Involvement of Opioid System and TRPM8/TRPA1 Channels in the Antinociceptive Effect of Spirulina platensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Plant Material and Extract Preparation
2.3. Total Phenolic Compound Determination
2.4. Flavonoid Compound Determination
2.5. Antioxidant Activity
2.6. Animals
2.7. Antinociceptive Activity
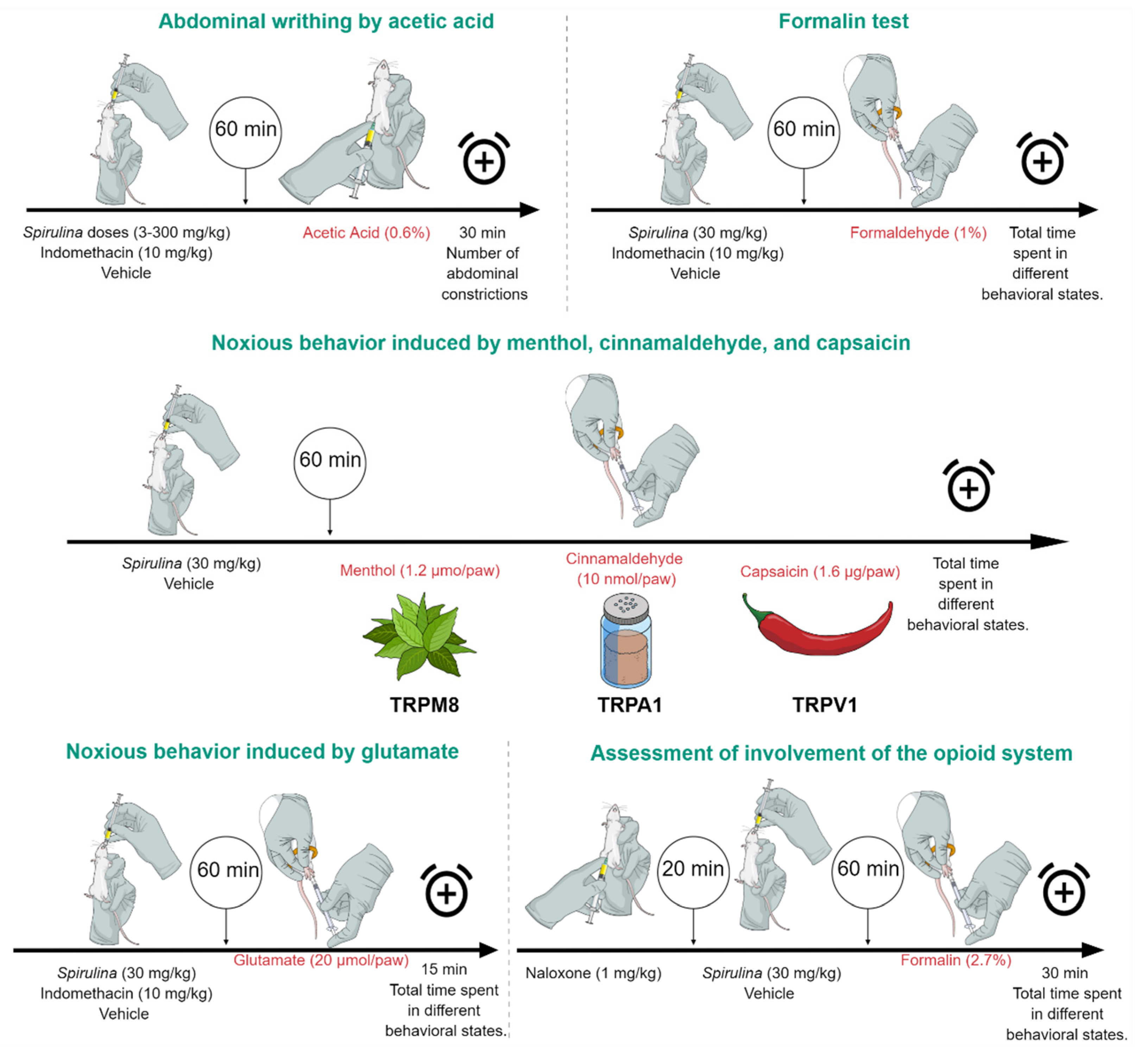
2.7.1. Abdominal Writhing by Intraperitoneal Injection of Acetic Acid
2.7.2. Formalin Test
2.8. Open Field and Rotarod Test
2.9. Noxious Behavior Induced by Menthol, Cinnamaldehyde, and Capsaicin
2.10. Noxious Behavior Induced by Glutamate
2.11. Assessment of Involvement of the Opioid System
2.12. Statistical Analysis
3. Results
3.1. Total Phenol and Flavonoids Contents, and Antioxidant Effect
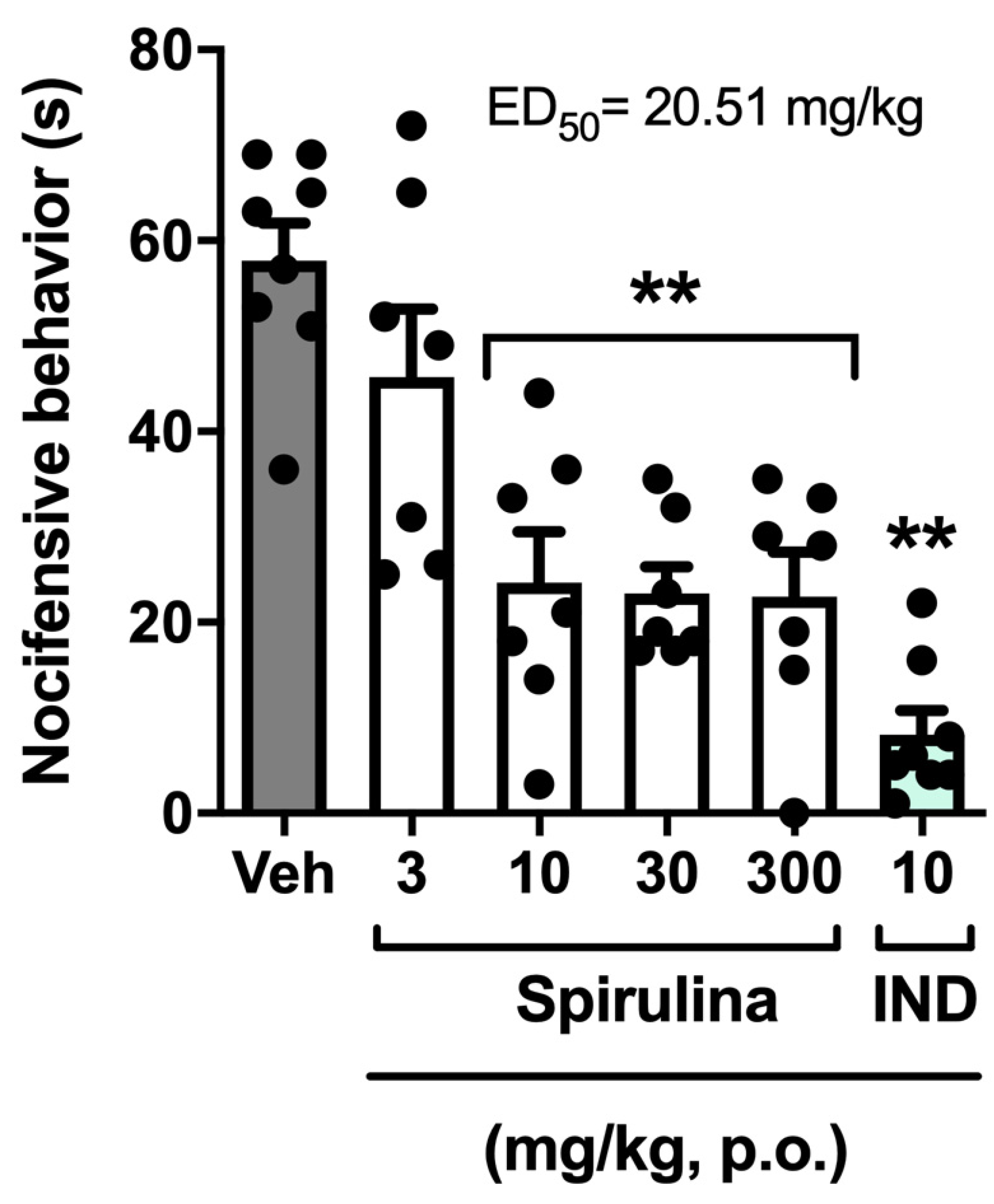
3.2. Nociceptive Behavior Induced by Acetic Acid
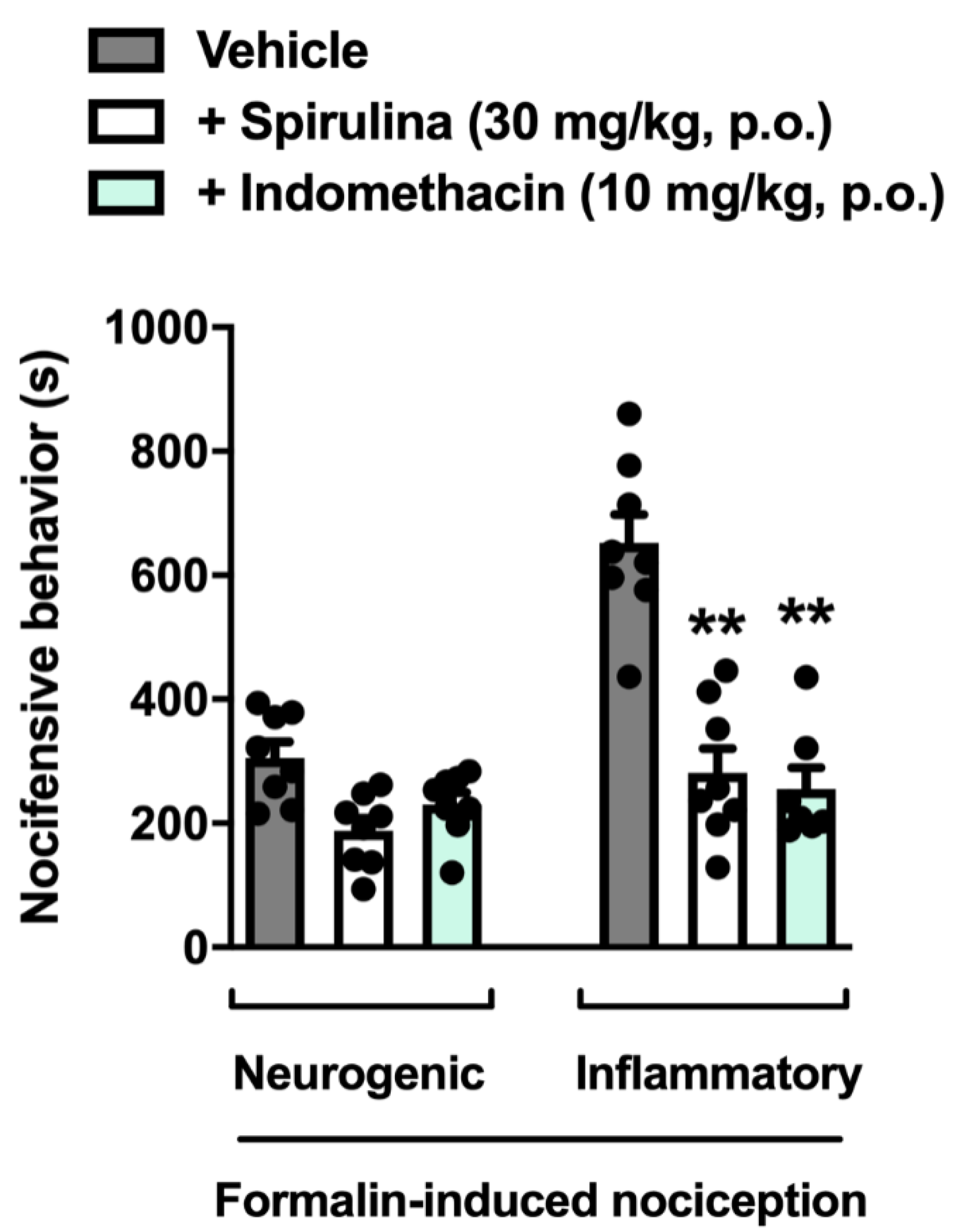
3.3. Nociceptive Behavior Induced by Formalin
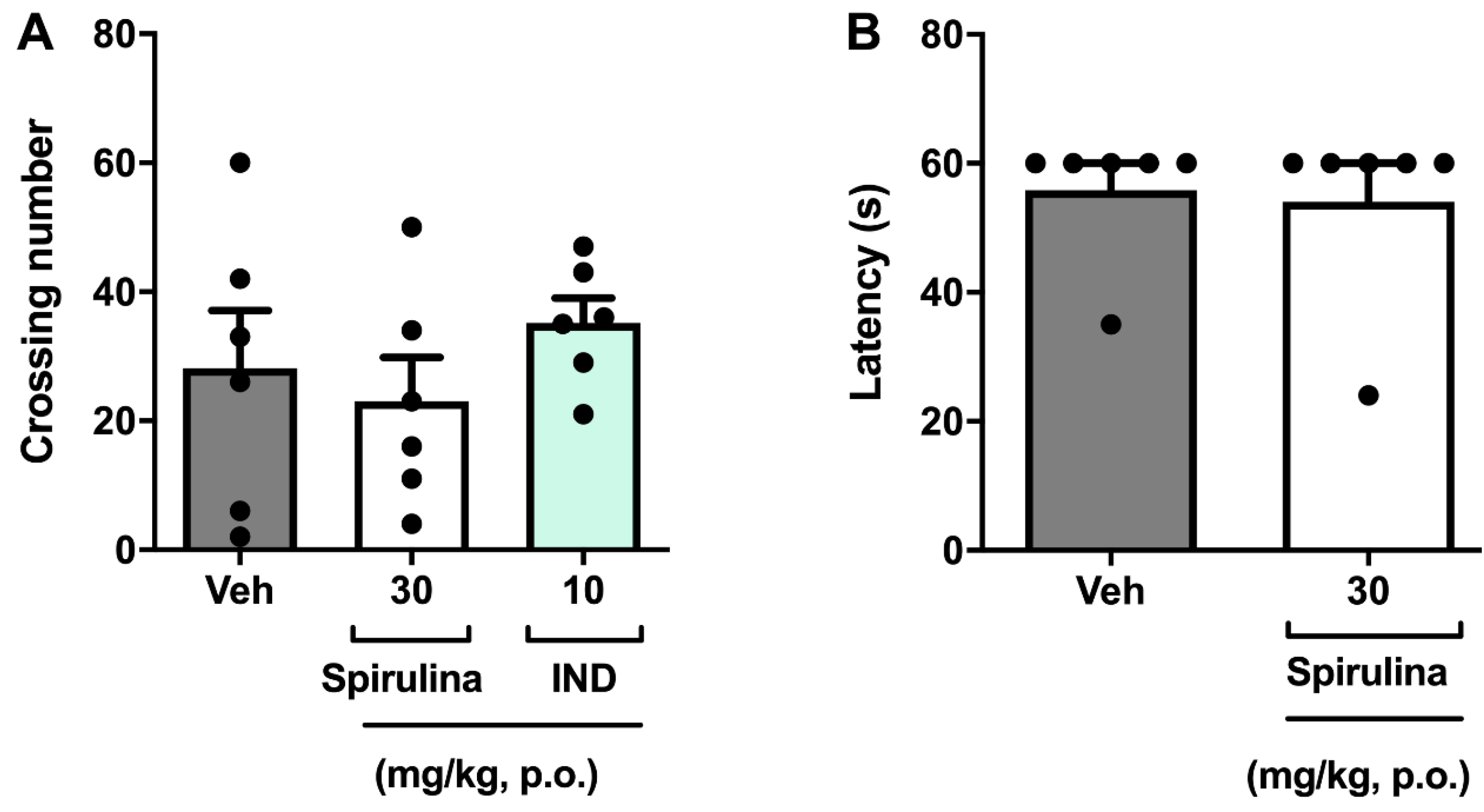
3.4. Assessment of Locomotor Activity
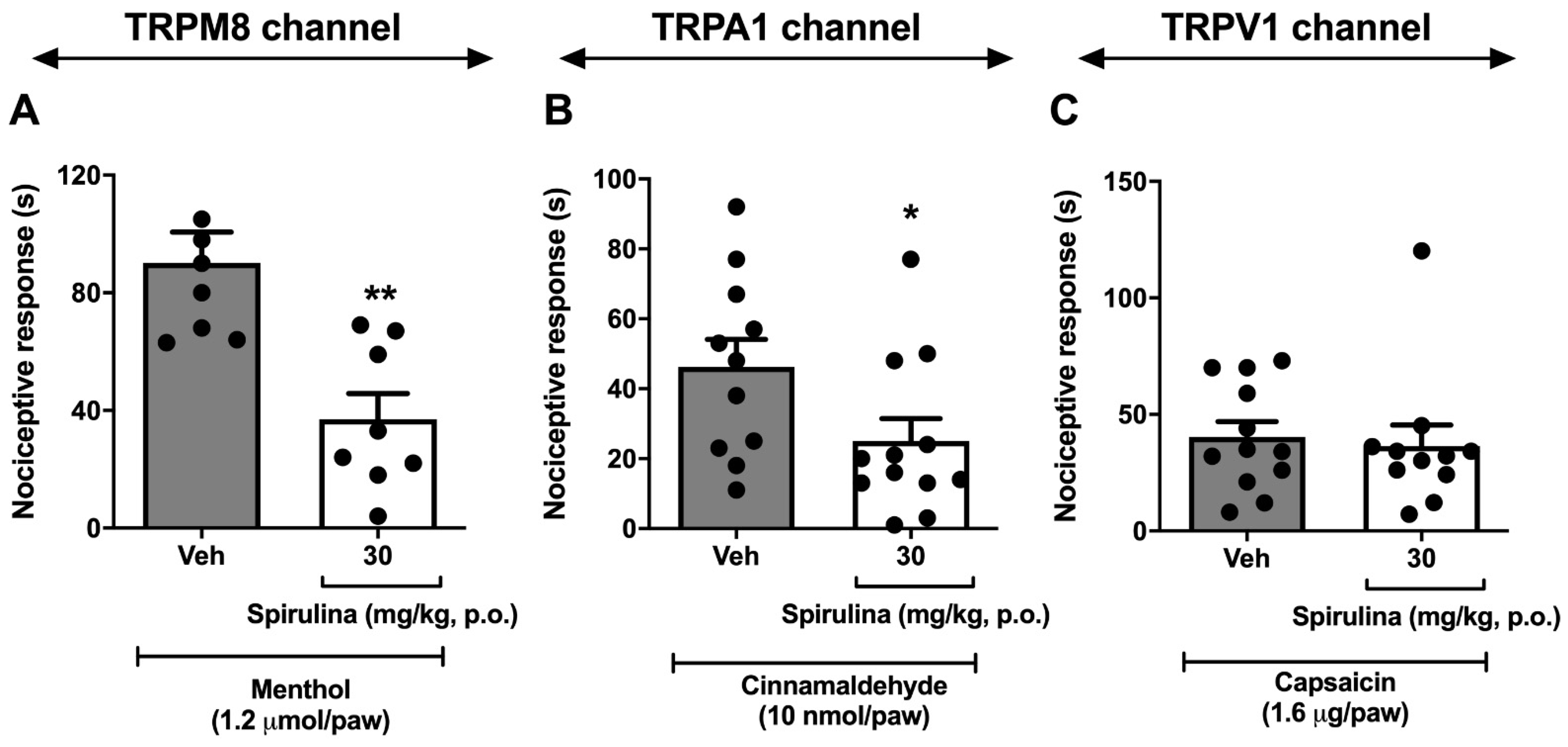
3.5. Nociceptive Behavior Induced by Menthol, Cinnamaldehyde, and Capsaicin
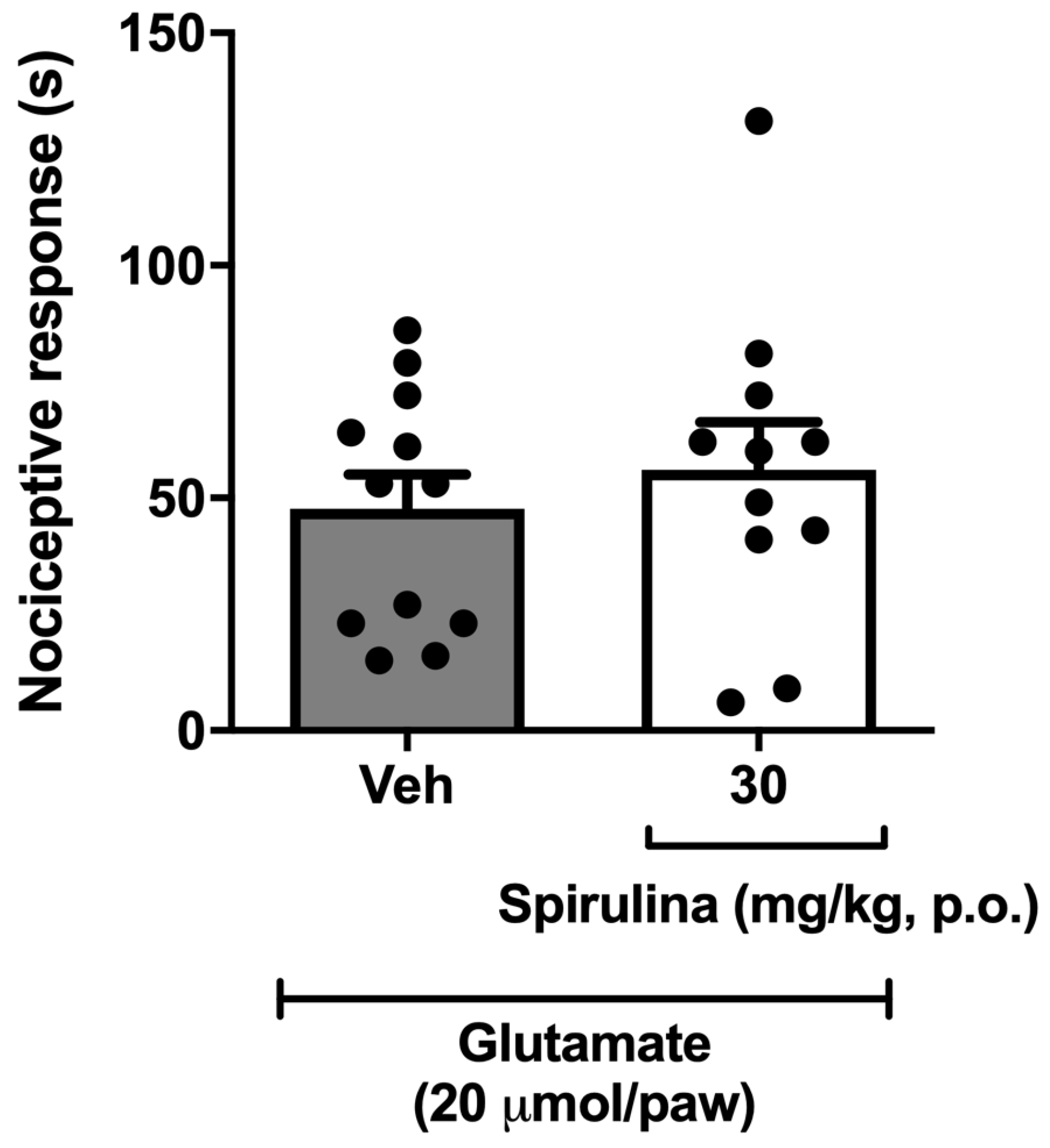
3.6. Nociceptive Behavior Induced by Glutamate
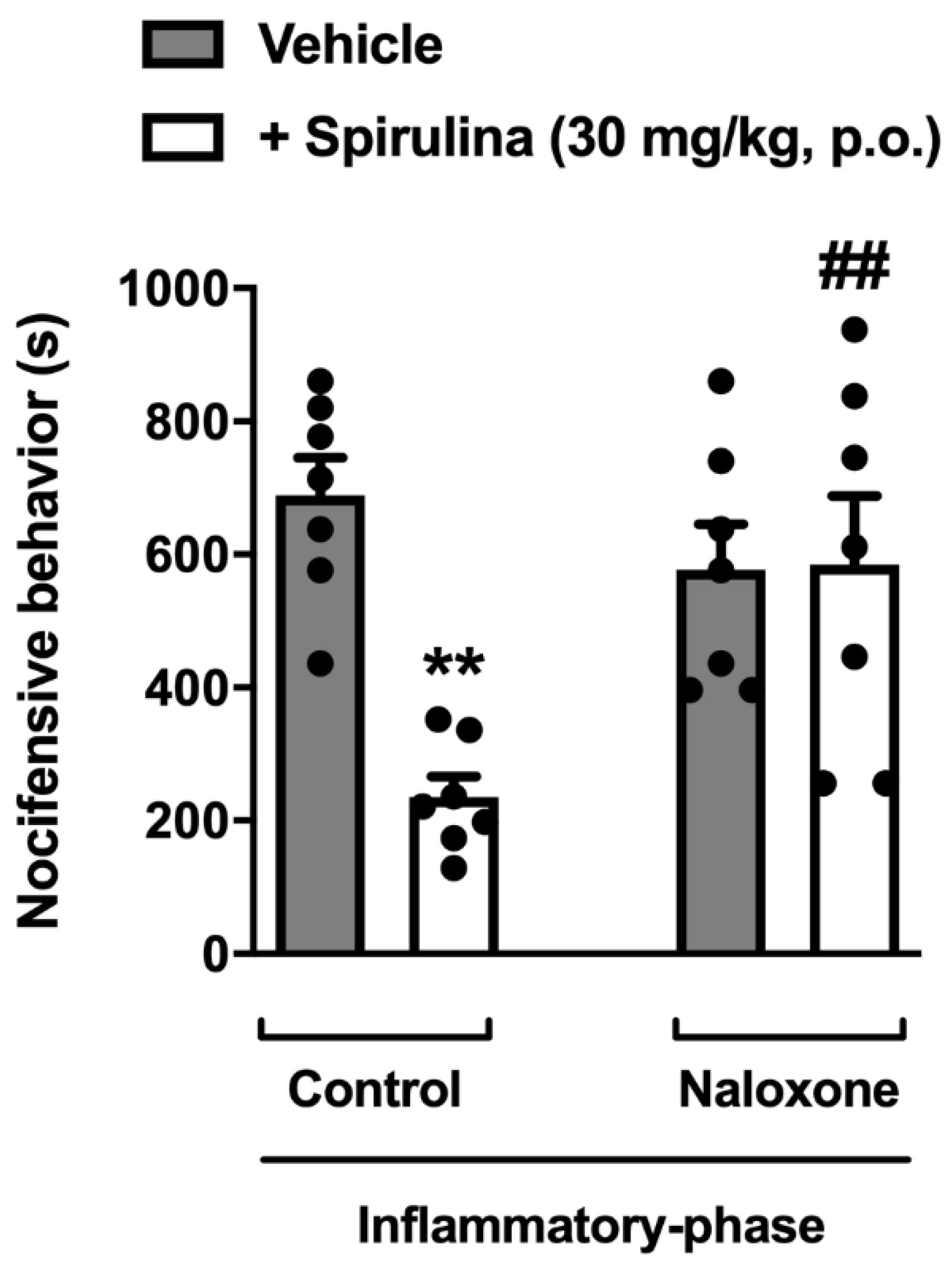
3.7. Assessment of Opioid System Involvement
4. Discussion
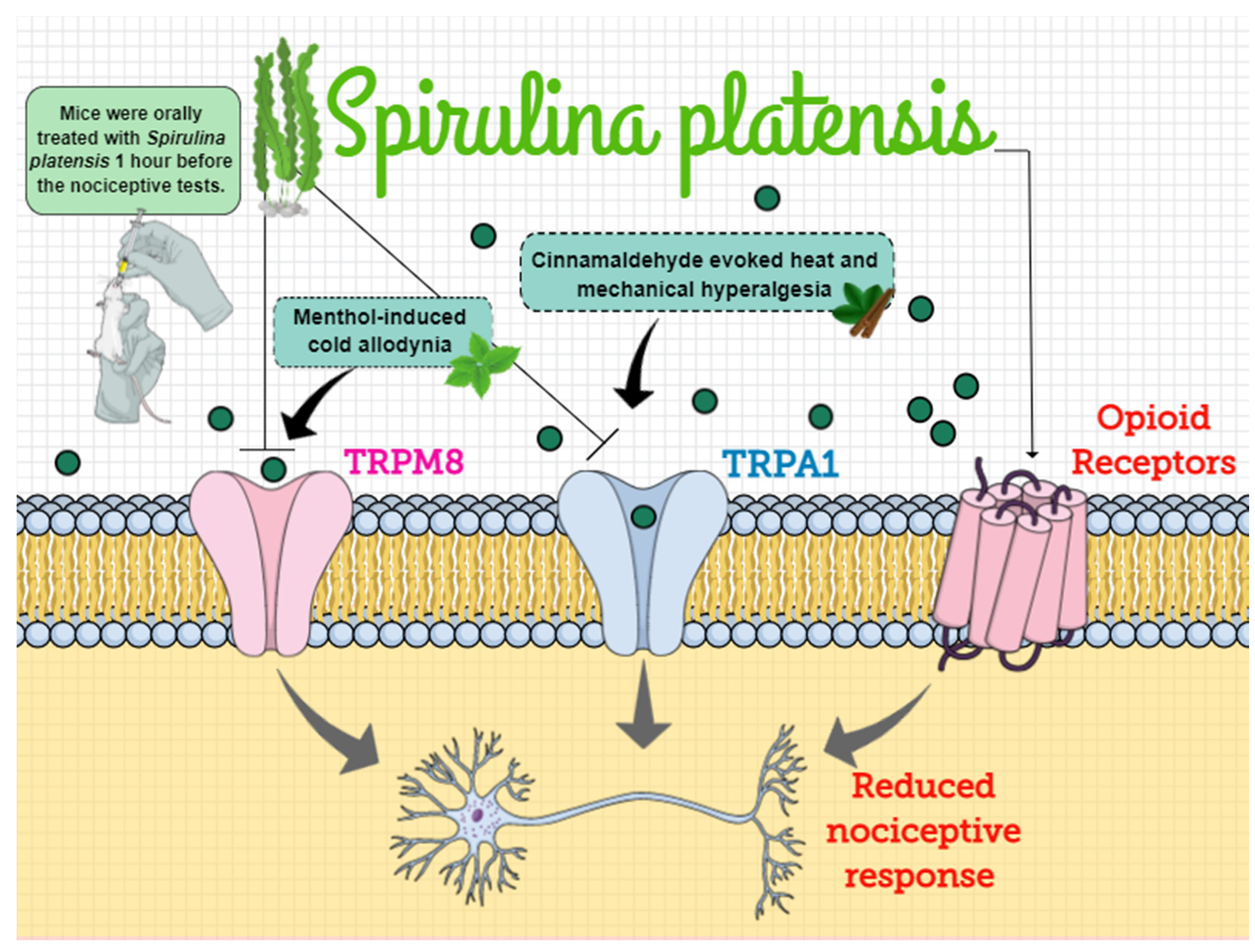
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granado-Lorencio, F.; Hernández-Alvarez, E. Functional foods and health effects: A nutritional biochemistry perspective. Curr. Med. Chem. 2016, 23, 2929–2957. [Google Scholar] [CrossRef]
- Henry, C.J. Functional foods. Eur. J. Clin. Nutr. 2010, 64, 657–659. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges—A position paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lepe, M.A.; Olivas-Aguirre, F.J.; Gómez-Miranda, L.M.; Hernández-Torres, R.P.; Manríquez-Torres, J.d.J.; Ramos-Jiménez, A. Systematic physical exercise and Spirulina maxima supplementation improve body composition, cardiorespiratory fitness, and blood lipid profile: Correlations of a randomized double-blind controlled trial. Antioxidants 2019, 8, 507. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, T.W.; DiCarlo, S.E.; Pravenec, M.; Morris, C. Functional foods for augmenting nitric oxide activity and reducing the risk for salt-induced hypertension and cardiovascular disease in Japan. J. Cardiol. 2019, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Rastqar, A.; Keshvari, M. Functional food and cardiovascular disease prevention and treatment: A Review. J. Am. Coll. Nutr. 2018, 37, 429–455. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.T.; Fernandes, C.P.; Daleprane, J.B.; Alves, M.S.; Stien, D.; Dhammika Nanayakkara, N.P. Role of natural antioxidants from functional foods in neurodegenerative and metabolic disorders. Oxid. Med. Cell. Longev. 2018, 2018, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Koutelidakis, A.E. Functional foods and bioactive compounds: A Review of its possible role on weight management and obesity’s metabolic consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Graphic representation of the relationship between oxygen-consumption and characteristics of normal gait of the human male. Curr. Pharm. Des. 2016, 75, 305–314. [Google Scholar] [CrossRef]
- Mathur, M. Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-54528-8. [Google Scholar]
- Yasmeen, R.; Fukagawa, N.K.; Wang, T.T. Establishing health benefits of bioactive food components: A basic research scientist’s perspective. Curr. Opin. Biotechnol. 2017, 44, 109–114. [Google Scholar] [CrossRef]
- Shearer, G.C.; Savinova, O.V.; Harris, W.S. Fish Oil and Plasma Triglycerides. Biochim. Biophys. Acta 2012, 23, 843–851. [Google Scholar] [CrossRef]
- Baker, A.D.; Malur, A.; Barna, B.P.; Kavuru, M.S.; Malur, A.G.; Thomassen, M.J. PPARγ regulates the expression of cholesterol metabolism genes in alveolar macrophages. Biochem. Biophys. Res. Commun. 2010, 393, 682–687. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Li, T. Regulation of bile acid and cholesterol metabolism by PPARs. PPAR Res. 2009, 2009. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Danneskiold-Samsøe, N.B.; Dias de Freitas Queiroz Barros, H.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Maróstica Júnior, M.R. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef]
- Man, S.M. Inflammasomes in the gastrointestinal tract: Infection, cancer and gut microbiota homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 721–737. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larqué, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A. Antiviral functional foods and exercise lifestyle prevention of coronavirus. Nutrients 2020, 12, 2633. [Google Scholar] [CrossRef] [PubMed]
- Zeinalian, R.; Farhangi, M.A.; Shariat, A.; Saghafi-Asl, M. The effects of Spirulina Platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: A randomized double blinded placebo controlled trial. BMC Complement. Altern. Med. 2017, 17, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, M.; Barzanti, V.; Carenini, G.; Gentili, P. Nutritional studies on Spirulina maxima. Acta Vitaminol. Enzym. 1984, 6, 295–304. [Google Scholar]
- De Freitas Brito, A.; Silva, A.S.; de Oliveira, C.V.C.; de Souza, A.A.; Ferreira, P.B.; de Souza, I.L.L.; da Cunha Araujo, L.C.; da Silva Félix, G.; de Souza Sampaio, R.; Tavares, R.L.; et al. Spirulina platensis prevents oxidative stress and inflammation promoted by strength training in rats: Dose-response relation study. Sci. Rep. 2020, 10, 6382. [Google Scholar] [CrossRef]
- Soheili, M.; Khosravi-Darani, K. The Potential Health Benefits of Algae and Micro Algae in Medicine: A Review on Spirulina platensis. Curr. Nutr. Food Sci. 2011, 7, 279–285. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Ali, M.S.; Madkour, F.F.; Elgendy, H. Oral spirulina platensis attenuates hyperglycemia and exhibits antinociceptive effect in streptozotocin-induced diabetic neuropathy rat model. J. Pain Res. 2020, 13, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K.M. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.A.V.; Joventino, I.P.; Joventino, F.P.; de Almeida, A.C.; Neves, K.R.T.; do Carmo, M.R.; Leal, L.K.A.M.; de Andrade, G.M.; de Barros Viana, G.S. Neuroprotective Activities of Spirulina platensis in the 6-OHDA Model of Parkinson’s Disease Are Related to Its Anti-Inflammatory Effects. Neurochem. Res. 2017, 42, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Abu-Taweel, G.M.; Mohsen, G.A.M.; Antonisamy, P.; Arokiyaraj, S.; Kim, H.J.; Kim, S.J.; Park, K.H.; Kim, Y.O. Spirulina consumption effectively reduces anti-inflammatory and pain related infectious diseases. J. Infect. Public Health 2019, 12, 777–782. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.R.; Graça, C.S.; de Assis, L.M.; de Souza-Soares, L.A. Uma abordagem sobre caracterização e avaliação do potencial antioxidante de extratos fenólicos de microalgas Spirulina sp. LEB-18 e Chlorella pyrenoidosa. Rev. Ciências Agrárias 2017, 40, 264–278. [Google Scholar] [CrossRef]
- da Bierhals, V.S.; Machado, V.G.; Echevenguá, W.O.; Costa, J.A.V.; Furlong, E.B. Compostos fenólicos totais, atividade antioxidante e antifúngica de multimisturas enriquecidas com a microalga Spirulina platensis. Rev. Inst. Adolfo Lutz 2009, 68, 42–48. [Google Scholar]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit; Elsevier: Amsterdam, The Netherlands, 2016; Volume 162, ISBN 2135415065. [Google Scholar]
- Singleton, S.; Rossi, J.; Singleton, V.; Singleton, V.; Rossi, J.; Singleton, V. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Dutra, R.C.; Leite, M.N.; Barbosa, N.R. Quantification of phenolic constituents and antioxidant activity of Pterodon emarginatus vogel seeds. Int. J. Mol. Sci. 2008, 9, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals; The National Academic Press: New York, NY, USA, 2011; Volume 21. [Google Scholar]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B.; Hess, S.C.; Messana, I.; Ferrari, F.; Yunes, R.A. Study of the antinociceptive action of the ethanolic extract and the triterpene 24-hydroxytormentic acid isolated from the stem bark of Ocotea suaveolens. Planta Med. 1999, 65, 50–55. [Google Scholar] [CrossRef]
- Martínez, V.; Coutinho, S.V.; Thakur, S.; Mogil, J.S.; Taché, Y.; Mayer, E.A. Differential effects of chemical and mechanical colonic irritation on behavioral pain response to intraperitoneal acetic acid in mice. Pain 1999, 81, 179–186. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Broadhurst, P.L. Experiments in Psychogenetics; Routledge Kegan Paul: London, UK, 1960; pp. 31–61. [Google Scholar]
- Johnson, J.; Pajarillo, E.A.B.; Taka, E.; Reams, R.; Son, D.S.; Aschner, M.; Lee, E. Valproate and sodium butyrate attenuate manganese-decreased locomotor activity and astrocytic glutamate transporters expression in mice. Neurotoxicology 2018, 64, 230–239. [Google Scholar] [CrossRef]
- Curzon, P.; Zhang, M.; Radek, R.; Fox, G. The behavioral assessment of sensorimotor processes in the mouse: Acoustic startle, sensory gating, locomotor activity, rotarod, and beam walking. Methods Behav. Anal. Neurosci. 2009, 2. [Google Scholar] [CrossRef]
- Simões, R.R.; Dos Santos Coelho, I.; Do Espírito Santo, C.C.; Morel, A.F.; Zanchet, E.M.; Santos, A.R.S. Oral treatment with methanolic extract of the root bark of Condalia buxifolia Reissek alleviates acute pain and inflammation in mice: Potential interactions with PGE2, TRPV1/ASIC and PKA signaling pathways. J. Ethnopharmacol. 2016, 185, 319–326. [Google Scholar] [CrossRef]
- Meotti, F.C.; dos Coelho, I.S.; Santos, A.R.S. The Nociception Induced by Glutamate in Mice Is Potentiated by Protons Released into the Solution. J. Pain 2010, 11, 570–578. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [CrossRef] [PubMed]
- Barrot, M. Tests and models of nociception and pain in rodents. Neuroscience 2012, 211, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. Trpa1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Bernd, N.; Flockerzi, V. (Eds.) Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2, ISBN 9783642542152. [Google Scholar]
- Azhdari-Zarmehri, H.; Mohammad-Zadeh Prof, M.; Feridoni, M.; Nazeri, M. Termination of nociceptive bahaviour at the end of phase 2 of formalin test is attributable to endogenous inhibitory mechanisms, but not by opioid receptors activation. Basic Clin. Neurosci. 2014, 5, 48–54. [Google Scholar]
- Dall’Acqua, M.C.; Bonet, I.J.M.; Zampronio, A.R.; Tambeli, C.H.; Parada, C.A.; Fischer, L. The contribution of transient receptor potential ankyrin 1 (TRPA1) to the in vivo nociceptive effects of prostaglandin E2. Life Sci. 2014, 105, 7–13. [Google Scholar] [CrossRef]
- Nassini, R.; Fusi, C.; Materazzi, S.; Coppi, E.; Tuccinardi, T.; Marone, I.M.; De Logu, F.; Preti, D.; Tonello, R.; Chiarugi, A.; et al. The TRPA1 channel mediates the analgesic action of dipyrone and pyrazolone derivatives. Br. J. Pharmacol. 2015, 172, 3397–3411. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, G.; Pérez-Albiter, M.; Serrano-García, N.; Mares-Sámano, J.J.; Rojas, P. Spirulina maxima pretreatment partially protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Nutr. Neurosci. 2006, 9, 207–212. [Google Scholar] [CrossRef]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef]
- Horváth, G.; Kemény, Á.; Barthó, L.; Molnár, P.; Deli, J.; Szente, L.; Bozó, T.; Pál, S.; Sándor, K.; Szőke, É.; et al. Effects of some natural carotenoids on TRPA1- and TRPV1-Induced neurogenic inflammatory processes in vivo in the mouse skin. J. Mol. Neurosci. 2015, 56, 113–121. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ali Reza, A.S.M.; Rahaman, M.M.; Nasrin, M.S.; Rahat, M.R.U.; Islam, M.R.; Uddin, M.J.; Rahman, M.A. Evaluation of morning glory (Jacquemontia tamnifolia (L.) Griseb) leaves for antioxidant, antinociceptive, anticoagulant and cytotoxic activities. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 291–299. [Google Scholar] [CrossRef]
- Djiazet, S.; Kenfack, L.B.M.; Devi, P.B.; Nazareth, M.S.; Tchiégang, C.; Shetty, P.H. Phenolic profile, antioxidant and enzyme inhibitory activities of underutilized spices from Central Africa. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Meini, M.R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from Syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Kim, H.J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.S.; Kang, C.M.; Ferruzzi, M.G.; Ahn, M.J. Two classes of pigments, carotenoids and c-phycocyanin, in spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Rossato, M.F.; Tonello, R.; Hoffmeister, C.; Klafke, J.Z.; Rosa, F.; Pinheiro, K.V.; Pinheiro, F.V.; Boligon, A.A.; Athayde, M.L.; et al. Gallic acid functions as a TRPA1 antagonist with relevant antinociceptive and antiedematogenic effects in mice. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014, 387, 679–689. [Google Scholar] [CrossRef]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Gomez-Monterrey, I.; La Rana, G.; Gualillo, O.; Calignano, A.; et al. Characterization of New TRPM8 Modulators in Pain Perception. Int. J. Mol. Sci. 2019, 20, 5544. [Google Scholar] [CrossRef] [PubMed]
- Beccari, A.R.; Gemei, M.; Monte, M.L.; Menegatti, N.; Fanton, M.; Pedretti, A.; Bovolenta, S.; Nucci, C.; Molteni, A.; Rossignoli, A.; et al. Novel selective, potent naphthyl TRPM8 antagonists identified through a combined ligand-and structure-based virtual screening approach. Sci. Rep. 2017, 7, 10999. [Google Scholar] [CrossRef]
- De Caro, C.; Russo, R.; Avagliano, C.; Cristiano, C.; Calignano, A.; Aramini, A.; Bianchini, G.; Allegretti, M.; Brandolini, L. Antinociceptive effect of two novel transient receptor potential melastatin 8 antagonists in acute and chronic pain models in rat. Br. J. Pharmacol. 2018, 175, 1691–1706. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Daniels, R.L.; Palkar, R.; McCoy, D.D.; McKemy, D.D. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Julius, D. TRP Channels and Pain; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2013; Volume 29, ISBN 9781315152837. [Google Scholar]
- Chung, M.K.; Jung, S.J.; Oh, S.B. Role of TRP channels in pain sensation. Adv. Exp. Med. Biol. 2011, 704, 615–636. [Google Scholar] [CrossRef]
- Baron, R. Neuropathic Pain: A Clinical Perspective. Handb. Exp. Pharmacol. 2009, 16, 129–145. [Google Scholar] [CrossRef]
- Pereira, V.; Goudet, C. Emerging trends in pain modulation by metabotropic glutamate receptors. Front. Mol. Neurosci. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.M.; Rojas, C.; Wu, Y.W.; Slusher, B.S. The Role of Glutamate Signaling in Pain Processes and its Regulation by GCP II Inhibition. Curr. Med. Chem. 2012, 19, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.M.; Miller, K.E. Peripheral inhibition of glutaminase reduces carrageenan- induced Fos expression in the superficial dorsal horn of the rat Ernest. Neurosci Lett. 2010, 472, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- White, J.P.M.; Urban, L.; Nagy, I. TRPV1 Function in Health and Disease. Curr. Pharm. Biotechnol. 2010, 12, 130–144. [Google Scholar] [CrossRef]
- Jardín, I.; López, J.J.; Diez, R.; Sánchez-Collado, J.; Cantonero, C.; Albarrán, L.; Woodard, G.E.; Redondo, P.C.; Salido, G.M.; Smani, T.; et al. TRPs in pain sensation. Front. Physiol. 2017, 8, 392. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Savegnago, L.; Jacob, R.G.; Victoria, F.N.; Martinez, D.M. Antinociceptive effect of essential oils and their constituents: An update review. J. Braz. Chem. Soc. 2016, 27, 435–474. [Google Scholar] [CrossRef]
- Thompson, S.J.; Pitcher, M.H.; Stone, L.S.; Tarum, F.; Niu, G.; Chen, X.; Kiesewetter, D.O.; Schweinhardt, P.; Bushnell, M.C. Chronic neuropathic pain reduces opioid receptor availability with associated anhedonia in rat. Pain 2018, 159, 1856–1866. [Google Scholar] [CrossRef]
- Liang, X.; Liu, R.; Chen, C.; Ji, F.; Li, T. Opioid system modulates the immune function: A Review. Transl. Perioper. Pain Med. 2016, 1, 5–13. [Google Scholar]
- Marco, C.A.; Trautman, W.; Cook, A.; Mann, D.; Rasp, J.; Perkins, O.; Ballester, M. Naloxone use among emergency department patients with opioid overdose. J. Emerg. Med. 2018, 55, 64–70. [Google Scholar] [CrossRef]
- Santos, D.S.; Lauria, P.S.S.; Evangelista, A.F.; Azeredo, F.J.; Costa, J.A.V.; Soares, M.B.P.; Druzian, J.I.; Villarreal, C.F. Beyond inflammation: centrally mediated antinociceptive properties of Spirulina platensis LEB-18 biomass via the opioid system. J. Funct. Foods 2020, 72, 104083. [Google Scholar] [CrossRef]
- Ruiz-Miyazawa, K.W.; Staurengo-Ferrari, L.; Mizokami, S.S.; Domiciano, T.P.; Vicentini, F.T.M.C.; Camilios-Neto, D.; Pavanelli, W.R.; Pinge-Filho, P.; Amaral, F.A.; Teixeira, M.M.; et al. Quercetin inhibits gout arthritis in mice: Induction of an opioid-dependent regulation of inflammasome. Inflammopharmacology 2017, 25, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.; Roman-Campos, D.; Lotufo, C.M.; Duarte, H.L.; Souza, G.R.; Verri, W.A.; Funez, M.I.; Dias, Q.M.; Schivo, I.R.; Domingues, A.C.; et al. Morphine peripheral analgesia depends on activation of the PI3Kγ/AKT/nNOS/NO/KATP signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 4442–4447. [Google Scholar] [CrossRef] [PubMed]
- Tatem, K.S.; Quinn, J.L.; Phadke, A.; Yu, Q.; Gordish-Dressman, H.; Nagaraju, K. Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J. Vis. Exp. 2014, 51785. [Google Scholar] [CrossRef]
- Zimcikova, E.; Simko, J.; Karesova, I.; Kremlacek, J.; Malakova, J. Behavioral effects of antiepileptic drugs in rats: Are the effects on mood and behavior detectable in open-field test? Seizure 2017, 52, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Pigatto, G.R.; Silva, C.S.; Parizotto, N.A. Photobiomodulation therapy reduces acute pain and inflammation in mice. J. Photochem. Photobiol. B Biol. 2019, 196, 111513. [Google Scholar] [CrossRef]
- Souza, G.V.; Simas, A.S.; Bastos-Pereira, A.L.; Frois, G.R.A.; Ribas, J.L.C.; Verdan, M.H.; Kassuya, C.A.L.; Stefanello, M.E.; Zampronio, A.R. Antinociceptive activity of the ethanolic extract, fractions, and aggregatin d isolated from sinningia aggregata Tubers. PLoS ONE 2015, 10. [Google Scholar] [CrossRef][Green Version]
- Radić, Z.; Sit, R.K.; Garcia, E.; Zhang, L.; Berend, S.; Kovarik, Z.; Amitai, G.; Fokin, V.V.; Barry Sharpless, K.; Taylor, P. Mechanism of interaction of novel uncharged, centrally active reactivators with OP-hAChE conjugates. Chem. Biol. Interact. 2013, 203, 67–71. [Google Scholar] [CrossRef]
- Rodrigues de Carvalho, A.M.; Vasconcelos, L.F.; Moura Rocha, N.F.; Vasconcelos Rios, E.R.; Dias, M.L.; Maria de França Fonteles, M.; Gaspar, D.M.; Barbosa Filho, J.M.; Chavez Gutierrez, S.J.; Florenço de Sousa, F.C. Antinociceptive activity of Riparin II from Aniba riparia: Further elucidation of the possible mechanisms. Chem. Biol. Interact. 2018, 287, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Nguelefack, T.B.; Dutra, R.C.; Paszcuk, A.F.; de Andrade, E.L.; Calixto, J.B. TRPV1 channel inhibition contributes to the antinociceptive effects of Croton macrostachyus extract in mice. BMC Complement. Altern. Med. 2015, 15, 293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, F.; Dadkhah, M.; Moradi-Kor, N.; Obeidavi, Z. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy Dovepress effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 375. [Google Scholar] [CrossRef] [PubMed]
| Nutritional Values | Per 100 g | * RIA/** NRV |
|---|---|---|
| Energy | 1364 kJ/326 Kcal | |
| Lipids | 1 g | |
| -of which saturated | 0.5 g | |
| Carbohydrates | 13.1 g | |
| -of which sugars | <0.1 g | |
| Fiber | 5.1 g | |
| Proteins | 65.9 g | 133% * |
| Salt | 0.9 g | |
| Potassium | 1040 mg | 52% ** |
| Calcium | 332.5 mg | 42% ** |
| Iron | 83.2 mg | 594% ** |
| Vitamin B6 (Pyridoxine) | 18.5 mg | 1314% ** |
| Vitamin B12 (Cobalamin) | 170 μg | 6800% ** |
| Vitamin E | 12.7 mg | 105% ** |
| Spirulina platensis | ||||
|---|---|---|---|---|
| Phenolic compounds (μg/mL) | Mean ± SD | |||
| -Gallic acid | 100.89 | 102.95 | 102.05 | 102.0 ± 1.03 |
| -Chlorogenic acid | 125.15 | 141.25 | 125.65 | 130.7 ± 9.15 |
| Flavonoids contents (μg/mL) | ||||
| -Quercetin | 624.23 | 611.42 | 605.00 | 613.6 ± 9.79 |
| -Catechin | 230.19 | 230.77 | 207.31 | 222.8 ± 13.38 |
| Antioxidant activity | ||||
| -Trolox (μg/mL) | 132.76 | 120.86 | 140.86 | 131.5 ± 10.06 |
| -Scavenging activity (%) | 42.17 | 40.16 | 43.55 | 41.96 ± 1.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, M.A.; Vasconcelos, A.; Gonçalves, E.C.D.; Ferrarini, E.G.; Vieira, G.B.; Cicia, D.; Cola, M.; Capasso, R.; Dutra, R.C. Involvement of Opioid System and TRPM8/TRPA1 Channels in the Antinociceptive Effect of Spirulina platensis. Biomolecules 2021, 11, 592. https://doi.org/10.3390/biom11040592
Freitas MA, Vasconcelos A, Gonçalves ECD, Ferrarini EG, Vieira GB, Cicia D, Cola M, Capasso R, Dutra RC. Involvement of Opioid System and TRPM8/TRPA1 Channels in the Antinociceptive Effect of Spirulina platensis. Biomolecules. 2021; 11(4):592. https://doi.org/10.3390/biom11040592
Chicago/Turabian StyleFreitas, Mariana A., Amanda Vasconcelos, Elaine C. D. Gonçalves, Eduarda G. Ferrarini, Gabriela B. Vieira, Donatella Cicia, Maíra Cola, Raffaele Capasso, and Rafael C. Dutra. 2021. "Involvement of Opioid System and TRPM8/TRPA1 Channels in the Antinociceptive Effect of Spirulina platensis" Biomolecules 11, no. 4: 592. https://doi.org/10.3390/biom11040592
APA StyleFreitas, M. A., Vasconcelos, A., Gonçalves, E. C. D., Ferrarini, E. G., Vieira, G. B., Cicia, D., Cola, M., Capasso, R., & Dutra, R. C. (2021). Involvement of Opioid System and TRPM8/TRPA1 Channels in the Antinociceptive Effect of Spirulina platensis. Biomolecules, 11(4), 592. https://doi.org/10.3390/biom11040592









