Therapeutic Potential of Brassinosteroids in Biomedical and Clinical Research
Abstract
:1. Introduction
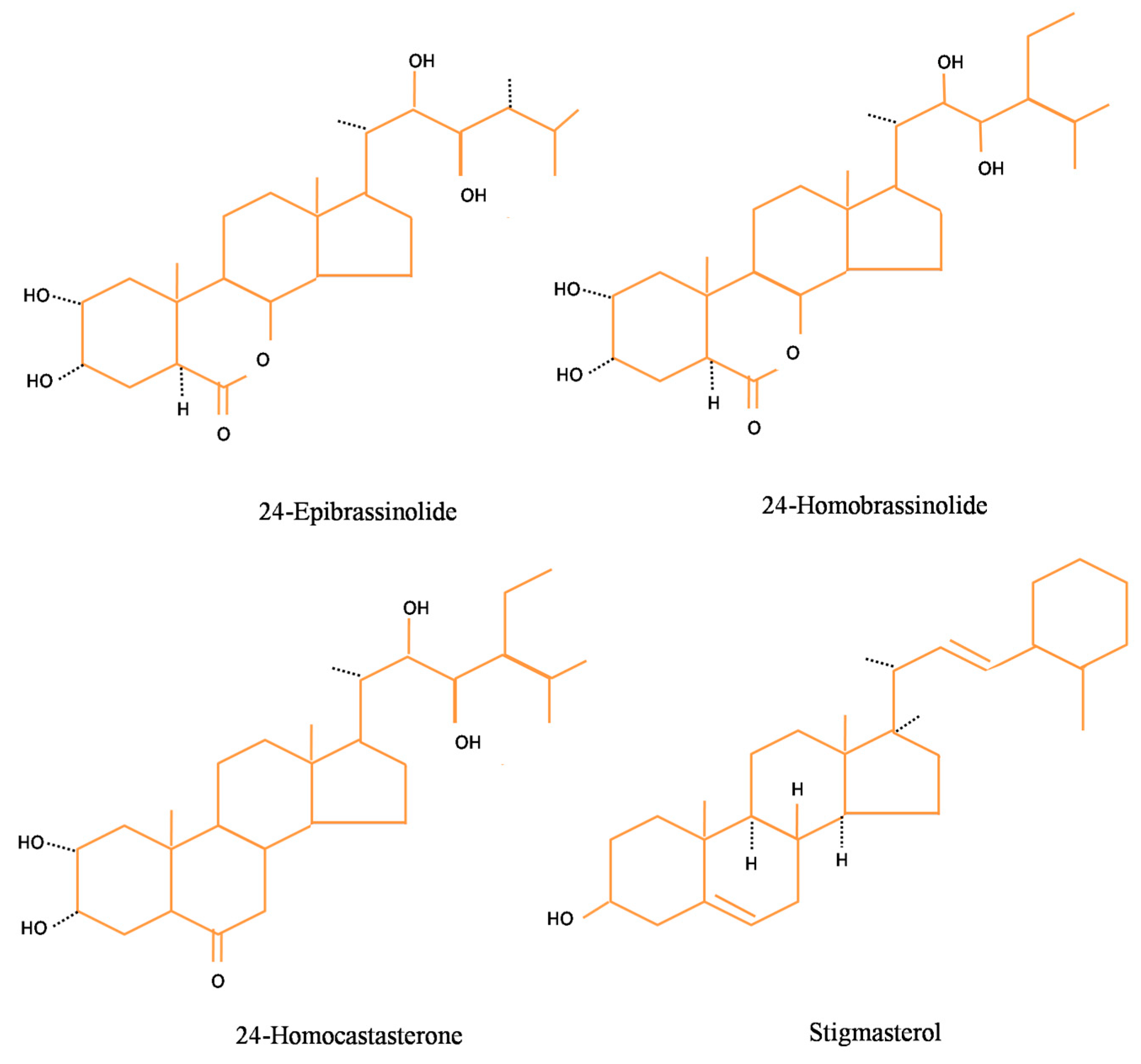
2. Dietary Sources of Phytosteroids in Plant
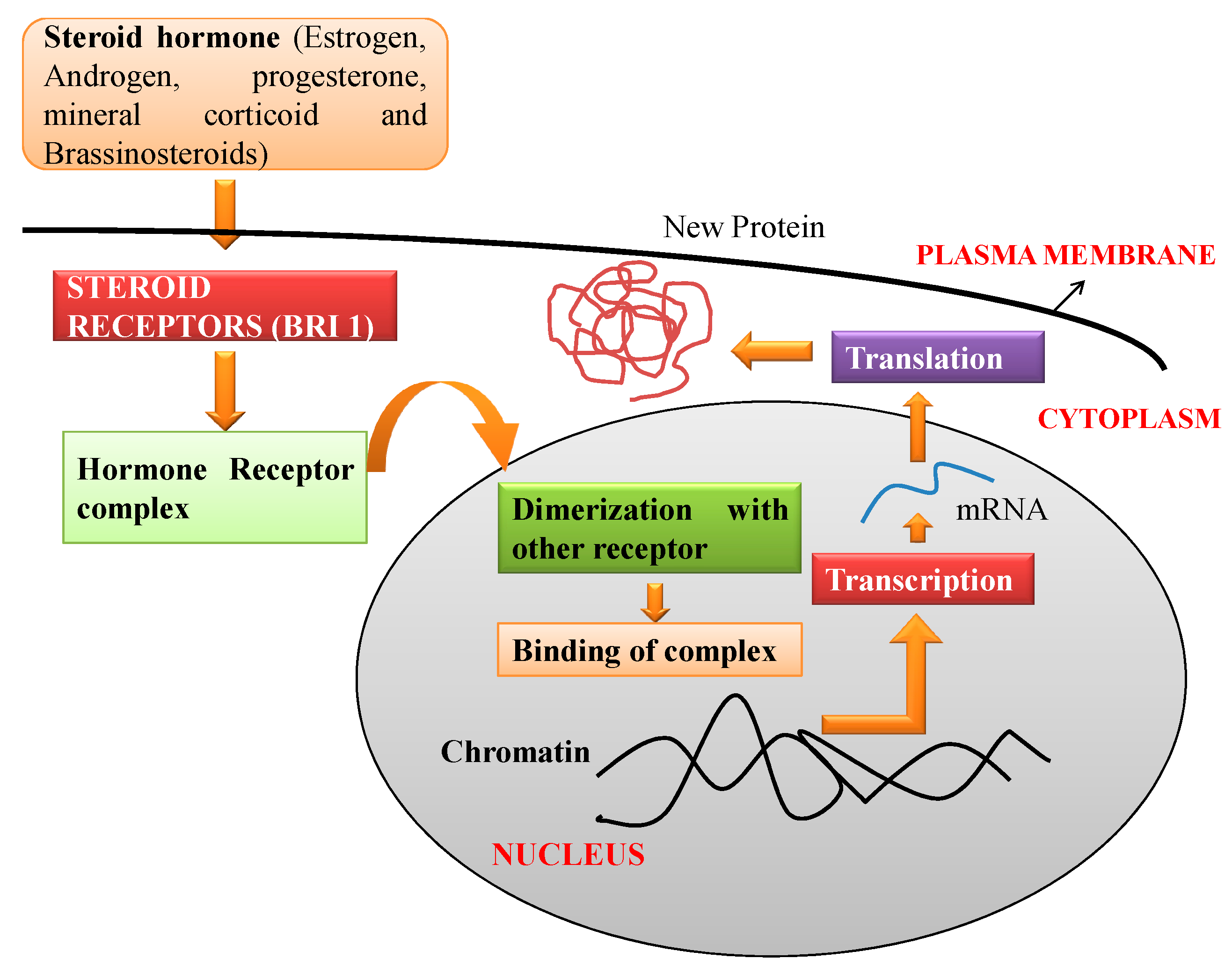
3. Mechanism of Action of BRs
4. Therapeutic Role of BRs
4.1. Anticancerous/Antiproliferative Activities
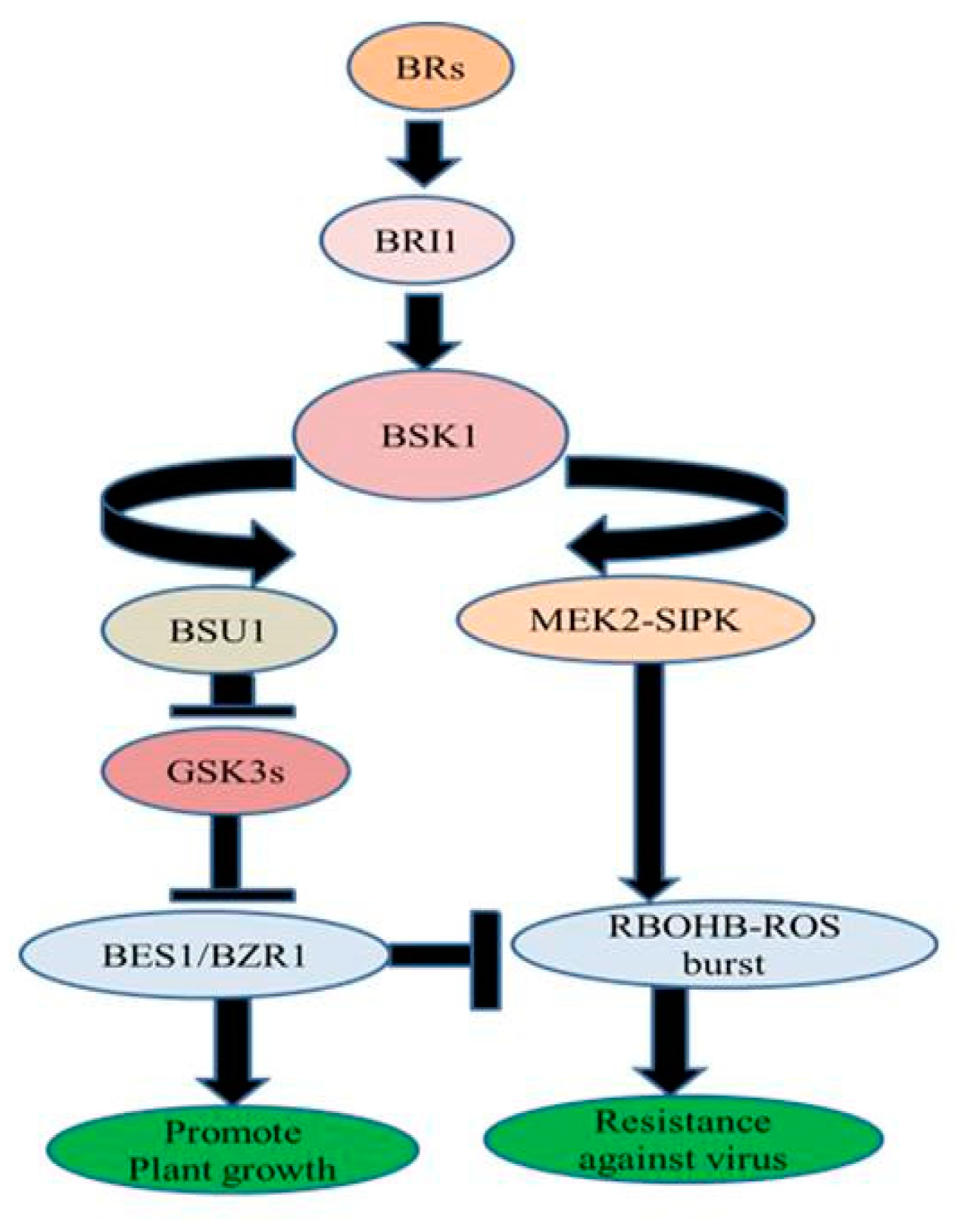
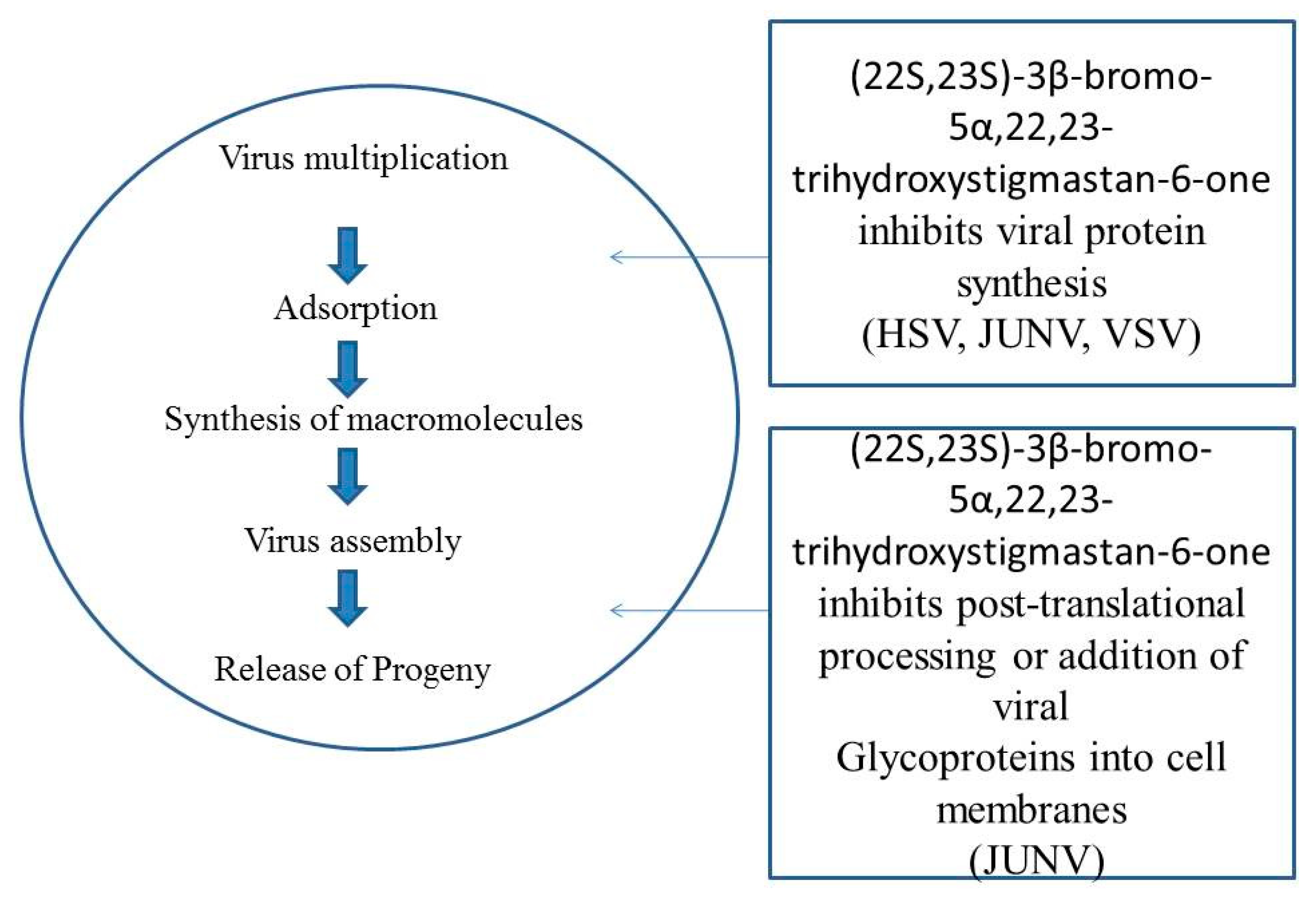
4.2. Antiviral Activities
4.3. Antiherpetic Activities
4.4. Antifungal and Antibacterial Activities
4.5. Anti-Inflammatory Activities
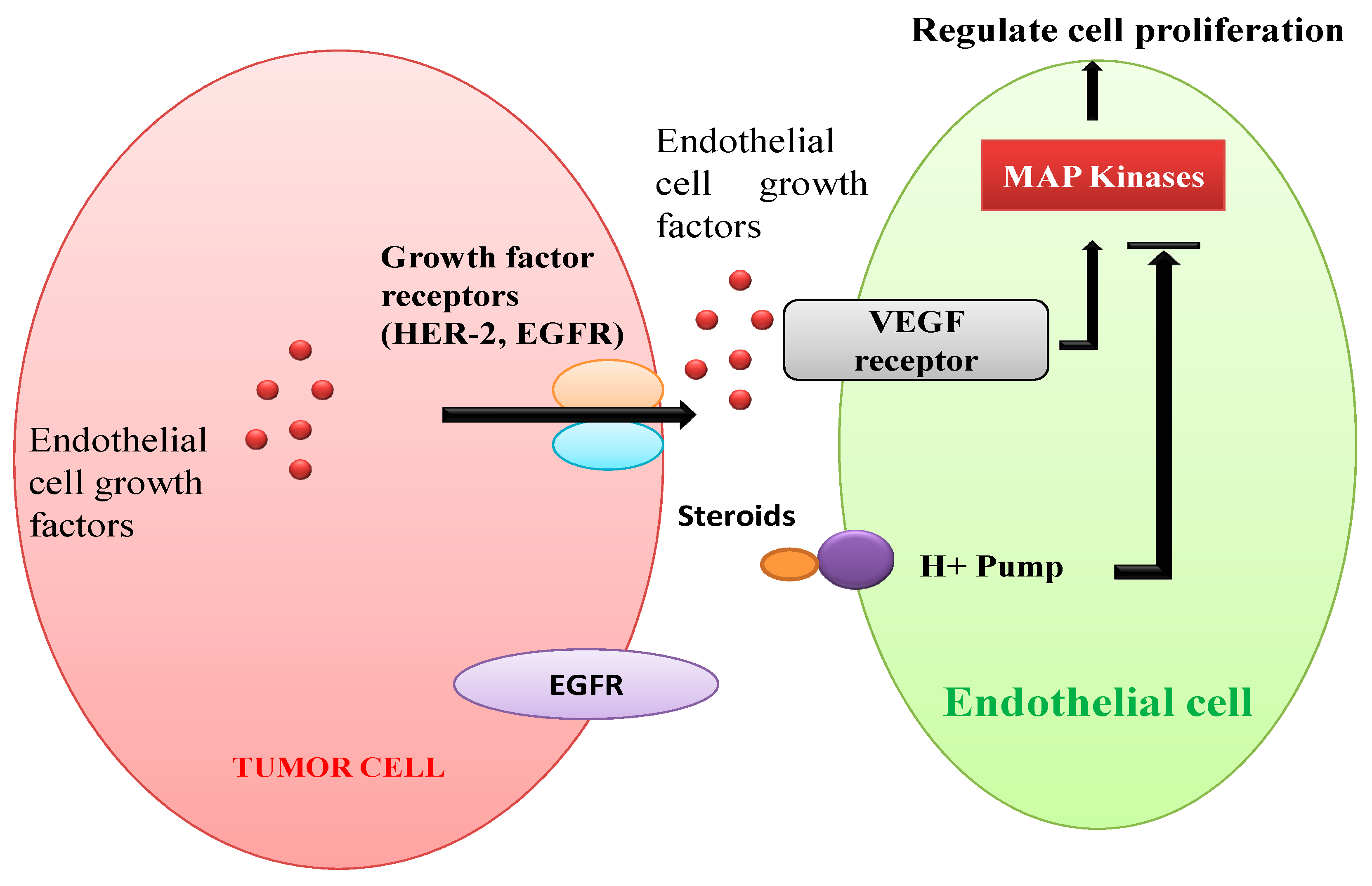
4.6. Antiangiogenic Activities and Antigenotoxic
4.7. Anticholesteromic Action
4.8. Ecdysteroidal Activities
4.9. Anabolic Activities
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Harborne, J.B. Flavonoid profiles in the compositae. In The Biology and Chemistry of the Compositae; Heywood, V.H., Harborne, J.B., Turner, B.L., Eds.; Academic Press: London, UK, 1977; pp. 359–384. [Google Scholar]
- Park, J.; Lee, Y.; Martinoia, E.; Geisler, M. Plant hormone transporters: What we know and what we would like to know. BMC Biol. 2017, 15, 93. [Google Scholar] [CrossRef] [Green Version]
- Janeczko, A.; Skoczowski, A. Mammalian sex hormones in plants. Folia Histochem. Cytobiol. 2005, 43, 71–79. [Google Scholar]
- Tarkowská, D. Plants are Capable of Synthesizing Animal Steroid Hormones. Molecules 2019, 24, 2585. [Google Scholar] [CrossRef] [Green Version]
- Czerpak, R.; Szamrej, I.K. The effect of β-estradiol and corticosteroids on chlorophylls and carotenoids content in Wolffia arrhiza(L.) Wimm. (Lemnaceae) growing in municipal bialystok tap water. Pol. J. Environ. Stud. 2003, 12, 677–684. [Google Scholar]
- Szamrej, I.K.; Czerpak, R. The effect of sex steroids and corticosteroids on the content of soluble proteins, nucleic acids and reducing sugars in Wolffia arrhiza(L.) Wimm. (Lemnaceae). Pol. J. Environ. Stud. 2004, 13, 565–571. [Google Scholar]
- Shpakovski, G.V.; Spivak, S.G.; Berdichevets, I.N.; Babak, O.G.; Kubrak, S.V.; Kilchevsky, A.V.; Aralov, A.V.; Slovokhotov, I.Y.; Shpakovski, D.G.; Baranova, E.N.; et al. A key enzyme of animal steroidogenesis can function in plants enhancing their immunity and accelerating the processes of growth and development. BMC Plant Biol. 2017, 17, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspi, E.; Lewis, D.O.; Platak, D.M.; Thimann, K.V.; Winter, A. Biosynthesis of plant sterols. Conversion of cholesterol to pregnenolone in Digitalis purpurea. Experientia 1966, 22, 506–507. [Google Scholar] [CrossRef]
- Caspi, E.; Lewis, D.O. Progesterone: Its possible role in the biosynthesis of cardenolides in Digitalis lanata. Science 1967, 156, 519–520. [Google Scholar] [CrossRef]
- Sonawane, P.D.; Heinig, U.; Panda, S.; Gilboa, N.S.; Yona, M.; Kumar, S.P.; Alkan, N.; Unger, T.; Bocobza, S.; Pliner, M.; et al. Short-chain dehydrogenase/reductase governs steroidal specialized metabolites structural diversity and toxicity in the genus Solanum. Proc. Natl. Acad. Sci. 2018, 115, E5419–E5428. [Google Scholar] [CrossRef] [Green Version]
- Bennett, R.D.; Heftmann, E. Biosynthesis of pregnenolone from cholesterol in Haplopappusheterophyllus. Phytochemistry 1966, 5, 747–754. [Google Scholar] [CrossRef]
- Bennett, R.; Heftmann, E.; Winter, B. Conversion of sitosterol to progesterone by Digitalis Lanata. Naturwissenschaften 1969, 56, 463. [Google Scholar] [CrossRef]
- Caspi, E.; Hornby, G.M. Biosynthesis of plant sterols-III. Mechanism of saturation on ring B in pregnenolone during its conversion to digitoxigenin in Digitalis lanata. Phytochemistry 1968, 7, 423–427. [Google Scholar] [CrossRef]
- Bennett, R.D.; Heftmann, E.; Joly, R.A. Biosynthesis of diosgenin from 26 hydroxy cholesterol in dioscorea. Phytochemistry 1970, 9, 349–353. [Google Scholar] [CrossRef]
- Heftmann, E. Functions of sterols in plants. Lipids 1971, 6, 128–133. [Google Scholar] [CrossRef]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D., Jr.; Steffens, G.L.; Flippen-Anderson, J.L.; Carter Cook, J., Jr. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Yokota, T.; Arima, M.; Takahashi, N. Castasterone, a new phytosterol with planthormone potency from chestnut insect gall. Tetrahedron Lett. 1982, 23, 1275–1278. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Arora, H.K.; Nagar, P.K.; Thukral, A.K. Brassinosteroids-A novel group of plant hormones. In Plant Molecular Physiology-Current Scenario and Future Projections; Trivedi, P.C., Ed.; Aaviskar Publisher: Jaipur, India, 2006; pp. 58–84. [Google Scholar]
- Bhardwaj, R.; Kaur, S.; Nagar, P.K.; Arora, H.K. Isolation and characterization of brassinosteroids from immature seeds of Camellia sinensis(O) Kuntze. Plant Growth Regul. 2007, 53, 1–5. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. Brassinosteriods: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Netto, A.B.; Schaefer, S.; Galagovsky, L.R.; Ramirez, J.A. Brassinosteroid-driven modulation of stem elongation and apical dominance: Applications in micropropagation. In Brassinosteroids; Springer: Dordrecht, The Netherlands, 2003; pp. 129–157. [Google Scholar]
- Nakamura, A.; Higuchi, K.; Goda, H.; Fujiwara, M.T.; Sawa, S.; Koshiba, T.; Shimada, Y.; Yoshida, S. Brassinolide induces IAA5, IAA19, and DR5, a synthetic auxin response element in Arabidopsis, implying a cross talk point of brassinosteroid and auxin signaling. Plant Physiol. 2003, 133, 1843–1853. [Google Scholar] [CrossRef]
- Khripach, V.A.; Zhabinskii, V.N.; de Groot, A. Twenty years of Brassinosteriods: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000, 86, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Clouse, S.D. Brassinosteroids. Arab. Book Am. Soc. Plant Biol. 2011, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, P. Brassinosteroid-Mediated Stress Responses. J. Plant Growth Regul. 2003, 22, 289–297. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Bali, S.; Khanna, K.; Bakshi, P.; Sharma, P.; Sharma, A.; Verma, V.; Ohri, P.; Mir, B.A.; Kaur, R.; et al. A Current Scenario on Role of Brassinosteroids in Plant Defense Triggered in Response to Biotic Challenges. In Brassinosteroids: Plant Growth and Development; Springer: Singapore, 2019; pp. 367–388. [Google Scholar]
- Xia, X.J.; Huang, L.F.; Zhou, Y.H.; Mao, W.H.; Shi, K.; Wu, J.X.; Asami, T.; Chen, Z.; Yu, J.Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A. Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch. Environ. Contam. Toxicol. 2010, 60, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Divi, U.K.; Rahman, T.; Krishna, P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Sharma, I.; Bhardwaj, R.; Pati, P.K. Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J. Plant Growth Regul. 2015, 34, 509–518. [Google Scholar] [CrossRef]
- Swaczynova, J.; Sisa, M.; Hnilickova, J.; Kohout, L.; Strnad, M. Synthesis, biological, immunological and anticancer properties of a new brassinosteroid ligand. Pol. J. Chem. 2006, 80, 629–636. [Google Scholar]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [Green Version]
- Peres, A.L.G.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malíkova, J.; Swaczynova, J.; Kolar, Z.; Strnad, M. Anticancer and antiproliferative activity of natural brassinosteroids. Phytochemistry 2008, 69, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Oklestkova, J.; Hoffmannova, L.; Steigerova, J.; Kohout, L.; Kolar, Z.; Strnad, M. Natural Brassinosteroids for Use for Treating Hyperproliferation, Treating Proliferative Diseases and Reducing Adverse Effects of Steroid Dysfunction in Mammals, Pharmaceutical Composition and Its Use. U.S. Patent No. 20100204460, 20 August 2008. [Google Scholar]
- Obakan, P.; Barrero, C.; Coker-Gurkan, A.; Arisan, E.D.; Merali, S.; Palavan-Unsal, N. SILAC-based mass spectrometry analysis reveals that epibrassinolide induces apoptosis via activating endoplasmic reticulum stress in prostate cancer cells. PLoS ONE 2015, 10, e0135788. [Google Scholar] [CrossRef]
- Esposito, D.; Rathinasabapathy, T.; Schmidt, B.; Shakarjian, M.P.; Komarnytsky, S.; Raskin, I. Acceleration of cutaneous wound healing by brassinosteroids. Wound Repair Regen. 2013, 21, 688–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Han, Z.; Chai, J. Q&A: What are brassinosteroids and how do they act in plants? BMC Biol. 2016, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Fedina, E.; Yarin, A.; Mukhitova, F.; Blufard, A.; Chechetkin, I. Brassinosteroid induced changes of lipid composition in leaves of Pisum sativum L. during senescence. Steroids 2017, 117, 25–28. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A. Phytoestrogens, phytosteroids and saponins in vegetables: Biosynthesis, functions, health effects and practical applications. Adv. Food Nutr. Res. 2019, 90, 351–421. [Google Scholar]
- Pavlovic, I.; Petrik, I.; Tarkowska, D.; Lepedus, H.; Bok, V.V.; Brkanac, S.R. Correlations between phytohormones and drought tolerance in selected Brassica crops: Chinese cabbage, white cabbage and kale. Int. J. Mol. Sci. 2018, 19, 2866. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.; Yokota, T.; Adam, G.; Takahashi, N. Castasterone and brassinoliddein Raphanus sativus seeds. Phytochemistry 1991, 30, 364–365. [Google Scholar] [CrossRef]
- Han, J.-H.; Yang, Y.-X.; Feng, M.-Y. Contents of phytosterols in vegetables andfruits commonly consumed in China. Biomed. Environ. Sci. 2008, 21, 449–453. [Google Scholar] [CrossRef]
- Piironen, V.; Toivo, J.; Puupponen-Pimi€a, R.; Lampi, A.-M. Plant sterols in vegetables, fruits and berries. J. Sci. Food Agric. 2003, 83, 330–337. [Google Scholar] [CrossRef]
- Zubay, G. In vitrosynthesis of protein in microbial systems. Annu. Rev. Genet. 1973, 7, 267–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Wang, Z.Y. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Biol. 2010, 61, 681–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–55. [Google Scholar] [CrossRef]
- Rarova, L.; Zahler, S.; Liebl, J.; Krystof, V.; Sedlak, D.; Bartunek, P.; Kohout, L.; Strnad, M. Brassinosteroids inhibit in vitro angiogenesis in human endothelial cells. Steroids 2012, 77, 1502–1509. [Google Scholar] [CrossRef]
- Steigerova, J.; Oklestkova, J.; Levkova, M.; Rarova, L.; Kolar, Z.; Strnad, M. Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chem. Interactions 2010, 188, 487–496. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Ramírez, J.A.; Talarico, L.B.; Galagovsky, L.R.; Coto, C.E. Antiviral activity of natural and synthetic brassinosteroids. Curr. Med. Chem. Anti-Infect. Agents 2004, 3, 163–179. [Google Scholar] [CrossRef]
- Sasse, J.M. Physiological actions of brassinosteroids: An update. J. Plant Growth Regul. 2003, 22, 276–288. [Google Scholar] [CrossRef]
- Michelini, F.M.; Zorrilla, P.; Robello, C.; Alche, L.E. Immunomodulatory activity of an anti-HSV-1 synthetic stigmastaneanalog. Bioorganic Med. Chem. 2013, 21, 560–568. [Google Scholar] [CrossRef]
- Mehtiev, A.R.; Misharin, A.Y. Biological activity of phytosterols and their derivatives. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2008, 2, 1–17. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal plants: their use in anticancer treatment. Int. J. Pharma. Sci. Res. 2015, 6, 4103. [Google Scholar] [CrossRef]
- Kaushik, P.; Pahwa, P.; Kaushik, P.P. A Comprehensive Review on Medicinal Plants with Anticancer Activity. Global J. Pharma Edu. Res. 2018, 3. [Google Scholar] [CrossRef]
- Zhabinskii, V.N.; Khripach, N.B.; Khripach, V.A. Steroid plant hormones: effects outside plant kingdom. Steroids 2015, 97, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; Michelini, F.M.; Galagovsky, L.R.; Berra, A.; Alche, L.E. Antiangiogenic Brassinosteroid Compounds. U.S. Patent 2013088400, 17 November 2015. [Google Scholar]
- Denmeade, S.R.; Lin, X.S.; Isaacs, J.T. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate 1996, 28, 251–265. [Google Scholar] [CrossRef]
- Weisburger, J.H. Worldwide prevention of cancer and other chronic diseases based on knowledge of mechanisms. Mutat. Res. Mol. Mech. Mutagen. 1998, 402, 331–337. [Google Scholar] [CrossRef]
- Parl, F.F. Estrogens, Estrogen Receptor and Breast Cancer; IOS Press: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Franek, F.; Eckschlager, T.; Kohout, L. 24-Epibrassinolide at subnanomolar concentrations modulates growth and production characteristics of a mouse hybridoma. Collect. Czechoslov. Chem. Commun. 2003, 68, 2190–2200. [Google Scholar] [CrossRef]
- Steigerova, J.; Rarova, L.; Oklestkova, J.; Krizova, K.; Levkova, M.; Svachova, M.; Kolar, Z.; Strnad, M. Mechanisms of natural brassinosteroid-induced apoptosis of prostate cancer cells. Food Chem. Toxicol. 2012, 50, 4068–4076. [Google Scholar] [CrossRef]
- Obakan, P.; Arisan, E.D.; Calcabrini, A.; Agostinelli, E.; Bolkent, S.; Palavan-Unsal, N. Activation of polyamine catabolic enzymes involved in diverse responses against epibrassinolide-induced apoptosis in LNCaP and DU145 prostate cancer cell lines. Amino Acids 2014, 46, 553–564. [Google Scholar] [CrossRef]
- Wu, Y.D.; Lou, Y.J. Brassinolide, a plant sterol from pollen of Brassica napus L., induces apoptosis in human prostate cancer PC-3 cells. Pharmazie 2007, 62, 392–395. [Google Scholar]
- Coskun, D.; Obakan, P.; Arisan, E.D.; Çoker-Gürkan, A.; Palavan-Ünsal, N. Epibrassinolide alters PI3K/MAPK signaling axis via activating Foxo3a-induced mitochondria-mediated apoptosis in colon cancer cells. Exp. Cell Res. 2015, 338, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Rárová, L.; Sedlák, D.; Oklestkova, J.; Steigerová, J.; Liebl, J.; Zahler, S.; Bartůněk, P.; Kolář, Z.; Kohout, L.; Kvasnica, M.; et al. The novel brassinosteroid analog BR4848 inhibits angiogenesis in human endothelial cells and induces apoptosis in human cancer cells in vitro. J. Steroid Biochem. Mol. Biol. 2018, 178, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.R.; Elbert, T.; Keri, R.S. Labelling of brassinosteroids by isotopes of hydrogen and carbon. RSC Adv. 2015, 5, 39726–39745. [Google Scholar] [CrossRef] [Green Version]
- Calil, I.P.; Fontes, E.P. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2016, 119, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Liebrand, T.W.H.; van den Burg, H.A.; Joosten, M.H.A.J. Two for all: Receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 2014, 19, 123–132. [Google Scholar] [CrossRef]
- Kørner, C.J.; Klauser, D.; Niehl, A.; Domínguez-Ferreras, A.; Chinchilla, D.; Boller, T.; Heinlein, M.; Hann, D.R. The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol. Plant Microbe Interact. 2013, 26, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Nakashita, H.; Yasuda, M.; Nitta, T.; Asami, T.; Fujioka, S.; Arai, Y.; Sekimata, K.; Takatsuto, S.; Yamaguchi, I.; Yoshida, S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003, 33, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Gou, X.; He, K.; Xi, D.; Du, J.; Lin, H.; Li, J. BAK1 and BKK1 in Arabidopsis thaliana confer reduced susceptibility to turnip crinkle virus. Eur. J. Plant Pathol. 2010, 127, 149–156. [Google Scholar] [CrossRef]
- Zhang, D.W.; Deng, X.G.; Fu, F.Q.; Lin, H.H. Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta 2015, 241, 875–885. [Google Scholar] [CrossRef]
- Deng, X.G.; Zhu, T.; Peng, X.J.; Xi, D.H.; Guo, H.; Yin, Y.; Zhang, D.W.; Lin, H.H. Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci. Rep. 2016, 6, 20579. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, M.B.; Castilla, V. Antiviral properties of brassinosteroids. In Brassinosteroids: Practical Applications in Agriculture and Human Health; Bentham Science Publishers: Sharjah, UAE, 2012; pp. 57–71. [Google Scholar]
- Wachsman, M.B.; Ramirez, J.A.; Galagovsky, L.R.; Coto, C.E. Antiviral activity of brassinosteroids derivatives against measles virus in cell cultures. Antivir. Chem. Chemother. 2002, 13, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Romanutti, C.; Castilla, V.; Coto, C.E.; Wachsman, M.B. Antiviral effect of a synthetic brassinosteroid on the replication of vesicular stomatitis virus in Vero cells. Int. J. Antimicrob. Agents 2007, 29, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Castilla, V.; Larzabal, M.; Sgalippa, N.A.; Wachsman, M.B.; Coto, C.E. Antiviral mode of action of a synthetic brassinosteroid against Junin virus replication. Antivir. Res. 2005, 68, 88–95. [Google Scholar] [CrossRef]
- Zou, L.J.; Deng, X.G.; Zhang, L.E.; Zhu, T.; Tan, W.R.; Muhammad, A.; Zhu, L.J.; Zhang, C.; Zhang, D.W.; Lin, H.H. Nitric oxide as a signaling molecule in brassinosteroid-mediated virus resistance to Cucumber mosaic virus in Arabidopsis thaliana. Physiol. Plant. 2018, 163, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Petrera, E.; Níttolo, A.G.; Alche, L.E. Antiviral Action of Synthetic Stigmasterol Derivatives on Herpes Simplex Virus Replication in Nervous Cells In Vitro. BioMed Res. Int. 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wickham, S.; Carr, D.J. Molecular mimicry versus bystander activation: Herpetic stromal keratitis. Autoimmunity 2004, 37, 393–397. [Google Scholar] [CrossRef]
- Toma, H.S.; Murina, A.T.; Areaux, R.G., Jr.; Neumann, D.M.; Bhattacharjee, P.S.; Foster, T.P.; Kaufman, H.E.; Hill, J.M. Ocular HSV-1 latency, reactivation and recurrent disease. Semin. Ophthalmol. 2008, 23, 249–273. [Google Scholar] [CrossRef]
- Jiang, X.; Chentoufi, A.A.; Hsiang, C.; Carpenter, D.; Osorio, N.; BenMohamed, L.; Fraser, N.W.; Jones, C.; Wechsler, S.L. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J. Virol. 2011, 85, 2325–2332. [Google Scholar] [CrossRef] [Green Version]
- Sabah, M.; Mulcahy, J.; Zeman, A. Herpes simplex encephalitis. Br. Med. J. 2012, 344, e3166. [Google Scholar] [CrossRef]
- Kamei, S.; Sekizawa, T.; Shiota, H.; Mizutani, T.; Itoyama, Y.; Takasu, T.; Hirayanagi, K. Evaluation of combination therapy using aciclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1544–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, S.; Taira, N.; Ishihara, M.; Sekizawa, T.; Morita, A.; Miki, K.; Itoyama, Y. Prognostic value of cerebrospinal fluid cytokine changes in herpes simplex virus encephalitis. Cytokine 2009, 46, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Castilla, V.; Ramirez, J.; Coto, C.E. Plant and animal steroids a new hope to search for antiviral agents. Curr. Med. Chem. 2010, 17, 1858–1873. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Teme Centurion, O.M.; Gros, E.G.; Galagovsky, L.R. Synthesis and bioactivity evaluation of brassinosteroid analogs. Steroids 2000, 65, 329–337. [Google Scholar] [CrossRef]
- Lan, W.; Petznick, A.; Heryati, S.; Rifada, M.; Tong, L. Nuclear Factor-κB: Central regulator in ocular surface inflammation and diseases. Ocul. Surf. 2012, 10, 137–148. [Google Scholar] [CrossRef]
- Berra, A.; Michelini, F.M.; Ramirez, J.; Galagovsky, L.; Alche, L. In vitro and in vivo Anti-Herpetic and Anti-Inflammatory Activities of a New Synthetic Brassinosteroid Analogue. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5519. [Google Scholar]
- Michelini, F.M.; Ramirez, J.A.; Berrac, A.; Galagovsky, L.R.; Alche, L.E. Anti-herpetic and anti-inflammatory activities of two new synthetic 22,23-dihydroxylated stigmastane derivatives. J. Steroid Biochem. Mol. Biol. 2008, 111, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Van Furth, R.; Cohn, Z.A.; Hirsch, J.G.; Humphrey, J.H.; Spector, W.G.; Langevoort, H.L. The mononuclear phagocyte system: A new classification of macrophages, monocytes, and their precursor cells. Bull. World Heal. Organ. 1972, 46, 845–852. [Google Scholar]
- Ramirez, S.H.; Reichenbach, N.L.; Fan, S.; Rom, S.; Merkel, S.F.; Wang, X.; Ho, W.Z.; Persidsky, Y. Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. J. Leukoc. Biol. 2013, 93, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Khripach, V.A.; Zhabinskii, V.N.; Konstantinova, O.V.; Khripach, N.B.; Antonchik, A.V.; Antonchik, A.P.; Schneider, B. Preparation of (25R)- and (25S)-26-functionalized steroids as tools for biosynthetic studies of cholic acids. Steroids 2005, 70, 551–562. [Google Scholar] [CrossRef]
- Khripach, V.A.; Sviridov, O.V.; Litvinovskaya, R.P.; Pryadko, A.G.; Drach, S.V.; Zhabinskii, V.N. Analysis of brassinosteroids. Pol. J. Chem. 2006, 80, 651–654. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Hendricks, R.L.; Charukamnoetkanok, P. Management of Herpes Simplex Virus Stromal Keratitis: An Evidence-based Review. Surv. Ophthalmol. 2009, 54, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Michelini, F.M.; Ramirez, J.A.; Berra, A.; Galagovsky, L.R.; Alche, L.E. In vitroand in vivoantiherpetic activity of three new synthetic brassinosteroid analogues. Steroids 2004, 69, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pshenichnaya, L.A.; Khripach, V.A.; Volynetz, A.P.; Prokkhorchik, R.A.; Manzhelesova, N.E.; Morozik, G.V. Brassinosteroids and resistance of barley plants to leaf diseases. In Problems of Experimental Botany; Parfenov, V.I., Ed.; Byelorussian Science: Minsk, Belarus, 1997; pp. 210–217. [Google Scholar]
- Roth, U.; Friebe, A.; Schnabl, H. Resistance induction in plants by a brassinosteroid-containing extract of LychnisViscaria L. Z. Naturfor. J. Biosci. 2000, 55, 552–559. [Google Scholar]
- Bibi, N.; Ahmed, I.M.; Fan, K.; Dawood, M.; Li, F.; Yuan, S.; Wang, X. Role of brassinosteroids in alleviating toxin-induced stress of Verticillium dahliae on cotton callus growth. Environ. Sci. Pollut. Res. 2017, 24, 12281–12292. [Google Scholar] [CrossRef] [PubMed]
- Bibi, N.; Fan, K.; Dawood, M.; Nawaz, G.; Yuan, S.; Xuede, W. Exogenous application of epibrassinolide attenuated Verticillium wilt in upland cotton by modulating the carbohydrates metabolism, plasma membrane ATPases and intracellular osmolytes. Plant Growth Regul. 2014, 73, 155. [Google Scholar] [CrossRef]
- Zhua, Z.; Zhanga, Z.; Qina, G.; Tiana, S. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2010, 56, 50–55. [Google Scholar] [CrossRef]
- Ali, S.S.; Kumar, G.S.; Khan, M.; Doohan, F.M. Brassinosteroid enhances resistance to fusarium diseases of barley. Phytopathology 2013, 103, 1260–1267. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.J.; Tai, S.S.K.; Peng, C.C.; Tzen, J.T.C. Steroleosin, a sterol-binding dehydrogenase in seed oil bodies. Plant Physiol. 2002, 128, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Churikova, V.V.; Vladimirova, I.N. Effect of epibrassinolide on activity of enzymes of oxidative metabolism of cucumber in peronosporousepiphytotia conditions. In Plant Growth and Development Regulators, Moscow; Khripach, V.A., Zhabinskii, V.N., de Groot, A.E., Eds.; Academic Press: Moscow, USA, 1997. [Google Scholar]
- Korableva, N.P.; Platonova, T.A.; Dogonadze, M.Z.; Evsunina, A.S. Brassinolide effect on growth of apical meristems, ethylene production, and abscisic acid content in potato tubers. Biol. Plant 2002, 45, 39–43. [Google Scholar] [CrossRef]
- Hoshi, T.; Yamada, K.; Fuji, S.; Furuya, H.; Yoshizawa, Y.; Oh, K. Antifungal activity of brassinosteroid biosynthesis inhibitors yucaizol derivatives against Magnaportheoryzae. Can. J. Pure Appl. Sci. 2015, 9, 3333–3338. [Google Scholar]
- Hoshi, T.; Yamada, K.; Yoshizawa, Y.; Oh, K. Structure-activity relationship study for fungicidal activity of 1-(4-phenoxymethyl-2-phenyl-(1,3)dioxolan-2-ylmethyl)-1H-1,2,4-triazole derivatives against rice blast. J. Plant Prot. Res. 2015, 55, 383–388. [Google Scholar] [CrossRef]
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Long, L.; Zhu, L.F.; Xu, L.; Gao, W.H.; Sun, L.Q.; Liu, L.L.; Zhang, X.L. Proteomic and virus-induced gene silencing (VIGS) analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol. Cell. Proteom. 2013, 12, 3690–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.S.; Gunupuru, L.R.; Kumar, G.S.; Khan, M.; Scofield, S.; Nicholson, P.; Doohan, F.M. Plant disease resistance is augmented in uzu barley lines modified in the brassinosteroid receptor BRI1. BMC Plant Biol. 2014, 14, 227. [Google Scholar] [CrossRef] [Green Version]
- Vleesschauwer, D.; Van Buyten, E.; Satoh, K.; Balidion, J.; Mauleon, R.; Choi, I.R.; Vera-Cruz, C.; Kikuchi, S.; Höfte, M. Brassinosteroids antagonize gibberellin-and salicylate-mediated root immunity in rice. Plant Physiol. 2012, 158, 1833–1846. [Google Scholar] [CrossRef] [Green Version]
- Skoczowski, A.; Janeczko, A.; Gullner, G.; Tóbias, I.; Kornas, A.; Barna, B. Response of brassinosteroid-treated oilseed rape cotyledons to infection with the wild type and HR-mutant of Pseudomonas syringae or with P. fluorescence. J. Therm. Anal. Calorim. 2010, 104, 131–139. [Google Scholar] [CrossRef]
- Schumacher, K.; Chory, J. Brassinosteroid signal transduction: Still casting the actors. Curr. Opin. Plant Biol. 2000, 3, 79–84. [Google Scholar] [CrossRef]
- Konstantopoulos, K. Editorial Hot Topic: Molecular biology-pathophysiology of inflammation and autoinflammation. Curr. Drug Target Inflammation Allergy 2005, 4, 1–39. [Google Scholar] [CrossRef]
- Patel, S.S.; Savjani, J.K. Systematic review of plant steroids as potential anti-inflammatory agents: Current status and future perspectives. J. Phytopharmacol. 2015, 4, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Alché, L.E.; Michelini, F.M. Antiherpetic and Anti-Inflammatory Activities of Novel Synthetic Brassinosteroids Analogs. In Brassinosteroids: Practical Applications in Agriculture and Human Health; Pereira-Netto, A.B., Ed.; Bentham Science Publishers: Curitiba-PR, Brazil, 2012; pp. 72–83. [Google Scholar]
- Moreno-Anzurez, N.E.; Marquina, S.; Alvarez, L.; Zamilpa, A.; Castillo-España, P.; Perea-Arango, I.; Torres, P.N.; Herrera-Ruiz, M.; García, E.R.D.; García, J.T.; et al. A Cytotoxic and Anti-inflammatory Campesterol Derivative from Genetically Transformed Hairy Roots of Lopeziaracemosa Cav. (Onagraceae). Molecules 2017, 22, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, D.; Tuazon, M.; Henderson, G.C.; Komarnytsky, S.; Raskin, I. Brassinosteroid enhances C57BL/6J mice treadmill endurance. FASEB J. 2012, 26, 1121–1128. [Google Scholar] [CrossRef]
- Raskin, I.; Esposito, D.; Komarnytsky, S.; Rathinasabapathy, T.; Rojo Castillo, L. Methods of Producing and using Brassinosteroids to Promote Growth, Repair, and Maintenance of Skeletal Muscle and Skin. U.S. Patent AU2011343970A1, 21 June 2012. [Google Scholar]
- Quiñones, J.P.; García, Y.C.; Curiel, H.; Covas, C.P. Microspheres of chitosan for controlled delivery of brassinosteroids with biological activity as chemicals. Carbohydr. Polym. 2010, 80, 915–921. [Google Scholar] [CrossRef]
- Ferrari, I.; Michelini, F.; Berra, M.; Alche, L.; Aguinaga, H.; Berra, A. In vitro and in vivo Anti-Adenovirus and Anti-Inflammatory Activities of a New Synthetic Brassinosteroid Analogue. Inves. Ophthalmol. Vis. Sci. 2009, 50, 3098. [Google Scholar] [CrossRef]
- Shen, X.; Hong, F.; Nguyen, V.A.; Gao, B. IL-10 attenuates IFN- K-activated STAT1 in the liver: Involvement of SOCS2 and SOCS3. FEBS J. 2000, 480, 132–136. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Liu, Z.; Huang, J.; Liu, T. Therapeutic effect of 28-homobrassinolide on leukotriene synthesis in leukemia cells. Cytotechnology 2017. [Google Scholar] [CrossRef]
- Song, L.; Qu, D.; Zhang, Q.; Jiang, J.; Zhou, H.; Jiang, R.; Li, Y.; Zhang, Y.; Yan, H. Phytosterol esters attenuate hepatic steatosis in rats with nonalcoholic fatty liver disease rats fed a high-fat diet. Sci. Rep. 2017, 7, 41604. [Google Scholar] [CrossRef]
- Athithan, V.; Ramesh, R.; Srikumar, K. 28-Homocastasterone: A Novel Dietary Phyto Keto Oxysterol Modulating Testicular Steroid Metabolism AndLxrMrna Expression in Diabetic Rat. Int. J. Pharm. Pharm. Sci. 2018, 10, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Pillay, S.; Byrne, H.M.; Maini, P.K. Modeling angiogenesis: A discrete to continuum description. Phys. Rev. E. 2017, 95, 012410. [Google Scholar] [CrossRef] [Green Version]
- Panibrat, O.V.; Zhabinskii, V.N.; Khripach, V.A. Anticancer Potential of Brassinosteroids. In Brassinosteroids: Plant Growth and Development; Springer: Singapore, 2019; pp. 389–406. [Google Scholar]
- Oklestkova, J.; Rarova, L.; Kvasnica, M.; Strnad, M. Brassinosteroids: Synthesis and biological activities. Phytochem. Rev. 2015, 14, 1053–1072. [Google Scholar] [CrossRef]
- Akhtar, J.; Tiwari, V.; Oh, M.J.; Kovacs, M.; Jani, A.; Kovacs, S.K.; Valyi-Nagy, T.; Shukla, D. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Investigative ophthalmology & visual science. Investig. Opthalmology Vis. Sci. 2008, 49, 4026–4035. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Agarwal, R. Tumor angiogenesis: A potential target in cancer control by phytochemicals. Curr. Cancer Drug Targets. 2003, 3, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Singh, R.P. Tumor angiogenesis—A potential target in cancer chemoprevention. Food Chem. Toxicol. 2008, 46, 1334–1345. [Google Scholar] [CrossRef]
- Pietras, R.J.; Weinberg, O.K. Antiangiogenic steroids in human cancer therapy. Adv. Access Pub. 2005, 2, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Winer, J.; Burton, T.; Rowland, A.; Siegel, M.; Phillips, H.S.; Terrell, T.; A Keller, G.; Levinson, A.D. Expression of vascular endothelial growth factor does not promote transformation but confers a growth advantage in vivo to Chinese Hanster Ovary cells. J. Clin. Investig. 1993, 91, 160–170. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, G.; Struman, I.; Martial, J.; Weiner, R. Activation of mitogen activated protein kinases by vascular endothelial growth factor and basic fibroblast growth factor in capillary endothelial cells is inhibited by the antiangiogenic factor 16-kd N-terminal fragment of prolactin. Proc. Natl. Acad. Sci. USA 1995, 92, 6374–6378. [Google Scholar] [CrossRef] [Green Version]
- Eckhardt, S.G. Angiogenesis inhibitors as cancer therapy. Hosp. Pract. 1999, 34, 63–84. [Google Scholar] [CrossRef]
- Akhter, S.; Nath, S.K.; Tse, C.M.; William, J.; Zasloff, M.; Donowitz, M. Squalamine, a novel cationic steroid, specifically inhibits the brushborder Na+/H+ exchanger isoform NHE3. Am. J. Physiol. Content 1999, 276, 136–144. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sharma, I.; Kanwar, M.; Handa, N.; Kapoor, D. Current Scenario of Applications of Brassinosteroids in Human Welfare. In Brassinosteroids: Practical Applications in Agriculture and Human Health; Pereira-Netto, A.B., Ed.; Bentham ebooks: Brazil, 2012; pp. 3–15. Available online: https://www.researchgate.net/publication/286318186_Current_Scenario_of_Applications_of_Brassinosteroids_in_Human_Welfare (accessed on 9 April 2020).
- Michelini, F.M.; Lombardi, M.G.; Bueno, C.A.; Berra, A.; Sales, M.E.; Alché, L.E. Synthetic stigmasterol derivatives inhibit capillary tube formation, herpetic corneal neovascularization and tumor induced angiogenesis: Antiangiogenic stigmasterol derivatives. Steroids 2016, 115, 160–168. [Google Scholar] [CrossRef]
- Hoffmannová, L. A Study of Molecular and Cellular Activities of Brassinosteroids and their Derivatives. Available online: https://theses.cz/id/nqsrld/130025-215872175.pdf (accessed on 5 April 2020).
- Bajguz, A.; Bajguz, A.J.; Tryniszewska, E.A. Recent advances in medicinal applications of brassinosteroids, a group of plant hormones. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 33–49. [Google Scholar]
- Onatskiy, N.M.; Marchenko, A.I.; Mikhina, L.V. Technical Report for Evaluation of Mutagenic Activity of Epibrassinolide (Active Ingredient of Epin) in Ames Test, Chromosome Aberrations and in Micronuclear Tests; Scientific Research Centre of Toxicologie and Hygienic Regulation of Biopreparations of Russia: Serpukhov, Russia, 1997. [Google Scholar]
- Howell, W.M.; Keller, G.E.; Kirkpatrick, J.D.; Jenkins, R.L.; Hunsinger, R.N.; McLaughlin, E.W. Effects of the plant steroidal hormone, 24-epibrassinolide, on the mitotic index and growth of onion (Allium cepa) root tips. Genet. Mol. Res. 2007, 6, 50–58. [Google Scholar] [PubMed]
- Sondhi, N.P.; Bhardwaj, R.; Kaur, S.; Kumar, N.; Singh, B. Isolation of 24-epibrassinolide from leaves of Aegle marmelos and evaluation of its antigenotoxicity employing Allium cepa chromosomal aberration assay. Plant Growth Regul. 2008, 54, 217–224. [Google Scholar] [CrossRef]
- Sondhi, N.; Bhardwaj, R.; Kaur, S.; Chandel, M.; Kumar, N.; Singh, B. Inhibition of H2O2-induced DNA damage in single cell gel electrophoresis assay (comet assay) by castasterone isolated from leaves of Centellaasiatica. Health 2010, 2, 595. [Google Scholar] [CrossRef] [Green Version]
- Moghadasian, M.H. Pharmacological properties of plant sterols in vivo andin vitro observations. Life Sci. 2000, 67, 605–615. [Google Scholar] [CrossRef]
- Schroepfer, G.J. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef]
- Vriet, C.; Russinova, E.; Reuzeau, C. From squalene to brassinolide: The steroid metabolic and signaling pathways across the plant kingdom. Mol. Plant 2013, 6, 1738–1757. [Google Scholar] [CrossRef] [Green Version]
- Schaller, H. The role of sterols in plant growth and development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Kashtanova, N.N.; Duchovskis, P. Changes in maize seedlings growth and membrane permeability under the effect of epibrassinolide and heavy metals. Russ. Agric. Sci. 2013, 39, 307–310. [Google Scholar] [CrossRef]
- Khripach, V.; Altsivanovich, K.; Zhabinskii, V.; Samusevich, M. Method for Decreasing Cholesterol Level in Blood. U.S. Patent US6998397B2, 14 February.
- Kitron, A.; Pergamentz, R. Brassinosteroids for use in treating prostatic hyperplasia and androgenic alopecia. U.S. Patent WO2010064242A1, 10 June 2010. [Google Scholar]
- Statsenko, E.A.; Korolevich, M.P.; Seregkina, T.V.; Paramonova, N.A.; Ostapenko, V.A.; Ryibkina, I.L. Methods of correction of lipid metabolism in athletes. Voennaya Medicina (Mil. Med.) 2008, 9, 102–104. [Google Scholar]
- Statsenko, E.A. Prophylactic and Correction of Functional State Among Athletes of High Qualifying Categories under Training Process. Ph.D. Thesis, Federal Scientific Center of Physical Culture and Sport, Moscow, Russia, 2013. [Google Scholar]
- Mohan, R.; Heyman, R.A. Orphan nuclear receptor modulators. Curr. Top. Med. Chem. 2003, 3, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Koolman, J. Ecdysteroid receptors of the blowfly Calliphoravicina: Partial purification and characterization of ecdysteroid binding. Mol. Cell. Endocrinol. 1988, 57, 239–249. [Google Scholar] [CrossRef]
- Smagghe, G.; Decombel, L.; Carton, B.; Voigt, B.; Adam, G.; Tirry, L. Action of brassinosteroids in the cotton leaf worm Spodopteralittoralis. Insect Biochem. Mol. Biol. 2002, 32, 199–204. [Google Scholar] [CrossRef]
- Richter, K.; Adam, G.; Vorbrodt, H.M. Inhibiting effect of 22S,23S-homobrassinolide on the moult of the cockroach Periplanetaamericana (L.) (Orthopt, Blattidae). J. Appl. Èntomol. 1987, 103, 532–534. [Google Scholar] [CrossRef]
- Spindler, K.D.; Spindler-Barth, M.; Turberg, A. Action of brassinosteroids on the epithelial cell line from Chironomus tentans. Zeitschrift für Naturforschung C 1992, 47, 280–284. [Google Scholar] [CrossRef]
- Lehmann, M.; Vorbrodt, H.M.; Adam, G.; Koolman, J. Antiecdysteroid activity of brassinosteroids. Experientia 1988, 44, 355–356. [Google Scholar] [CrossRef]
- Sobek, L.; Bohm, G.A.; Penzlin, H. Ecdysteroid receptors in last instar larvae of the wax moth Galleria mellonella L. Insect Biochem. Mol. Biol. 1993, 23, 125–129. [Google Scholar] [CrossRef]
- Davison, G.P.; Restrepo, R.; Martinez, G.; Coll, F.; Leon, O.S. Effects of a brassinosteroid analogue to mosquito larvae. Ecotoxicol. Environ. Saf. 2003, 56, 419–424. [Google Scholar] [CrossRef]
- Dinan, L.; Bourne, P.C.; Meng, Y.; Sarker, S.D.; Toletino, R.; Whiting, P. Assessment of natural products in the Drosophila melanogaster BII cell bioassay for ecdysteroid agonist and antagonist activities. Cell. Mol. Life Sci. 2001, 58, 321–342. [Google Scholar] [CrossRef]
- Decombel, L.; Tirry, L.; Smagghe, G. Action of 24-epibrassinolide on a cell line of the beet armyworm, Spodopteraexigua. Arch. Insect Biochem. Physiol. 2005, 58, 145–156. [Google Scholar] [CrossRef]
- Oh, K.; Kamada, H.; Yamada, K.; Yoshizawa, Y. Mosquito Larvicidal Activity of Triazole Type Brassinosteroid Biosynthesis Inhibitors. Int. J. Biosci. Biochem. Bioinform. 2016, 6, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Slama, K.; Lafont, R. Insect hormones—Ecdysteroids: Their presence and actions in vertebrates. Eur. J. Entomol. 1995, 92, 355–377. [Google Scholar]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect Sci. 2003, 3, 1–30. [Google Scholar] [CrossRef]
- Jones, A.; Pruessner, J.C.; McMillan, M.R.; Jones, R.W.; Kowalik, G.T.; Steeden, J.A.; Williams, B.; Taylor, A.M.; Muthurangu, V. Physiological adaptations to chronic stress in healthy humans–why might the sexes have evolved different energy utilisation strategies? J. Physiol. 2016, 594, 4297–4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheiko, I.P.; Budevich, A.I.; Khripach, V.A.; Smuneva, V.K.; Lebedev, S.G. Method of Increasing the Fertility of the Bull Sperm-Producer. Pat. 10768, 31 March 2008. [Google Scholar]
- Esposito, D.; Komarnytsky, S.; Shapses, S.; Raskin, I. Anabolic effect of plant brassinosteroid. FASEB J. 2011, 25, 3708–3719. [Google Scholar] [CrossRef] [Green Version]
| Viruses | (% Inhibition) | |
|---|---|---|
| 28-homocastaterone | Brassinolide | |
| Poliovirus type 1 (RNA virus) | 85 | 96 |
| Vesicular stomatitis Virus Indiana strain (RNA virus) | 23 | 100 |
| Herpes simplex virus-1 F strain (tk+) (DNA virus) | 50 | 96 |
| Herpes simplex virus-1 B2006 strain (tk−) (DNA virus) | 35 | 100 |
| Herpes simplex virus-1 G strain (tk+) (DNA virus) | 48 | 98 |
| Junin virus IV 4454 strain (RNA virus) | 79 | 74 |
| Tacaribe virus TRLV 11573 strain (RNA virus) | 99 | 55 |
| Pichinde virus AN3739 strain (RNA virus) | 98 | 67 |
| Measles virus Brasil/001/91 (RNA virus) | 50 | 100 |
| S. No. | Class of Compounds | Model System | Health Effects | Mechanism of Action | References |
|---|---|---|---|---|---|
| 1. | Brassinosteroids (28-HomoCS and 24-EpiBL) | Human Breast Cancer Cell Line, Human Prostate Cancer Cell Line | Antiproliferative activity, pro-apoptotic activity, and cell growth inhibitory responses in several human cell lines with no effect on non-tumor cell growth | Cell blockade and apoptosis of both hormone-sensitive and insensitive human breast cancer | [51,65] |
| 2. | Brassinosteroids (24-EpiBL and 24-EpiCS) | Human Cancer Cell Line | Antiproliferative, anticancer, antiangiogenic, antiviral, and antibacterial properties in the animal system | Inhibit replication of viruses in confluent with human cell culture, including cytotoxic effects in various types of cancer cells but normal human cells | [134] |
| 3. | Brassinosteroids (28-HomoCS) | RNA and DNA Viruses | Antiviral effect against RNA and DNA viruses | Limiting virus protein synthesis and mature viral particle formation | [79] |
| 4. | Stigmasterols [(22S,23S)-22,23-dihydroxystigmast-4-en-3-one, (22S,23S)-22,23-dihydroxystigmasta-1,4-dien-3-one] | Murine Macrophage Cell Line | Immuno-modulatory and neuro-protective activity | Blocked HSV-1 induced activation of NFαB by inhibiting its translocation to the nucleus of infected conneal and conjunctival cells in vitro, as well as significantly reduced the secretion of TNF-α infected NHC cells | [95] |
| 5. | Brassinosteroids (28-HomoBL and 24-EpiBL) | Human Cancer Cell Line | Anticancer bioactivities in various cell lines i.e., CEM (T-Lymophoblastic Leukemia), A549 (lung carcinoma), MCF-7 (breast carcinoma), LNCaP (prostate cancer) etc. | All these cells were found non-viable in response to 4-fold dilution for 72 h of IC50 value of BRs observed from Calcein AM assay | [36] |
| 6. | Brassinosteroids (24-EpiBL) | Human Prostate Cancer Cell Line | In vitro antiproliferative effect in the animal cell lines | Cytotoxicity in PC-3 cells activating polyamines catabolic machinery in prostate cancer cells | [66,67] |
| 7. | Brassinosteroids (24-EpiBL) | Human Colon Cancer Cell Line | Mitochondria-regulated cell death in colon cancer cells | Upregulation of Foxo3a and protein tryokinase Src p38, after the activation of P13K/AKT | [68] |
| 8. | Brassinosteroids (24-EpiBL) | Plant (Arabidopsis thaliana) | Amelioration of Turnip crinkle virus infection in Arabidopsis thaliana, BAK1 or BKK1 are essential components | Increase in activity of antioxidant enzymes and subsequent gene expression and also lowered photosystem deterioration | [76] |
| 9. | Brassinolide | Plant (Nicotiana benthamiana) | Increased resistance of Nicotiana benthamiana against TMV | BES1/BZR1 suppressed RBOHB-dependent ROS generation regulated by MEK2-SIPK signaling network | [78] |
| 10. | Brassinosteroid [(22S,23S)-3beta-bromo-5alpha,22,23-trihydroxystigmastan-6-one] | Vero Cell Line (virus growth) | Antiviral activity against Junin virus (JV) | Detrimental effect on RNA replication of trihydroxystigmastan-6-one and lately result in formation of viral glycoproteins | [82] |
| 11. | Brassinosteroids [(22S,23S)-3β-bromo-5α,22,23-trihydroxystigmastan-6-one] | Herpes Simplex Virus Cell Lines (Vero Cells) | Hampering the herpes simplex virus (HSV) type 1 replication in Vero cells by affecting the later stages of virus multiplication | Inhibition of the expression of HSV antigen and reduced the production of HSV late protein | [93] |
| 12. | Brassinosteroids (24-EpiBL) | Plants (Cucumis sativus) | 24-EpiBL minimized fungal initiated ROS and increased resistance towards Fusarium | Reduced Fusarium wilt in cucumber and enhanced their antioxidant and phenolic levels in roots | [114] |
| 13. | Brassinosteroid analogs [22S, 23S]-3β-bromo-5α, 22, 23-trihydroxystigmastan-6-one, [22S, 23S]-3β-5α, 22, 23-tetrahydroxystigmastan-6-one and [22S, 23S]-5α-fluoro-3β, 22, 23-tetrahydroxystigmastan-6-one | Herpes Simplex Virus Cell Lines (Vero Cells) | Anti-inflammatory activity and inflammation were lowered in three consecutive days | These analogues act against HSV-1 multiplication and exhibited anti-inflammatory potential in vero cells | [52] |
| 14. | Brassinosteroid (28-HomoBL Keto-isomers) | Human Conjuctival Cell Lines | Antiglycemic activity in diabetic rats | Improved RBC, WBC, platelets, hemoglobin levels, and reduced cellular damage | [127] |
| 15. | Stigmasterone derivatives [22S,23S]-22,23-dihydroxystigmast-4-en-3-one (Compound 1) and [22S,23S]-3β-bromo 5α,22,23-trihydroxystigmastan-6-one (Compound 2) | Human umbilical vein endothelial cells (HUVEC) | Prevents in vivo and in vitro angiogenesis, reduces the VEGF expression and also corneal neovascularization when applied during herpetic stromal keratitis | Retardation in migration of cells migration in human umbilical vein endothelial cells (HUVEC) and capillary tube-like structure formation | [143] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur Kohli, S.; Bhardwaj, A.; Bhardwaj, V.; Sharma, A.; Kalia, N.; Landi, M.; Bhardwaj, R. Therapeutic Potential of Brassinosteroids in Biomedical and Clinical Research. Biomolecules 2020, 10, 572. https://doi.org/10.3390/biom10040572
Kaur Kohli S, Bhardwaj A, Bhardwaj V, Sharma A, Kalia N, Landi M, Bhardwaj R. Therapeutic Potential of Brassinosteroids in Biomedical and Clinical Research. Biomolecules. 2020; 10(4):572. https://doi.org/10.3390/biom10040572
Chicago/Turabian StyleKaur Kohli, Sukhmeen, Abhay Bhardwaj, Vinay Bhardwaj, Anket Sharma, Namarta Kalia, Marco Landi, and Renu Bhardwaj. 2020. "Therapeutic Potential of Brassinosteroids in Biomedical and Clinical Research" Biomolecules 10, no. 4: 572. https://doi.org/10.3390/biom10040572
APA StyleKaur Kohli, S., Bhardwaj, A., Bhardwaj, V., Sharma, A., Kalia, N., Landi, M., & Bhardwaj, R. (2020). Therapeutic Potential of Brassinosteroids in Biomedical and Clinical Research. Biomolecules, 10(4), 572. https://doi.org/10.3390/biom10040572








