The Effect of Herbal Medicinal Products on Psoriasis-Like Keratinocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Extracts
2.2. Antibodies and Reagents
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Cell Proliferation (BrdU Assay, Roche)
2.6. RNA Extraction and RT-PCR
2.7. Immunohistochemistry
2.8. Immunofluorescence
2.9. IL-6 and IL-8 ELISA
2.10. Psoriasis Ex Vivo Model
2.11. Statistical Analysis
3. Results
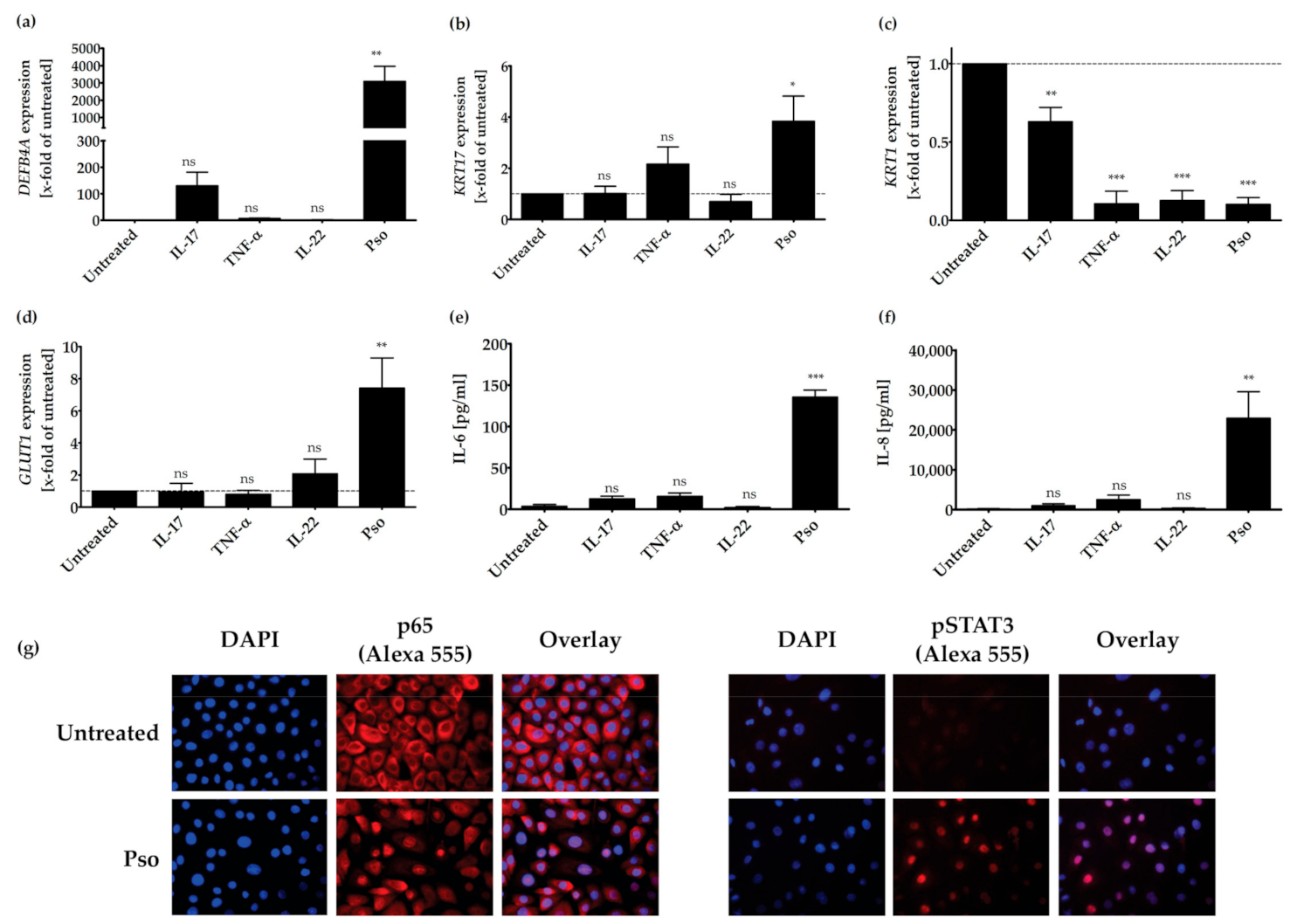
3.1. In Vitro Psoriasis Model
3.2. Screening of Plant Extracts for Anti-Psoriatic Effects In Vitro
3.3. Pathways Involved in the Anti-Psoriatic Effect of Curcuma amada (CA), Humulus lupulus (HL) and Hypericum perforatum (HP)
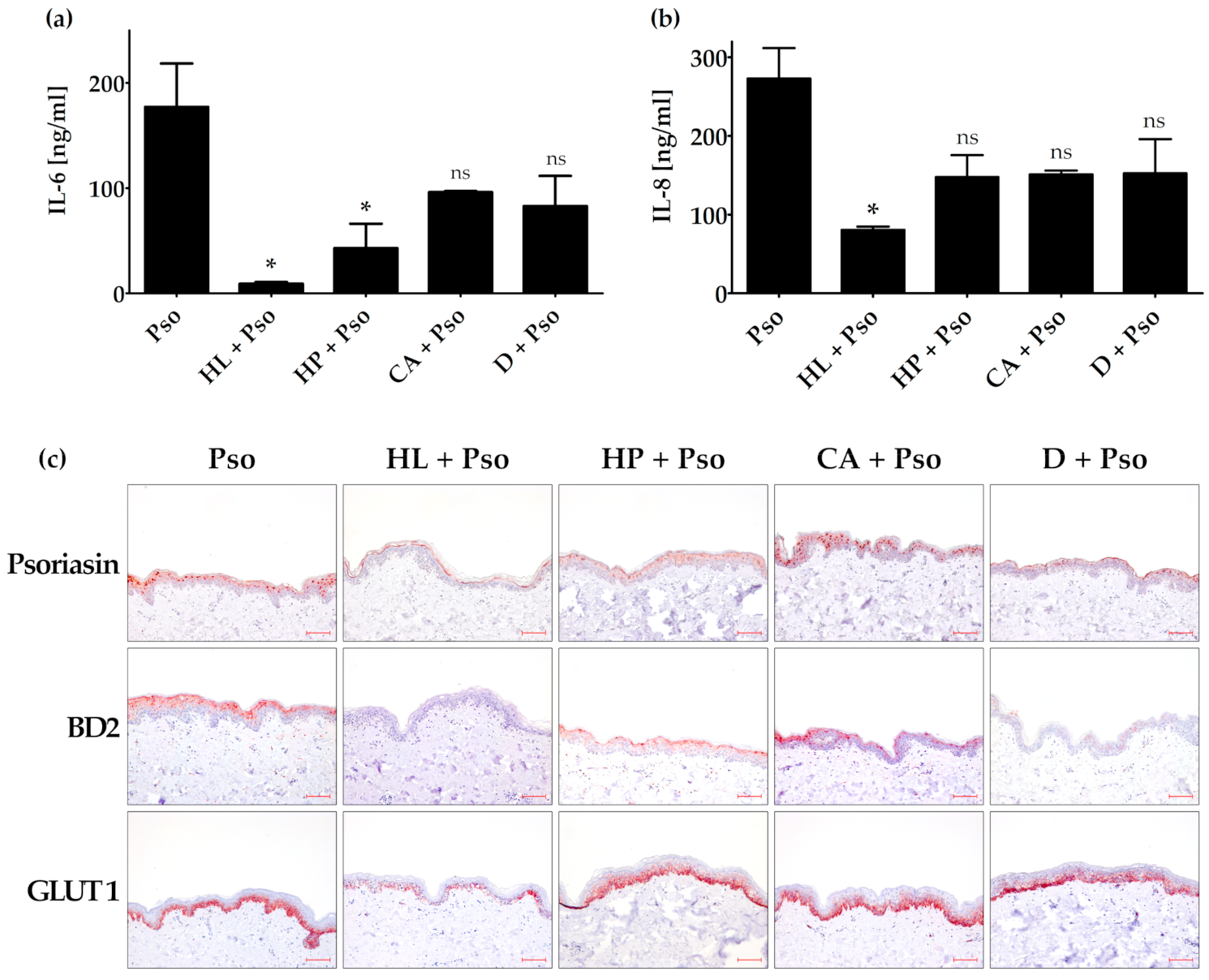
3.4. Effects of Humulus lupulus, Hypericum perforatum and Curcuma amada in an Ex Vivo Psoriasis Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melnikova, I. Psoriasis market. Nat. Rev. Drug Discov. 2009, 8, 767–768. [Google Scholar] [CrossRef]
- Benezeder, T.; Wolf, P. Resolution of plaque-type psoriasis: What is left behind (and reinitiates the disease). Semin. Immunopathol. 2019, 41, 633–644. [Google Scholar] [CrossRef]
- Hoffjan, S.; Stemmler, S. On the role of the epidermal differentiation complex in ichthyosis vulgaris, atopic dermatitis and psoriasis. Br. J. Dermatol. 2007, 157, 441–449. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Cui, T.; Dang, E.; Wang, G. Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17. J. Investig. Dermatol. 2017, 137, 2168–2176. [Google Scholar] [CrossRef]
- Herster, F.; Bittner, Z.; Archer, N.K.; Dickhöfer, S.; Eisel, D.; Eigenbrod, T.; Knorpp, T.; Schneiderhan-Marra, N.; Löffler, M.W.; Kalbacher, H.; et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Furue, K.; Yamamura, K.; Tsuji, G.; Mitoma, C.; Uchi, H.; Nakahara, T.; Kido-Nakahara, M.; Kadono, T.; Furue, M. Highlighting Interleukin-36 Signalling in Plaque Psoriasis and Pustular Psoriasis. Acta Derm. Venereol. 2018, 98, 5–13. [Google Scholar] [CrossRef]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef]
- Bai, X.; Yu, C.; Yang, L.; Luo, Y.; Zhi, D.; Wang, G.; Dang, E. Anti-psoriatic properties of paeoniflorin: Suppression of the NF-kappaB pathway and Keratin 17. Eur. J. Dermatol. EJD 2020, 30, 243–250. [Google Scholar] [CrossRef]
- Sampson, J.; Raman, A.; Karlsen, G.; Navsaria, H.; Leigh, I. In vitro keratinocyte antiproliferant effect of Centella asiatica extract and triterpenoid saponins. Phytomedicine 2001, 8, 230–235. [Google Scholar] [CrossRef]
- Liu, M.; Dai, Y.; Li, Y.; Luo, Y.; Huang, F.; Gong, Z.; Meng, Q. Madecassoside Isolated fromCentella asiaticaHerbs Facilitates Burn Wound Healing in Mice. Planta Medica 2008, 74, 809–815. [Google Scholar] [CrossRef]
- Masola, B.; Oguntibeju, O.O.; Oyenihi, A.B. Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed. Pharmacother. 2018, 101, 447–457. [Google Scholar] [CrossRef]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Dańczak-Pazdrowska, A.; Brzezińska, M. Centella asiaticain Dermatology: An Overview. Phytother. Res. 2014, 28, 1117–1124. [Google Scholar] [CrossRef]
- Vishnupriya, P.; Padma, V.V. A Review on the Antioxidant and Therapeutic Potential of Bacopa monnieri. React. Oxyg. Species 2017, 3. [Google Scholar] [CrossRef]
- Simpson, T.; Pase, M.P.; Stough, C. Bacopa monnierias an Antioxidant Therapy to Reduce Oxidative Stress in the Aging Brain. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Pytel, D.; Wieczfińska, J.; Szemraj, J.; Śliwiński, T. Induction of apoptosis by in vitro and in vivo plant extracts derived from Menyanthes trifoliata L. in human cancer cells. Cytotechnology 2019, 71, 165–180. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Sitarek, P.; Skała, E.; Rijo, P.; Andrade, J.M.; Synowiec, E.; Szemraj, J.; Krajewska, U.; Śliwiński, T. An Evaluation of the DNA-Protective Effects of Extracts from Menyanthes trifoliata L. Plants Derived from In Vitro Culture Associated with Redox Balance and Other Biological Activities. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Yang, H.-X.; Li, Z.-H.; Wang, G.-K.; Feng, T.; Liu, J.-K. Anti-inflammatory lupane triterpenoids from Menyanthes trifoliata. J. Asian Nat. Prod. Res. 2018, 21, 597–602. [Google Scholar] [CrossRef]
- Xie, X.; Li, H.; Wang, Y.; Wan, Z.; Luo, S.; Zhao, Z.; Liu, J.; Wu, X.; Li, X.; Li, X. Therapeutic effects of gentiopicroside on adjuvant-induced arthritis by inhibiting inflammation and oxidative stress in rats. Int. Immunopharmacol. 2019, 76, 105840. [Google Scholar] [CrossRef]
- Gendrisch, F.; Nováčková, A.; Sochorová, M.; Haarhaus, B.; Vávrová, K.; Schempp, C.M.; Wölfle, U. Gentiana lutea Extract Modulates Ceramide Synthesis in Primary and Psoriasis-Like Keratinocytes. Molecules 2020, 25, 1832. [Google Scholar] [CrossRef]
- Xiao, H.; Sun, X.; Liu, R.; Chen, Z.; Lin, Z.; Yang, Y.; Zhang, M.; Liu, P.; Quan, S.; Huang, H. Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol. Res. 2020, 151, 104559. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Yang, L.; Luo, M.; Han, L.; Lu, Y.; Shi, Q.; Wang, Y.; Liang, Q. Gentiopicroside (GENT) protects against sepsis induced by lipopolysaccharide (LPS) through the NF-κB signaling pathway. Ann. Transl. Med. 2019, 7, 731. [Google Scholar] [CrossRef]
- Bhat, A.A.; Prabhu, K.S.; Kuttikrishnan, S.; Krishnankutty, R.; Babu, J.; Mohammad, R.M.; Uddin, S. Potential therapeutic targets of Guggulsterone in cancer. Nutr. Metab. 2017, 14, 23. [Google Scholar] [CrossRef]
- Shishodia, S.; Azu, N.; Rosenzweig, J.A.; Jackson, D.A. Guggulsterone for Chemoprevention of Cancer. Curr. Pharm. Des. 2015, 22, 294–306. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Banik, K.; Bordoloi, D.; Harsha, C.; Sailo, B.L.; Padmavathi, G.; Roy, N.K.; Gupta, S.C.; Aggarwal, B.B. Googling the Guggul (Commiphora and Boswellia) for Prevention of Chronic Diseases. Front. Pharmacol. 2018, 9, 686. [Google Scholar] [CrossRef]
- Ramachandran, C.; Nair, S.M.; Quirrin, K.-W.; Melnick, S.J. Hypolipidemic Effects of a Proprietary Commiphora Mukul Gum Resin Extract and Medium-Chain Triglyceride Preparation (GU-MCT810). J. Evid. Based Integr. Med. 2013, 18, 248–256. [Google Scholar] [CrossRef]
- Chen, W.; Becker, T.; Qian, F.; Ring, J. Beer and beer compounds: Physiological effects on skin health. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 142–150. [Google Scholar] [CrossRef]
- Van Cleemput, M.; Cattoor, K.; de Bosscher, K.; Haegeman, G.; de Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-Derived Bitter Acids as Multipotent Bioactive Compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S.; Murata, M. Evaluation for Safety of Antioxidant Chemopreventive Agents. Antioxid. Redox Signal. 2005, 7, 1728–1739. [Google Scholar] [CrossRef]
- Weber, N.; Biehler, K.; Schwabe, K.; Haarhaus, B.; Quirin, K.-W.; Frank, U.; Schempp, C.M.; Wölfle, U. Hop Extract Acts as an Antioxidant with Antimicrobial Effects against Propionibacterium acnes and Staphylococcus aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Schempp, C. Topical Application of St. John’s Wort (Hypericum perforatum). Planta Med. 2013, 80, 109–120. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Toma, V.A.; Sevastre, B.; Hanganu, D.; Vlase, L.; Benedec, D.; Oniga, I.; Baldea, I.; Olteanu, D.; Moldovan, R.; et al. Characterization and biological effects of Hypericum extracts on experimentally-induced—Anxiety, oxidative stress and inflammation in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2019, 69. [Google Scholar] [CrossRef]
- You, M.-K.; Kim, H.-J.; Kook, J.H.; Kim, H.-A. St. John’s Wort Regulates Proliferation and Apoptosis in MCF-7 Human Breast Cancer Cells by Inhibiting AMPK/mTOR and Activating the Mitochondrial Pathway. Int. J. Mol. Sci. 2018, 19, 966. [Google Scholar] [CrossRef]
- Müller, M.; Essin, K.; Hill, K.; Beschmann, H.; Rubant, S.A.; Schempp, C.M.; Gollasch, M.; Boehncke, W.-H.; Harteneck, C.; Müller, W.E.; et al. Specific TRPC6 Channel Activation, a Novel Approach to Stimulate Keratinocyte Differentiation. J. Biol. Chem. 2008, 283, 33942–33954. [Google Scholar] [CrossRef]
- Leuner, K.; Kraus, M.; Woelfle, U.; Beschmann, H.; Harteneck, C.; Boehncke, W.-H.; Schempp, C.M.; Müller, W.E. Reduced TRPC Channel Expression in Psoriatic Keratinocytes Is Associated with Impaired Differentiation and Enhanced Proliferation. PLoS ONE 2011, 6, e14716. [Google Scholar] [CrossRef]
- Ramachandran, C.; Portalatin, G.; Quirin, K.-W.; Escalon, E.; Khatib, Z.; Melnick, S.J. Inhibition of AKT signaling by supercritical CO2 extract of mango ginger (Curcuma amada Roxb.) in human glioblastoma cells. J. Complement. Integr. Med. 2015, 12, 307–315. [Google Scholar] [CrossRef]
- Win, N.N.; Ito, T.; Ngwe, H.; Win, Y.Y.; Prema; Okamoto, Y.; Tanaka, M.; Asakawa, Y.; Abe, I.; Morita, H. Labdane diterpenoids from Curcuma amada rhizomes collected in Myanmar and their antiproliferative activities. Fitoterapia 2017, 122, 34–39. [Google Scholar] [CrossRef]
- Jatoi, S.A.; Kikuchi, A.; Gilani, S.A.; Watanabe, K.N. Phytochemical, pharmacological and ethnobotanical studies in mango ginger (Curcuma amada Roxb.; Zingiberaceae). Phytotherapy Res. 2007, 21, 507–516. [Google Scholar] [CrossRef]
- Ramachandran, C.; Quirin, K.-W.; Escalon, E.A.; Lollett, I.V.; Melnick, S.J. Therapeutic Effect of Supercritical CO2 Extracts of Curcuma Species with Cancer Drugs in Rhabdomyosarcoma Cell Lines. Phytotherapy Res. 2015, 29, 1152–1160. [Google Scholar] [CrossRef]
- Raduner, S.; Majewska, A.; Chen, J.-Z.; Xie, X.-Q.; Hamon, J.; Faller, B.; Altmann, K.-H.; Gertsch, J. Alkylamides from Echinacea Are a New Class of Cannabinomimetics. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef]
- Aarland, R.C.; Bañuelos-Hernández, A.E.; Fragoso-Serrano, M.; Sierra-Palacios, E.D.C.; de León-Sánchez, F.D.; Pérez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wu, Y.; Wang, B.; Chen, X.; Xu, X.; Chen, H.; Li, W.; Xu, X. Echinacea pupurea extracts promote murine dendritic cell maturation by activation of JNK, p38 MAPK and NF-κB pathways. Dev. Comp. Immunol. 2017, 73, 21–26. [Google Scholar] [CrossRef]
- Nath, S.; Pathak, B. Phytochemical and Pharmacological Characteristics of Wrightiatinctoria: A Review. Available online: /paper/Phytochemical-and-Pharmacological-Characteristics-%3A-Nath-Pathak/9f0ce9270365efb25ed698eeeff06c4e920ee67a (accessed on 11 January 2021).
- Srivastava, R. A review on phytochemical, pharmacological, and pharmacognostical profile of Wrightia tinctoria: Adulterant of kurchi. Pharmacogn. Rev. 2014, 8, 36–44. [Google Scholar] [CrossRef]
- Babu, M. Pharmacological Evaluation of Wrightia Tinctoria—A Review. Sci. Rep. 2015, 5, 1–15. [Google Scholar]
- Rao, B.; Rajeswari, D.; Devarakonda, R.; Battu, H. Phytochemical and Pharmacological Studies on Wrightia Tinctoria. World J. Pharm. Pharm. Sci. 2019. [Google Scholar] [CrossRef]
- Sehgal, V.N.; Verma, P.; Khurana, A. Anthralin/dithranol in dermatology. Int. J. Dermatol. 2014, 53, e449–e460. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.; Banerjee, S.; Negi, A.S.; Shanker, K. Determination of anti-tubercular agent in mango ginger (Curcuma amada Roxb.) by reverse phase HPLC-PDA-MS. Food Chem. 2012, 131, 375–379. [Google Scholar] [CrossRef]
- Rheinwatd, J.G.; Green, H. Seria cultivation of strains of human epidemal keratinocytes: The formation keratinizin colonies from single cell is. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Pfaff, C.M.; Marquardt, Y.; Fietkau, K.; Baron, J.M.; Lüscher, B. The psoriasis-associated IL-17A induces and cooperates with IL-36 cytokines to control keratinocyte differentiation and function. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Muromoto, R.; Hirao, T.; Tawa, K.; Hirashima, K.; Kon, S.; Kitai, Y.; Matsuda, T. IL-17A plays a central role in the expression of psoriasis signature genes through the induction of IκB-ζ in keratinocytes. Int. Immunol. 2016, 28, 443–452. [Google Scholar] [CrossRef]
- Teunissen, M.B.; Bos, J.D.; Koomen, C.W.; Malefyt, R.D.W.; Wierenga, E.A. Interleukin-17 and Interferon-γ Synergize in the Enhancement of Proinflammatory Cytokine Production by Human Keratinocytes. J. Investig. Dermatol. 1998, 111, 645–649. [Google Scholar] [CrossRef]
- Wolk, K.; Haugen, H.S.; Xu, W.; Witte, E.; Waggie, K.; Anderson, M.; Baur, E.V.; Witte, K.; Warszawska, K.; Philipp, S.; et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-γ are not. J. Mol. Med. 2009, 87, 523–536. [Google Scholar] [CrossRef]
- Ettehadi, P.; Greaves, M.W.; Wallach, D.; Aderka, D.; Camp, R.D.R. Elevated tumour necrosis factor-alpha (TNF-α) biological activity in psoriatic skin lesions. Clin. Exp. Immunol. 2008, 96, 146–151. [Google Scholar] [CrossRef]
- De Jongh, G.J.; Zeeuwen, P.L.; Kucharekova, M.; Pfundt, R.; van der Valk, P.G.; Blokx, W.; Dogan, A.; Hiemstra, P.S.; van de Kerkhof, P.C.; Schalkwijk, J. High Expression Levels of Keratinocyte Antimicrobial Proteins in Psoriasis Compared with Atopic Dermatitis. J. Investig. Dermatol. 2005, 125, 1163–1173. [Google Scholar] [CrossRef]
- Kolbinger, F.; Loesche, C.; Valentin, M.-A.; Jiang, X.; Cheng, Y.; Jarvis, P.; Peters, T.; Calonder, C.; Bruin, G.; Polus, F.; et al. β-Defensin 2 is a responsive biomarker of IL-17A–driven skin pathology in patients with psoriasis. J. Allergy Clin. Immunol. 2017, 139, 923–932. [Google Scholar] [CrossRef]
- Zanet, J.; Freije, A.; Ruiz, M.; Coulon, V.; Sanz, J.R.; Chiesa, J.; Gandarillas, A. A Mitosis Block Links Active Cell Cycle with Human Epidermal Differentiation and Results in Endoreplication. PLoS ONE 2010, 5, e15701. [Google Scholar] [CrossRef]
- Zhang, Z.; Zi, Z.; Lee, E.E.; Zhao, J.; Contreras, D.C.; South, A.P.; Abel, E.D.; Chong, B.F.; Vandergriff, T.; Hosler, G.A.; et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat. Med. 2018, 24, 617–627. [Google Scholar] [CrossRef]
- Hodeib, A.A.-H.; Neinaa, Y.M.-H.; Zakaria, S.S.; Alshenawy, H.A.-S. Glucose transporter-1 (GLUT-1) expression in psoriasis: Correlation with disease severity. Int. J. Dermatol. 2018, 57, 943–951. [Google Scholar] [CrossRef]
- Hiebert, P.; Werner, S. Targeting metabolism to treat psoriasis. Nat. Med. 2018, 24, 537–539. [Google Scholar] [CrossRef]
- Neuner, P.; Urbanski, A.; Trautinger, F.; Möller, A.; Kirnbauer, R.; Kapp, A.; Schöpf, E.; Schwarz, T.; Luger, T.A. Increased IL-6 Production by Monocytes and Keratinocytes in Patients with Psoriasis. J. Investig. Dermatol. 1991, 97, 27–33. [Google Scholar] [CrossRef]
- Gillitzer, R.; Berger, R.; Mielke, V.; Müller, C.; Wolff, K.; Stingl, G. Upper Keratinocytes of Psoriatic Skin Lesions Express High Levels of NAP-1/IL-8 mRNA In Situ. J. Investig. Dermatol. 1991, 97, 73–79. [Google Scholar] [CrossRef]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 81–102. [Google Scholar] [CrossRef]
- D’Amico, F.; Trovato, C.; Skarmoutsou, E.; Rossi, G.A.; Granata, M.; Longo, V.; Gangemi, P.; Pettinato, M.; Mazzarino, M.C. Effects of adalimumab, etanercept and ustekinumab on the expression of psoriasin (S100A7) in psoriatic skin. J. Dermatol. Sci. 2015, 80, 38–44. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, M.; Zhang, L.-J. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nam, S.; Hong, I.K.; Kim, H.; Yang, H.; Cho, H.-J. Antiproliferation of keratinocytes and alleviation of psoriasis by the ethanol extract of Artemisia capillaris. Phytother. Res. 2018, 32, 923–932. [Google Scholar] [CrossRef]
- Thaçi, D.; Augustin, M.; Krutmann, J.; Luger, T. Importance of basic therapy in psoriasis. J. Dtsch. Dermatol. Ges. 2015, 13, 415–418. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Mishra, A.; Prabhu, S. Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: Its evaluation using curcumin. Eur. J. Pharmacol. 2017, 813, 33–41. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin Inhibits Imiquimod-Induced Psoriasis-Like Inflammation by Inhibiting IL-1beta and IL-6 Production in Mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Leu, Y.-L.; Yang, S.-H.; Chen, H.-W.; Wang, C.-T.; Pang, J.-H.S. Anti-psoriatic effects of indigo naturalis on the proliferation and differentiation of keratinocytes with indirubin as the active component. J. Dermatol. Sci. 2009, 54, 168–174. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Sah, S.K.; Yang, H.S.; Lee, J.H.; Shin, J.; Kim, T.-Y. Rhododendrin inhibits toll-like receptor-7-mediated psoriasis-like skin inflammation in mice. Exp. Mol. Med. 2017, 49, e349. [Google Scholar] [CrossRef]
- Li, H.-J.; Wu, N.-L.; Lee, G.-A.; Hung, C.-F. The Therapeutic Potential and Molecular Mechanism of Isoflavone Extract against Psoriasis. Sci. Rep. 2018, 8, 6335. [Google Scholar] [CrossRef]
- Soboleva, A.G.; Zolotarenko, A.D.; Sobolev, V.V.; Bruskin, S.A.; Piruzian, E.S.; Mezentsev, A.V. Genetically predetermined limitation in HaCaT cells that affects their ability to serve as an experimental model of psoriasis. Russ. J. Genet. 2014, 50, 1081–1089. [Google Scholar] [CrossRef]
- Leigh, I.; Navsaria, H.; Purkis, P.; McKay, I.; Bowden, P.; Riddle, P. Keratins (Kl6 and Kl7) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br. J. Dermatol. 1995, 133, 501–511. [Google Scholar] [CrossRef]
- Zhang, W.; Dang, E.; Shi, X.; Jin, L.; Feng, Z.; Hu, L.; Wu, Y.; Wang, G. The Pro-Inflammatory Cytokine IL-22 Up-Regulates Keratin 17 Expression in Keratinocytes via STAT3 and ERK1/2. PLoS ONE 2012, 7, e40797. [Google Scholar] [CrossRef]
- Shi, X.; Jin, L.; Dang, E.; Chang, T.; Feng, Z.; Liu, Y.; Wang, G. IL-17A Upregulates Keratin 17 Expression in Keratinocytes through STAT1- and STAT3-Dependent Mechanisms. J. Investig. Dermatol. 2011, 131, 2401–2408. [Google Scholar] [CrossRef]
- De Jong, E.M.G.J.; van Vlijmen, I.M.M.J.; van Erp, P.E.J.; Ramaekers, F.C.S.; Troyanovski, S.M.; van de Kerkhof, P.C.M. Keratin 17: A useful marker in anti-psoriatic therapies. Arch. Dermatol. Res. 1991, 283, 480–482. [Google Scholar] [CrossRef]
- Radoja, N.; Komine, M.; Jho, S.H.; Blumenberg, M.; Tomic-Canic, M. Novel Mechanism of Steroid Action in Skin through Glucocorticoid Receptor Monomers. Mol. Cell. Biol. 2000, 20, 4328–4339. [Google Scholar] [CrossRef]
- Gilfix, B.M.; Eckert, R.L. Coordinate control by vitamin A of keratin gene expression in human keratinocytes. J. Biol. Chem. 1985, 260, 14026–14029. [Google Scholar] [CrossRef]
- Bonnekoh, B.; Böckelmann, R.; Ambach, A.; Gollnick, H. Dithranol and Dimethylfumarate Suppress the Interferon-γ-Induced Up-Regulation of Cytokeratin 17 as a Putative Psoriasis Autoantigen in vitro. Ski. Pharmacol. Physiol. 2001, 14, 217–225. [Google Scholar] [CrossRef]
- Jin, L.; Wang, G. Keratin 17: A Critical Player in the Pathogenesis of Psoriasis. Med. Res. Rev. 2014, 34, 438–454. [Google Scholar] [CrossRef]
- Chang, T.; Sun, L.; Wang, Y.; Wang, D.; Li, W.; Li, C.; Gao, T.; Liu, Y.; Wang, G. Inhibition of keratin 17 expression with antisense and RNAi strategies: Exploring novel therapy for psoriasis. Exp. Dermatol. 2011, 20, 555–560. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Wei, J.; Chen, H.; Lu, Y.; Li, L.; Han, L.; Lu, C. Gallic acid inhibits the expression of keratin 16 and keratin 17 through Nrf2 in psoriasis-like skin disease. Int. Immunopharmacol. 2018, 65, 84–95. [Google Scholar] [CrossRef]
- Niyonsaba, O.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I. Antimicrobial Peptides Human β-Defensins Stimulate Epidermal Keratinocyte Migration, Proliferation and Production of Proinflammatory Cytokines and Chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.A.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nat. Cell Biol. 2006, 445, 648–651. [Google Scholar] [CrossRef]
- Sano, S.; Chan, K.S.; Carbajal, S.; Clifford, J.L.; Peavey, M.; Kiguchi, K.; Itami, S.; Nickoloff, B.J.; di Giovanni, J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 2005, 11, 43–49. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X. The role of PI3K/AKT/FOXO signaling in psoriasis. Arch. Dermatol. Res. 2019, 311, 83–91. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, M.; Mangal, S.; Agrawal, U.; Vyas, S.P. Capsaicin-loaded vesicular systems designed for enhancing localized delivery for psoriasis therapy. Artif. Cell Nanomed. Biotechnol. 2014, 44, 1–10. [Google Scholar] [CrossRef]
- Choonhakarn, C.; Busaracome, P.; Sripanidkulchai, B.; Sarakarn, P. A prospective, randomized clinical trial comparing topical aloe vera with 0.1% triamcinolone acetonide in mild to moderate plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 168–172. [Google Scholar] [CrossRef]
- Di Nardo, V.; Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Barygina, V.; Lotti, J.; Daaboul, F.; Lotti, T. Use of Curcumin in Psoriasis. Open Access Maced. J. Med. Sci. 2018, 6, 218–220. [Google Scholar] [CrossRef]
- Hashemian, F.; Mansouri, P.; Mirafzal, S.; Najafizadeh, P.; Safaei-Naraghi, Z.; Salehi-Surmaghi, M. The impact of topical Saint John’s Wort (Hypericum perforatum) treatment on tissue tumor necrosis factor-alpha levels in plaque-type psoriasis: A pilot study. J. Postgrad. Med. 2017, 63, 215–220. [Google Scholar] [CrossRef]
- Lin, Y.-K.; See, L.-C.; Huang, Y.-H.; Chang, Y.-C.; Tsou, T.-C.; Lin, T.-Y.; Lin, N.-L. Efficacy and safety of Indigo naturalis extract in oil (Lindioil) in treating nail psoriasis: A randomized, observer-blind, vehicle-controlled trial. Phytomedicine 2014, 21, 1015–1020. [Google Scholar] [CrossRef]
- Bernstein, S.; Donsky, H.; Gulliver, W.; Hamilton, D.; Nobel, S.; Norman, R. Treatment of Mild to Moderate Psoriasis with Reliéva, a Mahonia aquifolium Extract—A Double-Blind, Placebo-Controlled Study. Am. J. Ther. 2006, 13, 121–126. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Topically Used Herbal Products for the Treatment of Psoriasis—Mechanism of Action, Drug Delivery, Clinical Studies. Planta Med. 2016, 82, 1447–1455. [Google Scholar] [CrossRef]
- Hoffmann, J.; Gendrisch, F.; Schempp, C.M.; Wölfle, U. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines 2020, 8, 27. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Chang, C.-J.; Chang, Y.-C.; Wong, W.-R.; Chang, S.-C.; Pang, J.-H.S. Clinical Assessment of Patients with Recalcitrant Psoriasis in a Randomized, Observer-Blind, Vehicle-Controlled Trial Using Indigo Naturalis. Arch. Dermatol. 2008, 144, 1457–1464. [Google Scholar] [CrossRef]
- Najafizadeh, P.; Hashemian, F.; Mansouri, P.; Farshi, S.; Surmaghi, M.S.; Chalangari, R. The evaluation of the clinical effect of topical St Johns wort (Hypericum perforatum L.) in plaque type psoriasis vulgaris: A pilot study. Australas. J. Dermatol. 2012, 53, 131–135. [Google Scholar] [CrossRef]
- Hawley-Nelson, P.; Vousden, K.H.; Hubbert, N.L.; Lowy, D.R.; Schiller, J.T. HPV16 E6 and E7 Proteins Cooperate to Immortalize Human Foreskin Keratinocytes. EMBO J. 1989, 8, 3905–3910. [Google Scholar]
- Sprenger, A.; Küttner, V.; Biniossek, M.L.; Gretzmeier, C.; Boerries, M.; Mack, C.; Has, C.; Bruckner-Tuderman, L.; Dengjel, J. Comparative Quantitation of Proteome Alterations Induced by Aging or Immortalization in Primary Human Fibroblasts and Keratinocytes for Clinical Applications. Mol. BioSyst. 2010, 6, 1579–1582. [Google Scholar] [CrossRef]


| Plant Plant Family | Used in | Extract and Solubility | Lead Substance | Possible Anti-Psoriatic Effects | |
|---|---|---|---|---|---|
| Asiatic pennywort (Centella asiatica) Apiaceae | TCM AM | HPE extract of dried leaves Soluble in PBS | Triterpenoid-Saponines: Asiaticoside (1.1%), Madecassosid (6.4%), DER 7/1 | 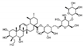 Asiaticoside | Anti-inflammatory [9,10] Anti-oxidant [10,11] Anti-proliferative [9,12] Wound healing induction [9,12] |
| Brahmi (Bacopa monniera L.) Scrophulariaceae | AM | HPE extract of dried leaves Soluble in ethanol (70%) | Steroid-Saponine: Bacosides (6.7%), DER 8/1 | 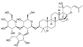 Bacoside A3 | Anti-inflammatory [13] Anti-oxidant [13,14] Adaptogene [14] |
| Buckbean (Menyanthes trifoliata L.) Menyanthaceae | TCM | HPE extract of dried leaves Soluble in PBS | Secoiridoidglycoside: Dihydroforliamenthin (≈1%), DER: 2.5/1 (for 50% extract dissolved in ethanol) |  | Anti-inflammatory [15] Anti-oxidant [15] Anti-proliferative [16] Inhibition of NO production [17] |
| Gentian (Gentiana lutea) Gentianaceae | OMM | HPE extract of roots Soluble in PBS | Bitter glycoside: Gentiopicroside (15.9%); DER ≈ 1.8/1 (for 50% extract dissolved in ethanol) | 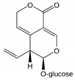 | Anti-inflammatory [18,19,20,21] Anti-oxidative [18] Inhibition of NF-κB [18,20,21] Induction of ceramide synthesis [19] |
| Guggul (Commiphora mukul) Burceraceae | AM | Supercritical Co-solvent extract with CO2 + EtOH (95 + 5) of tree gum Soluble in ethanol (70%) | Phytosteroid: Guggulsterone (2%); DER: 3.5/1 (for 50% extract dissolved in MCT-oil) | 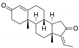 | Anti-inflammatory [22,23,24] Inhibition of lipid peroxidation [23] Anti-proliferative [22,23,24] Inhibition of AKT + NF-κB [22,23,24] Inhibition of mTOR [25] |
| Hop (Humulus lupulus) Cannabaceae | OMM AM | Supercritical CO2 extract of dried hop cones Soluble in DMSO | Bitter hop acids: Humulones (48%), Lupulones (24%) DER ≈ 4/1 | 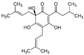 | Anti-inflammatory [26,27,28] Anti-oxidant [26,27,28,29] Anti-proliferative [26,27,28] Inhibition of NF-κB + AP-1 [27] |
| St. John’s wort (Hypericum perforatum) Hypericaceae | OMM | Supercritical CO2 extract of dried branch tips with flowers Soluble in DMSO | Polyprenylated phloroglycinol derivative: Hyperforin (30%), DER ≈ 20/1 | 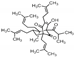 | Anti-inflammatory [30,31] Anti-oxidant [30,31] Anti-proliferative [30,31] AMPK/mTOR inhibition [32] Differentiation [33,34] |
| Mango ginger (Curcuma amada) Zingiberaceae | AM | Supercritical CO2 extract of rhizoma Soluble in ethanol (100%) | Diterpenoid: (E)-Labda-8(17),12-diene-15,16-dial (LDD): 42%; DER: 33/1 |  | Anti-inflammatory [35] Anti-oxidative [36,37] Anti-proliferative [35,36,38] Inhibition of AKT [38] Pro-apoptotic [35,38] |
| Purple coneflower (Echinacea purpurea) Asteracea | NAM | Supercritical CO2 extract of the dried roots Soluble in ethanol (100%) | Total alkylamides (24.3%), total sterols: 4.8%, DER ≈ 125/1 | 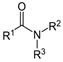 | Anti-inflammatory [39,40] Anti-oxidative [40] Anti-proliferative [40] Inhibition of NF-κB [39,41] Promotion of DC maturation [41] Cannabinomimetics [39] |
| Sweet indraja (Writhia tinctoria) Apocynaceae | AM | Supercritical CO2 extract of seeds Soluble in DMSO | Triterpenes: α und β Amyrin: 0.7%, total sterols 5.4%, DER: 5.2/1 | 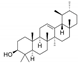 α-Amyrin | Anti-inflammatory [42,43,44,45] Anti-oxidative [42,45] Anti-ulcer activity [43,45] Anti-dandruff properties [45] |
| Dithranol originally from the Araroba tree (Vataireopsis araroba) Fabaceae | First synthesized in 1916 by the German pharmaceutical company Bayer Stock solution in DMSO | Anthracene compound: Dithranol (1,8-dihydroxy-9anthrone) |  | Anti-inflammatory [46] Antioxidant [46] Anti-proliferative [46] Useful in psoriasis [46] Side effects: e.g., skin irritation and discoloration of the skin | |
| Plant Extract | Cell Viability HPK, EC80 | Anti-Proliferative Effect Psoriasis-Like HPK, EC50 | Inhibition of IL-6 Pso-Like HPK, EC50 | Inhibition of IL-8 Pso-Like HPK, EC50 |
|---|---|---|---|---|
| Hop (Humulus lupulus) | 0.22 ± 1.15 µg/ml | 0.36 ± 0.02 µg/ml | 0.28 ± 0.26 µg/ml | 0.34 ± 0.44 µg/ml |
| St John’s wort (Hypericum perforatum) | 0.35 ± 1.08 µg/ml | 0.70 ± 0.03 µg/ml | 0.14 ± 0.12µg/ml | 0.28 ± 0.32 µg/ml |
| Mango ginger (Curcuma amada) | 2.12 ± 1.16 µg/ml | 2.33 ± 0.63 µg/ml | 1.08 ± 0.38 µg/ml | 1.38 ± 0.67 µg/ml |
| Control | ||||
| Dithranol | 0.05 ± 0.02 µg/ml | 0.03 ± 0.001 µg/ml | 0.01 ± 0.01 µg/ml | 0.02 ± 0.01 µg/ml |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gendrisch, F.; Haarhaus, B.; Krieger, N.; Quirin, K.-W.; Schempp, C.M.; Wölfle, U. The Effect of Herbal Medicinal Products on Psoriasis-Like Keratinocytes. Biomolecules 2021, 11, 371. https://doi.org/10.3390/biom11030371
Gendrisch F, Haarhaus B, Krieger N, Quirin K-W, Schempp CM, Wölfle U. The Effect of Herbal Medicinal Products on Psoriasis-Like Keratinocytes. Biomolecules. 2021; 11(3):371. https://doi.org/10.3390/biom11030371
Chicago/Turabian StyleGendrisch, Fabian, Birgit Haarhaus, Nina Krieger, Karl-Werner Quirin, Christoph M. Schempp, and Ute Wölfle. 2021. "The Effect of Herbal Medicinal Products on Psoriasis-Like Keratinocytes" Biomolecules 11, no. 3: 371. https://doi.org/10.3390/biom11030371
APA StyleGendrisch, F., Haarhaus, B., Krieger, N., Quirin, K.-W., Schempp, C. M., & Wölfle, U. (2021). The Effect of Herbal Medicinal Products on Psoriasis-Like Keratinocytes. Biomolecules, 11(3), 371. https://doi.org/10.3390/biom11030371






