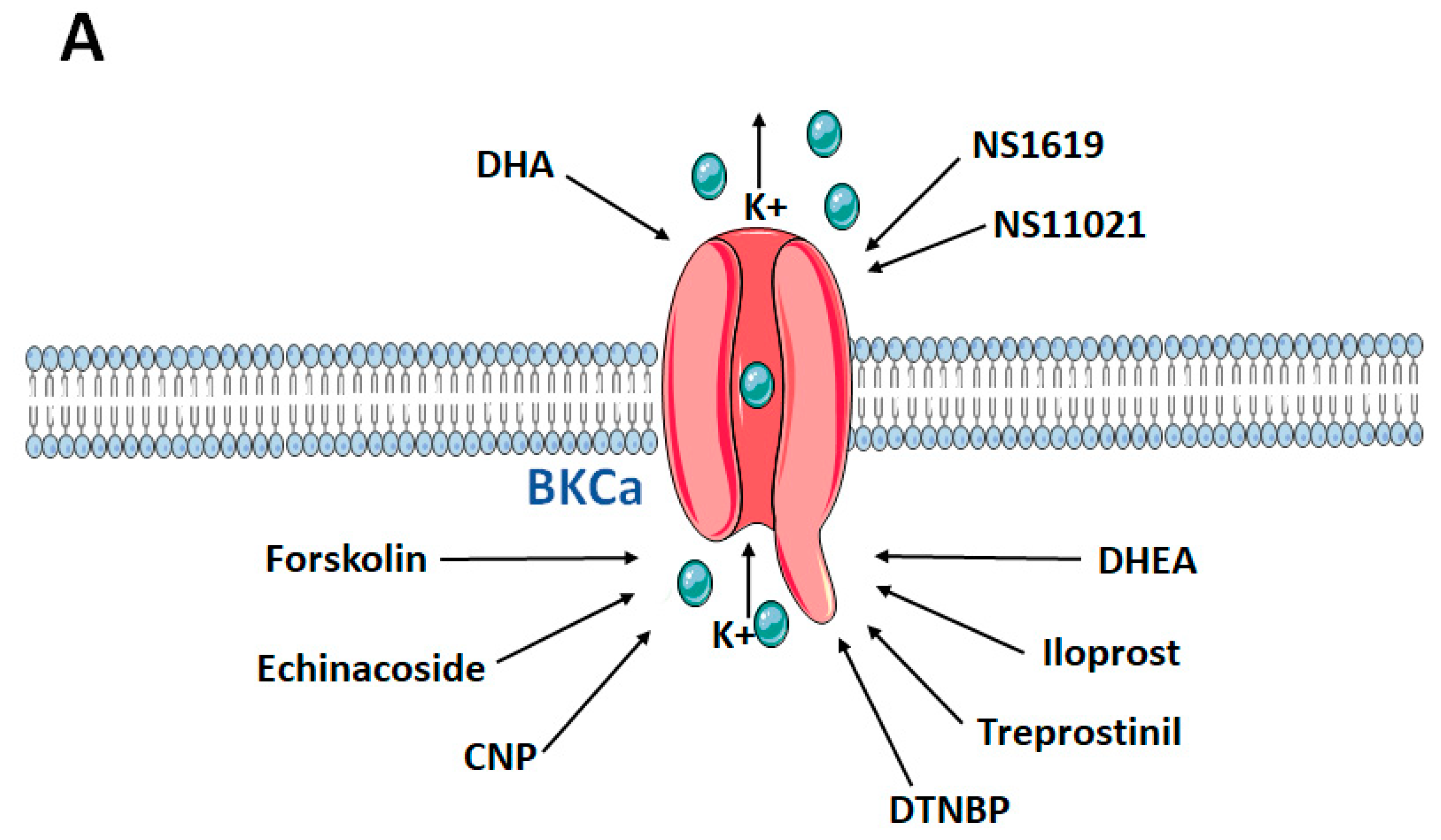
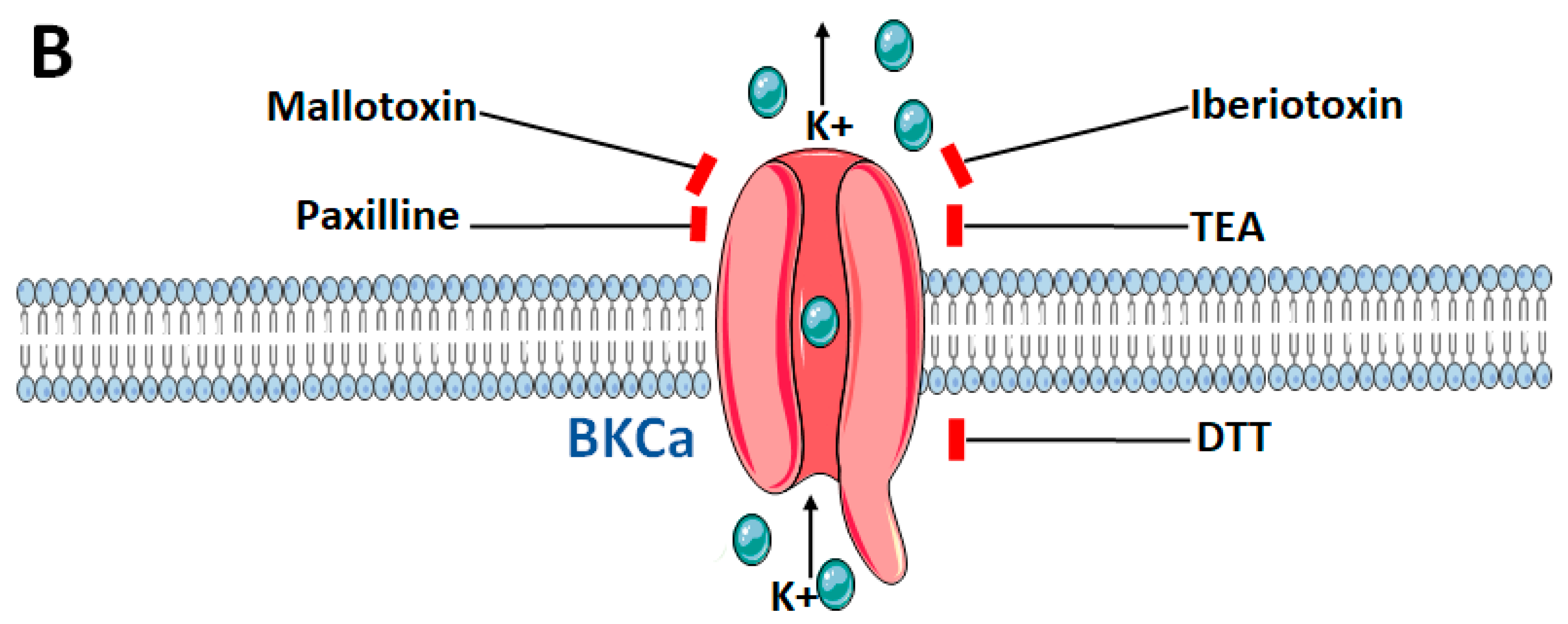
| Compound | (Disease) Model | Effect on BKCa | Effects in the Model | Potential Mechanisms | References |
|---|---|---|---|---|---|
| NS1619 and C-type natriuretic peptide | Normotensive rats | Activation | Enhanced endothelium-dependent PA ring dilation and PA pressure reduction ex vivo; hyperpolarised and increased NO production in PMVECs in vivo | Direct activation of the channel in PMVECs | [63] |
| Compound X | Monocrotaline (MCT)-induced pulmonary arterial hypertension (PAH) rat model | Activation | Reduced pulmonary vascular remodelling, pulmonary flow resistance, RV hypertrophy and afterload in PAH model in vivo strongly vasodilated PA rings ex vivo | Direct activation of the channel in PASMCs | [64] |
| Docosahexaenoic acid (DHA) | IPAH patients normotensive rats chronic hypoxia-induced PH mouse model Kcnma1−/− mouse | Activation | Reduced RV pressure in the PH animal model to normal in vivo enhanced endothelium-dependent PA ring dilation and PA pressure reduction ex vivo; dose-dependently activated BKCa current and hyperpolarised human IPAH-PASMCs to normal in vivo | Direct activation of the channel in PASMCs | [57] |
| Dehydroepiandrosterone (DHEA) | Chronic hypoxia-induced PH rat model | Activation | Reduced RV pressure, RV wall thickness and PA remodelling in the PH animal model in vivo restored the PA-pressure response to acute hypoxia in the PH animal model in vivo. Decreased intracellular [Ca2+] under hypoxia in PASMCs in vitro Increased BKCa channel activity and expression in PA in the PH animal model in vitro | Dual effect: (i) Activation of the channel in PA by changing the redox balance toward a more oxidative state (ii) Upregulated BKCa mRNA and protein in PASMCs of the chronic hypoxic PH model | [60] |
| Echinacoside | Normotensive rats | Activation | Reduced noradrenaline-induced contraction of PA in extracellular [Ca2+]-dependent manner ex vivo | Activated NO-cGMP-PKG pathway with subsequent hyperpolarisation and decrease of intracellular free [Ca2+] in PASMCs | [59] |
| Forskolin and cAMP activators | Fawn-hooded rat employed in chronic hypoxia-induced PH rat model | Activation | Increased open probability of BKCa channels in fawn-hooded PH animal PASMCs in vitro | Direct activation of the channel in PASMCs | [50] |
| NS1619 | Monocrotaline (MCT)-induced PAH rat model | Activation | Reduced RV pressure, carbon monoxide and improved oxygenation in the PAH animal model in vivo reduced PDGF-induced PASMC proliferation in vitro | Direct activation of the channel | [53] |
| JAK2 inhibitors | IPF patients bleomycin-induced lung fibrosis and PH rat model | Activation | Reduced RV pressure and PA Remodelling in vivo Reduced V/Q mismatch in animal model in vivo Promoted relaxant and anticontractile effects on IPF-PA ex vivo Inhibited effect of TGFβ1-induced loss of endothelial markers and upregulation of the PA remodelling markers in vitro Activated BKCa current in vitro | Unknown | [65] |
| Iloprost and treprostinil | Primary human PASMCs | Activation | Enhanced PA ring dilation ex vivo Enhanced BKCa current in vitro | (PKA)-induced phosphorylation of BKCa | [47] |
| NS1619 | Chronic hypoxia-induced PH rat model | Activation | Enhanced NO-dependent PA pressure reduction ex vivo | Direct activation of the channel | [52] |
| Compound | (Disease) Model | Effect on BKCa | Effects in the Model | Potential Mechanisms | References |
|---|---|---|---|---|---|
| Knocking down KCNMB1 | Chronic hypoxia-induced PH mouse model employing Kcnmb1−/− mouse | Inhibition | Increased pulmonary vascular response to acute and chronic hypoxia and increased RV pressure in vivo Increased focal complex expression in PASMCs in vitro | Downregulates BKCa channels (mRNA, protein and function) in PASMCs | [77] |
| Knocking down HIF-1α or KCNMB1 | Subacute hypoxia in hPASMCs | Inhibits upregulation of KCNMB1 in response to hypoxia | Potentiated the hypoxic-mediated increase in [Ca2+]i in vitro Strongly vasodilated PA rings ex vivo | Downregulates BKCa channels (mRNA, protein and function) in PASMCs | [79] |
| Overexpression or inhibition of miR-29b | Healthy and IPAH PASMCs | Inhibition or activation, respectively | Decreased BKCa channel currents and downregulated KCNMB1 in normal PASMCs in vitro Increased BKCa channel activity and upregulated KCNMB1 in IPAH-PASMC in vitro | Downregulation or activation of the channel in PASMCs | [80] |
| Upregulated KCNMB1 | IPF fibroblasts | Activation | Increased BKCa channel activity in vitro | Upregulated BKCa mRNA and protein in IPF fibroblasts | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guntur, D.; Olschewski, H.; Enyedi, P.; Csáki, R.; Olschewski, A.; Nagaraj, C. Revisiting the Large-Conductance Calcium-Activated Potassium (BKCa) Channels in the Pulmonary Circulation. Biomolecules 2021, 11, 1629. https://doi.org/10.3390/biom11111629
Guntur D, Olschewski H, Enyedi P, Csáki R, Olschewski A, Nagaraj C. Revisiting the Large-Conductance Calcium-Activated Potassium (BKCa) Channels in the Pulmonary Circulation. Biomolecules. 2021; 11(11):1629. https://doi.org/10.3390/biom11111629
Chicago/Turabian StyleGuntur, Divya, Horst Olschewski, Péter Enyedi, Réka Csáki, Andrea Olschewski, and Chandran Nagaraj. 2021. "Revisiting the Large-Conductance Calcium-Activated Potassium (BKCa) Channels in the Pulmonary Circulation" Biomolecules 11, no. 11: 1629. https://doi.org/10.3390/biom11111629
APA StyleGuntur, D., Olschewski, H., Enyedi, P., Csáki, R., Olschewski, A., & Nagaraj, C. (2021). Revisiting the Large-Conductance Calcium-Activated Potassium (BKCa) Channels in the Pulmonary Circulation. Biomolecules, 11(11), 1629. https://doi.org/10.3390/biom11111629








