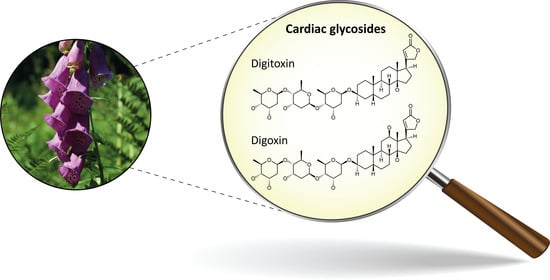
Cardiac Glycosides as Immune System Modulators
Abstract
1. Introduction
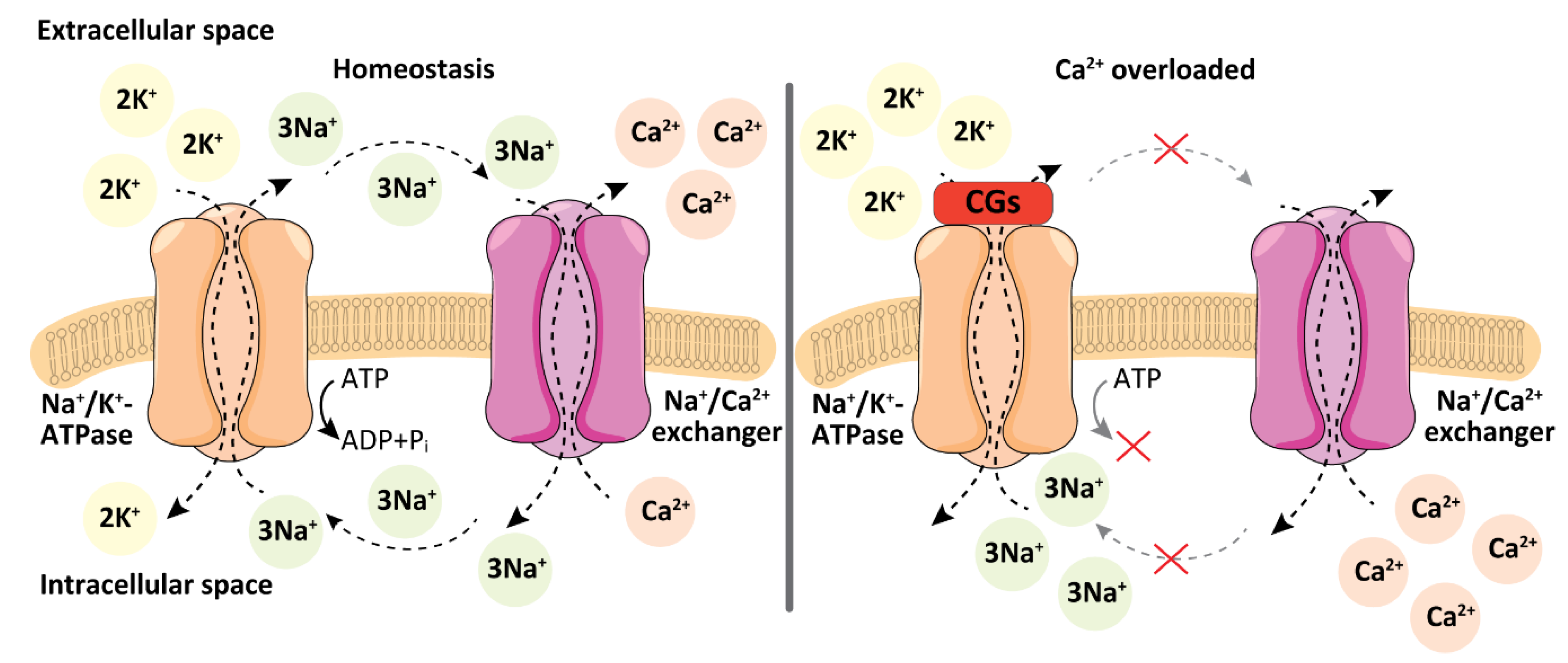
2. Cellular Targets of Cardiac Glycosides
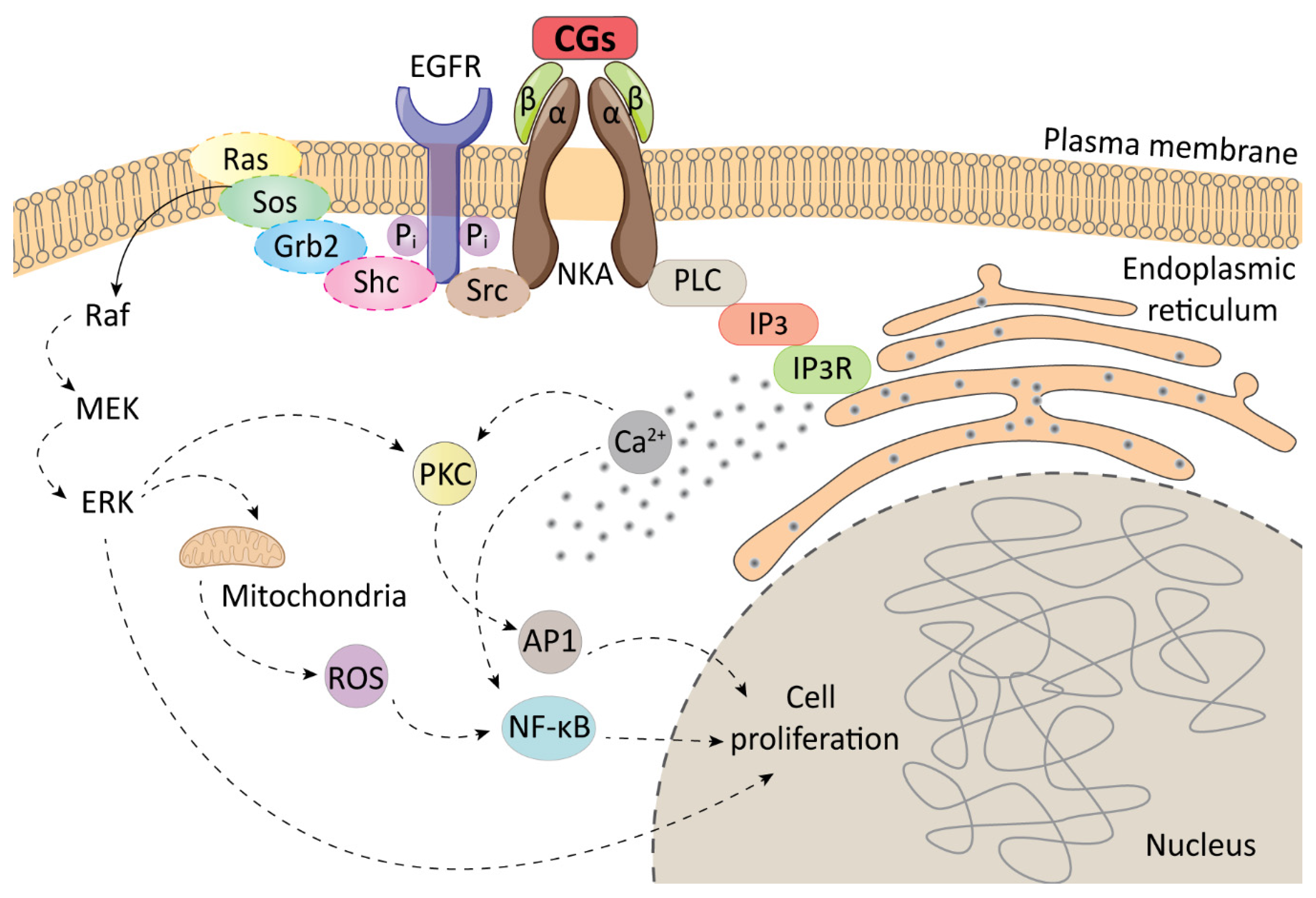
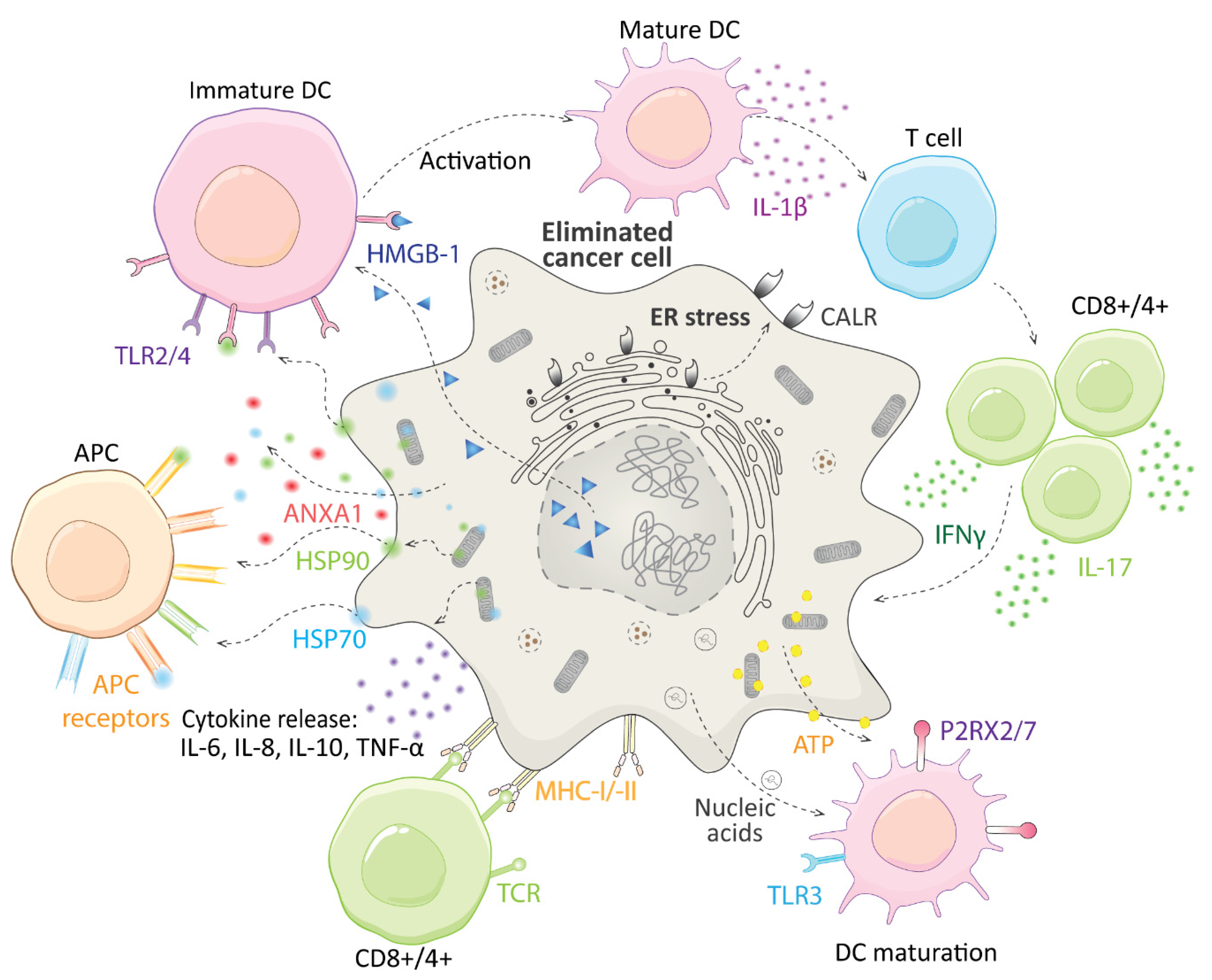
3. Immunogenic Cell Death and CGs
4. Immunomodulatory Action of CGs in Cancer Treatment
5. Immunomodulatory Action of CGs in Treatment of Autoimmune and Inflammatory Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A549 | Human cells from non–small-cell lung carcinoma |
| AAA | Abdominal aortic aneurysm |
| ANXA1 | Annexin A1 |
| AP1 | Transcription factor |
| APC | Antigen-presenting cells |
| APOBEC3F DNA | dC->dU-editing enzyme |
| ATP | Adenosine triphosphate |
| B16F10 | Mouse cells from melanoma |
| Cal27 | Human cells from oral squamous carcinoma |
| CALR | Calreticulin |
| CD | Cluster of differentiation |
| CF | Cystic fibrosis |
| CG | Cardiac glycosides |
| DAMP | Damage-associated molecular patterns |
| DC | Dendritic cell |
| EGFR | Epidermal growth factor receptor |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FDA | US Food and Drug Administration |
| GFP | Green fluorescent protein |
| GRB2 | Growth factor receptor-bound protein 2 |
| HCC | Human cells from hepatocellular carcinoma |
| HCT 116 | Human cells colon adenocarcinoma |
| HeLa | Human cells from cervical carcinoma |
| HepG2 | Human cells from hepatocellular carcinoma |
| HMGB-1 | High-mobility group box 1 |
| HSP70: HSP90 | Heat shock proteins 70 and 90 |
| ICD | Immunogenic cell death |
| IFNγ | Interferon γ |
| IL | Interleukin |
| IP3 | Inositol triphosphate |
| IP3R | Inositol triphosphate receptor |
| LNCaP | Human cells from prostate carcinoma |
| MDA-MB 231 | Human cells from breast carcinoma |
| MEK | Mitogen-activated protein kinase kinase |
| MHC-I/-II | Major histocompatibility complex I/II |
| NASH | Non-alcoholic steatohepatitis |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NKA | Na+/K+-ATPase |
| P2RX2/7 | P2X purinoceptor 2/7 |
| PCD | Programmed cell death |
| PERK | Protein kinase R-like ER kinase |
| PKC | Protein kinase C |
| PKM2 | Pyruvate kinase M2 |
| PLC | Phospholipase C |
| Raf | Serine/threonine kinase |
| Ras | Rat sarcoma protein |
| Rcd | Regulated cell death |
| RORγt | Receptor-related orphan receptor γ thymus |
| ROS | Reactive oxygen species |
| SHC | Src homology 2 domain-containing transforming protein 1 |
| SOS | Son of Sevenless |
| Src | Non-receptor tyrosine kinase |
| TLR | Toll-like receptor |
| TNF α | Tumor necrosis factor α |
| U-2 OS | Human cells derived from osteosarcoma |
| WEHI-3 | Human leukemia cells |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Bejcek, J.; Spiwok, V.; Kmonickova, E.; Ruml, T.; Rimpelova, S. Cardiac glycosides: On their therapeutic potential for cancer treatment. Chem. Listy 2021, 115, 4–12. [Google Scholar]
- Garcia, I.J.P.; de Oliveira, G.C.; Valadares, J.M.D.; Banfi, F.F.; Andrade, S.N.; Freitas, T.R.; Filho, E.D.S.M.; Santos, H.D.L.; Vieira, G.M.; Chaves, M.H.; et al. New bufadienolides extracted from Rhinella Marina inhibit Na,K-ATPase and induce apoptosis by activating caspases 3 and 9 in human breast and ovarian cancer cells. Steroids 2019, 152, 108490. [Google Scholar] [CrossRef]
- Geng, X.; Wang, F.; Tian, D.; Huang, L.; Streator, E.; Zhu, J.; Kurihara, H.; He, R.; Yao, X.; Zhang, Y.; et al. Cardiac glycosides inhibit cancer through Na/K-ATPase-dependent cell death induction. Biochem. Pharmacol. 2020, 182, 114226. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, N.; Rasul, A.; Hussain, G.; Anwar, H.; Shah, M.A.; Sarfraz, I.; Riaz, A.; Batool, R.; Shahbaz, M.; Hussain, A.; et al. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem. Toxicol. 2020, 143, 111570. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.L.; Zou, Y.J.; Lou, J.C.; Xing, J.S.; Wang, X.; Zou, S.; Ma, B.B.; Ding, Y.; Zhang, B. The sodium pump α1 subunit regulates bufalin sensitivity of human glioblastoma cells through the p53 signaling pathway. Cell Biol. Toxicol. 2019, 35, 521–539. [Google Scholar] [CrossRef]
- Leu, W.J.; Wang, C.T.; Hsu, J.L.; Chen, I.S.; Chang, H.S.; Guh, J.H. Ascleposide, a natural cardenolide, induces anticancer signaling in human castration-resistant prostatic cancer through Na+/K+-ATPase internalization and tubulin acetylation. Prostate 2020, 80, 305–318. [Google Scholar] [CrossRef]
- Michalak, K.; Rárová, L.; Kubala, M.; Štenclová, T.; Strnad, M.; Wicha, J. Synthesis and evaluation of Na+/K+-ATP-ase inhibiting and cytotoxic in vitro activities of oleandrigenin and its selected 17 beta-(butenolidyl)-and 17 beta-(3-furyl)- analogues. Eur. J. Med. Chem. 2020, 202, 112520. [Google Scholar] [CrossRef]
- Reddy, D.; Ghosh, P.; Kumavath, R. Strophanthidin attenuates MAPK, PI3K/AKT/mTOR, and Wnt/beta-catenin signaling pathways in human cancers. Front. Oncol. 2020, 9, 1469. [Google Scholar] [CrossRef]
- Reddy, D.; Kumavath, R.; Barh, D.; Azevedo, V.; Ghosh, P. Anticancer and antiviral properties of cardiac glycosides: A review to explore the mechanism of actions. Molecules 2020, 25, 3596. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.; Kumavath, R.; Tan, T.Z.; Ampasala, D.R.; Kumar, A.P. Peruvoside targets apoptosis and autophagy through MAPK Wnt/beta-catenin and PI3K/AKT/mTOR signaling pathways in human cancers. Life Sci. 2020, 241, 117147. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ribas, H.T.; Heath, K.; Wu, S.; Ren, J.; Shriwas, P.; Chen, X.; Johnson, M.E.; Cheng, X.; Burdette, J.E.; et al. Na+/K+-ATPase-targeted cytotoxicity of (+)-digoxin and several semisynthetic derivatives. J. Nat. Prod. 2020, 83, 638–648. [Google Scholar] [CrossRef]
- Rimpelová, S.; Zimmermann, T.; Drašar, P.B.; Dolenský, B.; Bejček, J.; Kmoníčková, E.; Cihlářová, P.; Gurská, S.; Kuklíková, L.; Hajdůch, M.; et al. Steroid glycosides hyrcanoside and deglucohyrcanoside: On isolation, structural identification, and anticancer activity. Foods 2021, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.F.Z.; Menegaz, D.; Dagostin, A.L.A.; Persich, L.; Rocha, S.C.; Ramos, A.C.P.; Cortes, V.F.; Fontes, C.F.L.; de Pádua, R.M.; Munkert, J.; et al. Cytotoxicity of glucoevatromonoside alone and in combination with chemotherapy drugs and their effects on Na+,K+-ATPase and ion channels on lung cancer cells. Mol. Cell. Biochem. 2021, 476, 1825–1848. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.Y.; Kim, S.; Choi, I.; Kim, S.H.; Shum, D.; Heo, J.; Kim, A.R.; Kim, K.M.; Seo, H.R. Inhibitors of Na+/K+ ATPase exhibit antitumor effects on multicellular tumor spheroids of hepatocellular carcinoma. Sci. Rep. 2020, 10, 5318. [Google Scholar] [CrossRef]
- Sun, X.; Ng, T.T.H.; Sham, K.W.Y.; Zhang, L.; Chan, M.T.V.; Wu, W.K.K.; Cheng, C.H.K. Bufalin, a traditional chinese medicine compound, prevents tumor formation in two murine models of colorectal cancer. Cancer Prev. Res. 2019, 12, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, L.; Tong, Y.; Li, L.; Liu, Y.; Gao, W.Q. Proscillaridin A slows the prostate cancer progression through triggering the activation of endoplasmic reticulum stress. Cell Cycle 2020, 19, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Q.; Zhang, S.; Liu, H.; Zhao, B.; Du, B.; Wang, W.; Lin, P.; Zhang, Z.; Zhong, Y.; et al. Digoxin enhances the anticancer effect on non-small cell lung cancer while reducing the cardiotoxicity of adriamycin. Front. Pharmacol. 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Bhusare, B.P.; John, C.K.; Bhatt, V.P.; Nikam, T.D. In vitro propagation of Digitalis lanata Ehrh. through direct shoot regeneration—A source of cardiotonic glycosides. Ind. Crop Prod. 2018, 121, 313–319. [Google Scholar] [CrossRef]
- Curfman, G. Digitalis glycosides for heart rate control in atrial fibrillation. JAMA 2020, 324, 2508. [Google Scholar] [CrossRef]
- Fujino, T.; Kuroda, M.; Matsuo, Y.; Kubo, S.; Tamura, C.; Sakamoto, N.; Mimaki, Y.; Hayakawa, M. Cardenolide glycosides from the seeds of Digitalis purpurea exhibit carcinoma-specific cytotoxicity toward renal adenocarcinoma and hepatocellular carcinoma cells. Biosci. Biotechnol. Biochem. 2015, 79, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kirmizibekmez, H.; Masullo, M.; Festa, M.; Capasso, A.; Piacente, S. Steroidal glycosides with antiproliferative activities from Digitalis trojana. Phytother. Res. 2014, 28, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.E.; He, D.N.; Yang, P.; Johansen, M.; Newman, R.A.; Lo, D.C. In vitro and in vivo neuroprotective activity of the cardiac glycoside oleandrin from Nerium oleander in brain slice-based stroke models. J. Neurochem. 2011, 119, 805–814. [Google Scholar] [CrossRef]
- Balderas-López, J.L.; Barbonetti, S.; Pineda-Rosas, E.L.; Tavares-Carvalho, J.C.; Navarrete, A. Cardiac glycosides from Cascabela thevetioides by HPLC-MS analysis. Rev. Bras. Farmacogn. 2019, 29, 441–444. [Google Scholar] [CrossRef]
- Kohls, S.; Scholz-Böttcher, B.M.; Teske, J.; Zark, P.; Rullkötter, J. Cardiac glycosides from yellow oleander (Thevetia peruviana) seeds. Phytochemistry 2012, 75, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.S.; Khatoon, N.; Begum, S.; Farooq, A.D.; Qamar, K.; Bhatti, H.A.; Ali, S.K. Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity. Phytochemistry 2012, 77, 238–244. [Google Scholar] [CrossRef]
- Mohammadi, S.; French, S.S.; Neuman-Lee, L.A.; Durham, S.L.; Kojima, Y.; Mori, A.; Brodie, E.D.; Savitzky, A.H. Corticosteroid responses of snakes to toxins from toads (bufadienolides) and plants (cardenolides) reflect differences in dietary specializations. Gen. Comp. Endocrinol. 2017, 247, 16–25. [Google Scholar] [CrossRef]
- Qi, J.; Zulfiker, A.H.M.; Li, C.; Good, D.; Wei, M.Q. The Development of toad toxins as potential therapeutic agents. Toxins 2018, 10, 336. [Google Scholar] [CrossRef]
- Buckalew, V.M. Endogenous digitalis-like factors: An overview of the history. Front. Endocrinol. 2015, 6, 49. [Google Scholar] [CrossRef]
- Buckalew, V.M. Role of endogenous digitalis-like factors in the clinical manifestations of severe preeclampsia: A systematic review. Clin. Sci. 2018, 132, 1215–1242. [Google Scholar] [CrossRef]
- Melero, C.P.; Medarde, M.; San Feliciano, A. A short review on cardiotonic steroids and their aminoguanidine analogues. Molecules 2000, 5, 51–81. [Google Scholar] [CrossRef]
- Bejček, J.; Spiwok, V.; Kmoníčková, E.; Rimpelová, S. Na+/K+-ATPase revisited: On its mechanism of action, role in cancer, and activity modulation. Molecules 2021, 26, 1905. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.W. Digitalis. Mechanisms of action and clinical use. N. Engl. J. Med. 1988, 318, 358–365. [Google Scholar] [CrossRef]
- Kepp, O.; Menger, L.; Vacchelli, E.; Adjemian, S.; Martins, I.; Ma, Y.; Sukkurwala, A.Q.; Michaud, M.; Galluzzi, L.; Zitvogel, L.; et al. Anticancer activity of cardiac glycosides. Oncoimmunology 2012, 1, 1640–1642. [Google Scholar] [CrossRef]
- Paula, S.; Tabet, M.R.; Ball, W.J. Interactions between cardiac glycosides and sodium/potassium-ATPase: Three-dimensional structure-activity relationship models for ligand binding to the E2-Pi form of the enzyme versus activity inhibition. Biochemistry. 2005, 44, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Lopina, O.D.; Tverskoi, A.M.; Klimanova, E.A.; Sidorenko, S.V.; Orlov, S.N. Ouabain-induced cell death and survival. Role of α1-Na,K-ATPase-mediated signaling and [Na+]i/[K+]i-dependent gene expression. Front. Phys. 2020, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.D.; Brickman, C.R.; Cottrill, C.L.; Shapiro, J.I.; Liu, J. The Na/K-ATPase Signaling: From specific ligands to general reactive oxygen species. Int. J. Mol. Sci. 2018, 19, 2600. [Google Scholar] [CrossRef]
- Cui, X.; Xie, Z. Protein interaction and Na/K-ATPase-mediated signal transduction. Molecules 2017, 22, 990. [Google Scholar] [CrossRef]
- Campia, I.; Gazzano, E.; Pescarmona, G.; Ghigo, D.; Bosia, A.; Riganti, C. Digoxin and ouabain increase the synthesis of cholesterol in human liver cells. Cell. Mol. Life Sci. 2009, 66, 1580–1594. [Google Scholar] [CrossRef]
- Manna, S.K.; Sreenivasan, Y.; Sarkar, A. Cardiac glycoside inhibits IL-8-induced biological responses by downregulating IL-8 receptors through altering membrane fluidity. J. Cell. Physiol. 2006, 207, 195–207. [Google Scholar] [CrossRef]
- Katz, A.; Lifshitz, Y.; Bab-Dinitz, E.; Kapri-Pardes, E.; Goldshleger, R.; Tal, D.M.; Karlish, S.J. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J. Biol. Chem. 2010, 285, 19582–19592. [Google Scholar] [CrossRef]
- Wansapura, A.N.; Lasko, V.; Xie, Z.; Fedorova, O.V.; Bagrov, A.Y.; Lingrel, J.B.; Lorenz, J.N. Marinobufagenin enhances cardiac contractility in mice with ouabain-sensitive alpha1 Na+-K+-ATPase. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1833–H1839. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Kennedy, D.J. Regulation of cardiac remodeling by cardiac Na+/K+-ATPase isoforms. Front. Physiol. 2016, 7, 382. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Fedorova, O.V.; Wei, W.; Rosen, H.; Horesh, N.; Ilani, A.; Lichtstein, D. Na+, K+-ATPase α isoforms and endogenous cardiac steroids in prefrontal cortex of bipolar patients and controls. Int. J. Mol. Sci. 2020, 21, 5912. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cai, T. Na+-K+--ATPase-mediated signal transduction: From protein interaction to cellular function. Mol. Interv. 2003, 3, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kutscher, L.M.; Shaham, S. Non-apoptotic cell death in animal development. Cell Death Differ. 2017, 24, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, A.H.L.A.N. Programmed cell death and tissue remodelling in plants. J. Exp. Bot. 2008, 59, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; De Laurenzi, V. Die for the community: An overview of programmed cell death in bacteria. Cell Death Dis. 2015, 6, e1609. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Buque Martinez, A.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Sun, F.; Cui, L.; Li, T.; Chen, S.; Song, J.; Li, D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J. Recept. Signal. Transduct. Res. 2019, 39, 208–214. [Google Scholar] [CrossRef]
- Kepp, O.; Zitvogel, L.; Kroemer, G. Lurbinectedin: An FDA-approved inducer of immunogenic cell death for the treatment of small-cell lung cancer. Oncoimmunology 2020, 9, 1795995. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Waxman, D.J. Medium dose intermittent cyclophosphamide induces immunogenic cell death and cancer cell autonomous type I interferon production in glioma models. Cancer Lett. 2020, 470, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Panaretakis, T.; Kepp, O.; Brockmeier, U.; Tesniere, A.; Bjorklund, A.C.; Chapman, D.C.; Durchschlag, M.; Joza, N.; Pierron, G.; van Endert, P.; et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009, 28, 578–590. [Google Scholar] [CrossRef]
- Garg, A.D.; Dudek, A.M.; Ferreira, G.B.; Verfaillie, T.; Vandenabeele, P.; Krysko, D.V.; Mathieu, C.; Agostinis, P. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 2013, 9, 1292–1307. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yang, W.; Zhou, Z.; Tian, R.; Lin, L.; Ma, Y.; Song, J.; Chen, X. Targeted scavenging of extracellular ROS relieves suppressive immunogenic cell death. Nat. Commun. 2020, 11, 4951. [Google Scholar] [CrossRef] [PubMed]
- Menger, L.; Vacchelli, E.; Adjemian, S.; Martins, I.; Ma, Y.; Shen, S.; Yamazaki, T.; Sukkurwala, A.Q.; Michaud, M.; Mignot, G.; et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci. Transl. Med. 2012, 4, 143ra99. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Chen, L.; Li, L.; Huang, Y. Restoration and enhancement of immunogenic cell death of cisplatin by coadministration with digoxin and conjugation to HPMA copolymer. ACS Appl. Mater. Interfaces 2020, 12, 1606–1616. [Google Scholar] [CrossRef]
- da Silva, J.M.C.; Campos, M.L.A.; Teixeira, M.P.; Faustino, R.D.S.; Aleixo, R.C.; Cavalcante, F.J.P.; Gomes, L.R.O.; de Albuquerque, L.Z.; Azevedo, A.D.N.; Cabral, V.R.; et al. Ouabain pre-treatment modulates B and T lymphocytes and improves survival of melanoma-bearing animals. Int. Immunopharmacol. 2020, 86, 106772. [Google Scholar] [CrossRef]
- Shih, Y.L.; Shang, H.S.; Chen, Y.L.; Hsueh, S.C.; Chou, H.M.; Lu, H.F.; Lee, M.Z.; Hou, H.T.; Chuang, Y.Y.; Lee, M.H.; et al. Ouabain promotes immune responses in WEHI-3 cells to generate leukemia mice through enhancing phagocytosis and natural killer cell activities in vivo. Environ. Toxicol. 2019, 34, 659–665. [Google Scholar] [CrossRef]
- Cavalcante-Silva, L.H.A.; Lima, E.D.; Carvalho, D.C.M.; de Sales-Neto, J.M.; Alves, A.K.D.A.; Galvao, J.G.F.M.; da Silva, J.S.D.F.; Rodrigues-Mascarenhas, S. Much more than a cardiotonic steroid: Modulation of inflammation by ouabain. Front. Physiol. 2017, 8, 895. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Chou, J.S.; Chen, Y.L.; Hsueh, S.C.; Chung, H.Y.; Lee, M.H.; Chen, C.P.; Lee, M.Z.; Hou, H.T.; Lu, H.F.; et al. Bufalin enhances immune responses in leukemic mice through enhancing phagocytosis of macrophage in vivo. In Vivo 2018, 32, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tao, Y.; Xu, X.; Cai, F.; Yu, Y.; Ma, L. Bufalin inhibits cell proliferation and migration of hepatocellular carcinoma cells via APOBEC3F induced intestinal immune network for IgA production signaling pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Spelmink, L.; Codemo, M.; Subramanian, K.; Pütsep, K.; Henriques-Normark, B.; Olliver, M. Cinobufagin modulates human innate immune responses and triggers antibacterial activity. PLoS ONE 2016, 11, e0160734. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Y.; An, N.; Zeng, S.; Wang, D.; Yu, L.; Zhu, T.; Zhang, T.; Cui, J.; Zhou, C.; et al. The effects of telocinobufagin isolated from Chan Su on the activation and cytokine secretion of immunocytes in vitro. Fundam. Clin. Pharmacol. 2009, 23, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; He, J.; Kisoh, K.; Hayashi, H.; Tanaka, S.; Si, N.; Zhao, H.Y.; Hirano, T.; Bian, B.; Takagi, N. Effects of active bufadienolide compounds on human cancer cells and CD4+CD25+Foxp3+ regulatory T cells in mitogen-activated human peripheral blood mononuclear cells. Oncol. Rep. 2016, 36, 1377–1384. [Google Scholar] [CrossRef]
- Huh, J.R.; Leung, M.W.L.; Huang, P.; Ryan, D.A.; Krout, M.R.; Malapaka, R.R.V.; Chow, J.; Manel, N.; Ciofani, M.; Kim, S.V.; et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 2011, 472, 486–490. [Google Scholar] [CrossRef]
- Karaś, K.; Sałkowska, A.; Walczak-Drzewiecka, A.; Ryba, K.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. The cardenolides strophanthidin, digoxigenin and dihydroouabain act as activators of the human ROR gamma/ROR gamma T receptors. Toxicol. Lett. 2018, 295, 314–324. [Google Scholar] [CrossRef]
- Karaś, K.; Sałkowska, A.; Sobalska-Kwapis, M.; Walczak-Drzewiecka, A.; Strapagiel, D.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. Digoxin, an overlooked agonist of RORγ/RORγT. Front. Pharmacol. 2018, 9, 1460. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Zhang, K.; Liao, Y.; Ye, P.; Wu, J.; Wang, Y.; Li, F.; Yao, Y.; Zhou, Y.; et al. Inhibiting the Th17/IL-17A-related inflammatory responses with digoxin confers protection against experimental abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2429–2438. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; López-Lázaro, M. The in vivo antitumor activity of cardiac glycosides in mice xenografted with human cancer cells is probably an experimental artifact. Oncogene 2014, 33, 2947–2948. [Google Scholar] [CrossRef]
- Tani, S.; Takano, R.; Tamura, S.; Oishi, S.; Iwaizumi, M.; Hamaya, Y.; Takagaki, K.; Nagata, T.; Seto, S.; Horii, T.; et al. Digoxin attenuates murine experimental colitis by downregulating Th17-related cytokines. Inflamm. Bowel. Dis. 2017, 23, 728–738. [Google Scholar] [CrossRef]
- Saeed, H.; Mateen, S.; Moin, S.; Khan, A.Q.; Owais, M. Cardiac glycoside digoxin ameliorates pro-inflammatory cytokines in PBMCs of rheumatoid arthritis patients in vitro. Int. Immunopharmacol. 2020, 82, 106331. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Mao, X.; Zhong, Y.; Liu, Y.; Zhao, X.; Yu, K.; Zhu, R.; Wei, Y.; Zhu, J.; Sun, H.; et al. Digoxin reduces atherosclerosis in apolipoprotein E-deficient mice. Br. J. Pharmacol. 2016, 173, 1517–1528. [Google Scholar] [CrossRef]
- Ouyang, X.; Han, S.N.; Zhang, J.Y.; Evangelos, D.; Nemeth, B.T.; Pacher, P.; Feng, D.; Bataller, R.; Cabezas, J.; Stärkel, P.; et al. Digoxin suppresses pyruvate kinase M2-promoted HIF-1α transactivation in steatohepatitis. Cell Metab. 2018, 27, 339–350.e3. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03559868?term=digoxin&recrs=abdf&draw=2&rank=7 (accessed on 14 March 2021).
- ClinicalTrials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04216693?term=digoxin&recrs=abdf&draw=2&rank=6 (accessed on 14 March 2021).
- Zeitlin, P.L.; Diener-West, M.; Callahan, K.A.; Lee, S.; Talbot, C.C.; Pollard, B.; Boyle, M.P.; Lechtzin, N. Digitoxin for airway inflammation in cystic fibrosis: Preliminary assessment of safety, pharmacokinetics, and dose Finding. Ann. Am. Thorac. Soc. 2017, 14, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhakeer, Z.; Hadeer, M.; Tuerxun, Z.; Tuerxun, K. Bufalin inhibits the inflammatory effects in asthmatic mice through the suppression of nuclear factor-kappa B activity. Pharmacology 2017, 99, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Galvão, J.G.F.M.; Cavalcante-Silva, L.H.A.; Carvalho, D.C.M.; Ferreira, L.K.D.P.; Monteiro, T.M.; Alves, A.F.; Ferreira, L.A.M.P.; Gadelha, F.A.A.F.; Piuvezam, M.R.; Rodrigues-Mascarenhas, S. Ouabain attenuates ovalbumin-induced airway inflammation. Inflamm. Res. 2017, 66, 1117–1130. [Google Scholar] [CrossRef]
- Jansson, D.; Dieriks, V.B.; Rustenhoven, J.; Smyth, L.C.D.; Scotter, E.; Aalderink, M.; Feng, S.; Johnson, R.; Schweder, P.; Mee, E.; et al. Cardiac glycosides target barrier inflammation of the vasculature, meninges and choroid plexus. Commun. Biol. 2021, 4, 260. [Google Scholar] [CrossRef]
- Škubník, J.; Jurášek, M.; Ruml, T.; Rimpelová, S. Mitotic poisons in research and medicine. Molecules 2020, 25, 4632. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current perspectives on taxanes: Focus on their bioactivity, delivery and combination therapy. Plants (Basel) 2021, 10, 569. [Google Scholar] [CrossRef] [PubMed]

| CG | Condition | Mechanism of Action | Reference |
|---|---|---|---|
| Digoxin, digitoxin, ouabain, bufalin gamabufotalin arenobufagin | Cancer | Induction of ICD | [57,60,63,66] |
| Cinobufagin | Microbial infection | Upregulating IL-1β | [64] |
| Digoxin | Abdominal aortic aneurysm | RORγt inhibition, suppressing Th17 differentiation | [70] |
| Rheumatoid arthritis | [73] | ||
| Colitis | [72] | ||
| Atherosclerosis | [70] | ||
| NASH | Suppressing hypoxia-induced factor 1 α, NFκB transcription, and ROS through binding to PKM2 | [74] | |
| Digitoxin | Cystic fibrosis | Suppressing NFκB, downregulating IL-8 | [78] |
| Bufalin, ouabain | Airway inflammation (asthma) | Suppressing NFκB | [79,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škubník, J.; Pavlíčková, V.; Rimpelová, S. Cardiac Glycosides as Immune System Modulators. Biomolecules 2021, 11, 659. https://doi.org/10.3390/biom11050659
Škubník J, Pavlíčková V, Rimpelová S. Cardiac Glycosides as Immune System Modulators. Biomolecules. 2021; 11(5):659. https://doi.org/10.3390/biom11050659
Chicago/Turabian StyleŠkubník, Jan, Vladimíra Pavlíčková, and Silvie Rimpelová. 2021. "Cardiac Glycosides as Immune System Modulators" Biomolecules 11, no. 5: 659. https://doi.org/10.3390/biom11050659
APA StyleŠkubník, J., Pavlíčková, V., & Rimpelová, S. (2021). Cardiac Glycosides as Immune System Modulators. Biomolecules, 11(5), 659. https://doi.org/10.3390/biom11050659