Bone Morphogenetic Proteins and Diabetic Retinopathy
Abstract
1. Introduction
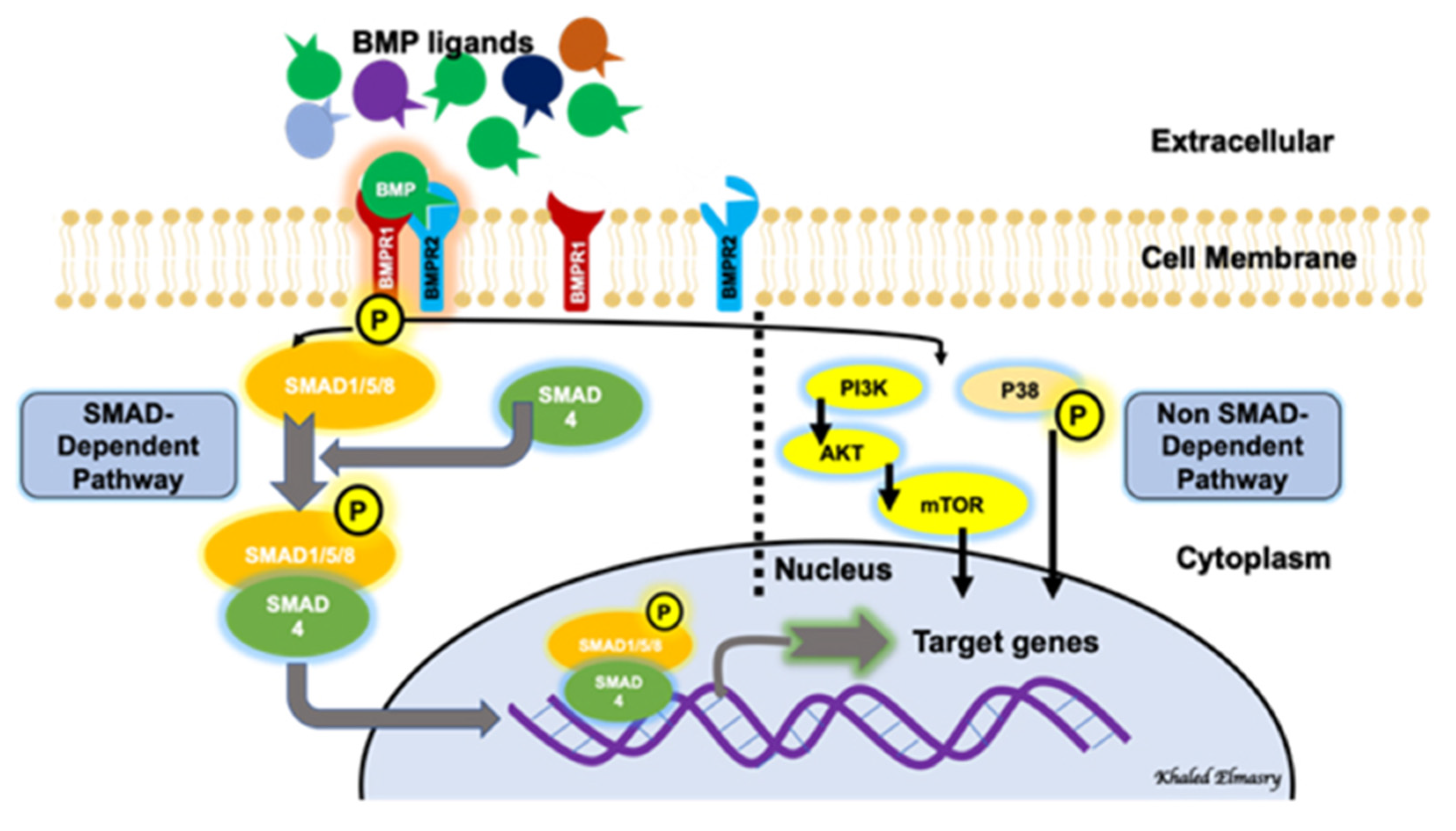
2. BMP Signaling Pathways
3. BMP Receptors
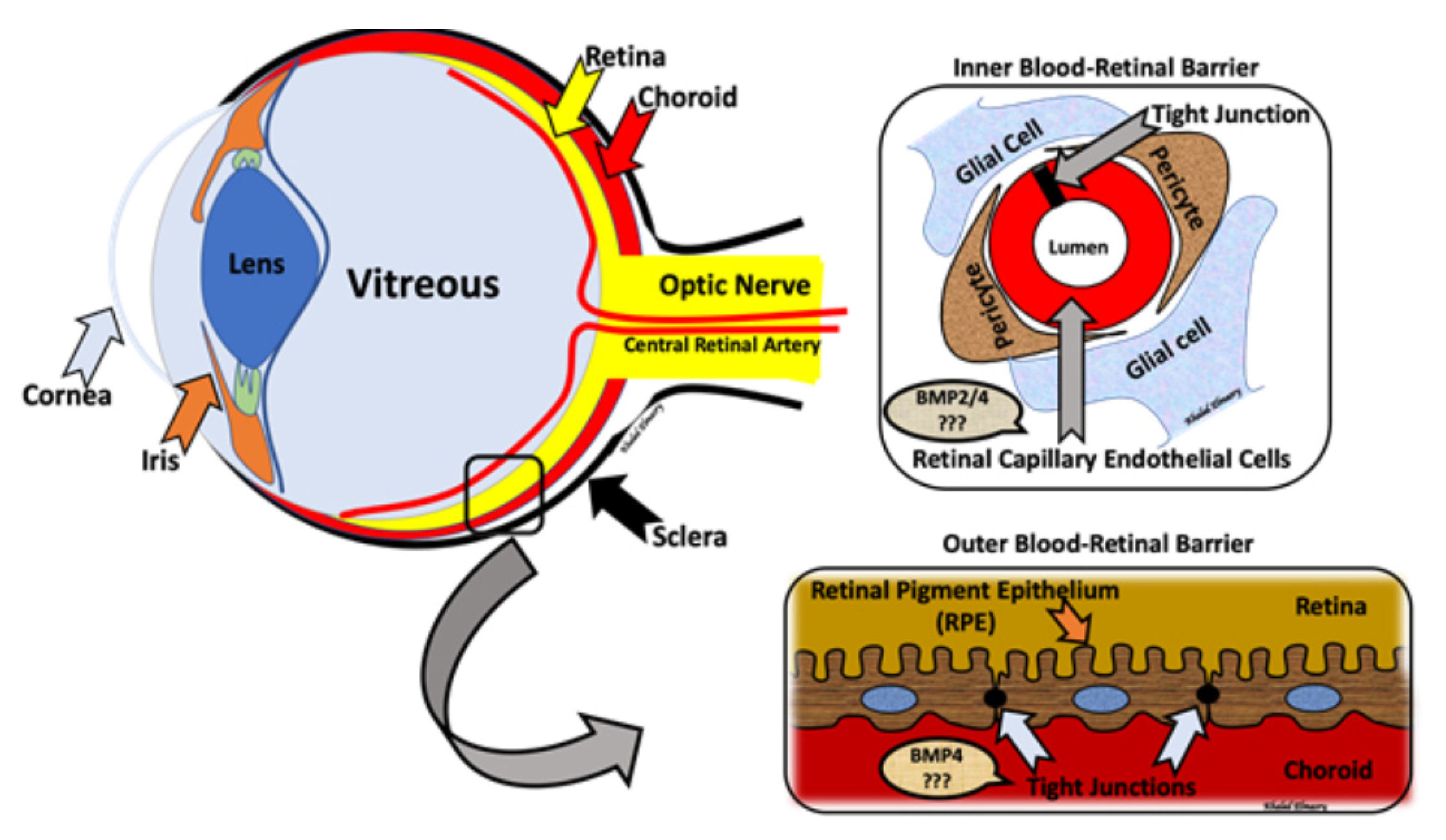
4. BMP Signaling Regulation and Endothelial Cell Function
5. BMPs and Retinal Development
6. BMP and Diabetes-Induced Vascular Complications
7. BMP and Diabetic Retinopathy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.H.; Campbell, J.; Holecamp, N.M.; Kiss, S.; Leowenstein, A.; Augustin, A.J.; Ma, J.; Ho, A.C.; Patel, V.; Whitcup, S.M.; et al. Early and Long-Term Responses to Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Ede-ma: Analysis of Protocol I Data. Am. J. Ophthalmol. 2016, 172, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.L.; Caballero, S.; Newell, C.K.; Steinmetz, R.L.; Watson, D.; Segal, M.S.; Harrison, J.; Scott, E.W.; Grant, M.B. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic reti-nopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch. Ophthalmol. 2004, 122, 1801–1807. [Google Scholar] [CrossRef]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]
- Haller, J.A.; Kuppermann, B.D.; Blumenkranz, M.S.; Williams, G.A.; Weinberg, D.V.; Chou, C.; Whitcup, S.M. Randomized Controlled Trial of an Intravitreous Dexamethasone Drug Delivery System in Patients With Diabetic Macular Edema. Arch. Ophthalmol. 2010, 128, 289–296. [Google Scholar] [CrossRef]
- Smithen, L.M.; Ober, M.D.; Maranan, L.; Spaide, R.F. Intravitreal triamcinolone acetonide and intraocular pressure. Am. J. Ophthalmol. 2004, 138, 740–743. [Google Scholar] [CrossRef]
- Callanan, D.G.; Gupta, S.; Boyer, D.S.; Ciulla, T.A.; Singer, M.A.; Kuppermann, B.D.; Ching-Chi, L.; Xiao-Yan, L.; Hollander, D.A.; Schiffman, R.M.; et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse dia-betic macular edema. Ophthalmology 2013, 120, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.-Y.; Cui, H.; Hashad, Y.; Whitcup, S.M. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Runkle, E.A.; Antonetti, D.A. The blood-retinal barrier: Structure and functional significance. Blood Brain Neural Barriers 2011, 686, 133–148. [Google Scholar]
- Liu, L.; Liu, X. Roles of Drug Transporters in Blood-Retinal Barrier. Adv. Exp. Med. Biol. 2019, 1141, 467–504. [Google Scholar]
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight Junctions of the Outer Blood Retina Barrier. Int. J. Mol. Sci. 2019, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- DeRubertis, F.R.; Craven, P.A.; Melham, M.F.; Salah, E.M. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: Evidence for reduced super-oxide-nitric oxide interaction. Diabetes 2004, 53, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes. Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect Biol. 2016, 8, a021899. [Google Scholar] [CrossRef]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-beta/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-beta Family. Cold Spring Harb Perspect Biol. 2017, 9, a022129. [Google Scholar] [CrossRef]
- Perera, N.; Ritchie, R.H.; Tate, M. The Role of Bone Morphogenetic Proteins in Diabetic Complications. ACS Pharmacol. Transl. Sci. 2020, 3, 11–20. [Google Scholar] [CrossRef]
- Heldin, C.H.; Miyazono, K. Ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef]
- Horbelt, D.; Denkis, A.; Knaus, P. A portrait of Transforming Growth Factor beta superfamily signalling: Background matters. Int. J. Biochem. Cell Biol. 2012, 44, 469–474. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, B.L.; Imamura, T.; Okadome, T.; Cox, G.N.; Yamashita, H.; Dijke, P.T.; Heldin, C.H.; Miyazono, K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 7632–7636. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A. Heldin, C.H. The regulation of TGFbeta signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2009, 147, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.B.; Beppu, H.; Kawai, N.; Li, E.; Bloch, K.D. Bone Morphogenetic Protein (BMP) Type II Receptor Deletion Reveals BMP Ligand-specific Gain of Signaling in Pulmonary Artery Smooth Muscle Cells. J. Biol. Chem. 2005, 280, 24443–24450. [Google Scholar] [CrossRef] [PubMed]
- Dijke, P.T.; Goumans, M.-J.; Pardali, E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008, 11, 79–89. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Mallet, C.; Mazerbourg, S.; Feige, J.J.; Bailly, S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endo-thelial cells. Blood 2007, 109, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Kirkbride, K.C.; Townsend, T.A.; Bruinsma, M.W.; Barnett, J.V.; Blobe, G.C. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J. Biol. Chem. 2008, 283, 7628–7637. [Google Scholar] [CrossRef]
- Barbara, N.P.; Wrana, J.L.; Letarte, M. Endoglin is an accessory protein that interacts with the signaling receptor complex of mul-tiple members of the transforming growth factor-beta superfamily. J. Biol. Chem. 1999, 274, 584–594. [Google Scholar] [CrossRef]
- Sedlmeier, G.; Sleeman, J.P. Extracellular regulation of BMP signaling: Welcome to the matrix. Biochem. Soc. Trans. 2017, 45, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kamiya, Y.; Imamura, T.; Miyazono, K.; Miyazawa, K. Selective Inhibitory Effects of Smad6 on Bone Morphogenetic Protein Type I Receptors. J. Biol. Chem. 2007, 282, 20603–20611. [Google Scholar] [CrossRef]
- Hanyu, A.; Ishidou, Y.; Ebisawa, T.; Shimanuki, T.; Imamura, T.; Miyazono, K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J. Cell Biol. 2001, 155, 1017–1027. [Google Scholar] [CrossRef]
- Dyer, L.A.; Pi, X.; Patterson, C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol. Metab. 2014, 25, 472–480. [Google Scholar] [CrossRef]
- Benn, A.; Hiepen, C.; Osterland, M.; Schütte, C.; Zwijsen, A.; Knaus, P. Role of bone morphogenetic proteins in sprouting angiogenesis: Differential BMP receptor-dependent signaling path-ways balance stalk vs. tip cell competence. FASEB J. 2017, 31, 4720–4733. [Google Scholar] [CrossRef]
- De Vinuesa, A.G.; Abdelilah-Seyfried, S.; Knaus, P.; Zwijsen, A.; Bailly, S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 2016, 27, 65–79. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Wang, Y.; Jiang, H.; Xu, X.; Zhang, C.; Li, D.; Xu, C.; Zhang, K.; Qi, Y.; et al. Bone morphogenetic protein 4 inhibits liposaccharide-induced inflammation in the airway. Eur. J. Immunol. 2014, 44, 3283–3294. [Google Scholar] [CrossRef]
- Helbing, T.; Arnold, L.; Wiltgen, G.; Hirschbihl, E.; Gabelmann, V.; Hornstein, A.; Esser, J.S.; Diehl, P.; Grundmann, S.; Busch, H.-J.; et al. Endothelial BMP4 Regulates Leukocyte Diapedesis and Promotes Inflammation. Inflammation. 2017, 40, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Pardali, E.; Makowski, L.M.; Leffers, M.; Borgscheiper, A.; Waltenberger, J. BMP-2 induces human mononuclear cell chemotaxis and adhesion and modulates monocyte-to-macrophage differentia-tion. J. Cell Mol. Med. 2018, 22, 5429–5438. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Q.; Xing, L.; Zhang, J.-H.; Zhao, M.; Horn, D.; Chan, J.; Boyce, B.F.; Harris, S.E.; Mundy, G.R.; Chen, D. NF-kappaB specifically activates BMP-2 gene expression in growth plate chondrocytes in vivo and in a chondrocyte cell line in vitro. J. Biol. Chem. 2003, 278, 29130–29135. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, I.; Arroyo-Camarena, U.D.; Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Dueñas-Andrade, Y.; Hernández-Ruiz, J.; Béjar, Y.L.; Zaga-Clavellina, V.; Morales-Montor, J.; Terrazas, L.I.; et al. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol. Lett. 2016, 176, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Moganti, K.; Li, F.; Schmuttermaier, C.; Riemann, S.; Klueter, H.; Gratchev, A.; Harmsen, M.C.; Kzhyshkowska, J. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology 2017, 222, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Bennett, B.J.; Wang, X.; Rosenfeld, M.E.; Giachelli, C.M.; Lusis, A.J.; Boström, K.I. Inhibition of Bone Morphogenetic Proteins Protects Against Atherosclerosis and Vascular Calcification. Circ. Res. 2010, 107, 485–494. [Google Scholar] [CrossRef]
- Boström, K.I.; Jumabay, M.; Matveyenko, A.; Nicholas, S.B.; Yao, Y. Activation of Vascular Bone Morphogenetic Protein Signaling in Diabetes Mellitus. Circ. Res. 2011, 108, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Derwall, M.S.; Malhotra, R.; Lai, C.S.; Beppu, Y.; Aikawa, E.; Seehra, J.S.; Zapol, W.M.; Bloch, K.D.; Yu, P.B. Inhibition of Bone Morphogenetic Protein Signaling Reduces Vascular Calcification and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-beta Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Chang, C. Agonists and Antagonists of TGF-beta Family Ligands. Cold Spring Harb Perspect Biol. 2016, 8, a021923. [Google Scholar] [CrossRef] [PubMed]
- Esser, J.S.; Rahner, S.; Deckler, M.; Bode, C.; Patterson, C.; Moser, M. Fibroblast Growth Factor Signaling Pathway in Endothelial Cells Is Activated by BMPER to Promote Angiogenesis. Arter. Thromb. Vasc. Biol. 2015, 35, 358–367. [Google Scholar] [CrossRef]
- Huan, C.; Yang, T.; Liang, J.; Xie, T.; Cheng, L.; Liu, N.; Kurkciyan, A.; Mena, J.M.; Wang, C.; Dai, H.; et al. Methylation-mediated BMPER expression in fibroblast activation in vitro and lung fibrosis in mice in vivo. Sci. Rep. 2015, 5, 14910. [Google Scholar] [CrossRef]
- Dyer, L.; Wu, Y.; Moser, M.; Patterson, C. BMPER-induced BMP signaling promotes coronary artery remodeling. Dev. Biol. 2014, 386, 385–394. [Google Scholar] [CrossRef]
- Helbing, T.; Wiltgen, G.; Hornstein, A.; Brauers, E.Z.; Arnold, L.; Bauer, A.; Esser, J.S.; Diehl, P.; Grundmann, S.; Fink, K.; et al. Bone Morphogenetic Protein-Modulator BMPER Regulates Endothelial Barrier Function. Inflammation 2017, 40, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Miralles, I.; Ren, R.; Moser, M.; Hartnett, M.E.; Patterson, C. Bone Morphogenetic Protein Endothelial Cell Precursor–Derived Regulator Regulates Retinal Angiogenesis In Vivo in a Mouse Model of Oxygen-Induced Retinopathy. Arter. Thromb. Vasc. Biol. 2011, 31, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.; Ren, R.; Pi, X.; Wu, Y.; Moreno, I.; Willis, M.; Moser, M.; Ross, M.; Podkowa, M.; Attisano, L.; et al. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J. Cell Biol. 2009, 184, 597–609. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Hussein, K.; Wang, F.; Wan, M.; Elmasry, K.; Elsherbiny, N.; Saleh, H.; Yu, P.B.; Tawfik, A.; Ibrahim, A.S. Bone Morphogenetic Protein-2 Induces Non-Canonical Inflammatory and Oxidative Pathways in Human Retinal Endothelial Cells. Front. Immunol. 2021, 11, 568795. [Google Scholar] [CrossRef]
- Mishina, Y.; Suzuki, A.; Ueno, N.; Behringer, R.R. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryo-genesis. Genes Dev. 1995, 9, 3027–3037. [Google Scholar] [CrossRef]
- Délot, E.C.; Bahamonde, M.E.; Zhao, M.; Lyons, K.M. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development 2003, 130, 209–220. [Google Scholar] [CrossRef]
- Khalaf, M.; Morera, J.; Bourret, A.; Reznik, Y.; Denoual, C.; Herlicoviez, M.; Mittre, H.; Benhaim, A. BMP system expression in GCs from polycystic ovary syndrome women and the in vitro effects of BMP4, BMP6, and BMP7 on GC steroidogenesis. Eur. J. Endocrinol. 2013, 168, 437–444. [Google Scholar] [CrossRef]
- Harris, R.E.; Russell, R.K. BMPR1A mutation-positive juvenile polyposis syndrome and atrial septal defect: Coincidence or associa-tion? BMJ Case Rep. 2019, 12, e229881. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, J.Y.; Di Wu, X.C.L.; Zhang, J.S.; Zhang, Z.Y. Mutually inductive interactions between the lens and retina require ALK3 functions during mouse embryonic devel-opment. Int. J. Ophthalmol. 2012, 5, 119–124. [Google Scholar] [PubMed]
- Todd, L.; Palazzo, I.; Squires, N.; Mendonca, N.; Fischer, A.J. BMP- and TGFbeta-signaling regulate the formation of Muller glia-derived progenitor cells in the avian retina. Glia 2017, 65, 1640–1655. [Google Scholar] [CrossRef]
- Pandit, T.; Jidigam, V.K.; Patthey, C.; Gunhaga, L. Neural retina identity is specified by lens-derived BMP signals. Development 2015, 142, 1850–1859. [Google Scholar] [CrossRef]
- Steinfeld, J.; Steinfeld, I.; Bausch, A.; Coronato, N.; Hampel, M.-L.; Depner, H.; Layer, P.G.; Vogel-Höpker, A. BMP-induced reprogramming of the neural retina into retinal pigment epithelium requires Wnt signalling. Biol. Open 2017, 6, 979–992. [Google Scholar] [CrossRef]
- Benn, A.; Alonso, F.; Mangelschots, J.; Génot, E.; Lox, M.; Zwijsen, A. BMP-SMAD1/5 Signaling Regulates Retinal Vascular Development. Biomolecules 2020, 10, 488. [Google Scholar] [CrossRef]
- Yan, X.; Atorf, J.; Ramos, D.; Thiele, F.; Weber, S.; Dalke, C.; Sun, M.; Puk, O.; Michel, D.; Fuchs, H.; et al. Mutation in Bmpr1b Leads to Optic Disc Coloboma and Ventral Retinal Gliosis in Mice. Investig. Opthalmology Vis. Sci. 2020, 61, 44. [Google Scholar] [CrossRef]
- Cau, E.; Ronsin, B.; Bessière, L.; Blader, P. A Notch-mediated, temporal asymmetry in BMP pathway activation promotes photoreceptor subtype diversification. PLoS Biol. 2019, 17, e2006250. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.A.; Mouillesseaux, K.P.; Chong, D.C.; Bautch, V.L. Developmental SMAD6 loss leads to blood vessel hemorrhage and disrupted endothelial cell junctions. Dev. Biol. 2018, 442, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Alsina-Sanchís, E.; García-Ibáñez, Y.; Figueiredo, A.M.; Riera-Domingo, C.; Figueras, A.; Matias-Guiu, X.; Casanovas, O.; Botella, L.M.; Pujana, M.A.; Riera-Mestre, A.; et al. ALK1 Loss Results in Vascular Hyperplasia in Mice and Humans Through PI3K Activation. Arter. Thromb. Vasc. Biol. 2018, 38, 1216–1229. [Google Scholar] [CrossRef]
- Ola, R.; Künzel, S.H.; Zhang, F.; Genet, G.; Chakraborty, R.; Pibouin-Fragner, L.; Martin, K.; Sessa, W.; Dubrac, A.; Eichmann, A. SMAD4 Prevents Flow Induced Arteriovenous Malformations by Inhibiting Casein Kinase 2. Circulation 2018, 138, 2379–2394. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Chong, D.C.; Ola, R.; Dunworth, W.P.; Meadows, S.; Ka, J.; Kaartinen, V.M.; Qyang, Y.; Cleaver, O.; Bautch, V.L.; et al. Alk2/ACVR1 and Alk3/BMPR1A Provide Essential Function for Bone Morphogenetic Protein-Induced Retinal Angi-ogenesis. Arter. Thromb. Vasc. Biol. 2017, 37, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, T.; Singh, R.R.; Gupta, S.; Surolia, A. Bone morphogenetic protein-7 (BMP-7) augments insulin sensitivity in mice with type II diabetes mellitus by potentiating PI3K/AKT pathway. BioFactors 2017, 43, 195–209. [Google Scholar] [CrossRef]
- Pauk, M.; Bordukalo-Niksic, T.; Brkljacic, J.; Paralkar, V.M.; Brault, A.L.; Dumic-Cule, I.; Borovecki, F.; Grgurevic, L.; Vukicevic, S. A novel role of bone morphogenetic protein 6 (BMP6) in glucose homeostasis. Acta Diabetol. 2018, 56, 365–371. [Google Scholar] [CrossRef]
- Hussein, K.A.; Choksi, K.; Akeel, S.; Ahmad, S.; Megyerdi, S.; El-Sherbiny, M.; Nawaz, M.; Abu El-Asrar, A.; Al-Shabrawey, M. Bone morphogenetic protein 2: A potential new player in the pathogenesis of diabetic retinopathy. Exp. Eye Res. 2014, 125, 79–88. [Google Scholar] [CrossRef]
- Koga, M.; Yamauchi, A.; Kanaoka, Y.; Jige, R.; Tsukamoto, A.; Teshima, N.; Nishioku, T.; Kataoka, Y. BMP4 is increased in the aortas of diabetic ApoE knockout mice and enhances uptake of oxidized low density lipoprotein into peritoneal macrophages. J. Inflamm. 2013, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sara, J.D.; Wang, F.-L.; Liu, L.-P.; Su, L.-X.; Zhe, J.; Wu, X.; Liu, J.-H. Increased plasma BMP-2 levels are associated with atherosclerosis burden and coronary calcification in type 2 diabetic patients. Cardiovasc. Diabetol. 2015, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akeel, S.; El-Awady, A.; Hussein, K.; El-Refaey, M.; Elsalanty, M.; Sharawy, M.; Al-Shabrawey, M. Recombinant bone morphogenetic protein-2 induces up-regulation of vascular endothelial growth factor and interleukin 6 in human pre-osteoblasts: Role of reactive oxygen species. Arch. Oral Biol. 2012, 57, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Bouletreau, P.J.; Warren, S.M.; Spector, J.A.; Peled, Z.M.; Gerrets, R.P.; Greenwald, J.A.; Longaker, M.T. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: Impli-cations for fracture healing. Plast. Reconstr. Surg. 2002, 109, 2384–2397. [Google Scholar] [CrossRef] [PubMed]
- Paine, S.K.; Basu, A.; Mondal, L.K.; Sen, A.; Choudhuri, S.; Choudhuri, I.H.; Saha, A.; Bhadhuri, G.; Mukherjee, A.; Bhattacharya, B.; et al. Association of vascular endothelial growth factor, transforming growth factor beta, and interferon gamma gene poly-morphisms with proliferative diabetic retinopathy in patients with type 2 diabetes. Mol. Vis. 2012, 18, 2749–2757. [Google Scholar] [PubMed]
- Guo, L.; Jiang, F.; Tang, Y.-T.; Si, M.-Y.; Jiao, X.-Y. The Association of Serum Vascular Endothelial Growth Factor and Ferritin in Diabetic Microvascular Disease. Diabetes Technol. Ther. 2014, 16, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Capitao, M.; Soares, R. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell Biochem. 2016, 117, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Hussein, K.; Wang, F.; Wan, M.; Saad, N.; Essa, M.; Kim, I.; Shakoor, A.; Owen, L.A.; DeAngelis, M.M.; et al. Bone Morphogenetic Protein (BMP)4 But Not BMP2 Disrupts the Barrier Integrity of Retinal Pigment Epithelia and Induces Their Migration: A Potential Role in Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2020, 9, 2293. [Google Scholar] [CrossRef]
- Vogt, R.R.; Unda, R.; Yeh, L.C.C.; Vidro, E.K.; Lee, J.C.; Tsin, A. T Bone morphogenetic protein-4 enhances vascular endothelial growth factor secretion by human retinal pigment epithe-lial cells. J. Cell Biochem. 2006, 98, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Li, H.; Yang, S.; Li, M.; Zhao, C.; Zhang, J.; Xu, G.; Wang, F. Inhibitory Effect of Bone Morphogenetic Protein 4 in Retinal Pigment Epithelial-Mesenchymal Transition. Sci. Rep. 2016, 6, 32182. [Google Scholar] [CrossRef] [PubMed]
- Akla, N.; Viallard, C.; Popovic, N.; Lora Gil, C.; Sapieha, P.; Larrivée, B. BMP9 (Bone Morphogenetic Protein-9)/Alk1 (Activin-Like Kinase Receptor Type I) Signaling Prevents Hyperglyce-mia-Induced Vascular Permeability. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1821–1836. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, D.; He, S.; Spee, C.; Ryan, S.J.; Hinton, D.R. Transcriptional regulation of bone morphogenetic protein 4 by tumor necrosis factor and its relationship with age-related macular degeneration. FASEB J. 2011, 25, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, D.; Sonoda, S.; He, S.; Spee, C.; Ryan, S.J.; Hinton, D.R. Over-expression of BMP4 inhibits experimental choroidal neovascularization by modulating VEGF and MMP-9. Angiogenesis 2012, 15, 213–227. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmasry, K.; Habib, S.; Moustafa, M.; Al-Shabrawey, M. Bone Morphogenetic Proteins and Diabetic Retinopathy. Biomolecules 2021, 11, 593. https://doi.org/10.3390/biom11040593
Elmasry K, Habib S, Moustafa M, Al-Shabrawey M. Bone Morphogenetic Proteins and Diabetic Retinopathy. Biomolecules. 2021; 11(4):593. https://doi.org/10.3390/biom11040593
Chicago/Turabian StyleElmasry, Khaled, Samar Habib, Mohamed Moustafa, and Mohamed Al-Shabrawey. 2021. "Bone Morphogenetic Proteins and Diabetic Retinopathy" Biomolecules 11, no. 4: 593. https://doi.org/10.3390/biom11040593
APA StyleElmasry, K., Habib, S., Moustafa, M., & Al-Shabrawey, M. (2021). Bone Morphogenetic Proteins and Diabetic Retinopathy. Biomolecules, 11(4), 593. https://doi.org/10.3390/biom11040593