Abstract
Li+/Eu3+ dual-doped calcium apatite analogues were fabricated using a microwave stimulated hydrothermal technique. XRPD, FT-IR, micro-Raman spectroscopy, TEM and SAED measurements indicated that obtained apatites are single-phased, crystallize with a hexagonal structure, have similar morphology and nanometric size as well as show red luminescence. Lithium effectively modifies the local symmetry of optical active sites and, thus, affects the emission efficiency. Moreover, the hydrodynamic size and surface charge of the nanoparticles have been extensively studied. The protein adsorption (lysozyme, LSZ; bovine serum albumin, BSA) on the nanoparticle surface depended on the type of cationic dopant (Li+, Eu3+) and anionic group (OH−, Cl−, F−) of the apatite matrix. Interaction with LSZ resulted in a positive zeta potential, and the nanoparticles had the lowest hydrodynamic size in this protein medium. The cytotoxicity assessment was carried out on the human osteosarcoma cell line (U2OS), murine macrophages (J774.E), as well as human red blood cells (RBCs). The studied apatites were not cytotoxic to RBCs and J774.E cells; however, at higher concentrations of nanoparticles, cytotoxicity was observed against the U2OS cell line. No antimicrobial activity was detected against Gram-negative bacteria with one exception for P. aeruginosa treated with Li+-doped fluorapatite.
1. Introduction
Synthetic nanoapatites are extensively studied in the area of biomedical applications due to their biocompatibility, high bioactivity and osteoconductive properties in vivo [1]. The biggest advantage of using them as a bioceramic or biomaterial compared to other materials, such as bioglass or glass–ceramic, is their chemical similarity to the inorganic component of the bone tissue. For example, synthetic hydroxyapatite has been applied clinically both as a dense, sintered cement and as a coating on metallic implants [2,3]. Nowadays, researchers are still developing many new areas of using the nanometric hydroxyapatite in orthopedics for drug release and bio-imaging [4,5,6,7]. An ideal diagnostic and therapeutic (theranostics) system should possess the ability to transport active pharmaceutical compounds to target cells or tissues and release them in a controlled manner simultaneously dealing with a real-time monitoring of treatment effect based on photoluminescence properties of rare earth ions (RE3+) [8].
In the present research, three analogues of apatite: hydroxyapatite (Ca10(PO4)6(OH)2; HAp), chlorapatite (Ca10(PO4)6Cl2; ClAp) and fluorapatite (Ca10(PO4)6F2; FAp) have been evaluated in terms of cytotoxicity in cell-culture studies. Moreover, these matrices have been modified (while maintaining the crystal structure) using cationic dopants, lithium (Li+) and europium (Eu3+) ions. Many metal cations can be substituted for calcium ions. Replacement of bivalent Ca2+ by other cations, e.g., monovalent (M+) or trivalent (M3+) ions, cause charge imbalance in the apatite lattice that can be neutralized by creating vacancy defects [8,9]. Whereas, the occurrence of simultaneous substitutions of both M+ and M3+ cations affects the reduction in vacancy defects, where M+ acting as a charge compensation ion [10,11]. Substitution of calcium ions by other cations modifies the cell parameters, which means a contraction or expansion of the lattice constants, depending on ionic radius and charge of the doped ions. The localization of specific ions in the apatitic structure can be generally correlated to their ionic radius. There is a possible substitution of the dopants in both cationic sites: Ca1 and Ca2 (see Figure 1), where the first one is smaller in volume than the second one [12]. It leads to the conclusion that a cation with a larger radius than a radius of Ca2+ rather should prefer to occupy the Ca2 site. However, our earlier experiments have shown that indeed for a low concentration range of smaller RE3+ ions would preferentially substitute the Ca1 site, the increase in dopant amount would lead to reverse dependence [10,13]. Probably, the repulsion between atoms in the Ca1 position would result in the enlargement of the c-axis, which is partially inhibited by accommodating metal atoms in the Ca2 site [14]. Moreover, not only the concentration effect but also the annealing temperature can affect the site preference of the RE3+ cations in the apatite lattice. The higher sintering temperature leads to a more preferential incorporation of Eu3+ ions at Ca2 crystallographic position, which is a consequence of the initial migration of Eu3+ from the Ca1 site by a diffusion process [15]. The thermal treatment at above 500 °C activates Ca2+ -vacancy-Eu3+ migration through the Ca1 column parallel to the c-axis allowing the Eu3+ ions (at Ca1 position) to diffuse through the apatite lattice until obtaining the final distribution approximately equal to 30% at the Ca1 site and 70% at the Ca2 site.

Figure 1.
The projection of the Ca10(PO4)6(OH)2 (a), Ca10(PO4)6Cl2 (b) and Ca10(PO4)6F2 (c) unit cells with the indication of the Ca1 and Ca2 coordination spheres.
In this work, the lithium cation was chosen because it shows (as confirmed in a mice model) a great potential to induce the formation of bone tissue as well as to reduce the risk of fracture in the Li+ ions-treated patients [16,17]. Our scientific group has shown that lithium ions can regulate the proliferative activity of mesenchymal stromal cells and their ability to differentiate into various types of cells [18]. From the physicochemical point of view, Li+ cation plays an important role as a charge compensator of trivalent lanthanide ion, thus promoting radiative transitions leading to an enhancement in the luminescence intensity [19]. Moreover, the quantum efficiency depends on the type of apatite matrix [20]. Rare earth ions substituted in FAp or ClAp lattice show higher quantum yield in relation to the HAp matrix, where the presence of OH− groups causes strong non-radiative migrations energy. Among of lanthanide ions group, Eu3+ cation was selected, because it can be used as a luminescent probe to study the local symmetry of the site(s) that it occupies in the apatite structure and as a high-sensitive, non-invasive tool for luminescence bio-detection in the range of visible light. So far, our scientific group has published research on the influence of Li+ ions on the structural and optical properties of FAp as well as Ca-Sr hydroxyapatites activated with Eu3+ ions, respectively [10,21].
For biomedical purposes, the toxicity of nanoparticles is a crucial factor in considering their potential in in vivo applications. Since these nanoparticles are expected to interact with living cells, it is important to design them in a way that avoids any adverse effects. The most significant is whether or not modified or unmodified nanoparticles will undergo biodegradation in the cellular environment and what kind of cellular responses will cause such degraded nanoparticles. While in vivo nanoparticles studies require comprehensive and stringent toxicological characterization, an in vitro pathway constitutes the first line of research needed for understanding the primary cytotoxic potential of the particles. This allows a high throughput selection of the most promising materials without unnecessary animal suffering. Typically, in vitro cytotoxicity studies are carried out using cell line models representative of the tissues expected to be the biological targets during in vivo exposure. Such studies are performed under specified conditions (particle concentration and time of exposure) and assess well-defined biological endpoints (e.g., metabolic rate or cell count) [22].
In the current research, the choice of J774.E cell line (murine macrophages) as the in vitro model was based on the fact that under in vivo conditions, macrophages form the primary line of response to particulate matter [23,24]. Thus, they are responsible for the distribution and clearance of nanoparticles and their agglomerates. Moreover, phagocyte interaction with nanoparticulate systems may elicit an inflammatory response that complicates the biocompatibility of some materials [25]. On the other hand, the choice of the human osteosarcoma cell line (U2OS) was due to the fact that it is a cancer cell line derived from bone tissue, which is rich in nanoapatites that play a pivotal role in the extracellular matrix formation. U2OS is an immortal cell line and, therefore, is easier and much more reproducible than primary cells. This cell line belongs to the most widely used models for in vitro studies on the bone tissue [26,27].
Moreover, to expand the evaluation of biocompatibility of the designed biomaterials, the influence of nanoapatites on human red blood cells (RBCs) as well as their interaction with bovine serum albumin (BSA) and lysozyme (LSZ) have been also investigated. Human RBC is the most numerous blood component and a valuable in vitro cell model for screening of biological activity of compounds, especially with the cell membrane-interacting activity [28]. Furthermore, the hemolytic activity of potential blood-contacting compound is an essential parameter in assessing its hemocompatibility [29]. Unfavorable interactions between the newly developed materials and RBCs’ membrane should be carefully analyzed in vitro to prevent any alteration of RBCs’ morphology and function in vivo. Therefore, hemocompatibility is one of the most important criteria that limit the clinical applicability of promising blood-contacting biomaterial. LSZ (cationic) and BSA (anionic) are characterized by opposite charge at pH 7 with isoelectric points 10.70–11.30 and 4.70–5.30, respectively, and are commonly used as model proteins in studies focusing on material–protein interaction. What is more, interactions of LSZ with nanoparticles are extensively studied also because of its antibacterial and possibly also anticancer effect [30,31].
Surgical procedures, for example, the placement of implants, often present certain health risks of bacterial infections through nosocomial strains such as Pseudomonas aeruginosa, Klebsiella pneumoniae and/or Escherichia coli [32,33]. Thus, an implant material should have antimicrobial properties to be protected by bacteria growth and, as a consequence, bacterial infection. It has been noted that the implant materials doped with various metals might exhibit antibacterial activity dependently on the ion concentration. It was shown that hydroxyapatite doped with Eu3+ or Li+ ions enhanced the bactericidal effect against Gram-positive strains [34,35].
In this paper, the multifunctional properties of nanoapatites structurally modified by Li+ and Eu3+ ions in comparison with un-doped apatite matrices have been investigated. The obtained materials were comprehensively characterized in terms of their physicochemical and biological properties. The innovation of proposed biomaterials can be associated with the possibility of using them for simultaneous regenerative medicine and in-vivo imaging. Therefore, our research program is dealing with biocompatibility and bioactivity of desired nanocrystalline HAp, ClAp and FAp doped with Li+ cation, which influence on cells activity and Eu3+ ion used as a bio-imaging probe. Cytotoxicity of obtained materials was evaluated in in vitro cell line models: U2OS, J774.E as well as RBCs to evaluate their hemocompatibility. The interactions of fabricated nanoapatites with BSA and LSZ were also investigated. In the context of the type of dopant, particle size, specific surface area and surface charge, the protein corona evaluation was discussed. Furthermore, the antibacterial properties of studied materials against Gram-negative strains (E. coli, P. aeruginosa and K. pneumoniae) were analyzed.
2. Materials and Methods
2.1. Preparation of Nanocrystalline Apatites
The nanoapatites: HAp, ClAp and FAp were obtained by a microwave-stimulated hydrothermal method using: Ca(NO3)2∙4H2O (99+% Acros Organics, Geel, Belgium), CaCl2∙2H2O (99.9% Alfa Aesar, Haverhill, MA, USA), (NH4)2HPO4 (≥98% Avantor Performance Materials Poland S.A, Gliwice, Poland), Eu2O3 (99.99% Alfa Aesar, Haverhill, MA, USA), Li2CO3 (99.9% Alfa Aesar, Haverhill, MA, USA) and NH4F (98% Alfa Aesar, Haverhill, MA, USA) as starting substrates and NH3∙H2O (99% was set to 2 mol% Li+ and 1 mol% Eu3+ in ratio to overall molar content of calcium cations). All reagents used depending on the type of matrix have been gathered in Table 1.

Table 1.
Reagents used for the synthesis of nanoapatites (+ means reagent used, − means reagent not used).
For example, to prepare hydroxyapatite co-doped with Eu3+ and Li+ (HAp: 1 mol% Eu3+, 2 mol% Li+), the stoichiometric amount of the Eu2O3 and Li2CO3 were digested in an excess of the HNO3 (ultrapure Avantor Performance Materials Poland S.A, Gliwice, Poland) to obtain water-soluble europium and lithium nitrates, which, then, were re-crystallized three times in order to remove the excess HNO3. Afterwards, an aqueous solution of diammonium hydrogen phosphate was added to the mixture of an aqueous solution of calcium, europium(III) and lithium nitrates. The pH of the suspension was adjusted to 10 by adding ammonia. The reaction condition was maintained at 200 °C for 1.5 h under autogenous pressure of 55 atm in the microwave reactor (ERTEC MV 02-02, Poland). Finally, the resultant precipitate was further dried at 90 °C and then, thermally treated under air atmosphere at 500 °C for 3 h (the heating rate was 3 °C/min).
2.2. Materials Characterization
The X-ray powder diffractograms (XRPD) were recorded on a PANalytical X’Pert Pro X-ray diffractometer (Malvern Panalytical Ltd., Royston, UK) equipped with Ni-filtered Cu Kα1 radiation (Kα1 = 1.54060 Å, V = 40 kV, I = 30 mA). The experimental patterns were compared with the Ca5(PO4)3(OH), Ca5(PO4)3Cl and Ca5(PO4)3F standards, respectively, derived from the Inorganic Crystal Structure Database (ICSD). The mean crystallite size was calculated according to the Scherer’s relation:
where D is the average crystallite size, λ denotes the X-ray radiation wavelength, β represents a full width at half-maximum of a diffraction line located at θ, and β0 represents a scan aperture of the diffractometer.
The crystallites size was also estimated with the help of Rietveld analysis [36] using an anisotropic approach and Maud 2.68 software [37].
High-resolution transmission electron microscopy (HRTEM) images were performed using a Philips CM-20 SuperTwin microscope (Eindhoven, The Netherlands) with a resolution of 0.25 nm at 200 kV. The sample for HRTEM was prepared by dispersing a small amount of specimen in methanol and putting a droplet of the suspension on a copper microscope gird covered with perforated carbon film. Manual particle size counting (100 measurements) was done by ImageJ software (National Institutes of Health and the Laboratory for Optical and Computational Instrumentation; University of Wisconsin, Madison, WI, USA) based on the known distance (scale bar). A histogram with normal distribution curve has been drawn up using the Origin data analysis software (OriginLab Corporation, Northampton, MA, USA).
The hydrodynamic size of the nanoparticles was determined by the Dynamic Light Scattering (DLS) method with a Zetasizer Nano ZS apparatus from Malvern Instruments operating under an He-Ne 633 nm laser and equipped with the Dispersion Technology Software for data collection and data analysis. The same apparatus was applied for measurements of zeta potential, using a capillary cell, by a combination of electrophoresis and laser doppler velocimetry. The starting concentration of nanoparticles in all prepared suspensions was around 500 µg·mL−1. Then, the suspensions were diluted with de-ionized water, 0.05% BSA or 0.05% LSZ water solution until the satisfactory concentration achieved reliable statistics results and excluded errors connected with too high or too low amounts of analyzed objects. Each measurement was repeated three times with fixed concentrations of particles. The hydrodynamic radius (rh) of the studied samples was determined using the Stokes–Einstein equation:
where KB is Boltzmann’s constant, T is temperature, Dt is particle diffusion coefficient, and η is solvent viscosity (H2O).
The zeta potential (z) of the particles in water suspension was determined based on Henry’s equation:
where UE is electrophoretic mobility, ε is dielectric constant, η is solvent viscosity (H2O), and f(Ka) is Henry’s function approximately equal to 1.5 (for aqueous suspension).
Overall chemical composition of obtained apatites was performed by Inductively Coupled Plasma Optical-Emission Spectrometry (ICP-OES) using Thermo Scientific iCAP 7000) spectrometer (Waltham, MA, USA). Moreover, the elemental analysis (except too light lithium) was carried out using a Scanning Electron Microscope FEI Nova NanoSEM 230 (Hillsboro, OR, USA) equipped with EDS spectrometer (EDAX PegasusXM4). Up to 10 measurements were made from different random areas to assure satisfactory statistics.
FT-IR spectra were recorded using a Thermo Scientific Nicolet iS50 FT-IR spectrometer (Thermo Waltham, MA, USA) over the wavenumbers 4000–500 cm−1 (spectral resolution was set to 4 cm−1) in KBr pellets at room temperature, and the background noise was corrected with pure KBr data.
Raman spectra in the range 100–1200 cm−1 were carried out using a micro-Raman system Renishaw InVia Raman spectrometer equipped with a confocal DM 2500 Leica optical microscope (Wotton-under-Edge, Gloucestershire, UK), diode laser (λexc = 830 nm) as an excitation source and thermoelectrically cooled charge-coupled device (CCD) as a detector.
All spectroscopy measurements were performed at room temperature. The emission spectra were recorded using FLS980 Fluorescence Spectrometer from Edinburgh Instruments (Kirkton Campus, UK) equipped with a 450 W Xenon lamp. The emission of a 300 mm focal length monochromator was in the Czerny–Turner configuration. The emission spectra were obtained with ruled grating, 1800 lines per mm blazed at 250 nm. The R928P side window photomultiplier tube from Hamamatsu was used as a detector. A microsecond flashlamp (mF2) was used for the measurements of luminescence decays and a Hamamatsu R928P photomultiplier (Hamamatsu City, Japan) was used as a detector. The effective emission lifetimes (τm) were estimated using the equation:
where I(t) is the luminescence intensity at time t corrected for the background, and the integrals are calculated over the range of 0 < t < tmax, where tmax >> τm.
2.3. Cytotoxicity Assessment in Osteosarcoma Cell Line and Murine Macrophage
2.3.1. Cell Lines and Culture
Cytotoxicity assessment was carried out on murine macrophage (J774.E) and human osteosarcoma (U2OS) cell lines. Both cell lines were cultured in RPMI-1640 medium (Institute of Immunology and Experimental Therapy, Wroclaw, Poland) supplemented with 10% fetal bovine serum (FBS, Gibco, Amarillo, TX, USA), L-glutamine (Sigma, Welwyn Garden City, UK) and antibiotics (penicillin and streptomycin, Sigma, Hamburg, Germany). Cytotoxicity was assessed by two assays that measure two different endpoints: MTT assay that determines the cellular metabolic activity and trypan blue exclusion assay (TBEA) that is based on counting the actual number of living cells. To exclude possible effects of soluble contaminants or free ionic compounds in the synthesized nanomaterials, the dispersions at the highest nanoparticle concentration were subjected to 2 h centrifugation (at 32,900× g), and the supernatants were added to cells as supernatant controls. The lack of adverse effects compared to medium controls indicates that all observed cytotoxicity can be attributed solely to the nanoparticles.
2.3.2. MTT Assay
For the MTT assay, cells were seeded in 96-well plates (TPP, Switzerland) at a density of 10 × 103 (J774.E) or 3 × 103 (U2OS) cells per well and pre-incubated at 37 °C for 24 h in a humidified atmosphere of 5% CO2. After that, nanoparticle dispersions were added. Stock dispersions of apatite nanoparticles were prepared based on a simplified version of the NANOGENOTOX dispersion protocol [38]. Nanoparticles were suspended in 0.05% bovine serum albumin (BSA) water solution and bath-sonicated at room temperature for 2 min. Next, the stock solutions were further diluted in 0.05% BSA, and dispersions in complete culture medium were prepared. Cells were exposed to the nanoparticle dispersions for 48 h (5% CO2, 37 °C). After that, the tetrazolium salt MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide] was added. After 2 h of incubation, cells were lysed and left for complete dissolution of purple-colored metabolite. After 24 h, the optical density (OD) was measured using of a microplate reader (Spark 10M, Tecan, Männedorf, Switzerland) at a wavelength of 570 nm (reference 630 nm). The OD of control cells was taken as 100%. The results were obtained from at least 3 independent experiments. To eliminate the possibility of artifacts due to particle interactions with either MTT or formazan, the suspensions were incubated with dispersions (100 µg/mL) in an acellular system. No interactions were observed.
2.3.3. Trypan Blue Exclusion Assay
For the TBEA assay, cells were seeded in 24-well plates (TPP, Switzerland) at a density of 50 × 103 (J774.E) or 20 × 103 (U2OS) cells per well and pre-incubated at 37 °C for 24 h in a humidified atmosphere of 5% CO2. After that, nanoparticle dispersions were added (see above). After 48 h of incubation, the culture medium was removed, and cells were washed with phosphate-buffered saline (PBS, Institute of Immunology and Experimental Therapy, Wroclaw, Poland) to remove dead and unattached cells. Next, the remaining cells were detached using 0.25% trypsin in EDTA (Sigma–Aldrich, Taufkirchen, Germany), mixed with 0.4% trypan blue solution (Sigma–Aldrich) and counted in the hemocytometer. In this assay, cells that do not stain blue are considered viable. Cell viability was calculated as a percent of control (treated as 100%). Each assay was made in triplicate, and the results are presented as a mean value (±SD) of three independent assays.
2.4. Evaluation of Hemolytic Activity in Human RBCs
2.4.1. Erythrocyte Preparation
Freshly human erythrocytes suspensions (hematocrit/Ht 65%) were purchased from the blood bank in Poznan according to the bilateral agreement No. ZP/907/1002/18. The erythrocytes were washed three times (3000 rpm, 10 min, +4 °C) in 7.4 pH phosphate-buffered saline (PBS—137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4) supplemented with 10 mM glucose. Following washing, RBCs were suspended in PBS buffer at 1.65 × 109 cells/mL (Ht = 15%), stored at 4 °C and used within 5 h.
2.4.2. Hemolysis Assays
Erythrocytes (1.65 × 108 cells/mL, Ht = 1.5%) were incubated in PBS buffer without and with nanoparticles tested (concentrations 0.1 mg/mL and 1 mg/mL) for 60 min at 37 °C in a shaking water bath. Samples with erythrocytes incubated in PBS buffer were taken as the controls. Each sample was repeated three times, and the experiments were repeated three times with RBC from different donors. After incubation, the samples were centrifuged (3000 rpm, 10 min, 4 °C), and released hemoglobin was determined spectrophotometrically at 540 nm (absorption A). The absorption corresponding to a complete hemolysis (absorption B) was acquired after centrifugation of cells treated with ice-cold distilled water. The hemolytic activity of compounds was, then, expressed as a percentage of hemolysis using the following formula:
2.4.3. Erythrocytes Sedimentation Rate under Nanomaterials Treatment
Erythrocytes (1.65 × 108 cells/mL, Ht = 1.5%) were incubated with PBS buffer without and with compounds tested (0.1 mg/mL and 1 mg/mL) in Eppendorf vials for 60 min, at 37 °C. After incubation, the erythrocytes sedimentation rate (ESR) was recorded using a digital camera.
2.4.4. Microscope Studies of Erythrocytes Shape Transformation
The following incubation as above, cells were fixed in 5% paraformaldehyde (PFA) plus 0.01% glutaraldehyde (GA) for 60 min at room temperature (RT). Fixed cells were washed by exchanging of supernatant with PBS buffer, settled on poly-L-lysine-treated (0.1 mg/mL, 10 min, RT) cover glasses and mounted on 80% glycerol. The cover slips were sealed with nail polish. A large number of cells in several separate experimental samples were studied using a Zeiss LSM 510 (AXIOVERT ZOOM) microscope (100 ×/1.4 aperture immersion oil objective, 10 × ocular). Images were acquired using the Zeiss LSM Image Browser program.
2.5. Bovine Serum Albumin and Lysozyme Adsorption Apatite Nanoparticles
Bovine serum albumin (BSA) and lysozyme (LSZ) from chicken white egg were obtained from Sigma. Nanoparticles were dispersed in 0.05% BSA or LSZ water solution at three concentrations: 2 mg/mL, 4 mg/mL and 8 mg/mL. After 180 s of bath sonication, the samples were incubated for 4 h at 37 °C under constant agitation. Afterwards, samples were centrifuged (10,000× g, 20 °C, 15 min), and the remaining protein concentration was measured in the supernatant using bicinchoninic acid (BCA) protein assay according to the manufacturer’s instruction (ThermoScientific). An additional standard curve for lysozyme was prepared. The results were normalized to blank samples (0.05% BSA or LSZ). The experiment was performed in triplicates.
2.6. Antibacterial Evaluation
Antibacterial activity of hydroxyapatites doped with Eu3+ and Li+ ions was tested using the following strains: E. coli ATCC 25922, E. coli ATCC 35218, K. pneumoniae ATCC 700603, P. aeruginosa PAO1 and P. aeruginosa ATCC 27853. The strains were cultivated overnight at 37 °C in LB (Luria Broth) medium, centrifuged and suspended in the saline (0.9% NaCl) to obtain optical density of 0.5 McFarland standard. Then, suspensions were diluted 10×, and 10 µL of cell suspension was transferred to 96-well polystyrene plates. The colloidal solutions of hydroxy-, fluor- and chlor-apatites doped with Eu3+ (1 mol%), Li+ (2 mol%) or Li+Eu3+ co-doped were diluted in the saline to obtain the final concentration of 100 µg/mL. The solutions (200 μL) were, then, transferred to the wells with bacterial suspensions and incubated for 24 h at 37 °C. The number of survived bacteria was evaluated by colony count (CFU/mL) on Muller–Hinton agar. Bacterial cells incubated with a salt solution were used as a control. Additionally, un-doped apatites were tested for antibacterial activity.
3. Results and Discussion
3.1. Physicochemical Characterization of Obtained Nanoapatites
According to the XRPD analysis (see Figure 2), the obtained materials are identified as hexagonal apatites belonged to the P63/m space group. The positions of the diffraction peaks correspond very well with the positions of the peaks ascribed to the reference standards of the Ca10(PO4)6(OH)2 (ICSD-26204), Ca10(PO4)6Cl2 (ICSD-24237) and Ca10(PO4)6F2 (ICSD-262707). No other peaks were detected, which indicates that the structure of the obtained apatites was stable even after simultaneous doping with ions with different charges (Eu3+ and Li+). The lack of secondary phases, impurities or amorphous forms confirm the formation of pure phase apatites and high crystallinity of the final products. The diffraction profiles are broadened, which is caused by a small mean crystallites size.
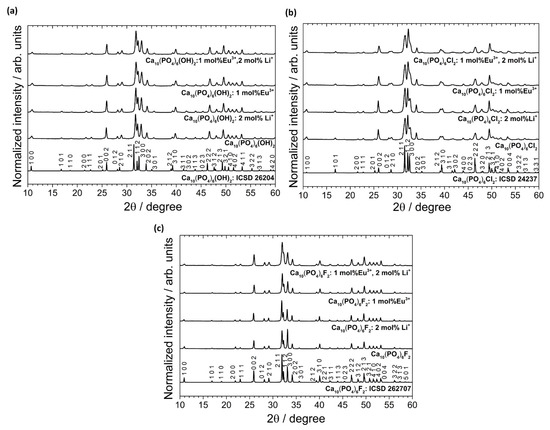
Figure 2.
XRPD patterns (with the indication of crystal planes) of the Ca10(PO4)6(OH)2 (a), Ca10(PO4)6Cl2 (b), Ca10(PO4)6F2 (c) un-doped as well as doped and co-doped with 1 mol% Eu3+ and 2 mol% Li+ ions, prepared at 500 °C.
The results of the crystallites size based on Scherer’s relation and Rietveld refinements (see Section 2.2. Materials Characterization) as well as calculated cell parameters were gathered in Table 2. It can be observed that the unit cell parameters decrease when the Eu3+ ions are doped. This result is expected, since europium ion (1.12 Å at CN9 and 1.01 Å at CN7) is smaller than the substituted calcium cations (1.18 Å at CN9 and 1.06 Å at CN7) [39]. The lithium cation is the smallest among them (0.92 Å at CN9). The a-axis parameter for all matrices slightly increases with a lithium doping. It can be related to the doping up to 2 mol% Li+ and creating cationic vacancies [10]. Above 2 mol% of Li+ concentration, a decreasing in the cell volume related to the incorporation of the interstitial Li+ into the crystal lattice was observed.

Table 2.
Unit cell parameters (a,c), cell volume (V), mean crystallites size calculated according to the Scherer (S) and Rietveld (R) method as well as refine factor (RW) for the Ca5(PO4)3Z (where Z—OH−, Cl−, F−) doped and co-doped with 1 mol% Eu3+ and 2 mol% Li+ powder prepared at 500 °C.
A lack of entrapped water affects the contraction of the a-axis observed in the precipitated apatites (water released between 200 °C and 400 °C was irreversibly lost, and the studied materials were sintered at 500 °C) [43]. This was later confirmed on the basis of the FT-IR spectra. In the case of HAp and Fap, lithium causes increasing cell volume. The expected opposite situation was observed in the case of ClAp. Moreover, the reduction in average crystallites size for co-doped apatites was found for both ClAp and FAp. The lack of clear trends suggests that the presence of various anions, i.e., OH−, Cl− and F−, in the apatite matrix, more precisely in the coordination sphere of calcium crystallographic positions, affects the obtaining cell parameters [12]. The substitution of OH− by F− towards a shift of (2 1 1) and (3 0 0) XRPD reflection to a higher 2θ angle (with the slight deviation in the (1 1 2) position) indicates a contraction of the a-axis dimensions (see Figure 3) [44,45]. By contrast, the value of a-axis parameter of the ClAp is significantly higher than for HAp (lower-angle shift of (2 1 1) and (3 0 0) planes), unlike in the case of FAp. On the other hand, the c-axis parameter increases with fluoride substitution; however, this change is much smaller than for a-axis parameter. All the results indicated that contraction and expansion of the a-axis parameter of the FAp and ClAp, respectively, are related to ion size mismatch (F−—1.32 Å < OH−—1.68 Å < Cl−—1.81 Å).
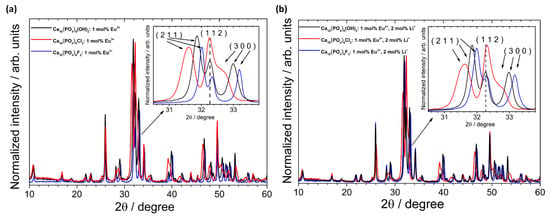
Figure 3.
XRPD patterns of the HAp, ClAp and FAp doped with 1 mol% Eu3+ (a) and co-doped with 1 mol% Eu3+ and 2 mol% Li+ (b) with the indication of lattice planes shift induced by various types of anions (inset).
The morphology and particle size of the apatites were determined using HRTEM technique. As it can be seen in Figure 4, all studied apatites have similar morphology (elongated nanorods) as well as similar average particle size (HAp: 30 nm × 40 nm, ClAp: 30 nm × 60 nm, FAp: 25 nm × 50 nm). The sample consists of well-crystallized nanograins with a tendency to agglomeration; however, the distribution of the grain size is relatively low. Furthermore, when the selected area electron diffraction (SAED) images reveal the absence of diffused rings and the appearance of intense spots forming a rings, the sample is polynanocrystalline [8]. The rings are present in the images and the spots corresponding to the crystallites (see Figure 4c). The labeled lattice spacing and crystal planes (hkl) (Figure 4b,c and Table S1, Supplementary Materials) correlate very well with the XRPD data.
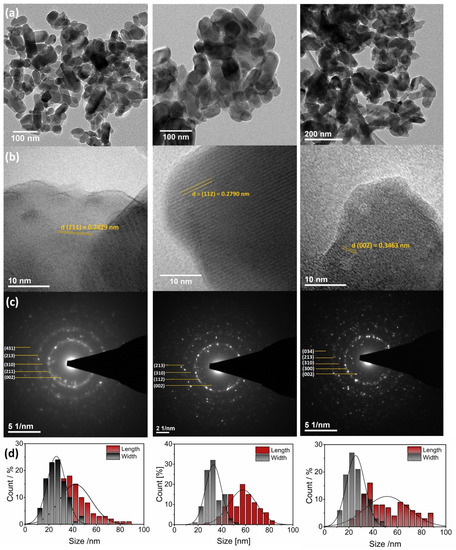
Figure 4.
TEM (a,b) and SAED (c) images of the Ca10(PO4)6(OH)2 (left), Ca10(PO4)6Cl2 (middle), Ca10(PO4)6F2 (right) co-doped with 1 mol% Eu3+ and 2 mol% Li+ prepared at 500 °C with the indication of lattice spacing and crystal planes (hkl). Histogram (d) of the grain size distribution (length and width diameters) of the apatites.
The grain size distributions estimated from TEM analysis were compared with those obtained from colloidal suspensions. The results of the hydrodynamic size measurements conducted on water dispersion of particles have been presented in Figure 5 and Table 3. The whole series of nanoapatites (HAp, ClAp, FAp) show a similar distribution of hydrodynamic size in the colloidal suspensions with the maxima at 164 nm for HAp: 1 mol% Eu3+, 2 mol% Li+, 164 nm in case of ClAp: 1 mol% Eu3+, 2 mol% Li+ and 154 nm for FAp: 1 mol% Eu3+, 2 mol% Li+. The difference in the grain size estimation between TEM and DLS is visible. When a dispersed particle moves through a liquid medium a thin, the electric dipole layer adheres to the particle surface, and therefore, this surface influence on intrinsic particle diameter. The thickness of the layer depends on various factors, such as the electrical conductivity of the liquid [46]. Moreover, the use of water on non-stabilizing agent treated particles promote their agglomeration leading directly to the extension of the hydrodynamic particle diameter. Consequently, the choice of dispersing agent is critical for certain biomedical applications. For instance, the way and extent of internalization will be different for single particles and for large agglomerates with an extensive shell called protein corona. The latter scenario usually leads to phagocytosis making macrophages the primary target for the agglomerating particles [47]. Nanomaterials exhibit a high specific surface area, and due to that, nanoparticles in biological fluids strongly adsorb protein on their surface. The composition and size of this protein corona determine how the cell will respond to such a protein interface and, thus, may affect the biodistribution of the nanoparticles and their cytotoxicity. The investigation into this scientific field shows that the formation of the protein corona on nanomaterials is time-dependent exposure. It has been found that corona formation occurs rapidly (<0.5 min) and over time does not transform in composition, but the amount of adsorbed protein changes significantly [48]. Rapid corona formation influence on nanoparticle uptake in the biological system, which ultimately affects the success of the nanomaterial-based therapy. Not only surface properties, but also nanoparticle size, determine the protein corona and both play a significant role in terms of nanosafety issues in a highly dynamic physiological system [49]. In this work, the interaction of studied nanoapatites with model proteins, albumin (BSA) and lysozyme (LSZ) was further discussed (see Section 3.2.3) and correlated with the results presented in Figure 6.

Figure 5.
Hydrodynamic size distribution of the nanoapatites water colloidal suspensions: Ca10(PO4)6(OH)2 (a), Ca10(PO4)6Cl2 (b) and Ca10(PO4)6F2 (c) co-doped with 1 mol% Eu3+ and 2 mol% Li+ ions. Presented values are the mean ± SD of three independent experiments.

Table 3.
Main hydrodynamic size of nanoapatites colloidal suspensions: Ca10(PO4)6(OH)2, Ca10(PO4)6Cl2 and Ca10(PO4)6F2 co-doped with 1 mol% Eu3+ and 2 mol% Li+ ions, measured in de-ionized water, 0.05% BSA and 0.05% LSZ based on DLS technique.
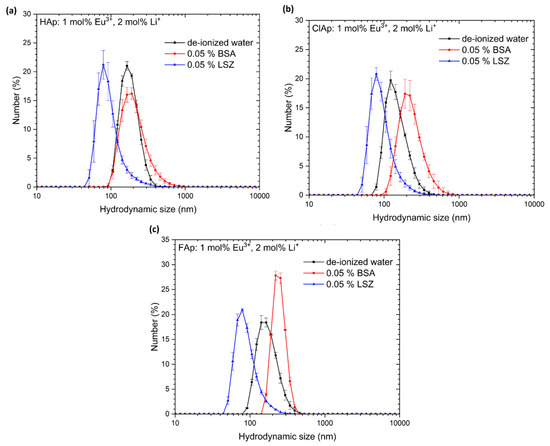
Figure 6.
Hydrodynamic size distribution of the nanoapatites (Hap—(a), ClAp—(b), Fap—(c)) co-doped with 1 mol% Eu3+ and 2 mol% Li+ ions in various colloidal suspensions: de-ionized water, 0.05% BSA and 0.05% LSZ. Presented values are the mean ±SD of three independent experiments.
One of the crucial parameters responsible for cell‒nanomaterial interaction is the surface charge of both elements. There is a potential between the surface of the particles and the dispersing liquid, which changes with distance from the surface of the particle. This potential measured for the slipping plane is called zeta potential (z) [50]. For clarity, the slipping plane is a boundary within the area of the diffusion layer. When the particle moves, the ions within that boundary move with it, but not any ions located outside that boundary. The measurements were made for two different dispersants, de-ionized water and phosphate-buffered saline (PBS). As it was shown in Table 4, in the case of PBS, the zeta potential reaches higher negative values, which could indicate grater particle stability. Generally, particles with an absolute zeta potential greater than 30 mV are considered stable; however, the stability strongly depends on their size and shape as well as the concentration of the suspension [50]. Particle size and charge are two major factors that play significant roles in the final properties of nanomaterials in biological systems, influencing on release from dosage forms as well as drug circulation and absorption into body membranes [51]. It is known that PBS with pH 7.4 is often used for the assessment of biomaterial in vitro, as its osmolarity and ion concentrations of the solutions are similar to those in the human body. Therefore, when PBS is used in the studies, the zeta potential is affected by not only pH, but also by ionic strength and compositions in the solution. The increase in zeta potential to higher negative values is expected due to an increase in PO43− concentration coming from the buffer. This means that PO43− is a potential-determining ion tightly adhered to the surface, which makes the apatite particles more negatively charged. X. Fan et al. [52] clearly showed the dependence of zeta potential on PO43−, Na+ and Cl− ions’ concentration (being a component of the PBS buffer). Although Na+ and Cl− are not potential-determining ions for calcium phosphates, they also influence on the final zeta potential through adjusting the ion strength in the solution. The authors also studied the use of simulated body fluid (SBF) as an alternative to PBS for the evaluation of biomaterial in vitro, which consequently led them to obtain a positive zeta potential (close to the zero point) of the calcium phosphates.

Table 4.
Zeta potentials of nanoapatites series in de-ionized water, PBS, BSA and LSZ suspensions.
It is already known that nanoparticles show a high affinity for cellular membranes mainly through electrostatic interactions between them. Cell membranes with a large negatively charged domains should repel negatively charged nanoparticles. However, when there is a possibility of existing cationic sites in the form of clusters, cells can adsorb negatively charged particles [53]. This electrostatic interaction can lead to bending of the membrane favoring endocytosis for cellular uptake [50]. Thus, by modifying the surface charge of the nanoparticles, it is possible to distribute and localize them to specific intracellular targets. For example, nanoparticles with a positive charge are preferentially taken up by usually charged negatively tumors and retained for a longer time interval compared to negatively charged or neutral particles [50]. This also applies to the protein corona, which positively (charged) modifies the surface of nanoparticles. The strategy of using a surface functionalization (changes the surface charge) of the nanoparticles may enhance their interaction with tumor cells, thus making the drug delivery system more effective.
EDS analysis was performed to confirm the elemental composition of the obtained nanoapatites (see Figure S1, Supplementary Materials). The resulting contents of the Eu3+, Cl− and F- ions (except Li+ ion, which is too light for this measuring technique) to the Ca2+ ions were in good agreement with their theoretical molar values. The lithium concentration was determined using the ICP-OES method, and the results are gathered in Table 5. The obtained values confirmed the stoichiometric composition of the synthesized apatites. The estimated molar ratio of cations to phosphorus ions has been very consistent with the theoretical value equal to 1.67.

Table 5.
The element content in the obtained hydroxyapatite measured by ICP-OES technique.
To obtain more insight into the structure of the obtained materials, the infrared spectra (FT-IR) were measured, and the results are shown in Figure 7. In the IR spectra, the absorption bands characteristic for the hydroxy-, chlor- and fluorapatites are visible. The typical bands of phosphate (PO43−) groups were recorded in the region around at 465–478 cm−1 (δ2—the doubly-degenerate bending), 549–620 cm−1 (δ4—the triply degenerate bending), 958–966 cm−1 (ν1—the symmetric non-degenerate stretching vibrations) and 1010–1137 cm−1 (ν3—the asymmetric triply degenerate stretching vibrations) [42,44]. The lack of vibrational transition that appears with the maximum at about 3442 cm−1 is associated with combined water loss during the annealing process at 500 °C. The observed stretching vibrational mode at 3572 cm−1 and vibrational mode at 633 cm−1 indicate the presence of OH− groups in hydroxyapatite structure. In the case of fluorapatite, the existence of active vibrations from OH− groups (belonged to FAp) were not observed, which indicates an ion exchange of OH− for F− during the synthesis of FAp. These OH− modes are also observed for chlorapatite; however, they are much less intense as in the case of HAp, which clearly shows that during the ClAp preparation process, some of OH− ions were not replaced with Cl− ions.
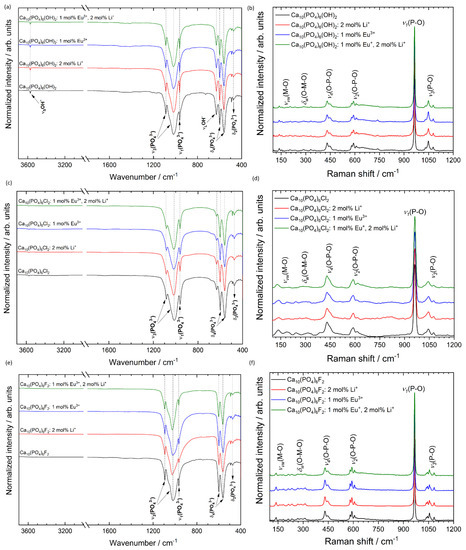
Figure 7.
FT-IR (left) and micro-Raman (right) spectra of the Ca10(PO4)6(OH)2 (a,b), Ca10(PO4)6Cl2 (c,d), Ca10(PO4)6F2 (e,f) co-doped with 1 mol% Eu3+ and 2 mol% Li+ ions, prepared at 500 °C.
To confirm the formation of pure phase apatite the micro-Raman spectroscopy was applied, and the results are gathered in Figure 7. The spectra of all studied materials contain four characteristic vibrational modes of the phosphate groups [54]. These bands are located at almost the same Raman shift for the same matrix but with different dopants, which indicates that the Li+ and Eu3+ ions at the selected concentration do not strongly affect the apatite structure. The bands at about 1077, 1047 and 1029 cm−1 were assigned to asymmetric ν3 (P–O) stretching. The most intense peak located at about 964 ± 2 cm−1 (depending on the type of matrix) corresponds to the symmetric stretching mode of the phosphate groups ν1 (PO43−). The vibrational bands at about 608, 591 and 580 cm−1 are attributed to the ν2 (PO43−) bending modes. The positions of about 447 and 430 cm−1 are associated with ν4 (PO43−) bending modes. The overlapping low-intensity bands in the frequency range of 350–100 cm−1 confirm the presence of metal–oxygen bonds (ν) M–O and (δ) O–M–O involved in the structure [10].
The apatites activated with rare earth ions show an intense photoluminescence and, therefore, could be used as a promising material for bio-detection. The red luminescence from the apatites doped and co-doped with Eu3+ and Li+ ions were presented in Figure 8a. A typical emission spectrum of Eu3+ ions located in hexagonal apatite lattice contains five emission bands centered at 575.3 nm (17,382.2 cm−1; 5D0 → 7F0), 591.8 nm (16,897.6 cm−1; 5D0 → 7F1), 612.0 nm (16,339.9 cm−1; 5D0 → 7F2), 653.0 nm (15,313.9 cm−1; 5D0 → 7F3) and 696.9 nm (14,349.3 cm−1; 5D0 → 7F4). The most intensive emission was observed for the hypersensitive 5D0 → 7F2 transition. The observation of three lines for the 5D0 → 7F0 transition reveals the presence of non-equivalent crystallographic sites that was previously described at the beginning of this section. It is possible to distinguished them with assigned C3 (3-fold rotation symmetry) and Cs (reflection symmetry) symmetries using so-called site-selective spectroscopy [55]. Throughout the use of this measuring technique, the preferential ion substitution can be evaluated particularly depending on the type and concentration of dopants. Consequently, it becomes possible to control the luminescence efficiency of novel designed biomaterials based on nanoapatites intended for future theranostic applications. In comparison with traditional using organic dyes, the biomaterials doped with RE3+ ions are characterized by narrow absorption/emission lines, long lifetimes of excited states as well as stable and efficient photoluminescence. The organic dyes are usually very fast photo-bleached and chemically degraded as well as their toxicity still being too high for safe medical use.
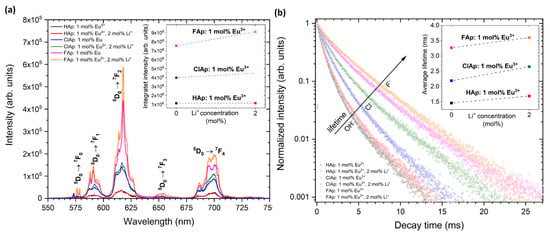
Figure 8.
Emission spectra (a) of Ca10(PO4)6(OH)2, Ca10(PO4)6Cl2, Ca10(PO4)6F2 doped with 1 mol% Eu3+ as well as co-doped with 1 mol% Eu3+ and 2 mol% Li+ ions under 395 nm excitation with the indication of 5D0 → 7FJ transitions in the range of 550–750 nm, detected at 300 K. The inset shows integral intensity of Eu3+ emission as a function of the Li+ ions’ concentration. Decay profiles (b) of Eu3+ ions in different types of apatite matrix, monitored at 618 nm according to the most intense electric dipole transition (5D0 → 7F2). The inset shows the average lifetimes of Eu3+ ions as a function of the Li+ ions’ concentration.
It is evident from Figure 8 that in the case of matrices with low phonon energies, such as FAp, the emission intensity (a) as well as luminescence lifetime (b) significantly increase. It is also found that upon Li+ ions co-doping, the enhanced luminescence of apatite: Eu3+ was observed. The substitution with Li+ cations strongly affects the crystal field symmetry. The luminescence enhancement induced by Li+ ions may be related to the creation of distortions around Eu3+ in apatite structure, mainly due to compensation of charge defects and occupation of Li+ ions in the interstitial sites (Li+ has a considerably smaller radius than Ca2+ or Eu3+) [19]. The possible charge compensation mechanisms were proposed in our previous paper [10,21]. In addition, it was shown that different concentrations of Li+ ions significantly affect the luminescence efficiency of Eu3+-doped apatites. The various Eu3+ distribution between available cationic sites depending on the concentration of Li+ cations was clearly demonstrated based on the Eu3+ ions’ luminescence behavior.
3.2. Biological Properties
3.2.1. Cytotoxicity Assessment in Osteosarcoma Cell Line
Results of the MTT assay are presented in Figure 9. The left column of graphs shows the metabolic activity of U2OS cells exposed to different concentrations of HAp, ClAp and FAp nanoparticles (from up to down). Neither un-doped nor doped HAp nanoparticles seem to elicit a cytotoxic response in osteosarcoma cells (upper left). On the other hand, both un-doped and doped ClAp nanoparticles seem to elicit some degree of cytotoxicity in osteosarcoma cells, but even at the highest concentration, the viability does not decrease below 50% (Figure 9, middle left). In case of FAp, both Eu− and Li+-doped particles visibly decreased cells’ metabolic activity. Surprisingly, the combination of both dopants seems not to cause any cytotoxicity. A high degree of variability precludes drawing firm conclusions, but it is apparent that signs of cytotoxicity appear only at the two highest concentrations. The right column of Figure 9 shows the results of the MTT test for J774.E macrophages. Intriguingly, in all types of apatite nanoparticles, the exposure is associated with an increase in metabolic activity in a concentration-dependent manner. Positive values in MTT assay are typically interpreted as a result of increased cell proliferation. Chen et al. [56] has indeed observed a HAp-induced increase in proliferation of osteoblast cells (MC3T3-E1), but the biologic effects of ClAp or FAp are much less studied. It is known, however, that in some cytotoxicity tests, false positive or false negative results are obtained. This may be due to dye–particle interactions [57] or as a result of changes in numerous enzymes, energy homeostasis or due to oxidative stress [58]. In the present study, cell-independent particle-dye interactions were excluded. To further elucidate whether the results of the MTT test really reflect cell proliferation, we performed the TBEA, an assay where the actual number of living cells is calculated. The results are shown in Figure 10.
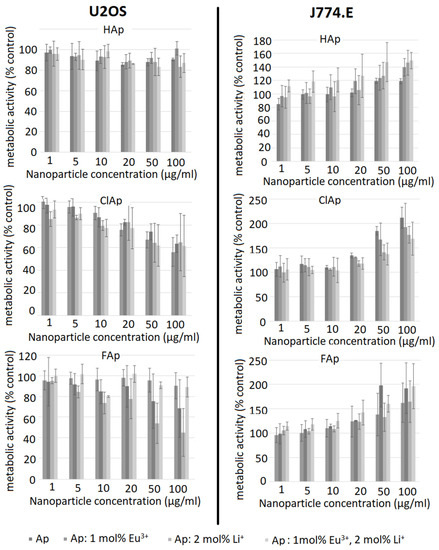
Figure 9.
Influence of un-doped nanoapatites (Ap‒ HAp, ClAp, FAp) as well as nanoapatites doped and co-doped with Eu3+/Li+ ions on metabolic activity of U2OS human osteosarcoma cells (left panel) and J774.E murine macrophages (right panel), determined by the MTT assay after 48 h exposure.
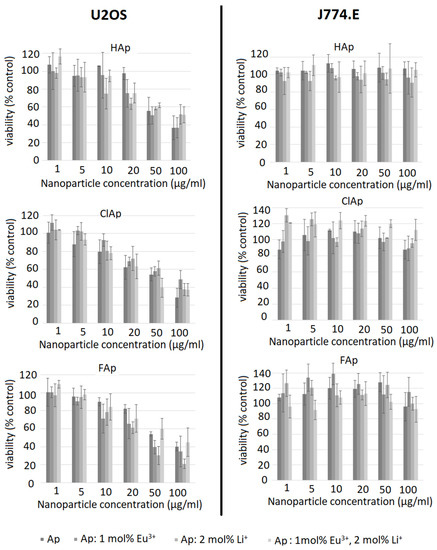
Figure 10.
Influence of un-doped nanoapatites (Ap‒HAp, ClAp, FAp) as well as nanoapatites doped and co-doped with Eu3+/Li+ ions on viability of U2OS human osteosarcoma cells (left panel) or J774.E murine macrophages (right panel) determined by the trypan blue exclusion assay (TBEA) after 48 h exposure.
A distinct concentration-dependent decrease in viability is seen in U2OS cells (Figure 10, left column). For the un-doped hydroxyapatite, cytotoxicity is observed at higher concentrations (50 µg/mL) compared to all doped nanoparticle types. Anti-proliferative effects of un-doped hydroxyapatite nanoparticles towards different cancer cell lines were described by several authors [59,60]. The mechanism involved is probably associated with particle internalization and subsequent apoptosis [61]. The effect of doping on the cytotoxicity of the nanoapatites used in this study is variable and inconclusive, as no clear trends were identified. In a study by Karthi et al. [62], murine fibroblast cell line L929 tolerated Nd3+- and Yb3+-doped FAp nanoparticles well (MTT assay). The discrepancy between MTT and TBEA results in the current study is probably associated with the metabolic alterations induced by nanoparticles that may mask and underestimate real cytotoxicity [58]. It is, therefore, important to use more than one test and different endpoints when assessing the biocompatibility of novel materials. In case of J774.E cells, TBEA results suggest no effect on the proliferation even at a very high concentration of 100 µg/mL. Although this suggests high biocompatibility of the nanoparticles under investigation, it should be kept in mind that the results of MTT may still provide important information on metabolic effects of the exposure to nanoparticles. Although not toxic, apatite particles may affect cell metabolism and energy homeostasis in macrophages. Under in vivo conditions, this stimulation might translate into proinflammatory effects or perhaps tissue remodeling.
It is concluded that apatite nanoparticles under investigation are not cytotoxic to J774.E macrophages but may induce slight cytotoxicity in U2OS osteosarcoma cell line at higher concentrations. In macrophages, the investigated nanoparticles may affect (induce) metabolic activity even at non-cytotoxic concentrations, which is probably related to phagocytosis and activation.
3.2.2. In Vitro Hemolytic Activity in Human RBCs
The red blood cells are the most abundant cellular component (99%) of the human circulatory system and are widely used as a cell model in the hemocompatibility studies of different compounds, including nanomaterials, with biomedical applications [63,64,65]. Therefore, new synthesized nanoapatites were evaluated in vitro for their interaction with RBCs’ membrane in the conventional optical microscopy and for their hemolytic activity. Compounds with hemolytic potential prompt the loss of RBCs’ membrane integrity resulting in the release of hemoglobin and in vivo can induce anemia. The results of all experiments clearly show the lack of harmful effect of nanoapatites on the molecular structure and permeability of RBCs’ membrane (i.e., no hemolytic activity—see Figure 11) as well as no effect on RBCs’ discoid shape (see Figure 12 and Table 6) and RBCs’ sedimentation rate (see Table 6 and Figure 13) up to a concentration of 1mg/mL. Whereas, in the case of a higher concentration used, slight aggregates of nanoapatites were detected as attached to the RBCs’ membrane (see Figure 12, arrows). A larger number of aggregates adsorbed on RBCs was observed for the un-doped HAp and ClAp. It should also be noted that RBCs’ incubation with nanoapatites results in their stronger aggregation (compare control RBCs’ images with RBCs’ nanoparticles-treated). The formation of RBCs’ aggregates irregular in size and shape was observed in the presence of many types of nanoparticles, including nanodiamonds [64] or lanthanide-doped core-shell nanomaterials [66]. It was shown that aggregation of RBCs affect their sedimentation pattern [67]. However, apart from RBCs’ aggregating effects observed in the conventional optical microscopy, the nanoapatites studied did not significantly modify the erythrocytes sedimentation rate at both concentrations, with the exception of ClAp (see Figure 13, white circle) at a concentration of 1 mg/mL.
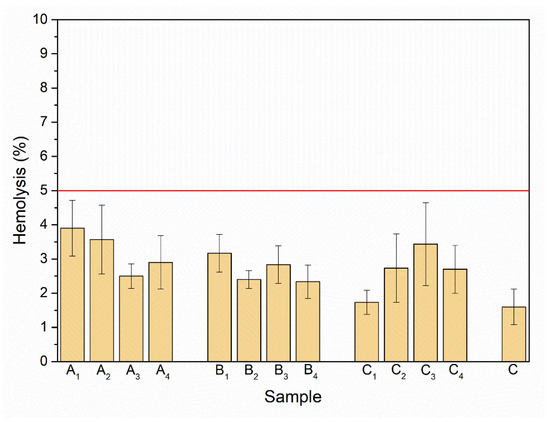
Figure 11.
Effect of the nanoapatites (1 mg/mL): HAp (A1), ClAp (B1) and FAp (C1) as well as nanoapatites doped with Li+ (A2, B2, C2), doped with Eu3+ (A3, B3, C3) and co-doped with Eu3+/Li+ ions (A4, B4, C4) on selective membrane permeability, i.e., hemolytic activity. Standard incubation conditions (PBS, 60 min, 37 °C; degree of hemolysis lower than 5% corresponding to a lack of hemolytic activity—red line), mean values ± SD, n = 9, C—control.
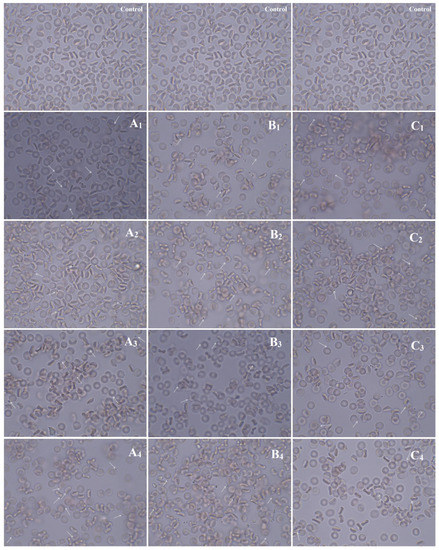
Figure 12.
Effect of the nanoapatites (1 mg/mL): HAp (A1), ClAp (B1) and FAp (C1) as well as nanoapatites doped with Li+ (A2,B2,C2), doped with Eu3+ (A3,B3,C3) and co-doped with Eu3+/Li+ ions (A4,B4,C4) on the human erythrocyte morphology observed in the light microscopy (60 min, 37 °C) at 100× magnification with immersion oil (10× ocular). Control – cells incubated in PBS buffer. The cells were fixed in a mixture of PFA 5% and GA 0.01%, rinsed 3× in PBS and closed in glycerol. Scale bar in control images is representative for all images presented: 10 µm. Arrows indicate slight aggregates of nanoapatites attached to the RBCs’ membrane.

Table 6.
The effect of the nanoapatites: HAp (A1), ClAp (B1) and FAp (C1) as well as nanoapatites doped with Li+ (A2, B2, C2), doped with Eu3+ (A3, B3, C3) and co-doped with Eu3+/Li+ (A4, B4, C4) ions on human erythrocytes (60 min, 37 °C) at the concentration of 1 mg/mL; at lower concentrations (0.1 mg/mL), no effect of the compounds on RBCs was detected.
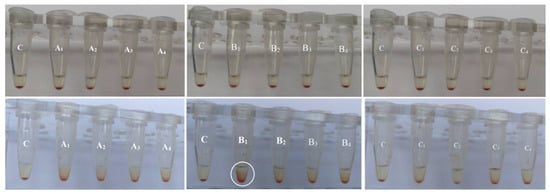
Figure 13.
Effect of the nanoapatites: HAp (A1), ClAp (B1) and FAp (C1) as well as nanoapatites doped with Li+ (A2, B2, C2), doped with Eu3+ (A3, B3, C3) and co-doped with Eu3+/Li+ (A4, B4, C4) ions on the human erythrocytes sedimentation rate at the concentration of 0.1 mg/mL (top) and 1 mg/mL (bottom) (60 min, 37 °C). C—PBS buffer, n = 3.
Detrimental interactions of nanomaterials with RBCs, including adsorption of compounds to RBCs’ membrane, RBCs’ aggregation, discocytic RBCs’ shape transformation into spherocytic (swollen, hemolytic cells) and increasing RBCs’ membrane permeability (inducing hemolysis), confirm incompatibility of compounds as blood contacting [66,68]. Our results strongly indicate that nanoapatites does not induce any harmful effects on human RBCs’ membrane structure and function in vitro during both short (60 min) and long (24 h) incubation. Therefore, biocompatibility of apatite nanoparticles studied is confirmed using hemolytic assay as a potent blood-contacting hemocompatible material for biomedical applications. It can be underlined that nanoapatites are attractive to potential medical applications, for example, in drug delivery, bio-imaging or theranostic applications. However, additional studies in vivo should be performed to assess their biocompatibility before considering any medical applications.
3.2.3. Bovine Serum Albumin and Lysozyme Interaction
In the experimental model used, apatites showed much lower affinity towards LSZ (remaining LSZ concentration was mostly around 90% compared to the initial solution) than towards BSA; however, some exceptions were observed (Figure 14). For instance, LSZ was adsorbed to a greater extent on FAp doped with Li+ ions and dual-doped with Li+ and Eu3+ ions and for the latter one. Moreover, LSZ was even undetectable (nd) in the supernatant after the incubation with the highest nanoparticles concentration. A higher affinity toward Li+ or Li+/Eu3+ co-doped nanoparticles is an interesting basis for developing optimal modifications of apatite nanoparticles to provide both antibacterial (LSZ breaks down peptidoglycan of the bacterial cell wall) and bone tissue formation-inducing properties.
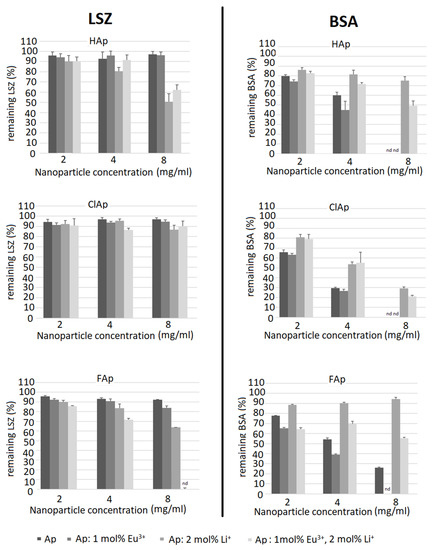
Figure 14.
Relative lysozyme (LSZ) (left panel) and bovine serum albumin (BSA) (right panel) adsorption to un-doped nanoapatites (Ap—HAp, ClAp, FAp) as well as nanoapatites doped and co-doped with Eu3+/Li+ ions. Nanoparticle dispersions in 0.05% LSZ and BSA were incubated for 4 h, centrifuged and the supernatants were assessed for protein concentration. Solutions of 0.05% LSZ and BSA were used as control (100% protein concentration).
An opposite tendency was observed for BSA adsorption—while in general, it adsorbed to a greater extent than LSZ, the lowest remaining protein concentration (thus, higher affinity) was observed for purified apatites or Eu3+ doped apatites. The addition of Li+ ions decreased protein BSA adsorption in contrary to the effect observed for LSZ. Another noteworthy observation was that ClAp showed the largest difference in the affinity towards LSZ and BSA, adsorbing very low concentrations of LSZ (regardless of Li+/Eu3+ doping) and the highest concentrations of BSA. Possible causes of this observation include the size of the chloride anion, causing the lower density of the negative charge and, thus, decreasing both the repelling of anionic (BSA) and attraction of cationic (LSZ) protein. Similarly, apatite containing strongly electronegative fluorine anion shows lower a tendency to adsorb BSA compared to ClAp. Observing the tendency that Li+ seems to enhance LSZ adsorption in contrast to BSA and the seemingly opposite influence of Eu3+ (or, at least no negative influence on BSA adsorption when compared to un-doped apatites), we can see that these interactions cannot be simply explained by the enrichment by cations. However, protein adsorption onto apatite surfaces relies on more factors, such as the inherent surface properties of materials, surface area, surface energy and hydrophobicity but also physiological conditions (including ionic strength) and protein–material interactions such as specific binding at Ca2+ and PO43− sites, non-specific binding through hydrogen bonding, etc. [69]. Thus, although as a general rule, positively charged nanoparticles attract proteins with an isoelectric point less than 5 (such as BSA) and negatively charged nanoparticles proteins with a higher isoelectric point [70], some authors observe that the polarity of the protein is not the main factor influencing the binding capability to apatite, e.g., showing similar profiles for BSA and LSZ [69].
Based on detailed research concentrating on the dynamics and kinetics of protein-nanoparticle interaction, other causes of those changes have to be taken into consideration, such as protein concentration, incubation time or even temperature [71]. Another possible cause of observed differences in protein adsorption might be the influence of adsorbed protein on nanoparticle aggregation, which can influence further binding of proteins by reducing the surface of the nanomaterial. This phenomenon was observed by other authors in studies on other types of nanoparticles, e.g., silica [72]. In the cited research, lysozyme was strongly bound by the particles causing their aggregation; however, we cannot exclude the importance of this kind of interaction in our study design, as we did not include solutions with different concentrations of proteins.
Interestingly, the DLS analysis of nanoparticles in protein solutions (see Figure 6) identical to those used for the adsorption test showed an increase in the size (both modal and average) of the nanoparticles dispersed in BSA solution, most probably reflecting the aggregation of nanoparticles and decreased size of the particles in LSZ solution (compared to de-ionized water), probably as a result of a positive effect of the LSZ on nanoparticles stability. In the LSZ adsorption test, LSZ seemed to be bound less effectively to the nanoparticles surface; however, we cannot exclude a potent impact on the stability of the nanoparticles even if small amount of LSZ is bound, which was not detectable in standard protein concentration assays. On the other hand, the design of the protein adsorption assay included centrifugation after the incubation period, so it is also possible that the LSZ binding to nanoparticles is weak and interrupted by further procedures of the test. It is to emphasize that tested nanoparticles showed weaker protein-binding activity, esp. BSA, compared to other particles, as shown in similar tests, for example, modified ferrite particles [73]. Thus, influencing the concentration of nanoparticles used for the test (in lower concentrations, no changes were noted in standard BCA assay). To address the issue in the context of the DLS results, advanced protein corona evaluation methods, for example, electron microscopy, would be necessary.
3.2.4. Antibacterial Evaluation
Since the apatite can enhance bone tissue proliferation, the replacement of bone loss with materials based on hydroxyapatite could facilitate bone regeneration [74]. Moreover, procedures of the implant re/placements often carry a risk of post-surgical infections. Thus, the potential antimicrobial properties of the implanted materials are of special interest.
The antibacterial activity of fluor-, hydroxy- and chlorapatites doped with 2 mol% Li+ and 1 mol% Eu3+ ions or both dopants were investigated against Gram-negative reference strains, commonly used for drug susceptibility testing or antimicrobial properties of materials (Figure 15).
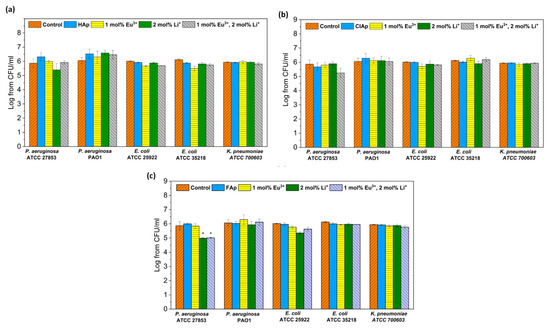
Figure 15.
Antibacterial effect of hydroxyapatites (a), chlorapatites (b) and fluorapatites (c) doped with Eu3+ and Li+ ions (concentration of 100 μg/mL) against Gram-negative strains: P. aeruginosa, E. coli and K. pneumoniae; mean ± SD, n = 3; *—statistically different from the control (p < 0.05).
No antibacterial activity of all tested apatites both doped and co-doped with europium or/and lithium was observed against tested strains, with only one exception for the P. aeruginosa ATCC27853 strain treated with fluorapatites doped with 2 mol% Li+ or 1 mol% Eu3+/2 mol% Li+ ions.
As investigated by Iconaru et al. [75], there was no antimicrobial activity of Eu3+-doped hydroxyapatites detected against E. coli 25922. Our previous study also showed no effect of Eu3+-doped hydroxyapatites and chlorapatites against Gram-negative bacteria [76,77]. However, these materials differed in the concentration of Eu3+ ions or contained other co-dopants, such as Sr2+ ions.
Apatites with lithium dopants have shown efficient osteoblast proliferation, but still, there is not enough data on their antimicrobial properties. Moghanian et al. [34] demonstrated a good antibacterial activity of Li+-doped hydroxyapatite bioactive glass against Gram-positive bacteria (MRSA).
4. Conclusions
Nanocrystalline apatite analogues un-doped, doped and co-doped with Li+ and Eu3+ ions were fabricated by using the microwave-assisted hydrothermal method. The XRPD, FT-IR and micro-Raman results have shown that all dopants have been successfully incorporated into the apatite structure causing changes in the lattice parameters. Co-doping with Li+ and Eu3+ ions caused significant enhancement of luminescence signal that might be useful in future bio-imaging applications. The nano-nature of all studied apatites was confirmed; however, the difference in the grain size was significant. LSZ was found to be a more efficient dispersant in water as compared to BSA. It was found that Li+ enhanced LSZ adsorption, whereas Eu3+ ions promoted BSA adsorption. FAp doped with both ions showed the highest affinity to LSZ, which may contribute to the antibacterial properties of this material being used as a bone graft substitute.
The biocompatibility of studied apatites was confirmed using U2OS, J774.E lines and human RBCs. The results indicated that nanoapatites do no induce any harmful effects on human RBCs’ membrane structure and function in vitro. A concentration-dependent decrease in cell viability was seen for the U2OS line, and the presence of Eu3+ or Li+ ions contributed to higher toxicity. The nanoparticle showed no adverse impact on J774.E cell proliferation, even at very high concentrations. Regarding antimicrobial properties, only a slight activity was detected for one P. aeruginosa representative treated with Li+ -doped fluorapatites, whereas the remaining compounds did not affect selected Gram-negative strains. The presented results suggest that the developed nanoapatite platforms may be a very attractive multimodal nanoagent for theranostic applications covering both regenerative as well as bio-imaging functions. However, extensive in vivo studies should be performed to assess their biocompatibility before considering any medical applications.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biom11091388/s1, Figure S1: EDS spectra of the (a) Ca10(PO4)6(OH)2, (b) Ca10(PO4)6Cl2 and (c) Ca10(PO4)6F2 co-doped with 1 mol% Eu3+ and 2 mol% Li+ ions, prepared at 500 °C with the quantitative analysis of elements (inset). Table S1: Lattice planes (hkl) assigned based on SAED images and ICSD data (Ca10(PO4)6(OH)2 (ICSD-26204), Ca10(PO4)6Cl2 (ICSD-24237) and Ca10(PO4)6F2 (ICSD-262707) of the Ca10(PO4)6(OH)2, Ca10(PO4)6Cl2 and Ca10(PO4)6F2 co-doped with 1 mol% Eu3+ and 2 mol% Li+ ions, prepared at 500 °C.
Author Contributions
Conceptualization, P.S. and R.J.W.; methodology, P.S., B.P., J.M., L.M., A.D.-J. and Z.D.-K.; investigation, P.S., B.P., M.T., J.M., L.M., A.P. and J.R.-S.; writing—original draft preparation, P.S., B.P., J.M., L.M. and A.P.; writing—review and editing, P.S., B.P., J.M., L.M., A.P., J.R.-S.; A.D-J., Z.D.-K. and R.J.W.; visualization, P.S., M.T., J.M., L.M. and J.R.-S.; supervision, P.S. and R.J.W.; funding acquisition, P.S. and R.J.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the National Science Centre, Poland (NCN) for financial support within the Projects “Preparation and investigation of multifunctional biomaterials based on nanoapatites for possible application in bone tumor treatment” (No. UMO-2017/27/N/ST5/02976) and “Preparation and characterization of biocomposites based on nanoapatites for theranostic” (No. UMO-2015/19/B/ST5/01330) as well as “Elaboration and characteristics of biocomposites with anti-virulent and anti-bacterial properties against Pseudomonas aeruginosa” (No. UMO-2016/21/B/NZ6/01157). Paulina Sobierajska received financial resources within the confines of financing the ETIUDA doctoral scholarship from the National Science Centre, Poland (No. UMO-2018/28/T/ST5/00326).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon request.
Acknowledgments
We are grateful to E. Bukowska for XRPD measurements, to K. Wiglusz for FT-IR spectra, to D. Szymanski for EDS spectra and to M. Malecka for TEM and SAED measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Beig, B.; Liaqat, U.; Niazi, M.F.K.; Douna, I.; Zahoor, M.; Niazi, M.B.K. Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings 2020, 10, 1249. [Google Scholar] [CrossRef]
- Arce, J.E.; Arce, A.E.; Aguilar, Y.; Yate, L.; Moya, S.; Rincón, C.; Gutiérrez, O. Calcium phosphate—Calcium titanate composite coatings for orthopedic applications. Ceram. Int. 2016, 42, 10322–10331. [Google Scholar] [CrossRef]
- Victor, S.P.; Paul, W.; Sharma, C.P. Drug Delivery Nanosystems for Biomedical Applications||Calcium Phosphate Nanoplatforms for Drug Delivery and Theranostic Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–179. ISBN 9780323509220. [Google Scholar]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.; Zhang, S.; Qiu, J.; Li, J.; Sang, Y.; Xia, H.; Jiang, H.; Claverie, J.; Liu, H. Eu/Tb codoped spindle-shaped fluorinated hydroxyapatite nanoparticles for dual-color cell imaging. Nanoscale 2016, 8, 11580–11587. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, P.; Wiglusz, R.J. Influence of the grain sizes on Stokes and anti-Stokes fluorescence in the Yb3+ and Tb3+ ions co-doped nanocrystalline fluorapatite. J. Alloys Compd. 2019, 785, 808–818. [Google Scholar] [CrossRef]
- Han, Y.; Wang, X.; Dai, H.; Li, S. Synthesis and luminescence of Eu3+ doped hydroxyapatite nanocrystallines: Effects of calcinations and Eu3+ content. J. Lumin. 2013, 135, 281–287. [Google Scholar] [CrossRef]
- Sobierajska, P.; Pazik, R.; Zawisza, K.; Renaudin, G.; Nedelec, J.-M.; Wiglusz, R.J. Effect of lithium substitution on the charge compensation, structural and luminescence properties of nanocrystalline Ca10(PO4)6F2 activated with Eu3+ ions. CrystEngComm 2016, 18, 3447–3455. [Google Scholar] [CrossRef]
- Sahoo, R.; Bhattacharya, S.K.; Debnath, R. A new type of charge compensating mechanism in Ca5(PO4)3F: Eu3+ phosphor. J. Solid State Chem. 2003, 175, 218–225. [Google Scholar] [CrossRef]
- Šupová, M. Substituted Hydroxyapatites for Biomedical Applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Pazik, R.; Nedelec, J.-M.; Wiglusz, R.J. Preferential site substitution of Eu3+ ions in Ca10(PO4)6Cl 2 nanoparticles obtained using a microwave stimulated wet chemistry technique. CrystEngComm 2014, 16, 5308–5318. [Google Scholar] [CrossRef]
- Bigi, A.; Boanini, E.; Capuccini, C.; Gazzano, M. Strontium-substituted hydroxyapatite nanocrystals. Inorg. Chim. Acta 2007, 360, 1009–1016. [Google Scholar] [CrossRef]
- Silva, F.R.O.; de Lima, N.B.; Bressiani, A.H.A.; Courrol, L.C.; Gomes, L. Synthesis, characterization and luminescence properties of Eu3+-doped hydroxyapatite nanocrystal and the thermal treatment effects. Opt. Mater. 2015, 47, 135–142. [Google Scholar] [CrossRef]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Reduced relative risk of fractures among users of lithium. Calcif. Tissue Int. 2005, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Clement-Lacroix, P.; Ai, M.; Morvan, F.; Roman-Roman, S.; Vayssiere, B.; Belleville, C.; Estrera, K.; Warman, M.L.; Baron, R.; Rawadi, G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 17406–17411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marycz, K.; Sobierajska, P.; Smieszek, A.; Maredziak, M.; Wiglusz, K.; Wiglusz, R.J. Li+ activated nanohydroxyapatite doped with Eu3+ ions enhances proliferative activity and viability of human stem progenitor cells of adipose tissue and olfactory ensheathing cells. Further perspective of nHAP:Li+, Eu3+ application in theranostics. Mater. Sci. Eng. C 2017, 78, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.K.; Rai, S.B. Role of Li+ ion in the luminescence enhancement of lanthanide ions: Favorable modifications in host matrices. RSC Adv. 2014, 4, 27039–27061. [Google Scholar] [CrossRef]
- Jagannathan, R.; Kottaisamy, M. Short-pulsed stimulated emission in the powders and Nd:Sr5(PO4)3F laser crystals. J. Phys. Condens. Matter 1995, 7, 8453–8466. [Google Scholar] [CrossRef]
- Sobierajska, P.; Wiglusz, R.J. Influence of Li+ ions on the physicochemical properties of nanocrystalline calcium-strontium hydroxyapatite doped with Eu3+ ions. New J. Chem. 2019, 43, 14908–14916. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Review Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.C.; Hauck, T.S.; Gómez-Aristizábal, A.; Chan, W.C. Exploring primary liver macrophages for studying quantum dot interactions with biological systems. Adv. Mater. 2010, 22, 2520–2524. [Google Scholar] [CrossRef] [PubMed]
- Nicolete, R.; dos Santos, D.F.; Faccioli, L.H. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int. Immunopharmacol. 2011, 11, 1557–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartsogiannis, V.; Ng, K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004, 228, 79–102. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem.-Biol. Interact. 2018, 279, 73–83. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Vellard, M. The enzyme as drug: Application of enzymes as pharmaceuticals. Curr. Opin. Biotechnol. 2003, 14, 444–450. [Google Scholar] [CrossRef]
- Murakami, F.; Sasaki, T.; Sugahara, T. Lysozyme stimulates immunoglobulin production by human-human hybridoma and human peripheral blood lymphocytes. Cytotechnology 1997, 24, 177–182. [Google Scholar] [CrossRef]
- Crémet, L.; Corvec, S.; Bémer, P.; Bret, L.; Lebrun, C.; Lesimple, B.; Miegeville, A.F.; Reynaud, A.; Lepelletier, D.; Caroff, N. Orthopaedic-implant infections by Escherichia coli: Molecular and phenotypic analysis of the causative strains. J. Infect. 2012, 64, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.B.; Osmon, D.R.; Steckelberg, J.M.; Sierra, R.J.; Walker, R.C.; Tande, A.J.; Berbari, E.F. Pseudomonas Prosthetic Joint Infections: A Review of 102 Episodes. J. Bone Jt. Infect. 2016, 1, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Moghanian, A.; Firoozi, S.; Tahriri, M.; Sedghi, A. A comparative study on the in vitro formation of hydroxyapatite, cytotoxicity and antibacterial activity of 58S bioactive glass substituted by Li and Sr. Mater. Sci. Eng. C 2018, 91, 349–360. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD: A friendly Java program for material analysis using diffraction. IUCr Comm. Powder Diffr. Newsl. 1999, 21, 14–15. [Google Scholar]
- Hartmann, N.B.; Jensen, K.A.; Baun, A.; Rasmussen, K.; Rauscher, H.; Tantra, R.; Cupi, D.; Gilliland, D.; Pianella, F.; Riego Sintes, J.M. Techniques and Protocols for Dispersing Nanoparticle Powders in Aqueous Media—Is there a Rationale for Harmonization? J. Toxicol. Environ. Health-Part B Crit. Rev. 2015, 18, 299–326. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Crystallogr. 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Hendricks, S.B.; Jefferson, M.E.; Mosley, V.M. The crystal structures of some natural and synthetic apatite-like substances. Z. Krist.-Cryst. Mater. 1932, 81, 352–369. [Google Scholar] [CrossRef]
- Lim, S.C.; Baikiea, T.; Pramana, S.S.; Smith, R.; White, T.J. Apatite metaprism twist angle (φ) as a tool for crystallochemical diagnosis. J. Solid State Chem. 2011, 184, 2978–2986. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Bonel, G.; Legros, R. Types of “H2O” in human enamel and in precipitated apatites. Calcif. Tissue Res. 1978, 26, 111–118. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Bian, M.; Zhao, J. Applied Surface Science Solution combustion method for synthesis of nanostructured hydroxyapatite, fluorapatite and chlorapatite. Appl. Surf. Sci. 2014, 314, 1026–1033. [Google Scholar] [CrossRef]
- Sader, M.S.; Lewis, K.; Soares, G.A.; Legeros, R.Z. Simultaneous Incorporation of Magnesium and Carbonate in Apatite: Effect on Physico-chemical Properties 2 Experimental Methods. Mater. Res. 2013, 16, 779–784. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science Principles and Applications; Academic Press: Cambridge, MA, USA, 1988; ISBN 9781483214085. [Google Scholar]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [Green Version]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part I). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Zhu, X.; Fan, H.; Li, D.; Xiao, Y.; Zhang, X. Protein Adsorption and Zeta Potentials of a Biphasic Calcium Phosphate Ceramic Under Various Conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82B, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Patila, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef] [Green Version]
- Nikolaev, A.; Kolesnikov, I.; Frank-Kamenetskaya, O.; Kuz’mina, M. Europium concentration effect on characteristics and luminescent properties of hydroxyapatite nanocrystalline powders. J. Mol. Struct. 2017, 1149, 323–331. [Google Scholar] [CrossRef]
- Karbowiak, M.; Hubert, S. Site-selective emission spectra of Eu3+:Ca5(PO4)3F2. J. Alloys Compd. 2000, 302, 87–93. [Google Scholar] [CrossRef]
- Chen, L.; Mccrate, J.M.; Lee, J.C.; Li, H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holder, A.L.; Goth-Goldstein, R.; Lucas, D.; Koshland, C.P. Particle-induced artifacts in the MTT and LDH viability assays. Chem. Res. Toxicol. 2012, 25, 1885–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, C.; Qian, J.; Wang, J.; Zhang, Y. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010, 31, 730–740. [Google Scholar] [CrossRef]
- Tang, W.; Yuan, Y.; Liu, C.; Wu, Y.; Lu, X.; Qian, J. Differential cytotoxicity and particle action of hydroxyapatite nanoparticles in human cancer cells. Nanomedicine 2014, 9, 397–412. [Google Scholar] [CrossRef]
- Masouleh, M.P.; Hosseini, V.; Pourhaghgouy, M.; Bakht, M.K. Calcium Phosphate Nanoparticles Cytocompatibility versus Cytotoxicity: A Serendipitous Paradox. Curr. Pharm. Des. 2017, 23, 2930–2951. [Google Scholar] [CrossRef] [PubMed]
- Karthi, S.; Kumar, G.A.; Sardar, D.K.; Santhosh, C.; Girija, E.K. Synthesis and characterization of Nd3+: Yb3+ co-doped near infrared sensitive fluorapatite nanoparticles as a bioimaging probe. Opt. Mater. 2018, 77, 39–47. [Google Scholar] [CrossRef]
- Grzyb, T.; Mrówczyńska, L.; Szczeszak, A.; Śniadecki, Z.; Runowski, M.; Idzikowski, B.; Lis, S. Synthesis, characterization, and cytotoxicity in human erythrocytes of multifunctional, magnetic, and luminescent nanocrystalline rare earth fluorides. J. Nanoparticle Res. 2015, 17, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avsievich, T.; Popov, A.; Bykov, A.; Meglinski, I. Mutual interaction of red blood cells influenced by nanoparticles. Sci. Rep. 2019, 9, 5147. [Google Scholar] [CrossRef] [PubMed]
- Czarniewska, E.; Mrówczyńska, L.; Jędrzejczak-Silicka, M.; Nowicki, P.; Trukawka, M.; Mijowska, E. Non-cytotoxic hydroxyl-functionalized exfoliated boron nitride nanoflakes impair the immunological function of insect haemocytes in vivo. Sci. Rep. 2019, 9, 14027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczeszak, A.; Ekner-Grzyb, A.; Runowski, M.; Szutkowski, K.; Mrówczyńska, L.; Kaźmierczak, Z.; Grzyb, T.; Dąbrowska, K.; Giersig, M.; Lis, S. Spectroscopic, structural and in vitro cytotoxicity evaluation of luminescent, lanthanide doped core@shell nanomaterials GdVO4:Eu3+5%@SiO2@NH2. J. Colloid Interface Sci. 2016, 481, 245–255. [Google Scholar] [CrossRef]
- Zhbanov, A.; Yang, S. Effects of Aggregation on Blood Sedimentation and Conductivity. PLoS ONE 2015, 10, e0129337. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.C.; Myerson, J.W.; Brenner, J.S.; Patel, P.N.; Anselmo, A.C.; Mitragotri, S.; Muzykantov, V. Nanoparticle Properties Modulate Their Attachment and Effect on Carrier Red Blood Cells. Sci. Rep. 2018, 8, 1615. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.H.; Loo, C.Y.; Van, K.L.; Zavgorodniy, A.V.; Rohanizadeh, R. Modulating protein adsorption onto hydroxyapatite particles using different amino acid treatments. J. R. Soc. Interface 2012, 9, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Laurent, S.; Tawil, N.; Yahia, L.H.; Mahmoudi, M. Protein-Nanoparticle Interactions; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–94. ISBN 978-3-642-37554-5. [Google Scholar]
- Uygun, M.; Uygun, D.A.; Altunbaş, C.; Akgöl, S.; Denizli, A. Dye Attached Nanoparticles for Lysozyme Adsorption. Sep. Sci. Technol. 2014, 49, 1270–1278. [Google Scholar] [CrossRef]
- Kumar, S.; Aswal, A.V. SANS study of Lysozyme vs. BSA protein adsorption on silica nanoparticles. AIP Conf. Proc. 2012, 1447, 181–182. [Google Scholar] [CrossRef] [Green Version]
- Zachanowicz, E.; Zięcina, A.; Mikołajczyk, P.A.; Rogacki, K.; Małecka, M.; Marycz, K.; Marędziak, M.; Poźniak, B.; Nowakowska, M.; Tikhomirov, M.; et al. Cytotoxic Effects of Co1–xMnxFe2O4 Ferrite Nanoparticles Synthesized under Non-Hydrolytic Conditions (Bradley’s Reaction)—In Vitro. Eur. J. Inorg. Chem. 2016, 2016, 5315–5323. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Motelica-Heino, M.; Predoi, D. Study on europium-doped hydroxyapatite nanoparticles by fourier transform infrared spectroscopy and their antimicrobial properties. J. Spectrosc. 2013, 2013, 284285. [Google Scholar] [CrossRef] [Green Version]
- Szyszka, K.; Rewak-Soroczynska, J.; Dorotkiewicz-Jach, A.; Ledwa, K.A.; Piecuch, A.; Giersig, M.; Drulis-Kawa, Z.; Wiglusz, R.J. Structural modification of nanohydroxyapatite Ca10(PO4)6(OH)2 related to Eu3+ and Sr2+ ions doping and its spectroscopic and antimicrobial properties. J. Inorg. Biochem. 2020, 203, 110884. [Google Scholar] [CrossRef] [PubMed]
- Wiglusz, R.J.; Drulis-Kawa, Z.; Pazik, R.; Zawisza, K.; Dorotkiewicz-Jach, A.; Roszkowiak, J.; Nedelec, J.M. Multifunctional lanthanide and silver ion co-doped nano-chlorapatites with combined spectroscopic and antimicrobial properties. Dalton Trans. 2015, 44, 6918–6925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).