Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum inerme; Characterization, Antimicrobial, and Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
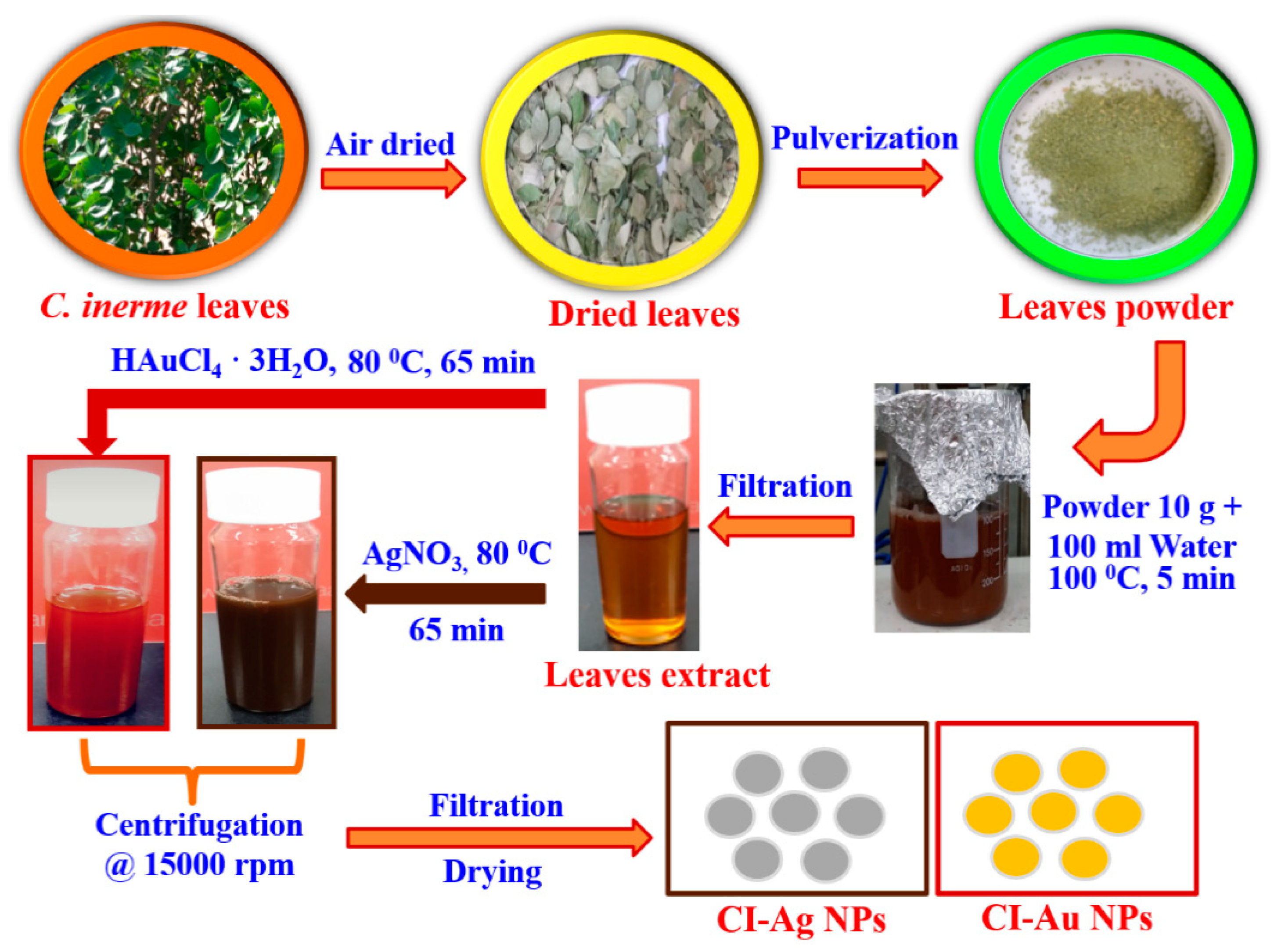
2.2. Plant Extract Preparation
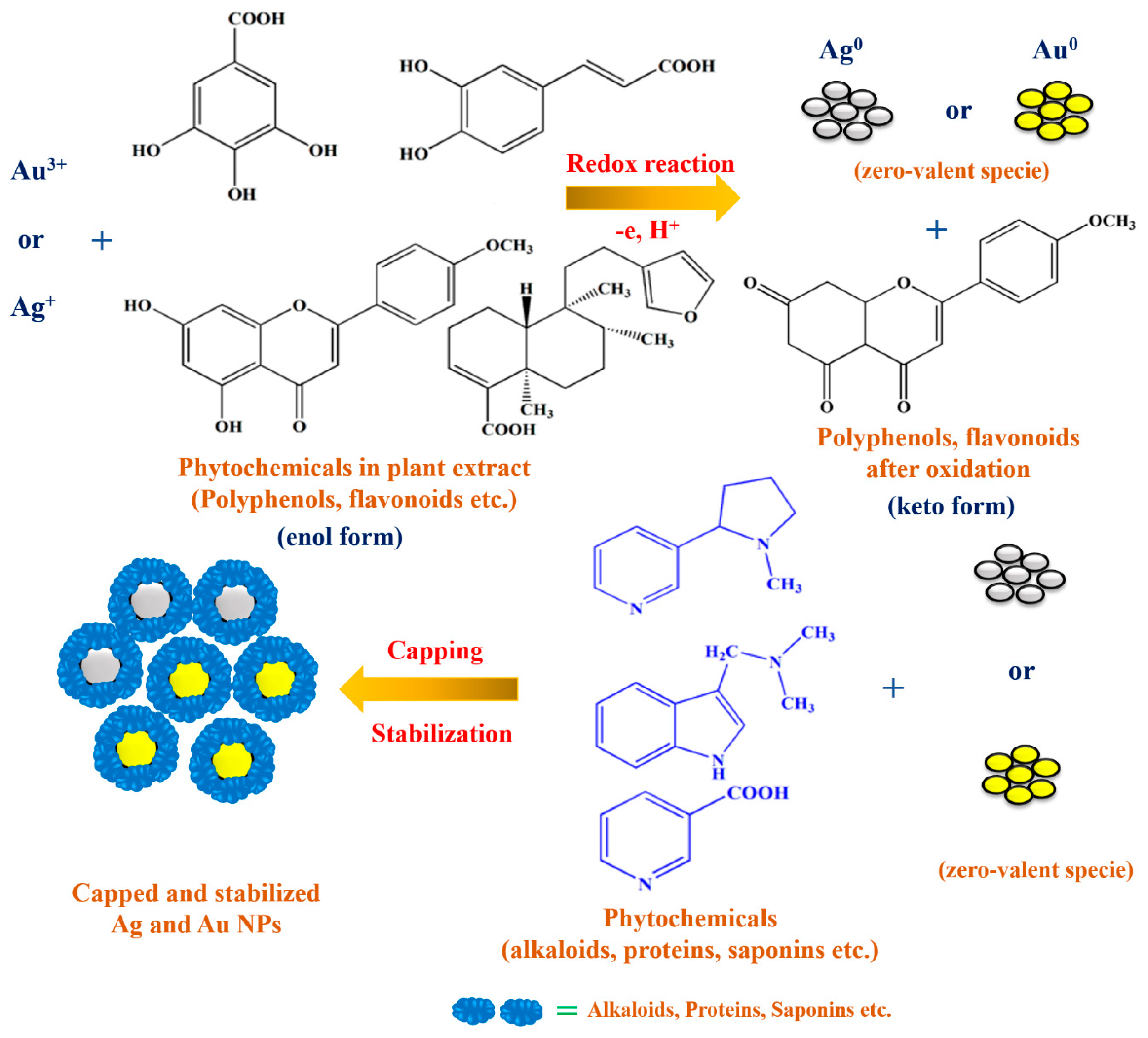
2.3. Synthesis of CI-Au and CI-Ag NPs
2.4. Characterizations
2.5. Antioxidant Activity
2.6. Antibacterial Propensity
2.7. Minimum Inhibitory Concentrations
2.8. Antimycotic Activity
2.9. Biofilm Inhibition Activity
2.10. FT-IR Analysis of Bacterial and Fungal Strains
2.11. Intracellular Reactive Oxygen Species (ROS) Analysis
2.12. Intracellular Glutathione (GSH) Investigation
2.13. Cytotoxicity Activity
2.14. Biocompatibility
2.15. Statistical Analysis
3. Results
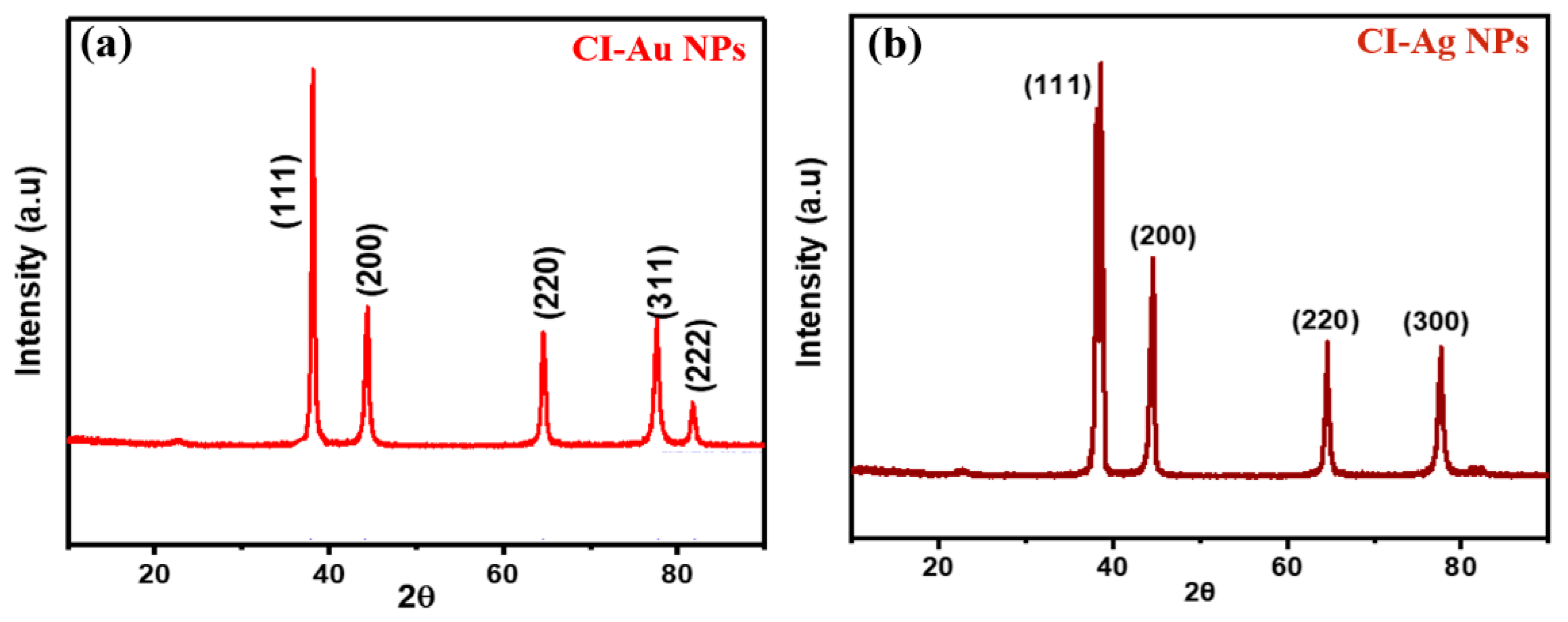
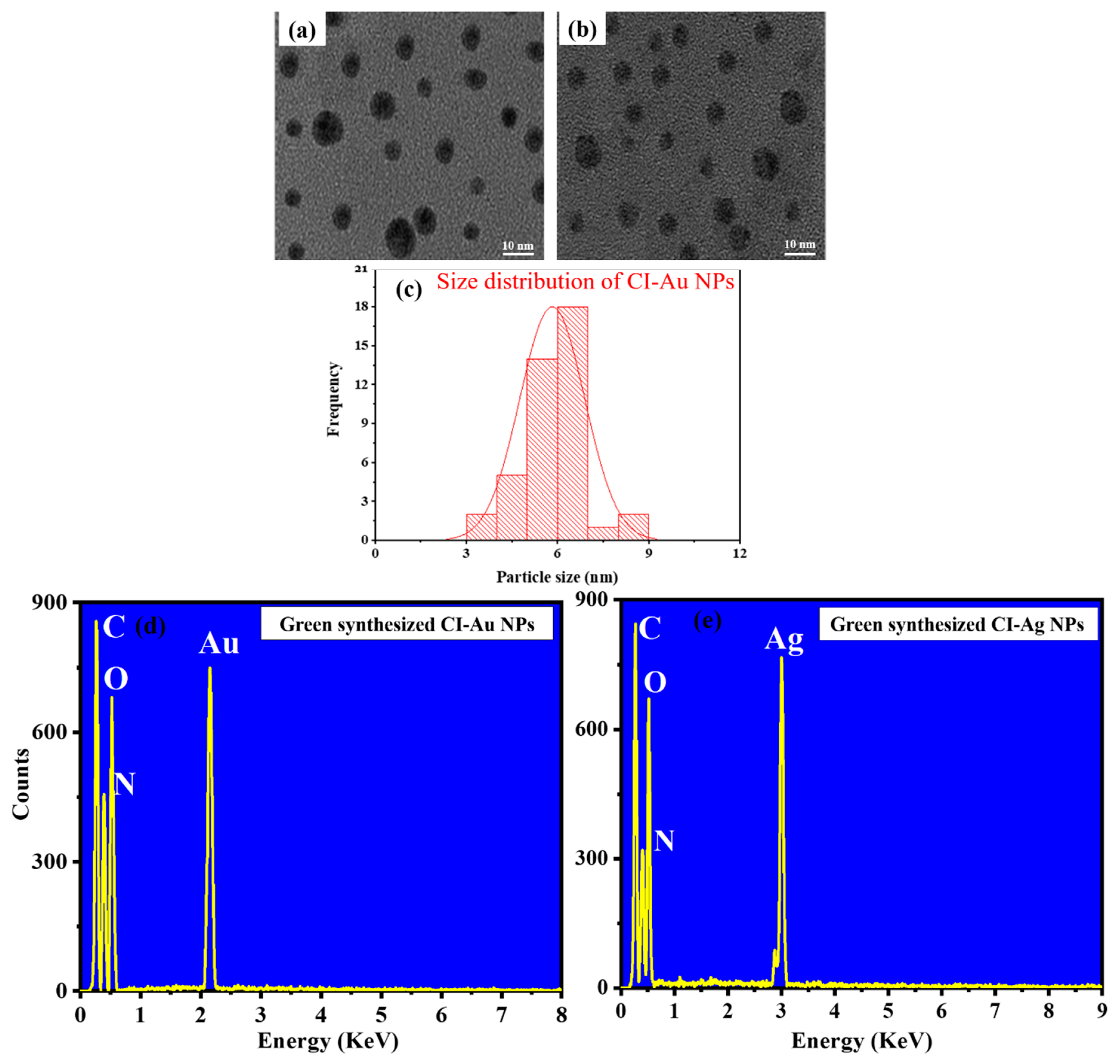
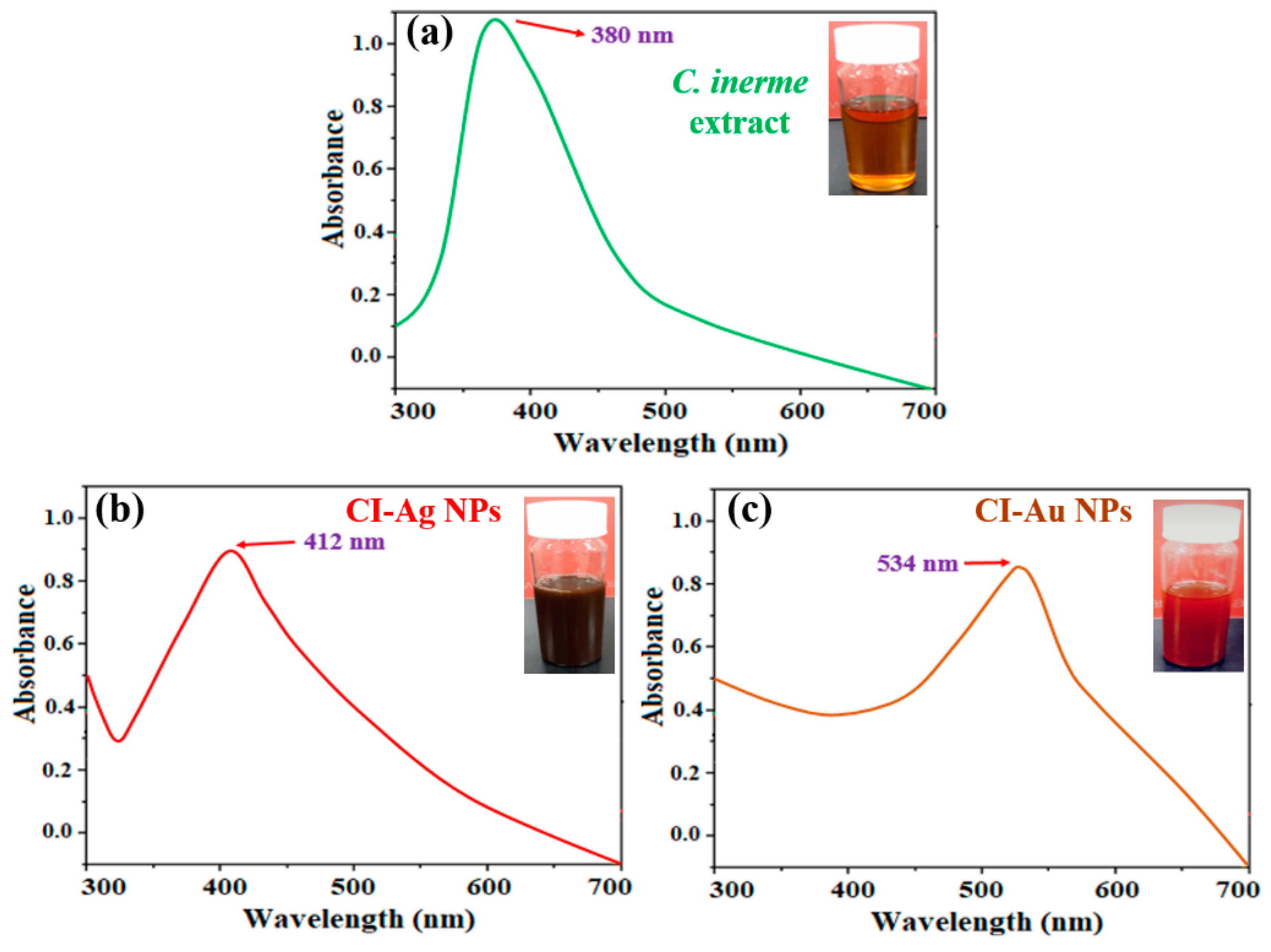
3.1. Compositions and Structures Analysis
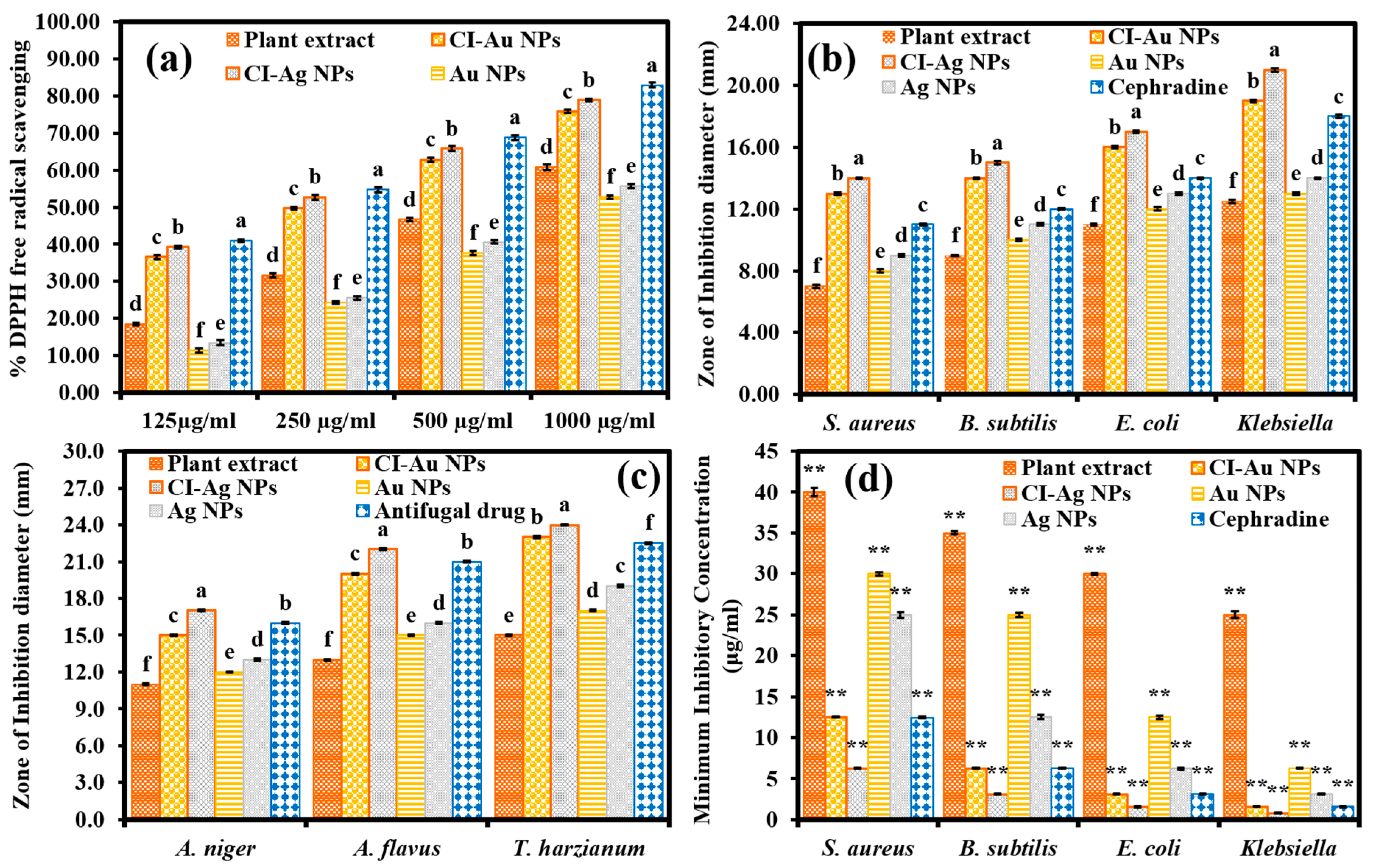
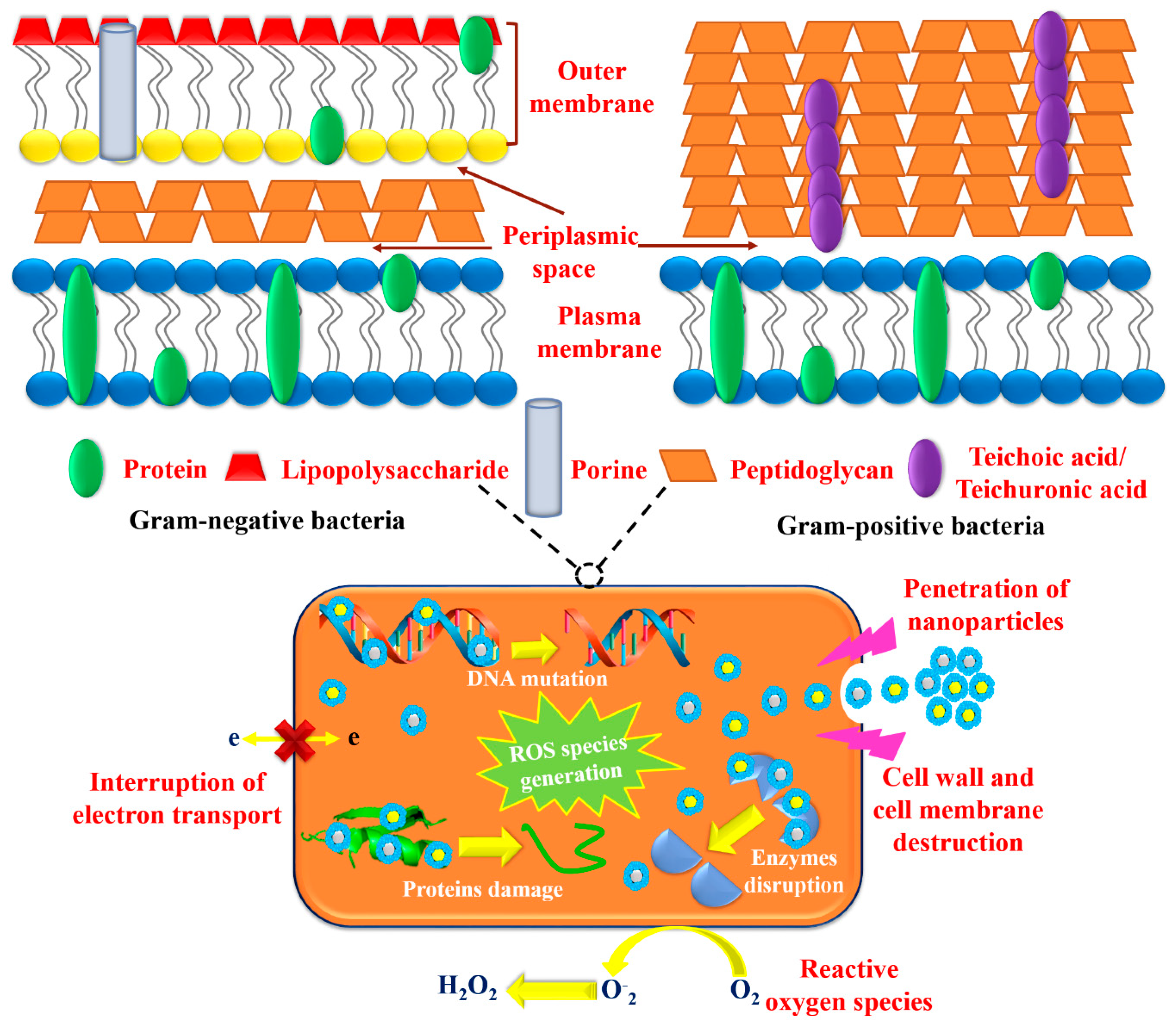
3.2. Antioxidant, Antibacterial, and Antimycotic Performance
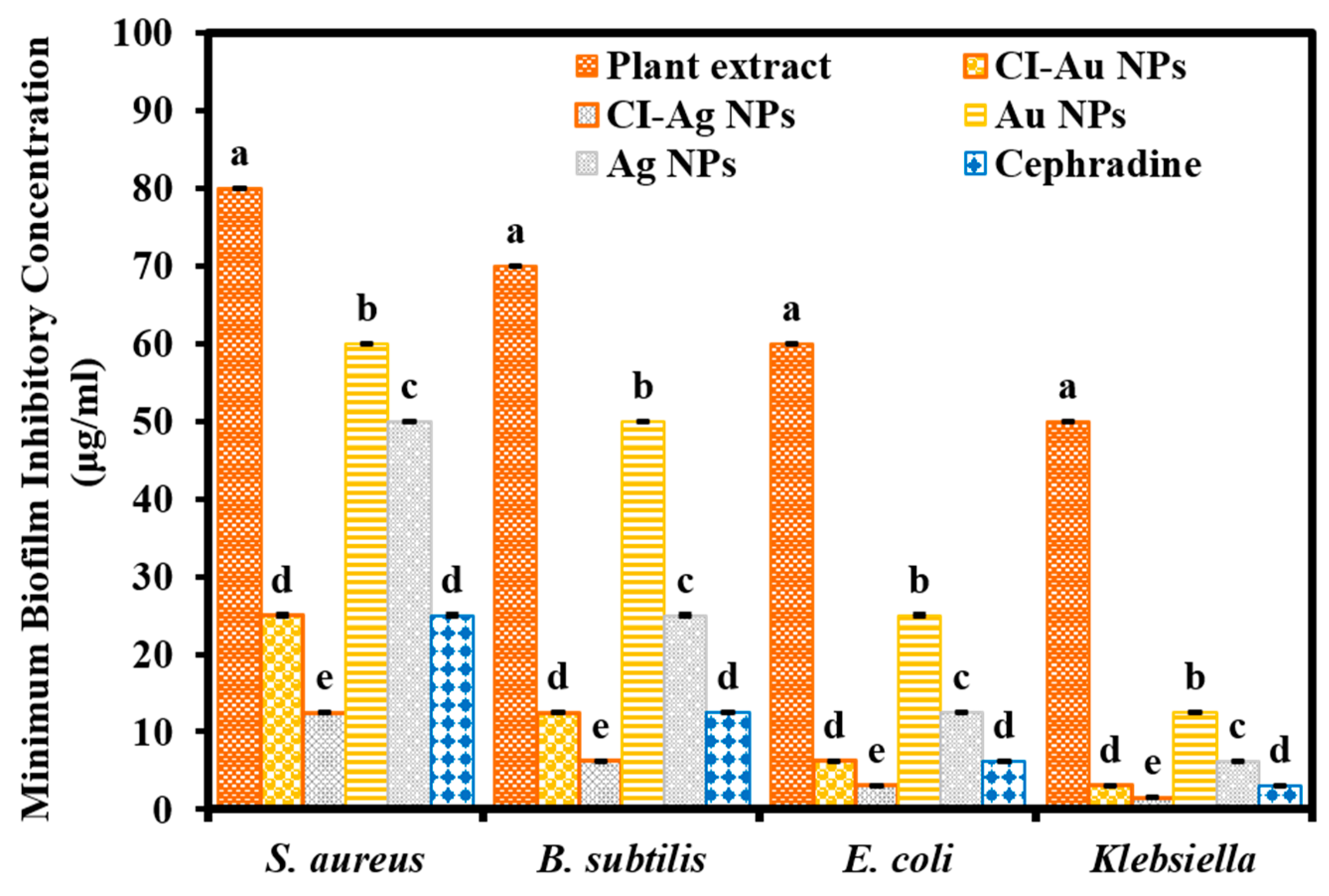
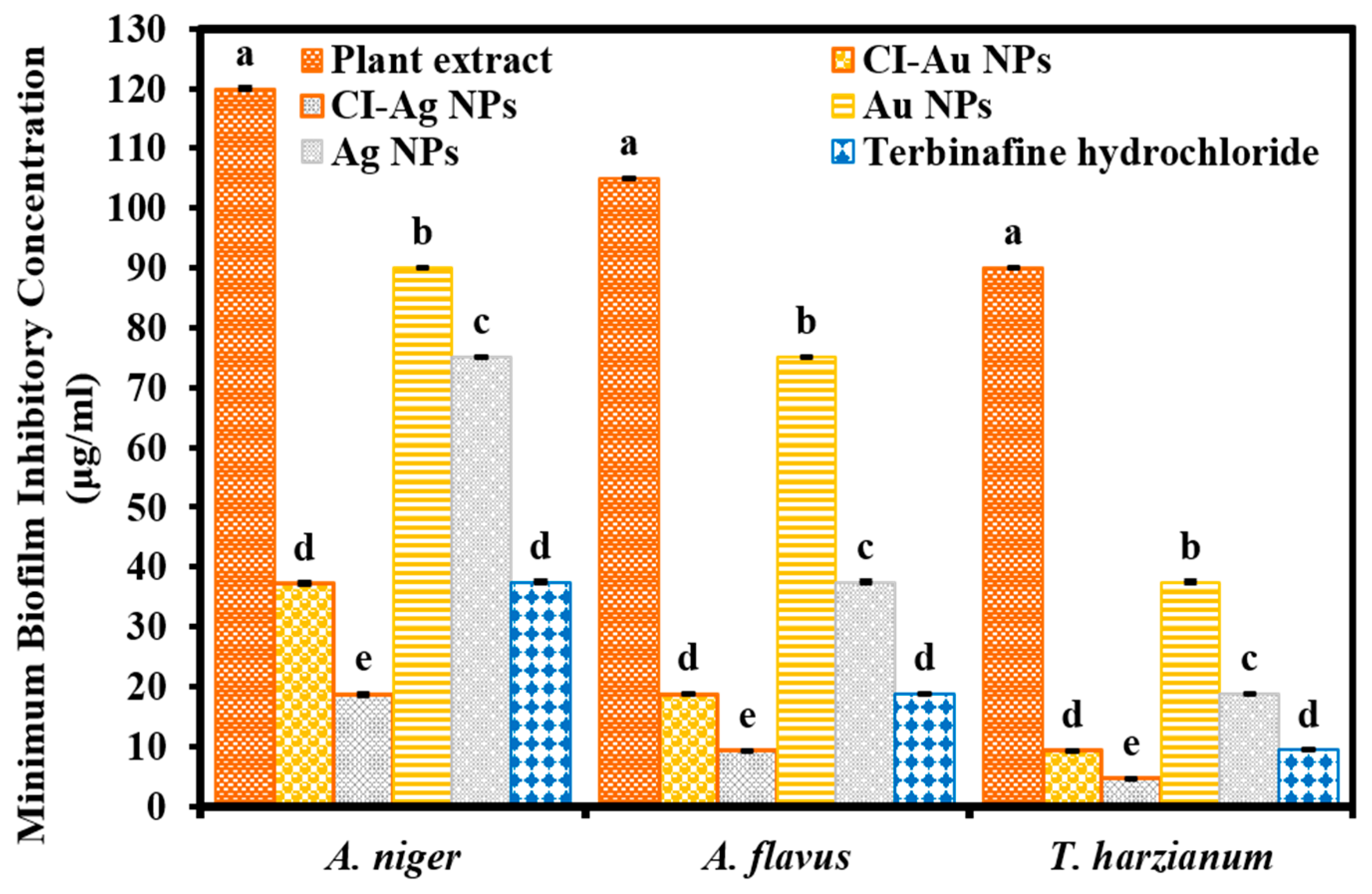
3.3. Biofilm Inhibition Activity
3.4. The Capability of ROS Generation
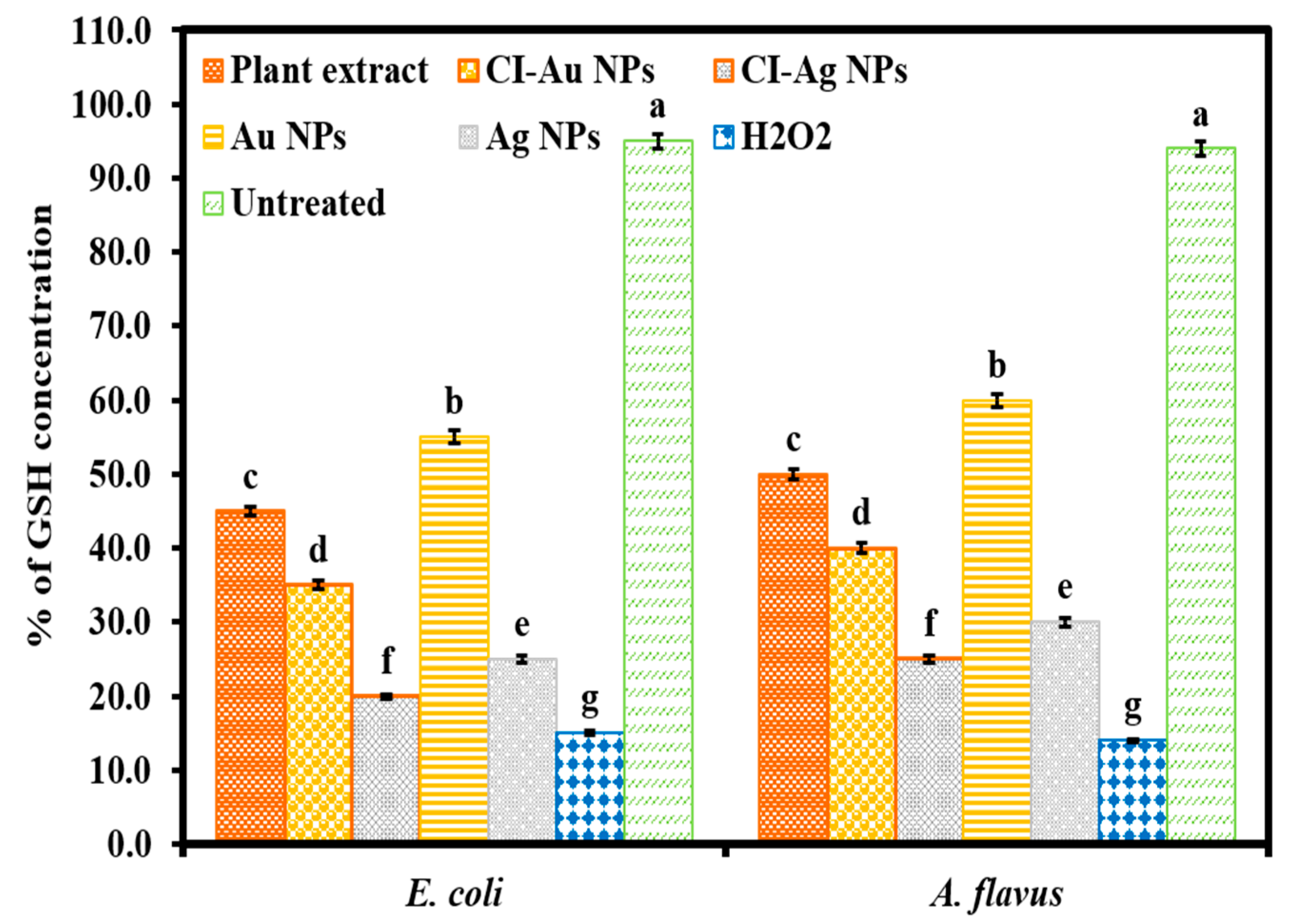
3.5. Measurement of GSH Concentration
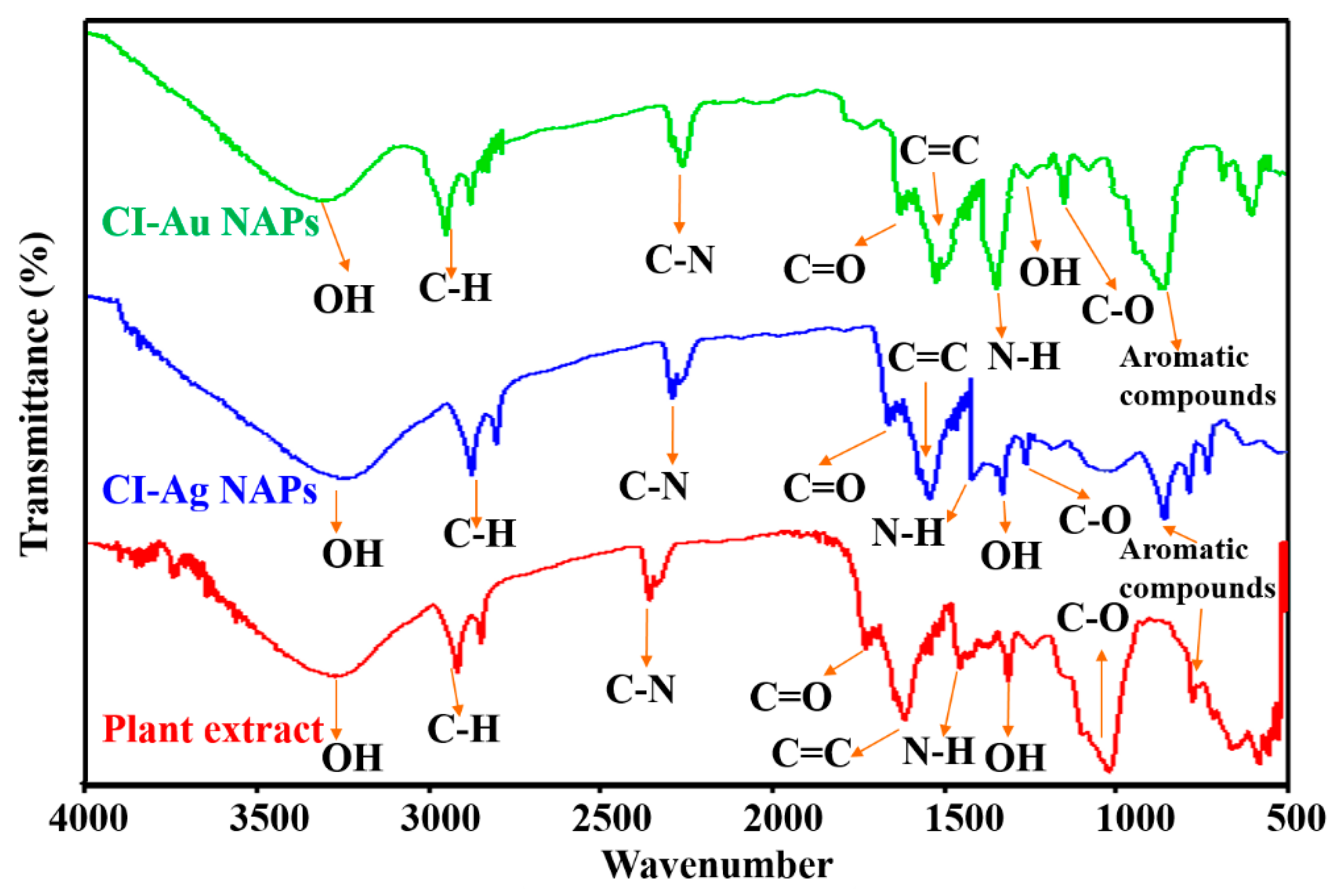
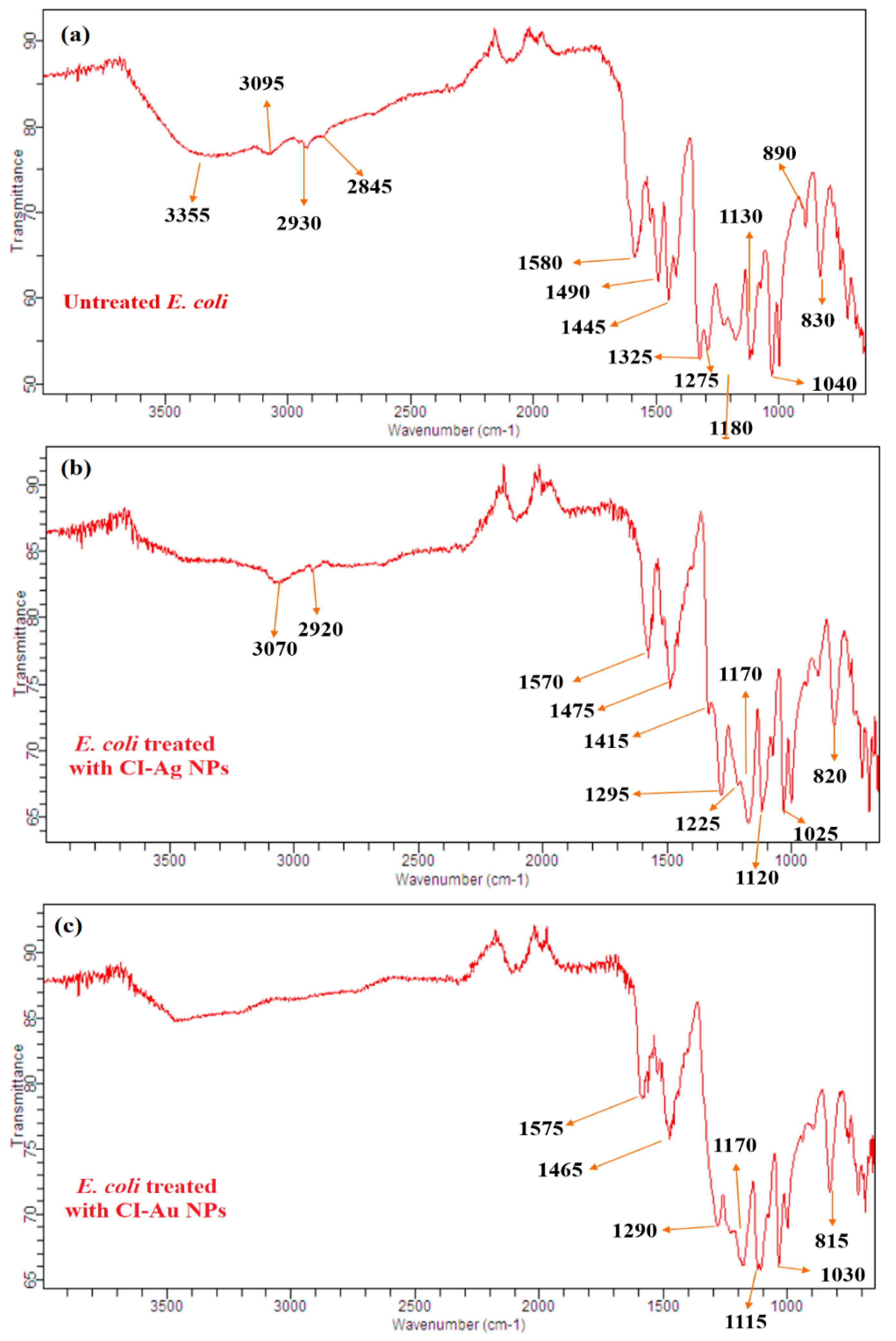
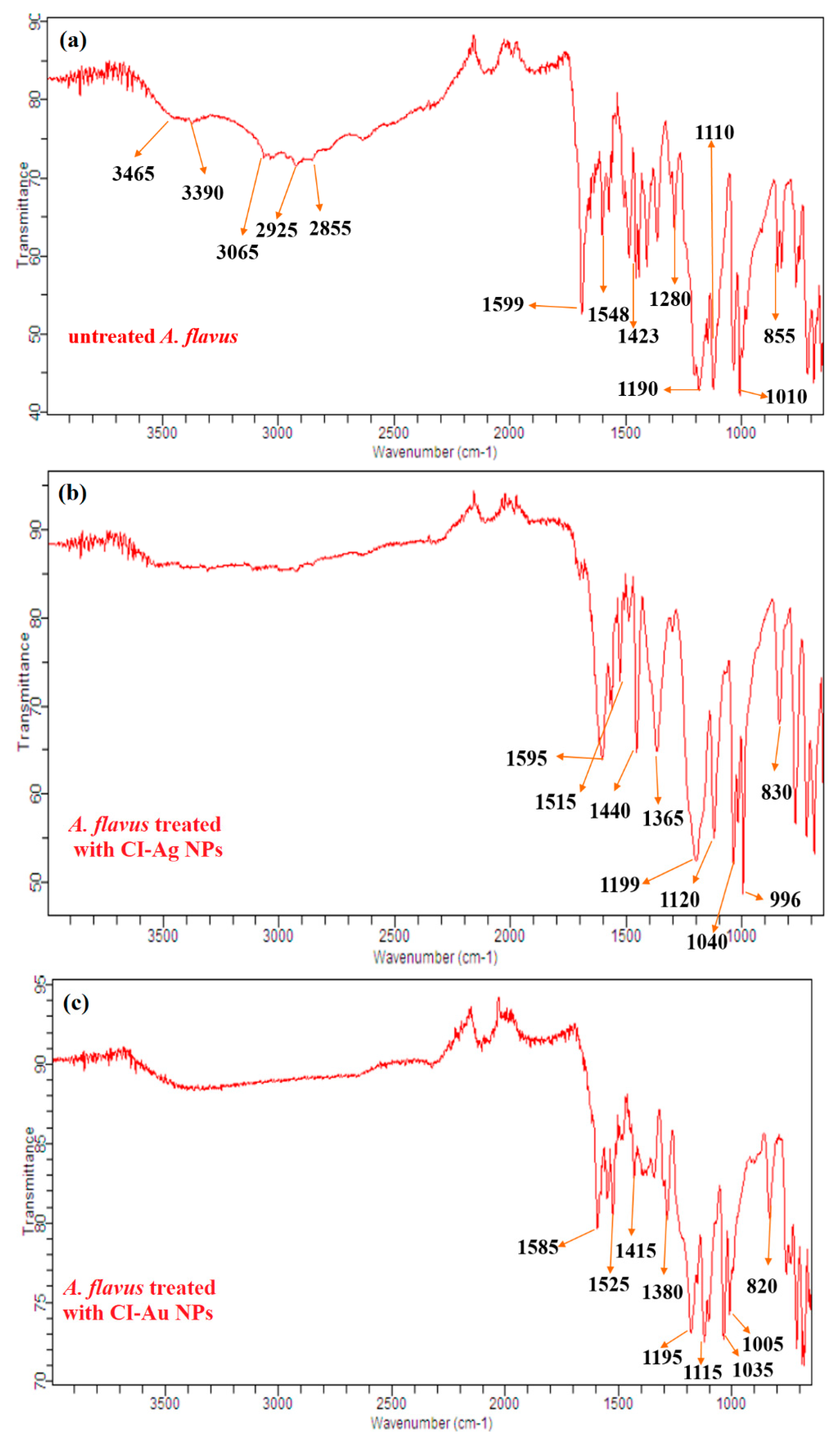
3.6. FTIR Analysis of Bacteria and Fungi
3.7. Cytotoxicity Study
3.8. Biocompatibility Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Balabanian, G.; Rose, M.; Manning, N.; Landman, D.; Quale, J. Effect of Porins and bla KPC Expression on Activity of Imipenem with Relebactam in Klebsiella pneumoniae: Can Antibiotic Combinations Overcome Resistance? Microb. Drug. Resist. 2018, 24, 877–881. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Slavin, M.A.; Schwarer, A.P.; Mijch, A.M. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 111–113. [Google Scholar] [PubMed]
- Aquino-Andrade, A.; Merida-Vieyra, J.; de la Garza, E.A.; Arzate-Barbosa, P.; Ranero, A.D. Carbapenemase-producing Enterobacteriaceae in Mexico: Report of seven non-clonal cases in a pediatric hospital. BMC Microbiol. 2018, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Arena, F.; Tascini, C.; Cannatelli, A.; De Angelis, L.H.; Fortunato, S.; Giani, T.; Menichetti, F.; Rossolini, G.M. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob. Agents Chemother. 2016, 60, 5612–5615. [Google Scholar] [CrossRef]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/detail/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 9 March 2020).
- Boomi, P.; Ganesan, R.M.; Poorani, G.; Prabu, H.G.; Ravikumar, S.; Jeyakanthan, J. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater. Sci. Eng. C 2019, 99, 202–210. [Google Scholar] [CrossRef]
- Parthiban, E.; Manivannan, N.; Ramanibai, R.; Mathivanan, N. Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol. Rep. 2019, 21, e00297. [Google Scholar] [CrossRef]
- Lian, S.; Diko, C.S.; Yan, Y.; Li, Z.; Zhang, H.; Ma, Q.; Qu, Y. Characterization of biogenic selenium nanoparticles derived from cell-free extracts of a novel yeast Magnusiomyces ingens. 3 Biotech 2019, 9, 221. [Google Scholar] [CrossRef]
- Ijaz, F.; Shahid, S.; Khan, S.A.; Ahmad, W.; Zaman, S. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activitie. Trop. J. Pharm. Res. 2017, 16, 743–753. [Google Scholar]
- Khan, S.A.; Noreen, F.; Kanwal, S.; Iqbal, A.; Hussain, G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater. Sci. Eng. C 2018, 82, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.A.; Iqbal, J.; Mahmood, T.; Ahmad, R.; Kanwal, S.; Afridi, S. Plant-mediated synthesis of nickel oxide nanoparticles (NiO) via Geranium wallichianum: Characterization and different biological applications. Mater. Res. Express 2019, 6, 0850a7. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Shahid, B.; Fatima, U.; Abbasi, S.A. Green Synthesis of MnO Nanoparticles Using Abutilon indicum Leaf Extract for Biological, Photocatalytic, and Adsorption Activities. Biomolecules 2020, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Bhat, M.P.; Udayashankar, A.C.; Lakshmeesha, T.R.; Geetha, N.; Jogaiah, S. Biosynthesis and characterization of Dillenia indica-mediated silver nanoparticles and their biological activity. Appl. Organomet. Chem. 2020, e5567. [Google Scholar] [CrossRef]
- Gharehyakheh, S.; Ahmeda, A.; Haddadi, A.; Jamshidi, M.; Nowrozi, M.; Zangeneh, M.M.; Zangeneh, A. Effect of gold nanoparticles synthesized using the aqueous extract of Satureja hortensis leaf on enhancing the shelf life and removing Escherichia coli O157: H7 and Listeria monocytogenes in minced camel’s meat: The role of nanotechnology in the food industry. Appl. Organomet. Chem. 2020, 34, e5492. [Google Scholar]
- Khan, S.A.; Rasool, N.; Riaz, M.; Nadeem, R.; Rashid, U.; Rizwan, K.; Zubair, M.; Bukhari, I.H.; Gulzar, T. Evaluation of antioxidant and cytotoxicity studies of Clerodendrum inerme. Asian J. Chem. 2013, 13, 7457–7462. [Google Scholar] [CrossRef]
- Ba Vinh, L.; Thi Minh Nguyet, N.; Young Yang, S.; Hoon Kim, J.; Thi Vien, L.; Thi Thanh Huong, P.; Van Thanh, N.; Xuan Cuong, N.; Hoai Nam, N.; Van Minh, C.; et al. A new rearranged abietane diterpene from Clerodendrum inerme with antioxidant and cytotoxic activities. Nat. Prod. Res. 2018, 32, 2001–2007. [Google Scholar] [CrossRef]
- Anandhi, K.; Ushadevi, T. Analysis of Phytochemical Constituents and Antibacterial Activities of Clerodendron inerme L. Against Some Selected Pathogens. Int. J. Biotechnol. Allied Fields 2013, 1, 387–393. [Google Scholar]
- Shahid, S.; Fatima, U.; Sajjad, R.; Khan, S.A. Bioinspired nanotheranostic agent: Zinc oxide; green synthesis and biomedical potential. Dig. J. Nanomater Bios. 2019, 14, 1023–1031. [Google Scholar]
- Iqbal, A.; Khan, Z.A.; Shahzad, S.A.; Usman, M.; Khan, S.A.; Fauq, A.H.; Bari, A.; Sajid, M.A. Synthesis of E-stilbene azomethines as potent antimicrobial and antioxidant agents. Turk. J. Chem. 2018, 42, 1518–1533. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci. Rep. 2015, 15, 9578. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. In Vitro 2010, 24, 872–878. [Google Scholar] [PubMed]
- Ajitha, B.; Reddy, Y.A.; Reddy, P.S. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C 2015, 49, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar]
- Alfuraydi, A.A.; Devanesan, S.; Al-Ansari, M.; AlSalhi, M.S.; Ranjitsingh, A.J. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B 2019, 192, 83–89. [Google Scholar] [CrossRef]
- Latha, D.; Sampurnam, S.; Arulvasu, C.; Prabu, P.; Govindaraju, K.; Narayanan, V. Biosynthesis and characterization of gold nanoparticle from Justicia adhatoda and its catalytic activity. Mater. Today Proc. 2018, 5, 8968–8972. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Masip, L.; Veeravalli, K.; Georgiou, G. The many faces of glutathione in bacteria. Antioxid. Redox Sign. 2006, 8, 753–762. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- Banerjee, M.; Mallick, S.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Heightened reactive oxygen species generation in the antimicrobial activity of a three component iodinated chitosan—silver nanoparticle composite. Langmuir 2010, 26, 5901–5908. [Google Scholar] [PubMed]
- Das, S.K.; Khan, M.M.; Guha, A.K.; Das, A.R.; Mandal, A.B. Silver-nano biohybride material: Synthesis, characterization and application in water purification. Bioresour. Technol. 2012, 124, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Khan, I.; Bhatti, Z.A.; Tang, F.; Peng, C. Fungal strain Aspergillus flavus F3 as a potential candidate for the removal of lead (II) and chromium (VI) from contaminated soil. Main Group Met. Chem. 2016, 39, 93–104. [Google Scholar] [CrossRef]
- Gué, M.; Dupont, V.; Dufour, A.; Sire, O. Bacterial swarming: A biochemical time-resolved FTIR—ATR study of Proteus mirabilis swarm-cell differentiation. Biochemistry 2001, 40, 11938–11945. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kurvet, I.; Kahru, A. Particle-cell contact enhances antibacterial activity of silver nanoparticles. PLoS ONE 2013, 8, e64060. [Google Scholar]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.; Chiu, J.F.; Che, C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Parandhaman, T.; Das, A.; Ramalingam, B.; Samanta, D.; Sastry, T.P.; Mandal, A.B.; Das, S.K. Antimicrobial behavior of biosynthesized silica–silver nanocomposite for water disinfection: A mechanistic perspective. J. Hazard. Mater. 2015, 290, 117–126. [Google Scholar]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483. [Google Scholar] [PubMed]
- Jalilian, F.; Chahardoli, A.; Sadrjavadi, K.; Fattahi, A.; Shokoohinia, Y. Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol. 2020. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C. Eco-friendly synthesis of silver nanoparticles using Umbrella plant, and evaluation of their photocatalytic and antibacterial activities. Nano-Met. Chem. 2020, 50, 389–399. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sarada, D.V. Gold and silver nanoparticles from Trianthema decandra: Synthesis, characterization, and antimicrobial properties. Int. J. Nanomed. 2012, 7, 5375. [Google Scholar]
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Asghar, M.A.; Iqbal, J.; Walker, G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT 2018, 90, 98–107. [Google Scholar] [CrossRef]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.J.; Cho, M.; Lee, S.M.; Park, J.H.; Oh, S.G.; Bang, K.S.; Oh, B.T. Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioproc. Biosystems Eng. 2014, 37, 1935–1943. [Google Scholar]
- Küp, F.Ö.; Çoşkunçay, S.; Duman, F. Biosynthesis of silver nanoparticles using leaf extract of Aesculus hippocastanum (horse chestnut): Evaluation of their antibacterial, antioxidant and drug release system activities. Mater. Sci. Eng. C 2020, 107, 110207. [Google Scholar]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Microwave assisted green synthesis of silver nanoparticles using leaf extract of elephantopus scaber and its environmental and biological applications. Artif. Cell Nanomed. B. 2018, 46, 795–804. [Google Scholar]
- Wang, L.; Wu, Y.; Xie, J.; Wu, S.; Wu, Z. Characterization, antioxidant and antimicrobial activities of green synthesized silver nanoparticles from Psidium guajava L. leaf aqueous extracts. Mater. Sci. Eng. C 2018, 86, 1–8. [Google Scholar] [CrossRef]
- Mata, R.; Bhaskaran, A.; Sadras, S.R. Green-synthesized gold nanoparticles from Plumeria alba flower extract to augment catalytic degradation of organic dyes and inhibit bacterial growth. Particuology 2016, 24, 78–86. [Google Scholar] [CrossRef]
- Asariha, M.; Chahardoli, A.; Karimi, N.; Gholamhosseinpour, M.; Khoshroo, A.; Nemati, H.; Shokoohinia, Y.; Fattahi, A. Green synthesis and structural characterization of gold nanoparticles from Achillea wilhelmsii leaf infusion and in vitro evaluation. Bull. Mater. Sci. 2020, 43, 57. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253. [Google Scholar] [PubMed]
- Zhaleh, M.; Zangeneh, A.; Goorani, S.; Seydi, N.; Zangeneh, M.M.; Tahvilian, R.; Pirabbasi, E. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl. Organomet. Chem. 2019, 33, e5015. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Saneei, S.; Zangeneh, A.; Toushmalani, R.; Haddadi, A.; Almasi, M.; Amiri-Paryan, A. Preparation, characterization, and evaluation of cytotoxicity, antioxidant, cutaneous wound healing, antibacterial, and antifungal effects of gold nanoparticles using the aqueous extract of Falcaria vulgaris leaves. Appl. Organomet. Chem. 2019, 33, e5216. [Google Scholar] [CrossRef]
- Hu, X.; Ahmeda, A.; Zangeneh, M.M. Chemical characterization and evaluation of antimicrobial and cutaneous wound healing potentials of gold nanoparticles using Allium saralicum RM Fritsch. Appl. Organomet. Chem. 2020, 34, e5484. [Google Scholar] [CrossRef]
- Piruthiviraj, P.; Margret, A.; Krishnamurthy, P.P. Gold nanoparticles synthesized by Brassica oleracea (Broccoli) acting as antimicrobial agents against human pathogenic bacteria and fungi. Appl. Nanosci. 2016, 6, 467–473. [Google Scholar]


| Materials (NPs) | Size (nm) | Plant Used | Antimicrobial Properties | References | ||
|---|---|---|---|---|---|---|
| Species | Conc. of NPs | ZOIs | ||||
| CI-Ag | 2–10 | C. inerme | E. coli | 250 µg/mL | 17 | This work |
| Ag | 8–20 | D. bulbifera | E. coli | 500 µg/mL | 15 | [42] |
| Ag | 8–50 | Allium ampeloprasum | E. coli | 300 µg/mL | 13 | [43] |
| Ag | 20 | Umbrella | E. coli | 250 µg/mL | 16 | [44] |
| Ag | 36–74 | Trianthema decandra | E. coli | 10 mg/mL | 15.5 | [45] |
| CI-Ag | 2–10 | C. inerme | S. aureus | 250 µg/mL | 14 | This work |
| Ag | 10–20 | Green and black tea | S. aureus | 1 mg/mL | 19–21 | [46] |
| Ag | 10–20 | Zingiber officinale | S. aureus | 0.1 mg/mL | 6.5 | [47] |
| Ag | 8–50 | Allium ampeloprasum | S. aureus | 300 µg/mL | 8 | [43] |
| Ag | 20 | Umbrella | S. aureus | 250 µg/mL | 12.7 | [44] |
| Ag | 36–74 | Trianthema decandra | S. aureus | 10 mg/mL | 13.5 | [45] |
| CI-Ag | 2–10 | C. inerme | K. pneumoniae | 250 µg/mL | 21 | This work |
| Ag | 8–20 | D. bulbifera | K. pneumoniae | 500 µg/mL | 15 | [42] |
| Ag | 20 | Umbrella | K. pneumoniae | 250 µg/mL | 13.1 | [44] |
| Ag | 50 | Aesculus hippocastanum | K. pneumoniae | 100 µg/mL | 12.5 | [48] |
| CI-Ag | 2–10 | C. inerme | B. subtilis | 250 µg/mL | 15 | This work |
| Ag | 37 | E. scaber | B. subtilis | 1 mg/mL | 16 | [49] |
| Ag | 20–25 | P. guajava | B. subtilis | 300 µg/mL | 19 | [50] |
| Ag | 10–20 | Zingiber officinale | B. subtilis | 0.1 mg/mL | 0 | [47] |
| Ag | 36–74 | Trianthema decandra | B. subtilis | 10 mg/mL | 12 | [45] |
| CI-Ag | 2–10 | C. inerme | A. flavus | 250 µg/mL | 22 | This work |
| Ag | 37 | E. scaber | A. flavus | 1 mg/mL | 12 | [49] |
| CI-Ag | 2–10 | C. inerme | A. niger | 250 µg/mL | 17 | This work |
| Ag | 20–25 | P. guajava | A. niger | 300 µg/mL | 18.79 | [50] |
| Materials (NPs) | Size (nm) | Plant Used | Antimicrobial Properties | References | ||
|---|---|---|---|---|---|---|
| Conc. of NPs | Species | ZOIs | ||||
| CI-Au | 3–9 | C. inerme | E. coli | 250 µg/mL | 16 | This work |
| Au | 15.6 | Plumeria alba | E. coli | 400 µg/mL | 16 | [51] |
| Au | 2.7–38.7 | Achillea wilhelmsii | E. coli | 300 µg/mL | 0 | [52] |
| Au | 20–140 | Citrullus lanatus | E. coli | 1000 µg/mL | 9.23 | [53] |
| Au | 33–65 | Trianthema decandra | E. coli | 10 mg/mL | 9.5 | [45] |
| Au | 40–45 | Gundelia tournefortii | E. coli | 2 mg/mL | 9.8 | [54] |
| Au | 40–45 | Falcaria vulgaris | E. coli | 4 mg/mL | 8.6 | [55] |
| Au | 40–45 | Allium saralicum | E. coli | 4 mg/mL | 10.8 | [56] |
| CI-Au | 3–9 | C. inerme | S. aureus | 250 µg/mL | 13 | This work |
| Au | 20–140 | Citrullus lanatus | S. aureus | 1000 µg/mL | 0 | [53] |
| Au | 33–65 | Trianthema decandra | S. aureus | 10 mg/mL | 14.5 | [45] |
| Au | 40–45 | Gundelia tournefortii | S. aureus | 2 mg/mL | 11.2 | [54] |
| Au | 40–45 | Falcaria vulgaris | S. aureus | 4 mg/mL | 13 | [55] |
| Au | 40–45 | Allium saralicum | S. aureus | 4 mg/mL | 11.6 | [56] |
| CI-Au | 3–9 | C. inerme | B. subtilis | 250 µg/mL | 14 | This work |
| Au | 2.7–38.7 | Achillea wilhelmsii | B. subtilis | 300 µg/mL | 11 | [52] |
| Au | 33–65 | Trianthema decandra | B. subtilis | 10 mg/mL | 9.5 | [45] |
| Au | 40–45 | Gundelia tournefortii | B. subtilis | 2 mg/mL | 14.2 | [54] |
| Au | 40–45 | Falcaria vulgaris | B. subtilis | 4 mg/mL | 14 | [55] |
| Au | 40–45 | Allium saralicum | B. subtilis | 4 mg/mL | 14.2 | [56] |
| CI-Au | 3–9 | C. inerme | A. niger | 250 µg/mL | 15 | This work |
| Au | 12–22 | Brassica oleracea | A. niger | 50 µg/mL | 9 | [57] |
| CI-Au | 3–9 | C. inerme | A. flavus | 250 µg/mL | 20 | This work |
| Au | 12–22 | Brassica oleracea | A. flavus | 50 µg/mL | 9 | [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.A.; Shahid, S.; Lee, C.-S. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum inerme; Characterization, Antimicrobial, and Antioxidant Activities. Biomolecules 2020, 10, 835. https://doi.org/10.3390/biom10060835
Khan SA, Shahid S, Lee C-S. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum inerme; Characterization, Antimicrobial, and Antioxidant Activities. Biomolecules. 2020; 10(6):835. https://doi.org/10.3390/biom10060835
Chicago/Turabian StyleKhan, Shakeel Ahmad, Sammia Shahid, and Chun-Sing Lee. 2020. "Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum inerme; Characterization, Antimicrobial, and Antioxidant Activities" Biomolecules 10, no. 6: 835. https://doi.org/10.3390/biom10060835
APA StyleKhan, S. A., Shahid, S., & Lee, C.-S. (2020). Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum inerme; Characterization, Antimicrobial, and Antioxidant Activities. Biomolecules, 10(6), 835. https://doi.org/10.3390/biom10060835