Role of Intestinal Alkaline Phosphatase in Innate Immunity
Abstract
:1. Introduction
2. IAP and Barrier Function
2.1. IAP Protects the Intestinal Barrier Integrity in Various Disease Models
2.2. IAP and Tight Junction Proteins (TJPs)
3. IAP, Inflammation and TLR4
4. Role of IAP in Maintaining Gut Microbial Homoeostasis
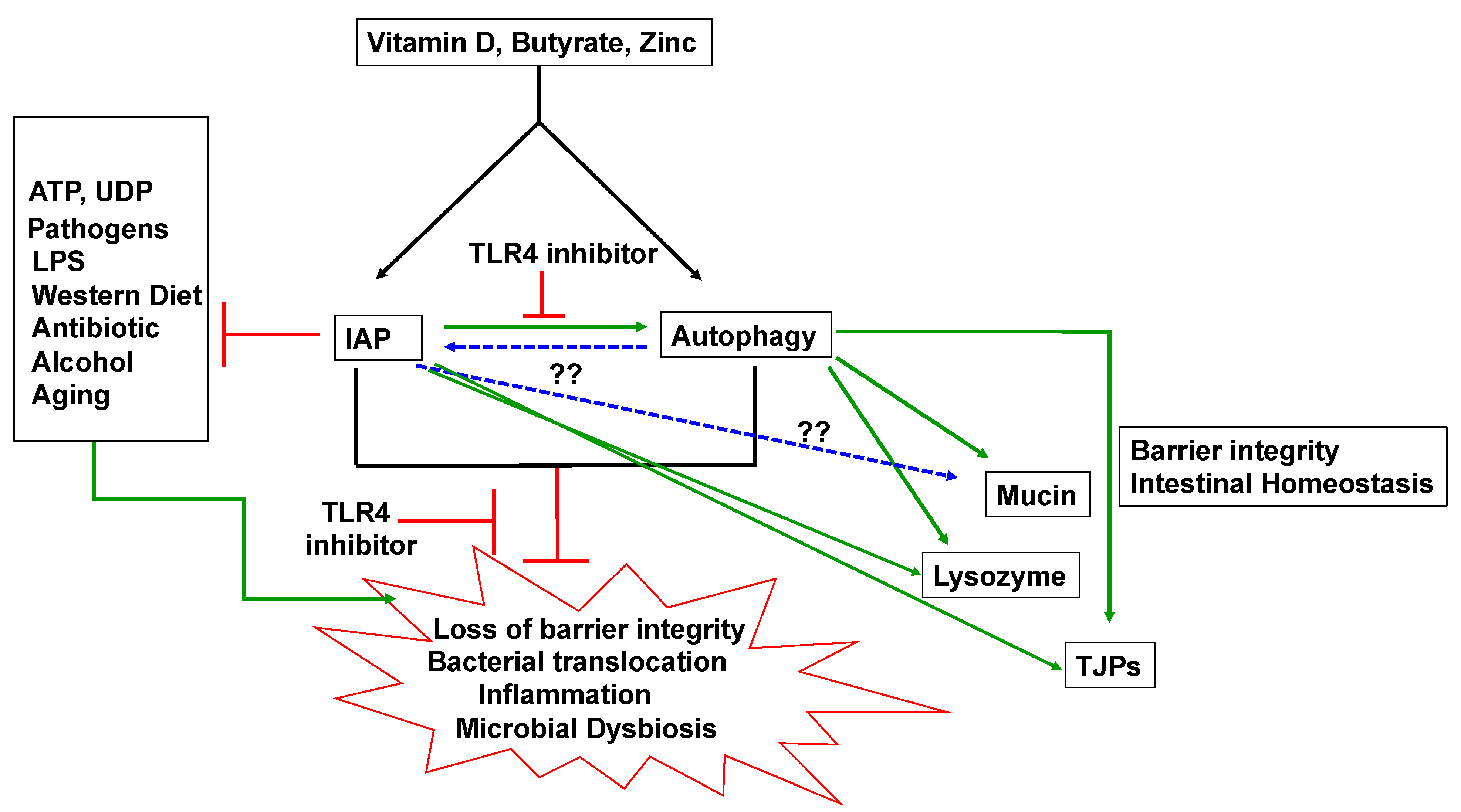
5. IAP and Autophagy
5.1. IAP and Autophagy and Barrier Function
5.2. IAP and Autophagy in Inflammation
5.3. IAP and Autophagy and Gut Microbes
5.4. IAP, Autophagy and Mucin
6. Inducers of IAP and Autophagy
6.1. Butyrate
6.2. Vitamin D
6.3. Zinc
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parlato, M.; Charbit-Henrion, F.; Pan, J.; Romano, C.; Duclaux-Loras, R.; Le Du, M.H.; Warner, N.; Francalanci, P.; Bruneau, J.; Bras, M.; et al. Human ALPI deficiency causes inflammatory bowel disease and highlights a key mechanism of gut homeostasis. EMBO Mol. Med. 2018, 10, e8483. [Google Scholar] [CrossRef]
- Kaliannan, K.; Hamarneh, S.R.; Economopoulos, K.P.; Nasrin Alam, S.; Moaven, O.; Patel, P.; Malo, N.S.; Ray, M.; Abtahi, S.M.; Muhammad, N.; et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 7003–7008. [Google Scholar] [CrossRef] [Green Version]
- Whitehouse, J.S.; Riggle, K.M.; Purpi, D.P.; Mayer, A.N.; Pritchard, K.A., Jr.; Oldham, K.T.; Gourlay, D.M. The protective role of intestinal alkaline phosphatase in necrotizing enterocolitis. J. Surg. Res. 2010, 163, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, F.; Adiliaghdam, F.; Cavallaro, P.M.; Hamarneh, S.R.; Tsurumi, A.; Hoda, R.S.; Munoz, A.R.; Dhole, Y.; Ramirez, J.M.; Liu, E.; et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 2020, 5, e134049. [Google Scholar] [CrossRef]
- Beumer, C.; Wulferink, M.; Raaben, W.; Fiechter, D.; Brands, R.; Seinen, W. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J. Pharmacol. Exp. Ther. 2003, 307, 737–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malo, M.S.; Moaven, O.; Muhammad, N.; Biswas, B.; Alam, S.N.; Economopoulos, K.P.; Gul, S.S.; Hamarneh, S.R.; Malo, N.S.; Teshager, A.; et al. Intestinal alkaline phosphatase promotes gut bacterial growth by reducing the concentration of luminal nucleotide triphosphates. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Mizumori, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 1223–1233. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Hu, D.; Huo, H.; Zhang, W.; Adiliaghdam, F.; Morrison, S.; Ramirez, J.M.; Gul, S.S.; Hamarneh, S.R.; Hodin, R.A. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. J. Am. Coll. Surg. 2016, 222, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Lalles, J.P. Intestinal alkaline phosphatase: Novel functions and protective effects. Nutr. Rev. 2014, 72, 82–94. [Google Scholar] [CrossRef]
- Singh, S.B.; Carroll-Portillo, A.; Coffman, C.; Ritz, N.L.; Lin, H.C. Intestinal Alkaline Phosphatase Exerts Anti-Inflammatory Effects Against Lipopolysaccharide by Inducing Autophagy. Sci. Rep. 2020, 10, 3107. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Cooper, R.C.; Sandhu, Y.K.; Kakiyama, G.; Korzun, W.J.; Ghosh, S. Over-Expression of Intestinal Alkaline Phosphatase Attenuates Atherosclerosis. Circ. Res. 2021, 128, 1646–1659. [Google Scholar] [CrossRef]
- Zarepour, M.; Bhullar, K.; Montero, M.; Ma, C.; Huang, T.; Velcich, A.; Xia, L.; Vallance, B.A. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect. Immun. 2013, 81, 3672–3683. [Google Scholar] [CrossRef] [Green Version]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Metta, V.; Leta, V.; Mrudula, K.R.; Prashanth, L.K.; Goyal, V.; Borgohain, R.; Chung-Faye, G.; Chaudhuri, K.R. Gastrointestinal dysfunction in Parkinson’s disease: Molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J. Neurol. 2021. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel. Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Cavallaro, P.M.; Kim, B.M.; Liu, T.; Wang, H.; Kuhn, F.; Adiliaghdam, F.; Liu, E.; Vasan, R.; Samarbafzadeh, E.; et al. A role for intestinal alkaline phosphatase in preventing liver fibrosis. Theranostics. 2021, 11, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Adiliaghdam, F.; Cavallaro, P.; Mohad, V.; Almpani, M.; Kuhn, F.; Gharedaghi, M.H.; Najibi, M.; Rahme, L.G.; Hodin, R.A. Targeting the gut to prevent sepsis from a cutaneous burn. JCI Insight. 2020, 5, e137128. [Google Scholar] [CrossRef]
- Hamarneh, S.R.; Kim, B.M.; Kaliannan, K.; Morrison, S.A.; Tantillo, T.J.; Tao, Q.; Mohamed, M.M.R.; Ramirez, J.M.; Karas, A.; Liu, W.; et al. Intestinal Alkaline Phosphatase Attenuates Alcohol-Induced Hepatosteatosis in Mice. Dig. Dis. Sci. 2017, 62, 2021–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Chen, S.W.; Zhu, J.; Zuo, S.; Ma, Y.Y.; Chen, Z.Y.; Zhang, J.L.; Chen, G.W.; Liu, Y.C.; Wang, P.Y. Intestinal alkaline phosphatase inhibits the translocation of bacteria of gut-origin in mice with peritonitis: Mechanism of action. PLoS ONE 2015, 10, e0124835. [Google Scholar] [CrossRef] [Green Version]
- Plaeke, P.; De Man, J.G.; Smet, A.; Malhotra-Kumar, S.; Pintelon, I.; Timmermans, J.P.; Nullens, S.; Jorens, P.G.; Hubens, G.; De Winter, B.Y. Effects of intestinal alkaline phosphatase on intestinal barrier function in a cecal ligation and puncture (CLP)-induced mouse model for sepsis. Neurogastroenterol Motil. 2020, 32, e13754. [Google Scholar] [CrossRef]
- Ghosh, S.S.; He, H.; Wang, J.; Korzun, W.; Yannie, P.J.; Ghosh, S. Intestine-specific expression of human chimeric intestinal alkaline phosphatase attenuates Western diet-induced barrier dysfunction and glucose intolerance. Physiol. Rep. 2018, 6, e13790. [Google Scholar] [CrossRef] [Green Version]
- Huo, J.Y.; Jiang, W.Y.; Yin, T.; Xu, H.; Lyu, Y.T.; Chen, Y.Y.; Chen, M.; Geng, J.; Jiang, Z.X.; Shan, Q.J. Intestinal Barrier Dysfunction Exacerbates Neuroinflammation via the TLR4 Pathway in Mice With Heart Failure. Front. Physiol. 2021, 12, 712338. [Google Scholar] [CrossRef]
- Furlan Freguia, C.; Marriott, A.; Gill, D.; Kaleko, M. Maternal treatment with oral intestinal alkaline phosphatase mitigates high fat diet-induced cognitive disorders in offspring mice. Behav. Brain. Res. 2020, 392, 112701. [Google Scholar] [CrossRef]
- Hamarneh, S.R.; Mohamed, M.M.; Economopoulos, K.P.; Morrison, S.A.; Phupitakphol, T.; Tantillo, T.J.; Gul, S.S.; Gharedaghi, M.H.; Tao, Q.; Kaliannan, K.; et al. A novel approach to maintain gut mucosal integrity using an oral enzyme supplement. Ann. Surg. 2014, 260, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Cohen, C.J.; Shieh, J.T.; Pickles, R.J.; Okegawa, T.; Hsieh, J.T.; Bergelson, J.M. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 2001, 98, 15191–15196. [Google Scholar] [CrossRef] [Green Version]
- Grbic, D.M.; Degagne, E.; Langlois, C.; Dupuis, A.A.; Gendron, F.P. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J. Immunol. 2008, 180, 2659–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielak, A.; Wojcik, D.; Mazur-Bialy, A.; Surmiak, M.; Bilski, J.; Targosz, A.; Magierowski, M.; Chmura, A.; Strzalka, M.; Krzysiek-Maczka, G.; et al. Intestinal Alkaline Phosphatase Combined with Voluntary Physical Activity Alleviates Experimental Colitis in Obese Mice. Involvement of Oxidative Stress, Myokines, Adipokines and Proinflammatory Biomarkers. Antioxidant 2021, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Kim, J.H.; Lee, C.; Im, J.P.; Kim, J.S. Intestinal alkaline phosphatase ameliorates experimental colitis via toll-like receptor 4-dependent pathway. Eur. J. Pharmacol. 2018, 820, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Gioannini, T.L.; Weiss, J.P. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol. Res. 2007, 39, 249–260. [Google Scholar] [CrossRef]
- Clarke, T.B.; Francella, N.; Huegel, A.; Weiser, J.N. Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium. Cell Host Microbe. 2011, 9, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Pawar, R.D.; Castrezana-Lopez, L.; Allam, R.; Kulkarni, O.P.; Segerer, S.; Radomska, E.; Meyer, T.N.; Schwesinger, C.M.; Akis, N.; Grone, H.J.; et al. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology 2009, 128, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Maynard, C.; Weinkove, D. The Gut Microbiota and Ageing. Subcell. Biochem. 2018, 90, 351–371. [Google Scholar] [PubMed]
- Malo, M.S.; Alam, S.N.; Mostafa, G.; Zeller, S.J.; Johnson, P.V.; Mohammad, N.; Chen, K.T.; Moss, A.K.; Ramasamy, S.; Faruqui, A.; et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 2010, 59, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, A.F.; Amoroso, T.L.; Knabel, S.J. Destruction of gram-negative food-borne pathogens by high pH involves disruption of the cytoplasmic membrane. Appl. Environ. Microbiol. 1994, 60, 4009–4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumori, M.; Ham, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D.; Akiba, Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J. Physiol. 2009, 587, 3651–3663. [Google Scholar] [CrossRef]
- De Lisle, R.C.; Mueller, R.; Boyd, M. Impaired mucosal barrier function in the small intestine of the cystic fibrosis mouse. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.N.; Yammine, H.; Moaven, O.; Ahmed, R.; Moss, A.K.; Biswas, B.; Muhammad, N.; Biswas, R.; Raychowdhury, A.; Kaliannan, K.; et al. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann. Surg. 2014, 259, 715–722. [Google Scholar] [CrossRef]
- Yang, W.H.; Heithoff, D.M.; Aziz, P.V.; Sperandio, M.; Nizet, V.; Mahan, M.J.; Marth, J.D. Recurrent infection progressively disables host protection against intestinal inflammation. Science 2017, 358. [Google Scholar] [CrossRef] [Green Version]
- Ostaff, M.J.; Stange, E.F.; Wehkamp, J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 2013, 5, 1465–1483. [Google Scholar] [CrossRef]
- Lapaquette, P.; Glasser, A.L.; Huett, A.; Xavier, R.J.; Darfeuille-Michaud, A. Crohn’s disease-associated adherent-invasive E. coli are selectively favoured by impaired autophagy to replicate intracellularly. Cell Microbiol. 2010, 12, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.B.; Lin, H.C. Autophagy counters LPS-mediated suppression of lysozyme. Innate. Immun. 2017, 23, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Grondin, J.; Banskota, S.; Khan, W.I. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 2019, 26, 19. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, C.; Zhao, W.; He, C.; Ding, J.; Dai, R.; Xu, K.; Xiao, L.; Luo, L.; Liu, S.; et al. Impaired Autophagy in Intestinal Epithelial Cells Alters Gut Microbiota and Host Immune Responses. Appl. Environ. Microbiol. 2018, 84, e00880-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 2016, 202, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Zahradnik-Bilska, J.; Brzozowski, B.; Magierowski, M.; Mach, T.; Magierowska, K.; Brzozowski, T. The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract. Mediat. Inflamm. 2017, 2017, 9074601. [Google Scholar] [CrossRef]
- Pan, H.H.; Zhou, X.X.; Ma, Y.Y.; Pan, W.S.; Zhao, F.; Yu, M.S.; Liu, J.Q. Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy. World J. Gastroenterol. 2020, 26, 4945–4959. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Sun, Y.; Han, F.; Hu, C.A.; Wu, Z. Amino acid deprivation disrupts barrier function and induces protective autophagy in intestinal porcine epithelial cells. Amino. Acids 2015, 47, 2177–2184. [Google Scholar] [CrossRef]
- Cheng, S.; Ma, X.; Geng, S.; Jiang, X.; Li, Y.; Hu, L.; Li, J.; Wang, Y.; Han, X. Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury. mSystems 2018, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Nighot, P.K.; Hu, C.A.; Ma, T.Y. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J. Biol. Chem. 2015, 290, 7234–7246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, P.; Das, P.; Verma, A.K.; Prakash, S.; Das, T.K.; Nag, T.C.; Ahuja, V.; Gupta, S.D.; Makharia, G.K. Are alterations of tight junctions at molecular and ultrastructural level different in duodenal biopsies of patients with celiac disease and Crohn’s disease? Virchows Arch. 2014, 465, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, T.S.; Crisan, T.O.; Oosting, M.; van de Veerdonk, F.L.; de Jong, D.J.; Philpott, D.J.; van der Meer, J.W.; Girardin, S.E.; Joosten, L.A.; Netea, M.G. Crohn’s disease-associated ATG16L1 polymorphism modulates pro-inflammatory cytokine responses selectively upon activation of NOD2. Gut 2011, 60, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Crisan, T.O.; Plantinga, T.S.; van de Veerdonk, F.L.; Farcas, M.F.; Stoffels, M.; Kullberg, B.J.; van der Meer, J.W.; Joosten, L.A.; Netea, M.G. Inflammasome-independent modulation of cytokine response by autophagy in human cells. PLoS ONE 2011, 6, e18666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giegerich, A.K.; Kuchler, L.; Sha, L.K.; Knape, T.; Heide, H.; Wittig, I.; Behrends, C.; Brune, B.; von Knethen, A. Autophagy-dependent PELI3 degradation inhibits proinflammatory IL1B expression. Autophagy 2014, 10, 1937–1952. [Google Scholar] [CrossRef] [Green Version]
- Verweij, W.R.; Bentala, H.; Huizinga-van der Vlag, A.; Miek van Loenen-Weemaes, A.; Kooi, K.; Meijer, D.K.; Poelstra, K. Protection against an Escherichia coli-induced sepsis by alkaline phosphatase in mice. Shock 2004, 22, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.T.; Yang, H.R.; Lo, H.C.; Hsieh, Y.C.; Tsai, S.C.; Hong, C.W.; Hsieh, C.H. Enhancing autophagy with activated protein C and rapamycin protects against sepsis-induced acute lung injury. Surgery 2013, 153, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, W.; Watanabe, E.; Fujimura, L.; Watanabe-Takano, H.; Yoshidome, H.; Swanson, P.E.; Tokuhisa, T.; Oda, S.; Hatano, M. Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit. Care 2013, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Watanabe, E.; Sakamoto, T.; Takasu, O.; Ikeda, T.; Ikeda, K.; Kotani, J.; Kitamura, N.; Sadahiro, T.; Tateishi, Y.; et al. Autophagy-related IRGM polymorphism is associated with mortality of patients with severe sepsis. PLoS ONE 2014, 9, e91522. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, F.; Chen, Y.; Zhang, W.; Lin, Y.; Cai, Y.; Yin, Z.; Tao, S.; Liao, Q.; Zhao, J.; et al. Association between genetic polymorphisms in the autophagy-related 5 gene promoter and the risk of sepsis. Sci. Rep. 2017, 7, 9399. [Google Scholar] [CrossRef]
- Johansson, M.E.; Sjovall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Bergstrom, K.; Shan, X.; Casero, D.; Batushansky, A.; Lagishetty, V.; Jacobs, J.P.; Hoover, C.; Kondo, Y.; Shao, B.; Gao, L.; et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science 2020, 370, 467–472. [Google Scholar] [CrossRef]
- Einerhand, A.W.; Renes, I.B.; Makkink, M.K.; van der Sluis, M.; Buller, H.A.; Dekker, J. Role of mucins in inflammatory bowel disease: Important lessons from experimental models. Eur J. Gastroenterol. Hepatol. 2002, 14, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Miyoshi, H.; Beatty, W.L.; Head, R.D.; Malvin, N.P.; Cadwell, K.; Guan, J.L.; Saitoh, T.; Akira, S.; Seglen, P.O.; et al. proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 2013, 32, 3130–3144. [Google Scholar] [CrossRef] [Green Version]
- Ke, P.; Shao, B.Z.; Xu, Z.Q.; Chen, X.W.; Liu, C. Intestinal Autophagy and Its Pharmacological Control in Inflammatory Bowel Disease. Front. Immunol. 2016, 7, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90 (Suppl. S4), 266–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnie, I.A.; Dwarakanath, A.D.; Taylor, B.A.; Rhodes, J.M. Colonic mucin synthesis is increased by sodium butyrate. Gut 1995, 36, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raqib, R.; Sarker, P.; Bergman, P.; Ara, G.; Lindh, M.; Sack, D.A.; Nasirul Islam, K.M.; Gudmundsson, G.H.; Andersson, J.; Agerberth, B. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. USA 2006, 103, 9178–9183. [Google Scholar] [CrossRef] [Green Version]
- Melo, A.D.; Silveira, H.; Bortoluzzi, C.; Lara, L.J.; Garbossa, C.A.; Preis, G.; Costa, L.B.; Rostagno, M.H. Intestinal alkaline phosphatase and sodium butyrate may be beneficial in attenuating LPS-induced intestinal inflammation. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Basson, M.D.; Hong, F. Tyrosine kinase inhibitors reverse butyrate stimulation of human Caco-2 intestinal epithelial cell alkaline phosphatase but not butyrate promotion of dipeptidyl dipeptidase. Cell Biol. Int. 1998, 22, 339–344. [Google Scholar] [CrossRef]
- Fukushima, K.; Sasaki, I.; Hasegawa, H.; Takahashi, K.; Naito, H.; Funayama, Y.; Matsuno, S. Sodium butyrate-induced liver-type alkaline phosphatase activity in a small intestinal epithelial cell line, IEC6. Dig. Dis. Sci. 1998, 43, 1116–1123. [Google Scholar] [CrossRef]
- Goldberg, R.F.; Austen, W.G., Jr.; Zhang, X.; Munene, G.; Mostafa, G.; Biswas, S.; McCormack, M.; Eberlin, K.R.; Nguyen, J.T.; Tatlidede, H.S.; et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc. Natl Acad. Sci. USA 2008, 105, 3551–3556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Carr, A.; Corner, G.A.; Togel, L.; Davalos-Salas, M.; Tran, H.; Chueh, A.C.; Al-Obaidi, S.; Chionh, F.; Ahmed, N.; et al. The intestinal epithelial cell differentiation marker intestinal alkaline phosphatase (ALPi) is selectively induced by histone deacetylase inhibitors (HDACi) in colon cancer cells in a Kruppel-like factor 5 (KLF5)-dependent manner. J. Biol. Chem. 2014, 289, 25306–25316. [Google Scholar] [CrossRef] [Green Version]
- Qiao, C.M.; Sun, M.F.; Jia, X.B.; Shi, Y.; Zhang, B.P.; Zhou, Z.L.; Zhao, L.P.; Cui, C.; Shen, Y.Q. Sodium butyrate causes alpha-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp. Cell. Res. 2020, 387, 111772. [Google Scholar] [CrossRef]
- Luo, S.; Li, Z.; Mao, L.; Chen, S.; Sun, S. Sodium butyrate induces autophagy in colorectal cancer cells through LKB1/AMPK signaling. J. Physiol. Biochem. 2019, 75, 53–63. [Google Scholar] [CrossRef]
- Parizadeh, S.M.; Jafarzadeh-Esfehani, R.; Hassanian, S.M.; Mottaghi-Moghaddam, A.; Ghazaghi, A.; Ghandehari, M.; Alizade-Noghani, M.; Khazaei, M.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Vitamin D in inflammatory bowel disease: From biology to clinical implications. Complement. Med. 2019, 47, 102189. [Google Scholar] [CrossRef]
- Meeker, S.; Seamons, A.; Maggio-Price, L.; Paik, J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 2016, 22, 933–948. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Z.; You, B.; Zhang, Y.L.; Yang, Z.C.; Chen, P.; Shi, Y.L.; Chen, Y.; Chen, Y.J.; Chen, J.; Peng, Y.Z. Effects of vitamin D3 on intestinal mucosal barrier of mice with severe burns. Zhonghua Shao Shang Za Zhi 2019, 35, 284–291. [Google Scholar]
- Zhao, H.; Zhang, H.; Wu, H.; Li, H.; Liu, L.; Guo, J.; Li, C.; Shih, D.Q.; Zhang, X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012, 12, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Thingholm, L.B.; Skieceviciene, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.A.; Ruhlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Halline, A.G.; Davidson, N.O.; Skarosi, S.F.; Sitrin, M.D.; Tietze, C.; Alpers, D.H.; Brasitus, T.A. Effects of 1,25-dihydroxyvitamin D3 on proliferation and differentiation of Caco-2 cells. Endocrinology 1994, 134, 1710–1717. [Google Scholar] [CrossRef]
- Nakaoka, K.; Yamada, A.; Noda, S.; Goseki-Sone, M. Vitamin D-restricted high-fat diet down-regulates expression of intestinal alkaline phosphatase isozymes in ovariectomized rats. Nutr. Res. 2018, 53, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.C. Vitamin D differentially regulates Salmonella-induced intestine epithelial autophagy and interleukin-1beta expression. World J. Gastroenterol. 2016, 22, 10353–10363. [Google Scholar] [CrossRef]
- Cousins, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef] [PubMed]
- Sikora, S.K.; Spady, D.; Prosser, C.; El-Matary, W. Trace elements and vitamins at diagnosis in pediatric-onset inflammatory bowel disease. Clin. Pediatr. 2011, 50, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Skrovanek, S.; DiGuilio, K.; Bailey, R.; Huntington, W.; Urbas, R.; Mayilvaganan, B.; Mercogliano, G.; Mullin, J.M. Zinc and gastrointestinal disease. World J. Gastrointest. Pathophysiol. 2014, 5, 496–513. [Google Scholar] [CrossRef]
- Siva, S.; Rubin, D.T.; Gulotta, G.; Wroblewski, K.; Pekow, J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2017, 23, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hering, N.A.; Schulzke, J.D. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig. Dis. 2009, 27, 450–454. [Google Scholar] [CrossRef]
- Cui, L.; Takagi, Y.; Wasa, M.; Iiboshi, Y.; Inoue, M.; Khan, J.; Sando, K.; Nezu, R.; Okada, A. Zinc deficiency enhances interleukin-1alpha-induced metallothionein-1 expression in rats. J. Nutr. 1998, 128, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Takenaka, T.; Inoue, T.; Sato, M.; Miyajima, Y.; Nodera, M.; Hanyu, M.; Ohno, Y.; Shibazaki, S.; Suzuki, H. Lipopolysaccharide-induced overproduction of nitric oxide and overexpression of iNOS and interleukin-1beta proteins in zinc-deficient rats. Biol. Trac. E Elem. Res. 2012, 145, 375–381. [Google Scholar] [CrossRef]
- Cho, Y.E.; Lomeda, R.A.; Ryu, S.H.; Sohn, H.Y.; Shin, H.I.; Beattie, J.H.; Kwun, I.S. Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr. Res. Pract. 2007, 1, 113–119. [Google Scholar] [CrossRef]
- Wang, W.; Van Noten, N.; Degroote, J.; Romeo, A.; Vermeir, P.; Michiels, J. Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Pieper, R.; Schunter, N.; Vahjen, W.; Zentek, J. Performance, organ zinc concentration, jejunal brush border membrane enzyme activities and mRNA expression in piglets fed with different levels of dietary zinc. Arch. Anim. Nutr. 2013, 67, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.S.; Langkamp-Henken, B.; Chang, S.M.; Shankar, M.N.; Cousins, R.J. Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc. Natl. Acad. Sci. USA 2011, 108, 20970–20975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.J.; Kim, H.N.; Kim, J.; Cho, D.H.; Kim, M.J.; Kim, Y.S.; Kim, Y.; Park, S.J.; Koh, J.Y. Zinc(II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals 2010, 23, 997–1013. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.B.; Lin, H.C. Role of Intestinal Alkaline Phosphatase in Innate Immunity. Biomolecules 2021, 11, 1784. https://doi.org/10.3390/biom11121784
Singh SB, Lin HC. Role of Intestinal Alkaline Phosphatase in Innate Immunity. Biomolecules. 2021; 11(12):1784. https://doi.org/10.3390/biom11121784
Chicago/Turabian StyleSingh, Sudha B., and Henry C. Lin. 2021. "Role of Intestinal Alkaline Phosphatase in Innate Immunity" Biomolecules 11, no. 12: 1784. https://doi.org/10.3390/biom11121784
APA StyleSingh, S. B., & Lin, H. C. (2021). Role of Intestinal Alkaline Phosphatase in Innate Immunity. Biomolecules, 11(12), 1784. https://doi.org/10.3390/biom11121784





