Abstract
Chronic inflammatory disorders are characterised by aberrant and exaggerated inflammatory immune cell responses. Modes of extrinsic cell death, apoptosis and necroptosis, have now been shown to be potent drivers of deleterious inflammation, and mutations in core repressors of these pathways underlie many autoinflammatory disorders. The receptor-interacting protein (RIP) kinases, RIPK1 and RIPK3, are integral players in extrinsic cell death signalling by regulating the production of pro-inflammatory cytokines, such as tumour necrosis factor (TNF), and coordinating the activation of the NOD-like receptor protein 3 (NLRP3) inflammasome, which underpin pathological inflammation in numerous chronic inflammatory disorders. In this review, we firstly give an overview of the inflammatory cell death pathways regulated by RIPK1 and RIPK3. We then discuss how dysregulated signalling along these pathways can contribute to chronic inflammatory disorders of the joints, skin, and gastrointestinal tract, and discuss the emerging evidence for targeting these RIP kinases in the clinic.
1. Introduction
Common chronic inflammatory and autoinflammatory diseases are caused by immune dysregulation, the aetiology of which involves a complex interplay between genetic and environmental factors that is incompletely understood [1]. Unmistakably, however, the underlying cause of distinct tissue pathologies is a failure to resolve inflammation arising from the excessive production of inflammatory cytokines and chemokines, as well as danger-associated molecular patterns (DAMPs) that are released from dying cells. Tumour necrosis factor (TNF) is the archetypal death ligand and a pivotal pathogenic cytokine in many common inflammatory diseases. Accordingly, a number of TNF antagonists, as well as other anti-inflammatory biological agents that dampen its activity, have met with considerable success in the clinic. Yet, many patients still fail to respond, or else develop severe adverse reactions to these therapies, highlighting the need for a both a greater understanding of TNF signalling on a cell-by-cell level and also the need for alternative strategies to target TNF signalling pathways in disease.
Over the last decade, the serine-threonine receptor-interacting protein (RIP) kinases -1 and -3 have emerged as key regulators of innate immunity via their integral roles in cell death signalling during cellular stress and following exposure to inflammatory and infectious stimuli [2,3,4]. RIPK1 has a critical scaffolding role in tumour necrosis factor receptor-1 (TNFR1) and Toll-like receptor 3/4 (TLR3/4) pro-inflammatory signalling [5,6,7], as well as a kinase-dependent role in both apoptotic and necroptotic cell death [8]. The carboxy-terminal death domain (DD) of RIPK1 can facilitate its interaction with other DD-containing proteins to promote formation of death receptor signalling complexes [9,10,11], while its RIP homotypic interaction motif (RHIM) domain (also shared with RIPK3) enables RIPK1/3 interactions with other cell death and/or immune adaptors [12], e.g., TIR domain-containing adaptor-inducing IFN-β (TRIF) [5,13] and the dsDNA receptor Z-DNA binding protein-1 (ZBP1; also known as DAI) [14].
TNF ligation of TNFR1 typically recruits RIPK1 into a membrane bound receptor signalling complex, referred to as complex I, that activates NF-κB-mediated transcription of pro-survival genes (Figure 1). Along with RIPK1, TNFR1-associated death domain protein (TRADD) is recruited to TNFR1 via their common DD [15,16]. The adaptor TNFR-associated factor 2 (TRAF2) subsequently binds to TRADD and recruits the cellular inhibitor of apoptosis proteins (cIAP)-1 and cIAP2 [17,18]. The E3 ubiquitin ligase activity of cIAP1/2 attaches K63- and K11-linked polyubiquitin chains to RIPK1, allowing for the recruitment of the linear ubiquitin chain assembly complex (LUBAC), which is a heterotrimeric complex made up of haeme-oxidized IRP2 ubiquitin ligase 1 (HOIL-1), HOIL-1-interacting protein (HOIP), and SHANK-associated RH domain-interacting protein (SHARPIN). Subsequent M1-linked ubiquitylation of complex I components by LUBAC stabilises the complex and promotes the TAK1- and IKK-dependent activation of canonical NF-κB transcription [19,20,21]. Post-translational modification of RIPK1 within complex I, as well as the selective cleavage of RIPK1 and RIPK3 (and cylindromatosis [CYLD]) by caspase-8, maintain TNF-mediated pro-survival signalling and block the transition to pro-apoptotic cell death complex II [22,23,24,25,26,27,28,29]. This critical pro-survival scaffolding role for RIPK1 is evidenced by the fact that RIPK1-deficient mice die shortly after birth due to systemic multi-organ inflammation mediated by unrestrained apoptosis and necroptosis [30,31,32,33,34,35,36].
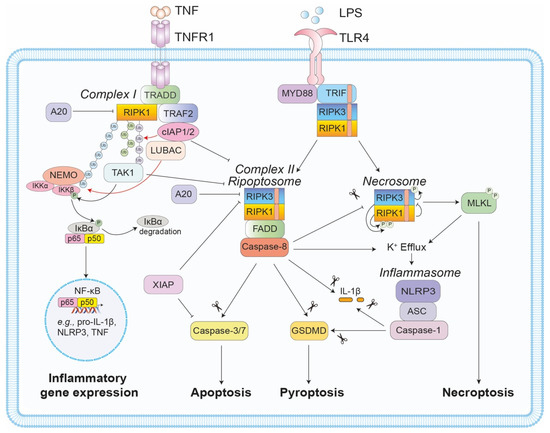
Figure 1.
Regulation of RIPK1- and RIPK3-mediated cell death and inflammation. TNF ligation of its receptor, TNFR1, typically induces complex 1 formation, where TNFR1 associates with RIPK1 and TRADD. TRADD subsequently interacts with TRAF2 to facilitate cIAP1 and LUBAC recruitment to the complex. The cIAPs and LUBAC ubiquitylate RIPK1 to facilitate a signalling cascade that induces pro-survival and pro-inflammatory gene expression. If either RIPK1 phosphorylation or ubiquitylation are impaired, FADD-RIPK1(RIPK3)-caspase-8 associate to form the pro-apoptotic complex II (or the ripoptosome). If caspase-8 activity is compromised, a further complex, termed the necrosome, forms in a RIPK1- and RIPK3 kinase-dependent manner. Within this complex, RIPK3-mediated phosphorylation and activation of MLKL induces necroptosis, a lytic inflammatory form of cell death. Of note, TLR4 ligation can also induce similar inflammatory (e.g., RIPK3-caspase-8-mediated cytokine transcription) and cell death events via RHIM-RHIM interactions between the adaptor TRIF and RIPK1/3. In addition to cell death and cytokine transcription, it is now appreciated that both the ripoptosome and the necrosome can promote inflammasome assembly, i.e., caspase-8 and MLKL activity can trigger NLRP3 inflammasome activation, via K+ efflux, while caspase-8 can also directly cleave IL-1β and GSDMD to induce inflammation.
Complex II, and the related ripoptosome complex that can form in the absence of death receptor ligation [37,38], are essentially comprised of the adaptor protein Fas-associated protein with death domain (FADD), RIPK1, RIPK3, and pro-caspase-8 (Figure 1). Known triggers of complex II/ripoptosome include cIAP loss, via the natural IAP antagonist second mitochondria-derived activator of caspases (Smac; also known as DIABLO), or genetic/chemical depletion [25,37,38,39,40], as well as loss/inhibition of TRAF2, TAK1, or IKK-β/γ [18,29,41]. Once formed, catalytically active caspase-8 within complex II triggers apoptosis by cleaving and activating the downstream effector caspases-3 and -7. Caspase-8 can also cleave and truncate Bid to engage the mitochondrial apoptosis pathway and amplify death in certain cell types (i.e., type II cells, including hepatocytes) [42,43,44]. It is worth mentioning, that while antagonism of X-linked IAP (XIAP) by Smac is thought to promote apoptosis indirectly by unleashing caspase-3, -7, and -9 activity, the ubiquitin E3 ligase activity of XIAP also critically represses TNFR1 and TLR3/4-induced cell death in myeloid cells [45,46,47,48,49].
When the activity of caspase-8 is compromised, either genetically or by viral/chemical inhibition, both TNFR1 and TLR-TRIF signalling can trigger a RIP kinase-dependent, caspase-independent form of lytic cell death called necroptosis (Figure 1) [50,51]. In the case of TNF signalling, loss of both the IAPs and caspase-8 activity promotes full-length RIPK1 and RIPK3 interactions via their RHIM domains to form a functional oligomeric amyloid structure, termed the necrosome [52]. Next, autophosphorylation of RIPK3 facilitates the recruitment of the terminal necroptotic effector, the pseudokinase mixed-lineage kinase domain-like (MLKL). Subsequent phosphorylation of the MLKL activation loop by RIPK3 triggers a conformational change that exposes its N-terminal 4-helix bundle (4HB) killing domain and promotes the assembly of pore-forming oligomers within the plasma membrane and the lytic release of the cellular contents, including inflammatory DAMPs, e.g., interleukin (IL)-1α, IL-33, high-mobility group box 1 (HMGB1), ATP, uric acid, and heat shock proteins (HSPs) [53,54,55,56,57,58]. Of note, while the kinase activity of RIPK1 is essential for TNF-mediated necroptosis, it is not required for TLR-TRIF signalling. Moreover, RIPK1 inhibits the necroptotic activity of RIPK3 in response to TLR-TRIF signalling [45], and its RHIM domain also acts as a brake on ZBP1-RIPK3-mediated necroptosis during development, via an unknown mechanism [59,60,61].
It has recently emerged that significant crosstalk exists between extrinsic cell death and inflammatory signalling, particularly with regard to the NOD-like receptor protein 3 (NLRP3) inflammasome (reviewed in [42,62]). The multimeric cytosolic NLRP3 inflammasome complex comprises the sensor protein NLRP3 that is triggered by a number of host and pathogen danger molecules and instigates stepwise inflammasome assembly via recruitment of the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) and pro-caspase-1. Active caspase-1 then cleaves pro-IL-1β and pro-IL-18 into their mature bioactive forms and activates the pore-forming effector, gasdermin D (GSDMD), to induce pyroptotic cell death. A number of studies have now highlighted how cell death machinery can regulate NLRP3 inflammasome activity via effects on transcriptional responses and post-translational events that facilitate complex assembly [63]. For example, TLR-induced NLRP3 inflammasome priming (i.e., induction of core inflammasome machinery NLRP3 and pro-IL-1β) and post-translational activity was suggested to be dependent on a FADD-caspase-8 complex in response to both canonical and non-canonical caspase-11 inflammasome stimuli [64]. Whilst the defective transcriptional pathway associated specifically with blunted enzymatic caspase-8 activity upon TLR ligation in Ripk3−/−Caspase-8−/−, Ripk3−/−Fadd−/−, and Fadd−/−Mlkl−/− bone marrow-derived macrophages (BMDM) and bone marrow-derived dendritic cells (BMDC) remains controversial [64,65,66,67,68], it is now appreciated that a FADD-RIPK1-caspase-8 scaffolding complex supports inflammasome responses [69,70], and that caspase-8 can also interact with ASC to coordinate cell death and IL-1β activation (in the absence of caspase-1) [71,72,73].
It is now also becoming increasingly clear that RIPK1 and RIPK3 can coordinate alternative and cell death-induced pathways to NLRP3 inflammasome and IL-1β activation [42,74,75] (Figure 1). Loss of key negative regulators of cell death, such as the IAPs, the NF-κB inhibitor and deubiquitinase A20, and TAK1, which promotes phosphorylation events that prevent RIPK1 death signalling [76,77], can also induce modes of RIPK1 (RIPK3)-FADD-caspase-8-mediated NLRP3 inflammasome activation [45,46,47,49,78,79,80,81,82,83,84,85]. For example, the potassium efflux (K+) associated with necroptotic cell death in IAP- and/or caspase-8-deficient cells can potently activate the NLRP3 inflammasome in a cell intrinsic manner [45,86,87]. Likewise, RIPK1 can act as a repressor of RIPK3/MLKL-mediated necroptotic activation of NLRP3 [45]. Additionally, TLR4-TRIF (or TNFR) signalling in IAP-depleted myeloid cells can induce the direct caspase-8-mediated proteolysis of IL-1β, as well as NLRP3 inflammasome activation that is dependent on K+ efflux linked to caspase-8-mediated apoptosis occurring upstream [45,78,88].
TNFR1 signalling via RIPK1 has been long recognised for its role in driving pathogenic pro-inflammatory cytokine and chemokine production in common diseases, such as Rheumatoid arthritis (RA), psoriasis, ankylosing spondylitis, and inflammatory bowel disease (IBD). Hence, TNF antagonists with different mechanisms of action have been used extensively, and with considerable success, to treat these disorders [89,90,91]. Intriguingly, recent evidence shows that pathology in a number of rare autoinflammatory syndromes is driven by excessive RIPK1/3-mediated cell death and inflammasome-associated IL-1β and IL-18 production [92]. Consequently, RIP kinase inhibitors have significant therapeutic potential beyond simply limiting TNF-induced inflammation. To date, small-molecule inhibitor development has preferentially focused on targeting the kinase activity of RIPK1 [93,94], as RIPK3 kinase inhibitor development has been thwarted by the fact they can promote apoptosis via a RIPK1-FADD-caspase-8-cFLIP complex. This phenomenon is analogous to the embryonic lethality observed in RIPK3 kinase dead (Ripk3D161N/D161N) mice [95,96]. Since knock-in mice expressing a kinase dead form of RIPK1 are healthy [96,97], it is predicted that inhibitors targeting the kinase activity of RIPK1 will be well tolerated and not disrupt its critical pro-survival scaffolding role. It is also worth acknowledging that, unlike RIPK1-deficient mice that die perinatally, patients with biallelic loss-of-function mutations in RIPK1 survive into early adulthood but do develop primary immunodeficiency typified by recurrent infections, as well as clinical manifestations including oral/skin lesions, arthritis, and early-onset IBD [98,99]. This partial redundancy in humans again highlights RIPK1 as a suitable druggable target for disease.
Current efforts to generate RIPK1 inhibitors have yielded small molecules that bind within the hydrophobic pocket of RIPK1, located between the N- and C-terminus of the kinase domain, and stabilise RIPK1 in an inactive conformation [100]. Pre-clinical studies using RIPK1 kinase dead mice and the first generation indole-hydantoin RIP1 kinase inhibitors, Necrostatin-1s (Nec-1s), and less-specific analogue Nec-1, have demonstrated the potential to target RIPK1 signalling in numerous injury (e.g., renal ischaemic reperfusion, hypoxic brain injury), acute (e.g., systemic inflammatory response syndrome), and chronic inflammatory disease models (e.g., multiple sclerosis) (comprehensively reviewed in [42,94,101]). Now, a number of more specific, small-molecule RIPK1 inhibitors have been developed by Glaxo-Smith Kline (GSK), Sanofi/Denali (DNL), Takeda, and Genentech, which are either undergoing pre-clinical testing or have entered early-phase clinical trials, including the benzoxazepinone GSK2982772 for the treatment of psoriasis, ulcerative colitis (UC), and RA, and brain-penetrant DNL747 for the treatment of the neurodegenerative disease Amyotrophic Lateral Sclerosis (ALS) [94,101,102].
This review will explore the evidence for a role for RIP kinase-regulated cell death and inflammation in rare autoinflammatory syndromes and how mechanistic insights from these conditions can be extrapolated into chronic inflammatory diseases of the gastrointestinal tract, joints, and skin (Table 1), which are triggered by ill-defined environmental and genetic factors. It will also address the therapeutic potential of targeting RIPK1 in the clinic to treat these disorders.

Table 1.
Spontaneous tissue pathologies linked to RIPK signalling.
2. Multi-Organ—Autoinflammatory Syndromes
To date, mutations in cell death regulatory genes, including TNFR1, XIAP, A20, OTULIN, LUBAC components (HOIL-1, HOIP and SHARPIN), RIPK1, and caspase-8, cause primary immunodeficiency, autoimmunity, and/or trigger autoinflammation reminiscent of NLRP3 autoactivating mutation-associated cryopyrin-associated periodic syndromes (CAPS). In the case of TNFR-associated periodic syndrome (TRAPs), XIAP deficiency (i.e., hemophagocytic lymphohistiocytosis), haploinsufficiency in A20 (HA20), LUBAC deficiency, otulipenia, and biallelic RIPK1 deficiency common overlapping clinical manifestations include recurrent fever and infection, arthritis and arthralgia/myalgia (i.e., joint and musculoskeletal pain), skin lesions, and intestinal inflammation and/or diarrhoea, as well as on occasion hepatosplenomegaly [63,98,103,104,105,106,107,108]. Often, mutation of these genes in mice causes embryonic or perinatal lethality associated with multi-organ inflammation and/or excessive cell death that precludes analysis of common disease manifestations, but cell-specific gene targeting is beginning to establish disease mechanisms and therapeutic targets [107,109,110,111,112,113]. Moreover, despite the variability in death modalities that may drive autoinflammation, therapeutics targeting TNF, IL-6, or IL-1β itself are comparatively effective, although responses can be unpredictable [114]. Hence, targeting upstream signalling pathways may yield a more uniform therapeutic responsiveness and this approach will be discussed in more detail below.
3. Joints—Rheumatoid Arthritis
Historically, RA development and chronicity have been linked to a lack of apoptosis due to the expression of intrinsic and extrinsic pro-survival proteins in inflamed synovial joints [115,116,117,118,119]. This prompted interest in examining the cell-specific survival requirements of pathogenic cells [120,121,122,123] and the repurposing and pre-clinical testing of anti-cancer small-molecule drugs (e.g., BH3 mimetics, pegylated TRAIL) to treat arthritis, albeit with limited to moderate therapeutic success [124,125,126,127]. In fact, the generation of conditional mutant mice lacking key extrinsic cell death regulatory machinery, namely A20 (A20LysM.cre), cIAP1/2 (cIap1LysM.crecIap2−/−), or XIAP/cIAP1/2 (cIap1LysM.creXiap−/−cIap2−/−) in myeloid cells, and caspase-8 inhibitor c-FLIP (c-flarCD11c.cre) in dendritic cells, revealed how perturbation of cell death signalling pathways can induce multi-organ inflammation characterised by severe inflammatory arthritis (Table 1). Moreover, a dominant inflammasome-associated IL-1β signature was observed in all arthritic mutant mice; with the exception of the myeloid-specific cIAP1/2 knockout mouse (that exhibited TNF as the main biomarker) [45,82,128,129]. Notably, pathogenicity of disease in these spontaneous arthritis models is complex as, in some cases, disease is rescued by NLRP3 inflammasome deficiency rather than TNF loss, and vice versa. Therefore, the contribution of apoptotic versus necroptotic signalling, particularly in IL-1β activation, remains ill defined. For example, while in vitro evidence suggests that NLRP3 inflammasome and IL-1β activation in the absence of A20 (encoded by TNFAIP3) is regulated by RIPK3-caspase-8 signalling, a recent study in A20LysM.cre mice showed that loss of RIPK3, MLKL, or the kinase activity of RIPK1, dampened IL-1β activity and alleviated arthritis symptoms [130].
Examination of genetic knockout mice lacking central cell death repressors, the IAPs, in experimental arthritis models has also revealed potential overlap between monogenic disease and inflammatory arthritis pathogenesis. Reconstitution of wild-type mice with bone marrow lacking XIAP/cIAP1/2 or cIAP1/2 in the myeloid compartment not only transferred a mild form of spontaneous arthritis but also promoted exaggerated innate immune cell-driven K/BxN serum-induced arthritic responses that were associated with enhanced IL-1β and TNF activity, respectively. Noting that single cIAP1-, cIAP2-, and XIAP-deficient, as well as XIAP/cIAP2 doubly-deficient mice behaved akin to wild-type animals [45]. Further mechanistic insight into how TNFR1 and TLR signalling may regulate inflammatory arthritis has also been gained using mice deficient in core cell death signalling machinery and kinase inhibitors. For example, in the acute K/BxN serum-induced arthritis model, signalling via TLR4-MyD88, as well as IL-1α and IL-1β activity, play a more crucial role in disease progression and chronicity than TNF itself [34]. Remarkably, studies examining Trif−/−, Ripk3−/−, Mlkl−/−, and Ripk3−/−caspase-8−/− (where caspase-8 is deleted on a RIPK3-deficient background to avoid lethal necroptotic signalling [43,44]) mice revealed that a TLR-TRIF-RIPK3-caspase-8 signalling axis regulates caspase-1-independent IL-1β secretion during the late macrophage-driven phase of this disease model. Surprisingly, MLKL was redundant for arthritis development or resolution in this context [45]. Fitting with the idea that dysregulated caspase-8 activity may drive disease progression, mice lacking the extrinsic death receptor Fas in myeloid cells also exhibited enhanced disease resolution, although this was not associated with a blunted IL-1β response [123]. Intriguingly, a further study showed that conditional deletion of caspase-8 in dendritic cells (Caspase-8CD11c.cre/+) exacerbated arthritis, whilst, conversely, myeloid cell-specific caspase-8 loss (Caspase-8LysM.cre/cre) enhanced disease resolution. Disease perturbations were suggested to be caused by altered RIPK3-mediated signalling and the development of innate immune cell populations, as co-deletion of RIPK3 reversed overall disease responses [131]. This work points to RIPK1/3-caspase-8 signalling having differential effects in specific myeloid populations, as previously evidenced by the distinct role for RIPK1 versus RIPK3 in repressing cytokine production in the Caspase-8CD11c.cre/+ and Caspase-8LysM.cre mice [132]. It is also worth noting that in our hands, Caspase-8LysM.cre/+ (hemizygous Cre) mice developed comparable, if not mildly enhanced, levels of arthritis compared to Caspase-8lox/lox mice (Lawlor KE, unpublished data), which is more reminiscent of the Caspase-8CD11c.cre/+ mice. Overall, differences in RIPK-caspase-8 signalling requirements in arthritis pathogenesis could be due to differential cell-specific effects, levels of Cre recombinase expression, absolute levels of caspase-8 that can act as scaffolding for multiple modes of cell death [45,69,70,87,132,133], as well as environmental and genetic differences (e.g., Caspase-8lox/lox derived on a 129 [131] versus C57BL/6 [45] background).
RIPK1 kinase inhibitors are an attractive therapeutic avenue for RA, as they may limit TNF-induced inflammation in addition to blocking cell death that also has the propensity to be pro-inflammatory. Indeed, two groups have documented the therapeutic efficacy of RIPK1 kinase inhibitors in acute and chronic arthritis models. In the case of chronic autoimmune collagen-induced arthritis, prophylactic treatment with the RIPK1 kinas inhibitor necrostatin-1s (Nec-1s) modestly reduced the incidence and severity of arthritis and was associated with reduced cytokine and necroptotic activity in the synovium, a skewing of the CD4+ T-cell response towards a TH2 and regulatory T-cell profile, and attenuated osteoclastogenesis [134]. In comparison, both RIPK1 kinase dead mice and mice receiving the RIPK1 kinase inhibitor, GNE684, were shown to have reduced acute innate immune cell-driven collagen antibody-induced arthritis (CAIA). This effect was directly attributable to GNE684 inhibiting TNF signalling, as blocking TNF using TNFR2-Fc caused an equivalent effect and failed to further dampen responses upon co-therapy [135]. On the surface, these results suggest that RIPK1 kinase inhibitors are likely to be as efficacious as TNF biologicals for the treatment of RA. However, a Phase II clinical trial in patients with moderate to severe RA using the first-in-class small-molecule RIPK1 inhibitor GSK2982772 (NCT02858492) failed to see significant clinical improvement beyond potential reductions in bone erosions [136]. It remains to be seen whether improved bioavailability, or treatment of mild RA cases, will reveal therapeutic efficacy.
4. Skin—Psoriasis and Dermatitis
RIPK1 has an established role in preventing apoptosis and necroptosis of murine epithelial cells, and Ripk1−/− neonates have elevated levels of inflammatory cytokines in their skin, as well as keratinocyte hyperplasia that is a hallmark of excessive cell death [30,35,36,59,61,137]. RIPK1 also appears to play a protective role in human keratinocytes, albeit less dominantly. Patients with biallelic mutations in RIPK1 can present with inflammatory skin lesions among a number of more debilitating pathologies (Table 1) [98,99]. In contrast, patients with cleavage-resistant RIPK1 autoinflammatory (CRIA) syndrome caused by heterozygous mutations in the caspase-8 cleavage site (D324) do not display a skin phenotype, although mice harbouring a homozygous mutation (Ripk1D325A/D325A) of this conserved site do exhibit skin hyperplasia [138]. In fact, a number of mouse models with dysregulated complex I signalling present with lethal inflammatory skin disorders, often linked to RIPK1 activity [139,140]. In humans, autoinflammatory skin disorders, such as psoriasis, are multi-factorial [141]. However, inflammation-associated keratinocyte cell death does appear to major contributor to disease pathology.
The heterotrimeric linear ubiquitin chain assembly complex (LUBAC; composed of HOIP that harbours the essential enzymatic activity, and HOIL-1 and SHARPIN that promote complex assembly and stability) coordinates NF-κB activation via linear ubiquitylation of RIPK1 and NEMO within complex I. As briefly touched on above, HOIL-1- and HOIP-deficient patients survive but present with immunodeficiency and multi-organ autoinflammation, including dermatitis [106,142,143]. In contrast, the absence of HOIL-1 or HOIP in mice, and the ensuing lack of linear ubiquitylation in complex I, results in early-mid-gestational lethality due to aberrant TNFR1-mediated endothelial apoptotic and necroptotic cell death that is only partially dependent on the kinase activity of RIPK1 [144]. Intriguingly, whilst combined MLKL and caspase-8 deficiency rescues the HOIL-1 and HOIP-deficient mice from embryonic lethality, co-deletion of RIPK3 and caspase-8 results in late-gestation lethality due to RIPK1-induced haematopoietic defects [144]. Examination of the role of LUBAC selectively in skin homeostasis, via conditional deletion of HOIL-1 and HOIP in mouse keratinocytes (keratin 14 promoter), revealed the onset of lethal postnatal dermatitis at 4–6 days-of-age, which was partially attributable to exaggerated TNFR1-mediated apoptotic caspase-8 activity but largely RIPK1-independent [145]. Interestingly, however, the skin lesions that developed in the absence of both TNFR1 and HOIL-1 were delayed by MLKL loss and completely rescued by RIPK1 kinase inhibition using GSK’547, suggesting a combination of RIPK1 kinase-dependent apoptosis and necroptosis is triggered in the absence of LUBAC and TNFR1 [145]. Additionally, in the absence of TNFR1, dermatitis was induced redundantly by TNF-related apoptosis-inducing ligand (TRAIL) or Fas (CD95), suggesting that multiple death receptors mediate skin disease in the absence of linear ubiquitylation signals [145].
In contrast to HOIL-1- and HOIP-deficiency, mice deficient in SHARPIN (Sharpincpdm/cpdm) are viable but present with severe multi-organ inflammation and progressive dermatitis with alopecia that develops from 3–4 weeks of age [146]. Viability is attributed to the fact that, in the absence of SHARPIN, decoration of TNFR1 signalling components with linear ubiquitin chains is merely reduced, rather than absent [144]. Dermatitis in Sharpincpdm/cpdm mice is driven by TNF/TNFR signalling in keratinocytes and is dependent on the kinase activity of RIPK1, and also partly dependent on NLRP3 inflammasome-mediated IL-1β activity and IL-1 receptor signalling [19,113,140,147,148,149,150]. Caspase-8 heterozygosity significantly delays dermatitis onset, while co-deletion of FADD (in the skin) and RIPK3 was required to entirely ablate skin inflammation, implying that inflammation is primarily driven by apoptosis, with a minor role for necroptosis [113,151]. Interestingly, in line with the late-gestation lethality observed in HOIL-1- and HOIP-deficient mice, Sharpincpdm/cpdmRipk3−/−caspase-8−/− mice exhibited perinatal lethality due to defects in haematopoiesis [113,144]. Mirroring genetic studies, small-molecule RIPK1 inhibitor GNE684 therapy in Sharpincpdm/cpdm mice with established dermatitis reduced inflammation and caspase-3 cleavage in the skin [135,152].
As discussed above, the inhibitor of apoptosis proteins (cIAP1, cIAP2, and XIAP) also regulate cell fate in a RIPK1 and/or RIPK3-dependent manner. Loss of IAP activity in the skin, via injection of a pan-IAP-targeting Smac mimetic (SM), Compound A or 911, leads to more extensive skin lesions than those observed in Sharpincpdm/cpdm mice. Noting that disease manifestations were linked to TNFR1 and/or FasL-mediated apoptotic cell death and inflammation, with necroptosis appearing to drive secondary damage [139]. Genetic loss of specific IAP family members was also found to trigger distinct skin phenotypes. For example, while cIap1K14.cre/K14.creXiap−/− mice develop a spongiotic dermatitis with psoriasis-like features affecting the ears and face from around 10 weeks of age, cIap1K14.cre/K14.crecIap2−/− mice become moribund at postpartum day 10 due to widespread dermatoses that, like the Sharpincpdm/cpdm mice, are prevented by Ripk1 heterozygosity [139]. In humans, IAPs are abundantly expressed in the skin and their levels are potently reduced by SM administration [153], so one could envisage unwanted inflammation could present during systemic therapies. However, SM have in general been well tolerated during Phase1/II clinical trials for haematological and solid cancer malignancies; with only high doses inducing adverse events (e.g., cytokine release syndrome, diarrhoea, vomiting, fatigue, anorexia, and occasionally a pruritic rash [93,154]).
Neutrophilic dermatoses, such as Sweet’s syndrome and pyoderma gangrenosum, are often associated with mutant forms of protein tyrosine phosphatase-6 (PTPN6) that trigger cell death-induced tissue inflammation [155,156]. In mice, conditional deletion of Ptpn6 in neutrophils is sufficient to initiate a severe IL-1α/β-mediated cutaneous inflammatory disease driven by both caspase-8-dependent apoptotic and RIPK3/MLKL-dependent necroptotic signalling, suggesting that mutant forms of PTPN6 may fail to restrict RIPK1 pro-death activity [157,158]. Remarkably, genetic loss of RIPK1, or its kinase activity (Ripk1D138N), actually accelerated disease onset in this context, suggesting divergent signalling in neutrophilic dermatoses compared to psoriasiform conditions caused by LUBAC and IAP mutations [158].
Despite genetic evidence that the IAPs and RIPK1 can repress inflammatory skin lesions, studies into RIPK1 and RIPK3 function in the context of psoriasis are limited. RIPK1 expression was reported to be decreased in lesions of plaque-type psoriasis patients, thereby sensitizing keratinocytes to TRAIL. Correspondingly, TRAIL neutralisation in an imiquimod (IMQ)-induced murine model of psoriasis improved skin inflammation and led to a decrease in inflammatory cytokines [159]. In contrast, a more recent study observed increased levels of RIPK1, RIPK3, MLKL, and phosphorylated (p)MLKL in psoriatic lesions from patients, suggesting that the skin pathology may be driven by necroptosis [160]. Consistent with this notion, treatment with the RIPK1 inhibitor, Nec-1s, or the MLKL inhibitor necrosulfonamide (NSA) reduced IMQ-induced psoriasis, with the caveats that NSA targets GSDMD rather than MLKL in mice, and the fact that a further study found no role genetically for the kinase activity of RIPK1 or RIPK3 [161,162]. Overall, the complexity of RIPK1’s role in skin inflammation suggests that caution should be taken when targeting RIPK1 in all hyperinflammatory disorders, or that rationally designed drugs targeting other cell death machinery may have merit [158]. Encouragingly, a recent Phase II clinical trial using RIPK1 inhibitor GSK2982772 in plaque-type psoriasis has shown therapeutic benefit but there is still a lack of data surrounding RIPK1 inhibitors and their long-term use in the clinic [163,164].
5. Gut—Inflammatory Bowel Disease
Inflammatory bowel disease (IBD), which includes the chronic relapsing inflammatory disorders Crohn’s Disease (CD) and ulcerative colitis (UC), is characterised by intestinal inflammation and epithelial cell injury affecting the gastrointestinal (GI) lining [165]. The GI tract harbours an enormous bacterial load and disruption of mucosal barrier integrity due to aberrant intestinal epithelial cell (IEC) death allows luminal bacteria to enter the lamina propria and trigger inflammation [166,167,168].
Germline loss-of-function mutations in XIAP pre-dispose males to very-early-onset (VEO)-IBD [169,170,171]. Additionally, approximately 50–70% of patients with X-linked lymphoproliferative disease 2 (XLP2), caused by mutations that limit XIAP expression/function, develop hemophagocytic lymphohistiocytosis (HLH) with symptoms that include hyperinflammation and early-onset chronic haemorrhagic colitis [172,173,174,175]. In addition to its critical role in suppressing ripoptosome formation and subsequent NLRP3 inflammasome activation in innate immune cells [45,46,47,48,49,78], XIAP also ubiquitylates the nucleotide-binding oligomerisation domain (NOD)-2 adaptor, RIPK2 [176]. NOD2 responds to the bacterial cell wall component, muramyl dipeptide (MDP), and signalling via this pathway has been implicated in IBD pathogenesis [177,178,179]. The fact that both XLP2 and very early-onset (VEO)-IBD XIAP variants confer increased sensitivity to cell death [180] suggests the possibility of a combined role for perturbed NOD2-RIPK2 signalling and RIPK1-mediated cell death in IBD pathogenesis.
TNFR1 signalling is unequivocally connected with IBD pathogenesis, as best demonstrated by the wide-spread use of anti-TNF therapeutics in IBD patients [181,182], and the clinical benefits observed in pre-clinical IBD models using drugs targeting downstream TLR/TNFR1 inflammatory signalling molecules [183]. Interestingly, despite a clear role for TNFR1 complex I signalling, evidence now suggests that TNFR1-mediated cell death also contributes to IBD (Table 1). Mutations in mice that derepress RIPK1-dependent apoptotic and/or necroptotic IEC death elicit severe chronic intestinal inflammation, akin to that seen in IBD in humans. For example, loss of IKK complex component NEMO in IECs (Villin cre) results in spontaneous colitis mediated by TNFR1 signalling and RIPK1 kinase-dependent cell death [135,184,185], while IKKβ protects against colitis induced by Clostridium difficile or dextran sodium sulphate (DSS) [186,187]. Loss of upstream TAK1 in IECs triggers TNFR1-dependent (presumably RIPK1-dependent) apoptosis and intestinal inflammation, although colitis and ileitis ultimately develop in the absence of TNFR1 [188]. Whilst deficiencies in IKK or NF-κB have not yet been reported in human IBD patients, patients with ecto-dermal dysplasia with immunodeficiency (EDA-ID) caused by hypomorphic NEMO mutations often suffer from colitis [189,190,191,192]. In addition, TNFAIP3, which encodes A20, is a susceptibility locus for CD and UC in humans [193]. Exactly how A20 expression contributes to IBD development is still unclear but, in contrast to other spontaneous diseases caused by conditional A20 loss, deletion of A20 in both IECs and myeloid cells is required to induce spontaneous colitis and ileitis, as well as apoptosis of crypt cells [194,195]. Different groups have also reported that overexpression of A20 in IECs is protective in lipopolysaccharide (LPS) and DSS-induced colitis models [196,197] but promotes RIPK1-dependent IEC death in response to TNF [198].
Both apoptotic caspase-8 and its adaptor protein FADD have been revealed to be vital to maintaining intestinal homeostasis [43,44,199,200]. Mice lacking FADD in IECs (FaddVillin.cre) develop severe colitis and ileitis, as well as Paneth cell loss [201]. Caspase-8Villin.cre mice also present with ileitis and Paneth cell loss, but colitis only develops in the presence of certain microbiota [202,203]. Colitis in both the FaddVillin.cre and Caspase-8Villin.cre mice is caused by RIPK1 kinase activity-dependent IEC necroptosis and is primarily mediated by TNFR1, with a minor role for ZBP1 [201,204,205]. In contrast, TNFR1 and ZBP1 act redundantly to trigger ileitis in the Caspase-8Villin.cre and FaddVillin.cre mice, which is also driven by necroptotic IEC death. It is worth noting that FADD-deficient IECs can also reportedly utilise RIPK3 scaffolding to undergo caspase-8-mediated apoptosis and a ‘pyroptotic-like’ death [204]. Surprisingly, humans with loss-of-function mutations in the CASP8 gene develop VEO-IBD that is refractory to TNF treatment [206], suggesting that ZBP1 (or possibly TLR signalling) may also drive intestinal inflammation in human patients. The importance of RIPK1 kinase activity in inflammation arising from loss FADD or caspase-8 loss underscores the therapeutic potential of targeting the kinase activity of RIPK1 in CASP8 mutant patients with VEO-IBD.
RIPK1 has a critical pro-survival scaffolding role and patients with biallelic mutations in RIPK1 present with early-onset IBD involving the upper and lower GI tract that is triggered by environmental insults [98,99,207]. It is clear from these studies that RIPK1-deficient human cells have aberrant TNFR and TLR signalling responses and a potential increase in the number of apoptotic bodies within crypts. However, how RIPK1 regulates intestinal homeostasis (i.e., IEC death versus cytokine production) remains ill defined. Interestingly, the presence of necrotic lesions in CD patients [202,208], combined with the fact that elevated pRIPK1, pRIPK3, and pMLKL levels in the terminal ileum and colon correlate with severe disease in both CD and UC patients [209,210,211,212], indicates that RIPK1-dependent necroptosis may contribute to pathology in human IBD. Indeed, pharmacological inhibition of RIPK1 kinase activity has been shown to be effective in the murine CD4+CD45RBHI T-cell transfer model of colitis and provided dose-dependent protection from inflammatory cell death in NemoVillin.cre/+ mice [213]. Yet, curiously, despite some groups reporting that MLKL-deficient mice are protected from DSS-induced colitis and colitis-associated tumourigenesis [214,215], other studies, including those using littermate control animals, found little-to-no role for RIPK1 kinase, RIPK3- or MLKL-dependent necroptotic signalling in DSS-induced colitis and/or colorectal cancer [162,216]. Nonetheless, RIPK1 may still mediate deleterious non-necroptotic signalling in IBD and other related disorders. In fact, pre-clinical studies have shown that the small-molecule RIPK1 kinase inhibitor, GSK2982772, blocks spontaneous cytokine production in explants derived from UC patients and reduces inflammation in a DSS-induced colitis model in mice [164,217]. It will be interesting to see the published results from a recently completed Phase II clinical trial examining the efficacy of GSK2982772 in UC patients [NCT02903966].
6. Conclusions
This review gives an overview of the role of RIPK1 and RIPK3 activity in inflammatory diseases associated with the skin, gut, lung, and joints. RIPK1 and RIPK3 are central regulators of inflammatory signalling and control multiple modalities of cell death downstream of death receptor and TLR ligation. As such, perturbations in RIPK-dependent signalling networks are a feature of many auto-inflammatory and chronic inflammatory disorders. Existing therapeutics largely focus on targeting pro-inflammatory cytokines, such as TNF, IL-1β, and IL-6. However, many patients experience treatment failures or severe adverse reactions, supporting the need for new drug development in this space. Small-molecule RIP kinase inhibitors are now emerging in the clinic. Although a large number of pre-clinical studies have shown that targeting RIPK1 kinase activity limits deleterious inflammation in models of acute and chronic inflammation [101], early results from Phase I/II clinical trials with RIPK1 kinase inhibitors have revealed that, although well tolerated, they may not hold up to their full therapeutic promise [136]. As such, much more information is required in order to fully understand the multi-faceted roles these kinases play in regulating cell fate and innate immunity during homeostasis, as well as during infectious and inflammatory disease in humans. Ultimately, this knowledge will enable better rational drug design and the development of co-therapeutic strategies that maximise clinical benefit whilst ameliorating the risk of treatment failures and unwanted side-effects.
Author Contributions
Writing—original draft preparation, M.S.; T.M.D.; K.E.L.; writing—review and editing, S.A.C. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the Australian National Health and Medical Research Coun-cil (NHMRC) through Project and Ideas grants to KEL (1145788, 1162765, 1181089) and a CJ Martin Biomedical Training Fellowship to SAC (1144014). KEL is an Australian Research Council (ARC) Future Fellow (FT190100266). This work was supported by operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASC | apoptosis-associated speck-like containing a CARD |
| CAIA | collagen antibody-induced arthritis |
| CAPS | cryopyrin-associated periodic syndromes |
| CARD | caspase activation and recruitment domain |
| caspase | cysteine-dependent aspartic acid-specific protease |
| CD | Crohn’s Disease |
| cFLIP | cellular FLICE-like inhibitory protein |
| cIAP | cellular inhibitor of apoptosis protein |
| CRIA | cleavage-resistant RIPK1-induced autoinflammatory syndrome |
| CYLD | cylindromatosis |
| DAI | DNA-dependent activator of IFN regulatory transcription factors |
| DAMP | damage-associated molecular pattern |
| DNA | deoxyribonucleic acid |
| EDA-ID | ecto-dermal dysplasia with immunodeficiency |
| ER | endoplasmic reticulum |
| GI | gastrointestinal |
| GSDMD | gasdermin D |
| HA20 | haploinsufficiency in A20 |
| HLH | hemophagocytic lymphohistiocytosis |
| HMGB1 | high-mobility group box 1 |
| HOIL-1 | haeme-oxidized IRP2 ubiquitin ligase 1 |
| HOIP | HOIL1-interacting protein |
| HSP | heat shock protein |
| IBD | inflammatory bowel disease |
| IAV | Influenza A virus |
| IEC | intestinal epithelial cell |
| IFN | interferon |
| IL | interleukin |
| IMQ | imiquimod |
| LOF | loss of function |
| LRR | leucine-rich repeat |
| LUBAC | linear ubiquitin chain assembly complex |
| MAPK | mitogen-activated protein kinase |
| MDP | muramyl dipeptide |
| MLKL | mixed lineage kinase domain-like |
| NEMO | NF-κB essential modulator (IKK) |
| NF-κB | nuclear factor-kappa B |
| NLR | NOD-like receptor |
| NLRP3 | NOD-like protein receptor 3 |
| NOD | nucleotide-binding oligomerization domain |
| NSA | necrosulfonamide |
| PAMP | pathogen-associated molecular pattern |
| PRR | pattern recognition receptor |
| RA | rheumatoid arthritis |
| RIPK | receptor-interacting protein kinase |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| RSV | respiratory syncytial virus |
| SHARPIN | SHANK-associated RH domain-interacting protein |
| PTPN6 | protein tyrosine phosphatase-6 |
| SMAC | second mitochondrial activator of caspases |
| SM | Smac mimetic |
| TAK1 | transforming growth factor—activated kinase |
| TLR | Toll-like receptor |
| TNF | tumour necrosis factor |
| TNFR | tumour necrosis factor receptor |
| TRADD | TNFR1-associated death domain protein |
| TRAF2 | TNF receptor-associated factor 2 |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TRAPS | TNFR-associated periodic syndrome |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| UC | ulcerative colitis |
| VEO | very-early onset |
| XIAP | X-linked inhibitor of apoptosis protein |
| XPL2 | X-linked lymphoproliferative disease 2 |
| ZBP1 | Z-DNA binding protein 1 |
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Humphries, F.; Yang, S.; Wang, B.; Moynagh, P.N. RIP kinases: Key decision makers in cell death and innate immunity. Cell Death Differ. 2015, 22, 225–236. [Google Scholar] [CrossRef]
- He, S.; Wang, X. RIP kinases as modulators of inflammation and immunity. Nat. Immunol. 2018, 19, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Eng, V.V.; Wemyss, M.A.; Pearson, J.S. The diverse roles of RIP kinases in host-pathogen interactions. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Meylan, E.; Burns, K.; Hofmann, K.; Blancheteau, V.; Martinon, F.; Kelliher, M.; Tschopp, J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat. Immunol. 2004, 5, 503–507. [Google Scholar] [CrossRef]
- Kelliher, M.A.; Grimm, S.; Ishida, Y.; Kuo, F.; Stanger, B.Z.; Leder, P. The Death Domain Kinase RIP Mediates the TNF-Induced NF-kB Signal. Immunity 1998, 8, 297–303. [Google Scholar] [CrossRef]
- Ting, A.T.; Pimentel-Muinos, F.X.; Seed, B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996, 15, 6189–6196. [Google Scholar] [CrossRef] [PubMed]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef]
- Hsu, H.; Huang, J.; Shu, H.B.; Baichwal, V.; Goeddel, D.V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 1996, 4, 387–396. [Google Scholar] [CrossRef]
- Park, Y.H.; Jeong, M.S.; Park, H.H.; Jang, S.B. Formation of the death domain complex between FADD and RIP1 proteins In Vitro. Biochim. Biophys. Acta 2013, 1834, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Stanger, B.Z.; Leder, P.; Lee, T.H.; Kim, E.; Seed, B. RIP: A novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 1995, 81, 513–523. [Google Scholar] [CrossRef]
- Sun, X.; Yin, J.; Starovasnik, M.A.; Fairbrother, W.J.; Dixit, V.M. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J. Biol. Chem. 2002, 277, 9505–9511. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Offermann, M.K. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J. Immunol. 2005, 174, 4942–4952. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.J.; Upton, J.W.; Mocarski, E.S. Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 2008, 181, 6427–6434. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Xiong, J.; Goeddel, D.V. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 1995, 81, 495–504. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF Receptor I-Mediated Apoptosis via Two Sequential Signaling Complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Shu, H.B.; Takeuchi, M.; Goeddel, D.V. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc. Natl. Acad. Sci. USA 1996, 93. [Google Scholar] [CrossRef]
- Vince, J.E.; Pantaki, D.; Feltham, R.; Mace, P.D.; Cordier, S.M.; Schmukle, A.C.; Davidson, A.J.; Callus, B.A.; Wong, W.W.; Gentle, I.E.; et al. TRAF2 Must Bind to Cellular Inhibitors of Apoptosis for Tumor Necrosis Factor (TNF) to Efficiently Activate NF-kB and to Prevent TNF-induced Apoptosis. J. Biol. Chem. 2009, 284, 35906–35915. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, B.; Cordier, S.M.; Schmukle, A.C.; Emmerich, C.H.; Rieser, E.; Haas, T.L.; Webb, A.I.; Rickard, J.A.; Anderton, H.; Wong, W.W.; et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 2011, 471, 591–596. [Google Scholar] [CrossRef]
- Haas, T.L.; Emmerich, C.H.; Gerlach, B.; Schmukle, A.C.; Cordier, S.M.; Rieser, E.; Feltham, R.; Vince, J.; Warnken, U.; Wenger, T.; et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 2009, 36, 831–844. [Google Scholar] [CrossRef]
- Tokunaga, F.; Nakagawa, T.; Nakahara, M.; Saeki, Y.; Taniguchi, M.; Sakata, S.; Tanaka, K.; Nakano, H.; Iwai, K. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature 2011, 471, 633–636. [Google Scholar] [CrossRef]
- Liu, Z.; Chan, F.K. Regulatory mechanisms of RIPK1 in cell death and inflammation. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Simpson, D.S.; Gabrielyan, A.; Feltham, R. RIPK1 ubiquitination: Evidence, correlations and the undefined. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Jaco, I.; Annibaldi, A.; Lalaoui, N.; Wilson, R.; Tenev, T.; Laurien, L.; Kim, C.; Jamal, K.; Wicky John, S.; Liccardi, G.; et al. MK2 Phosphorylates RIPK1 to Prevent TNF-Induced Cell Death. Mol. Cell 2017, 66, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, F.; Wang, X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008, 133, 693–703. [Google Scholar] [CrossRef]
- Hitomi, J.; Christofferson, D.E.; Ng, A.; Yao, J.; Degterev, A.; Xavier, R.J.; Yuan, J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008, 135, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Dynek, J.N.; Goncharov, T.; Dueber, E.C.; Fedorova, A.V.; Izrael-Tomasevic, A.; Phu, L.; Helgason, E.; Fairbrother, W.J.; Deshayes, K.; Kirkpatrick, D.S.; et al. c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J. 2010, 29, 4198–4209. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Aguileta, M.A.; Goossens, V.; Dubuisson, C.; Grootjans, S.; Dejardin, E.; Vandenabeele, P.; Bertrand, M.J. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013, 20, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, Y.; Jouan-Lanhouet, S.; Divert, T.; Theatre, E.; Bertin, J.; Gough, P.J.; Giansanti, P.; Heck, A.J.; Dejardin, E.; Vandenabeele, P.; et al. NF-kappaB-Independent Role of IKKalpha/IKKbeta in Preventing RIPK1 Kinase-Dependent Apoptotic and Necroptotic Cell Death during TNF Signaling. Mol. Cell 2015, 60, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Dannappel, M.; Vlantis, K.; Kumari, S.; Polykratis, A.; Kim, C.; Wachsmuth, L.; Eftychi, C.; Lin, J.; Corona, T.; Hermance, N.; et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014, 513, 90–94. [Google Scholar] [CrossRef]
- Dillon, C.P.; Weinlich, R.; Rodriguez, D.A.; Cripps, J.G.; Quarato, G.; Gurung, P.; Verbist, K.C.; Brewer, T.L.; Llambi, F.; Gong, Y.N.; et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 2014, 157, 1189–1202. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Daley-Bauer, L.P.; Thapa, R.J.; Mandal, P.; Berger, S.B.; Huang, C.; Sundararajan, A.; Guo, H.; Roback, L.; Speck, S.H.; et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc. Natl. Acad. Sci. USA 2014, 111, 7753–7758. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.J.; Cullen, S.P.; Clancy, D.; Martin, S.J. RIPK1 can function as an inhibitor rather than an initiator of RIPK3-dependent necroptosis. FEBS J. 2014, 281, 4921–4934. [Google Scholar] [CrossRef] [PubMed]
- Orozco, S.; Yatim, N.; Werner, M.R.; Tran, H.; Gunja, S.Y.; Tait, S.W.; Albert, M.L.; Green, D.R.; Oberst, A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014, 21, 1511–1521. [Google Scholar] [CrossRef]
- Rickard, J.A.; O’Donnell, J.A.; Evans, J.M.; Lalaoui, N.; Poh, A.R.; Rogers, T.; Vince, J.E.; Lawlor, K.E.; Ninnis, R.L.; Anderton, H.; et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 2014, 157, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Vereecke, L.; Bertrand, M.J.; Duprez, L.; Berger, S.B.; Divert, T.; Goncalves, A.; Sze, M.; Gilbert, B.; Kourula, S.; et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 2014, 513, 95–99. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Kellert, B.; Dimitrova, D.P.; Langlais, C.; Hupe, M.; Cain, K.; MacFarlane, M.; Hacker, G.; Leverkus, M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 2011, 43, 449–463. [Google Scholar] [CrossRef]
- Tenev, T.; Bianchi, K.; Darding, M.; Broemer, M.; Langlais, C.; Wallberg, F.; Zachariou, A.; Lopez, J.; MacFarlane, M.; Cain, K.; et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 2011, 43, 432–448. [Google Scholar] [CrossRef]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef]
- Verhagen, A.M.; Ekert, P.G.; Pakusch, M.; Silke, J.; Connolly, L.M.; Reid, G.E.; Moritz, R.L.; Simpson, R.J.; Vaux, D.L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000, 102, 43–53. [Google Scholar] [CrossRef]
- Mihaly, S.R.; Ninomiya-Tsuji, J.; Morioka, S. TAK1 control of cell death. Cell Death Differ. 2014, 21, 1667–1676. [Google Scholar] [CrossRef]
- Feltham, R.; Vince, J.E.; Lawlor, K.E. Caspase-8: Not so silently deadly. Clin. Transl. Immunol. 2017, 6, e124. [Google Scholar] [CrossRef]
- Oberst, A.; Dillon, C.P.; Weinlich, R.; McCormick, L.L.; Fitzgerald, P.; Pop, C.; Hakem, R.; Salvesen, G.S.; Green, D.R. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011, 471, 363–367. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Upton, J.W.; Long, A.B.; Livingston-Rosanoff, D.; Daley-Bauer, L.P.; Hakem, R.; Caspary, T.; Mocarski, E.S. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 2011, 471, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.E.; Khan, N.; Mildenhall, A.; Gerlic, M.; Croker, B.A.; D’Cruz, A.A.; Hall, C.; Kaur Spall, S.; Anderton, H.; Masters, S.L.; et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 2015, 6, 6282. [Google Scholar] [CrossRef] [PubMed]
- Yabal, M.; Muller, N.; Adler, H.; Knies, N.; Gross, C.J.; Damgaard, R.B.; Kanegane, H.; Ringelhan, M.; Kaufmann, T.; Heikenwalder, M.; et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014, 7, 1796–1808. [Google Scholar] [CrossRef]
- Wicki, S.; Gurzeler, U.; Wei-Lynn Wong, W.; Jost, P.J.; Bachmann, D.; Kaufmann, T. Loss of XIAP facilitates switch to TNFalpha-induced necroptosis in mouse neutrophils. Cell Death Dis. 2016, 7, e2422. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.E.; Feltham, R.; Yabal, M.; Conos, S.A.; Chen, K.W.; Ziehe, S.; Grass, C.; Zhan, Y.; Nguyen, T.A.; Hall, C.; et al. XIAP Loss Triggers RIPK3- and Caspase-8-Driven IL-1beta Activation and Cell Death as a Consequence of TLR-MyD88-Induced cIAP1-TRAF2 Degradation. Cell Rep. 2017, 20, 668–682. [Google Scholar] [CrossRef]
- Chen, K.W.; Lawlor, K.E.; von Pein, J.B.; Boucher, D.; Gerlic, M.; Croker, B.A.; Bezbradica, J.S.; Vince, J.E.; Schroder, K. Cutting Edge: Blockade of Inhibitor of Apoptosis Proteins Sensitizes Neutrophils to TNF- but Not Lipopolysaccharide-Mediated Cell Death and IL-1beta Secretion. J. Immunol. 2018, 200, 3341–3346. [Google Scholar] [CrossRef]
- He, S.; Liang, Y.; Shao, F.; Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl. Acad. Sci. USA 2011, 108, 20054–20059. [Google Scholar] [CrossRef]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef]
- Mompean, M.; Li, W.; Li, J.; Laage, S.; Siemer, A.B.; Bozkurt, G.; Wu, H.; McDermott, A.E. The Structure of the Necrosome RIPK1-RIPK3 Core, a Human Hetero-Amyloid Signaling Complex. Cell 2018, 173, 1244–1253. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Czabotar, P.E.; Hildebrand, J.M.; Lucet, I.S.; Zhang, J.G.; Alvarez-Diaz, S.; Lewis, R.; Lalaoui, N.; Metcalf, D.; Webb, A.I.; et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013, 39, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Jitkaew, S.; Zhao, J.; Chiang, H.C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.G.; Liu, Z.G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2014, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, Y.; Declercq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W.; et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014, 7, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.M.; Tanzer, M.C.; Lucet, I.S.; Young, S.N.; Spall, S.K.; Sharma, P.; Pierotti, C.; Garnier, J.M.; Dobson, R.C.; Webb, A.I.; et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl. Acad. Sci. USA 2014, 111, 15072–15077. [Google Scholar] [CrossRef] [PubMed]
- Shlomovitz, I.; Erlich, Z.; Speir, M.; Zargarian, S.; Baram, N.; Engler, M.; Edry-Botzer, L.; Munitz, A.; Croker, B.A.; Gerlic, M. Necroptosis directly induces the release of full-length biologically active IL-33 In Vitro and in an inflammatory disease model. FEBS J. 2019, 286, 507–522. [Google Scholar] [CrossRef]
- Newton, K.; Wickliffe, K.E.; Maltzman, A.; Dugger, D.L.; Strasser, A.; Pham, V.C.; Lill, J.R.; Roose-Girma, M.; Warming, S.; Solon, M.; et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature 2016, 540, 129–133. [Google Scholar] [CrossRef]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 2012, 11, 290–297. [Google Scholar] [CrossRef]
- Lin, J.; Kumari, S.; Kim, C.; Van, T.M.; Wachsmuth, L.; Polykratis, A.; Pasparakis, M. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 2016, 540, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Lawlor, K.E.; Murphy, J.M.; Vince, J.E. More to life than death: Molecular determinants of necroptotic and non-necroptotic RIP3 kinase signaling. Curr. Opin. Immunol. 2014, 26, 76–89. [Google Scholar] [CrossRef]
- Speir, M.; Lawlor, K.E. RIP-roaring inflammation: RIPK1 and RIPK3 driven NLRP3 inflammasome activation and autoinflammatory disease. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Gurung, P.; Anand, P.K.; Malireddi, R.K.; Vande Walle, L.; Van Opdenbosch, N.; Dillon, C.P.; Weinlich, R.; Green, D.R.; Lamkanfi, M.; Kanneganti, T.D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 2014, 192, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.; Lawlor, K.E.; Yu, E.C.; Mildenhall, A.L.; Moujalled, D.M.; Lewis, R.S.; Ke, F.; Mason, K.D.; White, M.J.; Stacey, K.J.; et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014, 15, 982–990. [Google Scholar] [CrossRef]
- Weng, D.; Marty-Roix, R.; Ganesan, S.; Proulx, M.K.; Vladimer, G.I.; Kaiser, W.J.; Mocarski, E.S.; Pouliot, K.; Chan, F.K.; Kelliher, M.A.; et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl. Acad. Sci. USA 2014, 111, 7391–7396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, C.; Zhang, H.; Zhao, Q.; Liu, Y.; Xu, C.; Xie, Q.; Wu, X.; Yu, X.; Zhang, J.; et al. MLKL and FADD Are Critical for Suppressing Progressive Lymphoproliferative Disease and Activating the NLRP3 Inflammasome. Cell Rep. 2016, 16, 3247–3259. [Google Scholar] [CrossRef]
- Philip, N.H.; DeLaney, A.; Peterson, L.W.; Santos-Marrero, M.; Grier, J.T.; Sun, Y.; Wynosky-Dolfi, M.A.; Zwack, E.E.; Hu, B.; Olsen, T.M.; et al. Activity of Uncleaved Caspase-8 Controls Anti-bacterial Immune Defense and TLR-Induced Cytokine Production Independent of Cell Death. PLoS Pathog. 2016, 12, e1005910. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Fernandes-Alnemri, T.; Rogers, C.; Mayes, L.; Wang, Y.; Dillon, C.; Roback, L.; Kaiser, W.; Oberst, A.; Sagara, J.; et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 2015, 6, 7515. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Wickliffe, K.E.; Maltzman, A.; Dugger, D.L.; Reja, R.; Zhang, Y.; Roose-Girma, M.; Modrusan, Z.; Sagolla, M.S.; Webster, J.D.; et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature 2019, 575, 679–682. [Google Scholar] [CrossRef]
- Antonopoulos, C.; Russo, H.M.; El Sanadi, C.; Martin, B.N.; Li, X.; Kaiser, W.J.; Mocarski, E.S.; Dubyak, G.R. Caspase-8 as an Effector and Regulator of NLRP3 Inflammasome Signaling. J. Biol. Chem. 2015, 290, 20167–20184. [Google Scholar] [CrossRef]
- Sagulenko, V.; Thygesen, S.J.; Sester, D.P.; Idris, A.; Cridland, J.A.; Vajjhala, P.R.; Roberts, T.L.; Schroder, K.; Vince, J.E.; Hill, J.M.; et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013, 20, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.S.; Gross, C.J.; Dreier, R.F.; Saller, B.S.; Mishra, R.; Gorka, O.; Heilig, R.; Meunier, E.; Dick, M.S.; Cikovic, T.; et al. The Inflammasome Drives GSDMD-Independent Secondary Pyroptosis and IL-1 Release in the Absence of Caspase-1 Protease Activity. Cell Rep. 2017, 21, 3846–3859. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Schmidt, T.; Schmid-Burgk, J.L.; Rapino, F.; Robertson, A.A.; Cooper, M.A.; Graf, T.; Hornung, V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 2016, 44, 833–846. [Google Scholar] [CrossRef]
- Moriwaki, K.; Bertin, J.; Gough, P.J.; Chan, F.K. A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. J. Immunol. 2015, 194, 1938–1944. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Delanghe, T.; Priem, D.; Wynosky-Dolfi, M.A.; Sorobetea, D.; Rojas-Rivera, D.; Giansanti, P.; Roelandt, R.; Gropengiesser, J.; Ruckdeschel, K.; et al. Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat. Commun. 2019, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.B.; Gropengiesser, J.; Fischer, J.; Novikova, L.; Deuretzbacher, A.; Lafera, J.; Schimmeck, H.; Czymmeck, N.; Ronkina, N.; Kotlyarov, A.; et al. p38(MAPK)/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection. Nat. Cell Biol. 2017, 19, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.E.; Wong, W.W.; Gentle, I.; Lawlor, K.E.; Allam, R.; O’Reilly, L.; Mason, K.; Gross, O.; Ma, S.; Guarda, G.; et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 2012, 36, 215–227. [Google Scholar] [CrossRef]
- Orning, P.; Weng, D.; Starheim, K.; Ratner, D.; Best, Z.; Lee, B.; Brooks, A.; Xia, S.; Wu, H.; Kelliher, M.A.; et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 2018, 362, 1064–1069. [Google Scholar] [CrossRef]
- Malireddi, R.K.S.; Gurung, P.; Mavuluri, J.; Dasari, T.K.; Klco, J.M.; Chi, H.; Kanneganti, T.D. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J. Exp. Med. 2018, 215, 1023–1034. [Google Scholar] [CrossRef]
- Duong, B.H.; Onizawa, M.; Oses-Prieto, J.A.; Advincula, R.; Burlingame, A.; Malynn, B.A.; Ma, A. A20 restricts ubiquitination of pro-interleukin-1beta protein complexes and suppresses NLRP3 inflammasome activity. Immunity 2015, 42, 55–67. [Google Scholar] [CrossRef]
- Vande Walle, L.; Van Opdenbosch, N.; Jacques, P.; Fossoul, A.; Verheugen, E.; Vogel, P.; Beyaert, R.; Elewaut, D.; Kanneganti, T.D.; van Loo, G.; et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 2014, 512, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Gurung, P.; Kesavardhana, S.; Samir, P.; Burton, A.; Mummareddy, H.; Vogel, P.; Pelletier, S.; Burgula, S.; Kanneganti, T.D. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Zheng, M.; Williams, E.P.; Malireddi, R.K.S.; Karki, R.; Banoth, B.; Burton, A.; Webby, R.; Channappanavar, R.; Jonsson, C.B.; Kanneganti, T.D. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J. Biol. Chem. 2020, 295, 14040–14052. [Google Scholar] [CrossRef]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef]
- Conos, S.A.; Chen, K.W.; De Nardo, D.; Hara, H.; Whitehead, L.; Núñez, G.; Masters, S.L.; Murphy, J.M.; Schroder, K.; Vaux, D.L.; et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl. Acad. Sci. USA 2017, 114, E961–E969. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.B.; Yang, S.H.; Toth, B.; Kovalenko, A.; Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 2013, 38, 27–40. [Google Scholar] [CrossRef]
- Knop, J.; Spilgies, L.M.; Rufli, S.; Reinhart, R.; Vasilikos, L.; Yabal, M.; Owsley, E.; Jost, P.J.; Marsh, R.A.; Wajant, H.; et al. TNFR2 induced priming of the inflammasome leads to a RIPK1-dependent cell death in the absence of XIAP. Cell Death Dis. 2019, 10, 700. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Blauvelt, A.; Thaci, D.; Leonardi, C.L.; Poulin, Y.; Drew, J.; Peterson, L.; Arendt, C.; Burge, D.; Reich, K. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J. Am. Acad. Dermatol. 2018, 79, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Blauvelt, A.; Paul, C.; Sofen, H.; Weglowska, J.; Piguet, V.; Burge, D.; Rolleri, R.; Drew, J.; Peterson, L.; et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: Results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). J. Am. Acad. Dermatol. 2018, 79, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, P.S.; Bissonnette, R.; Teixeira, H.D.; Valdecantos, W.C. Systematic review of efficacy of anti-tumor necrosis factor (TNF) therapy in patients with psoriasis previously treated with a different anti-TNF agent. J. Am. Acad. Dermatol. 2016, 75, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Seidelin, J.B.; LaCasse, E.C.; Nielson, O.H. SMAC mimetics and RIPK inhibitors as therapeutics for chronic inflammatory diseases. Sci. Signal. 2020, 13, eaax8295. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Hofmans, S.; Declercq, W.; Augustyns, K.; Vandenabeele, P. Inhibitors Targeting RIPK1/RIPK3: Old and New Drugs. Trends Pharmacol. Sci. 2020, 41, 209–224. [Google Scholar] [CrossRef]
- Mandal, P.; Berger, S.B.; Pillay, S.; Moriwaki, K.; Huang, C.; Guo, H.; Lich, J.D.; Finger, J.; Kasparcova, V.; Votta, B.; et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 2014, 56, 481–495. [Google Scholar] [CrossRef]
- Newton, K.; Dugger, D.L.; Wickliffe, K.E.; Kapoor, N. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 2014, 343, 1357–1360. [Google Scholar] [CrossRef]
- Polykratis, A.; Hermance, N.; Zelic, M.; Roderick, J.; Kim, C.; Van, T.M.; Lee, T.H.; Chan, F.K.M.; Pasparakis, M.; Kelliher, M.A. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 2014, 193, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Cuchet-Lourenco, D.; Eletto, D.; Wu, C.; Plagnol, V.; Papapietro, O.; Curtis, J.; Ceron-Gutierrez, L.; Bacon, C.M.; Hackett, S.; Alsaleem, B.; et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science 2018, 361, 810–813. [Google Scholar] [CrossRef]
- Li, Y.; Fuhrer, M.; Bahrami, E.; Socha, P.; Klaudel-Dreszler, M.; Bouzidi, A.; Liu, Y.; Lehle, A.S.; Magg, T.; Hollizeck, S.; et al. Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 970–975. [Google Scholar] [CrossRef]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef]
- Mifflin, L.; Ofengeim, D.; Yuan, J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat. Rev. Drug Discov. 2020, 19, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Weisel, K.; Scott, N.E.; Tompson, D.J.; Votta, B.J.; Madhavan, S.; Povey, K.; Wolstenholme, A.; Simeoni, M.; Rudo, T.; Richards-Peterson, L.; et al. Randomized clinical study of safety, pharmacokinetics, and pharmacodynamics of RIPK1 inhibitor GSK2982772 in healthy volunteers. Pharmacol. Res. Perspect. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Aksentijevich, I.; Galon, J.; Soares, M.; Mansfield, E.; Hull, K.; Oh, H.; Goldbach-Mansky, R.; Dean, B.; Athreya, A.; Reginato, J.; et al. The tumor-necrosis-factor receptor-associated periodic syndrome: New mutations in TNFRSF1A, ancestral origins, genotype-phenotype studies, and evidence for further genetic heterogeneity of periodic fevers. Am. J. Hum. Genet. 2001, 69, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Razaghian, A.; Alimadadi, H.; Shiari, R.; Shahrooei, M.; Parvaneh, N. Different phenotypes of the same XIAP mutation in a family: A case of XIAP deficiency with juvenile idiopathic arthritis. Pediatr. Blood Cancer 2019, 66, e27593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yu, X.; Demirkaya, E.; Deuitch, N.; Stone, D.; Tsai, W.L.; Kuehn, H.S.; Wang, H.; Yang, D.; Park, Y.H.; et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc. Natl. Acad. Sci. USA 2016, 113, 10127–10132. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Beck, D.B.; Kuehn, H.S.; Sampaio Moura, N.; Hoffmann, P.; Ibarra, M.; Stoddard, J.; Tsai, W.L.; Gutierrez-Cruz, G.; Gadina, M.; et al. Second Case of HOIP Deficiency Expands Clinical Features and Defines Inflammatory Transcriptome Regulated by LUBAC. Front. Immunol. 2019, 10, 479. [Google Scholar] [CrossRef]
- Damgaard, R.B.; Walker, J.A.; Marco-Casanova, P.; Morgan, N.V.; Titheradge, H.L.; Elliott, P.R.; McHale, D.; Maher, E.R.; McKenzie, A.N.J.; Komander, D. The Deubiquitinase OTULIN Is an Essential Negative Regulator of Inflammation and Autoimmunity. Cell 2016, 166, 1215–1230. [Google Scholar] [CrossRef]
- Tsuchida, N.; Kirino, Y.; Soejima, Y.; Onodera, M.; Arai, K.; Tamura, E.; Ishikawa, T.; Kawai, T.; Uchiyama, T.; Nomura, S.; et al. Haploinsufficiency of A20 caused by a novel nonsense variant or entire deletion of TNFAIP3 is clinically distinct from Behcet’s disease. Arthritis Res. Ther. 2019, 21, 137. [Google Scholar] [CrossRef]
- Moulin, M.; Anderton, H.; Voss, A.K.; Thomas, T.; Wong, W.W.; Bankovacki, A.; Feltham, R.; Chau, D.; Cook, W.D.; Silke, J.; et al. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 2012, 31, 1679–1691. [Google Scholar] [CrossRef]
- Onizawa, M.; Oshima, S.; Schulze-Topphoff, U.; Oses-Prieto, J.A.; Lu, T.; Tavares, R.; Prodhomme, T.; Duong, B.; Whang, M.I.; Advincula, R.; et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat. Immunol. 2015, 16, 618–627. [Google Scholar] [CrossRef]
- Turer, E.E.; Tavares, R.M.; Mortier, E.; Hitotsumatsu, O.; Advincula, R.; Lee, B.; Shifrin, N.; Malynn, B.A.; Ma, A. Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J. Exp. Med. 2008, 205, 451–464. [Google Scholar] [CrossRef]
- Peltzer, N.; Rieser, E.; Taraborrelli, L.; Draber, P.; Darding, M.; Pernaute, B.; Shimizu, Y.; Sarr, A.; Draberova, H.; Montinaro, A.; et al. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Rep. 2014, 9, 153–165. [Google Scholar] [CrossRef]
- Rickard, J.A.; Anderton, H.; Etemadi, N.; Nachbur, U.; Darding, M.; Peltzer, N.; Lalaoui, N.; Lawlor, K.E.; Vanyai, H.; Hall, C.; et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, B.E. Immunotherapeutic Biologic Agents in Autoimmune and Autoinflammatory Diseases. Immunol. Investig. 2015, 44, 777–802. [Google Scholar] [CrossRef]
- Matsumoto, S.; Müller-Ladner, U.; Gay, R.E.; Nishioka, K.; Gay, S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J. Rheumatol. 1996, 23, 1345–1352. [Google Scholar] [PubMed]
- Liu, H.; Huang, Q.; Shi, B.; Eksarko, P.; Temkin, V.; Pope, R.M. Regulation of Mcl-1 expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2006, 54, 3174–3181. [Google Scholar] [CrossRef] [PubMed]
- Dharmapatni, A.A.; Smith, M.D.; Findlay, D.M.; Holding, C.A.; Evdokiou, A.; Ahern, M.J.; Weedon, H.; Chen, P.; Screaton, G.; Xu, X.N.; et al. Elevated expression of caspase-3 inhibitors, survivin and xIAP correlates with low levels of apoptosis in active rheumatoid synovium. Arthritis Res. Ther. 2009, 11, R13. [Google Scholar] [CrossRef]
- Bai, S.; Liu, H.; Chen, K.H.; Eksarko, P.; Perlman, H.; Moore, T.L.; Pope, R.M. NF-kappaB-regulated expression of cellular FLIP protects rheumatoid arthritis synovial fibroblasts from tumor necrosis factor alpha-mediated apoptosis. Arthritis Rheum. 2004, 50, 3844–3855. [Google Scholar] [CrossRef]
- Liu, H.; Eksarko, P.; Temkin, V.; Haines, G.K., 3rd; Perlman, H.; Koch, A.E.; Thimmapaya, B.; Pope, R.M. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J. Immunol. 2005, 175, 8337–8345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rosloniec, E.; Price, J.; Boothby, M.; Chen, J. Constitutive expression of BCL-X(L) in the T lineage attenuates collagen-induced arthritis in Bcl-X(L) transgenic mice. Arthritis Rheum. 2002, 46, 514–521. [Google Scholar] [CrossRef]
- Zheng, B.; Marinova, E.; Switzer, K.; Wansley, D.; He, H.; Bheekha-Escura, R.; Behrens, T.W.; Han, S. Overexpression of Bcl(XL) in B cells promotes Th1 response and exacerbates collagen-induced arthritis. J. Immunol. 2007, 179, 7087–7092. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.E.; van Nieuwenhuijze, A.; Parker, K.L.; Drake, S.F.; Campbell, I.K.; Smith, S.D.; Vince, J.E.; Strasser, A.; Wicks, I.P. Bcl-2 overexpression ameliorates immune complex-mediated arthritis by altering FcgammaRIIb expression and monocyte homeostasis. J. Leukoc. Biol. 2013, 93, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Q.; Birkett, R.; Koessler, R.E.; Cuda, C.M.; Haines, G.K., 3rd; Jin, J.P.; Perlman, H.; Pope, R.M. Fas signaling in macrophages promotes chronicity in K/BxN serum-induced arthritis. Arthritis Rheumatol. 2014, 66, 68–77. [Google Scholar] [CrossRef]
- Bardwell, P.D.; Gu, J.; McCarthy, D.; Wallace, C.; Bryant, S.; Goess, C.; Mathieu, S.; Grinnell, C.; Erickson, J.; Rosenberg, S.H.; et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J. Immunol. 2009, 182, 7482–7489. [Google Scholar] [CrossRef] [PubMed]
- Dharmapatni, A.A.; Cantley, M.D.; Marino, V.; Perilli, E.; Crotti, T.N.; Smith, M.D.; Haynes, D.R. The X-Linked Inhibitor of Apoptosis Protein Inhibitor Embelin Suppresses Inflammation and Bone Erosion in Collagen Antibody Induced Arthritis Mice. Mediat. Inflamm. 2015, 2015, 564042. [Google Scholar] [CrossRef]
- Scatizzi, J.C.; Hutcheson, J.; Pope, R.M.; Firestein, G.S.; Koch, A.E.; Mavers, M.; Smason, A.; Agrawal, H.; Haines, G.K., 3rd; Chandel, N.S.; et al. Bim-Bcl-2 homology 3 mimetic therapy is effective at suppressing inflammatory arthritis through the activation of myeloid cell apoptosis. Arthritis Rheum. 2010, 62, 441–451. [Google Scholar] [CrossRef]
- Park, J.S.; Oh, Y.; Park, O.; Foss, C.A.; Lim, S.M.; Jo, D.G.; Na, D.H.; Pomper, M.G.; Lee, K.C.; Lee, S. PEGylated TRAIL ameliorates experimental inflammatory arthritis by regulation of Th17 cells and regulatory T cells. J. Control. Release 2017, 267, 163–171. [Google Scholar] [CrossRef]
- Matmati, M.; Jacques, P.; Maelfait, J.; Verheugen, E.; Kool, M.; Sze, M.; Geboes, L.; Louagie, E.; Mc Guire, C.; Vereecke, L.; et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat. Genet. 2011, 43, 908–912. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Perlman, H.; Birkett, R.; Doyle, R.; Fang, D.; Haines, G.K.; Robinson, W.; Datta, S.; Huang, Z.; Li, Q.Z.; et al. CD11c-mediated deletion of Flip promotes autoreactivity and inflammatory arthritis. Nat. Commun. 2015, 6, 7086. [Google Scholar] [CrossRef]
- Polykratis, A.; Martens, A.; Eren, R.O.; Shirasaki, Y.; Yamagishi, M.; Yamaguchi, Y.; Uemura, S.; Miura, M.; Holzmann, B.; Kollias, G.; et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat. Cell Biol. 2019, 21, 731–742. [Google Scholar] [CrossRef]
- Dominguez, S.; Montgomery, A.B.; Haines, G.K., 3rd; Bloomfield, C.L.; Cuda, C.M. The caspase-8/RIPK3 signaling axis in antigen presenting cells controls the inflammatory arthritic response. Arthritis Res. Ther. 2017, 19, 224. [Google Scholar] [CrossRef]
- Cuda, C.M.; Misharin, A.V.; Khare, S.; Saber, R.; Tsai, F.; Archer, A.M.; Homan, P.J.; Haines, G.K., 3rd; Hutcheson, J.; Dorfleutner, A.; et al. Conditional deletion of caspase-8 in macrophages alters macrophage activation in a RIPK-dependent manner. Arthritis Res. Ther. 2015, 17, 291. [Google Scholar] [CrossRef]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1beta Activation. Cell Rep. 2018, 25, 2339–2353. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Lee, S.H.; Kim, S.Y.; Ryu, J.; Kwon, J.Y.; Na, H.S.; Jung, K.; Moon, S.J.; Cho, M.L.; Min, J.K. RIPK1 inhibition attenuates experimental autoimmune arthritis via suppression of osteoclastogenesis. J. Transl. Med. 2019, 17, 84. [Google Scholar] [CrossRef]
- Patel, S.; Webster, J.D.; Varfolomeev, E.; Kwon, Y.C.; Cheng, J.H.; Zhang, J.; Dugger, D.L.; Wickliffe, K.E.; Maltzman, A.; Sujatha-Bhaskar, S.; et al. RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ. 2019, 27, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Weisel, K.; Berger, S.; Thorn, K.; Taylor, P.C.; Peterfy, C.; Siddall, H.; Tompson, D.; Wang, S.; Quattrocchi, E.; Burriss, S.W.; et al. A randomized, placebo-controlled experimental medicine study of RIPK1 inhibitor GSK2982772 in patients with moderate to severe rheumatoid arthritis. Arthritis Res. Ther. 2021, 23, 85. [Google Scholar] [CrossRef] [PubMed]
- Devos, M.; Tanghe, G.; Gilbert, B.; Dierick, E.; Verheirstraeten, M.; Nemegeer, J.; de Reuver, R.; Lefebvre, S.; De Munck, J.; Rehwinkel, J.; et al. Sensing of endogenous nucleic acids by ZBP1 induces keratinocyte necroptosis and skin inflammation. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Lalaoui, N.; Boyden, S.E.; Oda, H.; Wood, G.M.; Stone, D.L.; Chau, D.; Liu, L.; Stoffels, M.; Kratina, T.; Lawlor, K.E.; et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature 2019, 577, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Anderton, H.; Rickard, J.A.; Varigos, G.A.; Lalaoui, N.; Silke, J. Inhibitor of Apoptosis Proteins (IAPs) Limit RIPK1-Mediated Skin Inflammation. J. Investig. Dermatol. 2017, 137, 2371–2379. [Google Scholar] [CrossRef]
- Berger, S.B.; Kasparcova, V.; Hoffman, S.; Swift, B.; Dare, L.; Schaeffer, M.; Capriotti, C.; Cook, M.; Finger, J.; Hughes-Earle, A.; et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 2014, 192, 5476–5480. [Google Scholar] [CrossRef]
- Rendon, A.; Schakel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Boisson, B.; Laplantine, E.; Dobbs, K.; Cobat, A.; Tarantino, N.; Hazen, M.; Lidov, H.G.; Hopkins, G.; Du, L.; Belkadi, A.; et al. Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. J. Exp. Med. 2015, 212, 939–951. [Google Scholar] [CrossRef]
- Boisson, B.; Laplantine, E.; Prando, C.; Giliani, S.; Israelsson, E.; Xu, Z.; Abhyankar, A.; Israel, L.; Trevejo-Nunez, G.; Bogunovic, D.; et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 2012, 13, 1178–1186. [Google Scholar] [CrossRef]
- Peltzer, N.; Darding, M.; Montinaro, A.; Draber, P.; Draberova, H.; Kupka, S.; Rieser, E.; Fisher, A.; Hutchinson, C.; Taraborrelli, L.; et al. LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature 2018, 557, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Taraborrelli, L.; Peltzer, N.; Montinaro, A.; Kupka, S.; Rieser, E.; Hartwig, T.; Sarr, A.; Darding, M.; Draber, P.; Haas, T.L.; et al. LUBAC prevents lethal dermatitis by inhibiting cell death induced by TNF, TRAIL and CD95L. Nat. Commun. 2018, 9, 3910. [Google Scholar] [CrossRef]
- Seymour, R.E.; Hasham, M.G.; Cox, G.A.; Shultz, L.D.; Hogenesch, H.; Roopenian, D.C.; Sundberg, J.P. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes Immun. 2007, 8, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Deribe, Y.L.; Skanland, S.S.; Stieglitz, B.; Grabbe, C.; Franz-Wachtel, M.; van Wijk, S.J.; Goswami, P.; Nagy, V.; Terzic, J.; et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature 2011, 471, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.; Champagne, C.; Morizot, A.; Lapointe, J.M.; Saleh, M. The Inflammatory Caspases-1 and -11 Mediate the Pathogenesis of Dermatitis in Sharpin-Deficient Mice. J. Immunol. 2015, 195, 2365–2373. [Google Scholar] [CrossRef]
- Gurung, P.; Lamkanfi, M.; Kanneganti, T.D. Cutting edge: SHARPIN is required for optimal NLRP3 inflammasome activation. J. Immunol. 2015, 194, 2064–2067. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Sharma, B.R.; Kanneganti, T.D. Distinct role of IL-1beta in instigating disease in Sharpin(cpdm) mice. Sci. Rep. 2016, 6, 36634. [Google Scholar] [CrossRef]
- Kumari, S.; Redouane, Y.; Lopez-Mosqueda, J.; Shiraishi, R.; Romanowska, M.; Lutzmayer, S.; Kuiper, J.; Martinez, C.; Dikic, I.; Pasparakis, M.; et al. Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.D.; Kwon, Y.C.; Park, S.; Zhang, H.; Corr, N.; Ljumanovic, N.; Adedeji, A.O.; Varfolomeev, E.; Goncharov, T.; Preston, J.; et al. RIP1 kinase activity is critical for skin inflammation but not for viral propagation. J. Leukoc. Biol. 2020, 107, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Dees, E.C.; Olszanski, A.J.; Dhuria, S.V.; Sen, S.; Cameron, S.; Cohen, R.B. Phase I dose-escalation study of LCL161, an oral inhibitor of apoptosis proteins inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Morrish, E.; Brumatti, G.; Silke, J. Future Therapeutic Directions for Smac-Mimetics. Cells 2020, 9, 406. [Google Scholar] [CrossRef]
- Nesterovitch, A.B.; Gyorfy, Z.; Hoffman, M.D.; Moore, E.C.; Elbuluk, N.; Tryniszewska, B.; Rauch, T.A.; Simon, M.; Kang, S.; Fisher, G.J.; et al. Alteration in the gene encoding protein tyrosine phosphatase nonreceptor type 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. Am. J. Pathol. 2011, 178, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Lukens, J.R.; Vogel, P.; Johnson, G.R.; Kelliher, M.A.; Iwakura, Y.; Lamkanfi, M.; Kanneganti, T.D. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature 2013, 498, 224–227. [Google Scholar] [CrossRef]
- Abram, C.L.; Roberge, G.L.; Pao, L.I.; Neel, B.G.; Lowell, C.A. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity 2013, 38, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Speir, M.; Nowell, C.J.; Chen, A.A.; O’Donnell, J.A.; Shamie, I.S.; Lakin, P.R.; D’Cruz, A.A.; Braun, R.O.; Babon, J.J.; Lewis, R.S.; et al. Ptpn6 inhibits caspase-8- and Ripk3/Mlkl-dependent inflammation. Nat. Immunol. 2019, 21, 54–64. [Google Scholar] [CrossRef]
- Saito, N.; Honma, M.; Shibuya, T.; Iinuma, S.; Igawa, S.; Kishibe, M.; Ishida-Yamamoto, A. RIPK1 downregulation in keratinocyte enhances TRAIL signaling in psoriasis. J. Dermatol. Sci. 2018, 91, 79–86. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Liu, N.; Huang, Y.; Jin, Z.; Zhang, S.; Ming, Z.; Chen, H. Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis. 2020, 11, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Rathkey, J.K.; Zhao, J.; Liu, Z.; Chen, Y.; Yang, J.; Kondolf, H.C.; Benson, B.L.; Chirieleison, S.M.; Huang, A.Y.; Dubyak, G.R.; et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 2018, 3, eaat2738. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dugger, D.L.; Maltzman, A.; Greve, J.M.; Hedehus, M.; Martin-McNulty, B.; Carano, R.A.; Cao, T.C.; van Bruggen, N.; Bernstein, L.; et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016, 23, 1565–1576. [Google Scholar] [CrossRef]
- Weisel, K.; Berger, S.; Papp, K.; Maari, C.; Krueger, J.G.; Scott, N.; Tompson, D.; Wang, S.; Simeoni, M.; Bertin, J.; et al. Response to Inhibition of Receptor-Interacting Protein Kinase 1 (RIPK1) in Active Plaque Psoriasis: A Randomized Placebo-Controlled Study. Clin. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Harris, P.A.; Berger, S.B.; Jeong, J.U.; Nagilla, R.; Bandyopadhyay, D.; Campobasso, N.; Capriotti, C.A.; Cox, J.A.; Dare, L.; Dong, X.; et al. Discovery of a First-in-Class Receptor Interacting Protein 1 (RIP1) Kinase Specific Clinical Candidate (GSK2982772) for the Treatment of Inflammatory Diseases. J. Med. Chem. 2017, 60, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, R.; Duckworth, C.A.; Moussata, D.; Gloeckner, A.; Lim, L.G.; Goetz, M.; Pritchard, D.M.; Galle, P.R.; Neurath, M.F.; Watson, A.J. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012, 61, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Buhner, S.; Buning, C.; Genschel, J.; Kling, K.; Herrmann, D.; Dignass, A.; Kuechler, I.; Krueger, S.; Schmidt, H.H.; Lochs, H. Genetic basis for increased intestinal permeability in families with Crohn’s disease: Role of CARD15 3020insC mutation? Gut 2006, 55, 342–347. [Google Scholar] [CrossRef]
- Cifaldi, C.; Chiriaco, M.; Di Matteo, G.; Di Cesare, S.; Alessia, S.; De Angelis, P.; Rea, F.; Angelino, G.; Pastore, M.; Ferradini, V.; et al. Novel X-Linked Inhibitor of Apoptosis Mutation in Very Early-Onset Inflammatory Bowel Disease Child Successfully Treated with HLA-Haploidentical Hemapoietic Stem Cells Transplant after Removal of alphabeta(+) T and B Cells. Front. Immunol. 2017, 8, 1893. [Google Scholar] [CrossRef]
- Lekbua, A.; Ouahed, J.; O’Connell, A.E.; Kahn, S.A.; Goldsmith, J.D.; Imamura, T.; Duncan, C.N.; Kelsen, J.R.; Worthey, E.; Snapper, S.B.; et al. Risk-factors Associated With Poor Outcomes in VEO-IBD Secondary to XIAP Deficiency: A Case Report and Literature Review. J. Pediatr. Gastroenterol. Nutr. 2019, 69, e13–e18. [Google Scholar] [CrossRef] [PubMed]
- Serra, E.G.; Schwerd, T.; Moutsianas, L.; Cavounidis, A.; Fachal, L.; Pandey, S.; Kammermeier, J.; Croft, N.M.; Posovszky, C.; Rodrigues, A.; et al. Somatic mosaicism and common genetic variation contribute to the risk of very-early-onset inflammatory bowel disease. Nat. Commun. 2020, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Girardelli, M.; Arrigo, S.; Barabino, A.; Loganes, C.; Morreale, G.; Crovella, S.; Tommasini, A.; Bianco, A.M. The diagnostic challenge of very early-onset enterocolitis in an infant with XIAP deficiency. BMC Pediatr. 2015, 15, 208. [Google Scholar] [CrossRef]
- Latour, S.; Aguilar, C. XIAP deficiency syndrome in humans. Semin. Cell Dev. Biol. 2015, 39, 115–123. [Google Scholar] [CrossRef]
- Uhlig, H.H. Monogenic diseases associated with intestinal inflammation: Implications for the understanding of inflammatory bowel disease. Gut 2013, 62, 1795–1805. [Google Scholar] [CrossRef]
- Nielsen, O.H.; LaCasse, E.C. How genetic testing can lead to targeted management of XIAP deficiency-related inflammatory bowel disease. Genet. Med. 2017, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Stafford, C.A.; Lawlor, K.E.; Heim, V.J.; Bankovacki, A.; Bernardini, J.P.; Silke, J.; Nachbur, U. IAPs Regulate Distinct Innate Immune Pathways to Co-ordinate the Response to Bacterial Peptidoglycans. Cell Rep. 2018, 22, 1496–1508. [Google Scholar] [CrossRef]
- Negroni, A.; Stronati, L.; Pierdomenico, M.; Tirindelli, D.; Di Nardo, G.; Mancini, V.; Maiella, G.; Cucchiara, S. Activation of NOD2-mediated intestinal pathway in a pediatric population with Crohn’s disease. Inflamm. Bowel Dis. 2009, 15, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Stronati, L.; Negroni, A.; Merola, P.; Pannone, V.; Borrelli, O.; Cirulli, M.; Annese, V.; Cucchiara, S. Mucosal NOD2 expression and NF-kappaB activation in pediatric Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Stronati, L.; Negroni, A.; Pierdomenico, M.; D’Ottavio, C.; Tirindelli, D.; Di Nardo, G.; Oliva, S.; Viola, F.; Cucchiara, S. Altered expression of innate immunity genes in different intestinal sites of children with ulcerative colitis. Dig. Liver Dis. 2010, 42, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Chirieleison, S.M.; Marsh, R.A.; Kumar, P.; Rathkey, J.K.; Dubyak, G.R.; Abbott, D.W. Nucleotide-binding oligomerization domain (NOD) signaling defects and cell death susceptibility cannot be uncoupled in X-linked inhibitor of apoptosis (XIAP)-driven inflammatory disease. J. Biol. Chem. 2017, 292, 9666–9679. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L. Anti-TNF therapy in inflammatory bowel diseases: A huge review. Minerva Gastroenterol. Dietol. 2010, 56, 233–243. [Google Scholar]
- Liu, Z.; Kong, F.; Vallance, J.E.; Harmel-Laws, E.; Amarachintha, S.; Steinbrecher, K.A.; Rosen, M.J.; Bhattacharyya, S. Activation of TGF-beta activated kinase 1 promotes colon mucosal pathogenesis in inflammatory bowel disease. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef]
- Vlantis, K.; Wullaert, A.; Polykratis, A.; Kondylis, V.; Dannappel, M.; Schwarzer, R.; Welz, P.; Corona, T.; Walczak, H.; Weih, F.; et al. NEMO Prevents RIP Kinase 1-Mediated Epithelial Cell Death and Chronic Intestinal Inflammation by NF-kappaB-Dependent and -Independent Functions. Immunity 2016, 44, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Eckmann, L.; Miyamoto, Y.; Pothoulakis, C.; Karin, M.; Kagnoff, M.F. Epithelial cell I kappa B-kinase beta has an important protective role in Clostridium difficile toxin A-induced mucosal injury. J. Immunol. 2006, 177, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L.; Nebelsiek, T.; Fingerle, A.A.; Dann, S.M.; Mages, J.; Lang, R.; Robine, S.; Kagnoff, M.F.; Schmid, R.M.; Karin, M.; et al. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 15058–15063. [Google Scholar] [CrossRef] [PubMed]
- Kajino-Sakamoto, R.; Inagaki, M.; Lippert, E.; Akira, S.; Robine, S.; Matsumoto, K.; Jobin, C.; Ninomiya-Tsuji, J. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J. Immunol. 2008, 181, 1143–1152. [Google Scholar] [CrossRef]
- Fish, J.D.; Duerst, R.E.; Gelfand, E.W.; Orange, J.S.; Bunin, N. Challenges in the use of allogeneic hematopoietic SCT for ectodermal dysplasia with immune deficiency. Bone Marrow Transplant. 2009, 43, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Nishikomori, R.; Heike, T. Diagnosis and treatment in anhidrotic ectodermal dysplasia with immunodeficiency. Allergol. Int. 2012, 61, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.Y.; Levy, O.; Jabara, H.H.; Glickman, J.N.; Stoler-Barak, L.; Sachs, J.; Nurko, S.; Orange, J.S.; Geha, R.S. Allogeneic transplantation successfully corrects immune defects, but not susceptibility to colitis, in a patient with nuclear factor-kappaB essential modulator deficiency. J. Allergy Clin. Immunol. 2008, 122, 1113–1118. [Google Scholar] [CrossRef]
- Permaul, P.; Narla, A.; Hornick, J.L.; Pai, S.Y. Allogeneic hematopoietic stem cell transplantation for X-linked ectodermal dysplasia and immunodeficiency: Case report and review of outcomes. Immunol. Res. 2009, 44, 89–98. [Google Scholar] [CrossRef]
- Anderson, C.A.; Boucher, G.; Lees, C.W.; Franke, A.; D’Amato, M.; Taylor, K.D.; Lee, J.C.; Goyette, P.; Imielinski, M.; Latiano, A.; et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 2011, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Vereecke, L.; Vieira-Silva, S.; Billiet, T.; van Es, J.H.; Mc Guire, C.; Slowicka, K.; Sze, M.; van den Born, M.; De Hertogh, G.; Clevers, H.; et al. A20 controls intestinal homeostasis through cell-specific activities. Nat. Commun. 2014, 5, 5103. [Google Scholar] [CrossRef] [PubMed]
- Vereecke, L.; Sze, M.; Mc Guire, C.; Rogiers, B.; Chu, Y.; Schmidt-Supprian, M.; Pasparakis, M.; Beyaert, R.; van Loo, G. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J. Exp. Med. 2010, 207, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, L.E.; Lodolce, J.P.; Chang, J.E.; Schneider, J.R.; Grimm, W.A.; Bartulis, S.J.; Zhu, X.; Messer, J.S.; Murphy, S.F.; Reddy, N.; et al. TNFAIP3 maintains intestinal barrier function and supports epithelial cell tight junctions. PLoS ONE 2011, 6, e26352. [Google Scholar] [CrossRef]
- Rhee, L.; Murphy, S.F.; Kolodziej, L.E.; Grimm, W.A.; Weber, C.R.; Lodolce, J.P.; Chang, J.E.; Bartulis, S.J.; Messer, J.S.; Schneider, J.R.; et al. Expression of TNFAIP3 in intestinal epithelial cells protects from DSS- but not TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G220–G227. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonell, R.; Wong, J.; Kim, J.Y.; Close, L.A.; Boland, B.S.; Wong, T.L.; Harris, P.A.; Ho, S.B.; Das, S.; Ernst, P.B.; et al. Elevated A20 promotes TNF-induced and RIPK1-dependent intestinal epithelial cell death. Proc. Natl. Acad. Sci. USA 2018, 115, E9192–E9200. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diaz, S.; Dillon, C.P.; Lalaoui, N.; Tanzer, M.C.; Rodriguez, D.A.; Lin, A.; Lebois, M.; Hakem, R.; Josefsson, E.C.; O’Reilly, L.A.; et al. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity 2016, 45, 513–526. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, X.; McQuade, T.; Li, J.; Chan, F.K.; Zhang, J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 2011, 471, 373–376. [Google Scholar] [CrossRef]
- Welz, P.S.; Wullaert, A.; Vlantis, K.; Kondylis, V.; Fernandez-Majada, V.; Ermolaeva, M.; Kirsch, P.; Sterner-Kock, A.; van Loo, G.; Pasparakis, M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011, 477, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Gunther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.J.; Hedrick, S.M.; Tenzer, S.; Neurath, M.F.; et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335–339. [Google Scholar] [CrossRef]
- Stolzer, I.; Kaden-Volynets, V.; Ruder, B.; Letizia, M.; Bittel, M.; Rausch, P.; Basic, M.; Bleich, A.; Baines, J.F.; Neurath, M.F.; et al. Environmental Microbial Factors Determine the Pattern of Inflammatory Lesions in a Murine Model of Crohn’s Disease-Like Inflammation. Inflamm. Bowel Dis. 2020, 26, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, R.; Jiao, H.; Wachsmuth, L.; Tresch, A.; Pasparakis, M. FADD and Caspase-8 Regulate Gut Homeostasis and Inflammation by Controlling MLKL- and GSDMD-Mediated Death of Intestinal Epithelial Cells. Immunity 2020, 52, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Wittkopf, N.; Gunther, C.; Martini, E.; He, G.; Amann, K.; He, Y.W.; Schuchmann, M.; Neurath, M.F.; Becker, C. Cellular FLICE-like inhibitory protein secures intestinal epithelial cell survival and immune homeostasis by regulating caspase-8. Gastroenterology 2013, 145, 1369–1379. [Google Scholar] [CrossRef]
- Lehle, A.S.; Farin, H.F.; Marquardt, B.; Michels, B.E.; Magg, T.; Li, Y.; Liu, Y.; Ghalandary, M.; Lammens, K.; Hollizeck, S.; et al. Intestinal Inflammation and Dysregulated Immunity in Patients With Inherited Caspase-8 Deficiency. Gastroenterology 2019, 156, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Kim, C.A.; Pastorino, A.C.; Ceroni, J.; Lima, P.P.; de Barros Dorna, M.; Honjo, R.S.; Bertola, D.; Hamanaka, K.; Fujita, A.; et al. Primary immunodeficiency with chronic enteropathy and developmental delay in a boy arising from a novel homozygous RIPK1 variant. J. Hum. Genet. 2019. [Google Scholar] [CrossRef]
- Dourmashkin, R.; Davies, H.; Wells, C.; Shah, D.; Price, A.; O’Morain, J.; Levi, J. Epithelial patchy necrosis in Crohn’s disease. Hum. Pathol. 1989, 14, 643–648. [Google Scholar] [CrossRef]
- Pierdomenico, M.; Negroni, A.; Stronati, L.; Vitali, R.; Prete, E.; Bertin, J.; Gough, P.J.; Aloi, M.; Cucchiara, S. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am. J. Gastroenterol. 2014, 109, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Negroni, A.; Colantoni, E.; Pierdomenico, M.; Palone, F.; Costanzo, M.; Oliva, S.; Tiberti, A.; Cucchiara, S.; Stronati, L. RIP3 AND pMLKL promote necroptosis-induced inflammation and alter membrane permeability in intestinal epithelial cells. Dig. Liver Dis. 2017, 49, 1201–1210. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y.; Xue, L.; Sheng, Y.; Xu, L.; Xue, Y. Expression of receptor interacting protein 3 and mixed lineage kinase domain-like protein-key proteins in necroptosis is upregulated in ulcerative colitis. Ann. Palliat. Med. 2019, 8, 483–489. [Google Scholar] [CrossRef]
- Zhou, M.; He, J.; Shi, Y.; Liu, X.; Luo, S.; Cheng, C.; Ge, W.; Qu, C.; Du, P.; Chen, Y. ABIN3 Negatively Regulates Necroptosis-induced Intestinal Inflammation Through Recruiting A20 and Restricting the Ubiquitination of RIPK3 in Inflammatory Bowel Disease. J. Crohns Colitis. 2021, 15, 99–114. [Google Scholar] [CrossRef]
- Gobbetti, T.; Berger, S.B.; Fountain, K.; Slocombe, T.; Rowles, A.; Pearse, G.; Harada, I.; Bertin, J.; Haynes, A.C.; Beal, A.M. Receptor-interacting protein 1 kinase inhibition therapeutically ameliorates experimental T cell-dependent colitis in mice. Cell Death Dis. 2020, 11, 220. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, D.; Yang, Y.J.; Hu, G.Q.; Qin, X.X.; Du, C.T.; Chen, W. MLKL deficiency inhibits DSS-induced colitis independent of intestinal microbiota. Mol. Immunol. 2019, 107, 132–141. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, X.; Li, M.; Liu, Y.; Han, Y.; Zhang, X.; Li, X.M.; Wu, X.; Qin, J.; Fang, J.; et al. MLKL attenuates colon inflammation and colitis-tumorigenesis via suppression of inflammatory responses. Cancer Lett. 2019, 459, 100–111. [Google Scholar] [CrossRef]
- Alvarez-Diaz, S.; Preaudet, A.; Samson, A.L.; Nguyen, P.M.; Fung, K.Y.; Garnham, A.L.; Alexander, W.S.; Strasser, A.; Ernst, M.; Putoczki, T.L.; et al. Necroptosis is dispensable for the development of inflammation-associated or sporadic colon cancer in mice. Cell Death Differ. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, H.; Fan, C.; Qi, Q.; Yan, Y.; Wu, Y.; Feng, C.; Wu, B.; Gao, Y.; Zuo, J.; et al. RIPK1 inhibitor ameliorates colitis by directly maintaining intestinal barrier homeostasis and regulating following IECs-immuno crosstalk. Biochem. Pharmacol. 2020, 172, 113751. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).