Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Extract Preparation
2.3. Synthesis of Silver Nanoparticles
2.4. Characterization of Silver Nanoparticles
2.4.1. UV-Vis Spectroscopic Analysis
2.4.2. X-ray Diffraction (XRD) Analysis
2.4.3. Fourier Transform Infrared (FT-IR) Analysis
2.5. Free Radical Scavenging Activity
2.6. Analysis of Antibacterial Sensitivity Test
2.7. Determination of Minimum Inhibitory Concentration (MIC)
2.8. Statistical Analysis
3. Results
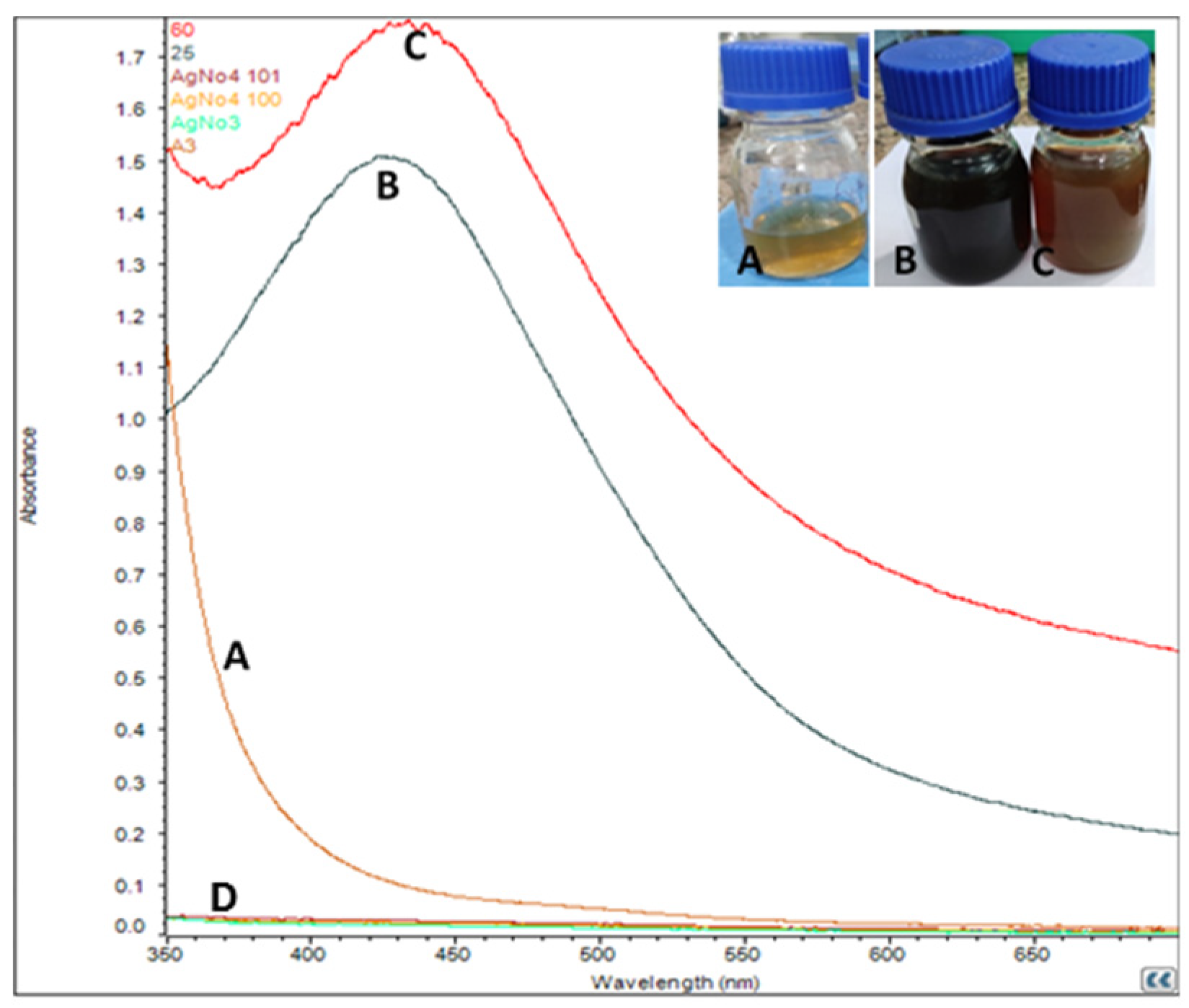
3.1. Formation of Silver Nanoparticles
3.2. UV-vis Spectroscopy Analysis
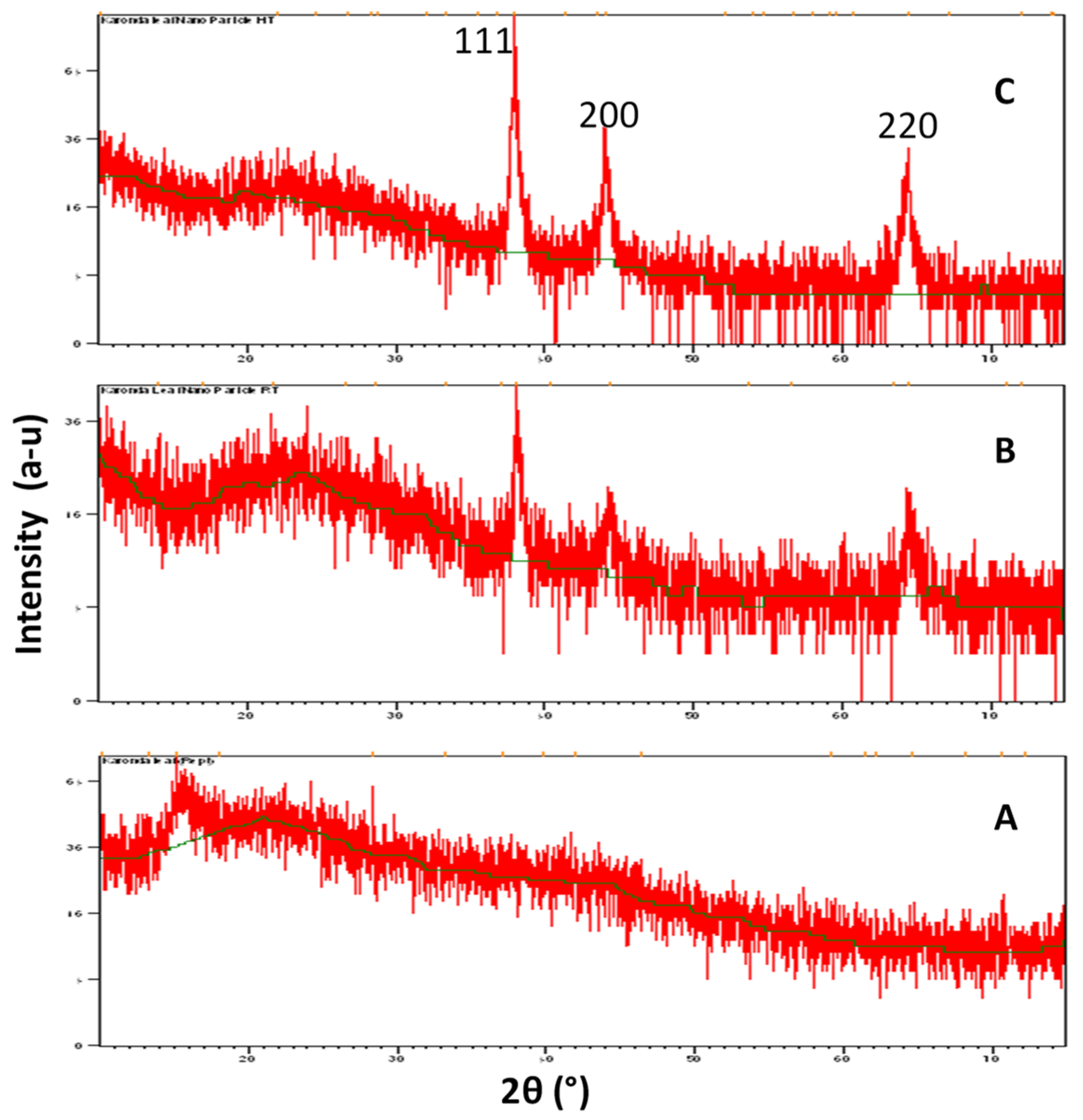
3.3. XRD Analysis
3.4. FT-IR Analysis
3.5. Antioxidant Activity
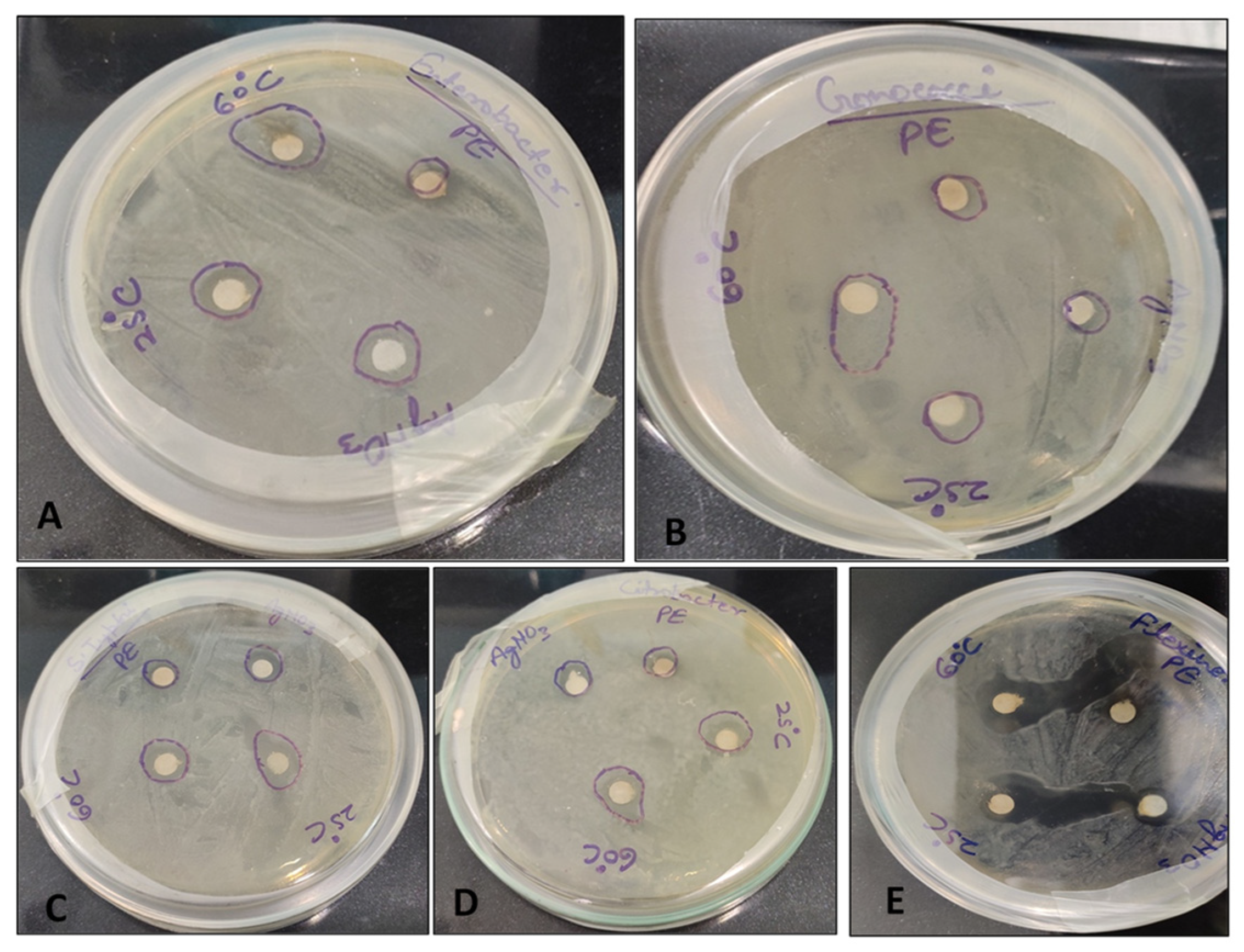
3.6. Antibacterial Sensitivity Test
3.7. Determination of Minimum Inhibitory Concentration (MIC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrecht, M.A.; Evan, C.W.; Raston, C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006, 8, 417–432. [Google Scholar] [CrossRef]
- Anjum, S.; Anjum, I.; Hano, C.; Kousar, S. Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Adv. 2019, 9, 40404–40423. [Google Scholar] [CrossRef]
- Saleem, K.; Khursheed, Z.; Hano, C.; Anjum, I.; Anjum, S. Applications of Nanomaterials in Leishmaniasis: A Focus on Recent Advances and Challenges. Nanomaterials 2019, 9, 1749. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Fazal, H.; Ahmad, N.; Ali, M.; Giglioli-Guivarch, N.; Hano, C. Nanomaterials for Cosmeceuticals: Nanomaterials-Induced Advancement in Cosmetics, Challenges, and Opportunities; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128222867. [Google Scholar]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem. Lett. Rev. 2018, 11, 492–502. [Google Scholar] [CrossRef]
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Zia, U.M.; Hano, C.; Abbasi, B.H. Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: A review. Int. J. Nanomed. 2020, 15, 5951. [Google Scholar] [CrossRef]
- Yu, D.G. Formation of colloidal silver nanoparticles stabilized by Na ± poly(gamma-glutamic acid)-silver nitrate complex via chemical reduction process. Colloids Surf. B 2007, 59, 171–178. [Google Scholar] [CrossRef]
- Mallick, K.; Witcombb, M.J.; Scurrella, M.S. Self-assembly of silver nanoparticles in a polymer solvent Formation of a nanochain through nanoscale soldering. Mater. Chem. Phys. 2005, 90, 221–224. [Google Scholar] [CrossRef]
- Khan, T.; Abbasi, B.H.; Afridi, M.S.; Tanveer, F.; Ullah, I.; Bashir, S.; Hano, C. Melatonin-enhanced biosynthesis of antimicrobial AgNPs by improving the phytochemical reducing potential of a callus culture of Ocimum basilicum L. var. thyrsiflora. RSC Adv. 2017, 7, 38699–38713. [Google Scholar] [CrossRef]
- Shah, M.; Nawaz, S.; Jan, H.; Uddin, N.; Ali, A.; Anjum, S.; Giglioli-Guivarc’H, N.; Hano, C.; Abbasi, B.H. Synthesis of bio-mediated silver nanoparticles from Silybum marianum and their biological and clinical activities. Mater. Sci. Eng. C 2020, 112, 110889. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Usman, H.; Khan, M.A.; Zia, M.; Hano, C.; Abbasi, B.H. Biogenic synthesis and characterization of antimicrobial and antiparasitic zinc oxide (ZnO) nanoparticles using aqueous extracts of the Himalayan Columbine (Aquilegia pubiflora). Front. Mater. 2020, 7, 249. [Google Scholar] [CrossRef]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2003, 14, 95–100. [Google Scholar] [CrossRef]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Minaeian, S.; Shahverdi, H.R.; Jamalifar, H.; Nohi, A.A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacter faecalis ia: A novel biological approach. Process Biochem. 2007, 42, 919–923. [Google Scholar] [CrossRef]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2000, 11, 37–44. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Rad. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Kumara, P.P.N.V.; Pammib, S.V.N.; Kollu, P.; Satyanarayana, K.V.V.; Shameema, U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti-bacterial activity. Ind. Crops Prod. 2014, 52, 562–566. [Google Scholar] [CrossRef]
- Phull, A.R.; Abbas, Q.; Ali, A.; Raza, H.; Kim, S.J.; Zia, M.; Haq, I.U. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliate. Future J. Pharm. Sci. 2016, 2, 31–36. [Google Scholar] [CrossRef]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mat. Sci. Eng. 2016, 62, 719–724. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Jiang, H.; Manolache, S.; Wong, A.C.L.; Denes, F.S. Plasma-enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J. Appl. Polym. Sci. 2004, 93, 1411–1422. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, B. Metal-Based Therapy in Traditional and Modern Medicine Systems. In Biomedical Applications of Metals, 1st ed.; Rai, M., Ingle, A.P., Medici, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 195–211. [Google Scholar]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russian Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Singh, R.; Tiwari, P.; Kumari, N.; Sharma, B. Biomedical Applications of Green Synthesized Nanoparticles. In Advances in Pharmaceutical Biotechnology, 1st ed.; Patra, J.K., Shukla, A.K., Das, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–245. [Google Scholar]
- Sawant, R.S.; Godghate, A.G. Comparative studies of phytochemical screening of Carissa carandas Linn. Asian J. Plant Sci. Res. 2013, 3, 21–25. [Google Scholar]
- Arif, M.; Kamal, M.; Jawaid, T.; Khalid, M.; Saini, K.S.; Kumar, A.; Ahmad, M. Carissa carandas Linn. (Karonda): An exotic minor plant fruit with immense value in nutraceuticals and pharmaceutical industries. Asian J. Biomed. Pharm. Sci. 2016, 6, 14–19. [Google Scholar]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, more than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Borchert, H.E.V.; Shevchenko, A.; Robert, I.; Mekis, A.; Kornowski, G.; Grabel, G.; Weller, H. Determination of Nanocrystal Sizes: A Comparison of TEM, SAXS, and XRD Studies of Highly Monodisperse CoPt3 Particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Singh, R.; Kumari, N. Sapindus mukorossi Gaertn.: Rich Source of Antioxidants and Reducing agents. Res. J. Chem. Environ. 2020, 24, 38–46. [Google Scholar]
- Singh, R.; Kumari, N. Comparative determination of phytochemicals and antioxidant activity from leaf and fruit of Sapindus mukorrossi Gaertn.—A valuable medicinal tree. Ind. Crops Prod. 2015, 73, 1–8. [Google Scholar] [CrossRef]
- Singh, R.; Kashyap, S.P.; Kumari, N.; Singh, M. Regeneration of soapnut tree through somatic embryogenesis and assessment of genetic fidelity through ISSR and RAPD markers. Physiol. Mol. Biol. Plants 2016, 22, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumari, N.; Gangwar, M.; Nath, G. Qualitative characterization of phytochemicals and in vitro antimicrobial evaluation of leaf extract of Couroupita guianensis Aubl.—A threatened medicinal tree. Int. J. Pharma. Pharma. Sci. 2015, 7, 212–215. [Google Scholar]
- Maiti, S.; Krishnan, D.; Barman, G.; Ghosh, S.K.; Laha, J.K. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J. Anal. Sci. Technol. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Biosynthesis of silver nanoparticles using citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta Part A 2011, 79, 594–598. [Google Scholar] [CrossRef]
- Rai, A.; Singh, A.; Ahmad, A.; Sastry, M. Role of Halide Ions and Temperature on the Morphology of Biologically Synthesized Gold Nanotriangles. Langmuir 2006, 22, 736–741. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Raghunandan, D.; Basavaraja, S.; Mahesh, B.; Balaji, S.; Manjunath, S.Y.; Venkataraman, A. Anti-cancer studies of noble metal nanoparticles synthesized using different plant extracts. Nanobiotechnology 2009, 5, 34–41. [Google Scholar] [CrossRef]
- Noginov, M.A.; Zhu, G.; Bahoura, M.; Adegoke, J.; Small, C.; Ritzo, B.A.; Drachev, V.P.; Shalaev, V.M. The effect of gain and absorption on surface plasmons in metal nanoparticles. Appl. Phys. B 2007, 86, 455–460. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Kathiravan, V.; Ravi, S.; Kumar, A.S.; Velmurugan, S.; Elumalai, K.; Khatiwada, C.P. Green synthesis of silver nanoparticles using Croton sparsi-florus morong leaf extract and their antibacterial and antifungal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 139, 200–205. [Google Scholar] [CrossRef]
- Kathiravan, V.; Ravi, S.; Kumar, A.S. Synthesis of silver nanoparti-cles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg, S.; Rancan, F.; Epple, M.; Loza, K.; Höppe, D.; Lagemann, J.; Vogt, A.; Kleuser, B.; Gerecke, C.; Meinke, M.C. Comparison of different methods to study effects of silver nanoparticles on the pro-and antioxidant status of human keratinocytes and fibroblasts. Methods 2016, 109, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Jain, N.; Pathak, A.; Singh, J.; Prasad, R.; Upadhyaya, C.P. Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J. Sol. Gel. Sci. Technol. 2018, 86, 682–689. [Google Scholar] [CrossRef]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf. B Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef] [PubMed]

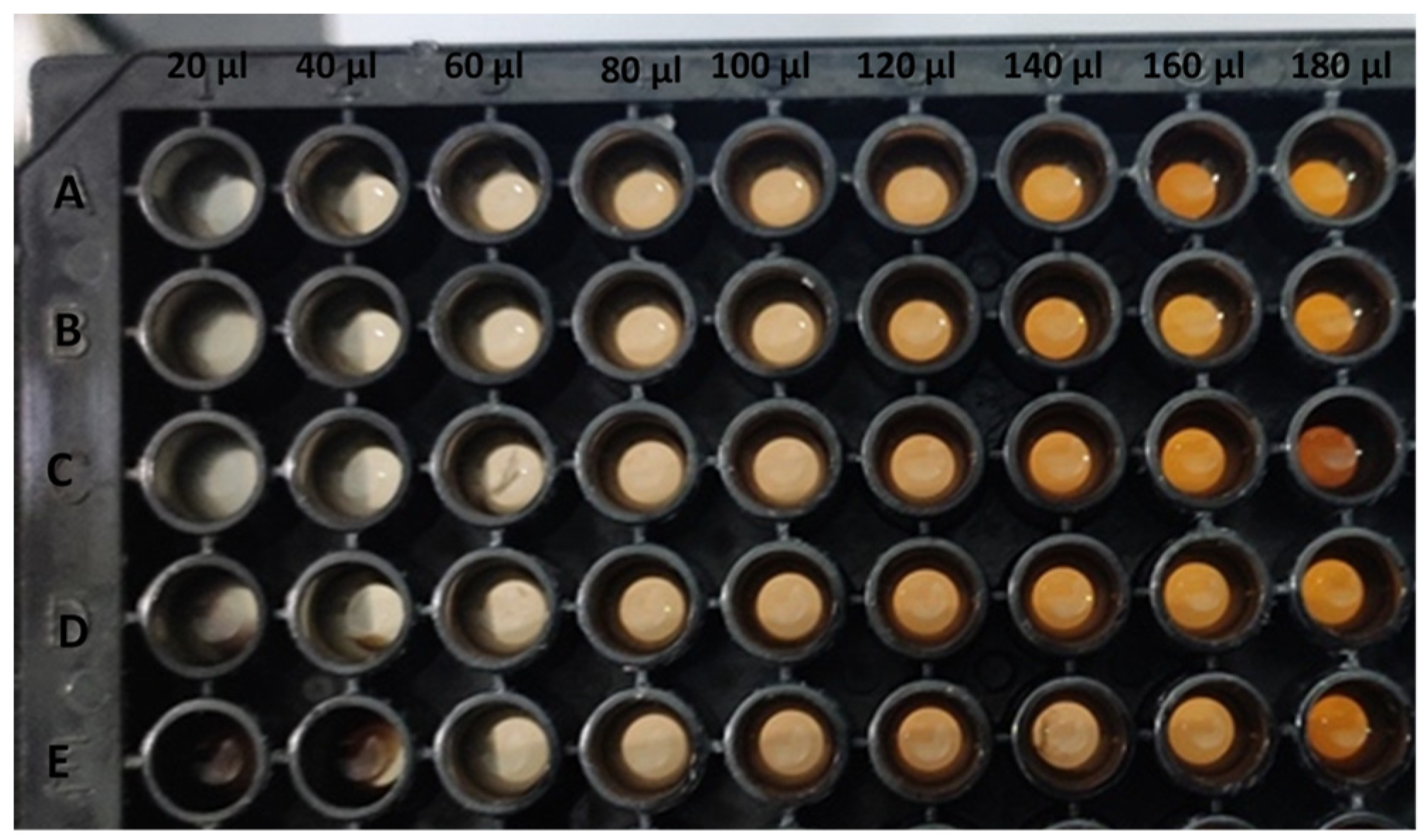
| Concentration (%NPs/mL) | AgNPs (25 °C) | AgNPs (60 °C) |
|---|---|---|
| 5 | 86.45 ± 0.38 cd | 76.69 ± 0.68 i |
| 10 | 88.89 ± 0.64 b | 85.54 ± 0.76 de |
| 15 | 90.30 ± 0.35 a | 87.12 ± 045 c |
| 20 | 82.67 ± 0.34 f | 84.62 ± 0.68 e |
| 25 | 80.35 ± 0.44 g | 82.12 ± 0.73 f |
| 30 | 76.02 ± 0.38 i | 78.58 ± 0.83 h |
| 35 | 72.84 ± 1.00 j | 76.26 ± 0.91 i |
| 40 | 70.53 ± 0.49 k | 74.25 ± 0.90 j |
| Bacteria | Growth Inhibition Zone (mm) | |||
|---|---|---|---|---|
| Plant Extract | AgNO3 | AgNPs (25 °C) | AgNPs (60 °C) | |
| Enterobacter faecalis | 7.0 ± 0.0 d | 9.0 ± 0.6 c | 10.0 ± 0.0 b | 16.0 ± 1.0 a |
| Gonococci spp. | 6.0 ± 0.0 d | 8.0 ± 0.00 c | 9.0 ± 0.0 b | 14.3 ± 0.6 a |
| Salmonella typhimurium | 8.0 ± 1.0 c | 10.0 ± 1.0 bc | 14.0 ± 0.0 a | 12.0 ± 1.0 b |
| Citrobacter spp. | 8.0 ± 1.0 c | 10.0 ± 0.0 b | 12.0 ± 1.0 a | 14.0 ± 1.0 a |
| Shigella flexneri | 8.0 ± 1.0 d | 11.0 ± 1.0 c | 24.0 ± 1.0 a | 21.3 ± 0.6 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Hano, C.; Nath, G.; Sharma, B. Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria. Biomolecules 2021, 11, 299. https://doi.org/10.3390/biom11020299
Singh R, Hano C, Nath G, Sharma B. Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria. Biomolecules. 2021; 11(2):299. https://doi.org/10.3390/biom11020299
Chicago/Turabian StyleSingh, Reetika, Christophe Hano, Gopal Nath, and Bechan Sharma. 2021. "Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria" Biomolecules 11, no. 2: 299. https://doi.org/10.3390/biom11020299
APA StyleSingh, R., Hano, C., Nath, G., & Sharma, B. (2021). Green Biosynthesis of Silver Nanoparticles Using Leaf Extract of Carissa carandas L. and Their Antioxidant and Antimicrobial Activity against Human Pathogenic Bacteria. Biomolecules, 11(2), 299. https://doi.org/10.3390/biom11020299









