The Polar Lipidome of Cultured Emiliania huxleyi: A Source of Bioactive Lipids with Relevance for Biotechnological Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Biomass Production
2.3. Moisture and Ash Determination
2.4. Total Sugar Content Determination
2.5. Neutral Sugars and Uronic Acids Analysis
2.6. Nitrogen Determination and Protein Estimation
2.7. Lipid Extraction
2.8. Glycolipids and Phospholipids Quantification
2.9. Fatty Acid Analysis by Gas Chromatography–Mass Spectrometry (GC–MS)
2.10. Hydrophilic Interaction Liquid Chromatography–Mass Spectrometry (HILIC–MS)
3. Results
3.1. Biomass Composition
3.2. Fatty Acid Profile
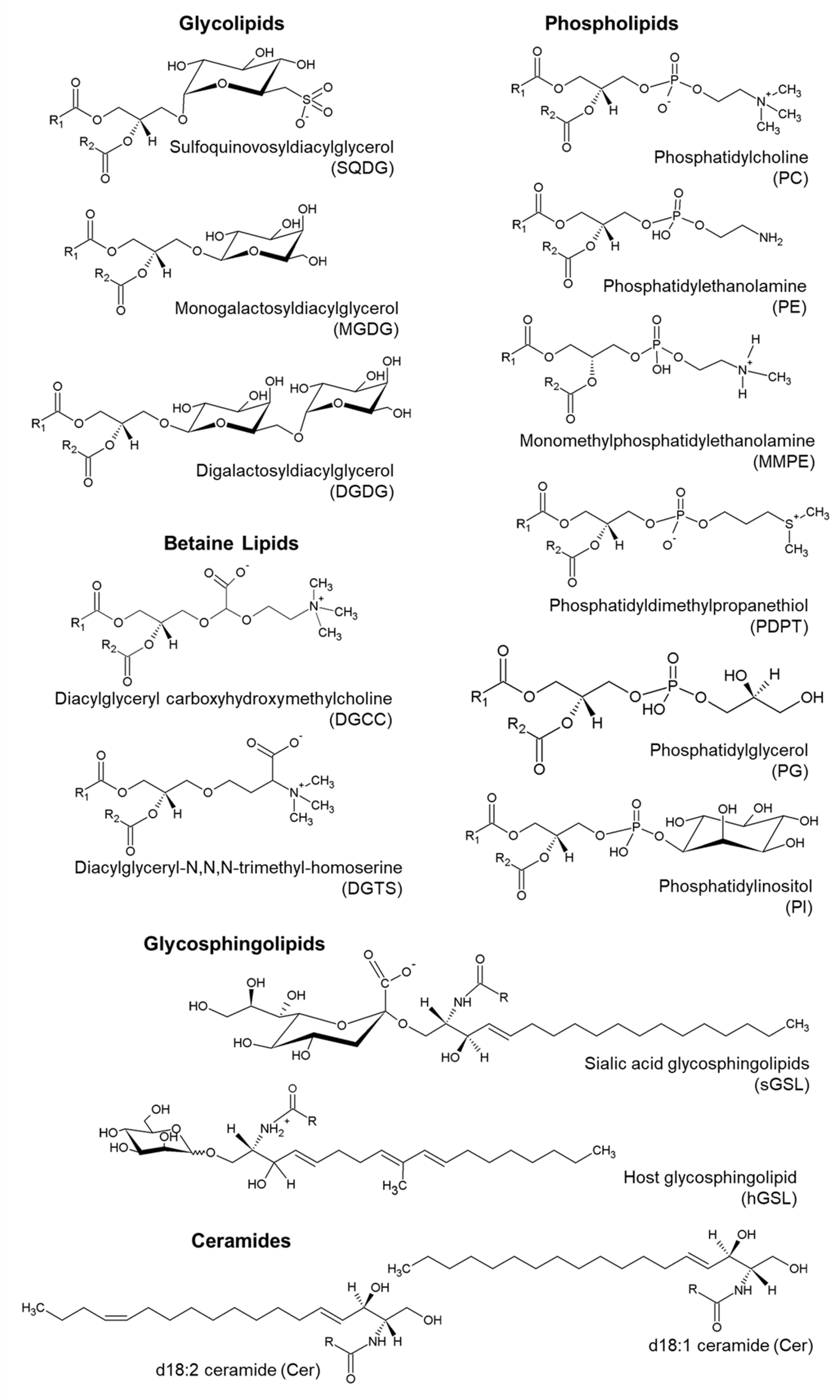
3.3. Identification of Polar Lipids by LC–MS
3.3.1. Glycerolipids
Glycolipids
Betaine Lipids
Phospholipids
3.3.2. Sphingolipids
Glycosphingolipids
Ceramides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Winter, A.; Henderiks, J.; Beaufort, L.; Rickaby, R.E.M.; Brown, C.W. Poleward expansion of the coccolithophore Emiliania huxleyi. J. Plankton Res. 2014, 36, 316–325. [Google Scholar] [CrossRef]
- Iglesias-Rodríguez, M.D.; Brown, C.W.; Doney, S.C.; Kleypas, J.; Kolber, D.; Kolber, Z.; Hayes, P.K.; Falkowski, P.G. Representing key phytoplankton functional groups in ocean carbon cycle models: Coccolithophorids. Glob. Biogeochem. Cycles 2002, 16, 47-1–47-20. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Jiménez Callejón, M.J.; Robles Medina, A.; González Moreno, P.A.; Esteban Cerdán, L.; Orta Guillén, S.; Molina Grima, E. Simultaneous extraction and fractionation of lipids from the microalga Nannochloropsis sp. for the production of EPA-rich polar lipid concentrates. J. Appl. Phycol. 2020, 32, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef]
- da Costa, E.; Silva, J.; Mendonça, S.H.; Abreu, M.H.; Domingues, M.R. Lipidomic approaches towards deciphering glycolipids from microalgae as a reservoir of bioactive lipids. Mar. Drugs 2016, 14, 101. [Google Scholar] [CrossRef] [Green Version]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef]
- Conte, M.H.; Volkman, J.K.; Eglinton, G. Lipid biomarkers of the Prymnesiophyceae. Haptophyte Algae 1994, 51, 351–377. [Google Scholar]
- Eltgroth, M.L.; Watwood, R.L.; Wolfe, G.V. Production and cellular localization of neutral long-chain lipids in the haptophyte algae Isochrysis galbana and Emiliania huxleyi. J. Phycol. 2005, 41, 1000–1009. [Google Scholar] [CrossRef]
- Boelen, P.; van Dijk, R.; Damsté, J.S.S.; Rijpstra, W.I.C.; Buma, A.G.J. On the potential application of polar and temperate marine microalgae for EPA and DHA production. AMB Express 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pond, D.W.; Harris, R.P. The lipid composition of the coccolithophore Emiliania huxleyi and its possible ecophysiological significance. J. Mar. Biol. Assoc. UK 1996, 76, 579–594. [Google Scholar] [CrossRef]
- Vardi, A.; Van Mooy, B.A.S.; Fredricks, H.F.; Popendorf, K.J.; Ossolinski, J.E.; Haramaty, L.; Bidle, K.D. Viral glycosphingolipids induce lytic infection and cell death in marine phytoplankton. Science 2009, 326, 861–865. [Google Scholar] [CrossRef]
- Schatz, D.; Rosenwasser, S.; Malitsky, S.; Wolf, S.G.; Feldmesser, E.; Vardi, A. Communication via extracellular vesicles enhances viral infection of a cosmopolitan alga. Nat. Microbiol. 2017, 2, 1485–1492. [Google Scholar] [CrossRef]
- Schleyer, G.; Shahaf, N.; Ziv, C.; Dong, Y.; Meoded, R.A.; Helfrich, E.J.N.; Schatz, D.; Rosenwasser, S.; Rogachev, I.; Aharoni, A.; et al. In plaque-mass spectrometry imaging of a bloom-forming alga during viral infection reveals a metabolic shift towards odd-chain fatty acid lipids. Nat. Microbiol. 2019, 4, 527–538. [Google Scholar] [CrossRef]
- Fulton, J.M.; Fredricks, H.F.; Bidle, K.D.; Vardi, A.; Kendrick, B.J.; DiTullio, G.R.; Van Mooy, B.A.S. Novel molecular determinants of viral susceptibility and resistance in the lipidome of Emiliania huxleyi. Environ. Microbiol. 2014, 16, 1137–1149. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, S.; Cai, W.; Jiang, H.; Lu, X.; Li, G.; Li, J.; Liu, J. Emerging lipidome patterns associated with marine Emiliania huxleyi-virus model system. Sci. Total Environ. 2019, 688, 521–528. [Google Scholar] [CrossRef]
- Vardi, A.; Haramaty, L.; Van Mooy, B.A.S.; Fredricks, H.F.; Kimmance, S.A.; Larsen, A.; Bidle, K.D. Host-virus dynamics and subcellular controls of cell fate in a natural coccolithophore population. Proc. Natl. Acad. Sci. USA 2012, 109, 19327–19332. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.L.; Fulton, J.M.; Brown, C.M.; Natale, F.; Van Mooy, B.A.S.; Bidle, K.D. Isolation and characterization of lipid rafts in Emiliania huxleyi: A role for membrane microdomains in host-virus interactions. Environ. Microbiol. 2014, 16, 1150–1166. [Google Scholar] [CrossRef]
- Hunter, J.E.; Frada, M.J.; Fredricks, H.F.; Vardi, A.; Van Mooy, B.A.S. Targeted and untargeted lipidomics of Emiliania huxleyi viral infection and life cycle phases highlights molecular biomarkers of infection, susceptibility, and ploidy. Front. Mar. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Malitsky, S.; Ziv, C.; Rosenwasser, S.; Zheng, S.; Schatz, D.; Porat, Z.; Ben-Dor, S.; Aharoni, A.; Vardi, A. Viral infection of the marine alga Emiliania huxleyi triggers lipidome remodeling and induces the production of highly saturated triacylglycerol. New Phytol. 2016, 210, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, A.R.P.; da Costa, E.; Silva, J.; Domingues, M.R.; Domingues, P. The effects of different extraction methods of lipids from Nannochloropsis oceanica on the contents of omega-3 fatty acids. Algal Res. 2019, 41. [Google Scholar] [CrossRef]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, E.; Melo, T.; Moreira, S.P.A.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, T.M.; Rego, M.A.; Domingues, P.; Calado, R.; et al. Valorization of lipids from Gracilaria sp. through lipidomics and decoding of antiproliferative and anti-inflammatory activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef] [Green Version]
- Rey, F.; Lopes, D.; Maciel, E.; Monteiro, J.; Skjermo, J.; Funderud, J.; Raposo, D.; Domingues, P.; Calado, R.; Domingues, M.R. Polar lipid profile of Saccharina latissima, a functional food from the sea. Algal Res. 2019, 39, 101473. [Google Scholar] [CrossRef]
- El Baz, F.K.; El Baroty, G.S.; Abd El Baky, H.H.; Abd El-Salam, O.I.; Ibrahim, E.A. Structural characterization and biological activity of sulfolipids from selected marine algae. Grasas y Aceites 2013, 64, 561–571. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; da Costa, E.; Melo, T.; Sulpice, R.; Cardoso, S.M.; Pitarma, B.; Pereira, R.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Seasonal plasticity of the polar lipidome of Ulva rigida cultivated in a sustainable integrated multi-trophic aquaculture. Algal Res. 2020, 49, 101958. [Google Scholar] [CrossRef]
- Da Costa, E.; Ricardo, F.; Melo, T.; Mamede, R.; Abreu, M.H.; Domingues, P.; Domingues, M.R.; Calado, R. Site-specific lipidomic signatures of sea lettuce (Ulva spp., chlorophyta) hold the potential to trace their geographic origin. Biomolecules 2020, 10, 489. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, J.P.; Rey, F.; Melo, T.; Moreira, A.S.P.; Arbona, J.F.; Skjermo, J.; Forbord, S.; Funderud, J.; Raposo, D.; Kerrison, P.D.; et al. The unique lipidomic signatures of Saccharina latissima can be used to pinpoint their geographic origin. Biomolecules 2020, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Mozes, A.; Florindo, C.; Polo, C.; Duarte, C.V.; Custádio, L.; Varela, J. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels 2011, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Dupré, C.; Burrows, H.D.; Campos, M.G.; Delattre, C.; Encarnação, T.; Fauchon, M.; Gaignard, C.; Hellio, C.; Ito, J.; Laroche, C.; et al. Microalgal biomass of industrial interest: Methods of characterization. In Handbook on Characterization of Biomass, Biowaste and Related By-Products; Springer International Publishing: Berlin, Germany, 2020; pp. 537–639. [Google Scholar]
- Liu, J.; Pan, Y.; Yao, C.; Wang, H.; Cao, X.; Xue, S. Determination of ash content and concomitant acquisition of cell compositions in microalgae via thermogravimetric (TG) analysis. Algal Res. 2015, 12, 149–155. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.E.; Heath, S.E.; Salmon, D.L.; Smirnoff, N.; Langer, G.; Taylor, A.R.; Brownlee, C.; Wheeler, G.L. An extracellular polysaccharide-rich organic layer contributes to organisation of the coccosphere in coccolithophores. Front. Mar. Sci. 2018, 5, 306. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Waldron, K.W.; Selvendran, R.R. Isolation and characterisation of cell wall polymers from olive pulp (Olea europaea L.). Carbohydr. Res. 1994, 252, 245–262. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Koch, A.K.; Käppeli, O.; Fiechter, A.; Reiser, J. Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J. Bacteriol. 1991, 173, 4212–4219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, W.W. The LipidWeb (Formerly the LipidHome)—An Alternative Lipid Library—Lipids, Fatty acids, Composition, Chemistry, Biochemistry, Mass Spectrometry, Blog. Available online: https://www.lipidhome.co.uk/ (accessed on 14 July 2020).
- Bell, B.M.; Daniels, D.G.H.; Fearn, T.; Stewart, B.A. Lipid compositions, baking qualities and other characteristics of wheat varieties grown in the U.K. J. Cereal Sci. 1987, 5, 277–286. [Google Scholar] [CrossRef]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric determination of phosphate esters in the presence and absence of orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef]
- Anjos, S.; Feiteira, E.; Cerveira, F.; Melo, T.; Reboredo, A.; Colombo, S.; Dantas, R.; Costa, E.; Moreira, A.; Santos, S.; et al. Lipidomics reveals similar changes in serum phospholipid signatures of overweight and obese pediatric subjects. J. Proteome Res. 2019, 18, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.; Alves, E.; Azevedo, V.; Martins, A.S.; Neves, B.; Domingues, P.; Calado, R.; Abreu, H.; Domingues, M.R. Lipidomics as a new approach for the bioprospecting of marine macroalgae–unraveling the polar lipid and fatty acid composition of Chondrus crispus. Algal Res. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Moreira, A.S.P.; Alves, E.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Decoding bioactive polar lipid profile of the macroalgae Codium tomentosum from a sustainable IMTA system using a lipidomic approach. Algal Res. 2015, 12, 388–397. [Google Scholar] [CrossRef]
- Murphy, R.C. Tandem Mass Spectrometry of Lipids; New Developments in Mass Spectrometry; The Royal Society of Chemistry, University of Colorado Denver: Aurora, CO, USA, 2015; ISBN 978-1-84973-827-9. [Google Scholar]
- Kato, M.; Sakai, M.; Adachi, K.; Ikemoto, H.; Sano, H. Distribution of betaine lipids in marine algae. Phytochemistry 1996, 42, 1341–1345. [Google Scholar] [CrossRef]
- Armada, I.; Hachero-Cruzado, I.; Mazuelos, N.; Ríos, J.L.; Manchado, M.; Cañavate, J.P. Differences in betaine lipids and fatty acids between Pseudoisochrysis paradoxa VLP and Diacronema vlkianum VLP isolates (Haptophyta). Phytochemistry 2013, 95, 224–233. [Google Scholar] [CrossRef]
- da Costa, E.; Azevedo, V.; Melo, T.; Rego, A.M.; Evtuguin, D.V.; Domingues, P.; Calado, R.; Pereira, R.; Abreu, M.H.; Domingues, M.R. High-Resolution Lipidomics of the Early Life Stages of the Red Seaweed Porphyra dioica. Molecules 2018, 23, 187. [Google Scholar] [CrossRef] [Green Version]
- Popendorf, K.J.; Fredricks, H.F.; Van Mooy, B.A.S. Molecular ion-independent quantification of polar glycerolipid classes in marine plankton using triple quadrupole MS. Lipids 2013, 48, 185–195. [Google Scholar] [CrossRef]
- Basconcillo, L.S.; Zaheer, R.; Finan, T.M.; McCarry, B.E. A shotgun lipidomics approach in Sinorhizobium meliloti as a tool in functional genomics. J. Lipid Res. 2009, 50, 1120–1132. [Google Scholar] [CrossRef] [Green Version]
- Degraeve-Guilbault, C.; Bréhélin, C.; Haslam, R.; Sayanova, O.; Marie-Luce, G.; Jouhet, J.; Corellou, F. Glycerolipid characterization and nutrient deprivation- associated changes in the green picoalga Ostreococcus tauri. Plant Physiol. 2017, 173, 2060–2080. [Google Scholar] [CrossRef] [Green Version]
- Liu, K. Characterization of ash in algae and other materials by determination of wet acid indigestible ash and microscopic examination. Algal Res. 2017, 25, 307–321. [Google Scholar] [CrossRef]
- Chairopoulou, M.A.; Kratzer, F.; Gross, R.; Herrmann, M.; Teipel, U. Influence of the Temperature on Coccolith-Containing Systems from Emiliania huxleyi Cultivations. Chem. Eng. Technol. 2020, 43, 904–912. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, G.W.; Yen, T.Q.; Leitch, M.A.; Wilson, G.R.; Brown, E.A.; Rider, D.A.; Reddy, C.M. Alkenones as renewable phase change materials. Renew. Energy 2019, 134, 89–94. [Google Scholar] [CrossRef]
- Conte, M.H.; Volkman, J.K. Lipid biomarkers of the Haptophyta. In The Haptophyte Algae; Green, J.C., Leadbeater, B.S.C., Eds.; Clarendon Press: Oxford, UK, 1994; pp. 351–377. [Google Scholar]
- Bell, M.V.; Pond, D. Lipid composition during growth of motile and coccolith forms of Emiliania huxleyi. Phytochemistry 1996, 41, 465–471. [Google Scholar] [CrossRef]
- Evans, C.; Pond, D.W.; Wilson, W.H. Changes in Emiliania huxleyi fatty acid profiles during infection with E. huxleyi virus 86: Physiological and ecological implications. Aquat. Microb. Ecol. 2009, 55, 219–228. [Google Scholar] [CrossRef]
- Sayanova, O.; Haslam, R.P.; Calerón, M.V.; López, N.R.; Worthy, C.; Rooks, P.; Allen, M.J.; Napier, J.A. Identification and functional characterisation of genes encoding the omega-3 polyunsaturated fatty acid biosynthetic pathway from the coccolithophore Emiliania huxleyi. Phytochemistry 2011, 72, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: A systematic review. Clin. Cardiol. 2009, 32, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Wang, B.G. Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J. Agric. Food Chem. 2004, 52, 4993–4997. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Ascherio, A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Siscovick, D.S.; Rimm, E.B. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005, 111, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, A.; Saotome, M.; Iguchi, K.; Maekawa, Y. Marine-derived omega-3 polyunsaturated fatty acids and heart failure: Current understanding for basic to clinical relevance. Int. J. Mol. Sci. 2019, 20, 4025. [Google Scholar] [CrossRef] [Green Version]
- Freitas, D.S.R.; Campos, M.M. Protective effects of omega-3 fatty acids in cancer-related complications. Nutrients 2019, 11, 945. [Google Scholar] [CrossRef] [Green Version]
- Rennie, K.L.; Hughes, J.; Lang, R.; Jebb, S.A. Nutritional management of rheumatoid arthritis: A review of the evidence. J. Hum. Nutr. Diet. 2003, 16, 97–109. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, J.P.; Parra-Cabrera, S.; Colditz, G.A.; Berkey, C.S.; Dwyer, J.T. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics 2000, 105, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Fonseca, J.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Dietary intake of α-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br. J. Nutr. 2011, 106, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Li, Y.-L.; Shen, W.-Z.; Rui, W.; Ma, X.-J.; Cen, Y.-Z. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. Bot. Mar. 2007, 50, 185–190. [Google Scholar] [CrossRef]
- Ohta, K.; Mizushima, Y.; Hirata, N.; Takemura, M.; Sugawara, F.; Matsukage, A.; Yoshida, S.; Sakaguchi, K. Sulfoquinovosyldiacylglycerol, KM043, a New Potent Inhibitor of Eukaryotic DNA Polymerases and HIV-Reverse Transcriptase Type 1 from a Marine Red Alga, Gigartina tenella. Chem. Pharm. Bull. (Tokyo) 1998, 46, 684–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Morimoto, T.; Nagatsu, A.; Murakami, N.; Sakakibara, J.; Tokuda, H.; Nishino, H.; Iwashima, A. Anti-tumour-promoting glyceroglycolipids from the green alga, Chlorella vulgaris. Phytochemistry 1995, 40, 1433–1437. [Google Scholar] [CrossRef]
- Canãvate, J.P.; Armada, I.; Riós, J.L.; Hachero-Cruzado, I. Exploring occurrence and molecular diversity of betaine lipids across taxonomy of marine microalgae. Phytochemistry 2016, 124, 68–78. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; McGinn, P.J. New diacylglyceryltrimethylhomoserines from the marine microalga Nannochloropsis granulata and their nitric oxide inhibitory activity. J. Appl. Phycol. 2013, 25, 1513–1521. [Google Scholar] [CrossRef]
- Miazek, K.; Lebecque, S.; Hamaidia, M.; Paul, A.; Danthine, S.; Willems, L.; Frederich, M.; De Pauw, E.; Deleu, M.; Richel, A.; et al. Sphingolipids: Promising lipid-class molecules with potential applications for industry. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 321–336. [Google Scholar]
- Li, Y.; Lou, Y.; Mu, T.; Ke, A.; Ran, Z.; Xu, J.; Chen, J.; Zhou, C.; Yan, X.; Xu, Q.; et al. Sphingolipids in marine microalgae: Development and application of a mass spectrometric method for global structural characterization of ceramides and glycosphingolipids in three major phyla. Anal. Chim. Acta 2017, 986, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.L.; Walters, K.S.; Drake, D.R.; Blanchette, D.R.; Dawson, D.V.; Brogden, K.A.; Wertz, P.W. Sphingoid bases are taken up by Escherichia coli and Staphylococcus aureus and induce ultrastructural damage. Skin Pharmacol. Physiol. 2012, 26, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becam, J.; Walter, T.; Burgert, A.; Schlegel, J.; Sauer, M.; Seibel, J.; Schubert-Unkmeir, A. Antibacterial activity of ceramide and ceramide analogs against pathogenic Neisseria. Sci. Rep. 2017, 7, 17627. [Google Scholar] [CrossRef] [PubMed]
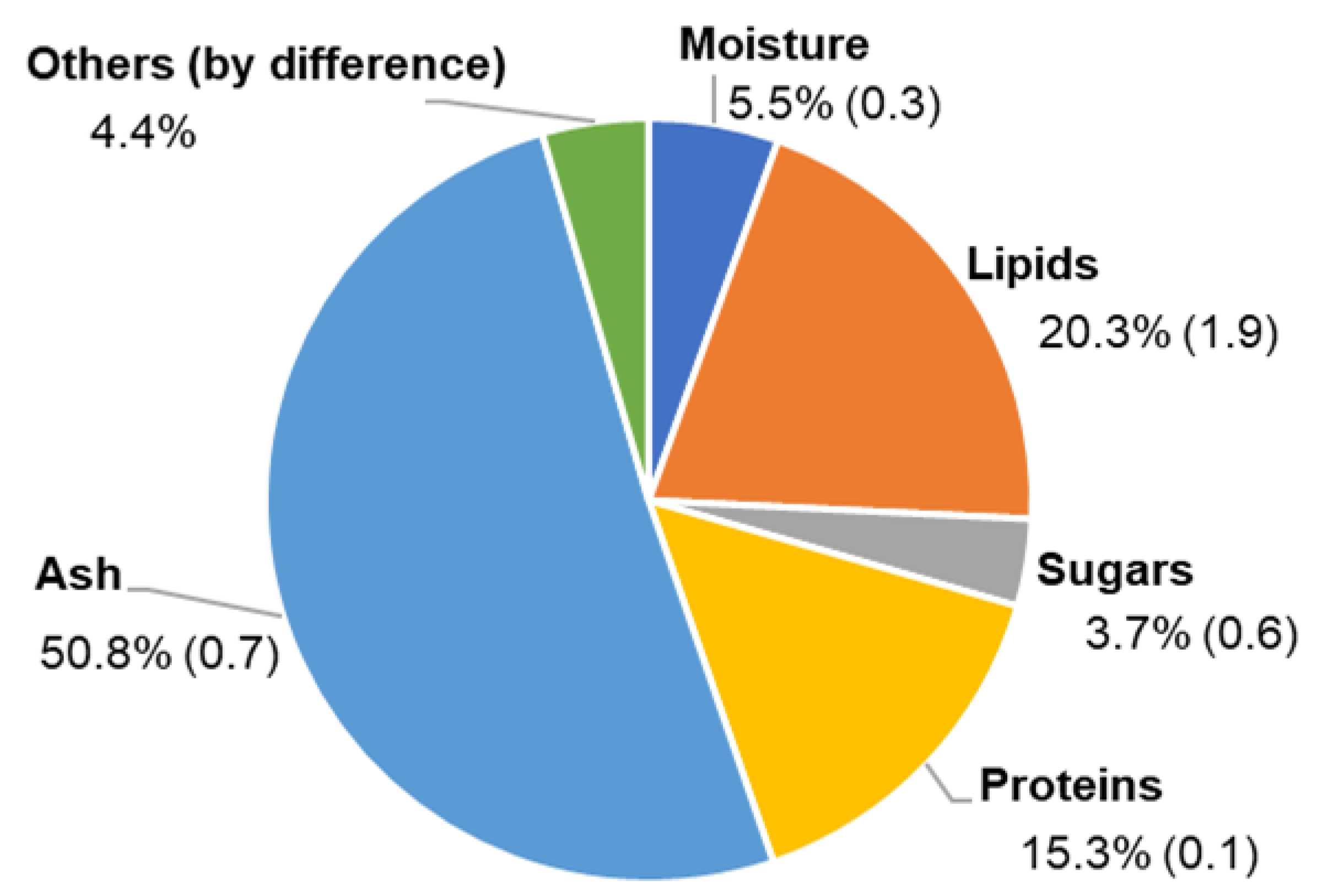
| Fatty Acids (% of Total FA) | Amount (±SD, n = 3) |
|---|---|
| 14:0 | 9.2 ± 1.4 |
| 13-methyl-14:0 (iso) | 2.4 ± 0.1 |
| 15:0 | 1.5 ± 0.2 |
| 16:0 | 17.1 ± 1.8 |
| 16:1n-9 | 0.7 ± 0.1 |
| 16:1n-7 | 1.4 ± 0.4 |
| 18:0 | 13.4 ± 1.6 |
| 18:1n-9 | 6.6 ± 0.2 |
| 18:1n-7 | 7.7 ± 0.3 |
| 18:3n-3 | 5.3 ± 0.2 |
| 18:4n-3 | 11.0 ± 0.4 |
| 18:5n-3 | 6.6 ± 0.5 |
| 22:6n-3 | 17.2 ± 2.7 |
| Σ SFA | 43.6 ± 2.5 |
| Σ MUFA | 16.4 ± 0.9 |
| Σ PUFA | 40.0 ± 3.2 |
| Total FA (µg mg−1 extract) | 85.1 ± 12.5 |
| Lipid Species (C:N) | Observed m/z | Theoretical m/z | Error (ppm) | Fatty Acyl Chain (s) | Formula |
|---|---|---|---|---|---|
| SQMG (14:0) | 527.2540 | 527.25261 | 2.6321 | 14:0 | C23H43O11S |
| SQMG (18:4) | 575.2541 | 575.25261 | 2.5218 | 18:4 | C27H43O11S |
| SQMG (22:6) | 627.2859 | 627.28391 | 3.0996 | 22:6 | C31H47O11S |
| SQDG (28:0) | 737.4534 | 737.4510 | 3.2395 | 14:0-14:0 | C37H69O12S |
| SQDG (30:0) | 765.4843 | 765.4823 | 2.5904 | 14:0-16:0 | C39H73O12S |
| SQDG (30:1) | 763.4668 | 763.4666 | 0.2376 | 14:0-16:1, 14:1-16:0 | C39H71O12S |
| SQDG (32:0) | 793.5124 | 793.5136 | −1.5073 | 16:0-16:0, 18:0-14:0 | C41H77O12S |
| SQDG (32:1) | 791.5006 | 791.4979 | 3.3639 | 14:0-18:1, 16:1-16:0 | C41H75O12S |
| SQDG (32:2) | 789.4825 | 789.4823 | 0.3421 | 14:0-18:2 | C41H73O12S |
| SQDG (32:3) | 787.4688 | 787.4666 | 2.8122 | 14:0-18:3 | C41H71O12S |
| SQDG (32:4) | 785.4545 | 785.4510 | 4.4448 | 14:0-18:4 | C41H69O12S |
| SQDG (32:5) | 783.4360 | 783.4353 | 0.8766 | 14:0-18:5 | C41H67O12S |
| SQDG (34:1) | 819.5288 | 819.5292 | −0.4555 | 16:0-18:1, 17:0-17:1 | C43H79O12S |
| SQDG (34:4) | 813.4846 | 813.4823 | 2.8541 | 16:0-18:4 | C43H73O12S |
| SQDG (34:6) | 809.4518 | 809.4510 | 1.0215 | 16:2-18:4 | C43H69O12S |
| SQDG (36:1) | 847.5594 | 847.5605 | −1.3057 | 22:1-14:0; 20:1-16:0 | C45H83O12S |
| SQDG (36:2) | 845.5466 | 845.5449 | 2.0742 | 16:0-20:2, 18:1-18:1 | C45H81O12S |
| SQDG (36:5) | 839.5003 | 839.4979 | 2.7927 | 18:1-18:4, 20:5-16:0, 22:5-14:0 | C45H75O12S |
| SQDG (36:6) | 837.4813 | 837.4823 | −1.2034 | 18:4-18:2, 14:0-22:6, 18:3-18:3 | C45H73O12S |
| SQDG (36:7) | 835.4692 | 835.4666 | 3.0794 | 18:3-18:4 | C45H71O12S |
| SQDG (36:8) | 833.4516 | 833.4510 | 0.7304 | 18:4-18:4 | C45H69O12S |
| SQDG (36:9) | 831.4378 | 831.4353 | 3.0652 | 18:4-18:5 | C45H67O12S |
| SQDG (38:2) | 873.5791 | 873.5761 | 3.4656 | 18:1-20:1 | C47H85O12S |
| SQDG (38:6) | 865.5158 | 865.5136 | 2.5672 | 16:0-22:6, 18:4-20:2, 18:3-20:3 | C47H77O12S |
| SQDG (38:9) | 859.4697 | 859.4666 | 3.5523 | 20:5-18:4 | C47H71O12S |
| SQDG (40:10) | 885.4853 | 885.4822 | 3.5017 | 18:4-22:6 | C49H73O12S |
| SQDG (44:12) | 937.5164 | 937.5136 | 3.0468 | 22:6-22:6 | C53H77O12S |
| Lipid Species (C:N) | Observed m/z | Theoretical m/z | Error (ppm) | Fatty Acyl Chain (s) | Formula |
|---|---|---|---|---|---|
| MGMG (14:0) | 482.3330 | 482.3329 | 0.1768 | 14:0 | C23H48NO9 |
| MGMG (16:0) | 510.3636 | 510.3642 | −1.1756 | * | C25H52NO9 |
| MGMG (18:1) | 536.3799 | 536.3799 | 0.1570 | * | C27H54NO9 |
| MGMG (18:3) | 532.3497 | 532.3486 | 2.0900 | * | C27H50NO9 |
| MGMG (18:4) | 530.3341 | 530.3329 | 2.2083 | 18:4 | C27H48NO9 |
| MGMG (18:5) | 528.3175 | 528.3173 | 0.5232 | * | C27H46NO9 |
| MGMG (20:5) | 556.3487 | 556.3486 | 0.2706 | * | C29H50NO9 |
| MGMG (22:6) | 582.3648 | 582.3642 | 1.0647 | * | C31H52NO9 |
| MGDG (28:0) | 692.5326 | 692.5307 | 2.7298 | * | C37H74NO10 |
| MGDG (32:1) | 746.5792 | 746.5777 | 2.0069 | 14:0-18:1 | C41H80NO10 |
| MGDG (32:3) | 742.5439 | 742.5464 | −3.4143 | * | C41H76NO10 |
| MGDG (32:4) | 740.5320 | 740.5307 | 1.8200 | * | C41H74NO10 |
| MGDG (34:1) | 774.6084 | 774.6090 | −0.7271 | * | C43H84NO10 |
| MGDG (36:2) | 800.6249 | 800.6252 | −0.3670 | * | C45H86NO10 |
| MGDG (36:4) | 796.5967 | 796.5933 | 4.2891 | * | C45H82NO10 |
| MGDG (36:8) | 788.5304 | 788.5313 | −1.0574 | 18:4-18:4 | C45H74NO10 |
| MGDG (36:9) | 786.5152 | 786.5156 | −0.4468 | * | C45H72NO10 |
| MGDG (36:10) | 784.5000 | 784.5000 | 0.0065 | 18:5-18:5 | C45H70NO10 |
| MGDG (38:5) | 822.6092 | 822.6095 | −0.3692 | * | C47H84NO10 |
| MGDG (38:6) | 820.5947 | 820.5939 | 0.9627 | * | C47H82NO10 |
| MGDG (38:9) | 814.5452 | 814.5469 | −2.0729 | * | C47H76NO10 |
| MGDG (40:10) | 840.5633 | 840.5626 | 0.9151 | * | C49H78NO10 |
| MGDG (40:11) | 838.5491 | 838.5469 | 2.6480 | * | C49H76NO10 |
| MGDG (40:3) | 854.6719 | 854.6721 | −0.2641 | * | C49H92NO10 |
| MGDG (44:12) | 892.5956 | 892.5939 | 1.9808 | * | C53H82NO10 |
| DGMG (18:5) | 690.3709 | 690.3701 | 1.1385 | * | C33H56NO14 |
| DGDG (28:0) | 854.5859 | 854.5841 | 2.1204 | * | C43H84NO15 |
| DGDG (32:1) | 908.6323 | 908.6310 | 1.4458 | 18:1-14:0 | C47H90NO15 |
| DGDG (36:5) | 956.6292 | 956.6310 | −1.8642 | * | C51H90NO15 |
| DGDG (36:6) | 954.6145 | 954.6154 | −0.9079 | * | C51H88NO15 |
| DGDG (36:8) | 950.5829 | 950.5841 | −1.2570 | * | C51H84NO15 |
| DGDG (36:10) | 946.5539 | 946.5528 | 1.1713 | 18:5-18:5 | C51H80NO15 |
| DGDG (40:11) | 1000.6029 | 1000.5997 | 3.2294 | * | C55H86NO15 |
| Lipid Species (C:N) | Observed m/z | Theoretical m/z | Error (ppm) | Fatty Acyl Chain (s) | Formula |
|---|---|---|---|---|---|
| MGTS (14:0) | 446.3483 | 446.3482 | 0.3670 | 14:0 | C24H48O6N |
| MGTS (20:5) | 520.3645 | 520.3638 | 1.3522 | 20:5 | C30H50O6N |
| DGTS (30:0) | 684.5791 | 684.5778 | 1.9458 | 15:0-15:0; 14:0-16:0 | C40H78O7N |
| DGTS (32:1) | 710.5940 | 710.5935 | 0.6954 | 18:1-14:0 | C42H80O7N |
| DGTS (32:2) | 708.5776 | 708.5778 | −0.2396 | 16:1-16:1 | C42H78O7N |
| DGTS (32:3) | 706.5613 | 706.5622 | −1.3216 | * | C42H76O7N |
| DGTS (32:7) | 698.5001 | 698.4996 | 0.7037 | * | C42H68O7N |
| DGTS (34:1) | 738.6252 | 738.6248 | 0.5559 | 16:0-18:1 | C44H84O7N |
| DGTS (34:4) | 732.5780 | 732.5778 | 0.2284 | * | C44H78O7N |
| DGTS (36:2) | 764.6416 | 764.6404 | 1.6039 | 18:1-18:1 | C46H86O7N |
| DGCC (36:6) | 772.5738 | 772.5727 | 1.4238 | 22:6-14:0 | C46H78O8N |
| DGCC (40:7) | 826.6216 | 826.6197 | 2.2985 | ** | C50H84O8N |
| DGCC (44:12) | 872.6045 | 872.6040 | 0.5730 | ** | C54H82O8N |
| BLL (38:6) | 830.5798 | 830.5782 | 1.9264 | ** | C48H80O10N |
| BLL (40:7) | 856.5946 | 856.5939 | 0.8172 | ** | C50H82O10N |
| Lipid Species (C:N) | Observed m/z | Theoretical m/z | Error (ppm) | Fatty Acyl Chain (s) | Formula |
|---|---|---|---|---|---|
| PC(30:0) | 706.5394 | 706.5387 | 0.9663 | 12:0-18:0 | C38H77NO8P |
| PC(30:3) | 700.4909 | 700.4917 | −1.2501 | * | C38H71NO8P |
| PC(36:2) | 786.6019 | 786.6013 | 0.7575 | 18:1-18:1 | C44H85NO8P |
| PC(36:3) | 784.5872 | 784.5856 | 2.0557 | * | C44H83NO8P |
| PC(36:6) | 778.5397 | 778.5387 | 1.3224 | 22:6-14:0 | C44H77NO8P |
| PC(37:2) | 800.6168 | 800.6169 | −0.1249 | ** | C45H87NO8P |
| PC(38:2) | 814.6326 | 814.6326 | −0.0365 | 18:1-20:1 | C46H89NO8P |
| PC(38:5) | 808.5837 | 808.5856 | −4.3696 | * | C46H83NO8P |
| PC(38:6) | 806.5718 | 806.5700 | 2.2120 | 22:6-16:0; 18:2-20:4; 18:1-20:5 | C46H81NO8P |
| PC(40:7) | 832.5865 | 832.5856 | 1.0146 | 22:6-18:1 | C48H83NO8P |
| PC(44:12) | 878.5718 | 878.5700 | 2.1064 | 22:6-22:6 | C52H81NO8P |
| LPC(14:0) | 468.3092 | 468.3090 | 0.4532 | ** | C22H47NO7P |
| LPC(16:0) | 496.3408 | 496.3403 | 0.9748 | ** | C24H51NO7P |
| LPC(18:1) | 522.3567 | 522.3560 | 1.3845 | ** | C26H53NO7P |
| LPC(22:6) | 568.3406 | 568.3403 | 0.4399 | ** | C30H51NO7P |
| PE(30:0) | 664.4923 | 664.4917 | 0.9125 | ** | C35H71NO8P |
| PE(30:1) | 662.4772 | 662.4761 | 1.7143 | ** | C35H69NO8P |
| PE(30:3) | 658.4435 | 658.4448 | −1.8979 | * | C35H65NO8P |
| PE(31:1)† | 676.4931 | 676.4917 | 2.0695 | ** | C36H71NO8P |
| PE(32:1) | 690.5065 | 690.5074 | −1.2180 | 16:0-16:1 | C37H73NO8P |
| PE(32:2) | 688.4952 | 688.4917 | 4.9833 | ** | C37H71NO8P |
| PE(32:6) | 680.4258 | 680.4291 | −4.8497 | * | C37H63NO8P |
| PE(34:1) | 718.5382 | 718.5387 | −0.7092 | 16:0-18:1; 14:0-20:1 | C39H77NO8P |
| PE(34:2) | 716.5239 | 716.5230 | 1.1764 | * | C39H75NO8P |
| PE(34:3) | 714.5087 | 714.5074 | 1.8055 | * | C39H73NO8P |
| PE(34:4) | 712.4914 | 712.4917 | −0.4003 | ** | C39H71NO8P |
| PE(36:2) | 744.5553 | 744.5543 | 1.2849 | 18:1-18:1 | C41H79NO8P |
| PE(36:3) | 742.5406 | 742.5387 | 2.5718 | ** | C41H77NO8P |
| PE(36:4) | 740.5240 | 740.5230 | 1.2744 | * | C41H75NO8P |
| PE(38:2) | 772.5848 | 772.5856 | −1.0554 | * | C43H83NO8P |
| PE(38:5) | 766.5373 | 766.5387 | −1.8029 | ** | C43H77NO8P |
| PE(38:6) | 764.5217 | 764.5230 | −1.6806 | * | C43H75NO8P |
| LPE(18:1) | 480.3096 | 480.3090 | 1.2613 | * | C23H47NO7P |
| MMPE (30:1)† | 676.4931 | 676.4917 | 2.0695 | ** | C36H71NO8P |
| PDPT (36:6) | 795.5009 | 795.4999 | 1.2571 | ** | C44H76O8PS |
| PDPT (38:6) | 823.5327 | 823.5311 | 1.9429 | ** | C46H80O8PS |
| PDPT (40:7) | 849.5469 | 849.5468 | 0.1177 | ** | C48H82O8PS |
| PDPT (44:12) | 895.5312 | 895.5312 | 0.0558 | * | C52H80O8PS |
| Lipid Species (C:N) | Observed m/z | Theoretical m/z | Error (ppm) | Fatty Acyl Chains | Formula |
|---|---|---|---|---|---|
| PG(30:0) | 693.4735 | 693.4707 | 4.0478 | 14:0-16:0 | C36H70O10P |
| PG(30:1) | 691.4569 | 691.4550 | 2.6592 | 14:0-16:1; 15:0-15:1; 16:0-14:1 | C36H68O10P |
| PG(32:0) | 721.5013 | 721.5020 | −0.8607 | 17:0-15:0; 16:0-16:0; 18:0-14:0 | C38H74O10P |
| PG(32:1) | 719.4885 | 719.4863 | 3.0933 | 16:1-16:0, 14:0-18:1,15:0-17:1, 17:0-15:1 | C38H72O10P |
| PG(32:2) | 717.4738 | 717.4707 | 4.3987 | 16:1-16:1 | C38H70O10P |
| PG(34:1) | 747.5200 | 747.5176 | 3.2058 | 16:0-18:1, 16:1-18:0 | C40H76O10P |
| PG(34:2) | 745.5042 | 745.5020 | 2.9900 | 16:1-18:1 | C40H74O10P |
| PG(36:2) | 773.5355 | 773.5333 | 2.8853 | 18:1-18:1 | C42H78O10P |
| PG(36:3) | 771.5194 | 771.5176 | 2.2813 | 18:1-18:2 | C42H76O10P |
| PG(36:7) | 763.4559 | 763.4550 | 1.1658 | 20:5-16:2 | C42H68O10P |
| PG(38:2) | 801.5670 | 801.5646 | 3.0267 | 18:1-20:1, 19:1-19:1 | C44H82O10P |
| PI(32:7) | 795.4113 | 795.4085 | 3.5930 | * | C41H64O13P |
| PI(38:6) | 881.5196 | 881.5180 | 1.8331 | 16:0-22:6 | C47H78O13P |
| Lipid Species (C:N) | Observed m/z | Theoretical m/z | Error (ppm) | Fatty Acyl Chain (s) | Formula |
|---|---|---|---|---|---|
| sGSL (d40:2) | 870.6685 | 870.6670 | 1.7228 | d18:2/22:0 | C49H92O11N |
| sGSL (d40:1) | 872.6829 | 872.6827 | 0.2292 | d18:1/22:0 | C49H94O11N |
| hGSL | 806.6159 | 806.6146 | 1.6117 | d19:3/h22:2 | C47H84O9N |
| Cer(d36:2) | 564.5363 | 564.5356 | 1.3198 | * | C36H70NO3 |
| Cer(d38:1) | 594.5827 | 594.5825 | 0.2838 | * | C38H76NO3 |
| Cer(d38:2) | 592.5672 | 592.5669 | 0.6268 | * | C38H74NO3 |
| Cer(d40:1) | 622.6143 | 622.6138 | 0.7211 | d18:1/22:0 | C40H80NO4 |
| Cer(d40:2) | 620.5975 | 620.5982 | −1.0393 | d18:2/22:0 | C40H78NO5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aveiro, S.S.; Melo, T.; Figueiredo, A.; Domingues, P.; Pereira, H.; Maia, I.B.; Silva, J.; Domingues, M.R.; Nunes, C.; Moreira, A.S.P. The Polar Lipidome of Cultured Emiliania huxleyi: A Source of Bioactive Lipids with Relevance for Biotechnological Applications. Biomolecules 2020, 10, 1434. https://doi.org/10.3390/biom10101434
Aveiro SS, Melo T, Figueiredo A, Domingues P, Pereira H, Maia IB, Silva J, Domingues MR, Nunes C, Moreira ASP. The Polar Lipidome of Cultured Emiliania huxleyi: A Source of Bioactive Lipids with Relevance for Biotechnological Applications. Biomolecules. 2020; 10(10):1434. https://doi.org/10.3390/biom10101434
Chicago/Turabian StyleAveiro, Susana S., Tânia Melo, Ana Figueiredo, Pedro Domingues, Hugo Pereira, Inês B. Maia, Joana Silva, M. Rosário Domingues, Cláudia Nunes, and Ana S. P. Moreira. 2020. "The Polar Lipidome of Cultured Emiliania huxleyi: A Source of Bioactive Lipids with Relevance for Biotechnological Applications" Biomolecules 10, no. 10: 1434. https://doi.org/10.3390/biom10101434
APA StyleAveiro, S. S., Melo, T., Figueiredo, A., Domingues, P., Pereira, H., Maia, I. B., Silva, J., Domingues, M. R., Nunes, C., & Moreira, A. S. P. (2020). The Polar Lipidome of Cultured Emiliania huxleyi: A Source of Bioactive Lipids with Relevance for Biotechnological Applications. Biomolecules, 10(10), 1434. https://doi.org/10.3390/biom10101434








