Hypoxia as a Driving Force of Pluripotent Stem Cell Reprogramming and Differentiation to Endothelial Cells
Abstract
1. Introduction
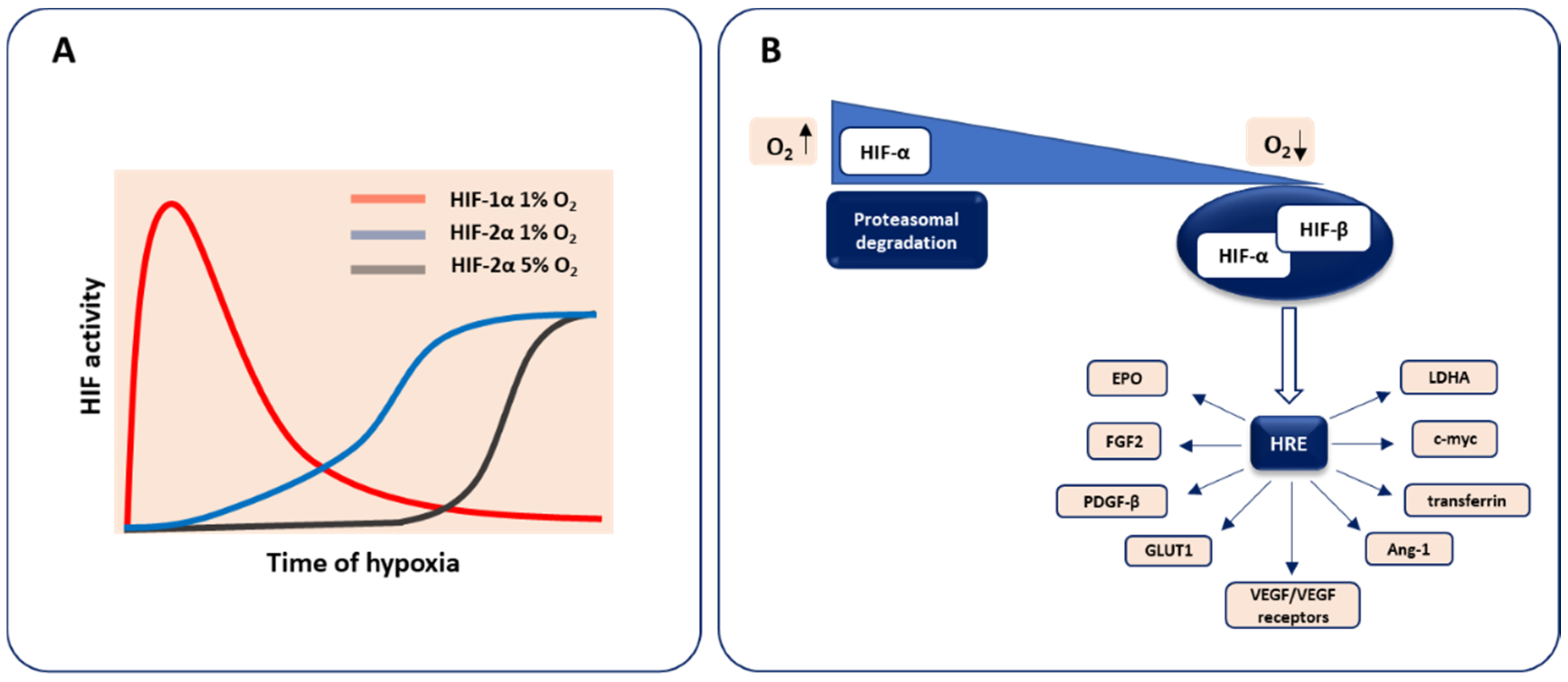
2. Molecular Basis of Oxygen Sensing
3. Hypoxia in Early Embryonic Development and Vasculature Formation
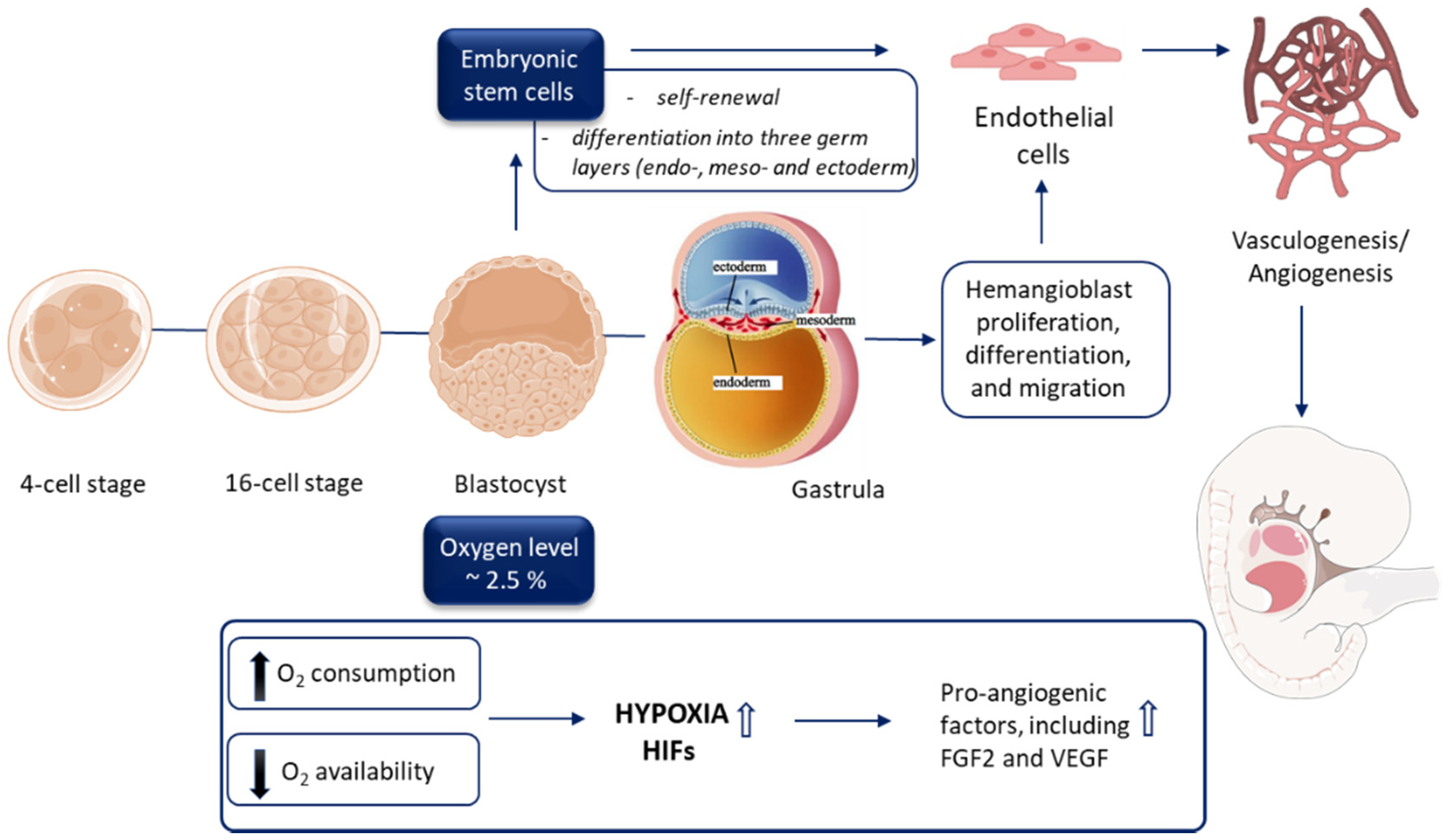
3.1. Early Embryogenesis Progresses in the Hypoxic Environment
3.2. ‘Physiological Hypoxia’ as a Driving Force of Vasculature Development
3.3. Vascular Development during Embryogenesis
3.4. Endothelial Cell Origin and Differentiation
3.5. Endothelial Cell Commitment and Autophagy
4. Hypoxia in the Derivation of Human Embryonic Stem Cells and Generation of induced Pluripotent Stem Cells
4.1. Hypoxia and hESC Culture
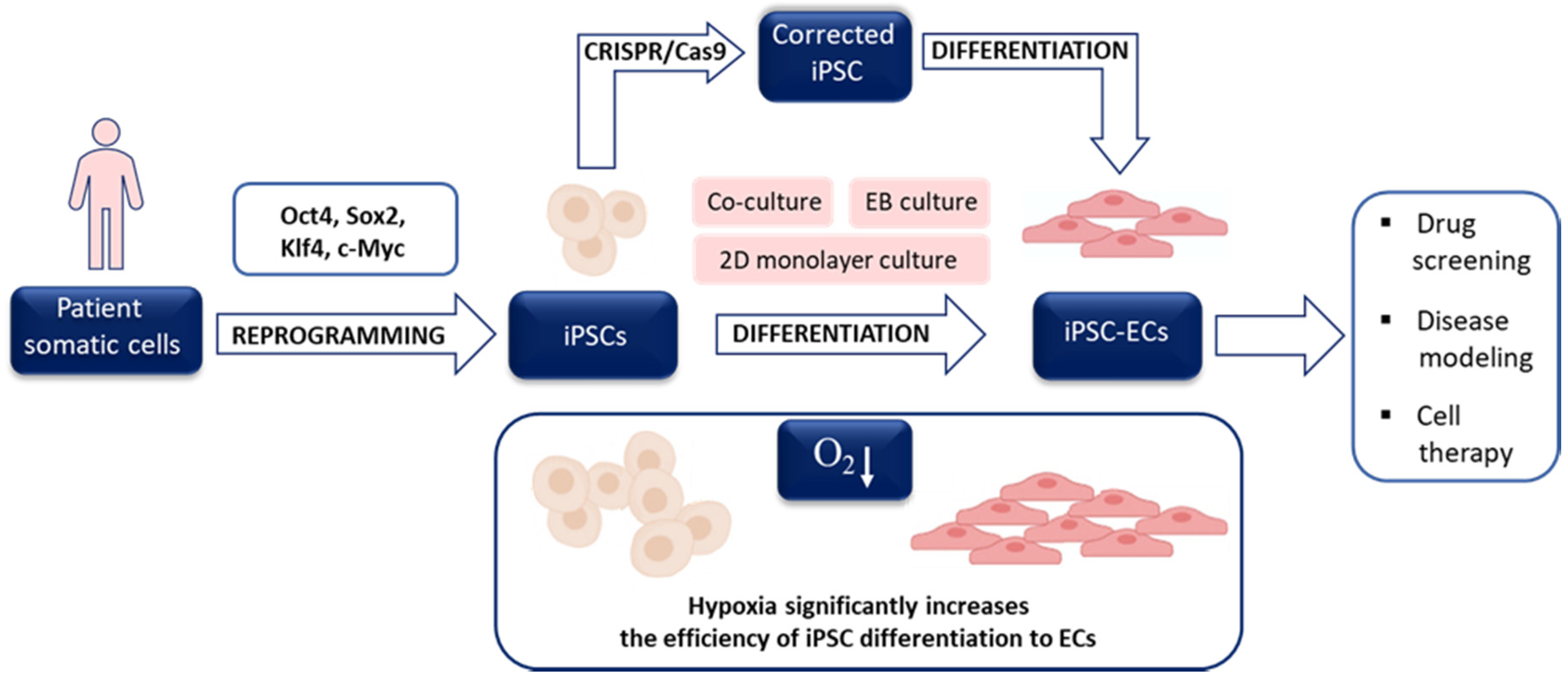
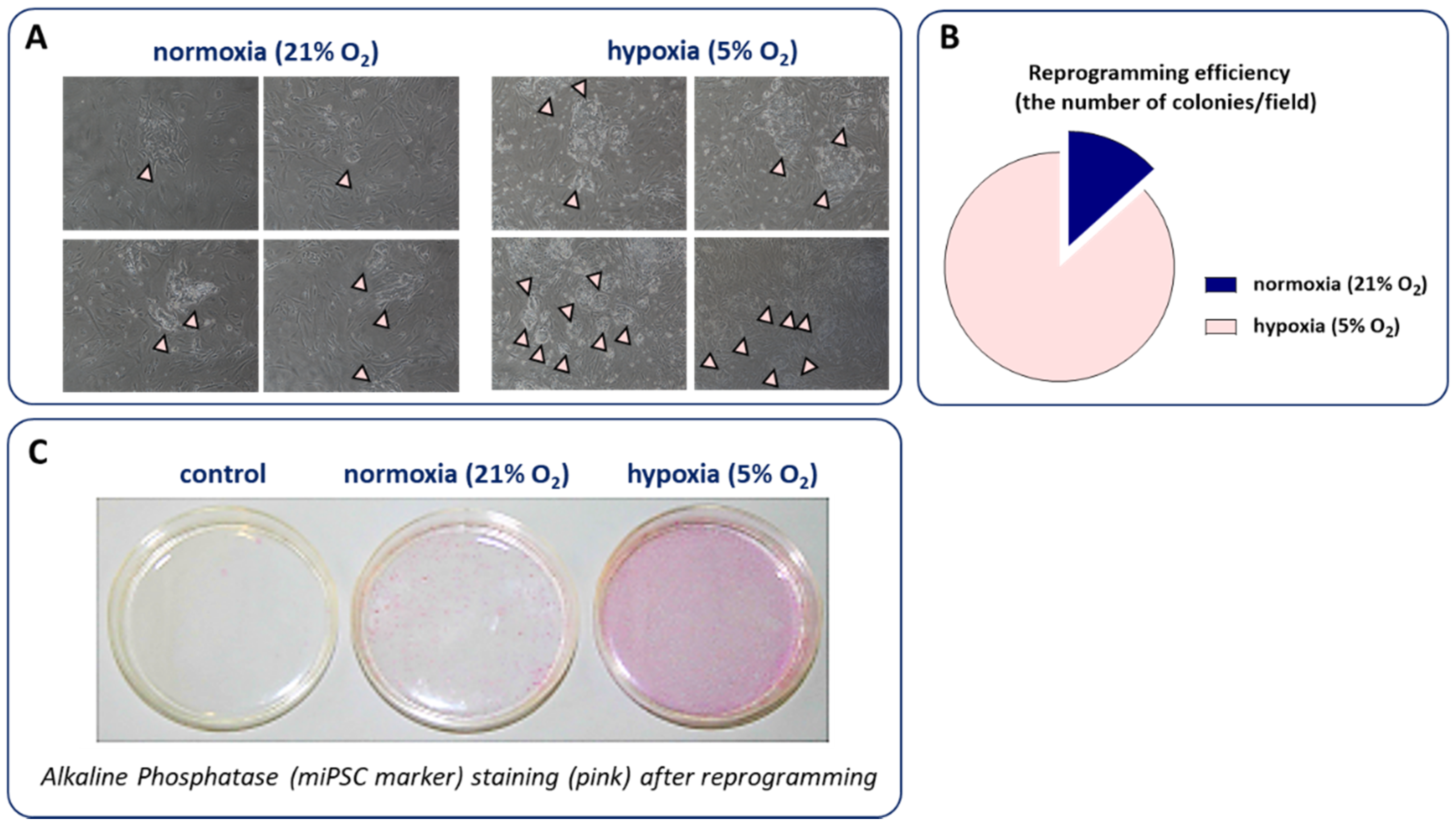
4.2. Hypoxia and the Generation of iPSCs
5. Hypoxia and Stem Cell Differentiation toward ECs
5.1. Hypoxia Facilitates Vascular Differentiation of Pluripotent Stem Cells
5.2. Initial Exposure to Hypoxia Is Crucial for Differentiation toward ECs
5.3. The Evidence for an Arterial Phenotype of ECs Differentiated under Hypoxic Conditions
5.4. The Role of VEGF in Hypoxia-Induced Differentiation toward ECs
5.5. HIF-1α as a Master Regulator of Hypoxia-Driven Differentiation toward ECs
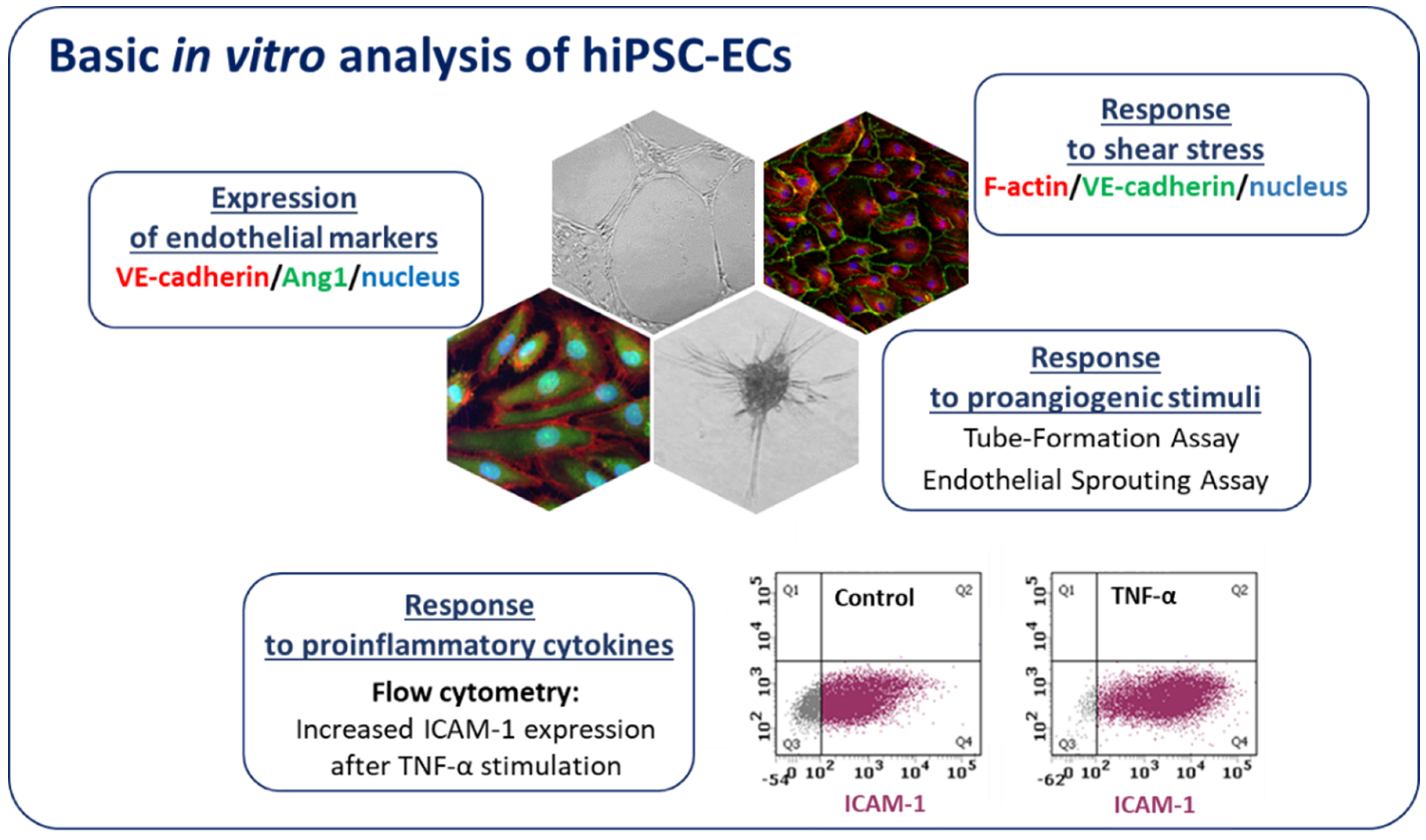
6. hiPSC-ECs in Disease Modeling
6.1. Monogenic Diseases
6.2. Complex Vascular Disease—Diabetes
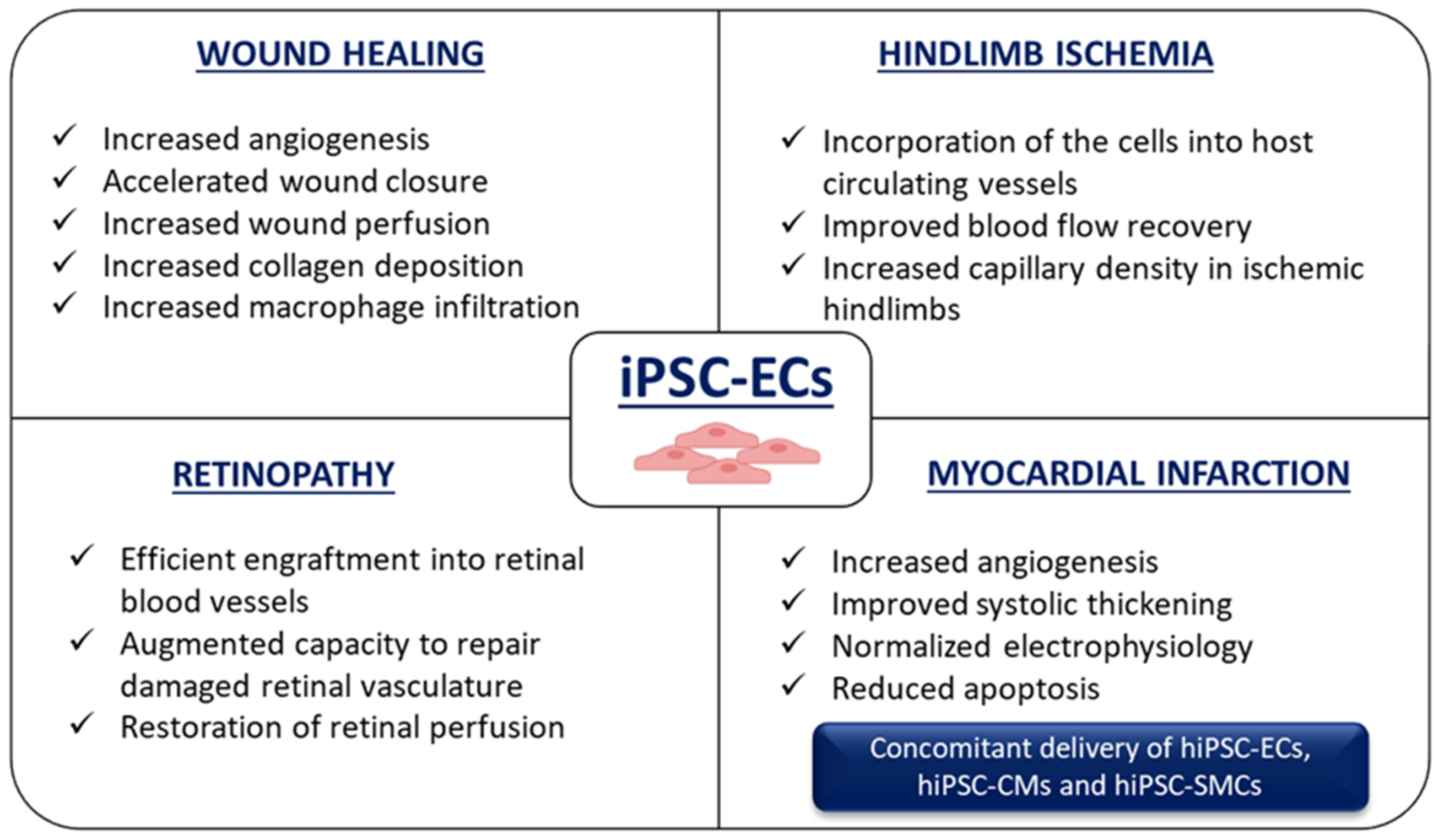
7. Application of hiPSC-ECs for Ischemia-Related Disorders
7.1. Wound Healing
7.2. Hindlimb Ischemia
7.3. Retinopathy
7.4. Myocardial Infarction
8. Limitations and Future Perspectives
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griffioen, A.W.; Bischoff, J. Oxygen sensing decoded: A Nobel concept in biology. Angiogenesis 2019, 22, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Pera, R.A.R.; Greely, H.T. Ethical and legal issues arising in research on inducing human germ cells from pluripotent stem cells. Cell Stem Cell 2013, 13, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Holmquist-Mengelbier, L.; Fredlund, E.; Löfstedt, T.; Noguera, R.; Navarro, S.; Nilsson, H.; Pietras, A.; Vallon-Christersson, J.; Borg, A.; Gradin, K.; et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 2006, 10, 413–423. [Google Scholar] [CrossRef]
- Patel, S.A.; Simon, M.C. Biology of Hypoxia-Inducible Factor-2α in Development and Disease. Cell Death Differ. 2008, 15, 628–634. [Google Scholar] [CrossRef]
- Duan, C. Hypoxia-inducible factor 3 biology: Complexities and emerging themes. Am. J. Physiol. Cell Physiol. 2016, 310, C260–C269. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 versus HIF-2—Is one more important than the other? Vasc. Pharm. 2012, 56, 245–251. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 and HIF-2 transcription factors—Similar but not identical. Mol. Cells 2010, 29, 435–442. [Google Scholar] [CrossRef]
- Larsen’s Human Embryology—5th Edition. Available online: https://www.elsevier.com/books/larsens-human-embryology/schoenwolf/978-1-4557-0684-6 (accessed on 8 July 2020).
- Hustin, J.; Schaaps, J.-P. Echocardiograhic and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am. J. Obs. Gynecol. 1987, 157, 162–168. [Google Scholar] [CrossRef]
- Marcelo Kathrina, L.; Goldie Lauren, C.; Hirschi Karen, K. Regulation of Endothelial Cell Differentiation and Specification. Circ. Res. 2013, 112, 1272–1287. [Google Scholar] [CrossRef] [PubMed]
- Olive, E.L.; Xiao, E.; Natale, D.R.; Fisher, S.A. Oxygen and lack of oxygen in fetal and placental development, feto–placental coupling, and congenital heart defects. Birth Defects Res. 2018, 110, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Bavister, B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Reproduction 1993, 99, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Batt, P.A.; Gardner, D.K.; Cameron, A.W. Oxygen concentration and protein source affect the development of preimplantation goat embryos in vitro. Reprod. Fertil. Dev. 1991, 3, 601–607. [Google Scholar] [CrossRef]
- Harvey, A.J.; Kind, K.L.; Pantaleon, M.; Armstrong, D.T.; Thompson, J.G. Oxygen-Regulated Gene Expression in Bovine Blastocysts. Biol. Reprod. 2004, 71, 1108–1119. [Google Scholar] [CrossRef]
- Pabon, J.E.; Findley, W.E.; Gibbons, W.E. The toxic effect of short exposures to the atmospheric oxygen concentration on early mouse embryonic development. Fertil. Steril. 1989, 51, 896–900. [Google Scholar] [CrossRef]
- Gomes Sobrinho, D.B.; Oliveira, J.B.A.; Petersen, C.G.; Mauri, A.L.; Silva, L.F.; Massaro, F.C.; Baruffi, R.L.; Cavagna, M.; Franco, J.G. IVF/ICSI outcomes after culture of human embryos at low oxygen tension: A meta-analysis. Reprod. Biol. Endocrinol. 2011, 9, 143. [Google Scholar] [CrossRef]
- Peng, Z.-F.; Shi, S.-L.; Jin, H.-X.; Yao, G.-D.; Wang, E.-Y.; Yang, H.-Y.; Song, W.-Y.; Sun, Y.-P. Impact of oxygen concentrations on fertilization, cleavage, implantation, and pregnancy rates of in vitro generated human embryos. Int. J. Clin. Exp. Med. 2015, 8, 6179–6185. [Google Scholar]
- Dumoulin, J.C.M.; Meijers, C.J.J.; Bras, M.; Coonen, E.; Geraedts, J.P.M.; Evers, J.L.H. Effect of oxygen concentration on human in-vitro fertilization and embryo culture. Hum. Reprod. 1999, 14, 465–469. [Google Scholar] [CrossRef]
- Shahbazi, M.N.; Jedrusik, A.; Vuoristo, S.; Recher, G.; Hupalowska, A.; Bolton, V.; Fogarty, N.N.M.; Campbell, A.; Devito, L.; Ilic, D.; et al. Self-organisation of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016, 18, 700–708. [Google Scholar] [CrossRef]
- Lee, Y.M.; Jeong, C.-H.; Koo, S.-Y.; Son, M.J.; Song, H.S.; Bae, S.-K.; Raleigh, J.A.; Chung, H.-Y.; Yoo, M.-A.; Kim, K.-W. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: A possible signal for vessel development. Dev. Dyn. 2001, 220, 175–186. [Google Scholar] [CrossRef]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998, 12, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bergeron, D.L.; Runge, A.; Adelman, D.M.; Gohil, M.; Simon, M.C. HIF-Dependent Hematopoietic Factors Regulate the Development of the Embryonic Vasculature. Dev. Cell 2006, 11, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Licht, A.H.; Müller-Holtkamp, F.; Flamme, I.; Breier, G. Inhibition of hypoxia-inducible factor activity in endothelial cells disrupts embryonic cardiovascular development. Blood 2006, 107, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.D.C.; Fryer, B.H.; Mack, F.A.; Compernolle, V.; Maltepe, E.; Adelman, D.M.; Carmeliet, P.; Simon, M.C. Hypoxia-Inducible Factors 1α and 2α Regulate Trophoblast Differentiation. Mol. Cell. Biol. 2005, 25, 10479–10491. [Google Scholar] [CrossRef]
- Burton, G.J. Oxygen, the Janus gas; its effects on human placental development and function. J. Anat. 2009, 215, 27–35. [Google Scholar] [CrossRef]
- Choi, K. The Hemangioblast: A Common Progenitor of Hematopoietic and Endothelial Cells. J. Hematother. Stem Cell Res. 2002, 11, 91–101. [Google Scholar] [CrossRef]
- Wilkinson, D.G.; Bhatt, S.; Herrmann, B.G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 1990, 343, 657–659. [Google Scholar] [CrossRef]
- Risau, W.; Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995, 11, 73–91. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, T.M.; Malik, A.B. Transcriptional Regulation of Endothelial Cell and Vascular Development. Circ. Res. 2013, 112, 1380–1400. [Google Scholar] [CrossRef]
- Millauer, B.; Wizigmann-Voos, S.; Schnürch, H.; Martinez, R.; Møller, N.P.H.; Risau, W.; Ullrich, A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72, 835–846. [Google Scholar] [CrossRef]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.-F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376, 62–66. [Google Scholar] [CrossRef]
- Suri, C.; Jones, P.F.; Patan, S.; Bartunkova, S.; Maisonpierre, P.C.; Davis, S.; Sato, T.N.; Yancopoulos, G.D. Requisite Role of Angiopoietin-1, a Ligand for the TIE2 Receptor, during Embryonic Angiogenesis. Cell 1996, 87, 1171–1180. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef]
- Kanno, S.; Oda, N.; Abe, M.; Terai, Y.; Ito, M.; Shitara, K.; Tabayashi, K.; Shibuya, M.; Sato, Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene 2000, 19, 2138–2146. [Google Scholar] [CrossRef]
- Cox, C.M.; Poole, T.J. Angioblast differentiation is influenced by the local environment: FGF-2 induces angioblasts and patterns vessel formation in the quail embryo. Dev. Dyn. 2000, 218, 371–382. [Google Scholar] [CrossRef]
- Palis, J.; Yoder, M.C. Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp. Hematol. 2001, 29, 927–936. [Google Scholar] [CrossRef]
- Xiong, J.-W. Molecular and developmental biology of the hemangioblast. Dev. Dyn. 2008, 237, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Afrikanova, I.; Chung, Y.S.; Zhang, W.J.; Arentson, E.; Fong, G.H.; Rosendahl, A.; Choi, K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development 2004, 131, 2749–2762. [Google Scholar] [CrossRef] [PubMed]
- Lugus, J.J.; Chung, Y.S.; Mills, J.C.; Kim, S.-I.; Grass, J.A.; Kyba, M.; Doherty, J.M.; Bresnick, E.H.; Choi, K. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development 2007, 134, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Lugus, J.J.; Park, C.; Ma, Y.D.; Choi, K. Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood 2009, 113, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Lancrin, C.; Sroczynska, P.; Stephenson, C.; Allen, T.; Kouskoff, V.; Lacaud, G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 2009, 457, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Yokomizo, T.; Zeigler, B.M.; Dzierzak, E.; Speck, N.A. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 2009, 457, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bergeron, D.L.; Runge, A.; Dahl, K.D.C.; Fehling, H.J.; Keller, G.; Simon, M.C. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development 2004, 131, 4623–4634. [Google Scholar] [CrossRef]
- Thompson, M.A.; Ransom, D.G.; Pratt, S.J.; MacLennan, H.; Kieran, M.W.; Detrich, H.W., III; Vail, B.; Huber, T.L.; Paw, B.; Brownlie, A.J.; et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Boil. 1998, 197, 248–269. [Google Scholar] [CrossRef]
- You, L.-R.; Lin, F.-J.; Lee, C.T.; DeMayo, F.J.; Tsai, M.-J.; Tsai, S.Y. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 2005, 435, 98–104. [Google Scholar] [CrossRef]
- Lawson, N.D.; Scheer, N.; Pham, V.N.; Kim, C.H.; Chitnis, A.B.; Campos-Ortega, J.A.; Weinstein, B.M. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 2001, 128, 3675–3683. [Google Scholar] [PubMed]
- Wang, H.-J.; Zhang, D.; Tan, Y.-Z.; Li, T. Autophagy in endothelial progenitor cells is cytoprotective in hypoxic conditions. Am. J. Physiol. Cell Physiol. 2012, 304, C617–C626. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Hilton, D.J.; Pease, S.; Willson, T.A.; Stewart, C.L.; Gearing, D.P.; Wagner, E.F.; Metcalf, D.; Nicola, N.A.; Gough, N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988, 336, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Mullen, A.C.; Wrana, J.L. TGF-β Family Signaling in Embryonic and Somatic Stem-Cell Renewal and Differentiation. Cold Spring Harb. Perspect. Biol. 2017, 9, a022186. [Google Scholar] [CrossRef]
- Najm, F.J.; Chenoweth, J.G.; Anderson, P.D.; Nadeau, J.H.; Redline, R.W.; McKay, R.D.G.; Tesar, P.J. Isolation of epiblast stem cells from pre-implantation mouse embryos. Cell Stem Cell 2011, 8, 318–325. [Google Scholar] [CrossRef]
- Lengner, C.J.; Gimelbrant, A.A.; Erwin, J.A.; Cheng, A.W.; Guenther, M.G.; Welstead, G.G.; Alagappan, R.; Frampton, G.M.; Xu, P.; Muffat, J.; et al. Derivation of Pre-X Inactivation Human Embryonic Stem Cells under Physiological Oxygen Concentrations. Cell 2010, 141, 872–883. [Google Scholar] [CrossRef]
- Ezashi, T.; Das, P.; Roberts, R.M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4783–4788. [Google Scholar] [CrossRef]
- Forsyth, N.R.; Musio, A.; Vezzoni, P.; Simpson, A.H.R.W.; Noble, B.S.; McWhir, J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells 2006, 8, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, N.R.; Kay, A.; Hampson, K.; Downing, A.; Talbot, R.; McWhir, J. Transcriptome alterations due to physiological normoxic (2% O2) culture of human embryonic stem cells. Regen. Med. 2008, 3, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Närvä, E.; Pursiheimo, J.-P.; Laiho, A.; Rahkonen, N.; Emani, M.R.; Viitala, M.; Laurila, K.; Sahla, R.; Lund, R.; Lähdesmäki, H.; et al. Continuous Hypoxic Culturing of Human Embryonic Stem Cells Enhances SSEA-3 and MYC Levels. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, U.; Czauderna, S.; Stachurska, A.; Tertil, M.; Nowak, W.; Kozakowska, M.; Poellinger, L.; Jozkowicz, A.; Loboda, A.; Dulak, J. Opposite effects of HIF-1α and HIF-2α on the regulation of IL-8 expression in endothelial cells. Free Radic. Biol. Med. 2011, 51, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Forristal, C.E.; Wright, K.L.; Hanley, N.A.; Oreffo, R.O.C.; Houghton, F.D. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction 2010, 139, 85–97. [Google Scholar] [CrossRef]
- Arthur, S.A.; Blaydes, J.P.; Houghton, F.D. Glycolysis Regulates Human Embryonic Stem Cell Self-Renewal under Hypoxia through HIF-2α and the Glycolytic Sensors CTBPs. Stem Cell Rep. 2019, 12, 728–742. [Google Scholar] [CrossRef]
- Forristal, C.E.; Christensen, D.R.; Chinnery, F.E.; Petruzzelli, R.; Parry, K.L.; Sanchez-Elsner, T.; Houghton, F.D. Environmental Oxygen Tension Regulates the Energy Metabolism and Self-Renewal of Human Embryonic Stem Cells. PLoS ONE 2013, 8, e62507. [Google Scholar] [CrossRef]
- Covello, K.L.; Simon, M.C.; Keith, B. Targeted replacement of hypoxia-inducible factor-1alpha by a hypoxia-inducible factor-2alpha knock-in allele promotes tumor growth. Cancer Res. 2005, 65, 2277–2286. [Google Scholar] [CrossRef]
- Covello, K.L.; Kehler, J.; Yu, H.; Gordan, J.D.; Arsham, A.M.; Hu, C.-J.; Labosky, P.A.; Simon, M.C.; Keith, B. HIF-2α regulates Oct-4: Effects of hypoxiaon stem cell function, embryonic development, and tumor growth. Genes Dev. 2006, 20, 557–570. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Takahashi, K.; Okita, K.; Ichisaka, T.; Yamanaka, S. Hypoxia Enhances the Generation of Induced Pluripotent Stem Cells. Cell Stem Cell 2009, 5, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Zhou, W.; Xing, Y.; Sperber, H.; Ferreccio, A.; Agoston, Z.; Kuppusamy, K.T.; Moon, R.T.; Ruohola-Baker, H. Hypoxia Inducible Factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 2014, 14, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.M.; Wu, J.C. Generation of Endothelial Cells From Human Pluripotent Stem Cells. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.M.; Hyun, J.S.; Cheng, K.T.; Vargas, M.; Mehta, D.; Ushio-Fukai, M.; Zou, L.; Pajcini, K.V.; Rehman, J.; Malik, A.B. Embryonic Stem Cell Differentiation to Functional Arterial Endothelial Cells through Sequential Activation of ETV2 and NOTCH1 Signaling by HIF1α. Stem Cell Rep. 2017, 9, 796–806. [Google Scholar] [CrossRef]
- Lee, S.-W.; Jeong, H.-K.; Lee, J.-Y.; Yang, J.; Lee, E.J.; Kim, S.-Y.; Youn, S.-W.; Lee, J.; Kim, W.J.; Kim, K.-W.; et al. Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol. Med. 2012, 4, 924–938. [Google Scholar] [CrossRef]
- Han, Y.; Kuang, S.-Z.; Gomer, A.; Ramirez-Bergeron, D.L. Hypoxia influences the vascular expansion and differentiation of embryonic stem cell cultures through the temporal expression of VEGF- receptors in an ARNT-dependent manner. Stem Cells 2010, 28, 799–809. [Google Scholar] [CrossRef]
- Prado-Lopez, S.; Conesa, A.; Armiñán, A.; Martínez-Losa, M.; Escobedo-Lucea, C.; Gandia, C.; Tarazona, S.; Melguizo, D.; Blesa, D.; Montaner, D.; et al. Hypoxia promotes efficient differentiation of human embryonic stem cells to functional endothelium. Stem Cells 2010, 28, 407–418. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, J.; Kim, H.E.; Lee, M.J.; Lee, K.I.; Yoo, E.G.; Jeon, Y.J.; Kim, D.-W.; Chae, J.-I.; Chung, H.M. Enhancement of differentiation efficiency of hESCs into vascular lineage cells in hypoxia via a paracrine mechanism. Stem Cell Res. 2011, 7, 173–185. [Google Scholar] [CrossRef]
- Cameron, C.M.; Harding, F.; Hu, W.-S.; Kaufman, D.S. Activation of hypoxic response in human embryonic stem cell-derived embryoid bodies. Exp. Biol. Med. (Maywood) 2008, 233, 1044–1057. [Google Scholar] [CrossRef]
- Kusuma, S.; Peijnenburg, E.; Patel, P.; Gerecht, S. Low oxygen tension enhances endothelial fate of human pluripotent stem cells. Arter. Thromb. Vasc. Biol. 2014, 34, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, J.; Xiao, M.; Zhou, T.; Shi, X. Role of Mir-155 in Controlling HIF-1α Level and Promoting Endothelial Cell Maturation. Sci. Rep. 2016, 6, 35316. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Torres, Á.; Ojeda, K.; Uribe, D.; Rocha, D.; Erices, J.; Niechi, I.; Ehrenfeld, P.; San Martín, R.; Quezada, C. The Adenosine A3 Receptor Regulates Differentiation of Glioblastoma Stem-Like Cells to Endothelial Cells under Hypoxia. Int. J. Mol. Sci. 2018, 19, 1228. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moon, S.-H.; Lee, S.-H.; Lee, D.-R.; Koh, G.-Y.; Chung, H.-M. Effective Isolation and Culture of Endothelial Cells in Embryoid Body Differentiated from Human Embryonic Stem Cells. Stem Cells Dev. 2007, 16, 269–280. [Google Scholar] [CrossRef]
- Cho, S.-W.; Moon, S.-H.; Lee, S.-H.; Kang, S.-W.; Kim, J.; Lim, J.M.; Kim, H.-S.; Kim, B.-S.; Chung, H.-M. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation 2007, 116, 2409–2419. [Google Scholar] [CrossRef]
- White, M.P.; Rufaihah, A.J.; Liu, L.; Ghebremariam, Y.T.; Ivey, K.N.; Cooke, J.P.; Srivastava, D. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells 2013, 31, 92–103. [Google Scholar] [CrossRef]
- Abaci, H.E.; Truitt, R.; Luong, E.; Drazer, G.; Gerecht, S. Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 2010, 298, C1527–C1537. [Google Scholar] [CrossRef]
- Diez, H.; Fischer, A.; Winkler, A.; Hu, C.-J.; Hatzopoulos, A.K.; Breier, G.; Gessler, M. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp. Cell Res. 2007, 313, 1–9. [Google Scholar] [CrossRef]
- Fish, J.E.; Wythe, J.D. The molecular regulation of arteriovenous specification and maintenance. Dev. Dyn. 2015, 244, 391–409. [Google Scholar] [CrossRef]
- Wythe, J.D.; Dang, L.T.H.; Devine, W.P.; Boudreau, E.; Artap, S.T.; He, D.; Schachterle, W.; Stainier, D.Y.R.; Oettgen, P.; Black, B.L.; et al. ETS factors regulate Vegf-dependent arterial specification. Dev. Cell 2013, 26, 45–58. [Google Scholar] [CrossRef]
- Kachamakova-Trojanowska, N.; Stepniewski, J.; Dulak, J. Human iPSCs-Derived Endothelial Cells with Mutation in HNF1A as a Model of Maturity-Onset Diabetes of the Young. Cells 2019, 8, 1440. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.; Marchetto, M.C.; Gage, F.H.; Mummery, C.L. Induced pluripotent stem cells: The new patient? Nat. Rev. Mol. Cell Biol. 2012, 13, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Drawnel, F.M.; Boccardo, S.; Prummer, M.; Delobel, F.; Graff, A.; Weber, M.; Gérard, R.; Badi, L.; Kam-Thong, T.; Bu, L.; et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep 2014, 9, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gil, C.-H.; Yoder, M.C. Differentiation, Evaluation, and Application of Human Induced Pluripotent Stem Cell–Derived Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2014–2025. [Google Scholar] [CrossRef] [PubMed]
- Barruet, E.; Morales, B.M.; Lwin, W.; White, M.P.; Theodoris, C.V.; Kim, H.; Urrutia, A.; Wong, S.A.; Srivastava, D.; Hsiao, E.C. The ACVR1 R206H mutation found in fibrodysplasia ossificans progressiva increases human induced pluripotent stem cell-derived endothelial cell formation and collagen production through BMP-mediated SMAD1/5/8 signaling. Stem Cell Res. Ther. 2016, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Hamauchi, S.; Shichinohe, H.; Uchino, H.; Yamaguchi, S.; Nakayama, N.; Kazumata, K.; Osanai, T.; Abumiya, T.; Houkin, K.; Era, T. Cellular Functions and Gene and Protein Expression Profiles in Endothelial Cells Derived from Moyamoya Disease-Specific iPS Cells. PLoS ONE 2016, 11, e0163561. [Google Scholar] [CrossRef]
- Sa, S.; Gu, M.; Chappell, J.; Shao, N.-Y.; Ameen, M.; Elliott, K.A.T.; Li, D.; Grubert, F.; Li, C.G.; Taylor, S.; et al. Induced Pluripotent Stem Cell Model of Pulmonary Arterial Hypertension Reveals Novel Gene Expression and Patient Specificity. Am. J. Respir. Crit. Care Med. 2017, 195, 930–941. [Google Scholar] [CrossRef]
- Gu, M.; Shao, N.-Y.; Sa, S.; Li, D.; Termglinchan, V.; Ameen, M.; Karakikes, I.; Sosa, G.; Grubert, F.; Lee, J.; et al. Patient-Specific iPSC-Derived Endothelial Cells Uncover Pathways that Protect against Pulmonary Hypertension in BMPR2 Mutation Carriers. Cell Stem Cell 2017, 20, 490–504.e5. [Google Scholar] [CrossRef]
- Theodoris, C.V.; Li, M.; White, M.P.; Liu, L.; He, D.; Pollard, K.S.; Bruneau, B.G.; Srivastava, D. Human Disease Modeling Reveals Integrated Transcriptional and Epigenetic Mechanisms of NOTCH1 Haploinsufficiency. Cell 2015, 160, 1072–1086. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, W.; Jiao, J.; Nielsen, J.B.; Mathis, M.R.; Heydarpour, M.; Lettre, G.; Folkersen, L.; Prakash, S.; Schurmann, C.; et al. Protein-altering and regulatory genetic variants near GATA4 implicated in bicuspid aortic valve. Nat. Commun. 2017, 8, 15481. [Google Scholar] [CrossRef]
- Roudnicky, F.; Lan, Y.; Friesen, M.; Dernick, G.; Zhang, J.D.; Staempfli, A.; Bordag, N.; Wagner-Golbs, A.; Christensen, K.; Ebeling, M.; et al. Modeling the Effects of Severe Metabolic Disease by Genome Editing of hPSC-Derived Endothelial Cells Reveals an Inflammatory Phenotype. Int. J. Mol. Sci. 2019, 20, 6201. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.-H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Forbes, J.M.; Fotheringham, A.K. Vascular complications in diabetes: Old messages, new thoughts. Diabetologia 2017, 60, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Eleftheriadou, M.; Kelaini, S.; Morrison, T.; González, M.V.; Caines, R.; Edwards, N.; Yacoub, A.; Edgar, K.; Moez, A.; et al. Targeting QKI-7 in vivo restores endothelial cell function in diabetes. Nat. Commun. 2020, 11, 3812. [Google Scholar] [CrossRef]
- Wallet, M.A.; Santostefano, K.E.; Terada, N.; Brusko, T.M. Isogenic Cellular Systems Model the Impact of Genetic Risk Variants in the Pathogenesis of Type 1 Diabetes. Front. Endocrinol. (Lausanne) 2017, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Carcamo-Orive, I.; Huang, N.F.; Quertermous, T.; Knowles, J.W. iPSC-derived endothelial cells in insulin resistance and metabolic syndrome. Arter. Thromb. Vasc. Biol. 2017, 37, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.L.; Song, S.-H.; Choi, K.-S.; Suh, W. Cooperation of endothelial and smooth muscle cells derived from human induced pluripotent stem cells enhances neovascularization in dermal wounds. Tissue Eng. Part A 2013, 19, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Clayton, Z.E.; Tan, R.P.; Miravet, M.M.; Lennartsson, K.; Cooke, J.P.; Bursill, C.A.; Wise, S.G.; Patel, S. Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model. Biosci. Rep. 2018, 38, BSR20180563. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.P.; Chan, A.H.P.; Lennartsson, K.; Miravet, M.M.; Lee, B.S.L.; Rnjak-Kovacina, J.; Clayton, Z.E.; Cooke, J.P.; Ng, M.K.C.; Patel, S.; et al. Integration of induced pluripotent stem cell-derived endothelial cells with polycaprolactone/gelatin-based electrospun scaffolds for enhanced therapeutic angiogenesis. Stem Cell Res. Ther. 2018, 9, 70. [Google Scholar] [CrossRef]
- Shen, Y.-I.; Cho, H.; Papa, A.E.; Burke, J.A.; Chan, X.Y.; Duh, E.J.; Gerecht, S. Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials 2016, 102, 107–119. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Huang, N.F.; Jamé, S.; Lee, J.C.; Nguyen, H.N.; Byers, B.; De, A.; Okogbaa, J.; Rollins, M.; Reijo-Pera, R.; et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arter. Thromb. Vasc. Biol. 2011, 31, e72–e79. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Sohn, Y.-D.; Andukuri, A.; Kim, S.; Byun, J.; Han, J.W.; Park, I.-H.; Jun, H.-W.; Yoon, Y.-S. Enhanced Therapeutic and Long-Term Dynamic Vascularization Effects of Human Pluripotent Stem Cell-Derived Endothelial Cells Encapsulated in a Nanomatrix Gel. Circulation 2017, 136, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.A.; Dewi, R.E.; Cai, L.; Hou, L.; Strassberg, Z.; Alcazar, C.A.; Heilshorn, S.C.; Huang, N.F. Protein-engineered hydrogels enhance the survival of induced pluripotent stem cell-derived endothelial cells for treatment of peripheral arterial disease. Biomater. Sci. 2018, 6, 614–622. [Google Scholar] [CrossRef]
- Huang, N.F.; Niiyama, H.; Peter, C.; De, A.; Natkunam, Y.; Fleissner, F.; Li, Z.; Rollins, M.D.; Wu, J.C.; Gambhir, S.S.; et al. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-H.; Ho, J.C.Y.; Chan, Y.-C.; Ng, J.H.L.; Au, K.-W.; Wong, L.-Y.; Siu, C.-W.; Tse, H.-F. Attenuation of Hind-Limb Ischemia in Mice with Endothelial-Like Cells Derived from Different Sources of Human Stem Cells. PLoS ONE 2013, 8, e57876. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Bhutto, I.; Zimmerlin, L.; Huo, J.S.; Nagaria, P.; Miller, D.; Rufaihah, A.J.; Talbot, C.; Aguilar, J.; Grebe, R.; et al. Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature. Circulation 2014, 129, 359–372. [Google Scholar] [CrossRef]
- Prasain, N.; Lee, M.R.; Vemula, S.; Meador, J.L.; Yoshimoto, M.; Ferkowicz, M.J.; Fett, A.; Gupta, M.; Rapp, B.M.; Saadatzadeh, M.R.; et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat. Biotechnol. 2014, 32, 1151–1157. [Google Scholar] [CrossRef]
- Cho, H.; Macklin, B.L.; Lin, Y.-Y.; Zhou, L.; Lai, M.J.; Lee, G.; Gerecht, S.; Duh, E.J. iPSC-derived endothelial cell response to hypoxia via SDF1a/CXCR4 axis facilitates incorporation to revascularize ischemic retina. JCI Insight 2020, 5, e131828. [Google Scholar] [CrossRef]
- Xiong, Q.; Hill, K.L.; Li, Q.; Suntharalingam, P.; Mansoor, A.; Wang, X.; Jameel, M.N.; Zhang, P.; Swingen, C.; Kaufman, D.S.; et al. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells 2011, 29, 367–375. [Google Scholar] [CrossRef]
- Ye, L.; Chang, Y.-H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014, 15, 750–761. [Google Scholar] [CrossRef]
- Masumoto, H.; Matsuo, T.; Yamamizu, K.; Uosaki, H.; Narazaki, G.; Katayama, S.; Marui, A.; Shimizu, T.; Ikeda, T.; Okano, T.; et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells 2012, 30, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Ikuno, T.; Takeda, M.; Fukushima, H.; Marui, A.; Katayama, S.; Shimizu, T.; Ikeda, T.; Okano, T.; Sakata, R.; et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci. Rep. 2014, 4, 6716. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.F.; Okogbaa, J.; Lee, J.C.; Jha, A.; Zaitseva, T.S.; Paukshto, M.V.; Sun, J.S.; Punjya, N.; Fuller, G.G.; Cooke, J.P. The modulation of endothelial cell morphology, function, and survival using anisotropic nanofibrillar collagen scaffolds. Biomaterials 2013, 34, 4038–4047. [Google Scholar] [CrossRef] [PubMed]
- Jazwa, A.; Florczyk, U.; Grochot-Przeczek, A.; Krist, B.; Loboda, A.; Jozkowicz, A.; Dulak, J. Limb ischemia and vessel regeneration: Is there a role for VEGF? Vasc. Pharm. 2016, 86, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Padgett, M.E.; McCord, T.J.; McClung, J.M.; Kontos, C.D. Methods for Acute and Subacute Murine Hindlimb Ischemia. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Saredy, J.; Yang, W.Y.; Sun, Y.; Lu, Y.; Saaoud, F.; Drummer, C.; Johnson, C.; Xu, K.; Jiang, X.; et al. Vascular Endothelial Cells and Innate Immunity. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e138–e152. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Lee, A.S.; Xu, D.; Plews, J.R.; Nguyen, P.K.; Nag, D.; Lyons, J.K.; Han, L.; Hu, S.; Lan, F.; Liu, J.; et al. Preclinical derivation and imaging of autologously transplanted canine induced pluripotent stem cells. J. Biol. Chem. 2011, 286, 32697–32704. [Google Scholar] [CrossRef]
- Paik, D.T.; Tian, L.; Lee, J.; Sayed, N.; Chen, I.Y.; Rhee, S.; Rhee, J.-W.; Kim, Y.; Wirka, R.C.; Buikema, J.W.; et al. Large-Scale Single-Cell RNA-Seq Reveals Molecular Signatures of Heterogeneous Populations of Human Induced Pluripotent Stem Cell-Derived Endothelial Cells. Circ. Res. 2018, 123, 443–450. [Google Scholar] [CrossRef]
- Uenishi, G.I.; Jung, H.S.; Kumar, A.; Park, M.A.; Hadland, B.K.; McLeod, E.; Raymond, M.; Moskvin, O.; Zimmerman, C.E.; Theisen, D.J.; et al. NOTCH signaling specifies arterial-type definitive hemogenic endothelium from human pluripotent stem cells. Nat. Commun. 2018, 9, 1828. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.S.; Kumar, A.; Slukvin, I.I. Functional Heterogeneity of Endothelial Cells Derived from Human Pluripotent Stem Cells. Stem Cells Dev. 2018, 27, 524–533. [Google Scholar] [CrossRef]
- Schaum, N.; Karkanias, J.; Neff, N.F.; May, A.P.; Quake, S.R.; Wyss-Coray, T.; Darmanis, S.; Batson, J.; Botvinnik, O.; Chen, M.B.; et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 2018, 562, 367–372. [Google Scholar] [CrossRef]
- Paik, D.T.; Chandy, M.; Wu, J.C. Patient and Disease–Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol. Rev. 2020, 72, 320–342. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Seppanen, E.; Chong, M.S.K.; Yeo, J.S.L.; Teo, E.Y.L.; Chan, J.K.Y.; Fisk, N.M.; Khosrotehrani, K. Prospective Surface Marker-Based Isolation and Expansion of Fetal Endothelial Colony-Forming Cells From Human Term Placenta. Stem Cells Transl. Med. 2013, 2, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, R.S.; Vadivel, A.; Zhong, S.; Zong, S.; McConaghy, S.; Ohls, R.; Yoder, M.C.; Thébaud, B. The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nat. Protoc. 2015, 10, 1697–1708. [Google Scholar] [CrossRef]
- Jia, J.; Ma, B.; Wang, S.; Feng, L. Therapeutic Potential of Endothelial Colony Forming Cells Derived from Human Umbilical Cord Blood. Curr. Stem Cell Res. Ther. 2019, 14, 460–465. [Google Scholar] [CrossRef]
- Paschalaki, K.E.; Randi, A.M. Recent Advances in Endothelial Colony Forming Cells Toward Their Use in Clinical Translation. Front. Med. 2018, 5, 295. [Google Scholar] [CrossRef]
| Cell Type | Hypoxia Level | Duration # | Scheme of the Experiment | Angiogenesis-Related Markers and/or Properties Increased vs. Normoxia (20% or 21% O2) | References |
|---|---|---|---|---|---|
| mESCs | 1% O2 | 7 days | Undifferentiated mESCs plated on collagen IV-coated plate and differentiated in ECDM containing Iscove’s Modified Dulbecco’s Medium (50%), 50% Ham’s F-12 medium (50%), supplemented with N2, B27 retinoic acid, 0.05% lipid-rich BSA, ascorbic acid (50 ng/mL), MTG (50 ng/mL), human VEGF (50 ng/mL), human FGF2 (10 ng/mL), and BMP4 (2 ng/mL) | CD144, PECAM1 mRNA and protein Percentage of CD144+PECAM1+ cell population HIF-1α protein Glut1, Pdk1, Pdk3, Pdk4, Ldha, Etv2, Ephb2, Notch1, Dll4 mRNA | [76] |
| hESCs | 1% O2 | 7 days | Undifferentiated hESCs plated on Matrigel-coated plate in Essential 8 medium. The next day, medium replaced with ECDM containing Iscove’s Modified Dulbecco’s Medium (50%), Ham’s F-12 medium (50%), insulin-transferrin-selenium-X, chemically defined lipid concentrate (1%), BSA (5 mg/mL), ascorbic acid (50 ng/mL), and 1-Thioglycerol (200 μM) supplemented with activin A (25ng/mL), BMP4 (10 ng/mL), VEGF (50 ng/mL), CHIR (1.5 μM) and incubated for 3 days. At days 3, 5, 7 medium replaced with serum-free differentiation medium supplemented with VEGF (50 ng/mL) and SB431542 (10 μM) | Percentage of CD144+PECAM1+ cell population | |
| hESCs | 1% and/or 5% O2 | up to 15 days | Undifferentiated hESCs cultured on ECM from human foreskin fibroblasts under hypoxic conditions in a foreskin-fibroblast conditioned medium composed of DMEM/F12, BME (100 µM), L-glutamine (1 mM), NEAA (100 mM), serum replacement (20%) without changing the media | Endothelial-like morphology of the cells VEGF, VEGFB, VEGFR2 mRNA ANGPTL4 mRNA and protein CD34 mRNA and protein, CD34+ cell population ECs phenotype: * CD34+ population: 57.7% VEGF+ population: 53.4% ANGPTL4+ population: 80.8% PECAM1+ population: 11.6% vWF+ population: 31.9% | [79] |
| As above but after 5–7 days cells plated on Matrigel and further cultured for 1–7 days | Sprouting of cellular aggregates expressing CD34 (24 h) Formation of cordlike structures expressing CD34 and VEGFR2 (3 days) More dense cordlike structures expressing PECAM1 and vWF (7 days) | ||||
| mEBs Derived from mESCs | 1% O2 | 3 days | After 3 days from mEBs formation, mEBs were kept under hypoxic conditions for 3 days for spontaneous differentiation | Vegf, Vegfr2, Pecam1, Acta2, Cd144, Vegfr1, Fgf2 mRNA HIF-1α, HIF-2α protein | [77] |
| 2 days | After 3 days from mEBs formation, mEBs were kept under hypoxic conditions for 2 days and seeded on a plate coated with 0.3% gelatin in DMEM/10% FBS. Next day, the culture medium was replaced with EGM2 medium supplemented with FBS (5%) and cells were incubated up to 14 days | Spreading of mEBs PECAM1+, CD144+ cells HIF-1α protein Vegf, Pecam1, Vegfr2, Cd144 mRNA Junctional distribution of PECAM1 exhibiting tubular and branching structure especially in the central region on 7 and 14 days after reattachment α-SMA, CD144 positive cells in the outgrowth region on later time points, 14 days after reattachment | |||
| mEBs Derived from mESCs | 3% O2 | 5 to 10 days | mEBs differentiated under hypoxic conditions in methylcellulose or in suspension for 5 to 9 days without exogenous VEGF | PECAM1+ cells | [78] |
| mEBs differentiated for 7 and 10 days | Adm, Ang1, Ang2, Vegfr2, Tie2, Tie1 mRNA (by day 7) Epo, Tie2, Tie1 mRNA (at day 10) | ||||
| Differentiation of mESCs into 10-day EBs in methylcellulose containing VEGF (25 ng/mL) and FGF2 (100 ng/mL). EBs were then replated in collagen-type-I gel matrix for 4 days | Increased percentage of highly angiogenic, sprouting cells | ||||
| As above but with a lower concentration of FGF2 (25 ng/mL) and VEGF (5 ng/mL or without this factor) | Elevated number of highly angiogenic, sprouting cells | ||||
| EBs differentiated in suspension or methylcellulose cultures in the absence of exogenous VEGF under hypoxic conditions up to 9 days | VEGFR2+mVEGFR1+ cells sVEGFR1 protein | ||||
| hEBs Derived from hESCs | 3% O2 | 7 days | hEBs transferred to hypoxic conditions 3 days after hEBs formation (cultured in DMEM/F12 medium supplemented with serum replacement (10%), L-glutamine (1 mM), NEAA (1%), BME (100 mM) without FGF2 treatment | VEGFR2, PECAM1, CD144, TIE2, FGFR1, PDGFBR mRNA HIF-1α, VEGF, FGF2, ANG1, PDGFB/PDGF-BB on mRNA and protein VEGF, FGF2, PDGF-BB and, to a lesser extent ANG1 on protein (secreted) Percentage of VEGFR1+, TIE2+, VEGFR2+, CD144+ and PECAM1+ cell populations | [80] |
| Cells plated on Matrigel after 7 days for additional 3 days | PECAM1+ and vWF+ cells spontaneously forming vessel-like structures Increased number of sprouts and, to a lesser extent, their length | ||||
| hEBs Derived from hESCs | 1% and 5% O2 | 7 days | Differentiating hEBs exposed to hypoxic conditions in a sealed 6.2-L modulator incubator; half-media changes occurred every 3 to 4 days as needed | HIF-1α protein VEGF, GLUT1 mRNA | [81] |
| hESCs and/or hiPSCs | 5% O2 | 6 days (primed) or 12 days (continuous) | hPSCs plated onto collagen IV-coated plates and cultured in a differentiation medium composed of α-MEM, FBS (10%) and BME (0.1 mM) Primed: cells attached in normoxic conditions for 4 hours and then subjected to hypoxia. On day 6, differentiated cells were collected, seeded on collagen-type-IV-coated plates in ECGM supplemented with FBS (2%) VEGF (50 ng/mL) and SB431542 (10 µM) for an additional 6 days Continuous: cells attached for 4 hours in normoxic conditions, and then subjected to continuous 5% O2 conditions | After 6 days: CD34, VEGFR2, CD56 mRNA Primed and continuous: CD144, PECAM1 mRNA; PECAM1+ cells; lectin binding, uptake of acLDL, tube formation on Matrigel Continuous: endothelial-like morphology with bundles of elongated cells and cobblestone area-forming cells; CD144+ cells; CD144 and PDGFRβ localized with CD144+ clusters surrounded by PDGFRβ+ cells | [82] |
| hESCs and/or hiPSCs | 1% and 5% O2 | up to 3 days | hESC and hiPSC cells grown on an inactivated mouse embryonic feeder layer in a growth medium consisting of 80% ES-DMEM/F12 supplemented with 20% KSR and FGF2 (4 and 10 ng/mL for hESCs and hiPSCs, respectively). For the experiment, cells were seeded on Matrigel-coated plates for feeder-free culturing in a conditioned medium supplemented with the same FGF2 concentrations above. Cells were allowed to attach in atmospheric oxygen for 24 h before the culture under low oxygen tension. | HIF-1α protein VEGF, ANG1, ANG2, GLUT1 mRNA | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podkalicka, P.; Stępniewski, J.; Mucha, O.; Kachamakova-Trojanowska, N.; Dulak, J.; Łoboda, A. Hypoxia as a Driving Force of Pluripotent Stem Cell Reprogramming and Differentiation to Endothelial Cells. Biomolecules 2020, 10, 1614. https://doi.org/10.3390/biom10121614
Podkalicka P, Stępniewski J, Mucha O, Kachamakova-Trojanowska N, Dulak J, Łoboda A. Hypoxia as a Driving Force of Pluripotent Stem Cell Reprogramming and Differentiation to Endothelial Cells. Biomolecules. 2020; 10(12):1614. https://doi.org/10.3390/biom10121614
Chicago/Turabian StylePodkalicka, Paulina, Jacek Stępniewski, Olga Mucha, Neli Kachamakova-Trojanowska, Józef Dulak, and Agnieszka Łoboda. 2020. "Hypoxia as a Driving Force of Pluripotent Stem Cell Reprogramming and Differentiation to Endothelial Cells" Biomolecules 10, no. 12: 1614. https://doi.org/10.3390/biom10121614
APA StylePodkalicka, P., Stępniewski, J., Mucha, O., Kachamakova-Trojanowska, N., Dulak, J., & Łoboda, A. (2020). Hypoxia as a Driving Force of Pluripotent Stem Cell Reprogramming and Differentiation to Endothelial Cells. Biomolecules, 10(12), 1614. https://doi.org/10.3390/biom10121614





