Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Plant Material
2.3. Preparation of Extracts
2.4. Phytochemical Profiling
UPLC-PDA-Q/TOF-MS Analysis of Polyphenols
2.5. Antioxidant Activity
2.5.1. ABTS Assay
2.5.2. DPPH Assay
2.6. Cytotoxic Activity
2.6.1. Cell Lines and Culture Medium
2.6.2. Assay for Cytotoxic Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Profiling
3.1.1. Identification of Phenolics in Salix alba Extracts
Phenolic Acid Derivatives
Flavanols and Procyanidins
Flavonols
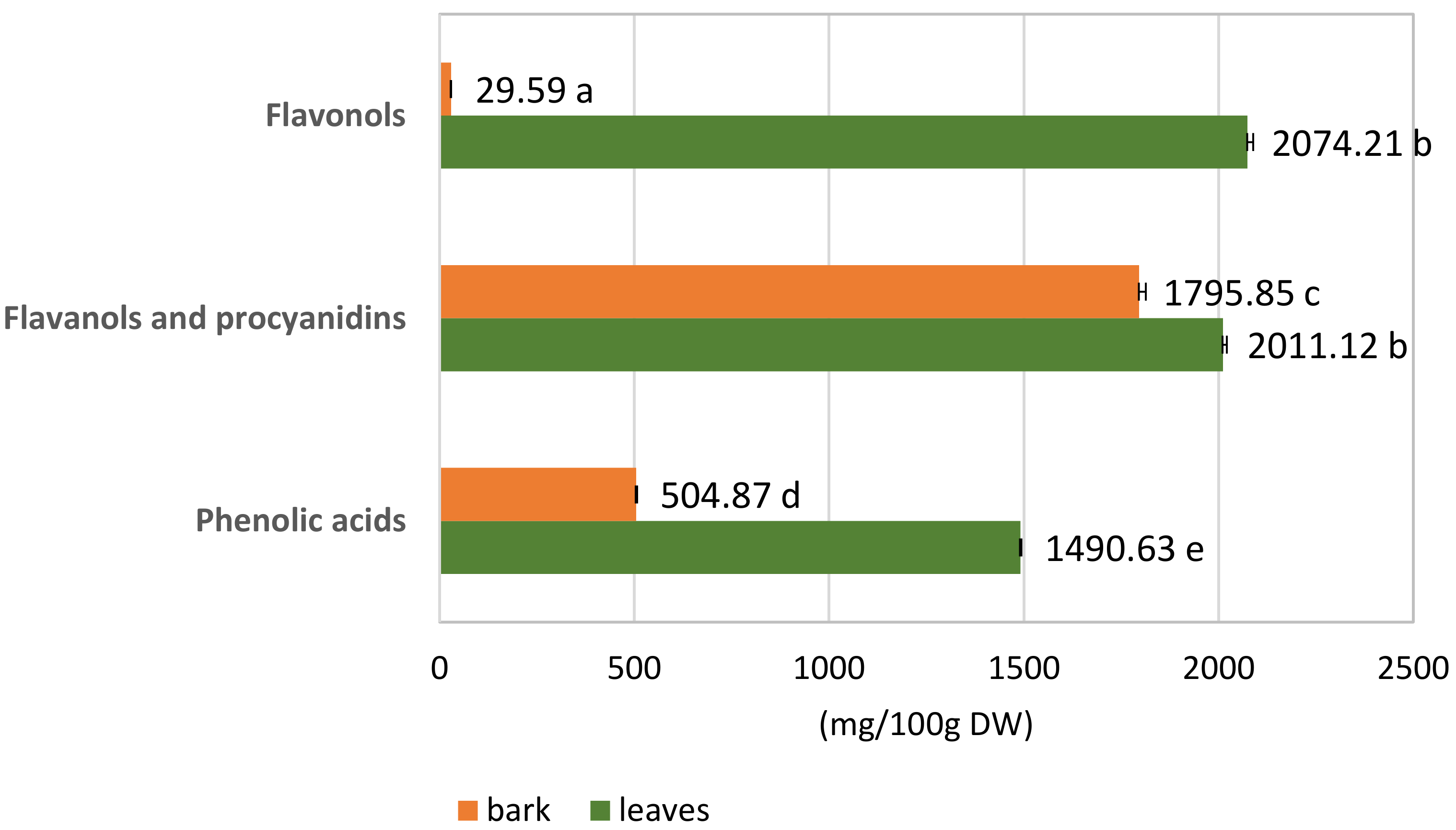
3.1.2. Quantitative Analysis of the S. alba Leaf and Bark Extracts
3.2. The Antioxidant Activity
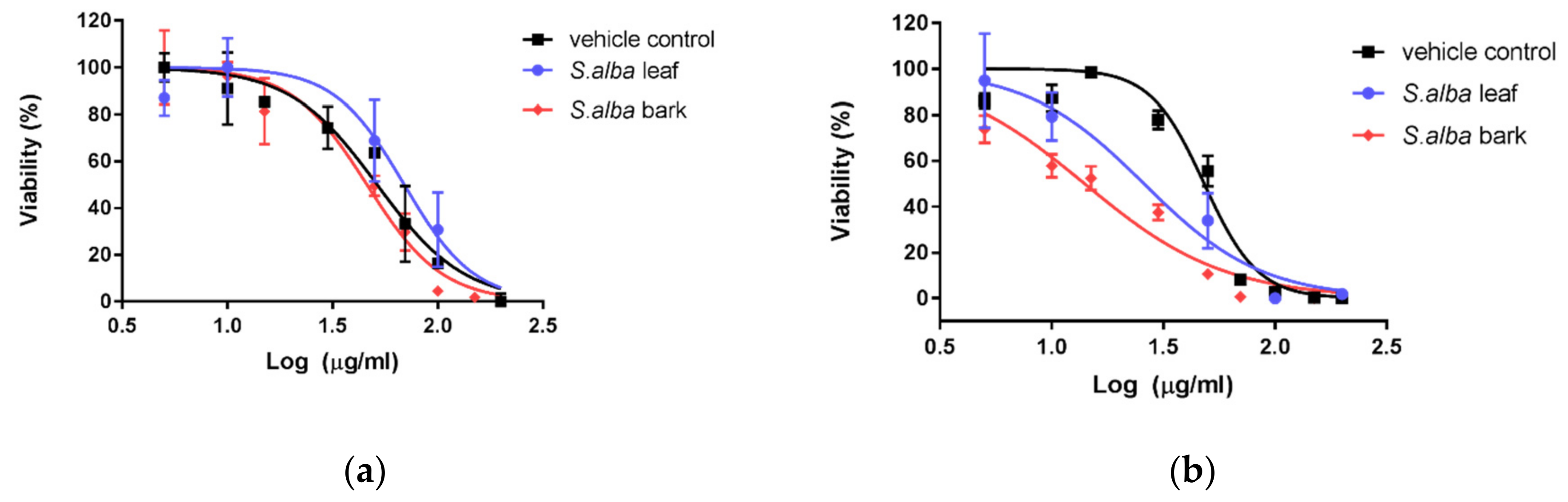
3.3. Cytotoxic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Braithwaite, M.C.; Tyagi, C.; Tomar, L.K.; Kumar, P.; Choonara, Y.E.; Pillay, V. Nutraceutical-based therapeutics and formulation strategies augmenting their efficiency to complement modern medicine: An overview. J. Funct. Foods 2014, 6, 82–99. [Google Scholar] [CrossRef]
- Rates, S.M. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Schmid, B.; Lüdtke, R.; Selbmann, H.K.; Kotter, R.; Tschirdewahn, B.; Schaffner, W.; Heide, L. Efficacy and tolerability of a standardized willow bark extract in patients with osteoarthritis: Randomized placebo-controlled, double blind clinical trial. Phytother. Res. 2001, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, J.G.; Mahdi, A.J.; Mahdi, A.J.; Bowen, I.D. The historical analysis of aspirin discovery, its relation to the willow tree and antiproliferative and anticancer potential. Cell Prolif. 2006, 39, 147–155. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Kelber, O.; Weiser, D.; Metz, J.; Kinscherf, R. In vitro anti-proliferative effects of the willow bark extract STW 33-I. Arzneimittelforschung 2010, 60, 330–335. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic content, antioxidant, antimicrobial and cytotoxic activities of ethanolic extract Salix alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef]
- Ishikado, A.; Sono, Y.; Matsumoto, M.; Robida-Stubbs, S.; Okuno, A.; Goto, M.; King, G.L.; Blackwell, T.K.; Makino, T. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans. Free Radic. Biol. Med. 2013, 65, 1506–1515. [Google Scholar] [CrossRef] [Green Version]
- Tanase, C.; Coșarcă, S.; Muntean, D.-L. A Critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [Green Version]
- Tanase, C.; Mocan, A.; Coșarcă, S.; Gavan, A.; Nicolescu, A.; Gheldiu, A.-M.; Vodnar, D.C.; Muntean, D.-L.; Crișan, O. Biological and chemical insights of beech (Fagus sylvatica L.) Bark: A source of bioactive compounds with functional properties. Antioxidants 2019, 8, 417. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Lauron-Moreau, A.; Pitre, F.E.; Argus, G.W.; Labrecque, M.; Brouillet, L. Phylogenetic relationships of american willows (Salix L., Salicaceae). PLoS ONE 2015, 10, e0138963. [Google Scholar] [CrossRef]
- Mohr, R. Weidenrinde: Salicis cortex. In Europaisches Arzneibuch 8. Ausgabe, 2. Nachtrag (Ph.Eur. 8.2); Amtliche deutsche Ausgabe, 1st ed.; Deutscher Apotheker Verlag: Stuttgart, Germany, 2015; pp. 2135–2136. [Google Scholar]
- Jürgenliemk, G.; Petereit, F.; Nahrstedt, A. flavan-3-ols and procyanidins from the bark of Salix purpurea L. Pharmazie 2007, 62, 231–234. [Google Scholar]
- Esatbeyoglu, T.; Wray, V.; Winterhalter, P. Dimeric procyanidins: Screening for B1 to B8 and semisynthetic preparation of B3, B4, B6, and B8 from a polymeric procyanidin fraction of white willow bark (Salix alba). J. Agric. Food Chem. 2010, 58, 7820–7830. [Google Scholar] [CrossRef] [PubMed]
- Wiesneth, S.; Petereit, F.; Jürgenliemk, G. Salix daphnoides: A screening for oligomeric and polymeric proanthocyanidins. Molecules 2015, 20, 13764–13779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyman, T.; Julkunen-Tiitto, R. Chemical variation within and among six northern willow species. Phytochemistry 2005, 66, 2836–2843. [Google Scholar] [CrossRef]
- Wiesneth, S.; Aas, G.; Heilmann, J.; Jürgenliemk, G. Investigation of the flavan-3-ol patterns in willow species during one growing-season. Phytochemistry 2018, 145, 26–39. [Google Scholar] [CrossRef]
- Karl, C.H.; Müller, G.; Pedersen, P.A. Flavonoide aus Salix alba. Die struktur des Terniflorins und eines weiteren acylflavonoides. Phytochemistry 1976, 15, 1084–1085. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.-C.; Baudelaire, É; Scher, J.; Dicko, A. Antioxidant and antiacetylcholinesterase activities of different granulometric classes of Salix alba (L) bark powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- Rutkowski, L. Klucz do Oznaczania Roślin Naczyniowych Polski Niżowej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2007; pp. 86, 88, 91. (In Polish) [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Guss, K.L.; Pavanni, S.; Prati, B.; Dazzi, L.; de Oliveira, J.P.; Nogueira, B.V.; Pereira, T.M.C.; Fronza, M.; Endringer, D.C.; Scherer, R. Ultrasound-assisted extraction of Achyrocline satureioides prevents contrast-induced nephropathy in mice. Ultrason. Sonochem. 2017, 37, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Domingues, R.M.A.; Oliveira, C.S.D.; Villaverde, J.J.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Secondary metabolites from Eucalyptus grandis wood cultivated in Portugal, Brazil and South Africa. Ind. Crop. Prod. 2017, 95, 357–364. [Google Scholar] [CrossRef]
- Varbanov, H.P.; Kuttler, F.; Banfi, D.; Turcatti, G.; Dyson, P.J. Repositioning approved drugs for the treatmentof problematic cancers using a screening approach. PLoS ONE 2017, 12, e0171052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavola, A.; Maukonen, M.; Julkunen-Tiitto, R. Variability in the composition of phenolic compounds in winter-dormant Salix pyrolifolia in relation to plant part and age. Phytochemistry 2018, 153, 102–110. [Google Scholar] [CrossRef]
- Thabti, I.; Elfalleh, W.; Hannachi, H.; Ferchichi, A.; Da Graca Campos, M. Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC–MS. J. Funct. Foods 2012, 4, 367–374. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Srećković, N.; Mišić, D.; Gašić, U.; Imbimbo, P.; Monti, D.M.; Mihailović, V. Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae)-A plant rich in rosmarinic acid. Ind. Crops Prod. 2020, 143, 111932. [Google Scholar] [CrossRef]
- Michel, P.; Owczarek, A.; Kosno, M.; Gontarek, D.; Matczak, M.; Olszewska, M.A. Variation in polyphenolic profile and in vitro antioxidant activity of eastern teaberry (Gaultheria procumbens L.) leaves following foliar development. Phytochem. Lett. 2017, 20, 356–364. [Google Scholar] [CrossRef]
- Matczak, M.; Marchelak, A.; Michel, P.; Owczarek, A.; Piszczan, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Sorbus domestica L. leaf extracts as functional products: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. J. Funct. Foods 2018, 40, 207–218. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Tawfeek, N.; Sobeh, M.; Hamdan, D.I.; Farrag, N.; Roxo, M.; El-Shazly, A.M.; Wink, M. Phenolic compounds from Populus alba L. and Salix subserrata Willd. (Salicaceae) counteract oxidative stress in Caenorhabditis elegans. Molecules 2019, 24, 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enayat, S.; Banerjee, S. Comparative antioxidant activity of extracts from leaves, bark and catkins of Salix aegyptiaca sp. Food Chem. 2009, 116, 23–28. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef]
- Park, Y.C.; Rimbach, G.; Saliou, C.; Valacchi, G.; Packer, L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2000, 465, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Pobłocka-Olech, L.; Krauze-Baranowska, M. SPE-HPTLC of procyanidins from the barks of different species and clones of Salix. J. Pharmaceut. Biomed. 2008, 48, 965–968. [Google Scholar] [CrossRef]
- Michel, P.; Dobrowolska, A.; Kicel, A.; Owczarek, A.; Bazylko, A.; Granica, S.; Piwowarski, J.P.; Olszewska, M.A. Polyphenolic profile, antioxidant and anti-inflammatory activity of eastern teaberry (Gaultheria procumbens L.) leaf extracts. Molecules 2014, 19, 20498–20520. [Google Scholar] [CrossRef] [Green Version]
- Karl, C.H.; Pedersen, P.A.; Schwarz, C.H. Ein neues flavonolacetylglucosid aus Salix viminalis. Phytochemistry 1977, 16, 1117. (In German) [Google Scholar] [CrossRef]
- Amel Zabihi, N.; Mahmoudabady, M.; Soukhtanloo, M.; Hayatdavoudi, P.; Beheshti, F.; Niazmand, S. Salix alba attenuated oxidative stress in the heart and kidney of hypercholesterolemic rabbits. Avicenna J. Phytomed. 2018, 8, 63–72. [Google Scholar]
- Ramos, P.A.B.; Moreirinha, C.; Silva, S.; Costa, E.M.; Veiga, M.; Coscueta, E.; Santos, S.A.O.; Almeida, A.; Pintado, M.M.; Freier, C.S.R.; et al. The health-promoting potential of Salix spp. bark polar extracts: Key insights on phenolic composition and in vitro bioactivity and biocompatibility. Antioxidants 2019, 8, 609. [Google Scholar] [CrossRef] [Green Version]
- Durak, A.; Gawlik-Dziki, U.; Sugier, D. Coffee enriched with willow (Salix purpurea and Salix myrsinifolia) bark preparation- interactions of antioxidative phytochemicals in a model system. J. Funct. Foods 2015, 18, 1106–1116. [Google Scholar] [CrossRef]
- Maistro, E.L.; Terrazzas, P.M.; Perazzo, F.F.; Gaivão, I.O.M.; Sawaya, A.C.H.F.; Rosa, P.C.P. Salix alba (white willow) medicinal plant presents genotoxic effects in human cultured leukocytes. J. Toxicol. Environ. Health 2019, 82, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
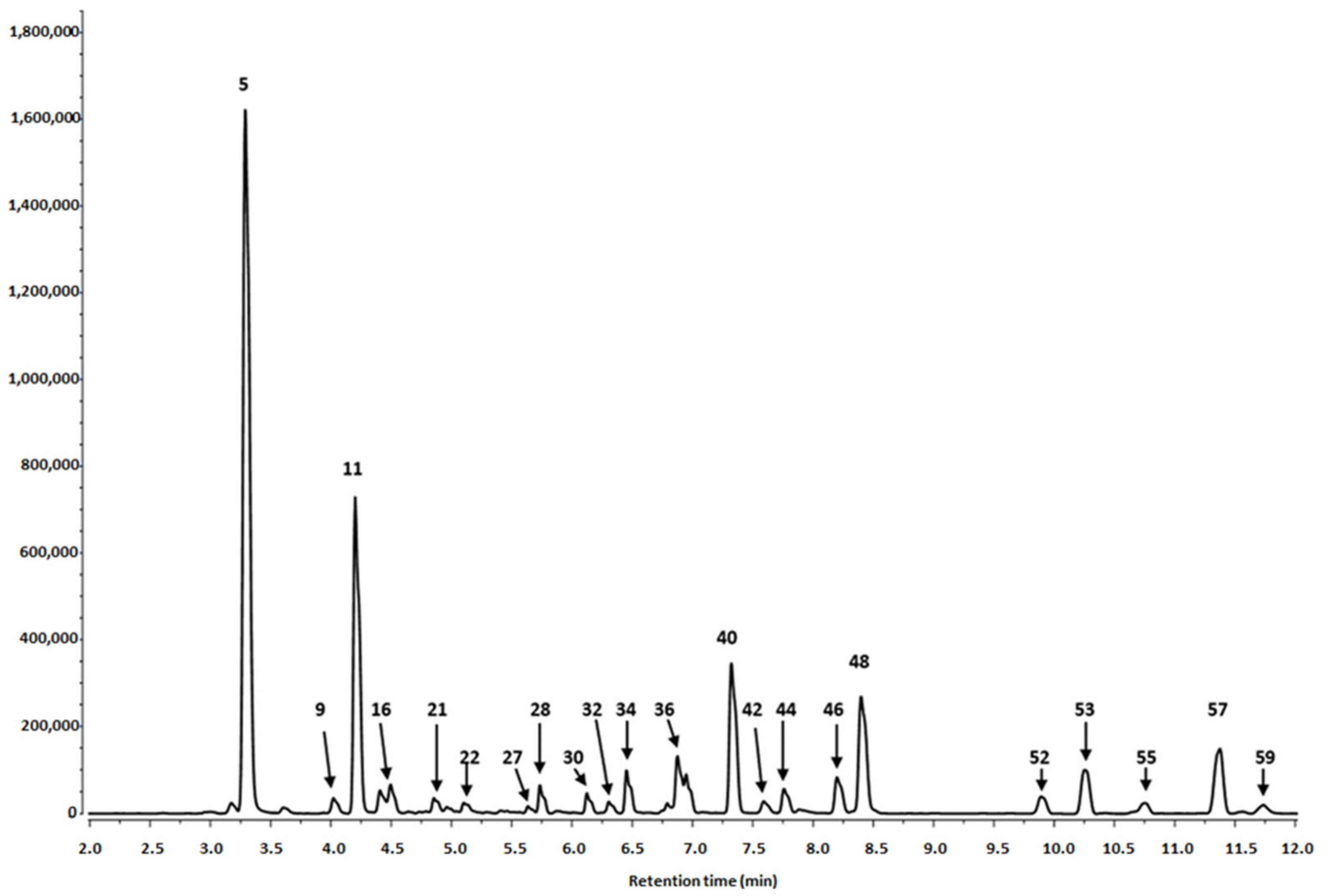
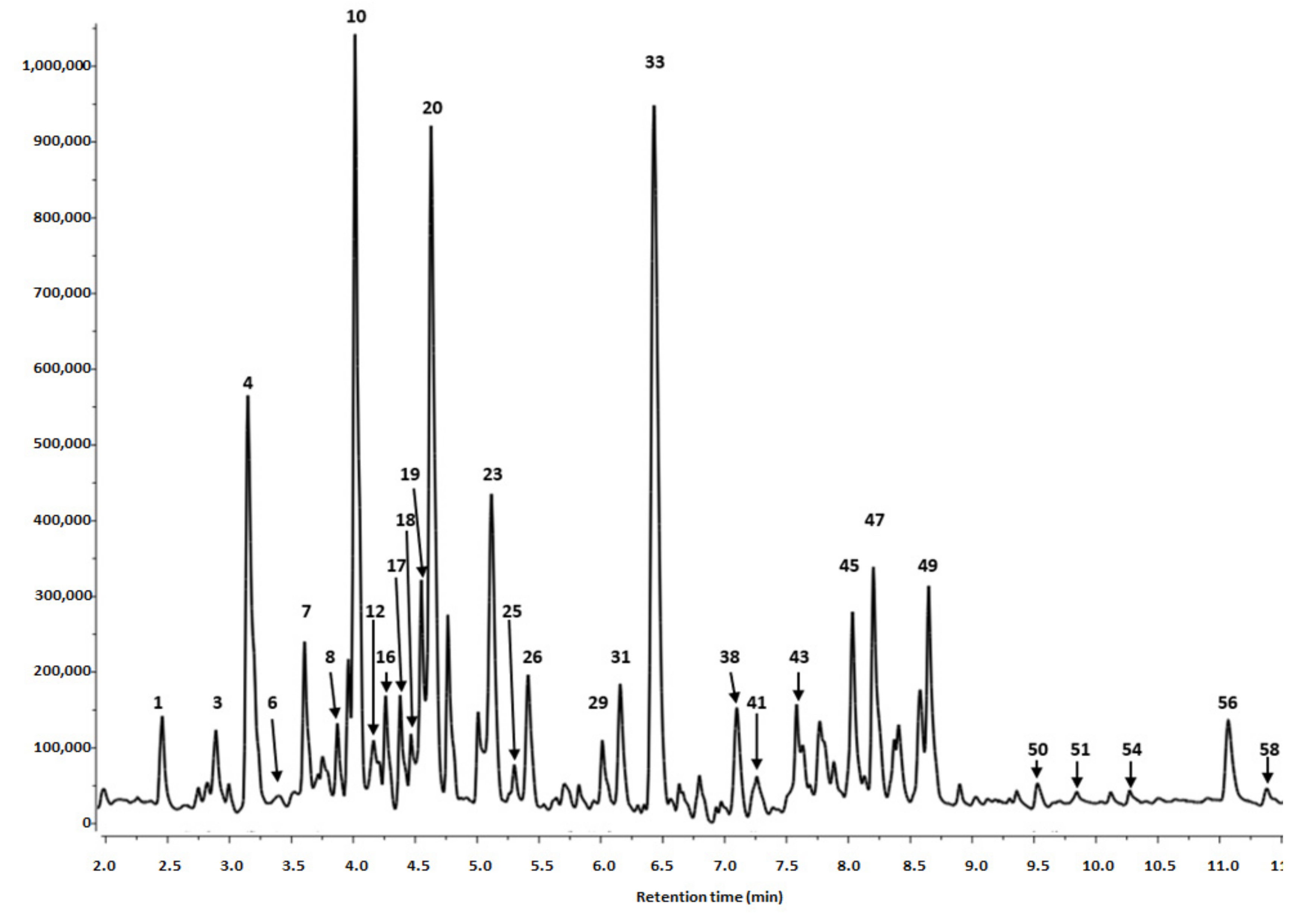
| Peak No | Retention Time (tr) | λmax (nm) | [M-H]− (m/z) a | MS/MS Fragments (m/z) a | Tentative Identification | Content (mg/100 g DW) c ± SD | |
|---|---|---|---|---|---|---|---|
| Leaves | Bark | ||||||
| Phenolic Acids | |||||||
| 1 | 2.51 | 324 | 341 | 179 | Caffeoylhexose I | 2.98 ± 0.07 | 57.71 ± 0.09 * |
| 2 | 2.72 | 324 | 341 | 179 | Caffeoylhexose II | n.d. | 24.71 ± 0.10 * |
| 3 | 2.76 | 320 | 487 | 308/179 | Caffeoyl hexose-deoxyhexoside I | n.d. | 140.00 ± 0.22 * |
| 5 | 3.26 | 320 | 487 | 308/179 | Caffeoyl hexose-deoxyhexoside II | 875.32 ± 1.18 * | n.d. |
| 9 | 4.04 | 324 | 353 | 191/179 | 1-O-Caffeoylquinic acid b | 117.43 ± 0.88 * | n.d. |
| 11 | 4.12 | 324 | 353 | 191/179 | 3-O-Caffeoylquinic acid b | 386.31 ± 1.26 | 41.96 ± 2.53 * |
| 13 | 4.17 | 324 | 341 | 179 | Caffeoylhexose III | n.d. | 240.49 ± 0.46 * |
| 15 | 4.24 | 325 | 707 | 353/191/179 | Caffeoylquinic acid dimer I | 60.63 ± 1.83 * | n.d. |
| 21 | 4.77 | 325 | 707 | 353/191/179 | Caffeoylquinic acid dimer II | 10.11 ± 0.75 * | n.d. |
| 24 | 5.14 | 324 | 353 | 191 | 5-O-Caffeoylquinic acid b | 37.85 ± 0.59 * | n.d. |
| Sum 1490.63 ± 2.86 | Sum 504.87 ± 2.58 * | ||||||
| Flavanols and Procyanidins | |||||||
| 4 | 3.12 | 280 | 593 | 407/289 | (Epi)catechin-(Epi)gallocatechin I | n.d. | 130.67 ± 1.16 * |
| 6 | 3.45 | 280 | 593 | 407/289 | (Epi)catechin-(Epi)gallocatechin II | n.d. | 10.09 ± 0.19 * |
| 7 | 3.69 | 280 | 593 | 407/289 | (Epi)catechin-(Epi)gallocatechin III | n.d. | 59.79 ± 5.79 * |
| 8 | 3.87 | 279 | 881 | 407/289 | A-type procyanidin dimer digallate | n.d. | 48.66 ± 0.50 * |
| 10 | 4.09 | 280 | 577 | 407/289 | A-type procyanidin dimer b I | n.d. | 224.33 ± 1.58 * |
| 12 | 4.16 | 281 | 577 | 407/289 | A-type procyanidin dimer b II | 241.31 ± 1.03 | 22.33 ± 0.57 * |
| 14 | 4.20 | 280 | 577 | 289 | A-type procyanidin dimer b III | n.d. | 9.22 ± 0.90 * |
| 16 | 4.30 | 277 | 865 | 577/287 | B-type procyanidin trimer I | 241.43 ± 1.16 | 22.90 ± 0.46 * |
| 17 | 4.39 | 277 | 865 | 577/289 | A-type procyanidin trimer I | n.d. | 25.87 ± 0.18 * |
| 18 | 4.48 | 281 | 577 | 407/289 | A-type procyanidin dimer b IV | n.d. | 14.27 ± 5.63 * |
| 19 | 4.55 | 280 | 289 | (+)-Catechin | n.d | 63.79 ± 2.60 * | |
| 20 | 4.74 | 280 | 289 | 245 | (−)-Epicatechin b | n.d. | 176.49 ± 14.02 * |
| 22 | 5.08 | 281 | 305 | 221/179 | Epigallocatechin b I | 319.52 ± 1.62 * | n.d. |
| 23 | 5.12 | 279 | 1169 | 865/577/289 | A-type procyanidin trimer digallate | n.d. | 99.25 ± 0.04 * |
| 25 | 5.30 | 277 | 865 | 577/289 | A-type procyanidin trimer II | n.d. | 15.07 ± 0.08 * |
| 26 | 5.45 | 277 | 865 | 577/287 | B-type procyanidin trimer II | n.d. | 40.79 ± 1.01 * |
| 27 | 5.64 | 280 | 865 | 577/287 | B-type procyanidin trimer III | 124.37 ± 9.44 * | n.d. |
| 28 | 5.75 | 280 | 305 | 221/219/179 | Epigallocatechin b II | 324.69 ± 0.88 * | n.d. |
| 29 | 6.07 | 281 | 1153 | 865/577/287 | B-type procyanidin tetramer I | n.d. | 25.54 ± 0.04 * |
| 30 | 6.10 | 277 | 865 | 577/287 | B-type procyanidin trimer IV | 246.46 ± 1.69 * | n.d. |
| 31 | 6.20 | 281 | 1154 | 577/287 | B-type procyanidin tetramer II | n.d. | 56.07 ± 1.51 * |
| 33 | 6.45 | 280 | 577 | 289 | A-type procyanidin dimer b V | n.d. | 198.24 ± 0.06 * |
| 34 | 6.47 | 281 | 1441 | 864/575/287 | B-type procyanidin pentamer | 118.11 ± 0.13 * | n.d. |
| 36 | 6.79 | 279 | 575 | 287 | B-type procyanidin dimer b I | 395.23 ± 0.73 * | n.d. |
| 38 | 7.14 | 280 | 465 | 289 | (Epi)catechin methyl-hexoside I | n.d. | 68.55 ± 1.12 * |
| 41 | 7.28 | 280 | 465 | 289 | (Epi)catechin methyl-hexoside II | n.d. | 56.78 ± 0.99 * |
| 43 | 7.60 | 280 | 1152 | 863/577/289 | A-type procyanidin tetramer | n.d | 33.45 ± 0.11 * |
| 45 | 8.08 | 280 | 577 | 407/289 | A-type procyanidin dimer b VI | n.d. | 94.91 ± 0.08 * |
| 47 | 8.17 | 280 | 865 | 577/289 | A-type procyanidin trimer III | n.d. | 103.61 ± 0.10 * |
| 49 | 8.68 | 280 | 465 | 289 | (Epi)catechin methyl-hexoside III | n.d. | 96.21 ± 1.10 * |
| 50 | 9.56 | 280 | 465 | 289 | (Epi)catechin methyl-hexoside IV | n.d. | 28.52 ± 0.07 * |
| 56 | 11.09 | 280 | 893 | 603/289 | (Epi)catechin-ethyl trimer | n.d. | 70.45 ± 0.9 * |
| Sum 2011.12 ± 9.92 | Sum 1795.85 ± 16.78 | ||||||
| Flavonols | |||||||
| 32 | 6.39 | 350 | 609 | 301 | Quercetin 3-O-rutinoside b | 92.91 ± 2.05 * | n.d. |
| 35 | 6.62 | 340 | 447 | 301 | Quercetin methyl-pentoside | 9.11 ± 0.39 * | n.d. |
| 37 | 7.13 | 340 | 651 | 446/301 | Quercetin acylated-deoxyhexoside I | 166.51 ± 1.79 * | n.d. |
| 39 | 7.18 | 340 | 651 | 447/301 | Quercetin acylated-deoxyhexoside II | 148.93 ± 1.02 * | n.d. |
| 40 | 7.22 | 351 | 623 | 315 | Isorhamnetin 3-O-rutinoside b | 459.80 ± 5.61 * | n.d. |
| 42 | 7.54 | 355 | 463 | 301 | Quercetin 3-O-galactoside b | 59.27 ± 0.24 * | n.d. |
| 44 | 7.70 | 355 | 463 | 301 | Quercetin 3-O-glucoside b | 98.43 ± 1.88 * | n.d. |
| 46 | 8.14 | 340 | 505 | 301 | Quercetin-acylated-hexoside I | 180.03 ± 0.99 * | n.d. |
| 48 | 8.43 | 340 | 505 | 463/301 | Quercetin-acylated-hexoside II | 413.14 ± 11.86 * | n.d. |
| 51 | 9.69 | 355 | 463 | 301 | Quercetin 3-O-hexoside | n.d. | 10.47 ± 0.06 * |
| 52 | 9.80 | 353 | 519 | 314/299 | Isorhamnetin-acylated-hexoside I | 4.65 ± 0.12 * | n.d. |
| 53 | 10.20 | 350 | 477 | 314 | Isoramnetin 3-O-galactoside b | 145.89 ± 2.11 * | n.d. |
| 54 | 10.27 | 350 | 423 | 287 | Kaempferol pentoside | n.d. | 6.58 ± 0.09 * |
| 55 | 10.63 | 350 | 519 | 314/299 | Isorhamnetin acylated-hexoside II | 32.62 ± 0.88 * | n.d. |
| 57 | 11.24 | 353 | 519 | 314/299 | Isorhamnetin-acylated-hexoside III | 234.36 ± 4.29 * | n.d. |
| 58 | 11.33 | 346 | 447 | 287 | Kaempferol 3-O-galactoside b | n.d. | 12.54 ± 0.07 * |
| 59 | 11.63 | 353 | 519 | 314/299 | Isorhamnetin-acylated-hexoside IV | 28.56 ± 1.18 * | n.d. |
| Sum2074.21 ± 14.50 | Sum29.59 ± 0.13 * | ||||||
| Sum of phenolic compounds | 5575.96 ± 17.80 | 2330.31 ± 16.98 * | |||||
| Plant Material | DPPH * | ABTS * |
|---|---|---|
| Bark | 13.51 ± 0.2 a | 21.50 ± 0.32 a |
| Leaf | 28.23 ± 0.6 b | 65.41 ± 0.27 b |
| Ascorbic acid (antioxidant standard) | 28.88± 0.21 b | 65.43± 0.22 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules 2020, 10, 1391. https://doi.org/10.3390/biom10101391
Piątczak E, Dybowska M, Płuciennik E, Kośla K, Kolniak-Ostek J, Kalinowska-Lis U. Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules. 2020; 10(10):1391. https://doi.org/10.3390/biom10101391
Chicago/Turabian StylePiątczak, Ewelina, Monika Dybowska, Elżbieta Płuciennik, Katarzyna Kośla, Joanna Kolniak-Ostek, and Urszula Kalinowska-Lis. 2020. "Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential" Biomolecules 10, no. 10: 1391. https://doi.org/10.3390/biom10101391
APA StylePiątczak, E., Dybowska, M., Płuciennik, E., Kośla, K., Kolniak-Ostek, J., & Kalinowska-Lis, U. (2020). Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules, 10(10), 1391. https://doi.org/10.3390/biom10101391









