Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective
Abstract
1. Introduction
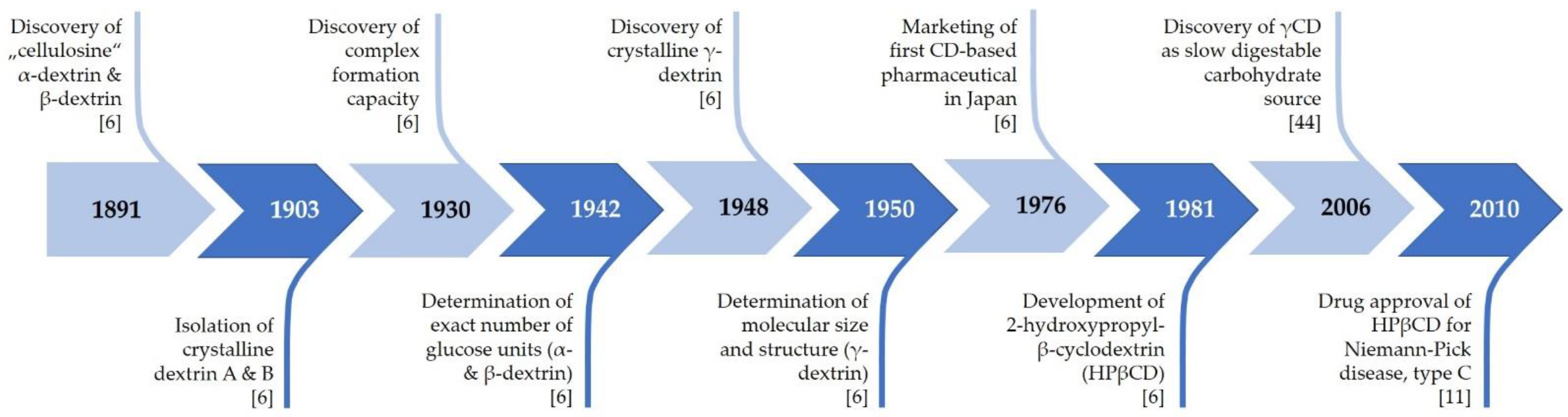
2. History of Cyclodextrins
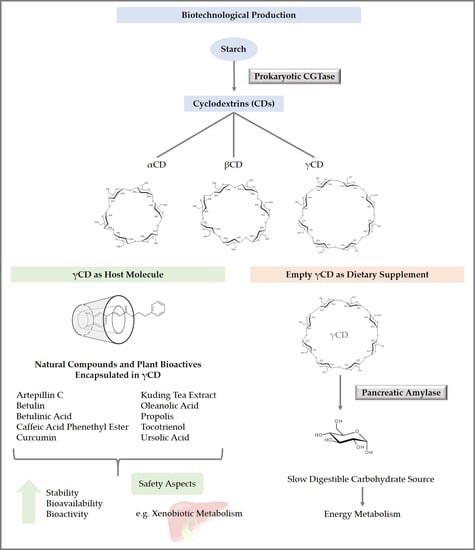
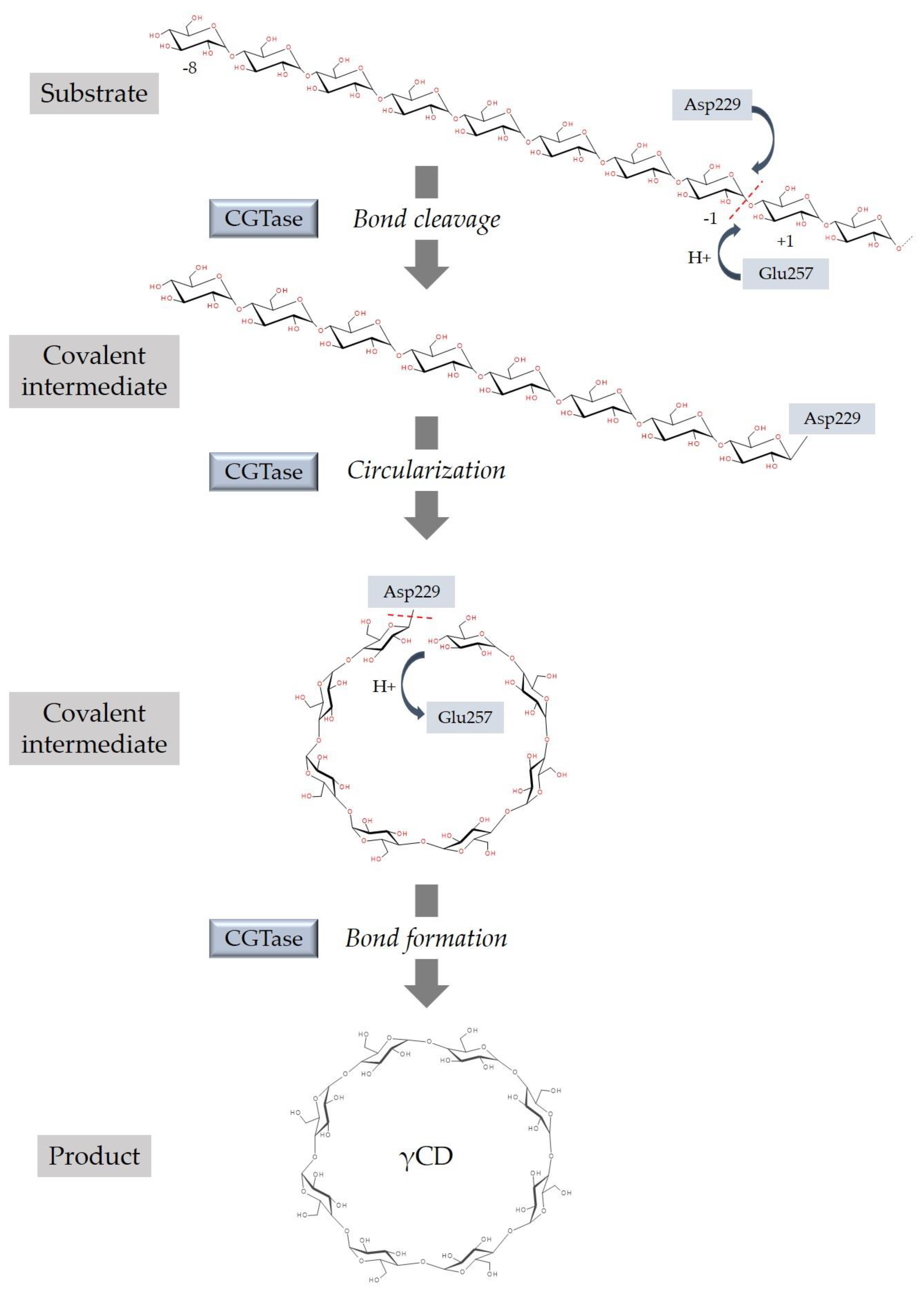
3. Synthesis of Cyclodextrins
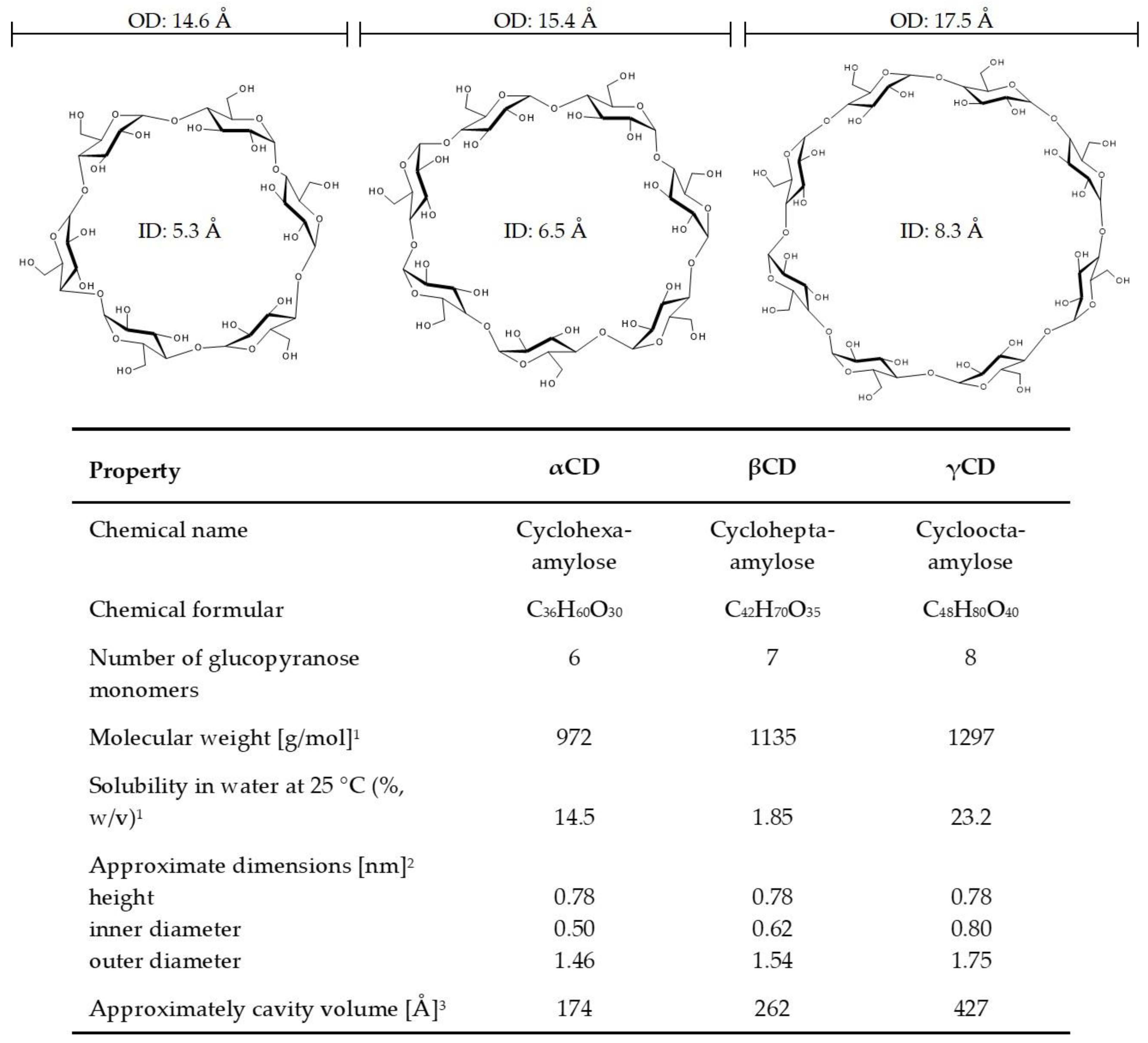
4. Properties of Cyclodextrins
5. Absorption, Distribution, Metabolism, and Excretion of Cyclodextrins
6. Safety of Cyclodextrins
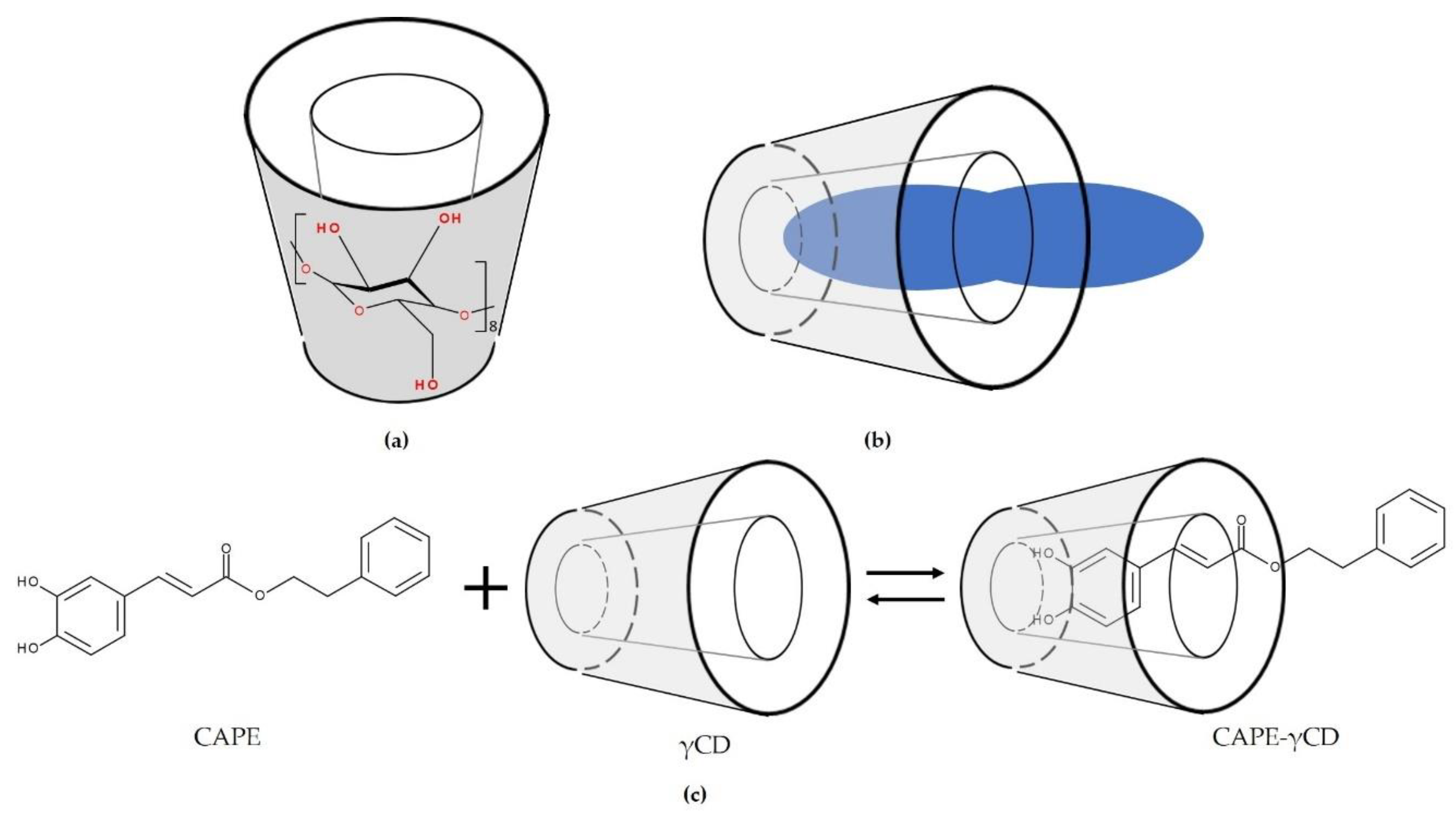
7. Cyclodextrin Inclusion Complexes
8. Application of Cyclodextrins
8.1. Inclusion of Tocotrienol in γ-Cyclodextrin Increased Its Bioavailability and Bioactivity
8.2. Pentacyclic Triterpenoids Encapsulated in γ-Cyclodextrin
8.3. Propolis, Propolis Extract, or Phytochemicals Isolated from Propolis Encapsulated in γ-Cyclodextrin
8.4. Increased Bioavailability of Curcumin by Complexation with γ-Cyclodextrin in Humans
8.5. Metabolic Activity of Empty γ-Cyclodextrin
9. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Wang, M.; Wang, F.; Gu, Z.; Du, G.; Wu, J.; Chen, J. γ-Cyclodextrin: A review on enzymatic production and applications. Appl. Microbiol. Biotechnol. 2007, 77, 245–255. [Google Scholar] [CrossRef]
- Del Valle, E. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Banerjee, U. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. γ-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Booij, L.H.D.J. Cyclodextrins and the emergence of sugammadex. Anaesthesia 2009, 64, 31–37. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- French, D. Preparation of Schardinger Dextrins. In Methods Enzymology; Elsevier: Amsterdam, The Netherlands, 1957; Volume 3, pp. 17–20. [Google Scholar]
- Pulley, A.O.; French, D. Studies on the Schardinger dextrins. XI: The isolation of new Schardinger dextrins. Biochem. Biophys. Res. Commun. 1961, 5, 11–15. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging medicines of the new millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Van Der Veen, B.A.; Van Alebeek, G.-J.W.M.; Uitdehaag, J.C.M.; Dijkstra, B.W.; Dijkhuizen, L. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur. J. Biochem. 2000, 267, 658–665. [Google Scholar] [CrossRef]
- Biwer, A.; Antranikian, G.; Heinzle, E. Enzymatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 2002, 59, 609–617. [Google Scholar] [CrossRef]
- Uitdehaag, J.C.; Mosi, R.; Kalk, K.H.; van der Veen, B.A.; Dijkhuizen, L.; Withers, S.G.; Dijkstra, B.W. X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the alpha-amylase family. Nat. Genet. 1999, 6, 432–436. [Google Scholar] [CrossRef]
- Goo, B.G.; Hwang, Y.J.; Park, J.K. Bacillus thuringiensis: A specific gamma-cyclodextrin producer strain. Carbohydr. Res. 2014, 386, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Cyclodextrins. Clin. Drug Investig. 1990, 2, 11–21. [Google Scholar] [CrossRef]
- Heredia, A.; Requena, G.; Garciasanchez, F. An approach for the estimation of the polarity of the β-cyclodextrin internal cavity. J. Chem. Soc. Chem. Commun. 1985, 1814–1815. [Google Scholar] [CrossRef]
- Kajtár, M.; Vikmon, M.; Morlin, E.; Szejtli, J. Aggregation of amphotericin B in the presence of γ-cyclodextin. Biopolym. Orig. Res. Biomol. 1989, 28, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Street, K.W.; Acree, W.E. Estimation of the effective dielectric constant of cyclodextrin cavities based on the fluorescence properties of pyrene-3-carboxaldehyde. Appl. Spectrosc. 1988, 42, 1315–1318. [Google Scholar] [CrossRef]
- Caira, M.R. Cyclodextrin inclusion of medicinal compounds for enhancement of their physicochemical and biopharmaceutical properties. Curr. Top. Med. Chem. 2019, 19, 2357–2370. [Google Scholar] [CrossRef]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. Biomed. Pharm. 2017, 88, 1122–1144. [Google Scholar] [CrossRef]
- Miyoshi, N.; Wakao, Y.; Tomono, S.; Tatemichi, M.; Yano, T.; Ohshima, H. The enhancement of the oral bioavailability of γ-tocotrienol in mice by γ-cyclodextrin inclusion. J. Nutr. Biochem. 2011, 22, 1121–1126. [Google Scholar] [CrossRef]
- Ishida, Y.; Gao, R.; Shah, N.; Bhargava, P.; Furune, T.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer activity in honeybee propolis: Functional insights to the role of caffeic acid phenethyl ester and its complex with γ-cyclodextrin. Integr. Cancer 2018, 17, 867–873. [Google Scholar] [CrossRef]
- Oprean, C.; Mioc, M.; Csányi, E.; Ambrus, R.; Bojin, F.; Tatu, C.; Cristea, M.; Ivan, A.; Danciu, C.; Dehelean, C.; et al. Improvement of ursolic and oleanolic acids’ antitumor activity by complexation with hydrophilic cyclodextrins. Biomed. Pharm. 2016, 83, 1095–1104. [Google Scholar] [CrossRef]
- Stella, V.J.; Rajewski, R.A. Cyclodextrins: Their future in drug formulation and delivery. Pharm. Res. 1997, 14, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Peng, Q.; Yong-Wei, W.; Dong-Sheng, Z.; Ming-Wan, Z.; Rui, L.; Nan, J. Host-guest interaction of β-cyclodextrin with isomeric ursolic acid and oleanolic acid: Physicochemical characterization and molecular modeling study. J. Biomed. Res. 2017, 31, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, É.; Vikmon, M.; Szente, L. Cyclodextrins in food technology and human nutrition: Benefits and limitations. Crit. Rev. Food Sci. Nutr. 2016, 56, 1981–2004. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- Kubota, Y.; Fukuda, M.; Muroguchi, M.; Koizumi, K. Absorption, distribution and excretion of BETA—Cyclodextrin and glucosyl- BETA.-cyclodextrin in rats. Biol. Pharm. Bull. 1996, 19, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Van Ommen, B.; De Bie, A.; Bär, A. Disposition of 14C-α-cyclodextrin in germ-free and conventional rats. Regul. Toxicol. Pharm. 2004, 39, 57–66. [Google Scholar] [CrossRef]
- De Bie, A.; Van Ommen, B.; Bar, A. Disposition of [14C]γ-Cyclodextrin in germ-free and conventional rats. Regul. Toxicol. Pharm. 1998, 27, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Frijlink, H.W.; Visser, J.; Hefting, N.R.; Oosting, R.; Meijer, D.K.F.; Lerk, C.F. The pharmacokinetics of β-cyclodextrin and hydroxypropyl-β-cyclodextrin in the rat. Pharm. Res. 1990, 7, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.; Newberne, P.; Young, V.; Bär, A. Safety assessment of γ-cyclodextrin. Regul. Toxicol. Pharm. 2004, 39, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Harangi, J.; Béke, G.; Harangi, M.; Mótyán, J.A. The digestable parent cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2011, 73, 335–339. [Google Scholar] [CrossRef]
- Lumholdt, L.R.; Holm, R.; Jørgensen, E.B.; Larsen, K.L. In Vitro investigations of α-amylase mediated hydrolysis of cyclodextrins in the presence of ibuprofen, flurbiprofen, or benzo[a]pyrene. Carbohydr. Res. 2012, 362, 56–61. [Google Scholar] [CrossRef]
- Frank, D.W.; Gray, J.E.; Weaver, R.N. Cyclodextrin nephrosis in the rat. Am. J. Pathol. 1976, 83, 367–382. [Google Scholar] [PubMed]
- Frijlink, H.W.; Eissens, A.C.; Hefting, N.R.; Poelstra, K.; Lerk, C.F.; Meijer, D.K.F. The effect of parenterally administered cyclodextrins on cholesterol levels in the rat. Pharm. Res. 1991, 8, 9–16. [Google Scholar] [CrossRef]
- Lina, B.; Bär, A. Subchronic oral toxicity studies with α-cyclodextrin in rats. Regul. Toxicol. Pharm. 2004, 39, 14–26. [Google Scholar] [CrossRef]
- Lina, B.; Bär, A. Subchronic (13-week) oral toxicity study of α-cyclodextrin in dogs. Regul. Toxicol. Pharm. 2004, 39, 27–33. [Google Scholar] [CrossRef]
- Amar, M.J.A.; Kaler, M.; Courville, A.B.; Shamburek, R.; Sampson, M.; Remaley, A.T. Randomized double blind clinical trial on the effect of oral α-cyclodextrin on serum lipids. Lipids Health Dis. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Bellringer, M.; Smith, T.; Read, R.; Gopinath, C.; Olivier, P. β-Cyclodextrin: 52-week toxicity studies in the rat and dog. Food Chem. Toxicol. 1995, 33, 367–376. [Google Scholar] [CrossRef]
- Til, H.; Bar, A. Subchronic (13-Week) oral toxicity study of γ-cyclodextrin in dogs. Regul. Toxicol. Pharm. 1998, 27, 159–165. [Google Scholar] [CrossRef]
- Waalkens-Berendsen, D.; Verhagen, F.; Bär, A. Embryotoxicity and teratogenicity study with γ-cyclodextrin in rats. Regul. Toxicol. Pharm. 1998, 27, 166–171. [Google Scholar] [CrossRef]
- Asp, M.L.; Hertzler, S.R.; Chow, J.; Wolf, B.W. Gamma-cyclodextrin lowers postprandial glycemia and insulinemia without carbohydrate malabsorption in healthy adults. J. Am. Coll. Nutr. 2006, 25, 49–55. [Google Scholar] [CrossRef]
- GRAS Notice 678, Alpha-cyclodextrin. Available online: https://www.fda.gov/media/101653/download (accessed on 6 November 2020).
- Wacker Biochem Corp. GRAS Notice 000046: GAMMA-CYCLODEXTRIN. Available online: http://wayback.archive-it.org/7993/20171031055850/https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM261675.pdf (accessed on 20 July 2020).
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharm. 2010, 62, 1607–1621. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U. Re-evaluation of β-cyclodextrin (E 459) as a food additive. EFSA J. 2016, 14, 257. [Google Scholar] [CrossRef]
- European Medicines Agency. Background Review for Cyclodextrins Used as Excipients; European Medicines Agency: London, UK, 2014. [Google Scholar]
- Hedges, A.R. Industrial applications of cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-F.; Song, C.-K.; Viernstein, H.; Unger, F.; Liang, Z.-S. Apoptosis of human breast cancer cells induced by microencapsulated betulinic acid from sour jujube fruits through the mitochondria transduction pathway. Food Chem. 2013, 138, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Şoica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupkó, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic acid in complex with a gamma-cyclodextrin derivative decreases proliferation and In Vivo tumor development of non-metastatic and metastatic B164A5 Cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef]
- Soica, C.; Oprean, C.; Borcan, F.; Danciu, C.; Trandafirescu, C.; Coricovac, D.; Crainiceanu, Z.; Dehelean, C.A.; Munteanu, M. The synergistic biologic activity of oleanolic and ursolic acids in complex with hydroxypropyl-γ-cyclodextrin. Molecules 2014, 19, 4924–4940. [Google Scholar] [CrossRef]
- Şoica, C.; Dehelean, C.; Danciu, C.; Wang, H.M.; Wenz, G.; Ambrus, R.; Bojin, F.; Anghel, M. Betulin complex in γ-cyclodextrin derivatives: Properties and antineoplasic activities in In Vitro and In Vivo tumor models. Int. J. Mol. Sci. 2012, 13, 14992–15011. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef]
- Friné, V.-C.; Hector, A.-P.; Manuel, N.-D.S.; Estrella, N.-D.; Antonio, G.J. Development and characterization of a biodegradable PLA food packaging hold monoterpene–cyclodextrin complexes against alternaria alternata. Polymers 2019, 11, 1720. [Google Scholar] [CrossRef]
- Al-Nasiri, G.; Cran, M.J.; Smallridge, A.J.; Bigger, S.W. Optimisation of β-cyclodextrin inclusion complexes with natural antimicrobial agents: Thymol, carvacrol and linalool. J. Microencapsul. 2017, 35, 26–35. [Google Scholar] [CrossRef]
- Das, S.; Gazdag, Z.; Szente, L.; Meggyes, M.; Horváth, G.; Lemli, B.; Kunsági-Máté, S.; Kuzma, M.; Kőszegi, T. Antioxidant and antimicrobial properties of randomly methylated β cyclodextrin—Captured essential oils. Food Chem. 2019, 278, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Wicochea-Rodríguez, J.D.; Chalier, P.; Ruiz, T.; Gastaldi, E. Active food packaging based on biopolymers and aroma compounds: How to design and control the release. Front. Chem. 2019, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Higueras, L.; López-Carballo, G.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Antimicrobial packaging of chicken fillets based on the release of carvacrol from chitosan/cyclodextrin films. Int. J. Food Microbiol. 2014, 188, 53–59. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, J.; Zhang, R.; Kong, R.; Lu, W.; Wang, X. Characterization and application of the microencapsulated carvacrol/sodium alginate films as food packaging materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhou, Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of chinese sea bass (Lateolabrax maculatus) during cold storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef] [PubMed]
- López–Gómez, A.; Ros–Chumillas, M.; Buendía-Moreno, L.; Martínez–Hernández, G.B. Active cardboard packaging with encapsulated essential oils for enhancing the shelf life of fruit and vegetables. Front. Nutr. 2020, 7, 559978. [Google Scholar] [CrossRef]
- López-Gómez, A.; Ros-Chumillas, M.; Buendía-Moreno, L.; Navarro-Segura, L.; Martínez-Hernández, G.B. Active cardboard box with smart internal lining based on encapsulated essential oils for enhancing the shelf life of fresh mandarins. Foods 2020, 9, 590. [Google Scholar] [CrossRef]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Uchida, T.; Ichikawa, T.; Watanabe, T.; Uekaji, Y.; Nakata, D.; Terao, K.; Yano, T. Complexation of tocotrienol with γ-cyclodextrin enhances intestinal absorption of tocotrienol in rats. Biosci. Biotechnol. Biochem. 2010, 74, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Kashima, N.; Fujikura, Y.; Komura, T.; Fujiwara, S.; Sakamoto, M.; Terao, K.; Nishikawa, Y. Development of a method for oral administration of hydrophobic substances to Caenorhabditis elegans: Pro-longevity effects of oral supplementation with lipid-soluble antioxidants. Biogerontology 2012, 13, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Schloesser, A.; Esatbeyoglu, T.; Piegholdt, S.; Dose, J.; Ikuta, N.; Okamoto, H.; Ishida, Y.; Terao, K.; Matsugo, S.; Rimbach, G.; et al. Dietary tocotrienol/γ-cyclodextrin complex increases mitochondrial membrane potential and ATP concentrations in the brains of aged mice. Oxidative Med. Cell. Longev. 2015, 2015, 789710. [Google Scholar] [CrossRef] [PubMed]
- Žaloudková, L.; Tichá, A.; Nekvindová, J.; Pavlíková, L.; Zadák, Z.; Živný, P. Different forms of ursolic acid and their effect on liver regeneration. Evid. Based Complement. Altern. Med. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Wüpper, S.; Fischer, A.; Lüersen, K.; Lucius, R.; Okamoto, H.; Ishida, Y.; Terao, K.; Rimbach, G. High dietary kuding tea extract supplementation induces hepatic xenobiotic-metabolizing enzymes—A 6-week feeding study in mice. Nutrients 2019, 12, 40. [Google Scholar] [CrossRef]
- Rimbach, G.; Fischer, A.; Schloesser, A.; Jerz, G.; Ikuta, N.; Ishida, Y.; Matsuzawa, R.; Matsugo, S.; Huebbe, P.; Terao, K.; et al. Anti-inflammatory properties of brazilian green propolis encapsulated in a γ-cyclodextrin complex in mice fed a western-type diet. Int. J. Mol. Sci. 2017, 18, 1141. [Google Scholar] [CrossRef]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C.; et al. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by γ cyclodextrin. J. Cancer 2016, 7, 1755–1771. [Google Scholar] [CrossRef]
- Bhargava, P.; Grover, A.; Nigam, N.; Kaul, A.; Doi, M.; Ishida, Y.; Kakuta, H.; Kaul, S.C.; Terao, K.; Wadhwa, R.; et al. Anticancer activity of the supercritical extract of Brazilian green propolis and its active component, artepillin C: Bioinformatics and experimental analyses of its mechanisms of action. Int. J. Oncol. 2018, 52, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Gutierrez, L.; Bordonaro, M.; Russo, D.; Anzelmi, F.; Hooven, J.T.; Cerra, C.; Lazarova, D.L. Effects of propolis and gamma-cyclodextrin on intestinal neoplasia in normal weight and obese mice. Cancer Med. 2016, 5, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef]
- Gai, L.; Cai, N.; Wang, L.; Xu, X.; Kong, X. Ursolic acid induces apoptosis via Akt/NF-κB signaling suppression in T24 human bladder cancer cells. Mol. Med. Rep. 2013, 7, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Qiu, H.; Zhang, X.; Guo, W.; Chen, W.; Tian, Y.; Fu, L.; Shi, D.; Cheng, J.; et al. Ursolic acid simultaneously targets multiple signaling pathways to suppress proliferation and induce apoptosis in colon cancer cells. PLoS ONE 2013, 8, e63872. [Google Scholar] [CrossRef] [PubMed]
- Jinhua, W. Ursolic acid: Pharmacokinetics processin vitroand In Vivo, a mini review. Arch. Pharm. 2019, 352, e1800222. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liu, Y.; Yang, Z.; Niu, R.; Gao, K.; Yang, B.; Liao, X.; Zhang, J. Solid inclusion complexes of oleanolic acid with amino-appended β-cyclodextrins (ACDs): Preparation, characterization, water solubility and anticancer activity. Mater. Sci. Eng. C 2016, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Münch, G.; Venigalla, M.; Gyengesi, E. Curcumin and apigenin-novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015, 10, 1181–1185. [Google Scholar] [CrossRef]
- Wupper, S.; Fischer, A.; Luersen, K.; Ipharraguerre, I.R.; Chikamoto, K.; Furune, T.; Ishida, Y.; Terao, K.; Rimbach, G. Effects of dietary gamma-cyclodextrin on voluntary activity and muscle strength in mice. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- Singhal, A.; Krystofiak, E.S.; Jerome, W.G.; Song, B. 2-Hydroxypropyl-gamma-cyclodextrin overcomes NPC1 deficiency by enhancing lysosome-ER association and autophagy. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Singhal, A.; Szente, L.; Hildreth, J.E.K.; Song, B. Hydroxypropyl-beta and -gamma cyclodextrins rescue cholesterol accumulation in Niemann–Pick C1 mutant cell via lysosome-associated membrane protein. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Davidson, C.D.; Fishman, Y.I.; Puskás, I.; Szemán, J.; Sohajda, T.; McCauliff, L.A.; Sikora, J.; Storch, J.; Vanier, M.T.; Szente, L.; et al. Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann. Clin. Transl. Neurol. 2016, 3, 366–380. [Google Scholar] [CrossRef]
- Soga, M.; Ishitsuka, Y.; Hamasaki, M.; Yoneda, K.; Furuya, H.; Matsuo, M.; Ihn, H.; Fusaki, N.; Nakamura, K.; Nakagata, N.; et al. HPGCD outperforms HPBCD as a potential treatment for niemann-pick disease type C during disease modeling with iPS cells. Stem Cells 2015, 33, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Hoque, S.; Kondo, Y.; Sakata, N.; Yamada, Y.; Fukaura, M.; Higashi, T.; Motoyama, K.; Arima, H.; Higaki, K.; Hayashi, A.; et al. Differential effects of 2-hydroxypropyl-cyclodextrins on lipid accumulation in Npc1-Null cells. Int. J. Mol. Sci. 2020, 21, 898. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.L.; Krus, K.L.; Lieberman, A.P. Lysosome and endoplasmic reticulum quality control pathways in niemann-pick type C disease. Brain Res. 2016, 1649, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.I.; Maxfield, F.R. Niemann-pick type C disease: Molecular mechanisms and potential therapeutic approaches. J. Neurochem. 2011, 116, 789–795. [Google Scholar] [CrossRef]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the impact of cavity size, occupancy, and substitutions on cytotoxicity and cholesterol homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef]
- Parvez, M.K.; Rishi, V. Herb-drug interactions and hepatotoxicity. Curr. Drug Metab. 2019, 20, 275–282. [Google Scholar] [CrossRef]
- Scorletti, E.; Bhatia, L.; McCormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D. On behalf of the Welcome study investigators effects of purified Eicosapentaenoic and Docosahexaenoic acids in nonalcoholic fatty liver disease: Results from the Welcome * study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, L. Allergy to honeybee not only stings. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 364–368. [Google Scholar] [CrossRef]
- Nissim, I.; Niv, M.Y.; Dagan-Wiener, A. The taste of toxicity: A quantitative analysis of bitter and toxic molecules. IUBMB Life 2017, 69, 938–946. [Google Scholar] [CrossRef] [PubMed]

| Substances and Cyclodextrins | Molar Ratio | Model Organism | Administration | Dose | Outcome | Authors |
|---|---|---|---|---|---|---|
| Tocotrienol (T3) and γ-cyclodextrin (γCD) | Wistar rats n = 5–8 | Oral | 13.9 mg T3 | Improved solubility and stability in the GIT; enhanced intestinal absorption of T3; increased plasma and tissue concentration of T3 | Ikeda et al. 2014 [67] | |
| T3-rich fraction (TRF) from rice bran and γCD | C57BL/6 mice n = 3 (bioavailability) n = 12–15 (bioactivity) | Oral | 2.79 mg TRF | 1.4-fold increase of AUC of T3 plasma concentration; improved bioavailability and physiological activity | Miyoshi et al. 2011 [22] | |
| T3 and γCD | Caenorhabditis elegans n = 30 worms per group/duplicates, performed two or three times | Oral | 26, 86, and 259 µg T3 | Prolonged lifespan | Kashima et al. 2012 [68] | |
| T3 and γCD | C57BL/6 mice n = 6–8 | Oral | 100 mg T3/kg diet | Increased mitochondrial membrane potential and ATP levels in aging brain | Schloesser et al. 2015 [69] | |
| Ursolic acid (UA) & oleanolic acid (OA) & 2-hydroxypropyl-γCD (HP-γCD) | SKH1 mice n = 6 | Dermal | 200 µL 2% aqueous solution | No modification in TWL; increased skin-pH; small change of erythema; small difference in stratum corneum moisture content; anti-tumor activity | Soica et al. 2014 [53] | |
| Betulin (Bet) and octakis-γCD | 1:1 | C57BL/6 mice n = 5 | Subcutaneous injection | 20 mg Bet/BW | Decreased tumor volume; decreased tumor weight | Soica et al. 2012 [54] |
| Betulinic acid (BA) and octakis-γCD | 1:1 | C57BL/6 mice n = 10 | Subcutaneous injection | 100 mg BA/kg | Decreased tumor volume; decreased tumor weight; decreased melanin, erythema, and TWL levels | Soica et al. 2014 [52] |
| UA and γCD | Wistar rats n = 6 | Intragastric | 20 mg UA | Increased liver regeneration; increased hepatocyte growth factor liver expression and plasma levels | Žaloudková et al. 2020 [70] | |
| Kuding tea extract (KTE) and γCD | 1:2 | C57BL/6 mice n = 8–10 | Oral | 7.12% KTE (comprising 0.15% UA) | Increased liver weight; elevated hepatic fat accumulation; increased hepatic PPARγ, CD36, CYP7A1, CYP3A, and GSTA1 levels; increased plasma cholesterol level | Wüpper et al. 2020 [71] |
| Brazilian green propolis supercritical extract (GPSE) and γCD | C57BL/6 mice n = 10 | Oral | 2.3 g/kg GPSE-γCD (providing 200 mg artepillin C/kg diet) | Decreased hepatic TNFα and Sap mRNA level; anti-inflammatory properties | Rimbach et al. 2017 [72] | |
| Caffeic acid phenethyl ester (CAPE) and γCD | 1:1 | Balb/c nude mice n = 9 | Oral or intraperitoneal | Decreased tumor volume; anti-metastatic properties | Wadhwa et al. 2016 [73] | |
| CAPE and γCD | A549 and HT1080 cells | Higher cytotoxicity; higher solubility in a mimicked intestinal environment | Ishida et al. 2018 [23] | |||
| GPSE and γCD | 1:1 | BALB/c nude mice n = 3 | Oral | GPSE-γCD (containing 3% artepillin C) | Decreased tumor growth | Bhargava et al. 2018 [74] |
| Propolis and γCD | C57BL/6J-ApcMin/+/J mice n = 5–6 | Oral | 8% propolis-γCD (comprising 6% γCD) and pure γCD | Decreased tumor development; decreased number of adenomas | Cho et al. 2016 [75] | |
| Curcuminoids and γCD | Humans n = 12 | Oral | 371.4 mg total curcuminoids (containing 346 mg curcumin) | Improved solubility and stability in the GIT; enhanced intestinal absorption of T3 increased plasma and tissue concentration of T3 | Purpura et al. 2018 [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. https://doi.org/10.3390/biom11030401
Wüpper S, Lüersen K, Rimbach G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules. 2021; 11(3):401. https://doi.org/10.3390/biom11030401
Chicago/Turabian StyleWüpper, Svenja, Kai Lüersen, and Gerald Rimbach. 2021. "Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective" Biomolecules 11, no. 3: 401. https://doi.org/10.3390/biom11030401
APA StyleWüpper, S., Lüersen, K., & Rimbach, G. (2021). Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules, 11(3), 401. https://doi.org/10.3390/biom11030401