The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization
Abstract
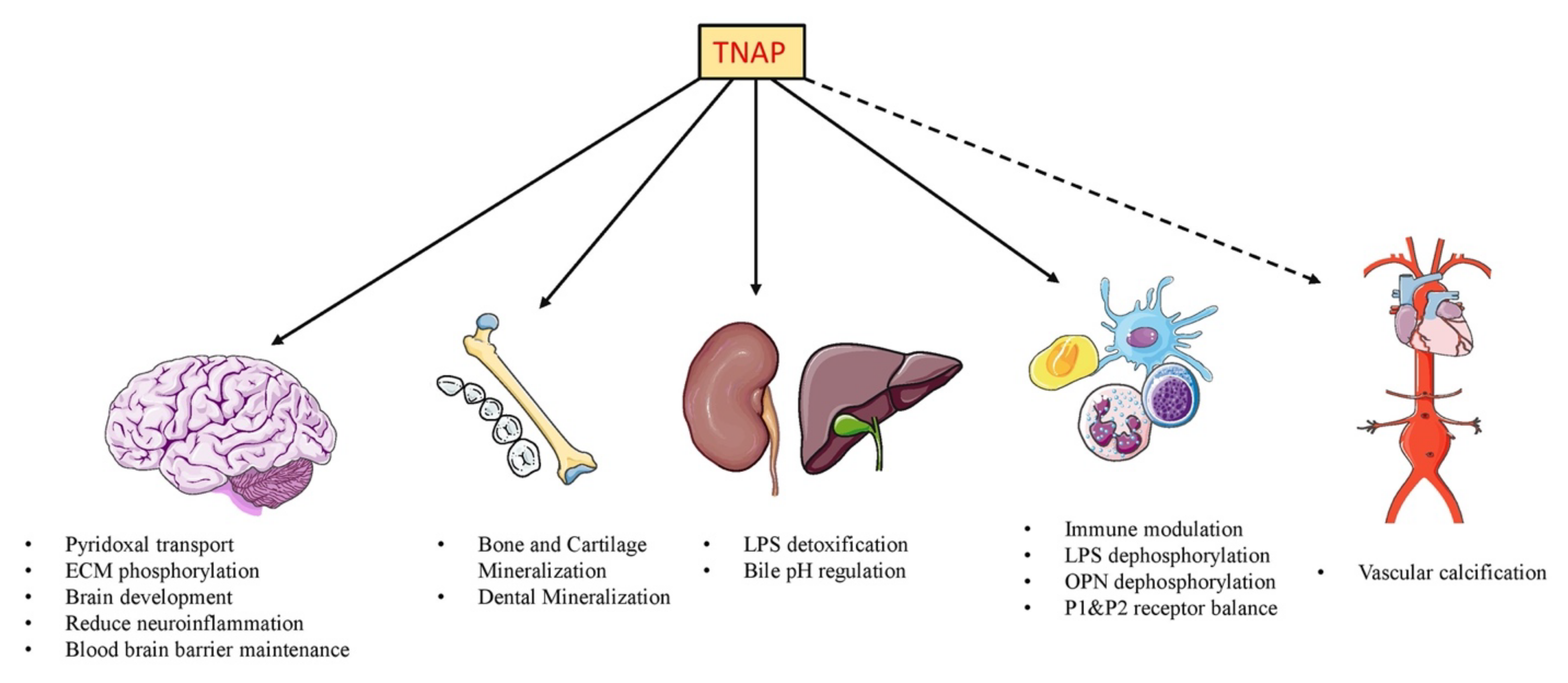
:1. Introduction
2. ALP Isoforms
2.1. Liver/Bone/Kidney Alkaline Phosphatase
2.2. Intestinal Alkaline Phosphatase
2.3. Placental Alkaline Phosphatase
2.4. Germ Cell Alkaline Phosphatase
3. TNAP
3.1. Gene Structure
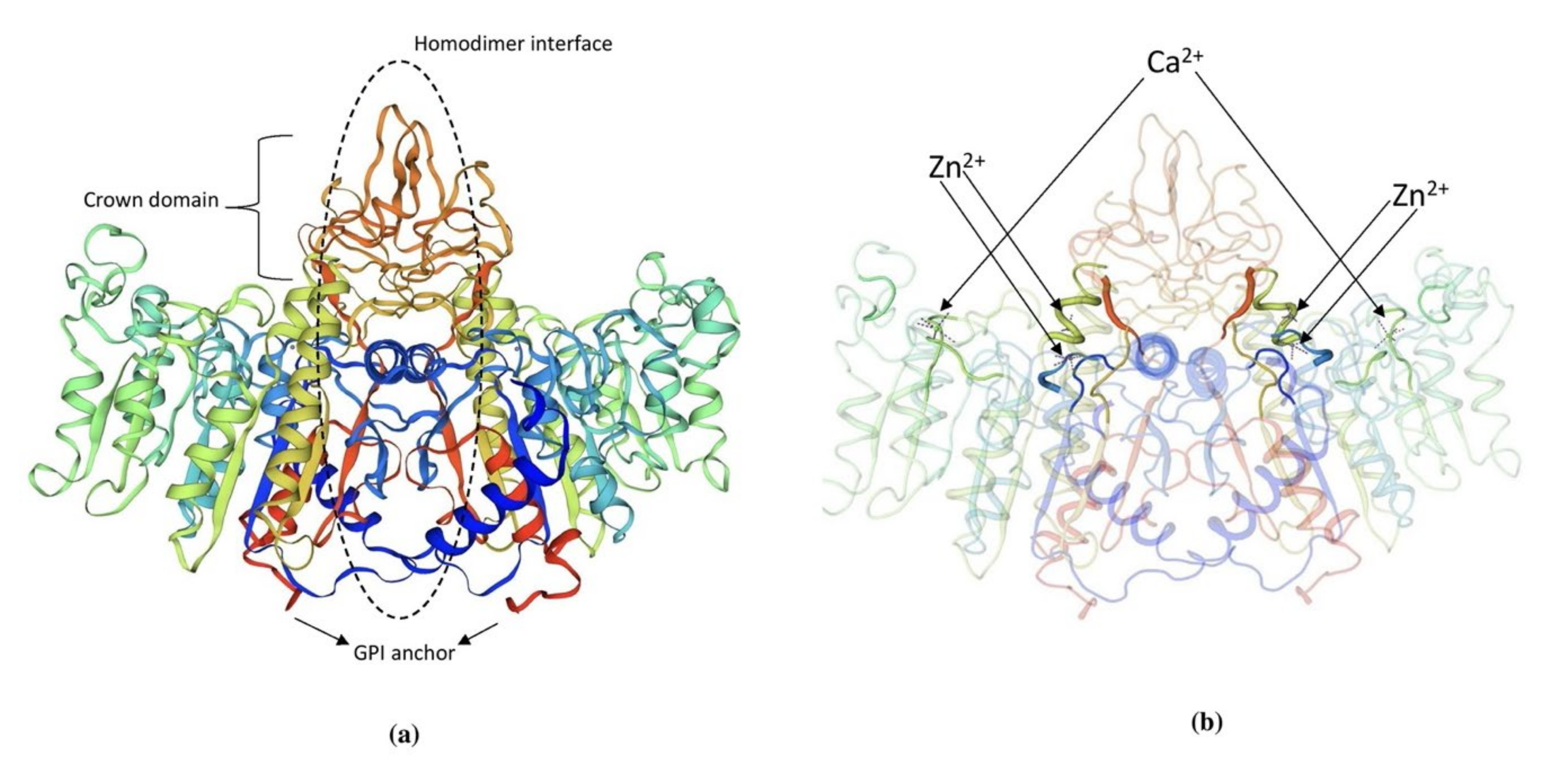
3.2. Protein Structure
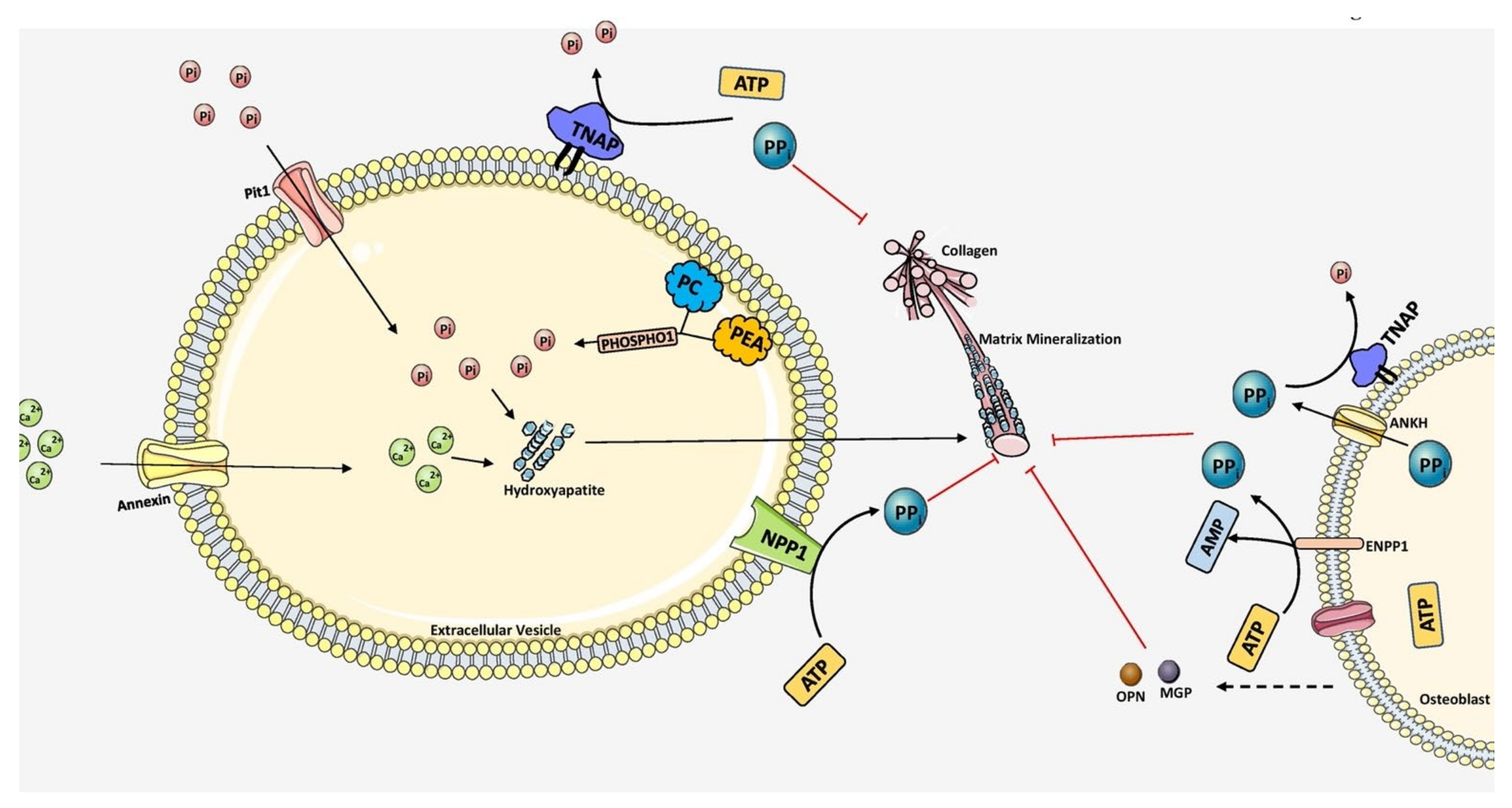
3.3. Mechanism of Mineralization-Promotion and Inhibition
3.4. Hypophosphatasia-Physiological Substrates of TNAP
3.5. Role of TNAP as An Anti-Inflammatory Enzyme
3.6. Role of TNAP in Central Nervous System
3.7. Hepatic Role of TNAP
3.8. Renal Role of TNAP
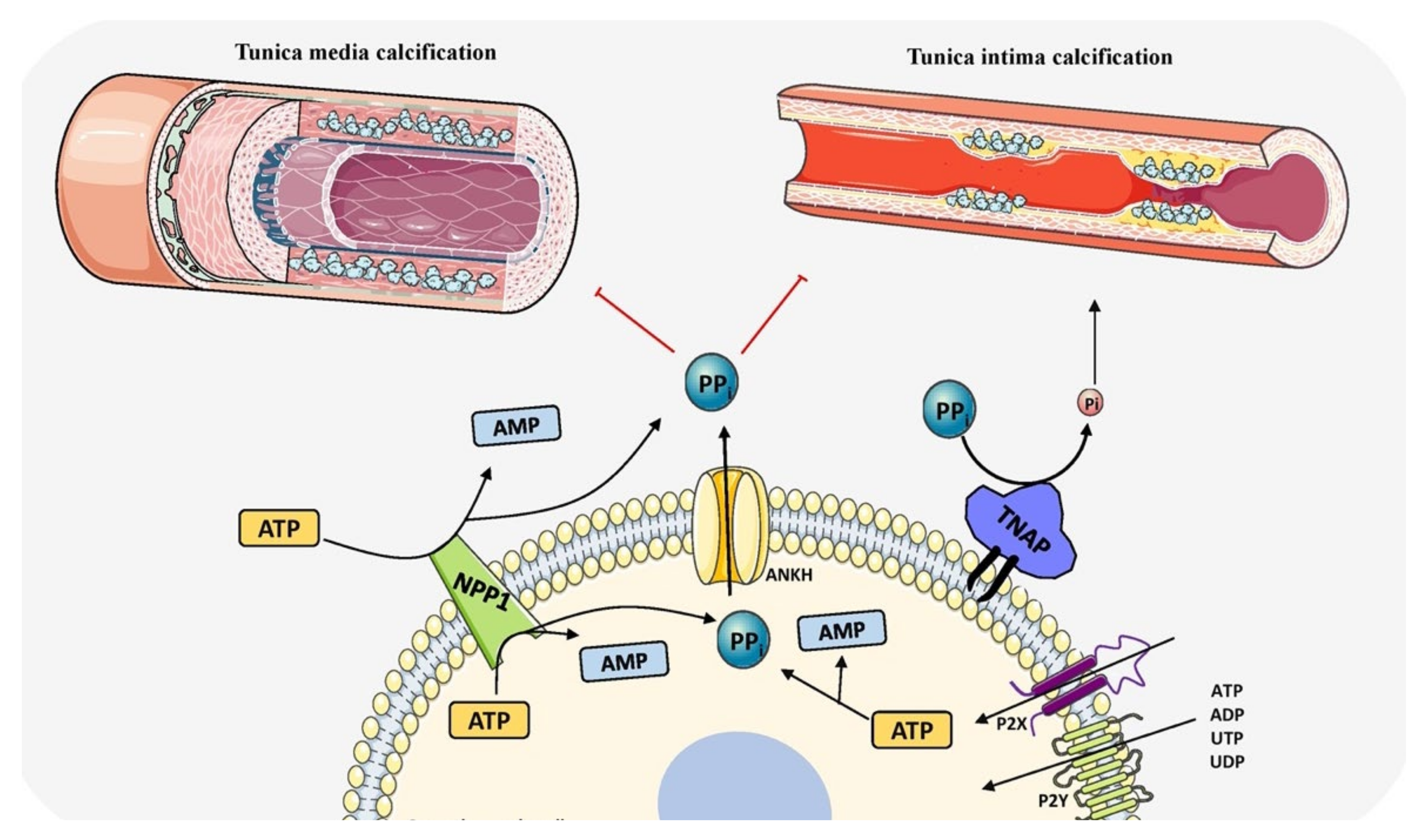
3.9. TNAP in Vascular Calcification
4. Pharmacological Inhibition of TNAP Needs Careful Consideration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T. Determinants of pathological mineralization. Curr. Opin. Rheumatol. 2006, 18, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T. Physiological and pathological mineralization: A complex multifactorial process. Curr. Opin. Orthop. 2007, 18, 425–427. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Egido, J. Phosphate, pyrophosphate, and vascular calcification: A question of balance. Eur. Heart J. 2015, 38, ehv605-1804. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, H.T.; Lansing, A.I.; Wheeler, P.A. Calcification of the Media of the Human Aorta and Its Relation to Intimal Arteriosclerosis, Ageing and Disease. Am. J. Pathol. 1944, 20, 665–687. [Google Scholar]
- Nicoll, R.; Henein, M.Y. The predictive value of arterial and valvular calcification for mortality and cardiovascular events. IJC Heart Vessel. 2014, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Cecelja, M.; Chowienczyk, P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc. Dis. 2012, 1, 1–10. [Google Scholar] [CrossRef]
- Shanahan, C.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial Calcification in Chronic Kidney Disease: Key Roles for Calcium and Phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Villa-Bellosta, R. Synthesis of Extracellular Pyrophosphate Increases in Vascular Smooth Muscle Cells during Phosphate-Induced Calcification. Arter. Thromb. Vasc. Biol. 2018, 38, 2137–2147. [Google Scholar] [CrossRef] [Green Version]
- Yamato, Y.; Matsukawa, M.; Mizukawa, H.; Yanagitani, T.; Yamazaki, K.; Nagano, A. Distribution of hydroxyapatite crystallite orientation and ultrasonic wave velocity in ring-shaped cortical bone of bovine femur. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 1298–1303. [Google Scholar] [CrossRef]
- Osaki, S.; Tohno, S.; Tohno, Y.; Ohuchi, K.; Takakura, Y. Determination of the orientation of collagen fibers in human bone. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2002, 266, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glimcher, M.J.; Muir, H. Recent studies of the mineral phase in bone and its possible linkage to the organic matrix by protein-bound phosphate bonds. Philos. Trans. R. Soc. B Biol. Sci. 1984, 304, 479–508. [Google Scholar] [CrossRef]
- Landis, W.; Song, M.; Leith, A.; McEwen, L.; McEwen, B.F. Mineral and Organic Matrix Interaction in Normally Calcifying Tendon Visualized in Three Dimensions by High-Voltage Electron Microscopic Tomography and Graphic Image Reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; De With, G.; Sommerdijk, N.A.J.M. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traub, W.; Arad, T.; Weiner, S. Origin of Mineral Crystal Growth in Collagen Fibrils. Matrix 1992, 12, 251–255. [Google Scholar] [CrossRef]
- Anderson, H.C. The role of matrix vesicles in physiological and pathological calcification. Curr. Opin. Orthop. 2007, 18, 428–433. [Google Scholar] [CrossRef]
- Van de Lest, C.H.A.; Vaandrager, A.B. Mechanism of cell-mediated mineralization. Curr. Opin. Orthop. 2007, 8, 434–443. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Nair, A.; Gautieri, A.; Chang, S.-W.; Buehler, M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nat. Commun. 2013, 4, 1724. [Google Scholar] [CrossRef] [Green Version]
- Le Du, M.-H.; Millán, J.L. Structural Evidence of Functional Divergence in Human Alkaline Phosphatases. J. Biol. Chem. 2002, 277, 49808–49814. [Google Scholar] [CrossRef] [Green Version]
- Al-Rashida, M.; Iqbal, J. Inhibition of Alkaline Phosphatase: An Emerging New Drug Target. Mini-Rev. Med. Chem. 2015, 15, 41–51. [Google Scholar] [CrossRef]
- Sebastián-Serrano, Á.; de Diego-García, L.; Martínez-Frailes, C.; Avila, J.; Zimmermann, H.; Millán, J.L.; Miras-Portugal, M.T.; Díaz-Hernández, M. Tissue-nonspecific Alkaline Phosphatase Regulates Purinergic Transmission in the Central Nervous System During Development and Disease. Comput. Struct. Biotechnol. J. 2015, 13, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Boechat, G.A.P.; Stinghen, S.T.; Custódio, G.; Pianovski, M.A.D.; Figueiredo, F.R.D.O.; Jenkins, J.; Zambetti, G.P.; Ribeiro, R.C.; Figueiredo, B.C. Placental Alkaline Phosphatase in Pediatric Adrenocortical Cancer. J. Pediatr. Hematol. 2011, 33, e149–e153. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P. Intestinal alkaline phosphatase: Novel functions and protective effects. Nutr. Rev. 2014, 72, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Zahradnik-Bilska, J.; Brzozowski, B.; Magierowski, M.; Mach, T.; Magierowska, K.; Brzozowski, T. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract. Mediat. Inflamm. 2017, 2017, 9074601. [Google Scholar] [CrossRef] [PubMed]
- Henthom, P.S.; Raducha, M.; Hadesch, T.; Weiss, M.J.; Harris, H. Sequence and characterization of the human intestinal alkaline phosphatase gene. J. Biol. Chem. 1988, 263, 12011–12019. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergote, I.B.; Ábeler, V.M.; Børmer, O.P.; Stigbrand, T.; Trope, C.; Nustad, K. CA125 and Placental Alkaline Phosphatase as Serum Tumor Markers in Epithelial Ovarian Carcinoma. Tumor Biol. 1992, 13, 168–174. [Google Scholar] [CrossRef]
- Llinas, P.; Stura, E.; Ménez, A.; Kiss, Z.; Stigbrand, T.; Millán, J.L.; Le Du, M.H. Structural Studies of Human Placental Alkaline Phosphatase in Complex with Functional Ligands. J. Mol. Biol. 2005, 350, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Zaher, D.M.; El-Gamal, M.I.; Omar, H.A.; Aljareh, S.N.; Al-Shamma, S.A.; Ali, A.J.; Zaib, S.; Iqbal, J. Recent advances with alkaline phosphatase isoenzymes and their inhibitors. Arch. Pharm. 2020, 353, e2000011. [Google Scholar] [CrossRef]
- Povinelli, C.M.; Knoll, B.J. Trace expression of the germ-cell alkaline phosphatase gene in human placenta. Placenta 1991, 12, 663–668. [Google Scholar] [CrossRef]
- Fishman, W.H. Clinical and biological significance of an isozyme tumor marker—PLAP. Clin. Biochem. 1987, 20, 387–392. [Google Scholar] [CrossRef]
- Orimo, H. The Mechanism of Mineralization and the Role of Alkaline Phosphatase in Health and Disease. J. Nippon. Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.; Ray, K.; Henthorn, P.S.; Lamb, B.; Kadesch, T.; Harris, H. Structure of the human liver/bone/kidney alkaline phosphatase gene. J. Biol. Chem. 1988, 263, 12002–12010. [Google Scholar] [CrossRef]
- Fedde, K.N.; Blair, L.; Silverstein, J.; Coburn, S.P.; Ryan, L.M.; Weinstein, R.S.; Waymire, K.; Narisawa, S.; Millan, J.L.; MacGregor, G.R.; et al. Alkaline Phosphatase Knock-Out Mice Recapitulate the Metabolic and Skeletal Defects of Infantile Hypophosphatasia. J. Bone Miner. Res. 1999, 14, 2015–2026. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Manoli, N.M.; Vimalraj, S.; Madhunapantula, S.V. Unmethylated promoter DNA correlates with p53 expression and apoptotic levels only in Vitamin B9 and B12 deficient megaloblastic anemia but not in non-megaloblastic anemia controls. Int. J. Biol. Macromol. 2018, 109, 76–84. [Google Scholar] [CrossRef]
- Whyte, M.P. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann. N. Y. Acad. Sci. 2010, 1192, 190–200. [Google Scholar] [CrossRef]
- Hessle, L.; Johnson, K.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmey, D.; Hessle, L.; Narisawa, S.; Johnson, K.A.; Terkeltaub, R.; Millán, J.L. Concerted Regulation of Inorganic Pyrophosphate and Osteopontin by Akp2, Enpp1, and Ank: An Integrated Model of the Pathogenesis of Mineralization Disorders. Am. J. Pathol. 2004, 164, 1199–1209. [Google Scholar] [CrossRef]
- Amadasi, A.; Bertoldi, M.; Contestabile, R.; Bettati, S.; Cellini, B.; Di Salvo, M.L.; Borri-Voltattorni, C.; Bossa, F.; Mozzarelli, A. Pyridoxal 5′-phosphate enzymes as targets for therapeutic agents. Curr. Med. Chem. 2007, 14, 1291–1324. [Google Scholar] [CrossRef] [PubMed]
- Waymire, K.G.; Mahuren, J.D.; Jaje, J.M.; Guilarte, S.R. Mice lacking tissue non–specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat. Genet. 1995, 11, 45–51. [Google Scholar] [CrossRef]
- Narisawa, S.; Wennberg, C.; Millán, J.L. Abnormal vitamin B6 metabolism in alkaline phosphatase knock-out mice causes multiple abnormalities, but not the impaired bone mineralization. J. Pathol. 2000, 193, 125–133. [Google Scholar] [CrossRef]
- Michigami, T.; Ohata, Y.; Fujiwara, M.; Mochizuki, H.; Adachi, M.; Kitaoka, T.; Kubota, T.; Sawai, H.; Namba, N.; Hasegawa, K.; et al. Clinical Practice Guidelines for Hypophosphatasia. Clin. Pediatr. Endocrinol. 2020, 29, 9–24. [Google Scholar] [CrossRef]
- Lei, W.; Ni, H.; Herington, J.; Reese, J.; Paria, B.C. Alkaline Phosphatase Protects Lipopolysaccharide-Induced Early Pregnancy Defects in Mice. PLoS ONE 2015, 10, e0123243. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wandler, A.M.; Postlethwait, J.H.; Guillemin, K. Dynamic Evolution of the LPS-Detoxifying Enzyme Intestinal Alkaline Phosphatase in Zebrafish and Other Vertebrates. Front. Immunol. 2012, 3, 314. [Google Scholar] [CrossRef] [Green Version]
- Sebastián-Serrano, Á.; de Diego-García, L.; Henshall, D.C.; Engel, T.; Diaz-Hernandez, M. Haploinsufficient TNAP Mice Display Decreased Extracellular ATP Levels and Expression of Pannexin-1 Channels. Front. Pharmacol. 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, P.S.; Doolittle, L.M.; Hickman-Davis, J.M.; Davis, I.C. ATP catabolism by tissue nonspecific alkaline phosphatase contributes to development of ARDS in influenza-infected mice. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L83–L92. [Google Scholar] [CrossRef]
- Zhang, Z.; Nam, H.; Crouch, S.; Hatch, N. Tissue Nonspecific Alkaline Phosphatase Function in Bone and Muscle Progenitor Cells: Control of Mitochondrial Respiration and ATP Production. Int. J. Mol. Sci. 2021, 22, 1140. [Google Scholar] [CrossRef] [PubMed]
- Narisawa, S.; Yadav, M.C.; Millán, J.L. In Vivo Overexpression of Tissue-Nonspecific Alkaline Phosphatase Increases Skeletal Mineralization and Affects the Phosphorylation Status of Osteopontin. J. Bone Miner. Res. 2013, 28, 1587–1598. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.C.; Huesa, C.; Narisawa, S.; Hoylaerts, M.F.; Moreau, A.; Farquharson, C.; Millán, J.L. Ablation of Osteopontin Improves the Skeletal Phenotype of Phospho1−/− Mice. J. Bone Miner. Res. 2014, 29, 2369–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addison, W.N.; Azari, F.; Sørensen, E.S.; Kaartinen, M.T.; McKee, M.D. Pyrophosphate Inhibits Mineralization of Osteoblast Cultures by Binding to Mineral, Up-regulating Osteopontin, and Inhibiting Alkaline Phosphatase Activity. J. Biol. Chem. 2007, 282, 15872–15883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, M.; Henthorn, P.S.; Lafferty, M.A.; Slaughter, C.; Raducha, M.; Harris, H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc. Natl. Acad. Sci. USA 1986, 83, 7182–7186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiledjian, M.; Kadesch, T. Analysis of the human liver/bone/kidney alkaline phosphatase promoter In Vivo and In Vitro. Nucleic Acids Res. 1990, 18, 957–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orimo, H.; Shimada, T. Regulation of the human tissue-nonspecific alkaline phosphatase gene expression by all-trans-retinoic acid in SaOS-2 osteosarcoma cell line. Bone 2005, 36, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H.; Shimada, T. Posttranscriptional modulation of the human tissue–nonspecific alkaline phosphatase gene expression by 1,25-dihydroxyvitamin D3 in MG-63 osteoblastic osteosarcoma cells. Nutr. Res. 2006, 26, 227–234. [Google Scholar] [CrossRef]
- Orimo, H.; Shimada, T. The role of tissue-nonspecific alkaline phosphatase in the phosphate-induced activation of alkaline phosphatase and mineralization in SaOS-2 human osteoblast-like cells. Mol. Cell. Biochem. 2008, 315, 51–60. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sañudo, C.; Sánchez-Verde, L.; García-Renedo, R.J.; Arozamena, J.; Riancho, J.A. Epigenetic regulation of alkaline phosphatase in human cells of the osteoblastic lineage. Bone 2011, 49, 830–838. [Google Scholar] [CrossRef]
- Knoll, B.J.; Rothblum, K.N.; Longley, M. Nucleotide sequence of the human placental alkaline phosphatase gene. Evolution of the 5′ flanking region by deletion/substitution. J. Biol. Chem. 1988, 263, 12020–12027. [Google Scholar] [CrossRef]
- Griffin, C.A.; Smith, M.; Henthorn, P.S.; Harris, H.; Weiss, M.; Raducha, M.; Emanuel, B.S. Human placental and intestinal alkaline phosphatase genes map to 2q34-q37. Am. J. Hum. Genet. 1987, 41, 1025–1034. [Google Scholar]
- Millán, J.L. Mammalian Alkaline Phosphatases: From Biology to Applications in Medicine and Biotechnology; Wiley-VCH GmbH & Co.: Weinheim, Germany, 2006. [Google Scholar]
- Pike, A.F.; Kramer, N.I.; Blaauboer, B.J.; Seinen, W.; Brands, R. A novel hypothesis for an alkaline phosphatase ‘rescue’ mechanism in the hepatic acute phase immune response. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 2044–2056. [Google Scholar] [CrossRef] [Green Version]
- Silvent, J.; Gasse, B.; Mornet, E.; Sire, J.-Y. Molecular Evolution of the Tissue-nonspecific Alkaline Phosphatase Allows Prediction and Validation of Missense Mutations Responsible for Hypophosphatasia. J. Biol. Chem. 2014, 289, 24168–24179. [Google Scholar] [CrossRef] [Green Version]
- Mornet, E.; Stura, E.; Lia-Baldini, A.-S.; Stigbrand, T.; Ménez, A.; Le Du, M.-H. Structural Evidence for a Functional Role of Human Tissue Nonspecific Alkaline Phosphatase in Bone Mineralization. J. Biol. Chem. 2001, 276, 31171–31178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosjean, O.; Koyama, I.; Goseki, M.; Roux, B.; Komoda, T. Human tissue non-specific alkaline phosphatases: Sugar-moiety-induced enzymic and antigenic modulations and genetic aspects. Biochem. J. 1997, 321, 297–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
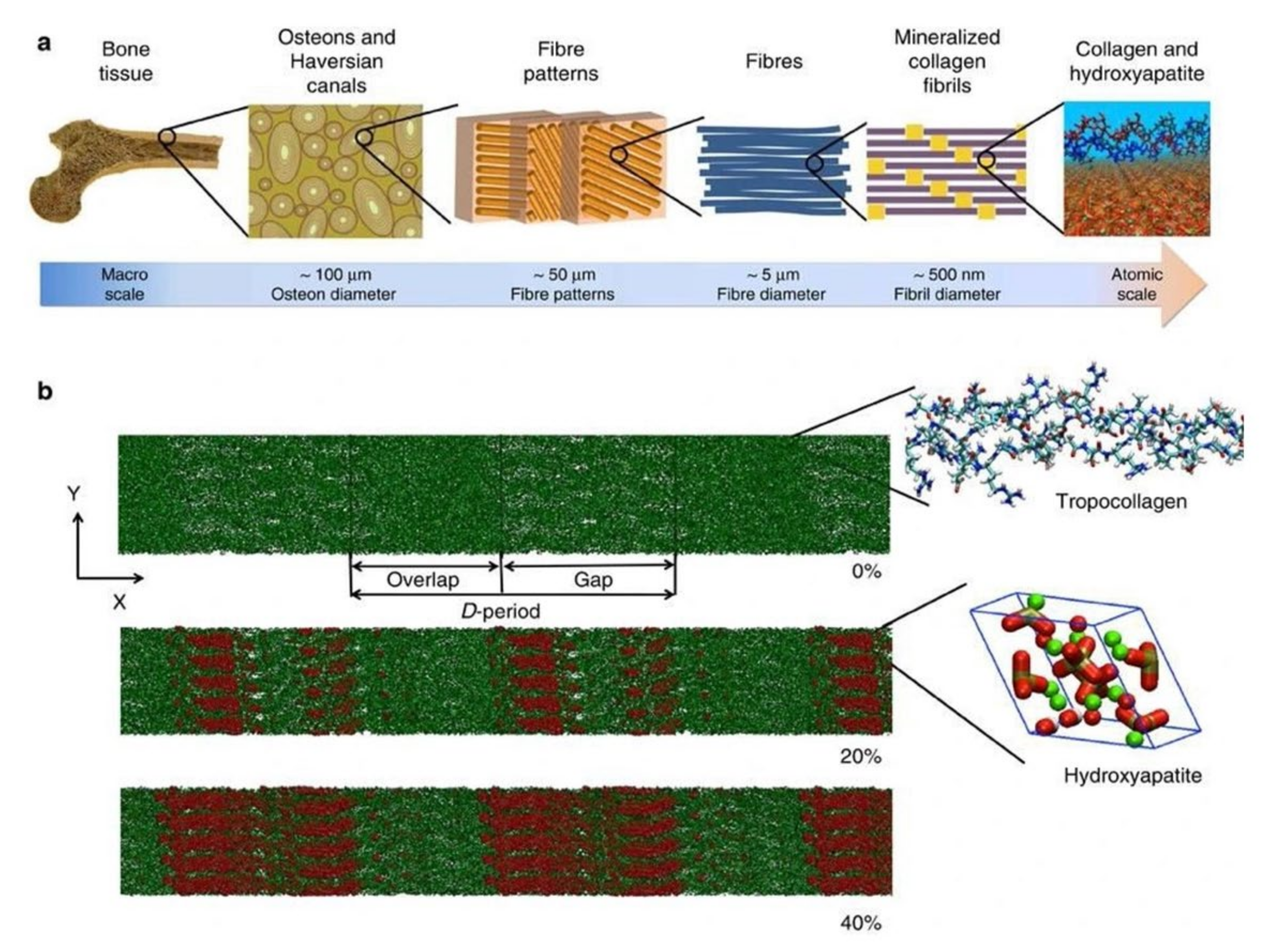
- Reznikov, N.; Steele, J.; Fratzl, P.; Stevens, M.M. A materials science vision of extracellular matrix mineralization. Nat. Rev. Mater. 2016, 1, 16041. [Google Scholar] [CrossRef] [Green Version]
- Davies, O.G.; Cox, S.C.; Azoidis, I.; McGuinness, A.; Cooke, M.; Heaney, L.; Davis, E.T.; Jones, S.W.; Grover, L.M. Osteoblast-Derived Vesicle Protein Content Is Temporally Regulated during Osteogenesis: Implications for Regenerative Therapies. Front. Bioeng. Biotechnol. 2019, 7, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaoutis, C.; Theocharis, S. The Role of Exosomes in Bone Remodeling: Implications for Bone Physiology and Disease. Dis. Markers 2019, 2019, 9417914. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Wuthier, R.E. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochim. Biophys. Acta Lipids Lipid Metab. 1975, 409, 128–143. [Google Scholar] [CrossRef]
- Bakhshian Nik, A.; Hutcheson, J.D.; Aikawa, E. Extracellular Vesicles as Mediators of Cardiovascular Calcification. Front. Cardiovasc. Med. 2017, 4, 78. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.; Narisawa, S.; Harmey, D.; Millán, J.L.; Farquharson, C. Functional Involvement of PHOSPHO1 in Matrix Vesicle-Mediated Skeletal Mineralization. J. Bone Miner. Res. 2007, 22, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Solomon, D.H.; Wilkins, R.J.; Meredith, D.; Browning, J.A. Characterisation of Inorganic Phosphate Transport in Bovine Articular Chondrocytes. Cell. Physiol. Biochem. 2007, 20, 099–108. [Google Scholar] [CrossRef]
- Golub, E.E. Role of matrix vesicles in biomineralization. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1592–1598. [Google Scholar] [CrossRef] [Green Version]
- Nahar, N.N.; Missana, L.; Garimella, R.; Tague, S.E.; Anderson, H.C. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), and noncollagenous matrix proteins. J. Bone Miner. Metab. 2008, 26, 514–519. [Google Scholar] [CrossRef]
- Ansari, S.; de Wildt, B.; Vis, M.; de Korte, C.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in Bone Mineralization and Potential Use as Therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, P.; Murer, H.; Biber, J. Type II Na+-Pi Cotransporters in Osteoblast Mineral Formation: Regulation by Inorganic Phosphate. Cell. Physiol. Biochem. 2007, 19, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, M.C.; Bottini, M.; Cory, E.; Bhattacharya, K.; Kuss, P.; Narisawa, S.; Sah, R.L.; Beck, L.; Fadeel, B.; Farquharson, C.; et al. Skeletal Mineralization Deficits and Impaired Biogenesis and Function of Chondrocyte-Derived Matrix Vesicles in Phospho1−/− and Phospho1/Pit1 Double-Knockout Mice. J. Bone Miner. Res. 2016, 31, 1275–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, S.J.; Stewart, A.J.; Sadler, P.J.; Farquharson, C. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem. J. 2004, 382, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.J.; Leong, D.T.K.; Farquharson, C. PLA 2 and ENPP6 may act in concert to generate phosphocholine from the matrix vesicle membrane during skeletal mineralization. FASEB J. 2018, 32, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Russell, R.G.G.; Straumann, F. Effect of Pyrophosphate on Hydroxyapatite and Its Implications in Calcium Homeostasis. Nat. Cell Biol. 1966, 212, 901–903. [Google Scholar] [CrossRef]
- Ho, A.M.; Johnson, M.D.; Kingsley, D.M. Role of the Mouse ank Gene in Control of Tissue Calcification and Arthritis. Science 2000, 289, 265–270. [Google Scholar] [CrossRef]
- Morava, E.; Kühnisch, J.; Drijvers, J.M.; Robben, J.H.; Cremers, C.; Van Setten, P.; Branten, A.; Stumpp, S.; De Jong, A.; Voesenek, K.; et al. Autosomal Recessive Mental Retardation, Deafness, Ankylosis, and Mild Hypophosphatemia Associated with a Novel ANKH Mutation in a Consanguineous Family. J. Clin. Endocrinol. Metab. 2011, 96, E189–E198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omelon, S.; Georgiou, J.; Henneman, Z.J.; Wise, L.M.; Sukhu, B.; Hunt, T.; Wynnyckyj, C.; Holmyard, D.; Bielecki, R.; Grynpas, M.D. Control of Vertebrate Skeletal Mineralization by Polyphosphates. PLoS ONE 2009, 4, e5634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omelon, S.; Ariganello, M.; Bonucci, E.; Grynpas, M.; Nanci, A. A Review of Phosphate Mineral Nucleation in Biology and Geobiology. Calcif. Tissue Int. 2013, 93, 382–396. [Google Scholar] [CrossRef] [Green Version]
- Jahnen-Dechent, W.; Schäfer, C.; Ketteler, M.; McKee, M.D. Mineral chaperones: A role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J. Mol. Med. 2007, 86, 379–389. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Heiss, A.; Schäfer, C.; Ketteler, M. Fetuin-A Regulation of Calcified Matrix Metabolism. Circ. Res. 2011, 108, 1494–1509. [Google Scholar] [CrossRef]
- Bozycki, L.; Mroczek, J.; Bessueille, L.; Mebarek, S.; Buchet, R.; Pikula, S.; Strzelecka-Kiliszek, A. Annexins A2, A6 and Fetuin-A Affect the Process of Mineralization in Vesicles Derived from Human Osteoblastic hFOB 1.19 and Osteosarcoma Saos-2 Cells. Int. J. Mol. Sci. 2021, 22, 3993. [Google Scholar] [CrossRef]
- Seto, J.; Busse, B.; Gupta, H.S.; Schäfer, C.; Krauss, S.; Dunlop, J.; Masic, A.; Kerschnitzki, M.; Zaslansky, P.; Boesecke, P.; et al. Accelerated Growth Plate Mineralization and Foreshortened Proximal Limb Bones in Fetuin-A Knockout Mice. PLoS ONE 2012, 7, e47338. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.M.X.; Smith, E.R.; Holt, S.G. The role of fetuin-A in mineral trafficking and deposition. BoneKEy Rep. 2015, 4, 672. [Google Scholar] [CrossRef] [Green Version]
- Heiss, A.; DuChesne, A.; Denecke, B.; Grötzinger, J.; Yamamoto, K.; Renné, T.; Jahnen-Dechent, W. Structural Basis of Calcification Inhibition by α2-HS Glycoprotein/Fetuin-A. J. Biol. Chem. 2003, 278, 13333–13341. [Google Scholar] [CrossRef] [Green Version]
- George, A.; Veis, A. Phosphorylated Proteins and Control over Apatite Nucleation, Crystal Growth, and Inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [Green Version]
- Beniash, E.; Deshpande, A.S.; Fang, P.A.; Lieb, N.S.; Zhang, X.; Sfeir, C.S. Possible role of DMP1 in dentin mineralization. J. Struct. Biol. 2011, 174, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Rios, H.F.; Myers, E.R.; Lu, Y.; Feng, J.Q.; Boskey, A.L. DMP1 depletion decreases bone mineralization in vivo: An FTIR imaging analysis. J. Bone Miner. Res. 2005, 20, 2169–2177. [Google Scholar] [CrossRef]
- Feng, J.Q.; Ward, L.M.; Liu, S.; Lu, Y.; Xie, Y.; Yuan, B.; Yu, X.; Rauch, F.; Davis, S.I.; Zhang, S.; et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006, 38, 1310–1315. [Google Scholar] [CrossRef]
- Gericke, A.; Qin, C.; Spevak, L.; Fujimoto, Y.; Butler, W.T.; Sørensen, E.S.; Boskey, A.L. Importance of Phosphorylation for Osteopontin Regulation of Biomineralization. Calcif. Tissue Int. 2005, 77, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Kaipatur, N.; Murshed, M.; McKee, M. Matrix Gla Protein Inhibition of Tooth Mineralization. J. Dent. Res. 2008, 87, 839–844. [Google Scholar] [CrossRef]
- Holm, E.; Gleberzon, J.S.; Liao, Y.; Sørensen, E.S.; Beier, F.; Hunter, G.K.; Goldberg, H.A. Osteopontin mediates mineralization and not osteogenic cell development In Vitro. Biochem. J. 2014, 464, 355–364. [Google Scholar] [CrossRef]
- Marinovich, R.; Soenjaya, Y.; Wallace, G.Q.; Zuskov, A.; Dunkman, A.; Foster, B.L.; Ao, M.; Bartman, K.; Lam, V.; Rizkalla, A.; et al. The role of bone sialoprotein in the tendon–bone insertion. Matrix Biol. 2016, 52–54, 325–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boskey, A.; Spevak, L.; Paschalis, E.; Doty, S.; McKee, M. Osteopontin Deficiency Increases Mineral Content and Mineral Crystallinity in Mouse Bone. Calcif. Tissue Int. 2002, 71, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A. Vascular Smooth Muscle Cells in the Pathogenesis of Vascular Calcification. Circ. Res. 2009, 104, 710–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nat. Cell Biol. 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; Nakano, Y.; Loisel, T.; Crine, P.; McKee, M.D. MEPE-ASARM Peptides Control Extracellular Matrix Mineralization by Binding to Hydroxyapatite: An Inhibition Regulated by PHEX Cleavage of ASARM. J. Bone Miner. Res. 2008, 23, 1638–1649. [Google Scholar] [CrossRef]
- Salmon, B.; Bardet, C.; Khaddam, M.; Naji, J.; Coyac, B.; Baroukh, B.; Letourneur, F.; Lesieur, J.; Decup, F.; Le Denmat, D.; et al. MEPE-Derived ASARM Peptide Inhibits Odontogenic Differentiation of Dental Pulp Stem Cells and Impairs Mineralization in Tooth Models of X-Linked Hypophosphatemia. PLoS ONE 2013, 8, e56749. [Google Scholar] [CrossRef]
- Gowen, L.C.; Petersen, D.N.; Mansolf, A.L.; Qi, H.; Stock, J.L.; Tkalcevic, G.T.; Simmons, H.A.; Crawford, D.T.; Chidsey-Frink, K.L.; Ke, H.Z.; et al. Targeted Disruption of the Osteoblast/Osteocyte Factor 45 Gene (OF45) Results in Increased Bone Formation and Bone Mass. J. Biol. Chem. 2003, 278, 1998–2007. [Google Scholar] [CrossRef] [Green Version]
- Quarles, L.D. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am. J. Physiol. Metab. 2003, 285, E1–E9. [Google Scholar] [CrossRef]
- Orimo, H. Hypophosphatasia: A systemic skeletal disorder caused by alkaline phosphatase deficiency. In Pathophysiology-Altered Physiological States; Gaze, D.C., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Whyte, M.P. Hypophosphatasia—Aetiology, nosology, pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2016, 12, 233–246. [Google Scholar] [CrossRef]
- Meah, F.; Basit, A.; Emanuele, N.; Emanuele, M.A. Hypophosphatasia: Review of Bone Mineral Metabolism, Pathophysiology, Clinical Presentation, Diagnosis, and Treatment. Clin. Rev. Bone Miner. Metab. 2017, 15, 24–36. [Google Scholar] [CrossRef]
- Di Mauro, S.; Manes, T.; Hessle, L.; Kozlenkov, A.; Pizauro, J.M.; Hoylaerts, M.F.; Millán, J.L. Kinetic Characterization of Hypophosphatasia Mutations with Physiological Substrates. J. Bone Miner. Res. 2002, 17, 1383–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whyte, M.P. Hypophosphatasia: Enzyme Replacement Therapy Brings New Opportunities and New Challenges. J. Bone Miner. Res. 2017, 32, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whyte, M.P.; Greenberg, C.R.; Salman, N.J.; Bober, M.; McAlister, W.H.; Wenkert, D.; Van Sickle, B.J.; Simmons, J.; Edgar, T.S.; Bauer, M.L.; et al. Enzyme-Replacement Therapy in Life-Threatening Hypophosphatasia. N. Engl. J. Med. 2012, 366, 904–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liedtke, D.; Hofmann, C.; Jakob, F.; Klopocki, E.; Graser, S. Tissue-Nonspecific Alkaline Phosphatase—A Gatekeeper of Physiological Conditions in Health and a Modulator of Biological Environments in Disease. Biomolecules 2020, 10, 1648. [Google Scholar] [CrossRef]
- Yadav, M.C.; Simão, A.M.S.; Narisawa, S.; Huesa, C.; McKee, M.D.; Farquharson, C.; Millán, J.L. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: A unified model of the mechanisms of initiation of skeletal calcification. J. Bone Miner. Res. 2011, 26, 286–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huesa, C.; Houston, D.; Kiffer-Moreira, T.; Yadav, M.C.; Millan, J.L.; Farquharson, C. The functional co-operativity of tissue-nonspecific alkaline phosphatase (TNAP) and PHOSPHO1 during initiation of skeletal mineralization. Biochem. Biophys. Rep. 2015, 4, 196–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terkeltaub, R. Physiologic and pathologic functions of the NPP nucleotide pyrophosphatase/phosphodiesterase family focusing on NPP1 in calcification. Purinergic Signal. 2006, 2, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stover, P.J.; Field, M. Vitamin B-6. Adv. Nutr. 2015, 6, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner-Sigl, S.; Haberlandt, E.; Mumm, S.; Scholl-Bürgi, S.; Sergi, C.; Ryan, L.; Ericson, K.L.; Whyte, M.P.; Högler, W. Pyridoxine-responsive seizures as the first symptom of infantile hypophosphatasia caused by two novel missense mutations (c.677T>C, p.M226T; c.1112C>T, p.T371I) of the tissue-nonspecific alkaline phosphatase gene. Bone 2007, 40, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Coburn, S.P.; Whyte, M.P. Role of phosphatases in the regulation of vitamin B-6 metabolism in hypophosphatasia and other disorders. In Current Topics in Nutrition and Disease Volume 19: Clinical and Physiological Application of Vitamin B-6; Leklem, J.E., Reynolds, R.D., Eds.; Alan R. Liss, Inc.: New York, NY, USA, 1988; pp. 65–93. [Google Scholar]
- Narisawa, S.; Hasegawa, H.; Watanabe, K.; Millán, J.L. Stage-specific expression of alkaline phosphatase during neural development in the mouse. Dev. Dyn. 1994, 201, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Fonta, C.; Negyessy, L. Subcellular biochemistry. In Neuronal Tissue-Nonspecific Alkaline Phosphatase (TNAP); Springer: Dordrecht, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Narisawa, S.; Fröhlander, N.; Millán, J.L. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev. Dyn. 1997, 208, 432–446. [Google Scholar] [CrossRef]
- Fedde, K.N.; Whyte, M.P. Alkaline phosphatase (tissue-nonspecific isoenzyme) is a phosphoethanolamine and pyridox-al-5′-phosphate ectophosphatase: Normal and hypophosphatasia fibroblast study. Am. J. Hum. Genet. 1990, 47, 767–775. [Google Scholar]
- Schiroli, D.; Ronda, L.; Peracchi, A. Kinetic characterization of the humanO-phosphoethanolamine phospho-lyase reveals unconventional features of this specialized pyridoxal phosphate-dependent lyase. FEBS J. 2014, 282, 183–199. [Google Scholar] [CrossRef] [Green Version]
- Hunter, G.K.; Kyle, C.L.; Goldberg, H.A. Modulation of crystal formation by bone phosphoproteins: Structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994, 300, 723–728. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, H.A.; Warner, K.J.; Li, M.C.; Hunter, G.K. Binding of Bone Sialoprotein, Osteopontin and Synthetic Polypeptides to Hydroxyapatite. Connect. Tissue Res. 2001, 42, 25–37. [Google Scholar] [CrossRef]
- Jono, S.; Peinado, C.; Giachelli, C.M. Phosphorylation of Osteopontin Is Required for Inhibition of Vascular Smooth Muscle Cell Calcification. J. Biol. Chem. 2000, 275, 20197–20203. [Google Scholar] [CrossRef] [Green Version]
- Harmey, D.; Johnson, K.A.; Zelken, J.; Camacho, N.P.; Hoylaerts, M.F.; Noda, M.; Terkeltaub, R.; Millán, J.L. Elevated Skeletal Osteopontin Levels Contribute to the Hypophosphatasia Phenotype in Akp2−/− Mice. J. Bone Miner. Res. 2006, 21, 1377–1386. [Google Scholar] [CrossRef]
- Garnero, P.; Delmas, P.D. Assessment of the serum levels of bone alkaline phosphatase with a new immunoradiometric assay in patients with metabolic bone disease. J. Clin. Endocrinol. Metab. 1993, 77, 1046–1053. [Google Scholar] [CrossRef]
- Nizet, A.; Cavalier, E.; Stenvinkel, P.; Haarhaus, M.; Magnusson, P. Bone alkaline phosphatase: An important biomarker in chronic kidney disease—Mineral and bone disorder. Clin. Chim. Acta 2020, 501, 198–206. [Google Scholar] [CrossRef]
- Pettengill, M.; Robson, S.; Tresenriter, M.; Millán, J.L.; Usheva, A.; Bingham, T.; Belderbos, M.; Bergelson, I.; Burl, S.; Kampmann, B.; et al. Soluble Ecto-5′-nucleotidase (5′-NT), Alkaline Phosphatase, and Adenosine Deaminase (ADA1) Activities in Neonatal Blood Favor Elevated Extracellular Adenosine. J. Biol. Chem. 2013, 288, 27315–27326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettengill, M.; Matute, J.D.; Tresenriter, M.; Hibbert, J.; Burgner, D.; Richmond, P.; Millán, J.L.; Ozonoff, A.; Strunk, T.; Currie, A.; et al. Human alkaline phosphatase dephosphorylates microbial products and is elevated in preterm neonates with a history of late-onset sepsis. PLoS ONE 2017, 12, e0175936. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Rolvien, T.; Linke, C.; Jandl, N.M.; Oheim, R.; Amling, M.; Barvencik, F. Outcome of Teriparatide Treatment on Fracture Healing Complications and Symptomatic Bone Marrow Edema in Four Adult Patients with Hypophosphatasia. JBMR Plus 2019, 3, e10215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whyte, M.P.; Wenkert, D.; McAlister, W.H.; Mughal, M.Z.; Freemont, A.J.; Whitehouse, R.; Baildam, E.M.; Coburn, S.P.; Ryan, L.M.; Mumm, S. Chronic Recurrent Multifocal Osteomyelitis Mimicked in Childhood Hypophosphatasia. J. Bone Miner. Res. 2009, 24, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Salini, V.; Andia, I. Tendons Involvement in Congenital Metabolic Disorders. Adv. Exp. Med. Biol. 2016, 920, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kapferer-Seebacher, I.; Foradori, L.; Zschocke, J.; Schilke, R. Rare Genetic Disorders Affecting the Periodontal Supporting Tissues in Adolescence. Front. Dent. Med. 2021, 2. [Google Scholar] [CrossRef]
- Bessueille, L.; Briolay, A.; Como, J.; Mebarek, S.; Mansouri, C.; Gleizes, M.; El Jamal, A.; Buchet, R.; Dumontet, C.; Matera, E.; et al. Tissue-nonspecific alkaline phosphatase is an anti-inflammatory nucleotidase. Bone 2020, 133, 115262. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Morbach, H.; Richl, P.; Stenzel, M.; Girschick, H.J. How can calcium pyrophosphate crystals induce inflammation in hypophosphatasia or chronic inflammatory joint diseases? Rheumatol. Int. 2008, 29, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Steiger, S.; Shi, C.; Anders, H. A guide to crystal-related and nano- or microparticle-related tissue responses. FEBS J. 2019, 287, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, Q.; Liu, Z.; Gao, C.; Wang, Z.; Xing, Z.; Song, J. Recombinant osteopontin provides protection for cerebral infarction by inhibiting the NLRP3 inflammasome in microglia. Brain Res. 2021, 1751, 147170. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Chirlaque, C.; Gámez-Belmonte, R.; Ocón, B.; Martínez-Moya, P.; Wirtz, S.; de Medina, F.S.; Martínez-Augustin, O. Tissue Non-specific Alkaline Phosphatase Expression is Needed for the Full Stimulation of T Cells and T Cell-Dependent Colitis. J. Crohns Coliti 2016, 11, 857–870. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, K.R.; Stokes, L.; Prince, L.; Marriott, H.; Meis, S.; Kassack, M.U.; Bingle, C.; Sabroe, I.; Surprenant, A.; Whyte, M.K.B. Inhibition of Neutrophil Apoptosis by ATP Is Mediated by the P2Y11Receptor. J. Immunol. 2007, 179, 8544–8553. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP Release Guides Neutrophil Chemotaxis via P2Y2 and A3 Receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef] [Green Version]
- Hümmeke-Oppers, F.; Hemelaar, P.; Pickkers, P. Innovative Drugs to Target Renal Inflammation in Sepsis: Alkaline Phosphatase. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Vorbrodt, A.W.; Lossinsky, A.S.; Wisniewski, H.M. Localization of Alkaline Phosphatase Activity in Endothelia of Developing and Mature Mouse Blood-Brain Barrier. Dev. Neurosci. 1986, 8, 1–13. [Google Scholar] [CrossRef]
- Bell, M.A.; Scarrow, W.G. Staining for microvascular alkaline phosphatase in thick celloidin sections of nervous tissue: Morphometric and pathological applications. Microvasc. Res. 1984, 27, 189–203. [Google Scholar] [CrossRef]
- Langer, D.; Ikehara, Y.; Takebayashi, H.; Hawkes, R.; Zimmermann, H. The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience 2007, 150, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Deracinois, B.; Lenfant, A.-M.; Dehouck, M.-P.; Flahaut, C. Tissue Non-specific Alkaline Phosphatase (TNAP) in Vessels of the Brain. Subcell. Biochem. 2015, 76, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Fonta, C.; Barone, P.; Martinez, L.R.; Négyessy, L. Rediscovering TNAP in the Brain: A Major Role in Regulating the Function and Development of the Cerebral Cortex. Subcell. Biochem. 2015, 76, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Demirbilek, H.; Alanay, Y.; Alikaşifoğlu, A.; Topçu, M.; Mornet, E.; Özön, A.; Kandemir, N. Hypophosphatasia Presenting with Pyridoxine-Responsive Seizures, Hypercalcemia, and Pseudotumor Cerebri: Case Report. J. Clin. Res. Pediatric Endocrinol. 2012, 4, 34–38. [Google Scholar] [CrossRef]
- Fukazawa, M.; Tezuka, J.; Sasazuki, M.; Masumoto, N.; Baba, H.; Doi, T.; Tsutsumi, Y.; Mizuno, Y.; Mihara, F.; Nakayama, H. Infantile hypophosphatasia combined with vitamin B6-responsive seizures and reticular formation lesions on magnetic resonance imaging: A case report. Brain Dev. 2018, 40, 140–144. [Google Scholar] [CrossRef]
- Ermonval, M.; Baudry, A.; Baychelier, F.; Pradines, E.; Pietri, M.; Oda, K.; Schneider, B.; Mouillet-Richard, S.; Launay, J.-M.; Kellermann, O. The Cellular Prion Protein Interacts with the Tissue Non-Specific Alkaline Phosphatase in Membrane Microdomains of Bioaminergic Neuronal Cells. PLoS ONE 2009, 4, e6497. [Google Scholar] [CrossRef] [Green Version]
- Vardy, E.R.; Kellett, K.; Cocklin, S.L.; Hooper, N. Alkaline Phosphatase Is Increased in both Brain and Plasma in Alzheimer’s Disease. Neurodegener. Dis. 2012, 9, 31–37. [Google Scholar] [CrossRef]
- Diaz-Hernandez, M.; Gómez-Ramos, A.; Rubio, A.; Gomez-Villafuertes, R.; Naranjo, J.; Miras-Portugal, M.T.; Avila, J. Tissue-nonspecific Alkaline Phosphatase Promotes the Neurotoxicity Effect of Extracellular Tau. J. Biol. Chem. 2010, 285, 32539–32548. [Google Scholar] [CrossRef] [Green Version]
- Brichacek, A.L.; Benkovic, S.A.; Chakraborty, S.; Nwafor, D.C.; Wang, W.; Jun, S.; Dakhlallah, D.; Geldenhuys, W.J.; Pinkerton, A.B.; Millán, J.L.; et al. Systemic inhibition of tissue-nonspecific alkaline phosphatase alters the brain-immune axis in experimental sepsis. Sci. Rep. 2019, 9, 18788. [Google Scholar] [CrossRef] [Green Version]
- Petty, G.W.; Brown, R.D., Jr.; Whisnant, J.P.; Sicks, J.D.; O’Fallon, W.M.; Wiebers, D.O. Ischemic Stroke Subtypes. Stroke 1999, 30, 2513–2516. [Google Scholar] [CrossRef] [Green Version]
- Ryu, W.-S.; Lee, S.-H.; Kim, C.K.; Kim, B.J.; Yoon, B.-W. Increased serum alkaline phosphatase as a predictor of long-term mortality after stroke. Neurology 2010, 75, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Ohara, T.; Minematsu, K.; Nagatsuka, K.; Toyoda, K. Predictors of Stroke Events in Patients with Transient Ischemic Attack Attributable to Intracranial Stenotic Lesions. Intern. Med. 2018, 57, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; You, S.; Chen, J.; Zhai, G.; Du, H.; Luo, Y.; Dong, X.; Cao, Y.; Liu, C.-F.; Zhang, Y. Serum Alkaline Phosphatase, Phosphate, and In-Hospital Mortality in Acute Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2018, 27, 257–266. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Xu, X.; Dong, M.; Jia, S.; Lu, C.; Wei, Y. Increased Serum Alkaline Phosphatase in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2018, 28, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshi, K.; Amizuka, N.; Oda, K.; Ikehara, Y.; Ozawa, H. Immunolocalization of tissue non-specific alkaline phosphatase in mice. Histochem. Cell Biol. 1997, 107, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Irie, M.; Iwata, K.; Nakane, H.; Yoshikane, M.; Koyama, Y.; Uehara, Y.; Takeyama, Y.; Kitamura, Y.; Sohda, T. Altered expression of alkaline phosphatase (ALP) in the liver of primary biliary cirrhosis (PBC) patients. Hepatol. Res. 2006, 35, 37–44. [Google Scholar] [CrossRef]
- Alvaro, D.; Benedetti, A.; Marucci, L.; Monache, M.D.; Monterubbianesi, R.; Di Cosimo, E.; Perego, L.; Macarri, G.; Glaser, S.; Le Sage, G.; et al. The function of alkaline phosphatase in the liver: Regulation of intrahepatic biliary epithelium secretory activities in the rat. Hepatology 2000, 32, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Mimura, Y.; Sakisaka, S.; Harada, M.; Sata, M.; Tanikawa, K. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology 1995, 109, 1969–1976. [Google Scholar] [CrossRef]
- Nouwen, E.J.; De Broe, M.E. Human intestinal versus tissue-nonspecific alkaline phosphatase as complementary urinary markers for the proximal tubule. Kidney Int. Suppl. 1994, 47, S43–S51. [Google Scholar]
- Moochhala, S.H.; Sayer, J.A.; Carr, G.; Simmons, N.L. Renal calcium stones: Insights from the control of bone mineralization. Exp. Physiol. 2008, 93, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Rutsch, F. Inherited Arterial Calcification Syndromes: Etiologies and Treatment Concepts. Curr. Osteoporos. Rep. 2017, 15, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Neven, E.; Millán, J.; Pinkerton, A.; D’Haese, P.; Verhulst, A. Chronic Kidney Disease-Induced Arterial Media Calcification in Rats Prevented by Tissue Non-Specific Alkaline Phosphatase Substrate Supplementation Rather Than Inhibition of the Enzyme. Pharmaceutics 2021, 13, 1138. [Google Scholar] [CrossRef] [PubMed]
- Jaminon, A.M.G.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Sci. Rep. 2020, 10, 6586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, T.M.; Fitzpatrick, L.A.; Inoue, D.; Qiao, J.-H.; Fishbein, M.C.; Detrano, R.C.; Shah, P.K.; Rajavashisth, T.B. Molecular, Endocrine, and Genetic Mechanisms of Arterial Calcification. Endocr. Rev. 2004, 25, 629–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervloet, M.; Cozzolino, M. Vascular calcification in chronic kidney disease: Different bricks in the wall? Kidney Int. 2017, 91, 808–817. [Google Scholar] [CrossRef]
- Greenland, P. Coronary Artery Calcium Score Combined with Framingham Score for Risk Prediction in Asymptomatic Individuals. Jama 2004, 291, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Patel, J.J.; Bourne, L.E.; Davies, B.K.; Arnett, T.R.; MacRae, V.E.; Wheeler-Jones, C.P.; Orriss, I.R. Differing calcification processes in cultured vascular smooth muscle cells and osteoblasts. Exp. Cell Res. 2019, 380, 100–113. [Google Scholar] [CrossRef]
- Alves, R.D.; Eijken, M.; van de Peppel, J.; van Leeuwen, J.P. Calcifying vascular smooth muscle cells and osteoblasts: Independent cell types exhibiting extracellular matrix and biomineralization-related mimicries. BMC Genom. 2014, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.C.; Leopold, J.A.; Loscalzo, J. Vascular Calcification. Circ. Res. 2006, 99, 1044–1059. [Google Scholar] [CrossRef] [Green Version]
- Roszkowska, M. Molecular Mechanisms of Vascular Smooth Muscle Cell Trans-Differentiation and Calcification in Atherosclerosis. Ph.D. Thesis, Université de Lyon, Lyon, France, 2018. [Google Scholar]
- Savinov, A.Y.; Salehi, M.; Yadav, M.C.; Radichev, I.; Millán, J.L.; Savinova, O.V. Transgenic Overexpression of Tissue-Nonspecific Alkaline Phosphatase (TNAP) in Vascular Endothelium Results in Generalized Arterial Calcification. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Romanelli, F.; Corbo, A.; Salehi, M.; Yadav, M.C.; Salman, S.; Petrosian, D.; Rashidbaigi, O.J.; Chait, J.; Kuruvilla, J.; Plummer, M.; et al. Overexpression of tissue-nonspecific alkaline phosphatase (TNAP) in endothelial cells accelerates coronary artery disease in a mouse model of familial hypercholesterolemia. PLoS ONE 2017, 12, e0186426. [Google Scholar] [CrossRef] [Green Version]
- Lomashvili, K.A.; Khawandi, W.; O’Neill, W.C. Reduced Plasma Pyrophosphate Levels in Hemodialysis Patients. J. Am. Soc. Nephrol. 2005, 16, 2495–2500. [Google Scholar] [CrossRef] [PubMed]
- Laurain, A.; Rubera, I.; Duranton, C.; Rutsch, F.; Nitschke, Y.; Ray, E.; Vido, S.; Sicard, A.; Lefthériotis, G.; Favre, G. Alkaline Phosphatases Account for Low Plasma Levels of Inorganic Pyrophosphate in Chronic Kidney Disease. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. New insights into endogenous mechanisms of protection against arterial calcification. Atherosclerosis 2020, 306, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, W.C.; Lomashvili, K.A.; Malluche, H.H.; Faugere, M.-C.; Riser, B.L. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011, 79, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, S.G.; Gahl, W.A.; Ferreira, C.R. Generalized Arterial Calcification of Infancy; University of Washington: Seattle, WA, USA, 2020. [Google Scholar]
- Rutsch, F.; Ruf, N.; Vaingankar, S.; Toliat, M.R.; Suk, A.; Höhne, W.; Schauer, G.; Lehmann, M.; Roscioli, T.; Schnabel, D.; et al. Mutations in ENPP1 are associated with ’idiopathic’ infantile arterial calcification. Nat. Genet. 2003, 34, 379–381. [Google Scholar] [CrossRef]
- Neven, E.G.; De Broe, M.E.; D’Haese, P.C. Prevention of vascular calcification with bisphosphonates without affecting bone mineralization: A new challenge? Kidney Int. 2009, 75, 580–582. [Google Scholar] [CrossRef] [Green Version]
- Fakhry, M.; Roszkowska, M.; Briolay, A.; Bougault, C.; Guignandon, A.; Diaz-Hernandez, J.I.; Diaz-Hernandez, M.; Pikuła, S.; Buchet, R.; Hamade, E.; et al. TNAP stimulates vascular smooth muscle cell trans-differentiation into chondrocytes through calcium deposition and BMP-2 activation: Possible implication in atherosclerotic plaque stability. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 643–653. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Wang, X.; Millán, J.L.; Dubyak, G.R.; O’Neill, W.C. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am. J. Physiol. Circ. Physiol. 2011, 301, H61–H68. [Google Scholar] [CrossRef]
- Narisawa, S.; Harmey, D.; Yadav, M.C.; O’Neill, W.C.; Hoylaerts, M.F.; Millán, J.L. Novel Inhibitors of Alkaline Phosphatase Suppress Vascular Smooth Muscle Cell Calcification. J. Bone Miner. Res. 2007, 22, 1700–1710. [Google Scholar] [CrossRef]
- Kiffer-Moreira, T.; Yadav, M.C.; Zhu, N.; Narisawa, S.; Sheen, C.; Stec, B.; Cosford, N.D.; Dahl, R.; Farquharson, C.; Hoylaerts, M.F.; et al. Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification. J. Bone Miner. Res. 2012, 28, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, L.G.; Rosay, B.; Czégé, D.; Fonta, C. Tetramisole and Levamisole Suppress Neuronal Activity Independently from Their Inhibitory Action on Tissue Non-specific Alkaline Phosphatase in Mouse Cortex. Subcell. Biochem. 2015, 76, 239–281. [Google Scholar] [CrossRef]
- Tani, T.; Fujiwara, M.; Orimo, H.; Shimizu, A.; Narisawa, S.; Pinkerton, A.B.; Millán, J.L.; Tsuruoka, S. Inhibition of tissue-nonspecific alkaline phosphatase protects against medial arterial calcification and improves survival probability in the CKD-MBD mouse model. J. Pathol. 2020, 250, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilham, D.; Tsujikawa, L.M.; Sarsons, C.D.; Halliday, C.; Wasiak, S.; Stotz, S.C.; Jahagirdar, R.; Sweeney, M.; Johansson, J.O.; Wong, N.C.; et al. Apabetalone downregulates factors and pathways associated with vascular calcification. Atherosclerosis 2019, 280, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Tsujikawa, L.M.; Fu, L.; Das, S.; Halliday, C.; Rakai, B.D.; Stotz, S.C.; Sarsons, C.D.; Gilham, D.; Daze, E.; Wasiak, S.; et al. Apabetalone (RVX-208) reduces vascular inflammation In Vitro and in CVD patients by a BET-dependent epigenetic mechanism. Clin. Epigenetics 2019, 11, 102. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, L.; Wen, X.; Gao, L.; Li, G.; Chang, G.; Qin, S.; Zhang, D. TNAP is a novel regulator of cardiac fibrosis after myocardial infarction by mediating TGF-β/Smads and ERK1/2 signaling pathways. EBioMedicine 2021, 67, 103370. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.-Y.; Liu, Z.-Q.; Jiang, D.; Wu, S.-Y.; Guo, Y.-Q.; Tao, H.-M.; Sun, M.; You, L.-N.; Qin, S.; et al. TNAP inhibition attenuates cardiac fibrosis induced by myocardial infarction through deactivating TGF-β1/Smads and activating P53 signaling pathways. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Opdebeeck, B.; Neven, E.; Millán, J.L.; Pinkerton, A.B.; D’Haese, P.C.; Verhulst, A. Pharmacological TNAP inhibition efficiently inhibits arterial media calcification in a warfarin rat model but deserves careful consideration of potential physiological bone formation/mineralization impairment. Bone 2020, 137, 115392. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekaran, S.; Vimalraj, S.; Thangavelu, L. The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization. Biomolecules 2021, 11, 1564. https://doi.org/10.3390/biom11111564
Sekaran S, Vimalraj S, Thangavelu L. The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization. Biomolecules. 2021; 11(11):1564. https://doi.org/10.3390/biom11111564
Chicago/Turabian StyleSekaran, Saravanan, Selvaraj Vimalraj, and Lakshmi Thangavelu. 2021. "The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization" Biomolecules 11, no. 11: 1564. https://doi.org/10.3390/biom11111564
APA StyleSekaran, S., Vimalraj, S., & Thangavelu, L. (2021). The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization. Biomolecules, 11(11), 1564. https://doi.org/10.3390/biom11111564





