Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors
Abstract
1. Introduction
2. Mesothelin as a Target
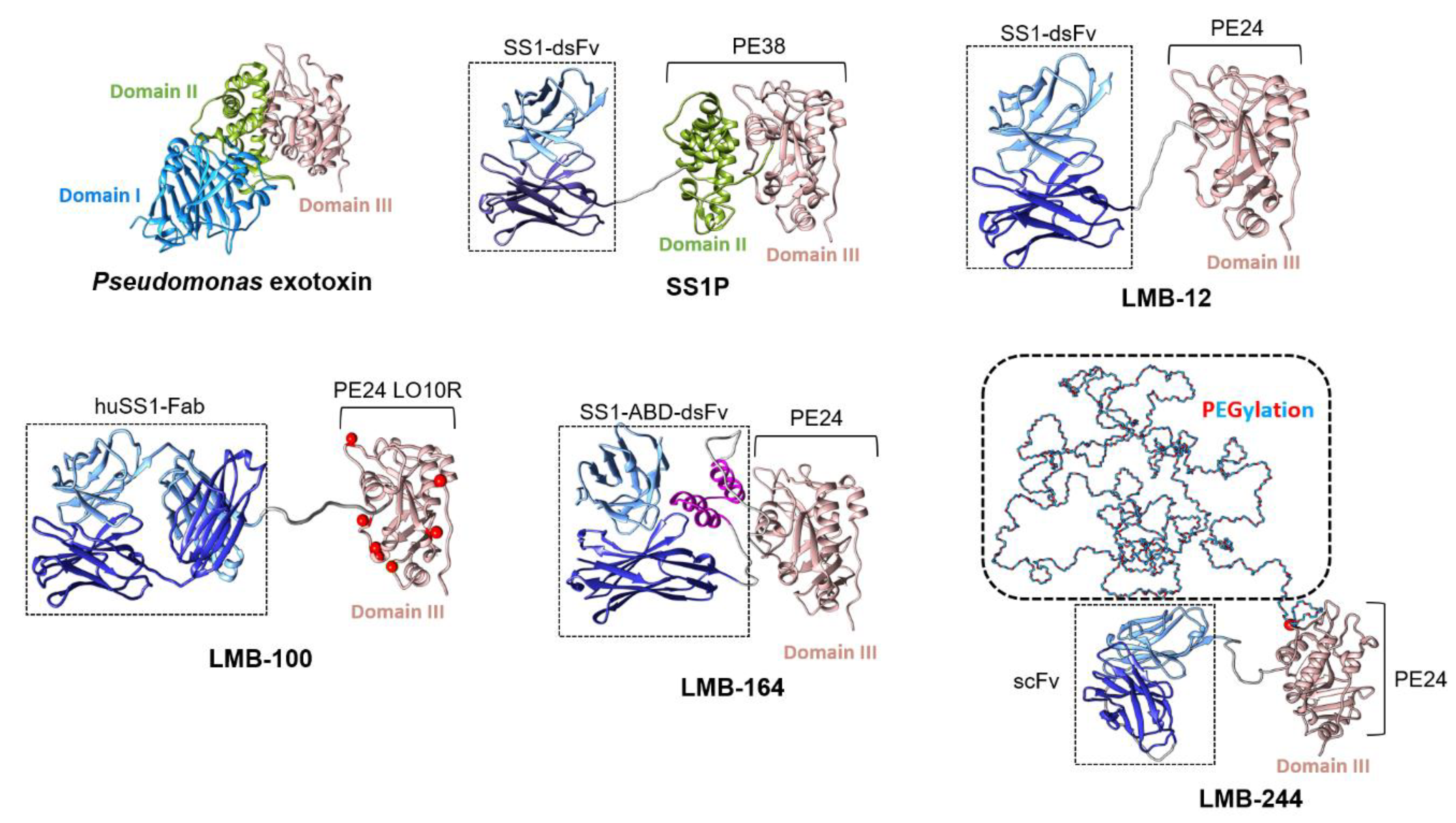
3. Pre-Clinical Development Pipeline
4. Clinical Experience
4.1. SS1P in the Clinic
4.2. LMB-100 for Mesothelioma Patients
4.3. LMB-100 for Pancreatic Cancer Patients
5. Overcoming Challenges
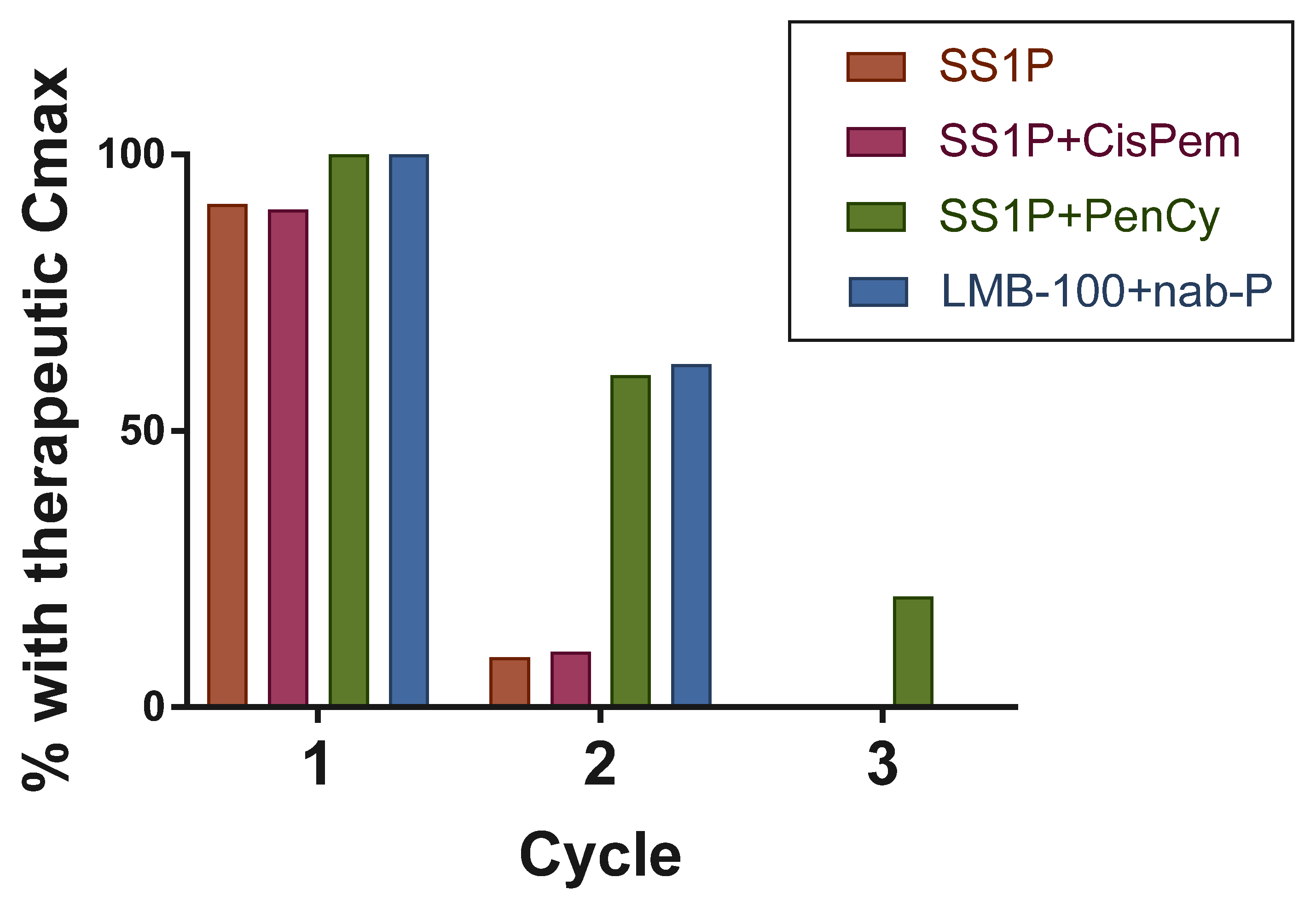
5.1. Anti-Drug Antibodies
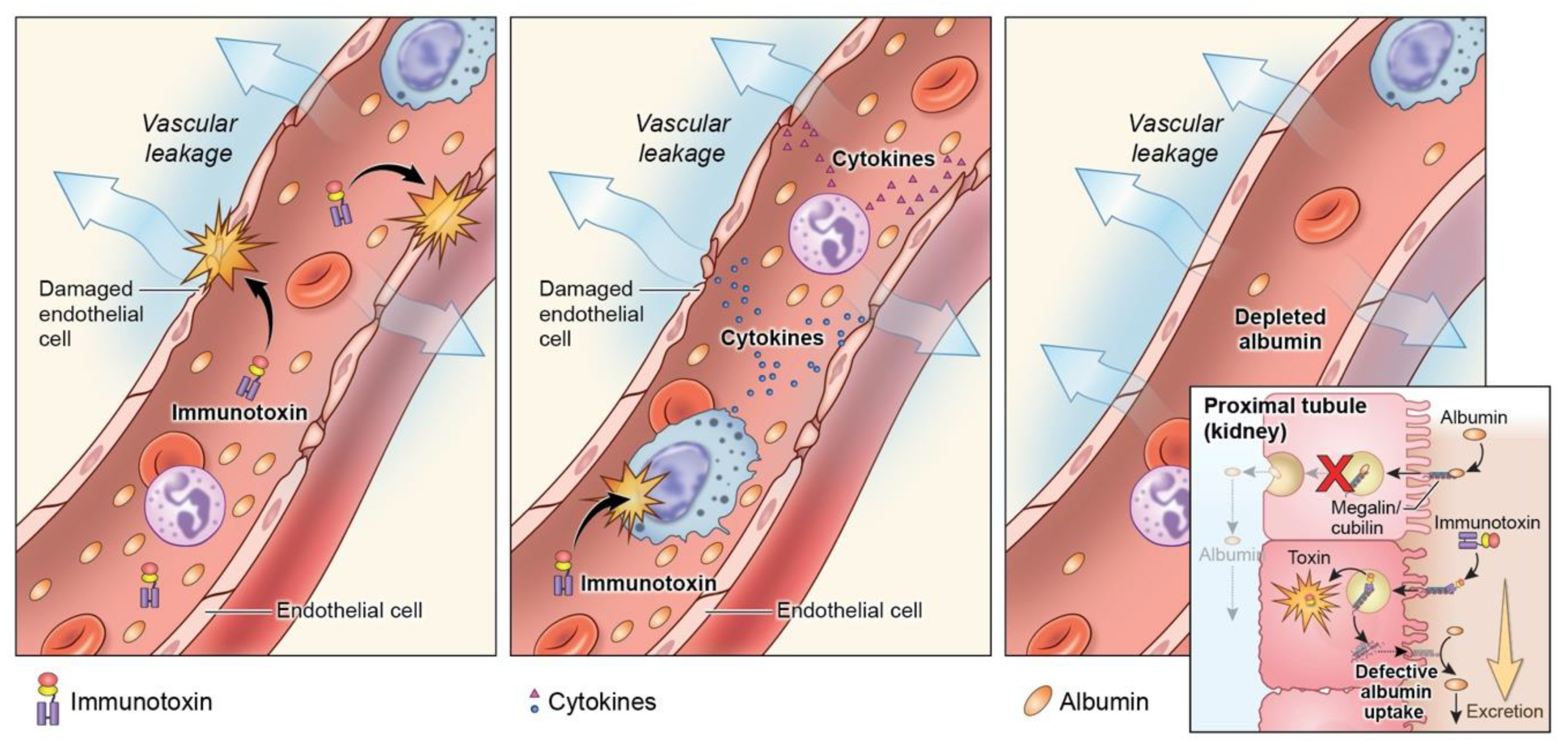
5.2. Toxicity
5.3. Delivery
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, K.; Pastan, I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesothiomas, and ovarian cancers. Proc. Natl. Acad. Sci. USA 1996, 93, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Bera, T.; Pastan, I. Mesothelin: A new target for immunotherapy. Clin. Cancer Res. 2004, 10, 3937–3942. [Google Scholar] [CrossRef]
- Argani, P.; Iacobuzio-Donahue, C.; Ryu, B.; Rosty, C.; Goggins, M.; Wilentz, R.E.; Murugesan, S.R.; Leach, S.D.; Jaffee, E.M.; Yeo, C.J.; et al. Mesothelin is overexpressed in the vast majority of ductal adenocarinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin. Cancer Res. 2001, 7, 3862–3868. [Google Scholar] [PubMed]
- Yu, L.; Feng, M.; Kim, H.; Phung, Y.; Kleiner, D.E.; Gores, G.J.; Qian, M.; Wang, X.W.; Ho, M. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J. Cancer 2010, 1, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.; Hsu, C.; Mao, T.; Wu, T.; Roden, R.; Wang, T.; Shih, I. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin. Cancer Res. 2006, 12, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Frierson, H.F.; Moskaluk, C.A.; Powell, S.M.; Zhang, H.; Cerilli, L.A.; Stoler, M.H.; Cathro, H.; Hampton, G.M. Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Hum. Pathol. 2003, 34, 605–609. [Google Scholar] [CrossRef]
- Han, S.H.; Joo, M.; Kim, H.; Chang, S. Mesothelin Expression in Gastric Adenocarcinoma and Its Relation to Clinical Outcomes. J. Pathol. Transl. Med. 2017, 51, 122–128. [Google Scholar] [CrossRef]
- Robinson, B.W.S.; Creaney, J.; Lake, R.A.; Nowak, A.; Musk, A.W.; de Klerk, N.; Winzell, P.; Hellström, K.E.; Hellström, I. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 2003, 362, 1612–1616. [Google Scholar] [CrossRef]
- Wang, L.; Niu, Z.; Zhang, L.; Liu, X.; Wang, X.; Li, F.; Wang, Y. Clinicopathological Significance of Mesothelin Expression in Invasive Breast Cancer. J. Int. Med. Res. 2012, 40, 909–916. [Google Scholar] [CrossRef]
- Bera, T.; Pastan, I. Mesothelin is not required for normal mouse development or reproduction. Mol. Cell. Biol. 2000, 20, 2902–2906. [Google Scholar] [CrossRef]
- Avula, L.R.; Rudloff, M.; El-Behaedi, S.; Arons, D.; Albalawy, R.; Chen, X.; Zhang, X.; Alewine, C. Mesothelin Enhances Tumor Vascularity in Newly Forming Pancreatic Peritoneal Metastases. Mol. Cancer Res. 2020, 18, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Jia, W.; Tang, Y.; Zhao, H.; Jiang, Y.; Sun, S. Mesothelin regulates growth and apoptosis in pancreatic cancer cells through p53-dependent and -independent signal pathway. J. Exp. Clin. Cancer Res. 2012, 31, 84. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Park, S.; Kim, M.H.; Nam, C.M.; Kim, H.; Choi, Y.Y.; Jung, M.K.; Choi, H.J.; Rha, S.Y.; Chung, H.C. Mesothelin expression is a predictive factor for peritoneal recurrence in curatively resected stage III gastric cancer. Oncologist 2019, 24, e1108–e1114. [Google Scholar] [CrossRef]
- Chen, S.H.; Hung, W.C.; Wang, P.; Paul, C.; Konstantopoulos, K. Mesothelin Binding to CA125/MUC16 Promotes Pancreatic Cancer Cell Motility and Invasion via MMP-7 Activation. Sci. Rep. 2013, 3, 1870. [Google Scholar] [CrossRef] [PubMed]
- Servais, E.L.; Colovos, C.; Rodriguez, L.; Bograd, A.J.; Nitadori, J.I.; Sima, C.; Rusch, V.W.; Sadelain, M.; Adusumilli, P.S. Mesothelin Overexpression Promotes Mesothelioma Cell Invasion and MMP-9 Secretion in an Orthotopic Mouse Model and in Epithelioid Pleural Mesothelioma Patients. Clin. Cancer Res. 2012, 18, 2478–2489. [Google Scholar] [CrossRef]
- Hassan, R.; Thomas, A.; Alewine, C.; Le, D.T.; Jaffee, E.M.; Pastan, I. Mesothelin immunotherapy for cancer: Ready for prime time? J. Clin. Oncol. 2016, 34, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Morello, A.; Sadelain, M.; Adusumilli, P. Mesothelin-targeted CARs: Driving T-cells to solid tumors. Cancer Discov. 2016, 6, 133–146. [Google Scholar] [CrossRef]
- Hassan, R.; Jennens, R.; Meerbeeck, J.P.V.; Nemunaitis, J.J.; Blumenschein, G.R.; Fennell, D.A.; Kindler, H.L.; Novello, S.; Elbi, C.; Walter, A.; et al. A pivotal randomized phase II study of anetumab ravtansine or vinorelbine in patients with advanced or metastatic pleural mesothelioma after progression on platinum/pemetrexed-based chemotherapy (NCT02610140). J. Clin. Oncol. 2016, 34, TPS8576. [Google Scholar] [CrossRef]
- Hassan, R.; Kindler, H.L.; Jahan, T.; Bazhenova, L.; Reck, M.; Thomas, A.; Pastan, I.; Parno, J.; O’Shannessy, D.J.; Fatato, P.; et al. Phase II Clinical Trial of Amatuximab, a Chimeric Antimesothelin Antibody with Pemetrexed and Cisplatin in Advanced Unresectable Pleural Mesothelioma. Clin. Cancer Res. 2014, 20, 5927–5936. [Google Scholar] [CrossRef]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V., Jr.; Greten, T.F.; Crocenzi, T.S.; Springett, G.M.; Morse, M.; Zeh, H.; Cohen, D.J.; Fine, R.L.; et al. A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. J. Clin. Oncol. 2014, 32, 177. [Google Scholar] [CrossRef]
- Li, M.; Bharadwaj, U.; Zhang, R.; Zhang, S.; Mu, H.; Fsiher, W.E.; Brunicardi, F.C.; Chen, C.; Yao, Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol. Cancer. Ther. 2008, 7, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Blythman, H.E.; Casellas, P.; Gros, O.; Gros, P.; Jansen, F.K.; Paolucci, F.; Pau, B.; Vidal, H. Immunotoxins: Hybrid molecules of monoclonal antibodies and a toxin subunit specifically kill tumour cells. Nature 1981, 290, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Spitler, L.E.; del Rio, M.; Khentigan, A.; Wedel, N.I.; Brophy, N.A.; Miller, L.L.; Harkonen, W.S.; Rosendorf, L.L.; Lee, H.M.; Mischak, R.P.; et al. Therapy of Patients with Malignant Melanoma Using a Monoclonal Antimelanoma Antibody-Ricin A Chain Immunotoxin. Cancer Res. 1987, 47, 1717–1723. [Google Scholar] [PubMed]
- Olsen, E.; Duvic, M.; Frankel, A.; Kim, Y.; Martin, A.; Vonderheid, E.; Jegasothy, B.; Wood, G.; Gordon, M.; Heald, P.; et al. Pivotal Phase III Trial of Two Dose Levels of Denileukin Diftitox for the Treatment of Cutaneous T-Cell Lymphoma. J. Clin. Oncol. 2001, 19, 376–388. [Google Scholar] [CrossRef]
- Zhou, H.; Marks, J.W.; Hittelman, W.N.; Yagita, H.; Cheung, L.H.; Rosenblum, M.G.; Winkles, J.A. Development and Characterization of a Potent Immunoconjugate Targeting the Fn14 Receptor on Solid Tumor Cells. Mol. Cancer Ther. 2011, 10, 1276–1288. [Google Scholar] [CrossRef]
- Tomé-Amat, J.; Olombrada, M.; Ruiz-de-la-Herrán, J.; Pérez-Gómez, E.; Andradas, C.; Sánchez, C.; Martínez, L.; Martínez-del-Pozo, Á.; Gavilanes, J.G.; Lacadena, J. Efficient in vivo antitumor effect of an immunotoxin based on ribotoxin α-sarcin in nude mice bearing human colorectal cancer xenografts. SpringerPlus 2015, 4, 168. [Google Scholar] [CrossRef]
- Pai, L.H.; Wittes, R.; Setser, A.; Willingham, M.C.; Pastan, I. Treatment of advanced solid tumors with immunotoxin LMB-1: An antibody linked to pseudomonas exotoxin. Nat. Med. 1996, 2, 350–353. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.; Gourgou-Bourgade, S.; Fouchardiere, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Robinson, B.W.S.; Musk, A.W.; Lake, R.A. Malignant mesothelioma. Lancet 2005, 366, 397–408. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): Preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef]
- Chowdhury, P.S.; Viner, J.L.; Beers, R.; Pastan, I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc. Natl. Acad. Sci. USA 1998, 95, 669–674. [Google Scholar] [CrossRef]
- Chang, K.; Pai, L.H.; Batra, J.K.; Pastan, I.; Willingham, M.C. Characterization of the Antigen (CAK1) Recognized by Monoclonal Antibody K1 Present on Ovarian Cancers and Normal Mesothelium. Cancer Res. 1992, 52, 181. [Google Scholar]
- Chang, K.; Pastan, I.; Willingham, M.C. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int. J. Cancer 1992, 50, 373–381. [Google Scholar] [CrossRef]
- Scholler, N.; Fu, N.; Yang, Y.; Ye, Z.; Goodman, G.E.; Hellström, K.E.; Hellström, I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc. Natl. Acad. Sci. USA 1999, 96, 11531–11536. [Google Scholar] [CrossRef]
- Zhang, Y.; Chertov, O.; Zhang, J.; Hassan, R.; Pastan, I. Cytotoxic Activity of Immunotoxin SS1P Is Modulated by TACE-Dependent Mesothelin Shedding. Cancer Res. 2011, 71, 5915–5922. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, J.A.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.F.; Huang, C.Y.; Chang, M.C.; Hu, Y.H.; Chiang, Y.C.; Chen, Y.L.; Hsieh, C.Y.; Chen, C.A. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian cancer. Br. J. Cancer 2009, 100, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Tang, L.H.; Klimstra, D.S.; Brennan, M.F.; Brody, J.R.; Rocha, F.G.; Jia, X.; Qin, L.; D’Angelica, M.I.; DeMatteo, R.P.; et al. A novel survival-based tissue microarray of pancreatic cancer validates MUC1 and mesothelin as biomarkers. PLoS ONE 2012, 7, e40157. [Google Scholar] [CrossRef]
- Nomura, R.; Fujii, H.; Abe, M.; Sugo, H.; Ishizaki, Y.; Kawasaki, S.; Hino, O. Mesothelin Expression Is a Prognostic Factor in Cholangiocellular Carcinoma. Int. Surg. 2013, 98, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Einama, T.; Kamachi, H.; Nishihara, H.; Shigenori, H.; Hiromi, K.; Kenta, K.; Ayami, S.; Munenori, T.; Kuniaki, O.; Shunji, M.; et al. Co-Expression of Mesothelin and CA125 Correlates With Unfavorable Patient Outcome in Pancreatic Ductal Adenocarcinoma. Pancreas 2011, 40, A1276–A1282. [Google Scholar] [CrossRef]
- Uehara, N.; Matsuoka, Y.; Tsubura, A. Mesothelin Promotes Anchorage-Independent Growth and Prevents Anoikis via Extracellular Signal-Regulated Kinase Signaling Pathway in Human Breast Cancer Cells. Mol. Cancer Res. 2008, 6, 186–193. [Google Scholar] [CrossRef]
- Bharadwaj, U.; Marin-Muller, C.; Li, M.; Chen, C.; Yao, Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis 2011, 32, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Scales, S.J.; Gupta, N.; Pacheco, G.; Firestein, R.; French, D.M.; Koeppen, H.; Rangell, L.; Barry-Hamilton, V.; Luis, E.; Chuh, J.; et al. An Antimesothelin-Monomethyl Auristatin E Conjugate with Potent Antitumor Activity in Ovarian, Pancreatic, and Mesothelioma Models. Mol. Cancer Ther. 2014, 13, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Wang, P.; Liang, S.; Iwaisako, K.; Liu, X.; Xu, J.; Zhang, M.; Sun, M.; Cong, M.; Karin, D.; et al. Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. J. Clin. Investig. 2017, 127, 1254–1270. [Google Scholar] [CrossRef]
- Hassan, R.; Viner, J.L.; Wang, Q.C.; Margulies, I.; Kreitman, R.J.; Pastan, I. Anti-Tumor Activity of K1-LysPE38QQR, an Immunotoxin Targeting Mesothelin, a Cell-Surface Antigen Overexpressed in Ovarian Cancer and Malignant Mesothelioma. J. Immunother. 2000, 23, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Lerner, M.R.; Benbrook, D.; Lightfoot, S.A.; Brackett, D.J.; Wang, Q.C.; Pastan, I. Antitumor Activity of SS(dsFv)PE38 and SS1(dsFv)PE38, Recombinant Antimesothelin Immunotoxins against Human Gynecologic Cancers Grown in Organotypic Culture. Clin. Cancer Res. 2002, 8, 3520–3526. [Google Scholar] [PubMed]
- Li, Q.; Verschraegen, C.F.; Mendoza, J.; Hassan, R. Cytotoxic activity of the recombinant anti-mesothelin immunotoxin, SS1 (dsFv)PE38, towards tumor cell lines established from ascites of patients with peritoneal mesothelioma. Anticancer Res. 2004, 24, 1327–1336. [Google Scholar] [PubMed]
- Zhang, Y.; Xiang, L.; Hassan, R.; Paik, C.H.; Carrasquillo, J.A.; Jang, B.; Le, N.; Ho, M.; Pastan, I. Synergistic Antitumor Activity of Taxol and Immunotoxin SS1P in Tumor-Bearing Mice. Clin. Cancer Res. 2006, 12, 4695–4701. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Broaddus, V.C.; Wilson, S.; Liewehr, D.J.; Zhang, J. Anti–Mesothelin Immunotoxin SS1P in Combination with Gemcitabine Results in Increased Activity against Mesothelin-Expressing Tumor Xenografts. Clin. Cancer Res. 2007, 13, 7166–7171. [Google Scholar] [CrossRef] [PubMed]
- Jawa, V.; Cousens, L.P.; Awwad, M.; Wakshull, E.; Kropshofer, H.; De Groot, A.S. T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clin. Immunol. 2013, 149, 534–555. [Google Scholar] [CrossRef]
- Brennan, F.R.; Morton, L.D.; Spindeldreher, S.; Kiessling, A.; Allenspach, R.; Hey, A.; Müller, P.; Frings, W.; Sims, J. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. mAbs 2010, 2, 233–255. [Google Scholar] [CrossRef]
- Cantor, J.R.; Yoo, T.H.; Dixit, A.; Iverson, B.L.; Forsthuber, T.G.; Georgiou, G. Therapeutic enzyme deimmunization by combinatorial T-cell epitope removal using neutral drift. Proc. Natl. Acad. Sci. USA 2011, 108, 1272–1277. [Google Scholar] [CrossRef]
- Cizeau, J.; Grenkow, D.M.; Brown, J.G.; Entwistle, J.; MacDonald, G.C. Engineering and Biological Characterization of VB6-845, an Anti-EpCAM Immunotoxin Containing a T-cell Epitope-depleted Variant of the Plant Toxin Bouganin. J. Immunother. 2009, 32, 574–584. [Google Scholar] [CrossRef]
- Mazor, R.; Eberle, J.A.; Hu, X.; Vassall, A.N.; Onda, M.; Beers, R.; Lee, E.C.; Kreitman, R.J.; Lee, B.; Baker, D.; et al. Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc. Natl. Acad. Sci. USA 2014, 111, 8571–8576. [Google Scholar] [CrossRef] [PubMed]
- Onda, M.; Beers, R.; Xiang, L.; Nagata, S.; Wang, Q.; Pastan, I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc. Natl. Acad. Sci. USA 2008, 105, 11311–11316. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Zhang, J.; Xiang, L.; Addissie, S.; Awuah, P.; Beers, R.; Hassan, R.; Pastan, I. Recombinant Immunotoxin with T-cell Epitope Mutations That Greatly Reduce Immunogenicity for Treatment of Mesothelin-Expressing Tumors. Mol. Cancer Ther. 2015, 14, 2789–2796. [Google Scholar] [CrossRef] [PubMed]
- Bauss, F.; Lechmann, M.; Krippendorff, B.; Staack, R.; Herting, F.; Festag, M.; Imhof-Jung, S.; Hesse, F.; Pompiati, M.; Kollmorgen, G.; et al. Characterization of a re-engineered, mesothelin-targeted Pseudomonas exotoxin fusion protein for lung cancer therapy. Mol. Oncol. 2016, 10, 1317–1329. [Google Scholar] [CrossRef]
- Liu, W.; Onda, M.; Lee, B.; Kreitman, R.J.; Hassan, R.; Xiang, L.; Pastan, I. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc. Natl. Acad. Sci. USA 2012, 109, 11782–11787. [Google Scholar] [CrossRef]
- Mazor, R.; Vassall, A.N.; Eberle, J.A.; Beers, R.; Weldon, J.E.; Venzon, D.J.; Tsang, K.Y.; Benhar, I.; Pastan, I. Identification and elimination of an immunodominant T-cell epitope in recombinant immunotoxins based on Pseudomonas exotoxin A. Proc. Natl. Acad. Sci. USA 2012, 109, E3597–E3603. [Google Scholar] [CrossRef]
- Oseroff, C.; Sidney, J.; Kotturi, M.F.; Kolla, R.; Alam, R.; Broide, D.H.; Wasserman, S.I.; Weiskopf, D.; McKinney, D.M.; Chung, J.L.; et al. Molecular Determinants of T Cell Epitope Recognition to the Common Timothy Grass Allergen. J. Immunol. 2010, 185, 943–955. [Google Scholar] [CrossRef]
- Mazor, R.; Onda, M.; Park, D.; Addissie, S.; Xiang, L.; Zhang, J.; Hassan, R.; Pastan, I. Dual B- and T-cell de-immunization of recombinant immunotoxin targeting mesothelin with high cytotoxic activity. Oncotarget 2016, 7, 29916–29926. [Google Scholar] [CrossRef]
- Weldon, J.E.; Xiang, L.; Zhang, J.; Beers, R.; Walker, D.A.; Onda, M.; Hassan, R.; Pastan, I. A Recombinant Immunotoxin against the Tumor-Associated Antigen Mesothelin Reengineered for High Activity, Low Off-Target Toxicity, and Reduced Antigenicity. Mol. Cancer Ther. 2013, 12, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Onda, M.; Pastan, I. Immunogenicity of therapeutic recombinant immunotoxins. Immnol. Rev. 2016, 270, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khanna, S.; Jiang, Q.; Alewine, C.; Miettinen, M.; Pastan, I.; Hassan, R. Efficacy of anti-mesothelin immunotoxin RG7787 plus Nab-Paclitaxel against mesothelioma patient-derived xenografts and mesothelin as a biomarker of tumor response. Clin. Cancer Res. 2017, 23, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Alewine, C.; Xiang, L.; Yamori, T.; Niederfellner, G.; Bosslet, K.; Pastan, I. Efficacy of RG7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancer. Mol. Cancer Ther. 2014, 13, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Kollmorgen, G.; Palme, K.; Seidl, A.; Scheiblich, S.; Birzele, F.; Wilson, S.; Clemens, C.; Voss, E.; Kaufmann, M.; Hirzel, K.; et al. A re-engineered immunotoxin shows promising preclinical activity in ovarian cancer. Sci. Rep. 2017, 7, 18086. [Google Scholar] [CrossRef] [PubMed]
- Kolyvas, E.; Rudloff, M.; Poruchynsky, M.; Landsman, R.; Hollevoet, K.; Venzon, D.; Alewine, C. Mesothelin-targeted immunotoxin RG7787 has synergistic anti-tumor activity when combined with taxanes. Oncotarget 2016, 8, 9189–9199. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.R.; Pastan, I.; FitzGerald, D.J. Combination treatments with the PKC inhibitor, enzastaurin, enhance the cytotoxicity of the anti-mesothelin immunotoxin, SS1P. PLoS ONE 2013, 8, e75576. [Google Scholar] [CrossRef]
- Leshem, Y.; O’Brien, J.; Liu, X.; Bera, T.; Terabe, M.; Berzofsky, J.A.; Bossenmaier, B.; Niederfellner, G.; Tai, C.; Reiter, Y.; et al. Combining local immunotoxins targeting mesothelin with CTLA-4 blockade synergistically eradicates murine cancer by promoting anti-cancer immunity. Cancer Immunol. Res. 2017, 5, 685–694. [Google Scholar] [CrossRef]
- Ali-Rahmani, F.; FitzGerald, D.J.; Martin, S.; Patel, P.; Prunotto, M.; Ormanoglu, P.; Thomas, C.; Pastan, I. Anticancer Effects of Mesothelin-Targeted Immunotoxin Therapy Are Regulated by Tyrosine Kinase DDR1. Cancer Res. 2016, 76, 1560–1568. [Google Scholar] [CrossRef]
- Liu, X.F.; Xiang, L.; Zhou, Q.; Carralot, J.; Prunotto, M.; Niederfellner, G.; Pastan, I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 10666–10671. [Google Scholar] [CrossRef]
- Liu, X.F.; Zhou, Q.; Hassan, R.; Pastan, I. Panbinostat decreases cFLIP and enhances killing of cancer cells by immunotoxin LMB-100 by stimulating the extrinsic apoptotic pathway. Oncotarget 2017, 8, 87307–87316. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.; Antignani, A.; Hewitt, S.M.; Gadina, M.; Alewine, C.; FitzGerald, D. Tofacitinib enhances delivery of antibody-based therapeutics to tumor cells through modulation of inflammatory cells. JCI Insight 2019, 4, e123281. [Google Scholar] [CrossRef]
- Onda, M.; Ghoreschi, K.; Steward-Tharp, S.; Thomas, C.; O’Shea, J.J.; Pastan, I.; FitzGerald, D. Tofacitinib Suppresses Antibody Responses to Protein Therapeutics in Murine Hosts. J. Immunol. 2014, 193, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Filpula, D.; Yang, K.; Basu, A.; Hassan, R.; Xiang, L.; Zhang, Z.; Wang, M.; Wang, Q.; Ho, M.; Beers, R.; et al. Releasable PEGylation of Mesothelin Targeted Immunotoxin SS1P Achieves Single Dosage Complete Regression of a Human Carcinoma in Mice. Bioconjugate Chem. 2007, 18, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Okada, R.; Kobayashi, H.; Nagaya, T.; Wei, J.; Zhou, Q.; Lee, F.; Bera, T.; Gao, Y.; Kuhlman, W.; et al. Site-Specific PEGylation of Anti-Mesothelin Recombinant Immunotoxins Increases Half-life and Antitumor Activity. Mol. Cancer Ther. 2020, 19, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Bera, T.K.; Liu, X.F.; Zhou, Q.; Onda, M.; Ho, M.; Tai, C.; Pastan, I. Recombinant immunotoxins with albumin-binding domains have long half-lives and high antitumor activity. Proc. Natl. Acad. Sci. USA 2018, 115, E3501–E3508. [Google Scholar] [CrossRef]
- Hassan, R.; Bullock, S.; Premkumar, A.; Kreitman, R.J.; Kindler, H.; Willingham, M.C.; Pastan, I. Phase 1 study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian and pancreatic cancers. Clin. Cancer Res. 2007, 13, 5144–5149. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Hassan, R.; FitzGerald, D.J.; Pastan, I. Phase I Trial of Continuous Infusion Anti-Mesothelin Recombinant Immunotoxin SS1P. Clin. Cancer Res. 2009, 15, 5274–5279. [Google Scholar] [CrossRef]
- Hassan, R.; Sharon, E.; Thomas, A.; Zhang, J.; Ling, A.; Miettinen, M.; Kreitman, R.J.; Steinberg, S.; Hollevoet, K.; Pastan, I. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with premetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with sereum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014, 120, 3311–3319. [Google Scholar]
- Hassan, R.; Miller, A.C.; Sharon, E.; Thomas, A.; Reynolds, J.C.; Ling, A.; Kreitman, R.J.; Miettinen, M.; Steinberg, S.; Fowler, D.H.; et al. Major Cancer Regressions in Mesothelioma After Treatment with an Anti-Mesothelin Immunotoxin and Immune Suppression. Sci. Transl. Med. 2013, 5, ra147–ra208. [Google Scholar] [CrossRef]
- Alewine, C.; Ahmad, M.; Peer, C.J.; Hu, Z.I.; Lee, M.; Yuno, A.; Kindrick, J.D.; Thomas, A.; Steinberg, S.; Trepel, J. Phase I/II study of the mesothelin-targeted immunotoxin LMB-100 with nab-paclitaxel for patients with advanced pancreatic adenocarcinoma. Clin. Cancer Res. 2020, 26, 828–836. [Google Scholar] [CrossRef]
- Mossoba, M.E.; Onda, M.; Taylor, J.; Massey, P.R.; Treadwell, S.; Sharon, E.; Hassan, R.; Pastan, I.; Fowler, D.H. Pentostatin Plus Cyclophosphamide Safely and Effectively Prevents Immunotoxin Immunogenicity in Murine Hosts. Clin. Cancer Res. 2011, 17, 3697–3705. [Google Scholar] [CrossRef]
- Sørensen, P.G.; Bach, F.; Bork, E.; Hansen, H.H. Randomized trial of doxorubicin versus cyclophosphamide in diffuse malignant pleural mesothelioma. Cancer Treat. Rep. 1985, 69, 1431–1432. [Google Scholar]
- Jiang, Q.; Ghafoor, A.; Mian, I.; Rathkey, D.; Thomas, A.; Alewine, C.; Sengupta, M.; Ahlman, M.A.; Zhang, J.; Morrow, B.; et al. Anti-Tumor Efficacy of Mesothelin Targeted Immunotoxin LMB-100 Plus Pembrolizumab in Mesothelioma Patients and Mouse Tumor Models. Sci. Transl. Med. 2020, in press. [Google Scholar] [CrossRef]
- Oratz, R.; Speyer, J.L.; Wernz, J.C.; Hochster, H.; Meyers, M.; Mischak, R.; Spitler, L.E. Antimelanoma monoclonal antibody-ricin A chain immunoconjugate (XMMME-001-RTA) plus cyclophosphamide in the treatment of metastatic malignant melanoma: Results of a phase II trial. J. Biol. Resp. Mod. 1990, 9, 345–354. [Google Scholar]
- Hassan, R.; Williams-Gould, J.; Watson, T.; Pai-Scherf, L.; Pastan, I. Pretreatment with Rituximab Does Not Inhibit the Human Immune Response against the Immunogenic Protein LMB-1. Clin. Cancer Res. 2004, 10, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, K.; Saria, E.A.; Schwartz, R.; Vlock, D.R.; Ackerman, S.; Wedel, N.; Kirkwood, J.M.; Jones, H.; Ernstoff, M.S. Phase I/II study of murine monoclonal antibody-ricin A chain (XOMAZYME-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J. Immunother. Emphasis Tumor Immunol. 1993, 13, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; King, E.M.; Onda, M.; Cuburu, N.; Addissie, S.; Crown, D.; Liu, X.; Kishimoto, T.K.; Pastan, I. Tolerogenic nanoparticles restore the antitumor activity of recombinant immunotoxins by mitigating immunogenicity. Proc. Natl. Acad. Sci. USA 2018, 115, E733–E742. [Google Scholar] [CrossRef]
- Manning, M.L.; Mason-Osann, E.; Onda, M.; Pastan, I. Bortezomib Reduces Pre-Existing Antibodies to Recombinant Immunotoxins in Mice. J. Immunol. 2015, 194, 1695–1701. [Google Scholar] [CrossRef]
- King, E.M.; Mazor, R.; Çuburu, N.; Pastan, I. Low-Dose Methotrexate Prevents Primary and Secondary Humoral Immune Responses and Induces Immune Tolerance to a Recombinant Immunotoxin. J. Immunol. 2018, 200, 2038–2045. [Google Scholar] [CrossRef]
- Kowalski, M.; Brazas, L.; Zaretsky, R.; Rasamoelisolo, M.; MacDonald, G.; Cuthbert, W.; Glover, N. A phase I study of VB6–845, an anti-EpCAM fusion protein targeting advanced solid tumours of epithelial origin: Preliminary results. J. Clin. Oncol. 2008, 26, 14663. [Google Scholar] [CrossRef]
- Jordaan, S.; Akinrinmade, O.A.; Nechreiner, T.; Cremer, C.; Naran, K.; Chetty, S.; Barth, S. Updates in the Development of ImmunoRNases for the Selective Killing of Tumor Cells. Biomedicines 2018, 6, 28. [Google Scholar] [CrossRef]
- Ruiz-de-la-Herrán, J.; Tomé-Amat, J.; Lazaro-Gorines, R.; Gavilanes, J.G. Inclusion of a Furin Cleavage Site Enhances Antitumor Efficacy against Colorectal Cancer Cells of Ribotoxin α-Sarcin or RNase T1-Based Immunotoxins. Toxins 2019, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Blumenschein, G.R., Jr.; Moore, K.N.; Santin, A.D.; Kindler, H.L.; Nemunaitis, J.J.; Seward, S.M.; Thomas, A.; Kim, S.K.; Rajagopalan, P.; et al. First-in-Human, Multicenter, Phase I Dose-Escalation and Expansion Study of Anti-Mesothelin Antibody–Drug Conjugate Anetumab Ravtansine in Advanced or Metastatic Solid Tumors. J. Clin. Oncol. 2020, 38, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Weekes, C.D.; Lamberts, L.E.; Borad, M.J.; Voortman, J.; McWilliams, R.R.; Diamond, J.R.; de Vries, E.G.E.; Verheul, H.M.; Lieu, C.H.; Kim, G.P.; et al. Phase I Study of DMOT4039A, an Antibody–Drug Conjugate Targeting Mesothelin, in Patients with Unresectable Pancreatic or Platinum-Resistant Ovarian Cancer. Mol. Cancer Ther. 2016, 15, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Brockstedt, D.G.; Nir-Paz, R.; Hampl, J.; Mathur, S.; Nemunaitis, J.; Sterman, D.H.; Hassan, R.; Lutz, E.; Moyer, B.; et al. A Live-Attenuated Listeria Vaccine (ANZ-100) and a Live-Attenuated Listeria Vaccine Expressing Mesothelin (CRS-207) for Advanced Cancers: Phase I Studies of Safety and Immune Induction. Clin. Cancer Res. 2012, 18, 858–868. [Google Scholar] [CrossRef]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef]
- Pai-Scherf, L.H.; Villa, J.; Pearson, D.; Watson, T.; Liu, E.; Willingham, M.C.; Pastan, I. Hepatotoxicity in Cancer Patients Receiving erb-38, a Recombinant Immunotoxin That Targets the erbB2 Receptor. Clin. Cancer Res. 1999, 5, 2311–2315. [Google Scholar]
- Vitetta, E. Immunotoxins and vascular leak syndrome. Cancer J. 2000, 6, S218. [Google Scholar]
- Siegall, C.B.; Liggitt, D.; Chace, D.; Mixan, B.; Sugai, J.; Davidson, T.; Steinitz, M. Characterization of vascular leak syndrome induced by the toxin component of Pseudomonas exotoxin-based immunotoxins and its potential inhibition with nonsteroidal anti-inflammatory drugs. Clin. Cancer Res. 1997, 3, 339–345. [Google Scholar]
- Liu, X.; Wei, J.; Zhou, Q.; Molitoris, B.A.; Sandoval, R.; Kobayashi, H.; Okada, K.; Nagaya, T.; Karim, B.; Butcher, D.; et al. Immunotoxin SS1P is rapidly removed by proximal tubule cells of kidney, whose damage contributes to albumin loss in urine. Proc. Natl. Acad. Sci. USA 2020, 117, 6086–6091. [Google Scholar] [CrossRef]
- Lindenberg, L.; Thomas, A.; Adler, S.; Mena, E.; Kurdziel, K.; Maltzman, J.; Wallin, B.; Hoffman, K.; Pastan, I.; Paik, C.H.; et al. Safety and biodistribution of 111In-amatuximab in patients with mesothelin expressing cancers using single photon emission computed tomography-computed tomography (SPECT-CT) imaging. Oncotarget 2015, 6, 4496–4504. [Google Scholar] [CrossRef]
- Lamberts, L.E.; Menke-van der Houven van Oordt, C.W.; ter Weele, E.J.; Bensch, F.; Smeenk, M.M.; Voortman, J.; Hoekstra, O.S.; Williams, S.P.; Fine, B.M.; Maslyar, D.; et al. ImmunoPET with Anti-Mesothelin Antibody in Patients with Pancreatic and Ovarian Cancer before Anti-Mesothelin Antibody–Drug Conjugate Treatment. Clin. Cancer Res. 2016, 22, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- El-Behaedi, S.; Landsman, R.; Rudloff, M.; Kolyvas, E.; Albalawy, R.; Zhang, X.; Bera, T.; Collins, K.; Kozlov, S.; Alewine, C. Protein Synthesis Inhibition Activity of Mesothelin Targeting Immunotoxin LMB-100 Decreases Concentrations of Oncogenic Signaling Molecules and Secreted Growth Factors. Toxins 2018, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Mason-Osann, E.; Hollevoet, K.; Niederfellner, G.; Pastan, I. Quantification of recombinant immunotoxin delivery to solid tumors allows for direct comparison of in vivo and in vitro results. Sci. Rep. 2015, 5, 10832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiang, L.; Hassan, R.; Pastan, I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 17099–17104. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, Q.; Tong, Z.; Xing, Y.; Xu, K.; Yijia Wang, J.; Li, W.; Zhao, J.; Zhao, L.; Hong, Z. HER2-specific immunotoxins constructed based on single-domain antibodies and the improved toxin PE24X7. Int. J. Pharm. 2020, 574, 118939. [Google Scholar] [CrossRef]
- Fleming, B.D.; Urban, D.J.; Hall, M.D.; Longerich, T.; Greten, T.F.; Pastan, I.; Ho, M. Engineered Anti-GPC3 Immunotoxin, HN3-ABD-T20, Produces Regression in Mouse Liver Cancer Xenografts Through Prolonged Serum Retention. Hepatology 2020, 71, 1696–1711. [Google Scholar] [CrossRef]
- Lázaro-Gorines, R.; Ruiz-de-la-Herrán, J.; Navarro, R.; Sanz, L.; Álvarez-Vallina, L.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. A novel Carcinoembryonic Antigen (CEA)-Targeted Trimeric Immunotoxin shows significantly enhanced Antitumor Activity in Human Colorectal Cancer Xenografts. Sci. Rep. 2019, 9, 11680. [Google Scholar] [CrossRef]
- Pak, Y.; Zhang, Y.; Pastan, I.; Lee, B. Antigen Shedding May Improve Efficiencies for Delivery of Antibody-Based Anticancer Agents in Solid Tumors. Cancer Res. 2012, 72, 3143–3152. [Google Scholar] [CrossRef]
- Zhang, Y.; Hansen, J.K.; Xiang, L.; Kawa, S.; Onda, M.; Ho, M.; Hassan, R.; Pastan, I. A Flow Cytometry Method to Quantitate Internalized Immunotoxins Shows that Taxol Synergistically Increases Cellular Immunotoxins Uptake. Cancer Res. 2010, 70, 1082–1089. [Google Scholar] [CrossRef]
- Awuah, P.; Bera, T.K.; Folivi, M.; Chertov, O.; Pastan, I. Reduced Shedding of Surface Mesothelin Improves Efficacy of Mesothelin-Targeting Recombinant Immunotoxins. Mol. Cancer Ther. 2016, 15, 1648–1655. [Google Scholar] [CrossRef]
| iTox | NCT | Trial Name | Location | Status | No. of Patients | ORR * | Ref |
|---|---|---|---|---|---|---|---|
| SS1P | 00066651 | Phase I Study of SS1(dsFv)-PE38 Anti-Mesothelin Immunotoxin in Advanced Malignancies: I.V. Infusion QOD Dosing | NCI | Closed | n = 34 20 mesothelioma 12 ovarian cancer 2 PDAC | 4/33 ^ | [85] |
| SS1P | 00006981 | Phase I Study of SS1(dsFv)-PE38 Anti-Mesothelin Immunotoxin in Advanced Malignancies: Continuous Infusion × 10 Days | NCI | Closed | n = 24 16 mesothelioma 7 ovarian 1 PDAC | 1/24 | [86] |
| SS1P | 01445392 | A Phase I, Single Center, Dose-Escalation Study of SS1(dsFv)PE38 Administered Concurrently with Pemetrexed and Cisplatin in Subjects with Unresectable Malignant Epithelial Pleural Mesothelioma | NCI | Closed | n = 24 mesothelioma | 12/20 | [87] |
| SS1P | 01362790 | A Pilot/Phase 2 Study of Pentostatin Plus Cyclophosphamide Immune Depletion to Decrease Immunogenicity of SS1P in Patients with Mesothelioma, Lung Cancer or Pancreatic Cancer | NCI | Closed | n = 11 mesothelioma | 3/10 | [88] |
| LMB-100 | 02317419 | Phase IA/IB, Open-Label, Multicenter, Multiple Ascending Dose Study Followed by an Extension Phase to Evaluate the Safety, Tolerability, Pharmacokinetics and Activity of R06927005, An Anti-Mesothelin (MSLN) Recombinant Cytolytic Fusion Protein (cFP), Administered Either Alone (Part A) or in Combination with Gemcitabine and Nab-Paclitaxel (Part B) in Patients with Mesothelin-Positive Metastatic and/or Locally Advanced Malignant Solid Tumors | Multicenter/multi-national: USA (NCI) Canada, Denmark France | Terminated | n = 15 7 mesothelioma 3 ovarian 3 PDAC 2 gastric | NR | - |
| LMB-100 | 02798536 | A Phase I Study of the Mesothelin-Targeted Immunotoxin LMB-100 With or Without Nab-Paclitaxel in Patients with Malignant Mesothelioma | NCI | Closed | n = 10 mesothelioma | NR | - |
| LMB-100 | 03436732 | A Phase I Study of the Mesothelin-Targeted Immunotoxin LMB-100 in Combination with SEL-110 in Subjects with Malignant Pleural of Peritoneal Mesothelioma | NCI | Terminated | n = 5 mesothelioma | NR | - |
| LMB-100 | 02810418 | A Phase I/II Study of Mesothelin-Targeted Immunotoxin LMB-100 Alone or in Combination with Nab-Paclitaxel in People with Previously Treated Metastatic and/or Locally Advanced Pancreatic Ductal Adenocarcinoma and Mesothelin Expressing Solid Tumors | NCI | Closed | n = 40 37 PDAC 1 rectal cancer 1 mesothelioma 1 ampullary | 1/40 | [89] |
| LMB-100 | 04034238 | A Phase I Study of Mesothelin-Targeted Immunotoxin LMB-100 in Combination with Tofacitinib in Persons with Previously Treated Pancreatic Adenocarcinoma, Cholangiocarcinoma and Other Mesothelin Expressing Solid Tumors | NCI | Recruiting | Up to 45 planned | NR | - |
| LMB-100 | 03644550 | Phase II Study of the Anti-Mesothelin Immunotoxin LMB-100 Followed by Pembrolizumab in Malignant Mesothelioma | NCI | Recruiting | Up to 100 planned | NR | - |
| LMB-100 | 04027946 | A Phase II Study of LMB-100 Followed by Pembrolizumab in the Treatment of Adults with Mesothelin-Expressing Non-Squamous Non-Small Cell Lung Cancer | NCI | Recruiting | Up to 38 planned | NR | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagerty, B.L.; Pegna, G.J.; Xu, J.; Tai, C.-H.; Alewine, C. Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors. Biomolecules 2020, 10, 973. https://doi.org/10.3390/biom10070973
Hagerty BL, Pegna GJ, Xu J, Tai C-H, Alewine C. Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors. Biomolecules. 2020; 10(7):973. https://doi.org/10.3390/biom10070973
Chicago/Turabian StyleHagerty, Brendan L., Guillaume J. Pegna, Jian Xu, Chin-Hsien Tai, and Christine Alewine. 2020. "Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors" Biomolecules 10, no. 7: 973. https://doi.org/10.3390/biom10070973
APA StyleHagerty, B. L., Pegna, G. J., Xu, J., Tai, C.-H., & Alewine, C. (2020). Mesothelin-Targeted Recombinant Immunotoxins for Solid Tumors. Biomolecules, 10(7), 973. https://doi.org/10.3390/biom10070973





