Tailoring the Health-Promoting Potential of Protein Hydrolysate Derived from Fish Wastes and Flavonoids from Yellow Onion Skins: From Binding Mechanisms to Microencapsulated Functional Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Flavonoids from Yellow Onion Skins
2.3. Fish Waste Material Preliminary Preparation
2.4. Enzymatic Hydrolysis of Fish Waste
2.5. Bioactive Peptides Identification and Characterization
2.6. Heat Treatment
2.7. Quenching of Bioactive Peptides with Yellow Onion Skins Extract
2.8. Microencapsulation of the Flavonoidic Extract in Bioactive Fish Peptides
2.9. Characterization of the Extract and of Microencapsulated Powders
2.10. Cell Culture and T
2.11. In Vitro Cytotoxicity Tests
2.12. Statistical Analysis
3. Results
3.1. Peptides Identification and Characterization
3.2. Characterization of the Flavonoid Extract from Onion Skins
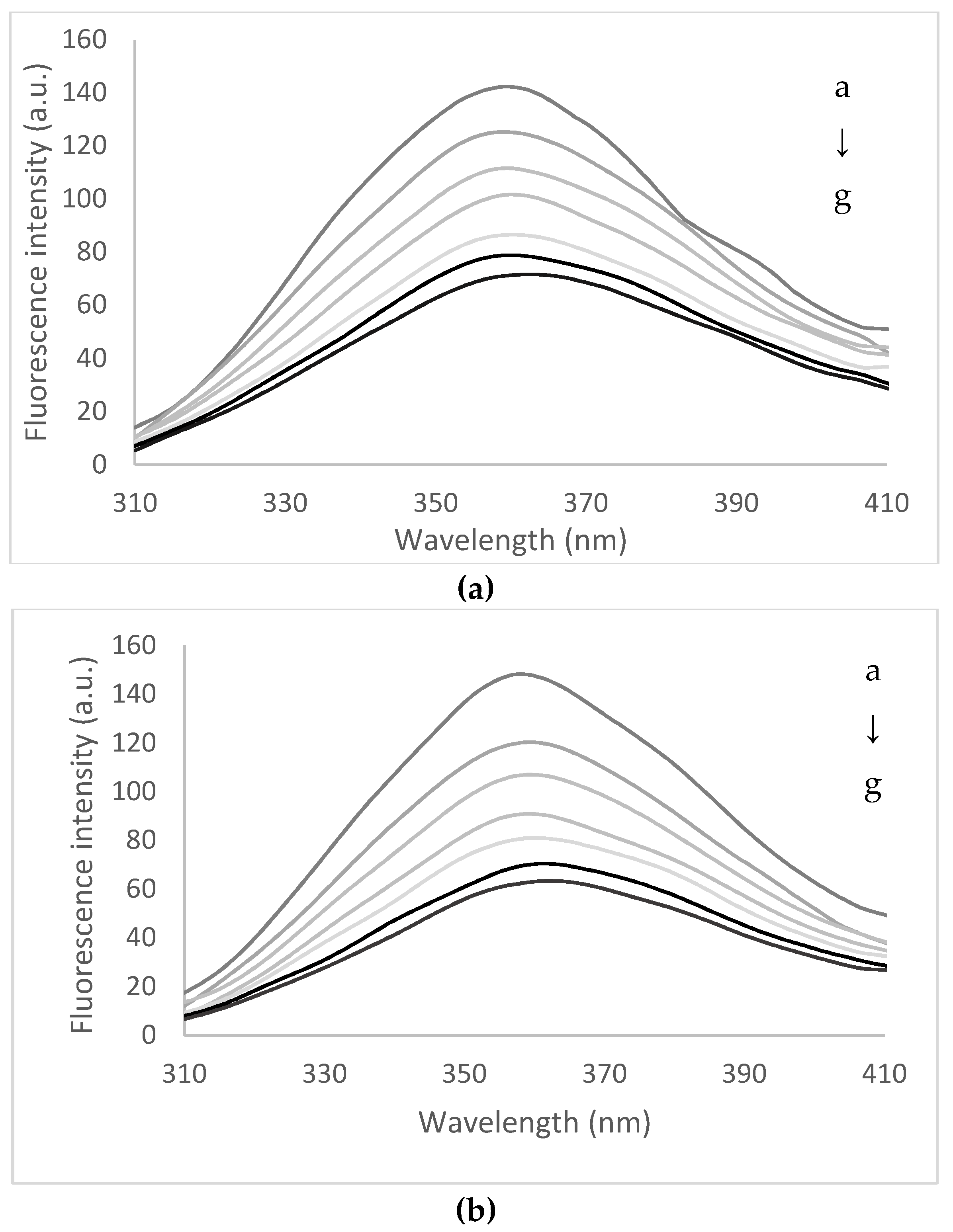
3.3. Quenching of Peptides Solutions with Flavonoids Extract
3.4. Microencapsulation of the Flavonoidic Extract in Bioactive Fish Peptides
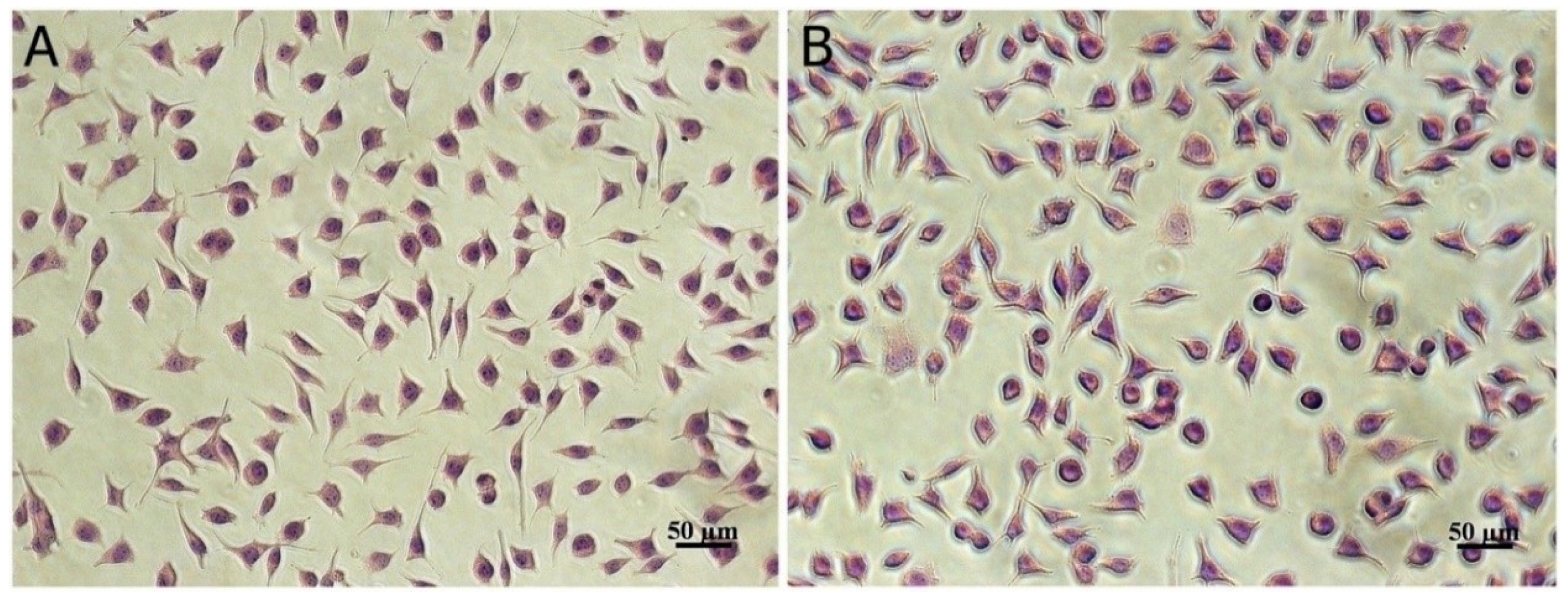
3.5. In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chalamaiah, M.; Kumar, B.D.; Hemalatha, R.; Jyothirmayi, T. (FPH): Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef] [PubMed]
- Nasri, R.; Younes, I.; Jridi, M.; Trigui, M.; Boutagef, A.; Nedjar-Arroume, N.; Dhulster, P.; Nasri, M.; Karra-Châabouni, M. ACE-inhibitory and antioxidative activities of Goby (Zosterissessorophiocephalus) (FPH): Effect on meat lipid oxidation. Food Res. Int. 2013, 54, 552–561. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ramakrishnan, V.V.; Brook, M.S.; Budge, S.M.; Dave, D. Fish processing wastes as a potential source of proteins, amino acids and oils: A critical review. J. Microb. Biochem. Technol. 2013, 5, 107–129. [Google Scholar]
- Galla, N.R.; Pamidighantam, P.R.; Akula, S.; Karakala, B. Functional properties and in vitro antioxidant activity of roe protein hydrolysates of Channastriatus and Labeohorita. Food Chem. 2012, 135, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Ghassem, M.; Arihara, K.; Babji, A.S.; Said, M.; Ibrahim, S. Purification and identification of ACE inhibitory peptides from Haruan (Channastriatus) myofibrillar protein hydrolysate using HPLC-ESI-TOF MS/MS. Food Chem. 2011, 129, 1770–1777. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Campone, L.; Celano, R.; Lisa Piccinelli, A.; Pagano, I.; Carabetta, S.; Sanzo, R.D.; Russo, M.; Ibañez, E.; Cifuentes, A.; Rastrelli, L. Response surface methodology to optimize supercritical carbon dioxide/co-solvent extraction of brown onion skin by-product as source of nutraceutical compounds. Food Chem. 2018, 269, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Boiangiu, R.S.; Mihasan, M.; Gorgan, L.D.; Stache, B.A.; Petre, B.A.; Hritcu, L. Cotinine and 6-hydroxy-L-nicotine reverses memory deficits and reduces oxidative stress in Aβ25-35-induced rat model of Alzheimer’s disease. Antioxidants (Basel) 2020, 9, 768. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of industrial onion wastes (Allium cepa L.): Dietary fibre and bioactive compounds. Plant. Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar]
- Suleria, H.A.R.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66. [Google Scholar] [CrossRef]
- Ly, T.N.; Hazama, C.; Shimoyamada, M.; Ando, H.; Kato, K.; Yamauchi, R. Antioxidative compounds from the outer scales of onion. J. Agric. Food Chem. 2005, 53, 8183–8189. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Inter. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condurache, N.N.; Aprodu, I.; Grigore-Gurgu, L.; Petre, B.A.; Enache, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Fluorescence spectroscopy and molecular modeling of anthocyanins binding to bovine lactoferrin peptides. Food Chem. 2020, 318, 126508. [Google Scholar] [CrossRef] [PubMed]
- Dumitrașcu, L.; Stănciuc, N.; Bahrim, G.E.; Aprodu, I. Insights into the binding of ferulic acid to the thermally treated xanthine oxidase. Luminescence 2016, 31, 1259–1266. [Google Scholar] [CrossRef]
- Horincar, G.; Aprodu, I.; Barbu, V.; Rapeanu, G.; Bahrim, G.E.; Stănciuc, N. Interactions of flavonoids from yellow onion skins with whey proteins: Mechanisms of binding and microencapsulation with different combinations of polymers. Spectrochim Acta A Mol. Biomol. Spectrosc. 2019, 215, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Milea, A.S.; Aprodu, I.; Vasile, A.M.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Widen the functionality of flavonoids from yellow onion skins through extraction and microencapsulation in whey proteins hydrolysates and different polymers. J. Food Eng. 2019, 251, 29–35. [Google Scholar] [CrossRef]
- Oancea, A.M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Rapeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT Food Sci. Technol. 2018, 95, 129–134. [Google Scholar] [CrossRef]
- ISO 10993-5:2009—Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009.
- Crăciunescu, O.; Gaspar, A.; Trif, M.; Moisei, M.; Oancea, A.; Moldovan, L.; Zarnescu, O. Preparation and characterization of a collagen-liposome-chondroitin sulfate matrix for inflammatory disorders treatment. J. Nanomat. 2014. [Google Scholar] [CrossRef]
- Dupree, E.J.; Jayathirtha, M.H.Y.; Petre, B.-A.; Mihasan, M.; Darie, C.C. A critical review of bottom-up proteomics: The good, the bad, and the future of this field. Proteomes 2020, 8, 14. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Humana Press: Tortowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Chakchouk-Mtibaa, A.; Elleuch, L.; Smaoui, S.; Najah, S.; Sellem, I.; Abdelkafi, S.; Mellouli, L. An antilisterial bacteriocin BacFL31 produced by Enterococcus faecium FL31 with a novel structure containing hydroxyproline residues. Anaerobe 2014, 27, 1–6. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Cleaner Prod. 2018, 183, 487–493. [Google Scholar] [CrossRef]
- Kim, S.W.; Ko, M.J.; Chung, M.S. Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J. Cleaner Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]
- Lakovicz, J.R. Principles of Fluorescence Spectroscopy; Springer Nature: Basel, Switzerland, 1999. [Google Scholar]
- Ciotta, E.; Prosposito, P.; Pizzoferrato, R. Positive curvature in Stern-Volmer plot described by a generalized model for static quenching. J. Lumin. 2019, 206, 518–522. [Google Scholar] [CrossRef]
- Tang, H.; Huang, L.; Zhao, D.; Sun, D.; Song, P. Interaction mechanism of flavonoids on bovine serum albumin: Insights from molecular property-binding affinity relationship. Spectrochim Acta A Mol. Biomol. Spectrosc. 2020, 239, 118519. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Zhang, G.W.; Hu, Y.T.; Ma, Y.D. Effect of luteolin on xanthine oxidase: Inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem. 2013, 141, 3766–3773. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, P.; Kong, L.; Xu, B. Microencapsulation of curcumin by spray drying and freeze drying. LWT Food Sci. Tech. 2020, 132, 109892. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Drying Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Chen, Z.; Liu, W.; Chen, H. Characterization and storage properties of a new microencapsulation of tea polyphenols. Ind. Crops Prod. 2016, 89, 152–156. [Google Scholar] [CrossRef]
- Zhang, X.; Yoon, H.J.; Kang, M.G.; Kim, G.J.; Shin, S.Y.; Baek, S.H.; Lee, J.G.; Bai, J.; Lee, S.Y.; Choi, M.J.; et al. Identification and evaluation of cytotoxicity of peptide liposome incorporated citron extracts in an in vitro system. Int. J. Mol. Sci. 2018, 19, 626. [Google Scholar] [CrossRef] [Green Version]
- Aprodu, I.; Milea, S.A.; Anghel, R.M.; Enachi, E.; Barbu, V.; Crăciunescu, O.; Râpeanu, G.; Bahrim, G.E.; Oancea, A.; Stănciuc, N. New functional ingredients based on microencapsulation of aqueous anthocyanin-rich extracts derived from black rice. Molecules 2019, 24, 3389. [Google Scholar] [CrossRef] [Green Version]
- Ghatak, D.; Iyyaswami, R. Selective encapsulation of quercetin from dry onion peel crude extract in reassembled casein particles. Food Bioprod. Proces. 2019, 115, 100–109. [Google Scholar] [CrossRef]

| Peptide Sequence | pI | MW | GRAVY | Instability Index |
|---|---|---|---|---|
| Peptides originating from collagen type I α1 | ||||
| 812-GEAGDNGAK-820 | 4.37 | 817.81 | −1.333 | −25.62 |
| 337-GEVGPQGAR-345 | 6.00 | 869.93 | −0.922 | 3.04 |
| 251-GHRGFSGLDGAK-262 | 8.75 | 1201.31 | −0.758 | −4.98 |
| 9-LALLLSATVLLAR-21 | 9.75 | 1353.71 | 2.031 | 9.23 |
| 1221-SLSQQIESIMSPDGTK-1236 | 4.37 | 1720.91 | −0.569 | 117.86 |
| 458-GEPGAAGGRGPPGERGAPGAR-478 | 9.51 | 1874.01 | −1.09 | 15.52 |
| 434-GEAGAQGVQGPPGPPGEEGKRGAR-457 | 6.23 | 2259.42 | −1.267 | 32.37 |
| 308-GNDGAAGAAGPPGPTGPAGPPGFPGGPGAK-337 | 5.84 | 2454.64 | −0.507 | 25.31 |
| 695-GDSGAPGAPGAQGPPGLQGMPGERGAAGLPGLK-727 | 6.07 | 2926.26 | −0.452 | 44.35 |
| 1384-AEGNSRFTYSVTEDGCTSHTGAWGKTVIDYK-1414 | 5.49 | 3381.63 | −0.716 | 19.36 |
| Peptides originating from collagen type I α2 | ||||
| 1109-ADQASLRAK-1117 | 8.79 | 959.07 | −0.778 | 20.86 |
| 1118-DYEVDATVK-1126 | 4.03 | 1039.11 | −0.689 | −19.39 |
| 1274-KAVLLQGSNDVELR-1287 | 6.07 | 1541.77 | −0.143 | 46.33 |
| 423-GPPGDAGRAGEPGLVGAR-440 | 6.07 | 1633.78 | −0.544 | 17.22 |
| T (°C) | KSV (104 mol−1) | Kq (1014 L/mol−1 s−1) | Kb (106 mol−1) | n |
|---|---|---|---|---|
| 25 | 9.03 ± 0.42 b,1 | 9.03 ± 0.42 b | 1.10 ± 0.11 a | 0.73 ± 0.08 a |
| 75 | 9.52 ± 0.34 b | 9.52 ± 0.34 b | 1.01 ± 0.03 a,b | 0.72 ± 0.01 a |
| 85 | 11.80 ± 0.43 a | 11.80 ± 0.43 a | 0.97 ± 0.02 a,b | 0.69 ± 0.02 a |
| 95 | 12.19 ± 0.61 a | 12.19 ± 0.61 a | 0.92 ± 0.01 b | 0.65 ± 0.05 a |
| T(K) | ΔHo (J·mol−1) | ΔSo (J·mol−1·K−1) | ΔGo (J·mol−1) | Ra |
|---|---|---|---|---|
| 298 | −251.02 ± 11.45 a | −0.73 ± 0.08 | −32.31 ± 1.25 | 0.92 |
| 348 | 4.37 ± 1.12 | |||
| 358 | 11.71 ± 0.97 | |||
| 368 | 19.05 ± 1.90 |
| Sample | Control | Microencapsulated Sample | H2O2 | ||||
|---|---|---|---|---|---|---|---|
| Concentration (µg/mL) | - | 50 | 100 | 500 | 1000 | 1500 | 1.7 |
| Cell viability (%) | 100 | 97.22 | 95.43 * | 93.10 ** | 90.77 ** | 89.96 ** | 7.97 ** |
| Degree of cytotoxicity | - | NC | NC | NC | NC | NC | C |
| SD | 1.17 | 1.48 | 1.94 | 1.95 | 1.62 | 2.19 | 0.41 |
| p | - | 0.063 | 0.025 | 0.006 | 0.001 | 0.002 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigore-Gurgu, L.; Crăciunescu, O.; Aprodu, I.; Bolea, C.A.; Iosăgeanu, A.; Petre, B.A.; Bahrim, G.E.; Oancea, A.; Stănciuc, N. Tailoring the Health-Promoting Potential of Protein Hydrolysate Derived from Fish Wastes and Flavonoids from Yellow Onion Skins: From Binding Mechanisms to Microencapsulated Functional Ingredients. Biomolecules 2020, 10, 1416. https://doi.org/10.3390/biom10101416
Grigore-Gurgu L, Crăciunescu O, Aprodu I, Bolea CA, Iosăgeanu A, Petre BA, Bahrim GE, Oancea A, Stănciuc N. Tailoring the Health-Promoting Potential of Protein Hydrolysate Derived from Fish Wastes and Flavonoids from Yellow Onion Skins: From Binding Mechanisms to Microencapsulated Functional Ingredients. Biomolecules. 2020; 10(10):1416. https://doi.org/10.3390/biom10101416
Chicago/Turabian StyleGrigore-Gurgu, Leontina, Oana Crăciunescu, Iuliana Aprodu, Carmen Alina Bolea, Andreea Iosăgeanu, Brîndușa Alina Petre, Gabriela Elena Bahrim, Anca Oancea, and Nicoleta Stănciuc. 2020. "Tailoring the Health-Promoting Potential of Protein Hydrolysate Derived from Fish Wastes and Flavonoids from Yellow Onion Skins: From Binding Mechanisms to Microencapsulated Functional Ingredients" Biomolecules 10, no. 10: 1416. https://doi.org/10.3390/biom10101416









