An Oral Rinse Active Matrix Metalloproteinase-8 Point-of-Care Immunotest May Be Less Accurate in Patients with Crohn’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Enrolment of Participants
2.2. Clinical Oral Examination
2.3. Application of an Oral Rinse aMMP-8 PoC Immunotest
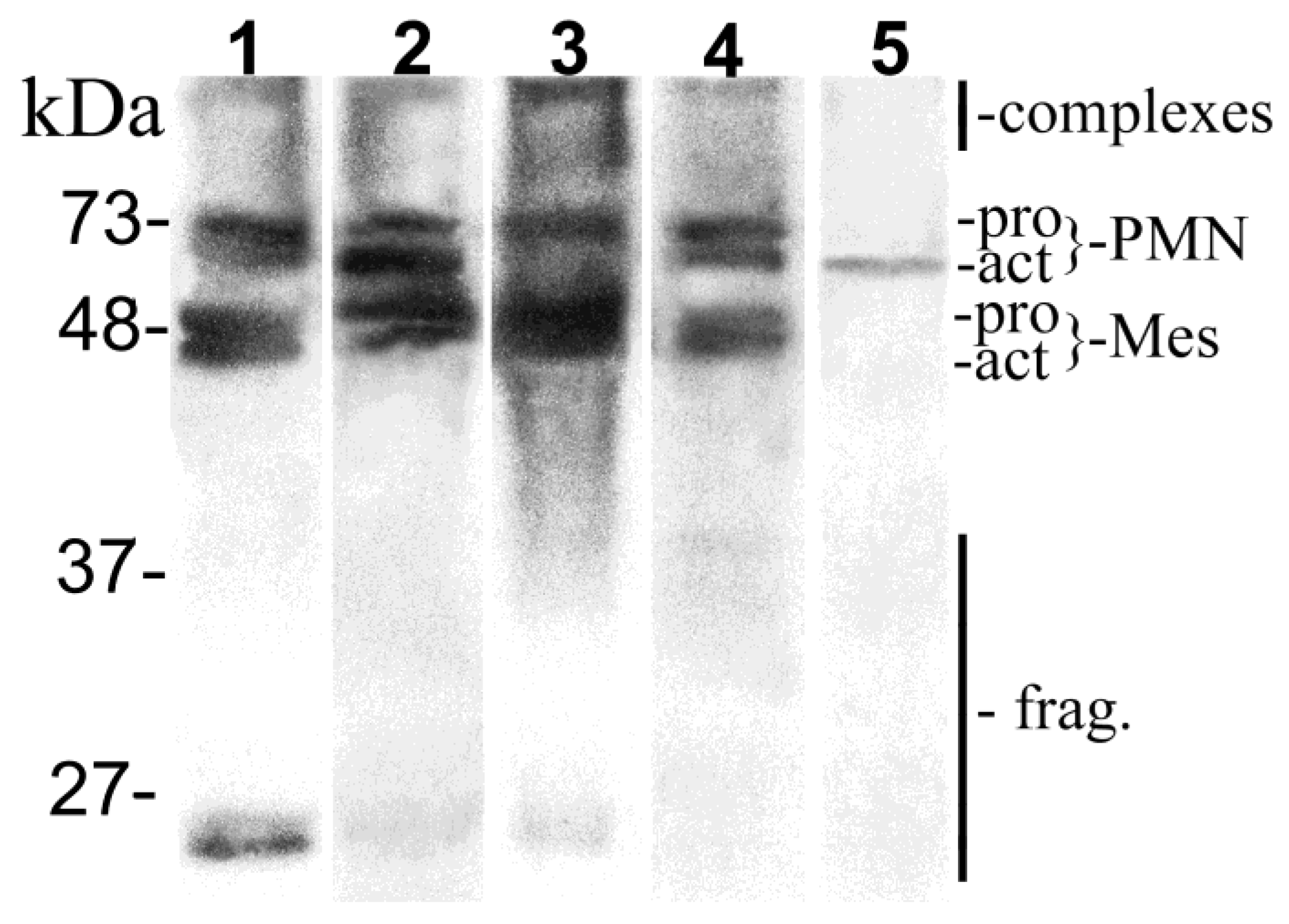
2.4. MMP-8 Western Immunoblot
2.5. Statistical Analyses
3. Results
3.1. Crohn’s Disease Status
3.2. Oral Health Status
3.3. Oral Rinse aMMP-8 PoC Immunotest
3.4. MMP-8 Immunoblot
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Conflicts of Interest
Abbreviations
References
- Papageorgiou, S.N.; Hagner, M.; Nogueira, A.V.B.; Franke, A.; Jäger, A.; Deschner, J. Inflammatory bowel disease and oral health: Systematic review and a meta-analysis. J. Clin. Periodontol. 2017, 44, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Habashneh, R.A.; Khader, Y.S.; Alhumouz, M.K.; Jadallah, K.; Ajlouni, Y. The association between inflammatory bowel disease and periodontitis among Jordanians: A case-control study. J. Periodontal. Res. 2012, 47, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.; de Barros, F.C.; Zaltman, C.; Carvalho, A.T.; Carneiro, A.J.; Fischer, R.G.; Gustafsson, A.; Figueredo, C.M. Prevalence of periodontitis and dmft index in patients with Crohn’s disease and ulcerative colitis. J. Clin. Periodontol. 2008, 35, 555–560. [Google Scholar] [CrossRef]
- Schütz, T.; Drude, C.; Paulisch, E.; Lange, K.P.; Lochs, H. Sugar intake, taste changes and dental health in Crohn’s disease. Dig. Dis. 2003, 21, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Said, H.S.; Suda, W.; Nakagome, S.; Chinen, H.; Oshima, K.; Kim, S.; Kimura, R.; Iraha, A.; Ishida, H.; Fujita, J.; et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res. 2014, 21, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Rautava, J.; Pinnell, L.J.; Vong, L.; Akseer, N.; Assa, A.; Sherman, P.M. Oral microbiome composition changes in mouse models of colitis. J. Gastroenterol. Hepatol. 2015, 30, 521–527. [Google Scholar] [CrossRef]
- Keskin, M.; Zeidán-Chuliá, F.; Gürsoy, M.; Könönen, E.; Rautava, J.; Gürsoy, U.K. Two cheers for Crohn’s disease and periodontitis: Beta-defensin-2 as an actionable target to intervene on two clinically distinct diseases. OMICS 2015, 19, 443–450. [Google Scholar] [CrossRef]
- Goethel, A.; Croitoru, K.; Philpott, D.J. The interplay between microbes and the immune response in inflammatory bowel disease. J. Physiol. 2018, 596, 3869–3882. [Google Scholar] [CrossRef]
- Somasundaram, R.; Nuij, V.J.; van der Woude, C.J.; Kuipers, E.J.; Peppelenbosch, M.P.; Fuhler, G.M. Peripheral neutrophil functions and cell signalling in Crohn’s disease. PLoS ONE 2013, 8, e84521. [Google Scholar] [CrossRef]
- Levine, A.P.; Segal, A.W. What is wrong with granulocytes in inflammatory bowel diseases? Dig. Dis. 2013, 31, 321–327. [Google Scholar] [CrossRef]
- Schmidt, J.; Weigert, M.; Leuschner, C.; Hartmann, H.; Raddatz, D.; Haak, R.; Mausberg, R.F.; Kottmann, T.; Schmalz, G.; Ziebolz, D. Active matrix metalloproteinase-8 and periodontal bacteria-interlink between periodontitis and inflammatory bowel disease? J. Periodontol. 2018, 89, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Koelink, P.J.; Overbeek, S.A.; Braber, S.; Morgan, M.E.; Henrkicks, P.A.J.; Roda, M.A.; Verspaget, H.W.; Wolfkamp, S.C.; te Velde, A.A.; Jones, C.W.; et al. Collagen degradation and neutrophilic infiltration: A vicious circle in inflammatory bowel disease. Gut 2014, 63, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Kinney, J.S.; Herr, A.E.; Braun, T.; Sugai, J.V.; Shelburne, C.A.; Rayburn, L.A.; Tran, H.M.; Singh, A.K.; Giannobile, W.V. Identification of pathogen and host-response markers correlated with periodontal disease. J. Periodontol. 2009, 80, 436–446. [Google Scholar] [CrossRef]
- Gürsoy, U.K.; Pussinen, P.J.; Salomaa, V.; Syrjäläinen, S.; Könönen, E. Cumulative use of salivary markers with an adaptive design improves detection of periodontal disease over fixed biomarker thresholds. Acta Odontol. Scand. 2018, 76, 493–496. [Google Scholar] [CrossRef]
- Sorsa, T.; Gürsoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gürsoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol. 2000 2016, 70, 142–163. [Google Scholar] [CrossRef]
- Ji, S.; Choi, Y. Point-of-care diagnosis of periodontitis using saliva: Technically feasible but still a challenge. Front. Cell Infect. Microbiol. 2015, 5, 65. [Google Scholar] [CrossRef]
- Mauramo, M.; Ramseier, A.M.; Mauramo, E.; Buser, A.; Tervahartiala, T.; Sorsa, T.; Waltimo, T. Associations of oral fluid MMP-8 with periodontitis in Swiss adult subjects. Oral Dis. 2018, 24, 449–455. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yan, H.; Huang, L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2018, 97, e9642. [Google Scholar] [CrossRef]
- Sorsa, T.; Alassiri, S.; Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Nwhator, S.O.; Gieselmann, D.-R.; Sakellari, D. Active MMP-8 (aMMP-8) as a grading and staging biomarker in the periodontitis classification. Diagnostics 2020, 10, 61, in press. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, A.M.; Nwhator, S.O.; Rathnayake, N.; Mäntylä, P.; Vatanen, P.; Sorsa, T. Pilot study on oral health status as assessed by an active matrix metalloproteinase-8 chairside mouthrinse test in adolescents. J. Periodontol. 2016, 87, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Ebersole, J.L.; Kryscio, R.J.; Danaher, R.J.; Dawson, D., 3rd; Al-Sabbagh, M.; Miller, C.S. Rapid assessment of salivary MMP-8 and periodontal disease using lateral flow immunoassay. Oral Dis. 2016, 22, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, I.T.; Heikkinen, A.M.; Siren, E.; Tervahartiala, T.; Gieselmann, D.R.; van der School, G.J.; van der Schoor, P.; Sorsa, T. Point-of-care/chairside aMMP-8 analytics of periodontal diseases activity and episodic progression. Diagnostics 2018, 8, 74. [Google Scholar] [CrossRef]
- Nwhator, S.O.; Ayanbadejo, P.O.; Umeizudike, K.A.; Opeodu, O.I.; Agbelusi, G.A.; Olamijulo, J.A.; Arowojolu, M.O.; Sorsa, T.; Babajide, B.S.; Opedun, D.O. Clinical correlates of a lateral-flow immunoassay rinse risk indicator. J. Periodontol. 2014, 85, 188–194. [Google Scholar] [CrossRef]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89, S237–S248. [Google Scholar] [CrossRef]
- Thia, K.; Faubion, W.A., Jr.; Loftus, E.V., Jr.; Persson, T.; Persson, A.; Sandborn, W.J. Short CDAI: Development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm. Bowel Dis. 2011, 17, 105–111. [Google Scholar] [CrossRef]
- Lankarani, K.B.; Sivandzadeh, G.R.; Hassanpour, S. Oral manifestation in inflammatory bowel disease: A review. World J. Gastroenterol. 2013, 19, 8571–8579. [Google Scholar] [CrossRef]
- Harty, S.; Fleming, P.; Rowland, M.; Crushell, E.; McDermott, M.; Drumm, B.; Bourke, B. A prospective study of the oral manifestations of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2005, 3, 886–891. [Google Scholar] [CrossRef]
- Sanz, M.; Bäumer, A.; Buduneli, N.; Dommisch, H.; Farina, R.; Könönen, E.; Linden, G.; Meyle, J.; Preshaw, P.M.; Quirynen, M.; et al. Effect of professional mechanical plaque removal on secondary prevention of periodontitis and the complications of gingival and periodontal preventive measures: Consensus report of group 4 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J. Clin. Periodontol. 2015, 42, S214–S220. [Google Scholar]
- American Academy of Periodontology task force report on the update to the 1999 classification of periodontal diseases and conditions. J. Periodontol. 2015, 86, 835–838. [CrossRef] [PubMed]
- Buduneli, E.; Mäntylä, P.; Emingil, G.; Tervahartiala, T.; Pussinen, P.; Barış, N.; Akıllı, A.; Atilla, G.; Sorsa, T. Acute myocardial infarction is reflected in salivary matrix metalloproteinase-8 activation level. J. Periodontol. 2011, 82, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, U.K.; Könönen, E.; Tervahartiala, T.; Gürsoy, M.; Pitkänen, J.; Torvi, P.; Suominen, A.L.; Pussinen, P.; Sorsa, T. Molecular forms and fragments of salivary MMP-8 in relation to periodontitis. J. Clin. Periodontol. 2018, 45, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Grössner-Schreiber, B.; Fetter, T.; Hedderich, J.; Kocher, T.; Schreiber, S.; Jepsen, S. Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: A case-control study. J. Clin. Periodontol. 2006, 33, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Järnerot, G.; Järnmark, I.; Nilsson, K. Consumption of refined sugar by patients with Crohn’s disease, ulcerative colitis, or irritable bowel syndrome. Scand. J. Gastroenterol. 1983, 18, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Indriolo, A.; Greco, S.; Ravelli, P.; Fagiuoli, S. What can we learn about biofilm/host interactions from the study of inflammatory bowel disease. J. Clin. Periodontol. 2011, 38, 36–43. [Google Scholar] [CrossRef]
- Stein, J.M.; Lammert, F.; Zimmer, V.; Granzow, M.; Reichert, S.; Schulz, S.; Ocklenburg, C.; Conrads, G. Clinical periodontal and microbiologic parameters in patients with Crohn’s disease with consideration of the CARD 15 genotype. J. Periodontol. 2010, 81, 535–545. [Google Scholar] [CrossRef]
- Flemmig, T.F.; Shanahan, F.; Miyasaki, K.T. Prevalence and severity of periodontal disease in patients with inflammatory bowel disease. J. Clin. Periodontol. 1991, 18, 690–697. [Google Scholar] [CrossRef]
- Rathnayake, N.; Akerman, S.; Klinge, B.; Lundegren, N.; Jansson, H.; Tryselius, Y.; Sorsa, T.; Gustafsson, A. Salivary biomarkers of oral health: A cross-sectional study. J. Clin. Periodontol. 2013, 40, 140–147. [Google Scholar] [CrossRef]
- Miller, C.S.; King, C.P., Jr.; Langub, M.C.; Kryscio, R.J.; Thomas, M.V. Salivary biomarkers of existing periodontal disease: A cross-sectional study. J. Am. Dent. Assoc. 2006, 137, 322–329. [Google Scholar] [CrossRef]
- Sexton, W.M.; Lin, Y.; Kryscio, R.J.; Dawson, D.R., 3rd; Ebersole, J.L.; Miller, C.S. Salivary biomarkers of periodontal disease in response to treatment. J. Clin. Periodontol. 2011, 38, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, U.K.; Könönen, E.; Pradhan-Palikhe, P.; Tervahartiala, T.; Pussinen, P.J.; Suominen-Taipale, L.; Sorsa, T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 2010, 37, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, I.T.; Sorsa, T.; van der Schoor, G.J.; Tervahartiala, T.; van der Schoor, P.; Gieselmann, D.R.; Heikkinen, A.M. Active matrix metalloproteinase-8 point-of-care (PoC)/chairside mouthrinse test vs. bleeding on probing in diagnosing subclinical periodontitis in adolescents. Diagnostics 2019, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Hanemaaijer, R.; Sorsa, T.; Konttinen, Y.T.; Ding, Y.; Sutinen, M.; Visser, H.; van Hinsbergh, V.W.; Helaakoski, T.; Kainulainen, T.; Rönkä, H.; et al. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J. Biol. Chem. 1997, 272, 31504–31509. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.P.; Sukhova, G.K.; Libby, P.; Gerdes, N.; Tang, N.; Horton, D.B.; Kilbride, M.; Breitbart, R.E.; Chun, M.; Schönbeck, U. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: A novel collagenolytic pathway suggested by transcriptional profiling. Circulation 2001, 104, 1899–1904. [Google Scholar] [CrossRef]
- Hedenbjörk-Lager, A.; Bjørndal, L.; Gustafsson, A.; Sorsa, T.; Tjäderhane, L.; Åkerman, S.; Ericson, D. Caries correlates strongly to salivary levels of matrix metalloproteinase-8. Caries Res. 2015, 49, 1–8. [Google Scholar] [CrossRef]
- Nascimento, F.D.; Minciotti, C.L.; Feraldeli, S.; Carrilho, M.R.; Pashely, D.F.; Tay, F.R.; Nader, H.B.; Salo, T.; Tjäderhane, L.; Tersariol, I.L. Cysteine cathepsins in human carious dentin. J. Dent. Res. 2011, 90, 506–511. [Google Scholar] [CrossRef]
- Räisänen, I.T.; Heikkinen, A.M.; Nwhator, S.O.; Umeizudike, K.A.; Tervahartiala, T.; Sorsa, T. On the diagnostic discrimination ability of mouthrinse and salivary aMMP-8 point-of-care testing regarding periodontal health and disease. Diagn. Microbiol. Infect. Dis. 2019, 95, 114871. [Google Scholar] [CrossRef]
- Huqot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cezard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef]
- Kirchner, A.; Jäger, J.; Krohn-Grimberghe, B.; Patschan, S.; Kottmann, T.; Schmalz, G.; Mausberg, R.F.; Haak, R.; Ziebolz, D. Active matrix metalloproteinase-8 and periodontal bacteria depending on periodontal status in patients with rheumatoid arthritis. J. Periodontal Res. 2017, 52, 745–754. [Google Scholar] [CrossRef]
- Serifova, X.; Ugarte-Berzal, E.; Opdenakker, G.; Vandooren, J. Homotrimeric MMP-9 is an active hitchhiker on alpha-2-macroglobulin partially escaping protease inhibition and internalization through LRP-1. Cell Mol. Life Sci. 2019. Oct 23, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Findings | Crohn’s Disease Group (N = 47) | Control Group (N = 41) | Difference P-Value |
|---|---|---|---|
| Gender (male %) | 23.4 | 29.3 | 0.629 |
| Age, years (mean, st.dev) | 46.4 (13.9) | 47.4 (13.2) | 0.734 |
| No. of teeth (mean, st.dev) | 27 (2.3) | 27.6 (2.4) | 0.192 |
| VPI % (mean, st.dev) | 23.8 (20.8) | 22.9 (18.7) | 0.828 |
| BOP % (mean, st.dev) | 32.7 (23.3) | 33.9 (28.6) | 0.820 |
| Individuals with PPD 4 mm (n, %) | 22 (46.8) | 13 (31.7) | 0.192 |
| Individuals with PPD 5–6 mm (n, %) | 1 (2.1) | 8 (19.5) | 0.011 |
| Individuals with PPD ≥ 7 mm (n, %) | 0 | 5 (12.2) | 0.019 |
| Individuals with REC ≥ 1 mm (n, %) | 33 (70.2) | 28 (68.3) | 1.000 |
| Individuals with REC ≥ 3 mm (n, %) | 14 (29.8) | 12 (29.3) | 0.073 |
| Periodontal diagnosis | 0.021 | ||
| Periodontally healthy (n, %) | 12 (25) | 15 (36.6) | |
| Gingivitis (n, %) | 20 (42.6) | 15 (36.6) | |
| Initial periodontitis (n, %) | 14 (29.8) | 4 (9.8) | |
| Moderate periodontitis (n, %) | 1 (2.1) | 2 (4.9) | |
| Severe periodontitis (n, %) | 0 (0) | 5 (12.2) | |
| Stimulated salivary flow, ml/min (mean, st.dev) | 1.42 (0.72) | 1.59 (0.90) | 0.313 |
| Periodontal Findings | Sensitivity/Spesificity | Crohn’s Disease Group | Control Group | Whole Population |
|---|---|---|---|---|
| (N = 47) | (N = 41) | (N = 88) | ||
| PPD 4 mm | Sensitivity | 54.5 | 84.6 | 65.7 |
| Specificity | 80 | 82.1 | 81.1 | |
| PPD 5–6 mm | Sensitivity | 100 | 87.5 | 88.9 |
| Specificity | 65.2 | 72.7 | 68.4 | |
| PPD ≥ 7 mm | Sensitivity | - | 100 | 100 |
| Specificity | - | 69.4 | 66.3 | |
| BOP ≥ 15% | Sensitivity | 37.1 | 57.7 | 45.9 |
| Specificity | 66.7 | 93.3 | 81.5 | |
| Furcation defect | Sensitivity | 40 | 66.7 | 54.5 |
| Specificity | 64.9 | 72.4 | 68.2 | |
| Attrition | Sensitivity | 52.4 | 38.9 | 46.2 |
| Specificity | 76.9 | 60.9 | 69.4 | |
| Periodontal diseases | Sensitivity | 35.3 | 57.7 | 45 |
| Specificity | 61.5 | 93.3 | 78.6 | |
| Periodontitis | Sensitivity | 60 | 90.9 | 73.1 |
| Specificity | 75 | 80 | 77.4 |
| Group | Periodontal Diseases | Periodontitis | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Crohn’s disease group (N = 47) | 0.9 (0.23–3.26), 0.84 | 1.3 (0.29–5.58), 0.752 | 4.5 (1.22–16.6), 0.024 | 5.2 (1.26–21.6), 0.022 |
| Control group (N = 41) | 19.1 (2.17–167), 0.08 | 20.4 (1.92–216), 0.012 | 40 (4.25–376), 0.001 | 54.3 (3.09–953), 0.006 |
| Whole population (N = 88) | 3.0 (1.06–8.45), 0.038 | 4.16 (1.34–12.9), 0.014 | 9.3 (3.25–26.6), <0.001 | 8.5 (2.88–25.3), <0.001 |
| Group | Complexes of MMP-8 | PMN | Mesenchymal | Fragments of MMP-8 | Total MMP-8 | |||
|---|---|---|---|---|---|---|---|---|
| Pro MMP-8 | aMMP-8 | Pro MMP-8 | aMMP-8 | |||||
| Crohn’s disease Group | + (N = 17) | 1.8 (0.6–4.1) | 0.4 (0.1–2.1) | 0.0 (0.0–1.2) | 0.6 (0.0–1.5) | 0.5 (0.0–1.6) | 0.1 (0.0–1.4) | 3.8 (1.0–9.5) |
| − (N = 30) | 1.5 (0.2–4.2) | 0.4 (0.0–1.1) | 0.1 (0.0–1.3) | 0.8 (0.0–1.6) | 0.8 (0.0–1.7) | 0.6 (0.0–0.5) | 3.7 (1.5–8.2) | |
| P | 0.330 | 0.877 | 0.708 | 0.303 | 0.061 | 0.192 | 0.982 | |
| Control Group | + (N = 16) | 1.4 (0.1–4.7) | 0.6 (0.0–1.5) | 0.4 (0.0–1.4) | 1.0 (0.1–1.8) | 0.9 (0.0–1.9) | 0.1 (0.0–2.4) | 4.9 (0.4–11.8) |
| − (N = 25) | 0.5 (0.0–3.7) | 0.2 (0.0–1.8) | 0.0 (0.0–1.0) | 0.6 (0.0–1.6) | 0.7 (0.0–1.7) | 0.0 (0.0–0.5) | 2.2 (0.2–7.6) | |
| P | 0.022 | 0.037 | 0.015 | 0.121 | 0.209 | 0.046 | 0.015 | |
| Whole Population | + (N = 33) | 1.6 (0.1–4.7) | 0.4 (0.0–2.1) | 0.1 (0.0–1.4) | 0.8 (0.0–1.8) | 0.2 (0.0–1.9) | 0.1 (0.0–2.4) | 3.9 (0.4–11.8) |
| − (N = 55) | 1.0 (0.0–4.2) | 0.3 (0.0–1.8) | 0.5 (0.0–1.3) | 0.7 (0.0–1.6) | 0.7 (0.0–1.7) | 0.1 (0.0–0.5) | 3.1 (0.2–8.2) | |
| P | 0.032 | 0.174 | 0.140 | 0.670 | 0.689 | 0.020 | 0.062 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rautava, J.; Gürsoy, U.K.; Kullström, A.; Könönen, E.; Sorsa, T.; Tervahartiala, T.; Gürsoy, M. An Oral Rinse Active Matrix Metalloproteinase-8 Point-of-Care Immunotest May Be Less Accurate in Patients with Crohn’s Disease. Biomolecules 2020, 10, 395. https://doi.org/10.3390/biom10030395
Rautava J, Gürsoy UK, Kullström A, Könönen E, Sorsa T, Tervahartiala T, Gürsoy M. An Oral Rinse Active Matrix Metalloproteinase-8 Point-of-Care Immunotest May Be Less Accurate in Patients with Crohn’s Disease. Biomolecules. 2020; 10(3):395. https://doi.org/10.3390/biom10030395
Chicago/Turabian StyleRautava, Jaana, Ulvi K. Gürsoy, Adrian Kullström, Eija Könönen, Timo Sorsa, Taina Tervahartiala, and Mervi Gürsoy. 2020. "An Oral Rinse Active Matrix Metalloproteinase-8 Point-of-Care Immunotest May Be Less Accurate in Patients with Crohn’s Disease" Biomolecules 10, no. 3: 395. https://doi.org/10.3390/biom10030395
APA StyleRautava, J., Gürsoy, U. K., Kullström, A., Könönen, E., Sorsa, T., Tervahartiala, T., & Gürsoy, M. (2020). An Oral Rinse Active Matrix Metalloproteinase-8 Point-of-Care Immunotest May Be Less Accurate in Patients with Crohn’s Disease. Biomolecules, 10(3), 395. https://doi.org/10.3390/biom10030395





