In Vitro and In Vivo Antioxidant Activity of Agave sisalana Agro-Industrial Residue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material
2.3. Extract Production
2.4. Chemical Characterization
2.4.1. Total Flavonoids
2.4.2. Total Saponins
2.4.3. Fourier Transform InfraRed (FTIR) Spectroscopy
2.5. In Vitro Antioxidant Activity
2.5.1. Total Antioxidant Capacity (TAC)
2.5.2. Reducing Power
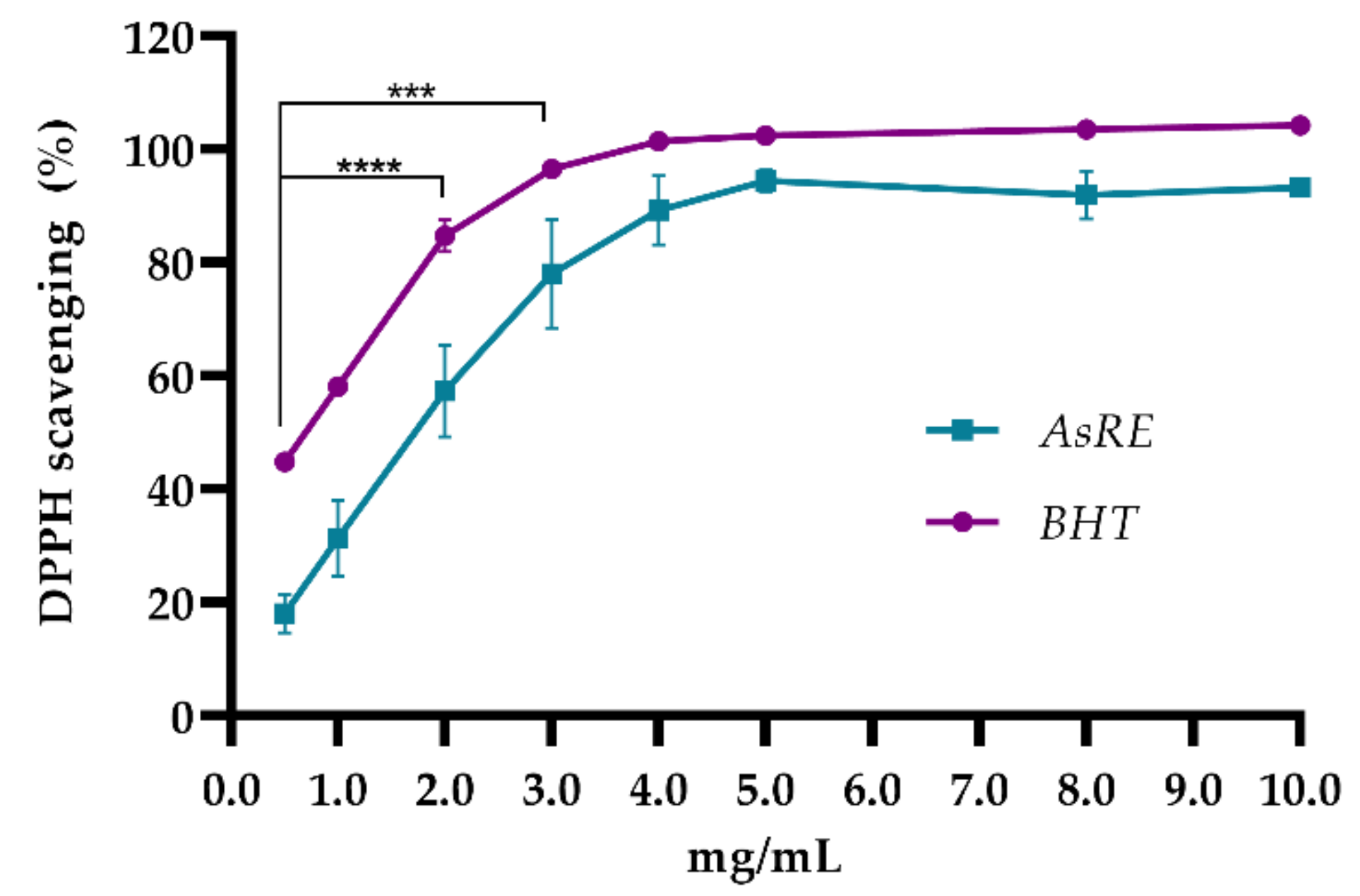
2.5.3. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Activity
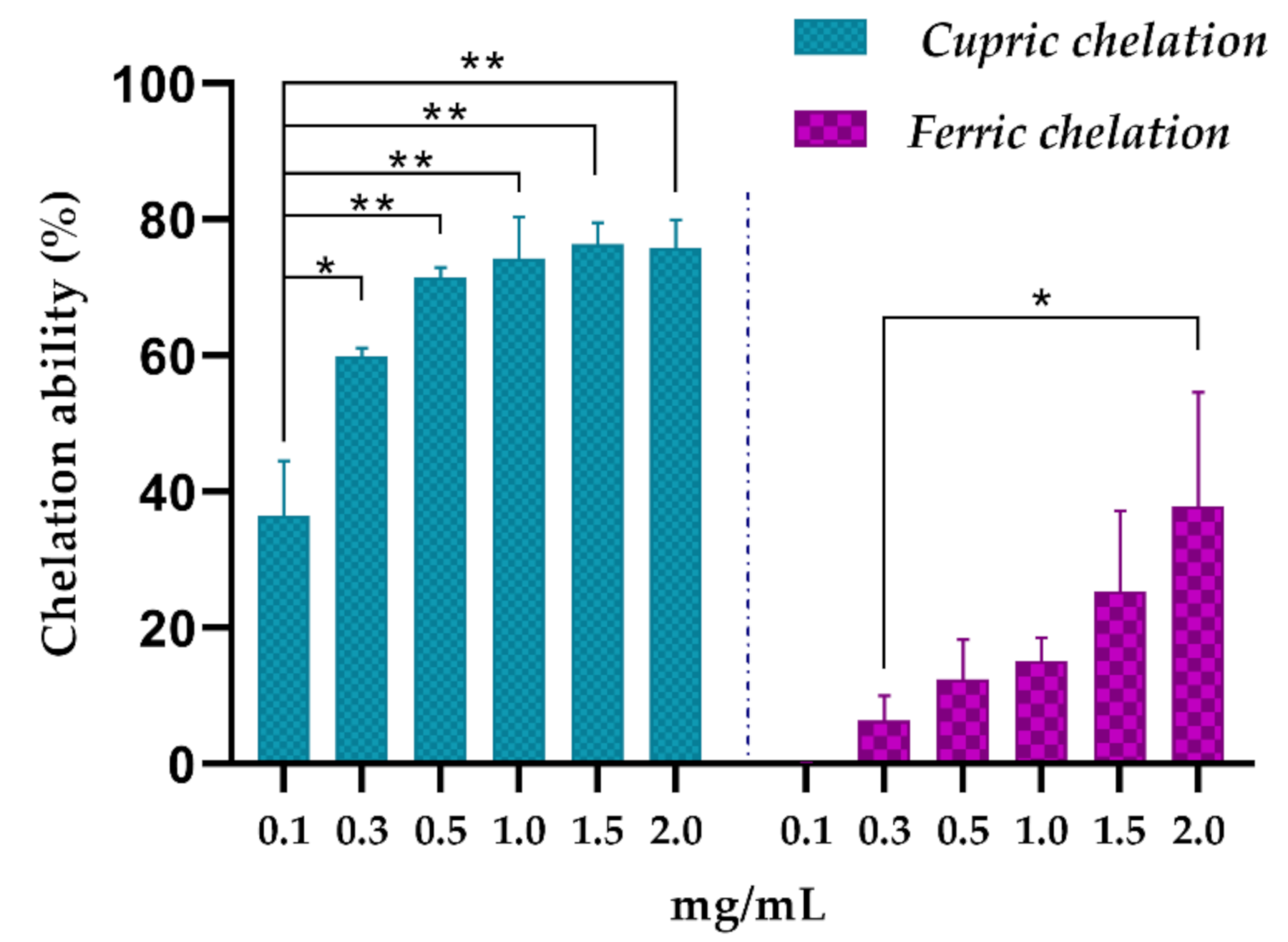
2.5.4. Cupric Chelating
2.5.5. Ferric Chelating
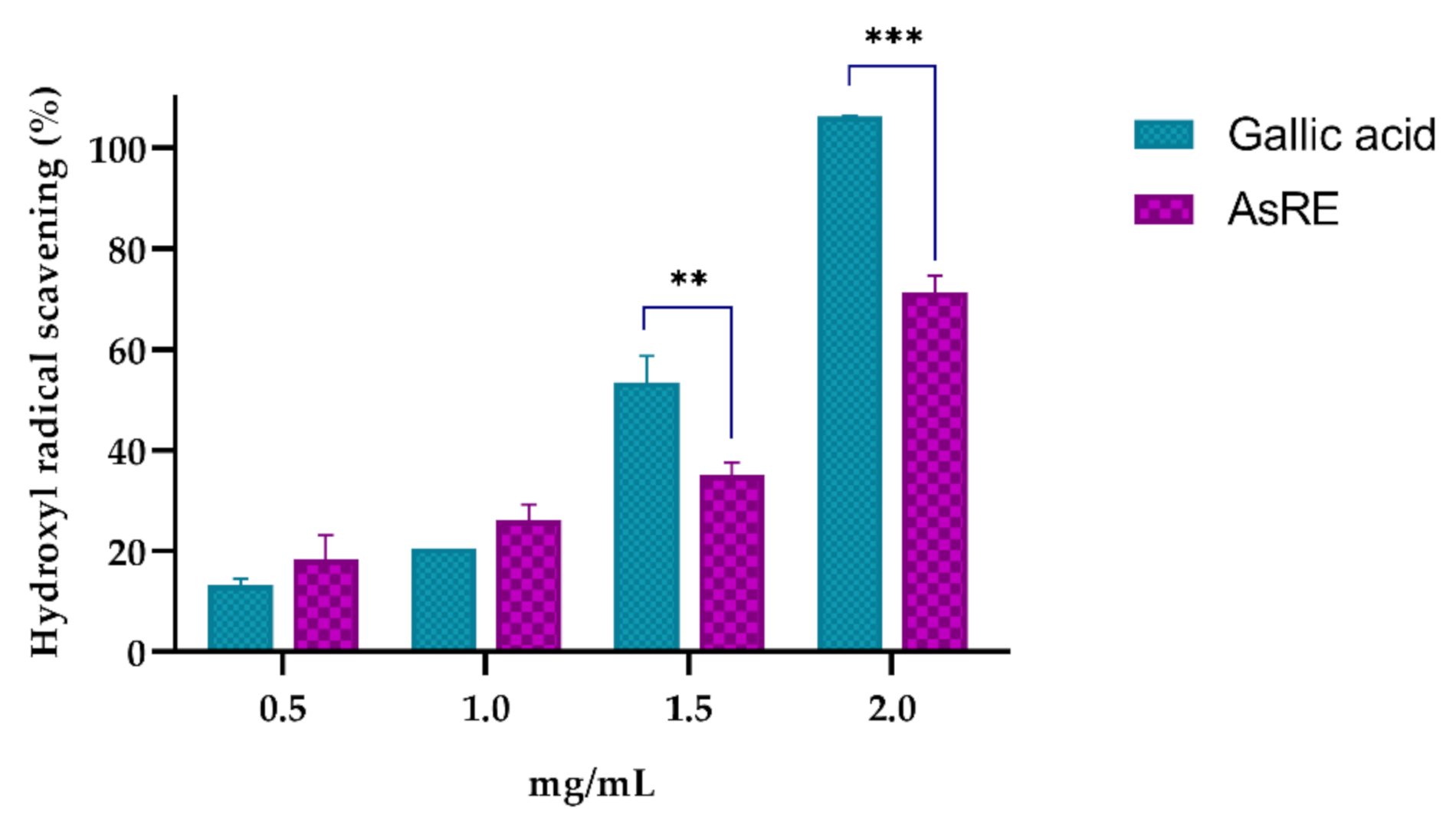
2.5.6. Hydroxyl Radical Scavenging Activity
2.6. Caenorhabditis elegans Maintenance Strains and Extract Treatment
2.7. Bacterial Growth Assay
2.8. Toxicity of Agave sisalana Extract Against C. elegans
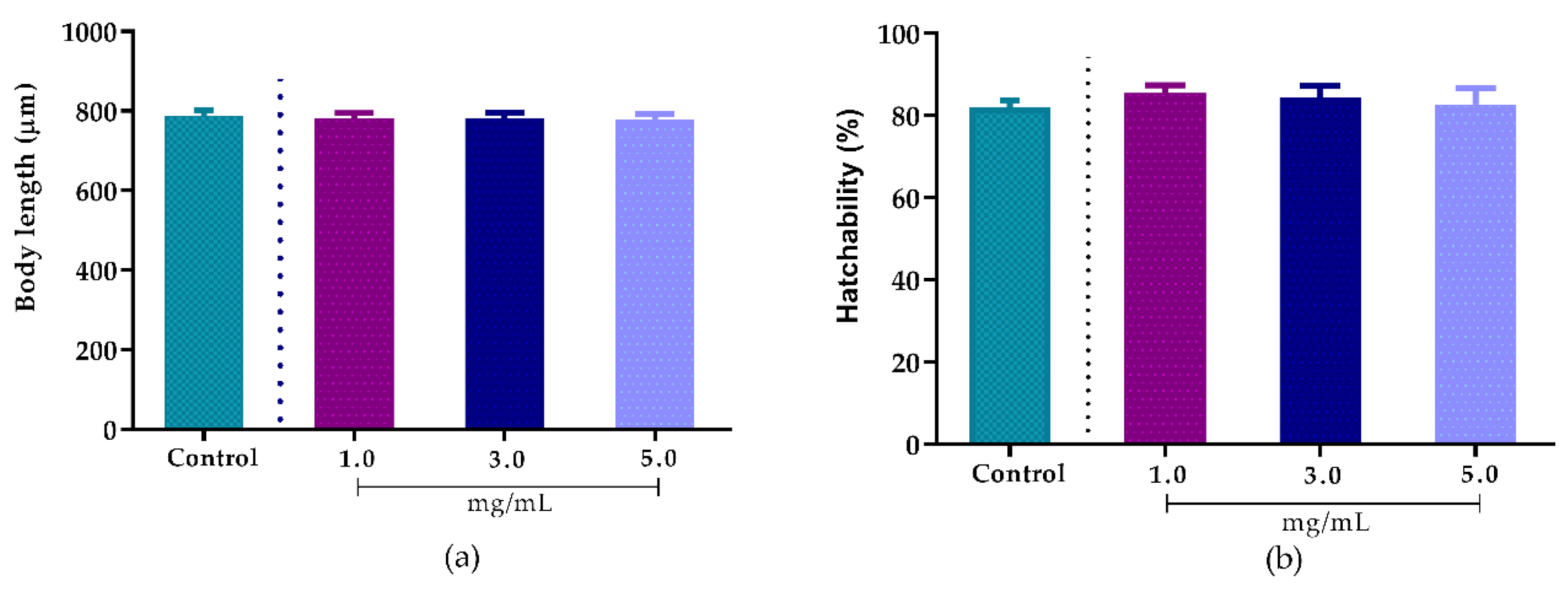
2.8.1. Effect of A. sisalana Agro-Industrial Residue Extract on Body Length
2.8.2. Effect of Agave sisalana Agro-Industrial Residue Extract on Hatchability
2.9. In Vivo Antioxidant Activity of Agave sisalana Agro-Industrial Residue Extract Using C. elegans as a Model Organism
2.9.1. Intracellular Accumulation of Reactive Oxygen Species (ROS) in C. elegans
2.9.2. Oxidative Stress Survival Assay
2.9.3. Expression Levels of the Oxidative Resistance Relative Gene
2.10. Statistical Analysis
3. Results
3.1. Chemical Characterization
3.2. In Vitro Antioxidant Activity
3.3. Bacterial Growth Assay
3.4. Toxicity of Agave sisalana Extract Against C. elegans
3.5. In Vivo Antioidant Activity
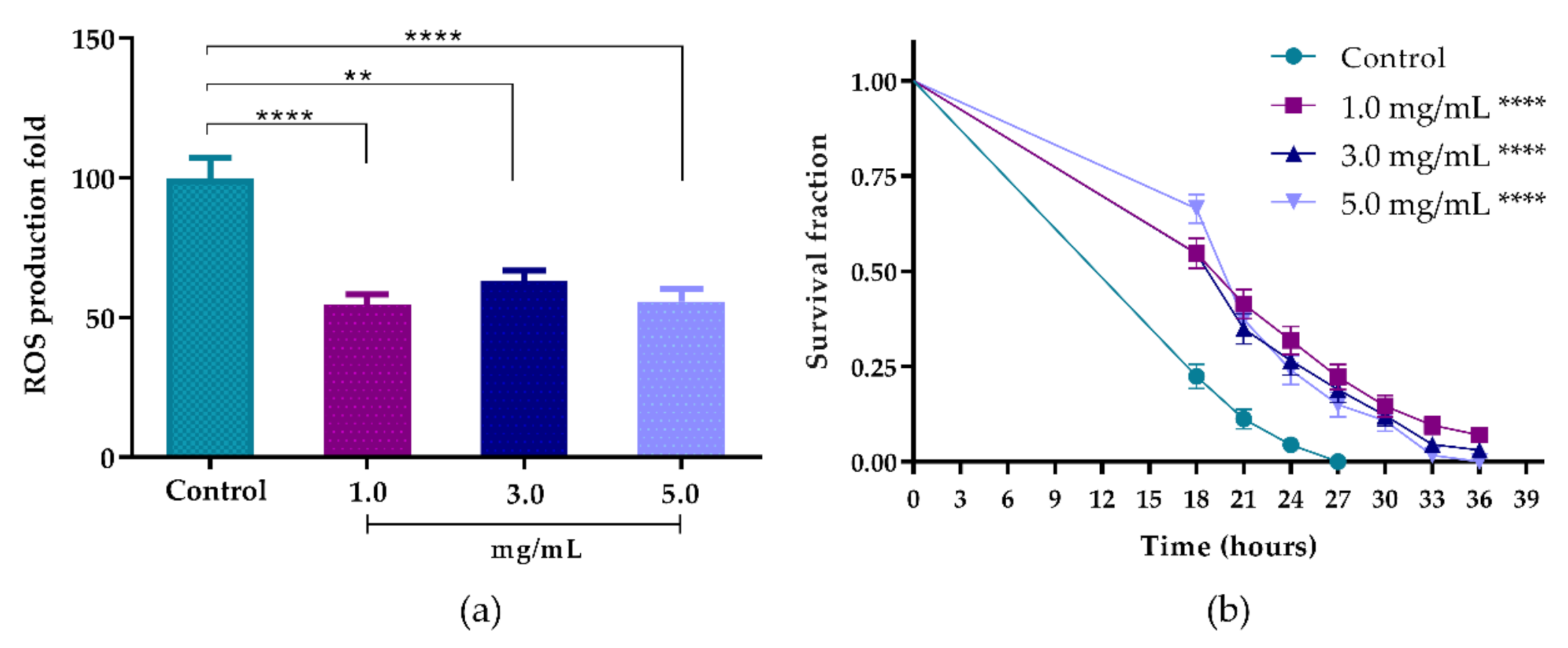
3.5.1. Intracellular Accumulation of ROS in C. elegans
3.5.2. Oxidative Stress Survival Assay
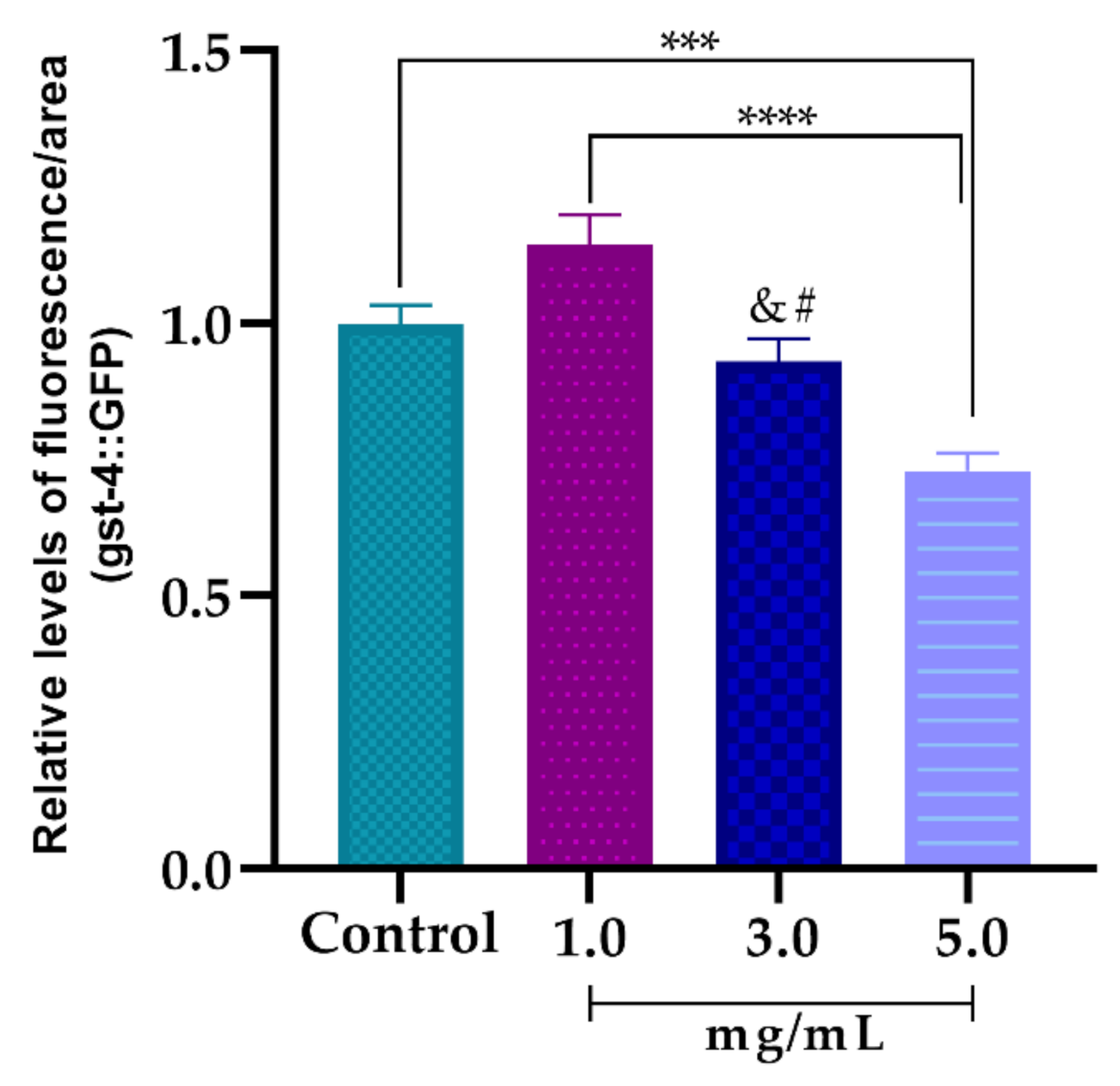
3.5.3. Effect of AsRE on the Expression Levels of the Oxidative Stress Relative Gene (gst-4::GFP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sidana, J.; Singh, B.; Sharma, O.P. Phytochemistry Saponins of Agave: Chemistry and bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Rodríguez, I.M.; del Río, J.C. Chemical composition of lipophilic extractives from sisal (Agave sisalana) fibers. Ind. Crops Prod. 2008, 28, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.D.G.; Vieira, I.J.C.; Braz-Filho, R.; Branco, A. Chemicals from Agave sisalana biomass: Isolation and identification. Int. J. Mol. Sci. 2015, 16, 8761–8771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of pectin from sisal waste. Carbohydr. Polym. 2014, 115, 732–738. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Lin, C. Isolation, structural characterization and antioxidant activity of a neutral polysaccharide from Sisal waste. Food Hydrocoll. 2014, 39, 10–18. [Google Scholar] [CrossRef]
- Martin, A.R.; Martins, M.A.; Mattoso, L.H.C.; Silva, O.R.R.F. Caracteriza̧ão química e estrutural de fibra de sisal da variedade Agave sisalana. Polimeros 2009, 19, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; Ávila-Reyes, J.A.; Uribe-Soto, J.N.; González-Valdez, L.S. The Phenols of the Genus Agave (Agavaceae). J. Biomater. Nanobiotechnol. 2013, 04, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, B.D.; Alviano, D.S.; Barreto, D.W.; Coelho, M.A.Z. Functional properties of saponins from sisal (Agave sisalana) and juá (Ziziphus joazeiro): Critical micellar concentration, antioxidant and antimicrobial activities. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 736–743. [Google Scholar] [CrossRef]
- Santos, J.D.G.; Branco, A. GC-MS Characterisation of Sapogenins from Sisal Waste and a Method to Isolate Pure Hecogenin. BioResources 2014, 9, 1325–1333. [Google Scholar] [CrossRef]
- Barreto, S.M.A.G.; Maia, M.S.; Benicá, A.M.; de Assis, H.R.B.S.; Leite-Silva, V.R.; da Rocha-Filho, P.A.; de Negreiros, M.M.F.; de Oliveira Rocha, H.A.; Ostrosky, E.A.; Lopes, P.S.; et al. Evaluation of in vitro and in vivo safety of the by-product of Agave sisalana as a new cosmetic raw material: Development and clinical evaluation of a nanoemulsion to improve skin moisturizing. Ind. Crops Prod. 2017, 108, 470–479. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Lin, C. Structural features, antioxidant and immunological activity of a new polysaccharide (SP1) from sisal residue. Int. J. Biol. Macromol. 2013, 59, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Grünz, G.; Haas, K.; Soukup, S.; Klingenspor, M.; Kulling, S.E.; Daniel, H.; Spanier, B. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev. 2012, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- De Oliveira Caland, R.B.; Cadavid, C.O.M.; Carmona, L.; Peña, L.; De Paula Oliveira, R. Pasteurized orange juice rich in carotenoids protects caenorhabditis elegans against oxidative stress and β-amyloid toxicity through direct and indirect mechanisms. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, B.C.; Roxo, M.; Borges, M.C.; Peixoto, H.; Crevelin, E.J.; Bertoni, B.W.; Contini, S.H.T.; Lopes, A.A.; França, S.C.; Pereira, A.M.S.; et al. Antioxidant Activity of an Aqueous Leaf Extract from Uncaria tomentosa and Its Major Alkaloids Mitraphylline and Isomitraphylline in Caenorhabditis elegans. Molecules 2019, 24, 3299. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Kang, N.; Li, Q.; Zhang, Y.; Liu, Y.; Tan, P. Study of the Effect of Neutral Polysaccharides from Rehmannia glutinosa on Lifespan of Caenorhabditis elegans. Molecules 2019, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, A.R.C.; da Silva Cordeiro, M.L.; Silva, L.M.P.; Cadavid, C.O.M.; de Oliveira Caland, R.B.; Fernandes-Negreiros, M.M.; Queiroz, M.F.; da Silva Barbosa, J.; Aragão, C.F.S.; Zucolotto, S.M.; et al. Myrciaria tenella (DC.) O. Berg (myrtaceae) leaves as a source of antioxidant compounds. Antioxidants 2019, 8, 310. [Google Scholar] [CrossRef] [Green Version]
- Artal-Sanz, M.; de Jong, L.; Tavernarakis, N. Caenorhabditis elegans: A versatile platform for drug discovery. Biotechnol. J. 2006, 1, 1405–1418. [Google Scholar] [CrossRef]
- Araldi, R.P.; dos Santos, M.O.; Barbon, F.F.; Manjerona, B.A.; Meirelles, B.R.; de Oliva Neto, P.; da Silva, P.I.; dos Santos, L.; Camargo, I.C.C.; de Souza, E.B. Analysis of antioxidant, cytotoxic and mutagenic potential of Agave sisalana Perrine extracts using Vero cells, human lymphocytes and mice polychromatic erythrocytes. Biomed. Pharmacother. 2018, 98, 873–885. [Google Scholar] [CrossRef]
- Chinedum, E.; Sanni, S.; Theressa, N.; Ebere, A. Effect of domestic cooking on the starch digestibility, predicted glycemic indices, polyphenol contents and alpha amylase inhibitory properties of beans (Phaseolis vulgaris) and breadfruit (Treculia africana). Int. J. Biol. Macromol. 2018, 106, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Use of micellar extraction and cloud point preconcentration for valorization of saponins from sisal (Agave sisalana) waste. Food Bioprod. Process. 2015, 94, 601–609. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, H.; Xu, Z.; Feng, S.; Yuan, M.; Huang, Y.; Liao, J.; Ding, C. Antioxidant and anti-aging activities and structural elucidation of polysaccharides from Panax notoginseng root. Process Biochem. 2019, 78, 189–199. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Anton, A. Colorimetric Estimation of Aluminum with Pyrocatechol Violet. Anal. Chem. 1960, 32, 725–726. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of Ferritin as a Lipid Oxidation Catalyst in Muscle Food. J. Agric. Food Chem. 1990, 674–677. [Google Scholar] [CrossRef]
- Sminorff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar]
- Brenner, S. Nature’s gift to science (Nobel lecture). ChemBioChem 2003, 4, 683–687. [Google Scholar] [CrossRef]
- Qu, Y.; Li, C.; Zhang, C.; Zeng, R.; Fu, C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016, 148, 345–353. [Google Scholar] [CrossRef]
- Wang, L.C.; Zhang, K.; Di, L.Q.; Liu, R.; Wu, H. Isolation and structural elucidation of novel homogenous polysaccharide from Mactra veneriformis. Carbohydr. Polym. 2011, 86, 982–987. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Del Castillo, L.; Webber, J.L.; Ho, T.T.M.; Ferri, J.K.; Krasowska, M.; Beattie, D.A. The influence of pH on the interfacial behaviour of Quillaja bark saponin at the air-solution interface. Colloids Surfaces B Biointerfaces 2019, 176, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.S.; Ali, M. Direct detection of saponins in crude extracts of soapnuts by FTIR. Nat. Prod. Res. 2015, 29, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, G.P.; Camara, R.B.G.; Queiroz, M.F.; Costa, M.S.S.P.; Santos, P.C.; Rocha, H.A.O.; Costa, L.S. Proteolysis, NaOH and ultrasound-enhanced extraction of anticoagulant and antioxidant sulfated polysaccharides from the edible seaweed, Gracilaria birdiae. Molecules 2014, 19, 18511–18526. [Google Scholar] [CrossRef] [Green Version]
- Galinari, É.; Sabry, D.A.; Sassaki, G.L.; Macedo, G.R.; Passos, F.M.L.; Mantovani, H.C.; Rocha, H.A.O. Chemical structure, antiproliferative and antioxidant activities of a cell wall α-D-mannan from yeast Kluyveromyces marxianus. Carbohydr. Polym. 2017, 157, 1298–1305. [Google Scholar] [CrossRef]
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic compound explorer: A mid-infrared spectroscopy database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin?s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Birch-Machin, M.A.; Bowman, A. Oxidative stress and ageing. Br. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Apea-bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef]
- Stojiljković, D.; Arsić, I.; Tadić, V. Extracts of wild apple fruit (Malus sylvestris (L.) Mill., Rosaceae) as a source of antioxidant substances for use in production of nutraceuticals and cosmeceuticals. Ind. Crops Prod. 2016, 80, 165–176. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhang, J.; Wang, X.; Hao, L.; Jia, L. Purification, in vitro antioxidant and in vivo anti-aging activities of exopolysaccharides by Agrocybe cylindracea. Int. J. Biol. Macromol. 2017, 102, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Sung, T.J.; Hu, Y.N.; Lu, H.Y.; Yang, L.C.; Cheng, K.C.; Lai, P.S.; Hsieh, C.W. Chemical analysis, moisture-preserving, and antioxidant activities of polysaccharides from Pholiota nameko by fractional precipitation. Int. J. Biol. Macromol. 2019, 131, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, W.; Zhu, Y.; Chen, Y.; Zhang, W.; Yu, P.; Mao, G.; Zhao, T.; Feng, W.; Yang, L.; et al. Structural elucidation and antioxidant activity a novel Se-polysaccharide from Se-enriched Grifola frondosa. Carbohydr. Polym. 2017, 161, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, R.; Dong, L.; Guo, J.; Deng, Y.; Yi, Y.; Zhang, M. Antioxidant and antiproliferative activities of polysaccharide fractions from litchi pulp. Food Funct. 2015, 6, 2598–2606. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Aquino-Martins, V.G.d.Q.; de Melo, L.F.M.; Silva, L.M.P.; de Lima, T.R.T.; Queiroz, M.F.; Viana, R.L.S.; Zucolotto, S.M.; Andrade, V.S.; Rocha, H.A.O.; Scortecci, K.C. In vitro antioxidant, anti-biofilm, and solar protection activities of melocactus zehntneri (Britton & rose) pulp extract. Antioxidants 2019, 8, 439. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods ? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Fidelis, G.P.; Viana, R.L.S.; Soeiro, V.C.; Da Silva, R.A.; Machado, D.; Costa, L.S.; Ferreira, C.V.; Rocha, H.A.O. Antioxidant and antiproliferative activities of methanolic extract from a neglected agricultural product: Corn cobs. Molecules 2014, 19, 5360–5378. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Cheng, Y.; Zhang, N.; Zhao, S.; Cui, H.; Zhou, H. Purification of flavonoids from Carex meyeriana Kunth based on AHP and RSM: Composition analysis, antioxidant, and antimicrobial activity. Ind. Crops Prod. 2020, 157, 112900. [Google Scholar] [CrossRef]
- Santos, J.D.G.; Branco, A.; Silva, A.F.; Pinheiro, C.S.R.; Neto, G.; Uetanabaro, A.P.T.; Queiroz, S.R.O.D.; Osuna, J.T. A Antimicrobial activity of Agave sisalana. Afr. J. Biotechnol. 2009, 8, 6181–6184. [Google Scholar] [CrossRef] [Green Version]
- Domingues, L.F.; Botura, M.B.; Da Cruz, A.C.F.G.; Yuki, C.C.; Da Silva, G.D.; Costa, M.S.; Murphy, G.; Moreira, E.L.T.; Meneses, I.D.S.; De Almeida, M.D.G.Á.R.; et al. Evaluation of anthelmintic activity of liquid waste of Agave sisalana (sisal) in goats. Rev. Bras. Parasitol. Vet. 2010, 19, 270–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botura, M.B.; dos Santos, J.D.G.; da Silva, G.D.; de Lima, H.G.; de Oliveira, J.V.A.; de Almeida, M.A.O.; Batatinha, M.J.M.; Branco, A. In vitro ovicidal and larvicidal activity of Agave sisalana Perr. (sisal) on gastrointestinal nematodes of goats. Vet. Parasitol. 2013, 192, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, H.; Roxo, M.; Silva, E.; Valente, K.; Braun, M.; Wang, X.; Wink, M. Bark extract of the amazonian tree endopleura uchi (humiriaceae) extends lifespan and enhances stress resistance in caenorhabditis elegans. Molecules 2019, 24, 915. [Google Scholar] [CrossRef] [Green Version]
- Roxo, M.; Peixoto, H.; Wetterauer, P.; Lima, E.; Wink, M. Piquiá Shells (Caryocar villosum): A Fruit by-Product with Antioxidant and Antiaging Properties in Caenorhabditis elegans. Oxid. Med. Cell Longev. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- De Bonomo, L.F.; Silva, D.N.; Boasquivis, P.F.; Paiva, F.A.; Guerra, J.F.D.C.; Martins, T.A.F.; De Jesus Torres, Á.G.; De Paula, I.T.B.R.; Caneschi, W.L.; Jacolot, P.; et al. Açaí (Euterpe oleracea Mart.) modulates oxidative stress resistance in Caenorhabditis elegans by direct and indirect mechanisms. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]

| Antioxidant Tests | IC5O Value (mg/mL) |
|---|---|
| Reducing power | 1.02 |
| DPPH radical scavenging | 1.44 |
| Cupric chelating | 0.16 |
| Ferric chelating | 2.76 |
| Hydroxyl radical scavenging | 1.61 |
| Concentrations (mg/mL) | Mean Survival (hours ± SEM) | % Mean Survival Time Variation vs. Untreated | p Value (Long Rank) Extract vs. Untreated | n 1 | |
|---|---|---|---|---|---|
| Control | 19.14 ± 0.19 | - | - | 165 (3) | |
| AsRE | 1.0 | 23.23 ± 0.49 | 21.37 | <0.0001 | 159 (3) |
| 3.0 | 22.55 ± 0.47 | 17.82 | <0.0001 | 154 (3) | |
| 5.0 | 22.66 ± 0.44 | 18.39 | <0.0001 | 152 (3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, S.M.A.G.; Cadavid, C.O.M.; Moura, R.A.d.O.; Silva, G.M.M.; Araújo, S.V.F.d.; Silva Filho, J.A.A.d.; Rocha, H.A.O.; Oliveira, R.d.P.; Giordani, R.B.; Ferrari, M. In Vitro and In Vivo Antioxidant Activity of Agave sisalana Agro-Industrial Residue. Biomolecules 2020, 10, 1435. https://doi.org/10.3390/biom10101435
Barreto SMAG, Cadavid COM, Moura RAdO, Silva GMM, Araújo SVFd, Silva Filho JAAd, Rocha HAO, Oliveira RdP, Giordani RB, Ferrari M. In Vitro and In Vivo Antioxidant Activity of Agave sisalana Agro-Industrial Residue. Biomolecules. 2020; 10(10):1435. https://doi.org/10.3390/biom10101435
Chicago/Turabian StyleBarreto, Stella Maria Andrade Gomes, Cesar Orlando Muñoz Cadavid, Rafael Amir de Oliveira Moura, Giovanna Melo Martins Silva, Samara Vitória Ferreira de Araújo, Jean Antônio Aderaldo da Silva Filho, Hugo Alexandre Oliveira Rocha, Riva de Paula Oliveira, Raquel Brandt Giordani, and Márcio Ferrari. 2020. "In Vitro and In Vivo Antioxidant Activity of Agave sisalana Agro-Industrial Residue" Biomolecules 10, no. 10: 1435. https://doi.org/10.3390/biom10101435
APA StyleBarreto, S. M. A. G., Cadavid, C. O. M., Moura, R. A. d. O., Silva, G. M. M., Araújo, S. V. F. d., Silva Filho, J. A. A. d., Rocha, H. A. O., Oliveira, R. d. P., Giordani, R. B., & Ferrari, M. (2020). In Vitro and In Vivo Antioxidant Activity of Agave sisalana Agro-Industrial Residue. Biomolecules, 10(10), 1435. https://doi.org/10.3390/biom10101435






