BRCA1-A and BRISC: Multifunctional Molecular Machines for Ubiquitin Signaling
Abstract
1. Introduction
1.1. K63-Linked Ubiquitination
1.2. Deubiquitination Complexes
1.3. Discovery of BRCA1-A and BRISC
1.4. Evolution of BRCA1-A and BRISC
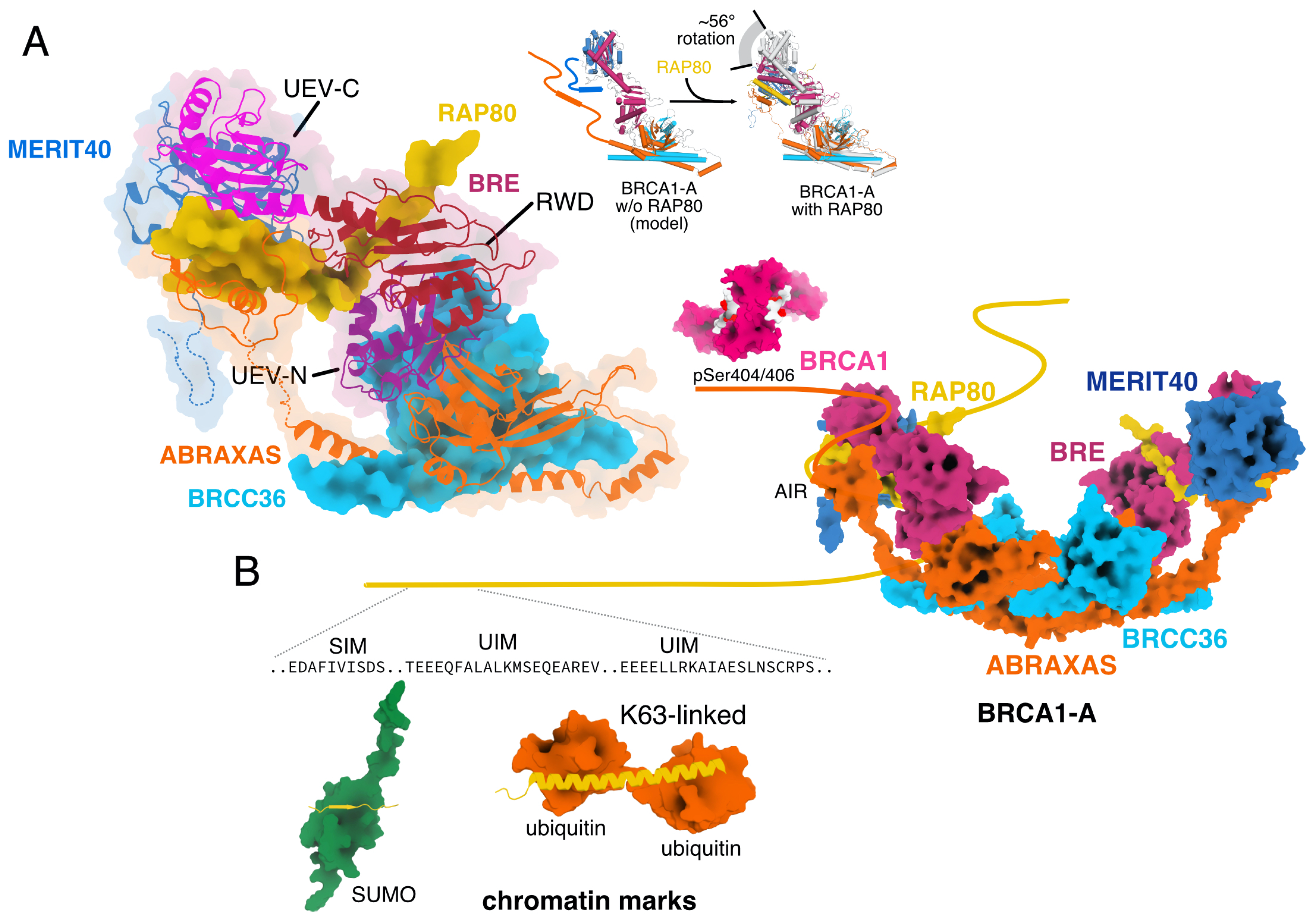
2. Subunits and Complex Assembly
2.1. Nomenclature
2.2. BRCC36
2.3. ABRAXAS
2.4. ABRO1
2.5. BRE
2.6. MERIT40
2.7. RAP80
2.8. Assembly of BRCA1-A and BRISC Complex
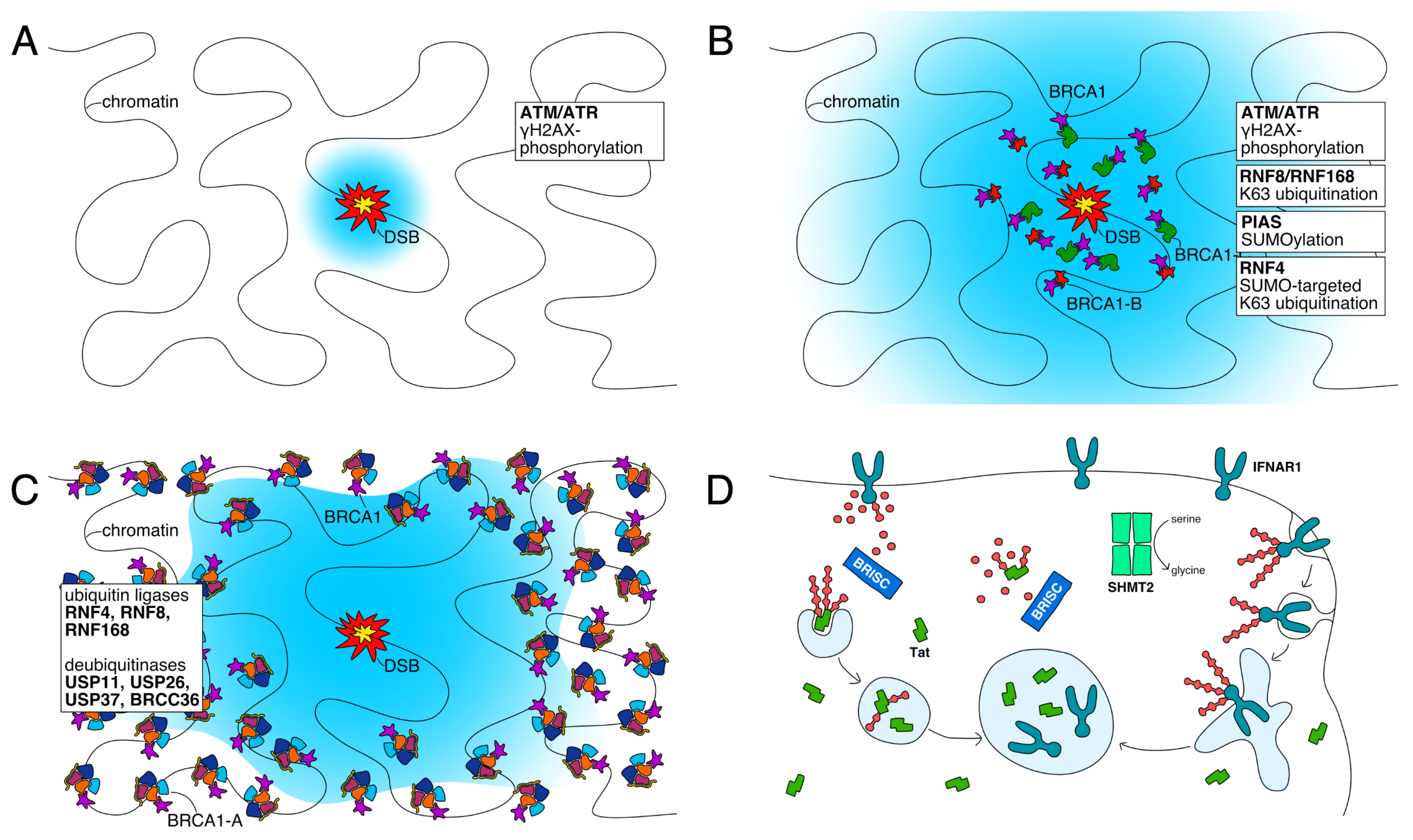
3. BRCA1-A Suppresses Homologous Recombination
3.1. Ubiquitin Marks Recruit BRCA1-A
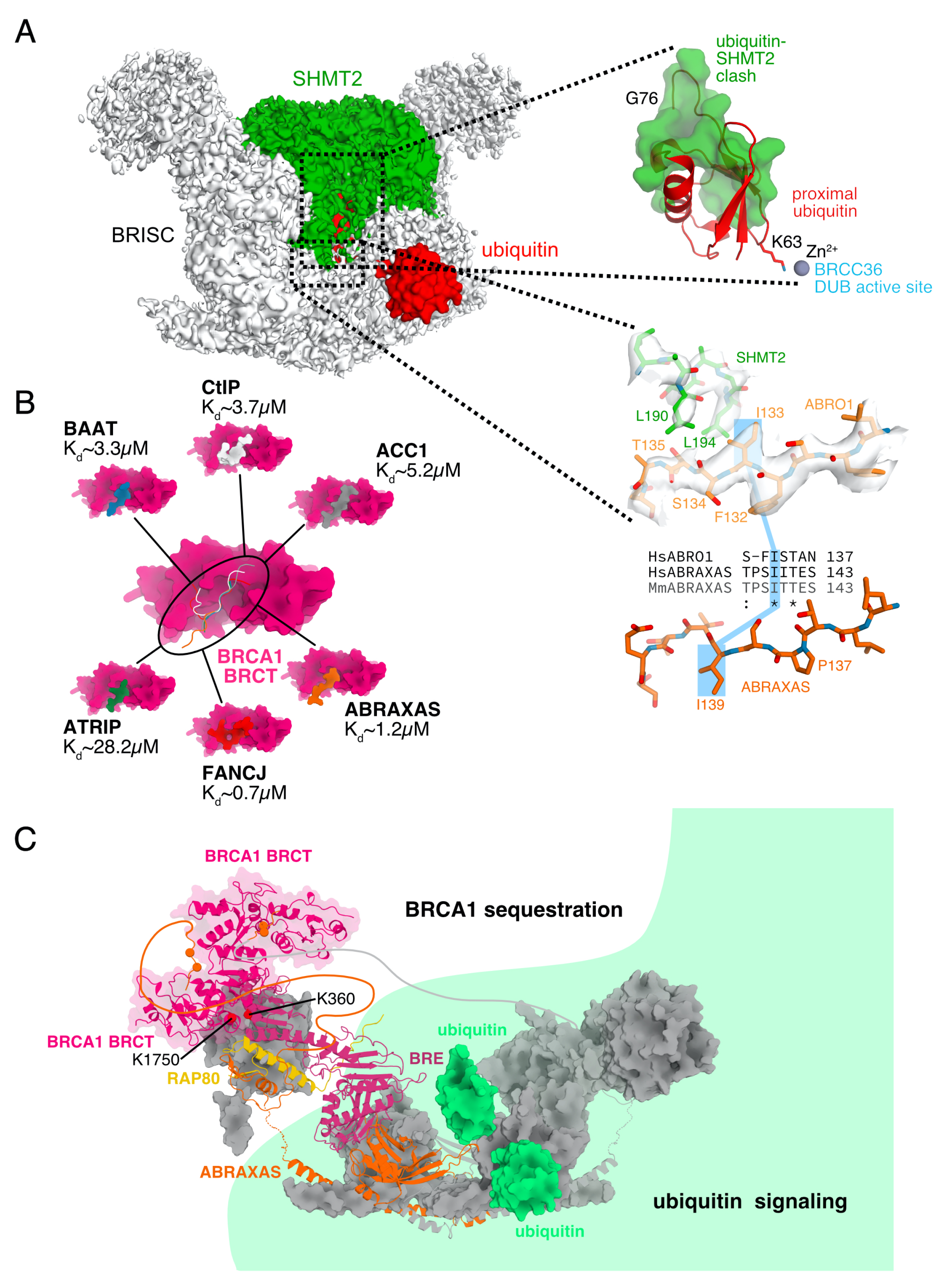
3.2. BRCA1 Sequestration by BRCA1-A
3.3. Ubiquitin Processing at DNA Repair Foci
4. BRISC Functions in Immune Response, Mitosis and Hematopoiesis
4.1. Interferon Response Control by BRISC-SHMT2
4.2. BRISC Counteracts Degradation of HIV-1 Tat
4.3. BRISC Functions in Mitosis
4.4. BRISC Controls JAK2 in Hematopoiesis
5. Specificity and Regulation of BRCC36
5.1. Activation by Assembly
5.2. Linkage Specificity
5.3. Length Selectivity
5.4. Mixed Chains
5.5. SHMT2 Inhibits BRISC
6. High-Affinity Binding of BRCA1
6.1. ABRAXAS-BRCA1 Complex Formation
6.2. BRCA1 Complexes
7. BRCA1-A and BRISC in Human Health
8. Summary and Outlook
Funding
Conflicts of Interest
Abbreviations
| DUB | Deubiquitinating enzyme |
| HR | Homologous Recombination |
| JAMM/MPN | JAB1/MPN/MOV34 metalloenzyme domain |
| NHEJ | Non-Homologous End Joining |
| PLP | pyridoxal 5’ -phosphate |
| RWD | RING finger and WD repeat containing proteins and DEXDc helicases |
| SIM | SUMO-Interacting Motif |
| UEV | Ubiquitin E2 Variant domain |
| UIM | Ubiquitin-Interacting Motif |
| VWA | Von Willebrand factor (vWF) type A domain |
References
- Komander, D.; Rape, M. The Ubiquitin Code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Kauko, A.; Lehto, K. Eukaryote specific folds: Part of the whole. Proteins Struct. Funct. Bioinform. 2018, 86, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Delley, C.L.; Müller, A.U.; Ziemski, M.; Weber-Ban, E. Prokaryotic Ubiquitin-Like Protein and Its Ligase/Deligase Enyzmes. J. Mol. Biol. 2017, 429, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Polge, C.; Uttenweiler-Joseph, S.; Leulmi, R.; Heng, A.E.; Burlet-Schiltz, O.; Attaix, D.; Taillandier, D. Deciphering the ubiquitin proteome: Limits and advantages of high throughput global affinity purification-mass spectrometry approaches. Int. J. Biochem. Cell Biol. 2013, 45, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Hospenthal, M.K.; Freund, S.M.V.; Komander, D. Assembly, analysis and architecture of atypical ubiquitin chains. Nat. Struct. Mol. Biol. 2013, 20, 555–565. [Google Scholar] [CrossRef]
- Ye, Y.; Blaser, G.; Horrocks, M.H.; Ruedas-Rama, M.J.; Ibrahim, S.; Zhukov, A.A.; Orte, A.; Klenerman, D.; Jackson, S.E.; Komander, D. Ubiquitin chain conformation regulates recognition and activity of interacting proteins. Nature 2012, 492, 266–270. [Google Scholar] [CrossRef]
- Bremm, A.; Freund, S.M.V.; Komander, D. Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nat. Struct. Mol. Biol. 2010, 17, 939–947. [Google Scholar] [CrossRef]
- Komander, D.; Reyes-Turcu, F.; Licchesi, J.D.F.; Odenwaelder, P.; Wilkinson, K.D.; Barford, D. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009, 10, 466–473. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Ciechanover, A. The Ubiquitin Code in the Ubiquitin-Proteasome System and Autophagy. Trends Biochem. Sci. 2017, 42, 873–886. [Google Scholar] [CrossRef]
- Erpapazoglou, Z.; Walker, O.; Haguenauer-Tsapis, R. Versatile Roles of K63-Linked Ubiquitin Chains in Trafficking. Cells 2014, 3, 1027–1088. [Google Scholar] [CrossRef]
- Clague, M.J.; Urbé, S. Endocytosis: The DUB version. Trends Cell Biol. 2006, 16, 551–559. [Google Scholar] [CrossRef]
- Leznicki, P.; Kulathu, Y. Mechanisms of regulation and diversification of deubiquitylating enzyme function. J. Cell Sci. 2017, 130, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Ritorto, M.S.; Ewan, R.; Perez-Oliva, A.B.; Knebel, A.; Buhrlage, S.J.; Wightman, M.; Kelly, S.M.; Wood, N.T.; Virdee, S.; Gray, N.S.; et al. Screening of DUB activity and specificity by MALDI-TOF mass spectrometry. Nat. Commun. 2014, 5, 4763. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Muniyappan, S.; Tran, N.N.; Park, H.; Lee, S.B.; Lee, B.H. Deubiquitination Reactions on the Proteasome for Proteasome Versatility. Int. J. Mol. Sci. 2020, 21, 5312. [Google Scholar] [CrossRef]
- Dong, Y.; Hakimi, M.A.; Chen, X.; Kumaraswamy, E.; Cooch, N.S.; Godwin, A.K.; Shiekhattar, R. Regulation of BRCC, a holoenzyme complex containing BRCA1 and BRCA2, by a signalosome-like subunit and its role in DNA repair. Mol. Cell 2003, 12, 1087–1099. [Google Scholar] [CrossRef]
- Kim, H.; Chen, J.; Yu, X. Ubiquitin-Binding Protein RAP80 Mediates BRCA1-Dependent DNA Damage Response. Science 2007, 316, 1202–1205. [Google Scholar] [CrossRef]
- Sobhian, B.; Shao, G.; Lilli, D.R.; Culhane, A.C.; Moreau, L.A.; Xia, B.; Livingston, D.M.; Greenberg, R.A. RAP80 Targets BRCA1 to Specific Ubiquitin Structures at DNA Damage Sites. Science 2007, 316, 1198–1202. [Google Scholar] [CrossRef]
- Wang, B.; Matsuoka, S.; Ballif, B.A.; Zhang, D.; Smogorzewska, A.; Gygi, S.P.; Elledge, S.J. Abraxas and RAP80 Form a BRCA1 Protein Complex Required for the DNA Damage Response. Science 2007, 316, 1194–1198. [Google Scholar] [CrossRef]
- Cooper, E.M.; Cutcliffe, C.; Kristiansen, T.Z.; Pandey, A.; Pickart, C.M.; Cohen, R.E. K63-specific deubiquitination by two JAMM/MPN+ complexes: BRISC-associated Brcc36 and proteasomal Poh1. EMBO J. 2009, 28, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Huang, J.; Chen, J. MERIT40 facilitates BRCA1 localization and DNA damage repair. Genes Dev. 2009, 23, 719–728. [Google Scholar] [CrossRef]
- Shao, G.; Patterson-Fortin, J.; Messick, T.E.; Feng, D.; Shanbhag, N.; Wang, Y.; Greenberg, R.A. MERIT40 controls BRCA1-Rap80 complex integrity and recruitment to DNA double-strand breaks. Genes Dev. 2009, 23, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hurov, K.; Hofmann, K.; Elledge, S.J. NBA1, a new player in the Brca1 A complex, is required for DNA damage resistance and checkpoint control. Genes Dev. 2009, 23, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kim, J.A.; Castillo, A.; Huang, M.; Liu, J.; Wang, B. NBA1/MERIT40 and BRE Interaction Is Required for the Integrity of Two Distinct Deubiquitinating Enzyme BRCC36-containing Complexes. J. Biol. Chem. 2011, 286, 11734–11745. [Google Scholar] [CrossRef] [PubMed]
- Zeqiraj, E.; Tian, L.; Piggott, C.A.; Pillon, M.C.; Duffy, N.M.; Ceccarelli, D.F.; Keszei, A.F.A.; Lorenzen, K.; Kurinov, I.; Orlicky, S.; et al. Higher-Order Assembly of BRCC36–KIAA0157 Is Required for DUB Activity and Biological Function. Mol. Cell 2015, 59, 970–983. [Google Scholar] [CrossRef]
- Rabl, J.; Bunker, R.D.; Schenk, A.D.; Cavadini, S.; Gill, M.E.; Abdulrahman, W.; Andrés-Pons, A.; Luijsterburg, M.S.; Ibrahim, A.F.M.; Branigan, E.; et al. Structural Basis of BRCC36 Function in DNA Repair and Immune Regulation. Mol. Cell 2019, 75, 483–497. [Google Scholar] [CrossRef]
- Walden, M.; Tian, L.; Ross, R.L.; Sykora, U.M.; Byrne, D.P.; Hesketh, E.L.; Masandi, S.K.; Cassel, J.; George, R.; Ault, J.R.; et al. Metabolic control of BRISC–SHMT2 assembly regulates immune signalling. Nature 2019, 570, 194–199. [Google Scholar] [CrossRef]
- Block-Schmidt, A.S.; Dukowic-Schulze, S.; Wanieck, K.; Reidt, W.; Puchta, H. BRCC36A is epistatic to BRCA1 in DNA crosslink repair and homologous recombination in Arabidopsis thaliana. Nucleic Acids Res. 2011, 39, 146–154. [Google Scholar] [CrossRef]
- Uhlén, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Wu, Q.; Paul, A.; Su, D.; Mehmood, S.; Foo, T.K.; Ochi, T.; Bunting, E.L.; Xia, B.; Robinson, C.V.; Wang, B.; et al. Structure of BRCA1-BRCT/Abraxas Complex Reveals Phosphorylation-Dependent BRCT Dimerization at DNA Damage Sites. Mol. Cell 2016, 61, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Elledge, S.J. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc. Natl. Acad. Sci. USA 2007, 104, 20759–20763. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.M.; Boeke, J.D.; Cohen, R.E. Specificity of the BRISC deubiquitinating enzyme is not due to selective binding to Lys63-linked polyubiquitin. J. Biol. Chem. 2010, 285, 10344–10352. [Google Scholar] [CrossRef] [PubMed]
- Mevissen, T.E.T.; Komander, D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 2017, 86, 159–192. [Google Scholar] [CrossRef] [PubMed]
- Lingaraju, G.M.; Bunker, R.D.; Cavadini, S.; Hess, D.; Hassiepen, U.; Renatus, M.; Fischer, E.S.; Thomä, N.H. Crystal structure of the human COP9 signalosome. Nature 2014, 512, 161–165. [Google Scholar] [CrossRef]
- Pathare, G.R.; Nagy, I.; Śledź, P.; Anderson, D.J.; Zhou, H.J.; Pardon, E.; Steyaert, J.; Förster, F.; Bracher, A.; Baumeister, W. Crystal structure of the proteasomal deubiquitylation module Rpn8-Rpn11. Proc. Natl. Acad. Sci. USA 2014, 111, 2984–2989. [Google Scholar] [CrossRef]
- Solyom, S.; Aressy, B.; Pylkäs, K.; Patterson-Fortin, J.; Hartikainen, J.M.; Kallioniemi, A.; Kauppila, S.; Nikkilä, J.; Kosma, V.M.; Mannermaa, A.; et al. Breast cancer-associated Abraxas mutation disrupts nuclear localization and DNA damage response functions. Sci. Transl. Med. 2012, 4, 122ra23. [Google Scholar] [CrossRef]
- Donaghy, R.; Han, X.; Rozenova, K.; Lv, K.; Jiang, Q.; Doepner, M.; Greenberg, R.A.; Tong, W. The BRISC deubiquitinating enzyme complex limits hematopoietic stem cell expansion by regulating JAK2 K63-ubiquitination. Blood 2019, 133, 1560–1571. [Google Scholar] [CrossRef]
- Hurley, J.H.; Lee, S.; Prag, G. Ubiquitin-binding domains. Biochem. J. 2006, 399, 361. [Google Scholar] [CrossRef]
- Hicke, L.; Schubert, H.L.; Hill, C.P. Ubiquitin-binding domains. Nat. Rev. Mol. Cell Biol. 2005, 6, 610–621. [Google Scholar] [CrossRef]
- Kyrieleis, O.J.P.; McIntosh, P.B.; Webb, S.R.; Calder, L.J.; Lloyd, J.; Patel, N.A.; Martin, S.R.; Robinson, C.V.; Rosenthal, P.B.; Smerdon, S.J. Three-Dimensional Architecture of the Human BRCA1-A Histone Deubiquitinase Core Complex. Cell Rep. 2016, 17, 3099–3106. [Google Scholar] [CrossRef]
- Anamika; Spyracopoulos, L. Molecular Basis for Phosphorylation-dependent SUMO Recognition by the DNA Repair Protein RAP80. J. Biol. Chem. 2016, 291, 4417–4428. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshikawa, A.; Mimura, H.; Yamashita, M.; Yamagata, A.; Fukai, S. Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by tandem UIMs of RAP80. EMBO J. 2009, 28, 2461–2468. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, X.; Lary, J.W.; Cole, J.L.; Walters, K.J. Defining how Ubiquitin Receptors hHR23a and S5a Bind Polyubiquitin. J. Mol. Biol. 2007, 369, 168–176. [Google Scholar] [CrossRef]
- Guettler, S.; LaRose, J.; Petsalaki, E.; Gish, G.; Scotter, A.; Pawson, T.; Rottapel, R.; Sicheri, F. Structural Basis and Sequence Rulesfor Substrate Recognition by Tankyrase Explain the Basis for Cherubism Disease. Cell 2011, 147, 1340–1354. [Google Scholar] [CrossRef] [PubMed]
- Vikrant; Sawant, U.U.; Varma, A.K. Role of MERIT40 in stabilization of BRCA1 complex: A protein-protein interaction study. Biochem. Biophys. Res. Commun. 2014, 446, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Anamika; Markin, C.J.; Rout, M.K.; Spyracopoulos, L. Molecular basis for impaired DNA damage response function associated with the RAP80 ΔE81 defect. J. Biol. Chem. 2014, 289, 12852–12862. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, C.M.; Berndsen, C.E.; Zhu, J.; Gupta, V.; Datta, A.; Greenberg, R.A.; Wolberger, C.; Matunis, M.J. RNF4-dependent hybrid SUMO-ubiquitin chains are signals for RAP80 and thereby mediate the recruitment of BRCA1 to sites of DNA damage. Sci. Signal. 2012, 5, ra88. [Google Scholar] [CrossRef]
- Hu, X.; Paul, A.; Wang, B. Rap80 Protein Recruitment to DNA Double-strand Breaks Requires Binding to Both Small Ubiquitin-like Modifier (SUMO) and Ubiquitin Conjugates. J. Biol. Chem. 2012, 287, 25510–25519. [Google Scholar] [CrossRef]
- Bian, C.; Wu, R.; Cho, K.; Yu, X. Loss of BRCA1-A complex function in RAP80 null tumor cells. PLoS ONE 2012, 7, e40406. [Google Scholar] [CrossRef]
- Patterson-Fortin, J.; Shao, G.; Bretscher, H.; Messick, T.E.; Greenberg, R.A. Differential Regulation of JAMM Domain Deubiquitinating Enzyme Activity within the RAP80 Complex. J. Biol. Chem. 2010, 285, 30971–30981. [Google Scholar] [CrossRef]
- Symington, L.S.; Gautier, J. Double-Strand Break End Resection and Repair Pathway Choice. Annu. Rev. Genet. 2011, 45, 247–271. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Baer, R.; Gautier, J. DNA double-strand break repair pathway choice and cancer. DNA Repair 2014, 19, 169–175. [Google Scholar] [CrossRef]
- Dantuma, N.P.; Pfeiffer, A. Real Estate in the DNA Damage Response: Ubiquitin and SUMO Ligases Home in on DNA Double-Strand Breaks. Front. Genet. 2016, 7, 58. [Google Scholar] [CrossRef]
- Nie, M.; Boddy, M. Cooperativity of the SUMO and Ubiquitin Pathways in Genome Stability. Biomolecules 2016, 6, 14. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef]
- Kakarougkas, A.; Jeggo, P.A. DNA DSB repair pathway choice: An orchestrated handover mechanism. Br. J. Radiol. 2014, 87, 20130685. [Google Scholar] [CrossRef]
- Smeenk, G.; Mailand, N. Writers, Readers, and Erasers of Histone Ubiquitylation in DNA Double-Strand Break Repair. Front. Genet. 2016, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Thorslund, T.; Ripplinger, A.; Hoffmann, S.; Wild, T.; Uckelmann, M.; Villumsen, B.; Narita, T.; Sixma, T.K.; Choudhary, C.; Bekker-Jensen, S.; et al. Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage. Nature 2015, 527, 389–393. [Google Scholar] [CrossRef]
- Mattiroli, F.; Vissers, J.H.A.; van Dijk, W.J.; Ikpa, P.; Citterio, E.; Vermeulen, W.; Marteijn, J.A.; Sixma, T.K. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell 2012, 150, 1182–1195. [Google Scholar] [CrossRef]
- Smeenk, G.; van Attikum, H. The Chromatin Response to DNA Breaks: Leaving a Mark on Genome Integrity. Annu. Rev. Biochem. 2013, 82, 55–80. [Google Scholar] [CrossRef]
- Galanty, Y.; Belotserkovskaya, R.; Coates, J.; Jackson, S.P. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 2012, 26, 1179–1195. [Google Scholar] [CrossRef] [PubMed]
- Kakarougkas, A.; Ismail, A.; Katsuki, Y.; Freire, R.; Shibata, A.; Jeggo, P.A. Co-operation of BRCA1 and POH1 relieves the barriers posed by 53BP1 and RAP80 to resection. Nucleic Acids Res. 2013, 41, 10298–10311. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Kastan, M.B. Repair versus Checkpoint Functions of BRCA1 Are Differentially Regulated by Site of Chromatin Binding. Cancer Res. 2015, 75, 2699–2707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, Y.; Scully, R.; Sobhian, B.; Xie, A.; Shestakova, E.; Livingston, D.M. RAP80-directed tuning of BRCA1 homologous recombination function at ionizing radiation-induced nuclear foci. Genes Dev. 2011, 25, 685–700. [Google Scholar] [CrossRef]
- Shao, G.; Lilli, D.R.; Patterson-Fortin, J.; Coleman, K.A.; Morrissey, D.E.; Greenberg, R.A. The Rap80-BRCC36 de-ubiquitinating enzyme complex antagonizes RNF8-Ubc13-dependent ubiquitination events at DNA double strand breaks. Proc. Natl. Acad. Sci. USA 2009, 106, 3166–3171. [Google Scholar] [CrossRef]
- Ng, H.M.; Wei, L.; Lan, L.; Huen, M.S.Y. The Lys 63-deubiquitylating Enzyme BRCC36 Limits DNA Break Processing and Repair. J. Biol. Chem. 2016, 291, 16197–16207. [Google Scholar] [CrossRef]
- Xu, M.; Moresco, J.J.; Chang, M.; Mukim, A.; Smith, D.; Diedrich, J.K.; Yates, J.R.; Jones, K.A. SHMT2 and the BRCC36/BRISC deubiquitinase regulate HIV-1 Tat K63-ubiquitylation and destruction by autophagy. PLoS Pathog. 2018, 14, e1007071. [Google Scholar] [CrossRef]
- Zheng, H.; Gupta, V.; Patterson-Fortin, J.; Bhattacharya, S.; Katlinski, K.; Wu, J.; Varghese, B.; Carbone, C.J.; Aressy, B.; Fuchs, S.Y.; et al. A BRISC-SHMT complex deubiquitinates IFNAR1 and regulates interferon responses. Cell Rep. 2013, 5, 180–193. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Katlinski, K.V.; Reichert, M.; Takano, S.; Brice, A.; Zhao, B.; Yu, Q.; Zheng, H.; Carbone, C.J.; Katlinskaya, Y.V.; et al. Triggering ubiquitination of IFNAR1 protects tissues from inflammatory injury. EMBO Mol. Med. 2014, 6, 384–397. [Google Scholar] [CrossRef]
- de Weerd, N.A.; Vivian, J.P.; Nguyen, T.K.; Mangan, N.E.; Gould, J.A.; Braniff, S.J.; Zaker-Tabrizi, L.; Fung, K.Y.; Forster, S.C.; Beddoe, T.; et al. Structural basis of a unique interferon-β signaling axis mediated via the receptor IFNAR1. Nat. Immunol. 2013, 14, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carvalho, L.P.; Bhattacharya, S.; Carbone, C.J.; Kumar, K.G.S.; Leu, N.A.; Yau, P.M.; Donald, R.G.K.; Weiss, M.J.; Baker, D.P.; et al. Mammalian Casein Kinase 1α and Its Leishmanial Ortholog Regulate Stability of IFNAR1 and Type I Interferon Signaling. Mol. Cell. Biol. 2009, 29, 6401–6412. [Google Scholar] [CrossRef]
- Kumar, K.G.S.; Krolewski, J.J.; Fuchs, S.Y. Phosphorylation and Specific Ubiquitin Acceptor Sites are Required for Ubiquitination and Degradation of the IFNAR1 Subunit of Type I Interferon Receptor. J. Biol. Chem. 2004, 279, 46614–46620. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.G.S.; Barriere, H.; Carbone, C.J.; Liu, J.; Swaminathan, G.; Xu, P.; Li, Y.; Baker, D.P.; Peng, J.; Lukacs, G.L.; et al. Site-specific ubiquitination exposes a linear motif to promote interferon-α receptor endocytosis. J. Cell Biol. 2007, 179, 935–950. [Google Scholar] [CrossRef]
- Lata, S.; Mishra, R.; Banerjea, A.C. Proteasomal Degradation Machinery: Favorite Target of HIV-1 Proteins. Front. Microbiol. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Li, L.; Wang, X.; Hong, R.; Zhang, Y.; Yang, H.; Lin, M.; Zhang, S.; He, Q.; Zheng, D.; et al. The deubiquitinating enzyme complex BRISC is required for proper mitotic spindle assembly in mammalian cells. J. Cell Biol. 2015, 210, 209–224. [Google Scholar] [CrossRef]
- Tripathi, E.; Smith, S. Cell cycle-regulated ubiquitination of tankyrase 1 by RNF8 and ABRO1/BRCC36 controls the timing of sister telomere resolution. EMBO J. 2017, 36, 503–519. [Google Scholar] [CrossRef]
- Feng, L.; Wang, J.; Chen, J. The Lys63-specific Deubiquitinating Enzyme BRCC36 Is Regulated by Two Scaffold Proteins Localizing in Different Subcellular Compartments. J. Biol. Chem. 2010, 285, 30982–30988. [Google Scholar] [CrossRef]
- Worden, E.J.; Padovani, C.; Martin, A. Structure of the Rpn11-Rpn8 dimer reveals mechanisms of substrate deubiquitination during proteasomal degradation. Nat. Struct. Mol. Biol. 2014, 21, 220–227. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshikawa, A.; Yamagata, A.; Mimura, H.; Yamashita, M.; Ookata, K.; Nureki, O.; Iwai, K.; Komada, M.; Fukai, S. Structural basis for specific cleavage of Lys63-linked polyubiquitin chains. Nature 2008, 455, 358–362. [Google Scholar] [CrossRef]
- Giardina, G.; Brunotti, P.; Fiascarelli, A.; Cicalini, A.; Costa, M.G.S.; Buckle, A.M.; di Salvo, M.L.; Giorgi, A.; Marani, M.; Paone, A.; et al. How pyridoxal 5’-phosphate differentially regulates human cytosolic and mitochondrial serine hydroxymethyltransferase oligomeric state. FEBS J. 2015, 282, 1225–1241. [Google Scholar] [CrossRef]
- Anderson, D.D.; Stover, P.J. SHMT1 and SHMT2 Are Functionally Redundant in Nuclear De novo Thymidylate Biosynthesis. PLoS ONE 2009, 4, e5839. [Google Scholar] [CrossRef]
- Shen, Y.; Tong, L. Structural Evidence for Direct Interactions between the BRCT Domains of Human BRCA1 and a Phospho-peptide from Human ACC1. Biochemistry 2008, 47, 5767–5773. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ladias, J.A.A. Structural Basis for the BRCA1 BRCT Interaction with the Proteins ATRIP and BAAT1. Biochemistry 2013, 52, 7618–7627. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.K.; Brown, R.S.; Birrane, G.; Ladias, J.A.A. Structural basis for cell cycle checkpoint control by the BRCA1-CtIP complex. Biochemistry 2005, 44, 10941–10946. [Google Scholar] [CrossRef]
- Shiozaki, E.N.; Gu, L.; Yan, N.; Shi, Y. Structure of the BRCT repeats of BRCA1 bound to a BACH1 phosphopeptide: Implications for signaling. Mol. Cell 2004, 14, 405–412. [Google Scholar] [CrossRef]
- Huyton, T.; Bates, P.A.; Zhang, X.; Sternberg, M.J.; Freemont, P.S. The BRCA1 C-terminal domain: Structure and function. Mutat. Res. 2000, 460, 319–332. [Google Scholar] [CrossRef]
- Yu, X.; Chini, C.C.S.; He, M.; Mer, G.; Chen, J. The BRCT domain is a phospho-protein binding domain. Science 2003, 302, 639–642. [Google Scholar] [CrossRef]
- Clapperton, J.A.; Manke, I.A.; Lowery, D.M.; Ho, T.; Haire, L.F.; Yaffe, M.B.; Smerdon, S.J. Structure and mechanism of BRCA1 BRCT domain recognition of phosphorylated BACH1 with implications for cancer. Nat. Struct. Mol. Biol. 2004, 11, 512–518. [Google Scholar] [CrossRef]
- Badgujar, D.C.; Sawant, U.; Vikrant; Yadav, L.; Hosur, M.V.; Varma, A.K. Preliminary crystallographic studies of BRCA1 BRCT-ABRAXAS complex. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 1401–1404. [Google Scholar] [CrossRef]
- Manke, I.A. BRCT Repeats As Phosphopeptide-Binding Modules Involved in Protein Targeting. Science 2003, 302, 636–639. [Google Scholar] [CrossRef]
- Vauquelin, G.; Charlton, S.J. Exploring avidity: Understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Br. J. Pharmacol. 2013, 168, 1771–1785. [Google Scholar] [CrossRef]
- Hunkeler, M.; Hagmann, A.; Stuttfeld, E.; Chami, M.; Guri, Y.; Stahlberg, H.; Maier, T. Structural basis for regulation of human acetyl-CoA carboxylase. Nature 2018, 558, 470–474. [Google Scholar] [CrossRef]
- Sartori, A.A.; Lukas, C.; Coates, J.; Mistrik, M.; Fu, S.; Bartek, J.; Baer, R.; Lukas, J.; Jackson, S.P. Human CtIP promotes DNA end resection. Nature 2007, 450, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jubb, H.; Blundell, T.L. Phosphopeptide interactions with BRCA1 BRCT domains: More than just a motif. Prog. Biophys. Mol. Biol. 2015, 117, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Paul, A.; Sun, B.; Huang, T.H.; Wang, Y.; Yazinski, S.A.; Tyler, J.; Li, L.; You, M.J.; Zou, L.; et al. The BRCA1-Interacting Protein Abraxas Is Required for Genomic Stability and Tumor Suppression. Cell Rep. 2014, 8, 807–817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, J.; Liu, C.; Chen, J.; Yu, X. RAP80 Protein Is Important for Genomic Stability and Is Required for Stabilizing BRCA1-A Complex at DNA Damage Sites in Vivo. J. Biol. Chem. 2012, 287, 22919–22926. [Google Scholar] [CrossRef]
- Xiao, L.; Lee, K.K.H. BRE facilitates skeletal muscle regeneration by promoting satellite cell motility and differentiation. Biol. Open 2016, 5, 100–111. [Google Scholar] [CrossRef][Green Version]
- Yin, Z.; Menendez, D.; Resnick, M.A.; French, J.E.; Janardhan, K.S.; Jetten, A.M. RAP80 Is Critical in Maintaining Genomic Stability and Suppressing Tumor Development. Cancer Res. 2012, 72, 5080–5090. [Google Scholar] [CrossRef]
- Vikrant; Kumar, R.; Yadav, L.R.; Nakhwa, P.; Waghmare, S.K.; Goyal, P.; Varma, A.K. Structural and Functional Implication of RAP80 ΔGlu81 Mutation. PLoS ONE 2013, 8, e72707. [Google Scholar]
- Nikkilä, J.; Coleman, K.A.; Morrissey, D.; Pylkas, K.; Erkko, H.; Messick, T.E.; Karppinen, S.M.; Amelina, A.; Winqvist, R.; Greenberg, R.A. Familial breast cancer screening reveals an alteration in the RAP80 UIM domain that impairs DNA damage response function. Oncogene 2009, 28, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Feng, W.; Lim, P.X.; Kass, E.M.; Jasin, M. Homology-Directed Repair and the Role of BRCA1, BRCA2, and Related Proteins in Genome Integrity and Cancer. Annu. Rev. Cancer Biol. 2018, 2, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Kass, E.M.; Lim, P.X.; Helgadottir, H.R.; Moynahan, M.E.; Jasin, M. Robust homology-directed repair within mouse mammary tissue is not specifically affected by Brca2 mutation. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Miskinyte, S.; Butler, M.G.; Hervé, D.; Sarret, C.; Nicolino, M.; Petralia, J.D.; Bergametti, F.; Arnould, M.; Pham, V.N.; Gore, A.V.; et al. Loss of BRCC3 deubiquitinating enzyme leads to abnormal angiogenesis and is associated with syndromic moyamoya. Am. J. Hum. Genet. 2011, 88, 718–728. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Gunasekaran, P.; Leung, K.; Bindal, N.; Boutselakis, H.; Ding, M.; Bamford, S.; Cole, C.; Ward, S.; et al. COSMIC: Exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015, 43, D805–D811. [Google Scholar] [CrossRef]
- Jin, G.; Mao, X.; Qiao, Z.; Chen, B.; Jin, F. RAP80 expression in breast cancer and its relationship with apoptosis in breast cancer cells. OncoTargets Ther. 2019, 12, 625–634. [Google Scholar] [CrossRef]
- The Australian Ovarian Cancer Study Group; The Australian Cancer Study (Ovarian Cancer); On behalf of the Ovarian Cancer Association Consortium; Bolton, K.L.; Tyrer, J.; Song, H.; Ramus, S.J.; Notaridou, M.; Jones, C.; Sher, T.; et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat. Genet. 2010, 42, 880–884. [Google Scholar]
- Noordermeer, S.M.; Wennemers, M.; Bergevoet, S.M.; Heijden, A.; Tönnissen, E.; Sweep, F.C.G.J.; Jansen, J.H.; Span, P.N.; Reijden, B.A. Expression of the BRCA1 complex member BRE predicts disease free survival in breast cancer. Breast Cancer Res. Treat. 2012, 135, 125–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noordermeer, S.M.; Monteferrario, D.; Sanders, M.A.; Bullinger, L.; Jansen, J.H.; van der Reijden, B.A. Improved classification of MLL-AF9-positive acute myeloid leukemia patients based on BRE and EVI1 expression. Blood 2012, 119, 4335–4337. [Google Scholar] [CrossRef][Green Version]
- Noordermeer, S.M.; Sanders, M.A.; Gilissen, C.; Tonnissen, E.; van der Heijden, A.; Dohner, K.; Bullinger, L.; Jansen, J.H.; Valk, P.J.M.; van der Reijden, B.A. High BRE expression predicts favorable outcome in adult acute myeloid leukemia, in particular among MLL-AF9-positive patients. Blood 2011, 118, 5613–5621. [Google Scholar] [CrossRef]
- Balgobind, B.V.; Zwaan, C.M.; Reinhardt, D.; Arentsen-Peters, T.J.C.M.; Hollink, I.H.I.M.; de Haas, V.; Kaspers, G.J.L.; de Bont, E.S.J.M.; Baruchel, A.; Stary, J.; et al. leu2010211a. Leukemia 2010, 24, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Katlinski, K.V.; Gui, J.; Katlinskaya, Y.V.; Ortiz, A.; Chakraborty, R.; Bhattacharya, S.; Carbone, C.J.; Beiting, D.P.; Girondo, M.A.; Peck, A.R.; et al. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer Cell 2017, 31, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Fiske, B.P.; Birsoy, K.; Freinkman, E.; Kami, K.; Possemato, R.L.; Chudnovsky, Y.; Pacold, M.E.; Chen, W.W.; Cantor, J.R.; et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 2015, 520, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors: Coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2016, 36, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kurdekar, V.; Giridharan, S.; Subbarao, J.; Nijaguna, M.B.; Periasamy, J.; Boggaram, S.; Shivange, A.V.; Sadasivam, G.; Padigaru, M.; Potluri, V.; et al. Structure-Guided Synthesis and Evaluation of Small-Molecule Inhibitors Targeting Protein–Protein Interactions of BRCA1 tBRCT Domain. ChemMedChem 2019, 14, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, J.; Kurdekar, V.; Jasti, S.; Nijaguna, M.B.; Boggaram, S.; Hurakadli, M.A.; Raina, D.; Kurup, L.M.; Chintha, C.; Manjunath, K.; et al. Targeting Phosphopeptide Recognition by the Human BRCA1 Tandem BRCT Domain to Interrupt BRCA1-Dependent Signaling. Cell Chem. Biol. 2018, 25, 677–690.e12. [Google Scholar] [CrossRef]
- Northall, S.; Ivančić-Baće, I.; Soultanas, P.; Bolt, E. Remodeling and Control of Homologous Recombination by DNA Helicases and Translocases that Target Recombinases and Synapsis. Genes 2016, 7, 52. [Google Scholar] [CrossRef]
- Ye, L.; Wang, C.; Hong, L.; Sun, N.; Chen, D.; Chen, S.; Han, F. Programmable DNA repair with CRISPRa/i enhanced homology-directed repair efficiency with a single Cas9. Cell Discov. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Fuchs, S.Y. Hope and Fear for Interferon: The Receptor-Centric Outlook on the Future of Interferon Therapy. J. Interferon Cytokine Res. 2013, 33, 211–225. [Google Scholar] [CrossRef]
- Sowa, M.E.; Bennett, E.J.; Gygi, S.P.; Harper, J.W. Defining the Human Deubiquitinating Enzyme Interaction Landscape. Cell 2009, 138, 389–403. [Google Scholar] [CrossRef]

| Name | Gene Names | Size (aa) | Size (kDa) | Domains | Associated with | Constitutive |
|---|---|---|---|---|---|---|
| ABRAXAS | ABRAXAS1, ABRA1, CCDC98, FAM175A, UNQ496/PRO1013 | 409 | 36.7 | JAMM/MPN, BRCA1 binding | BRCA1-A | subunit |
| ABRO1 | ABRAXAS2, FAM175B, KIAA0157 | 415 | 46.9 | JAMM/MPN, SHMT2 and LNK bdg. | BRISC | subunit |
| BRCC36 | BRCC3, C6.1A, CXorf53 | 316 | 36.0 | JAMM/MPN | BRCA1-A, BRISC | subunit |
| BRE | BABAM2, BRCC45 | 383 | 43.6 | UEV | BRCA1-A, BRISC | subunit |
| MERIT40 | BABAM1, C19orf62, NBA1, HSPC142 | 329 | 36.6 | VWA | BRCA1-A, BRISC | subunit |
| RAP80 | UIMC1, RXRIP110 | 719 | 79.7 | SIM, twin UIM, AIR | BRCA1-A | subunit |
| BRCA1 | RNF53 | 1863 | 207.7 | RING, BRCT | BRCA1-A | regulated binding |
| SHMT2 | GLYM | 483 | 53.5 | SHMT | BRISC | regulated binding |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabl, J. BRCA1-A and BRISC: Multifunctional Molecular Machines for Ubiquitin Signaling. Biomolecules 2020, 10, 1503. https://doi.org/10.3390/biom10111503
Rabl J. BRCA1-A and BRISC: Multifunctional Molecular Machines for Ubiquitin Signaling. Biomolecules. 2020; 10(11):1503. https://doi.org/10.3390/biom10111503
Chicago/Turabian StyleRabl, Julius. 2020. "BRCA1-A and BRISC: Multifunctional Molecular Machines for Ubiquitin Signaling" Biomolecules 10, no. 11: 1503. https://doi.org/10.3390/biom10111503
APA StyleRabl, J. (2020). BRCA1-A and BRISC: Multifunctional Molecular Machines for Ubiquitin Signaling. Biomolecules, 10(11), 1503. https://doi.org/10.3390/biom10111503





