Variations in Circulating Active MMP-9 Levels during Renal Replacement Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Active MMP-9
2.3. Assessment of Total MMP-9, Total TIMP-1, and MMP-9/TIMP-1 Interaction
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Dialysis Reduces Active MMP-9 Levels
3.3. Kidney Transplantation Increases Total and Active MMP-9 and Total TIMP-1 Levels, but Not MMP-9:TIMP-1 Protein Interactions
3.4. Active MMP-9 Positively Correlates with Arterial Stiffness in RRT Patients, but Not with Systolic Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamb, E.J.; Levey, A.S.; Stevens, P.E. The Kidney Disease Improving Global Outcomes (KDIGO) guideline update for chronic kidney disease: Evolution not revolution. Clin. Chem. 2013, 59, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hurtado, G.; Ruilope, L.M. Does cardiovascular protection translate into renal protection? Nat. Rev. Cardiol. 2014, 11, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Amann, K.; Shoji, T. The heart and vascular system in dialysis. Lancet 2016, 388, 276–284. [Google Scholar] [CrossRef]
- Valdivielso, J.M.; Rodríguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermúdez-López, M.; Sánchez-Niño, M.D.; Pérez-Fernández, M.; Ortiz, A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arter. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef] [PubMed]
- Lioufas, N.; Hawley, C.M.; Cameron, J.D.; Toussaint, N.D. Chronic Kidney Disease and Pulse Wave Velocity: A Narrative Review. Int. J. Hypertens 2019, 2019, 9189362. [Google Scholar] [CrossRef]
- Paloian, N.J.; Giachelli, C.M. A current understanding of vascular calcification in CKD. Am. J. Physiol. Ren. Physiol. 2014, 307, F891–F900. [Google Scholar] [CrossRef]
- Verbeke, F.; Van Biesen, W.; Honkanen, E.; Wikström, B.; Jensen, P.B.; Krzesinski, J.M.; Rasmussen, M.; Vanholder, R.; Rensma, P.L.; Investigators, C.S. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: Outcome of the calcification outcome in renal disease (CORD) study. Clin. J. Am. Soc. Nephrol. 2011, 6, 153–159. [Google Scholar] [CrossRef]
- Safar, M.E.; Blacher, J.; Pannier, B.; Guerin, A.P.; Marchais, S.J.; Guyonvarc’h, P.M.; London, G.M. Central pulse pressure and mortality in end-stage renal disease. Hypertension 2002, 39, 735–738. [Google Scholar] [CrossRef]
- Korogiannou, M.; Xagas, E.; Marinaki, S.; Sarafidis, P.; Boletis, J.N. Arterial Stiffness in Patients With Renal Transplantation; Associations With Co-morbid Conditions, Evolution, and Prognostic Importance for Cardiovascular and Renal Outcomes. Front. Cardiovasc. Med. 2019, 6, 67. [Google Scholar] [CrossRef]
- Litwin, M.; Wühl, E.; Jourdan, C.; Trelewicz, J.; Niemirska, A.; Fahr, K.; Jobs, K.; Grenda, R.; Wawer, Z.T.; Rajszys, P.; et al. Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J. Am. Soc. Nephrol. 2005, 16, 1494–1500. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Aakhus, S.; Dahl, K.; Widerøe, T.E. Cardiovascular morbidity and risk factors in renal transplant patients. Nephrol. Dial. Transpl. 1999, 14, 648–654. [Google Scholar] [CrossRef]
- Newby, A.C. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 2005, 85, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Olmo, H.; García-Prieto, C.F.; Álvarez-Llamas, G.; Barderas, M.G.; Vivanco, F.; Aranguez, I.; Somoza, B.; Segura, J.; Kreutz, R.; Fernández-Alfonso, M.S.; et al. Role of matrix metalloproteinase-9 in chronic kidney disease: A new biomarker of resistant albuminuria. Clin. Sci. (Lond) 2016, 130, 525–538. [Google Scholar] [CrossRef]
- Miljković, M.; Stefanović, A.; Bogavac-Stanojević, N.; Simić-Ogrizović, S.; Dumić, J.; Černe, D.; Jelić-Ivanović, Z.; Kotur-Stevuljević, J. Association of Pentraxin-3, Galectin-3 and Matrix Metalloproteinase-9/Timp-1 with Cardiovascular Risk in Renal Disease Patients. Acta Clin. Croat. 2017, 56, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kousios, A.; Kouis, P.; Panayiotou, A.G. Matrix Metalloproteinases and Subclinical Atherosclerosis in Chronic Kidney Disease: A Systematic Review. Int. J. Nephrol 2016, 2016, 9498013. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Pulido-Olmo, H.; Rodríguez-Sánchez, E.; Navarro-García, J.A.; Barderas, M.G.; Álvarez-Llamas, G.; Segura, J.; Fernández-Alfonso, M.; Ruilope, L.M.; Ruiz-Hurtado, G. Rapid, Automated, and Specific Immunoassay to Directly Measure Matrix Metalloproteinase-9-Tissue Inhibitor of Metalloproteinase-1 Interactions in Human Plasma Using AlphaLISA Technology: A New Alternative to Classical ELISA. Front. Immunol. 2017, 8, 853. [Google Scholar] [CrossRef]
- Navarro-García, J.A.; Rodríguez-Sánchez, E.; Aceves-Ripoll, J.; Abarca-Zabalía, J.; Susmozas-Sánchez, A.; González Lafuente, L.; Bada-Bosch, T.; Hernández, E.; Mérida-Herrero, E.; Praga, M.; et al. Oxidative Status before and after Renal Replacement Therapy: Differences between Conventional High Flux Hemodialysis and on-Line Hemodiafiltration. Nutrients 2019, 11, 2809. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Albert, E.; Giménez, E.; Ruiz-Merlo, T.; Parra, P.; López-Medrano, F.; San Juan, R.; Polanco, N.; Andrés, A.; Navarro, D.; et al. Monitoring of alphatorquevirus DNA levels for the prediction of immunosuppression-related complications after kidney transplantation. Am. J. Transpl. 2019, 19, 1139–1149. [Google Scholar] [CrossRef]
- Utrero-Rico, A.; Laguna-Goya, R.; Cano-Romero, F.; Chivite-Lacaba, M.; Gonzalez-Cuadrado, C.; Rodríguez-Sánchez, E.; Ruiz-Hurtado, G.; Serrano, A.; Fernández-Ruiz, M.; Justo, I.; et al. Early Posttransplant Mobilization of M-MDSC Correlates with Increase in Soluble Immunosuppressive Factors and Predicts Cancer in Kidney Recipients. Transplantation 2020. [Google Scholar] [CrossRef] [PubMed]
- Patrier, L.; Dupuy, A.M.; Granger Vallée, A.; Chalabi, L.; Morena, M.; Canaud, B.; Cristol, J.P. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional high flux hemodialysis. J. Nephrol. 2013, 26, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol Dial. Transpl. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef]
- Morad, A.A.; Bazaraa, H.M.; Abdel Aziz, R.E.; Abdel Halim, D.A.; Shoman, M.G.; Saleh, M.E. Role of online hemodiafiltration in improvement of inflammatory status in pediatric patients with end-stage renal disease. Iran. J. Kidney Dis. 2014, 8, 481–485. [Google Scholar] [PubMed]
- Ağbaş, A.; Canpolat, N.; Çalışkan, S.; Yılmaz, A.; Ekmekçi, H.; Mayes, M.; Aitkenhead, H.; Schaefer, F.; Sever, L.; Shroff, R. Hemodiafiltration is associated with reduced inflammation, oxidative stress and improved endothelial risk profile compared to high-flux hemodialysis in children. PLoS ONE 2018, 13, e0198320. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Bots, M.L.; Canaud, B.; Davenport, A.; Grooteman, M.P.; Kircelli, F.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Haemodiafiltration and mortality in end-stage kidney disease patients: A pooled individual participant data analysis from four randomized controlled trials. Nephrol. Dial. Transpl. 2016, 31, 978–984. [Google Scholar] [CrossRef]
- Nubé, M.J.; Peters, S.A.E.; Blankestijn, P.J.; Canaud, B.; Davenport, A.; Grooteman, M.P.C.; Asci, G.; Locatelli, F.; Maduell, F.; Morena, M.; et al. Mortality reduction by post-dilution online-haemodiafiltration: A cause-specific analysis. Nephrol. Dial. Transpl. 2017, 32, 548–555. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, J.T.; Park, K.S.; Kwon, Y.E.; Oh, H.J.; Yoo, T.H.; Kim, Y.L.; Kim, Y.S.; Yang, C.W.; Kim, N.H.; et al. Prognostic Value of Residual Urine Volume, GFR by 24-h Urine Collection, and eGFR in Patients Receiving Dialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 426–434. [Google Scholar] [CrossRef]
- van der Wal, W.M.; Noordzij, M.; Dekker, F.W.; Boeschoten, E.W.; Krediet, R.T.; Korevaar, J.C.; Geskus, R.B.; (NECOSAD), N.C.S.o.t.A.o.D.S.G. Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol. Dial. Transpl. 2011, 26, 2978–2983. [Google Scholar] [CrossRef]
- Hyodo, T.; Koutoku, N. Preservation of residual renal function with HDF. Contrib. Nephrol. 2011, 168, 204–212. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, E.; Navarro-García, J.A.; Aceves-Ripoll, J.; Álvarez-Llamas, G.; Segura, J.; Barderas, M.G.; Ruilope, L.M.; Ruiz-Hurtado, G. Association between renal dysfunction and metalloproteinase (MMP)-9 activity in hypertensive patients. Nefrologia 2019, 39, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.C.; Yang, C.W.; Hsieh, W.Y.; Chuang, W.H.; Lin, Y.C.; Lin, C.S. Decreases in plasma MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios in uremic patients during hemodialysis. Clin. Exp. Nephrol. 2016, 20, 934–942. [Google Scholar] [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Peripheral blood level alterations of MMP-2 and MMP-9 in patients with chronic kidney disease on conservative treatment and on hemodialysis. Clin. Biochem. 2011, 44, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Chou, F.P.; Chu, S.C.; Cheng, M.C.; Yang, S.F.; Cheung, W.N.; Chiou, H.L.; Hsieh, Y.S. Effect of hemodialysis on the plasma level of type IV collagenases and their inhibitors. Clin. Biochem. 2002, 35, 383–388. [Google Scholar] [CrossRef]
- Musiał, K.; Zwolińska, D.; Polak-Jonkisz, D.; Berny, U.; Szprynger, K.; Szczepańska, M. Soluble adhesion molecules in children and young adults on chronic hemodialysis. Pediatr. Nephrol. 2004, 19, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Libetta, C.; Esposito, P.; Borettaz, I.; Tinelli, C.; D’angelo, A.; Maffioli, P. Effects of two different dialytic treatments on inflammatory markers in people with end-stage renal disease with and without type 2 diabetes mellitus. Cytokine 2017, 92, 75–79. [Google Scholar] [CrossRef]
- Raikou, V.D.; Kardalinos, V.; Kyriaki, D. The Relationship of Residual Renal Function with Cardiovascular Morbidity in Hemodialysis Patients and the Potential Role of Monocyte Chemoattractant Protein-1. Kidney Dis. (Basel) 2018, 4, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Malyszko, J.S.; Koc-Zorawska, E.; Kozminski, P.; Mysliwiec, M. Neutrophil gelatinase-associated lipocalin in dialyzed patients is related to residual renal function, type of renal replacement therapy and inflammation. Kidney Blood Press Res. 2009, 32, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Mas, V.; Maluf, D.; Archer, K.; Yanek, K.; Mas, L.; King, A.; Gibney, E.; Massey, D.; Cotterell, A.; Fisher, R.; et al. Establishing the molecular pathways involved in chronic allograft nephropathy for testing new noninvasive diagnostic markers. Transplantation 2007, 83, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Cerrillos-Gutiérrez, J.I.; Miranda-Díaz, A.G.; Preciado-Rojas, P.; Gómez-Navarro, B.; Sifuentes-Franco, S.; Carrillo-Ibarra, S.; Andrade-Sierra, J.; Rojas-Campos, E.; Cueto-Manzano, A.M. The Beneficial Effects of Renal Transplantation on Altered Oxidative Status of ESRD Patients. Oxid Med. Cell Longev. 2016, 2016, 5757645. [Google Scholar] [CrossRef]
- Steubl, D.; Hettwer, S.; Vrijbloed, W.; Dahinden, P.; Wolf, P.; Luppa, P.; Wagner, C.A.; Renders, L.; Heemann, U.; Roos, M. C-terminal agrin fragment--a new fast biomarker for kidney function in renal transplant recipients. Am. J. Nephrol. 2013, 38, 501–508. [Google Scholar] [CrossRef]
- Simmons, E.M.; Langone, A.; Sezer, M.T.; Vella, J.P.; Recupero, P.; Morrow, J.D.; Ikizler, T.A.; Himmelfarb, J. Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation 2005, 79, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Shi, Y.; Ding, Y.; Liu, X.; Zou, H. Dramatic early event in chronic allograft nephropathy: Increased but not decreased expression of MMP-9 gene. Diagn. Pathol. 2013, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Andreucci, M.; Garofalo, C.; Faga, T.; Michael, A.; Ielapi, N.; Grande, R.; Sapienza, P.; Franciscis, S.; Mastroroberto, P.; et al. The Association of Matrix Metalloproteinases with Chronic Kidney Disease and Peripheral Vascular Disease: A Light at the End of the Tunnel? Biomolecules 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, G.; Aspelund, T.; Greve, A.M.; Eiriksdottir, G.; Acharya, T.; Thorgeirsson, G.; Harris, T.B.; Launer, L.J.; Gudnason, V.; Arai, A.E. Fibrosis as measured by the biomarker, tissue inhibitor metalloproteinase-1, predicts mortality in Age Gene Environment Susceptibility-Reykjavik (AGES-Reykjavik) Study. Eur. Heart J. 2017, 38, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.H.; Essalihi, R.; Bouvet, C.; Moreau, P. Evolution and modulation of age-related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovasc. Res. 2005, 66, 307–317. [Google Scholar] [CrossRef]
- Feng, S.; Wang, H.; Yang, J.; Hu, X.; Wang, W.; Liu, H.; Li, H.; Zhang, X. Kidney transplantation improves arterial stiffness in patients with end-stage renal disease. Int. Urol. Nephrol. 2020. [Google Scholar] [CrossRef]
- Hornum, M.; Clausen, P.; Idorn, T.; Hansen, J.M.; Mathiesen, E.R.; Feldt-Rasmussen, B. Kidney transplantation improves arterial function measured by pulse wave analysis and endothelium-independent dilatation in uraemic patients despite deterioration of glucose metabolism. Nephrol. Dial. Transpl. 2011, 26, 2370–2377. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Hae, R.; Spence, M.; Shea, B.; Agharazii, M.; Burns, K.D. A Systematic Review and Meta-analysis of Nonpharmacologic-based Interventions for Aortic Stiffness in End-Stage Renal Disease. Kidney Int. Rep. 2019, 4, 1109–1121. [Google Scholar] [CrossRef]
- Sidibé, A.; Fortier, C.; Desjardins, M.P.; Zomahoun, H.T.V.; Boutin, A.; Mac-Way, F.; De Serres, S.; Agharazii, M. Reduction of Arterial Stiffness After Kidney Transplantation: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
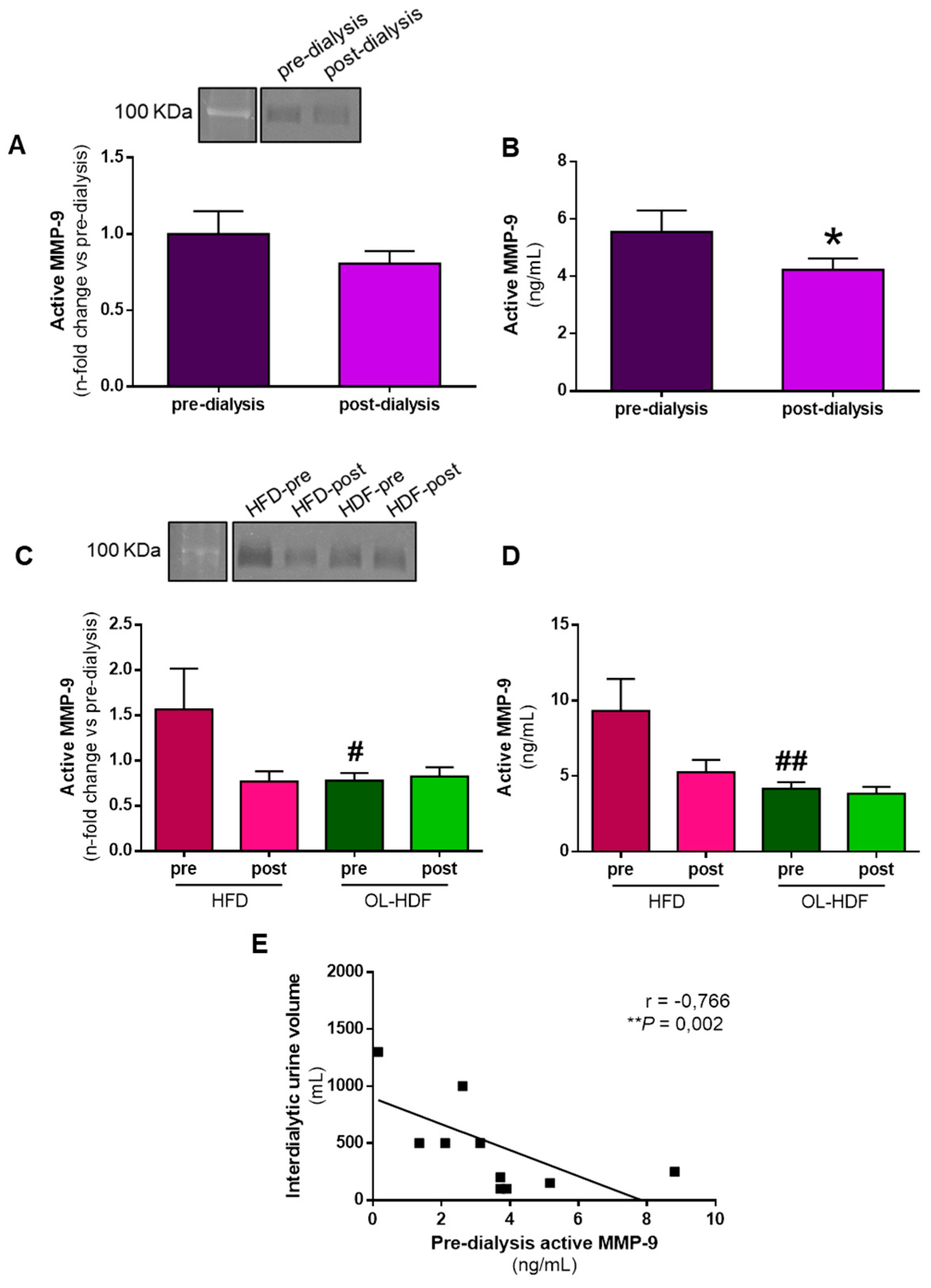
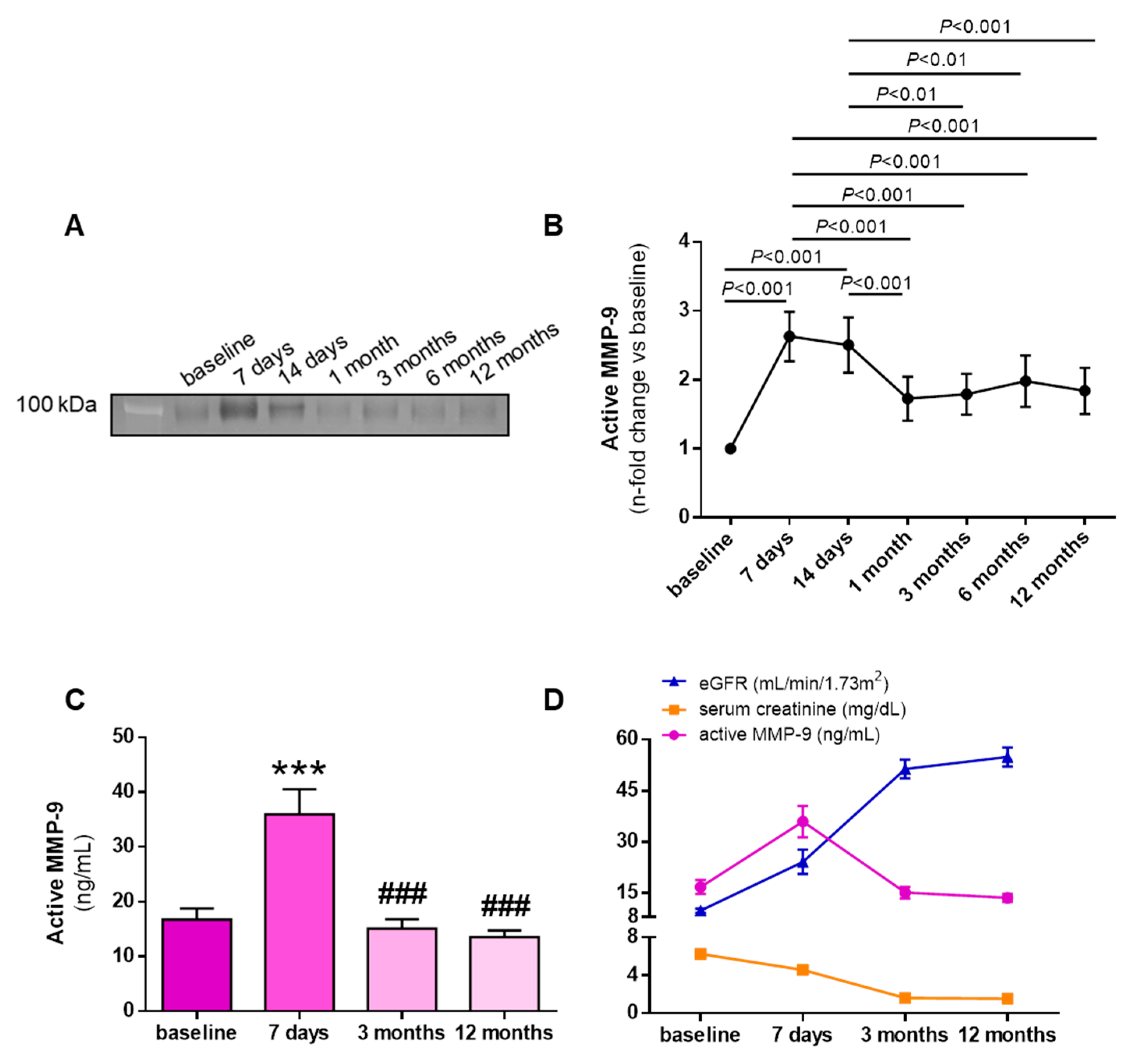
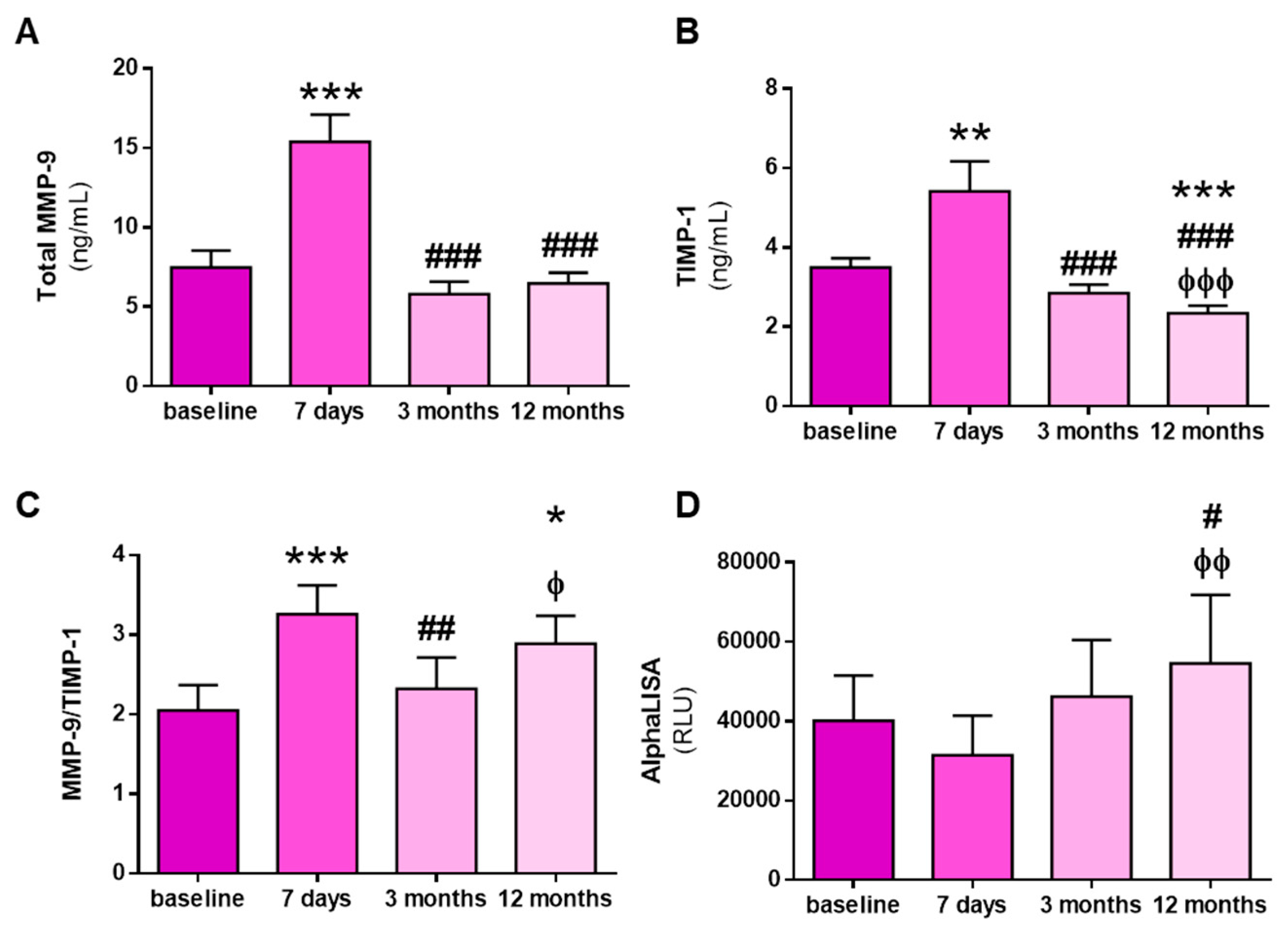
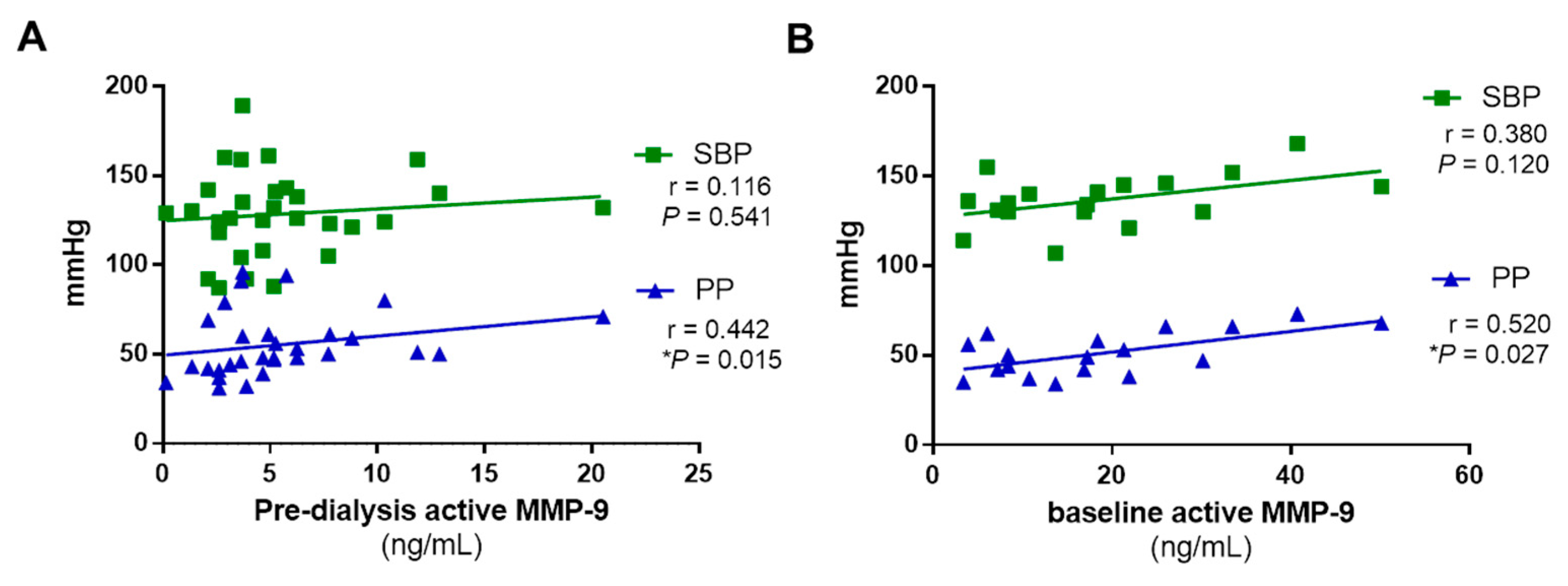
| All Patients (n = 32) | HFD Patients (n = 9) | OL-HDF Patients (n = 23) | p-Value | |
|---|---|---|---|---|
| Age (years) | 60.0 ± 16,4 | 64.7 ± 22.0 | 58.2 ± 13.8 | 0.322 |
| Male sex (n, %) | 16 (50) | 1 (11) | 15 (65) | 0.016 |
| Hypertension (n, %) | 25 (78) | 7 (78) | 18 (78) | 0.999 |
| SBP (mmHg) | 128.4 ± 23.0 | 126.2 ± 23.2 | 129.3 ± 23.4 | 0.739 |
| PP (mmHg) | 55.5 ± 17.5 | 58.4 ± 14.6 | 54.3 ± 18.7 | 0.556 |
| Diabetes mellitus (n, %) | 7 (22) | 2 (22) | 5 (22) | 0.999 |
| NT-proBNP (pg/mL) | 2565 ± 1735 | 2753 ± 1245 | 2509 ± 1881 | 0.769 |
| Dialysis-related parameters | ||||
| Serum creatinine (mg/dL) | 7.61 ± 2.22 | 7.16 ± 2.22 | 7.78 ± 2.25 | 0.486 |
| Serum albumin (g/dL) | 4.05 ± 0.43 | 3.91 ± 0.24 | 4.10 ± 0.48 | 0.259 |
| Potassium (mEq/L) | 5.15 ± 0.90 | 5.06 ± 0.78 | 5.19 ± 0.95 | 0.725 |
| Bicarbonate (mEq/L) | 21.3 ± 2.9 | 21.2 ± 3.8 | 21.3 ± 2.6 | 0.974 |
| Dialysis vintage (months) | 79.5 ± 74.3 | 31.4 ± 24.5 | 98.4 ± 79.1 | 0.020 |
| Kt/V | 1.63 ± 0.24 | 1.68 ± 0.25 | 1.61 ± 0.24 | 0.463 |
| eGFR (ml/min/1.73 m2) | 6.92 ± 3.00 | 6.48 ± 2.92 | 7.09 ± 3.08 | 0.614 |
| Residual diuresis (n, %) | 15 (52) | 5 (56) | 10 (43) | 0.699 |
| Interdialytic urine volume (mL) | 375 (163–875) | 250 (100–500) | 500 (175–1000) | 0.518 |
| Cause of CKD | 0.547 | |||
| Glomerulonephritis (n, %) | 7 (22) | 2 (22) | 5 (22) | |
| Diabetic nephropathy (n, %) | 5 (16) | 1 (11) | 4 (17) | |
| Polycystic kidney disease (n, %) | 4 (12) | 0 (0) | 4 (17) | |
| Hypertensive nephropathy (n, %) | 2 (6) | 0 (0) | 2 (9) | |
| Other or undetermined (n, %) | 14 (44) | 6 (67) | 8 (35) | |
| Medication | ||||
| ACEi/ARB (n, %) | 8 (25) | 2 (22) | 6 (26) | 0.999 |
| Diuretics (n, %) | 3 (9) | 0 (0) | 3 (13) | 0.541 |
| β-blockers (n, %) | 13 (41) | 5 (56) | 8 (35) | 0.427 |
| Cinacalcet (n, %) | 3 (9) | 0 (0) | 3 (13) | 0.541 |
| Paricalcitol (n, %) | 12 (38) | 5 (56) | 7 (30) | 0.253 |
| All Patients (n = 46) | |
|---|---|
| Age of the recipient (years) | 54.6 ± 15.8 |
| Male sex (n, %) | 27 (59) |
| Previous kidney transplant (n, %) | 4 (9) |
| Pretransplant dialysis (n, %) | 41 (89) |
| Dialysis vintage (months) | 21.0 ± 19.0 |
| SBP (mmHg) | 134.5 ± 14.8 |
| PP (mmHg) | 49.9 ± 12.9 |
| Serum creatinine (mg/dL) | 6.27 ± 2.56 |
| Serum albumin (g/dL) | 4.23 ± 0.52 |
| eGFR (mL/min/1.73 m2) | 9.82 ± 4.28 |
| Donor | |
| Male sex (n, %) | 25 (54) |
| Age (years) | 52.6 ± 14.6 |
| Living donor (n, %) | 5 (11) |
| Number of HLA-mismatches | 5 (4–5) |
| Cause of CKD | |
| IgA nephropathy | 9 (20) |
| Glomerulonephritis | 2 (4) |
| Diabetic nephropathy | 7 (15) |
| Polycystic kidney disease | 8 (17) |
| Hypertensive nephropathy | 3 (7) |
| Other or undetermined | 17 (37) |
| Induction therapy | 38 (83) |
| Maintenance immunosuppression | |
| Steroids | 46 (100) |
| Tacrolimus | 46 (100) |
| Mycophenolate mofetil/mycophenolic acid | 42 (91) |
| Cyclosporine A | 0 (0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sánchez, E.; Navarro-García, J.A.; Aceves-Ripoll, J.; Abarca-Zabalía, J.; Susmozas-Sánchez, A.; Bada-Bosch, T.; Hernández, E.; Mérida-Herrero, E.; Andrés, A.; Praga, M.; et al. Variations in Circulating Active MMP-9 Levels during Renal Replacement Therapy. Biomolecules 2020, 10, 505. https://doi.org/10.3390/biom10040505
Rodríguez-Sánchez E, Navarro-García JA, Aceves-Ripoll J, Abarca-Zabalía J, Susmozas-Sánchez A, Bada-Bosch T, Hernández E, Mérida-Herrero E, Andrés A, Praga M, et al. Variations in Circulating Active MMP-9 Levels during Renal Replacement Therapy. Biomolecules. 2020; 10(4):505. https://doi.org/10.3390/biom10040505
Chicago/Turabian StyleRodríguez-Sánchez, Elena, José Alberto Navarro-García, Jennifer Aceves-Ripoll, Judith Abarca-Zabalía, Andrea Susmozas-Sánchez, Teresa Bada-Bosch, Eduardo Hernández, Evangelina Mérida-Herrero, Amado Andrés, Manuel Praga, and et al. 2020. "Variations in Circulating Active MMP-9 Levels during Renal Replacement Therapy" Biomolecules 10, no. 4: 505. https://doi.org/10.3390/biom10040505
APA StyleRodríguez-Sánchez, E., Navarro-García, J. A., Aceves-Ripoll, J., Abarca-Zabalía, J., Susmozas-Sánchez, A., Bada-Bosch, T., Hernández, E., Mérida-Herrero, E., Andrés, A., Praga, M., Fernández-Ruiz, M., Aguado, J. M., Segura, J., Ruilope, L. M., & Ruiz-Hurtado, G. (2020). Variations in Circulating Active MMP-9 Levels during Renal Replacement Therapy. Biomolecules, 10(4), 505. https://doi.org/10.3390/biom10040505





