Anticancer Potential of Lichens’ Secondary Metabolites
Abstract
1. Introduction
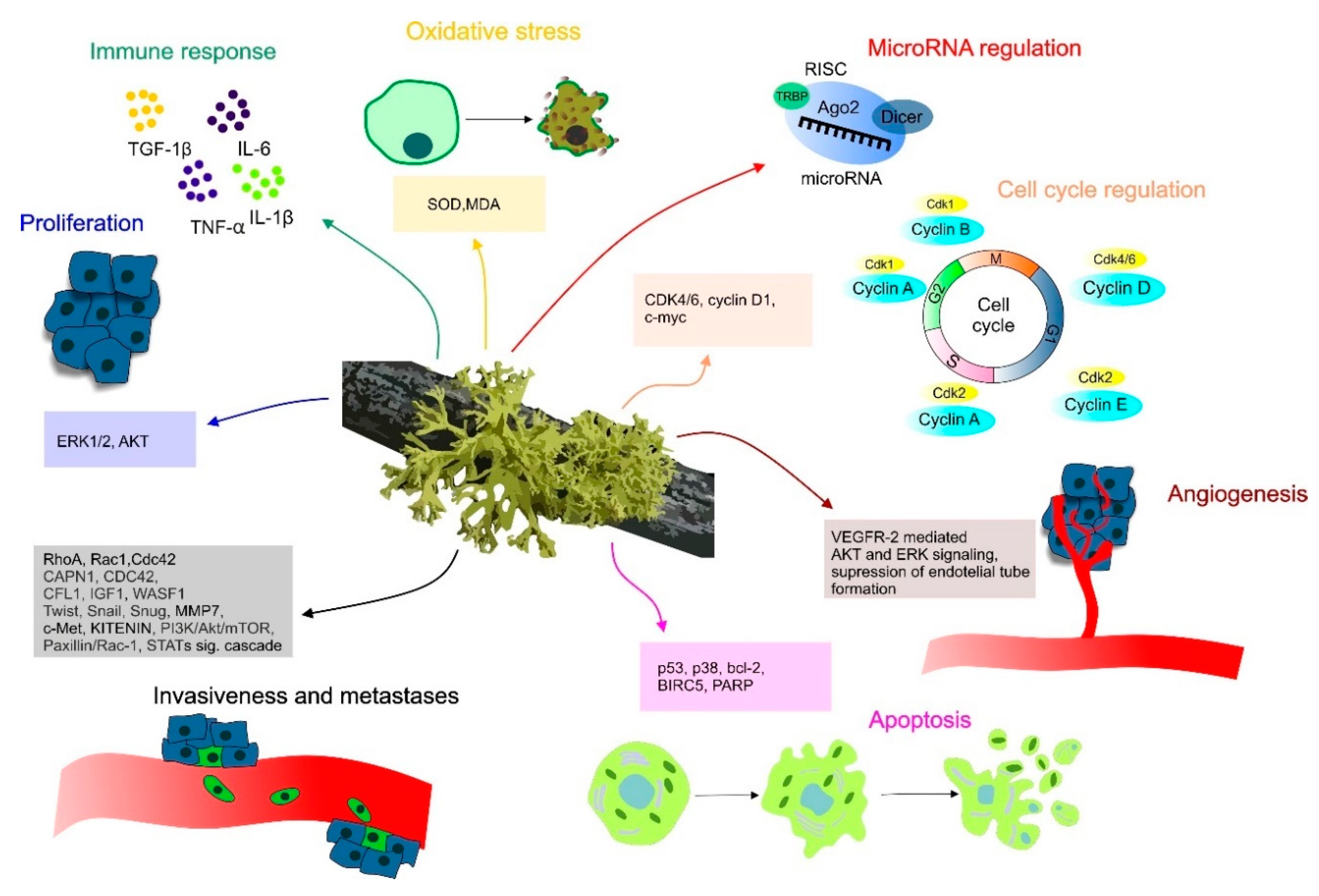
2. Molecular Mechanisms of Lichen Anticancer Potential
3. Anti-Neoplastic Effect of Lichens in Preclinical Research
3.1. In Vitro Evaluation of Anticancer Efficacy of Isolated Lichen Compounds
3.2. Combined Studies of Lichen Extracts and Isolated Lichen Compounds In Vitro
3.3. Determination of Lichens’ Extracts Effect Against Cancer Cells
4. Anticancer Effects of Lichens in Animal Models
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Patafio, F.M.; Brooks, S.C.; Wei, X.; Peng, Y.; Biagi, J.; Booth, C.M. Research output and the public health burden of cancer: Is there any relationship? Curr. Oncol. 2016, 23, 75. [Google Scholar] [CrossRef]
- Solár, P.; Ferenc, P.; Koval’, J.; Mikeš, J.; Solárová, Z.; Hrčková, G.; Fulton, B.L.; Fedoročko, P. Photoactivated Hypericin Induces Downregulation of HER2 Gene Expression. Radiat. Res. 2011, 175, 51–56. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. IJMS 2019, 20, 1749. [Google Scholar] [CrossRef]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef]
- Samec, M.; Liskova, A.; Kubatka, P.; Uramova, S.; Zubor, P.; Samuel, S.M.; Zulli, A.; Pec, M.; Bielik, T.; Biringer, K.; et al. The role of dietary phytochemicals in the carcinogenesis via the modulation of miRNA expression. J. Cancer Res. Clin. Oncol. 2019, 145, 1665–1679. [Google Scholar] [CrossRef]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Büsselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef]
- Uramova, S.; Kubatka, P.; Dankova, Z.; Kapinova, A.; Zolakova, B.; Samec, M.; Zubor, P.; Zulli, A.; Valentova, V.; Kwon, T.K.; et al. Plant natural modulators in breast cancer prevention: Status quo and future perspectives reinforced by predictive, preventive, and personalized medical approach. EPMA J. 2018, 9, 403–419. [Google Scholar] [CrossRef]
- Chen, V.; Staub, R.E.; Fong, S.; Tagliaferri, M.; Cohen, I.; Shtivelman, E. Bezielle Selectively Targets Mitochondria of Cancer Cells to Inhibit Glycolysis and OXPHOS. PLoS ONE 2012, 7, e30300. [Google Scholar] [CrossRef]
- Perez, A.T.; Arun, B.; Tripathy, D.; Tagliaferri, M.A.; Shaw, H.S.; Kimmick, G.G.; Cohen, I.; Shtivelman, E.; Caygill, K.A.; Grady, D.; et al. A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res. Treat. 2010, 120, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.; Shtivelman, E.; Perez, A.; Vogel, C.; Franco, S.; Tan Chiu, E.; Melisko, M.; Tagliaferri, M.; Cohen, I.; Shoemaker, M.; et al. Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res. Treat. 2007, 105, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, Q.; Yang, C.; Yu, P.; et al. Anti-Cancer Activities of Tea Epigallocatechin-3-Gallate in Breast Cancer Patients under Radiotherapy. CMM 2012, 12, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef]
- Jasek, K.; Kubatka, P.; Samec, M.; Liskova, A.; Smejkal, K.; Vybohova, D.; Bugos, O.; Biskupska-Bodova, K.; Bielik, T.; Zubor, P.; et al. DNA Methylation Status in Cancer Disease: Modulations by Plant-Derived Natural Compounds and Dietary Interventions. Biomolecules 2019, 9, 289. [Google Scholar] [CrossRef]
- Stanojković, T. Investigations of Lichen Secondary Metabolites with Potential Anticancer Activity. In Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Ranković, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 127–146. ISBN 978-3-319-13374-4. [Google Scholar]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Piovano, M.; Russo, A. Potential anticancer activity of lichen secondary metabolite physodic acid. Chem. Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef]
- White, P.; Oliveira, R.; Oliveira, A.; Serafini, M.; Araújo, A.; Gelain, D.; Moreira, J.; Almeida, J.; Quintans, J.; Quintans-Junior, L.; et al. Antioxidant Activity and Mechanisms of Action of Natural Compounds Isolated from Lichens: A Systematic Review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef]
- Yang, Y.; Nguyen, T.T.; Jeong, M.-H.; Crişan, F.; Yu, Y.H.; Ha, H.-H.; Choi, K.H.; Jeong, H.G.; Jeong, T.C.; Lee, K.Y.; et al. Inhibitory Activity of (+)-Usnic Acid against Non-Small Cell Lung Cancer Cell Motility. PLoS ONE 2016, 11, e0146575. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, Y.; Park, S.-Y.; Nguyen, T.T.; Seo, Y.-W.; Lee, K.H.; Lee, J.H.; Kim, K.K.; Hur, J.-S.; Kim, H. The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis. Sci. Rep. 2017, 7, 8136. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Dinh, M.H.; Chi, H.T.; Wang, S.-L.; Nguyen, Q.; Tran, T.D.; Nguyen, A.D. Antioxidant and cytotoxic activity of lichens collected from Bidoup Nui Ba National Park, Vietnam. Res. Chem. Intermed. 2019, 45, 33–49. [Google Scholar] [CrossRef]
- Taş, İ.; Han, J.; Park, S.-Y.; Yang, Y.; Zhou, R.; Gamage, C.D.B.; Van Nguyen, T.; Lee, J.-Y.; Choi, Y.J.; Yu, Y.H.; et al. Physciosporin suppresses the proliferation, motility and tumourigenesis of colorectal cancer cells. Phytomedicine 2019, 56, 10–20. [Google Scholar] [CrossRef]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Piotrowska, H.; Kucińska, M.; Murias, M.; Bylka, W. Cytotoxic activity of physodic acid and acetone extract from Hypogymnia physodes against breast cancer cell lines. Pharm. Biol. 2016, 54, 2480–2485. [Google Scholar] [CrossRef]
- Ristić, S.; Ranković, B.; Kosanić, M.; Stanojković, T.; Stamenković, S.; Vasiljević, P.; Manojlović, I.; Manojlović, N. Phytochemical study and antioxidant, antimicrobial and anticancer activities of Melanelia subaurifera and Melanelia fuliginosa lichens. J. Food Sci. Technol. 2016, 53, 2804–2816. [Google Scholar] [CrossRef]
- Paluszczak, J.; Kleszcz, R.; Studzińska-Sroka, E.; Krajka-Kuźniak, V. Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol. Cell. Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef]
- Yurdacan, B.; Egeli, U.; Eskiler, G.G.; Eryilmaz, I.; Cecener, G.; Tunca, B. The role of usnic acid-induced apoptosis and autophagy in hepatocellular carcinoma. Hum. Exp. Toxicol. 2019, 38, 201–215. [Google Scholar] [CrossRef]
- Singh, N.; Nambiar, D.; Kale, R.K.; Singh, R.P. Usnic Acid Inhibits Growth and Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma A549 Cells. Nutr. Cancer 2013, 65, 36–43. [Google Scholar] [CrossRef]
- Dinçsoy, A.B.; Duman, D.C. Changes in apoptosis-related gene expression profiles in cancer cell lines exposed to usnic acid lichen secondary metabolite. Turk. J. Biol. 2017, 41, 484–493. [Google Scholar] [CrossRef]
- Hong, J.-M.; Suh, S.-S.; Kim, T.; Kim, J.; Han, S.; Youn, U.; Yim, J.; Kim, I.-C. Anti-Cancer Activity of Lobaric Acid and Lobarstin Extracted from the Antarctic Lichen Stereocaulon alpnum. Molecules 2018, 23, 658. [Google Scholar] [CrossRef]
- Bessadóttir, M.; Skúladóttir, E.Á.; Gowan, S.; Eccles, S.; Ómarsdóttir, S.; Ögmundsdóttir, H.M. Effects of anti-proliferative lichen metabolite, protolichesterinic acid on fatty acid synthase, cell signalling and drug response in breast cancer cells. Phytomedicine 2014, 21, 1717–1724. [Google Scholar] [CrossRef]
- El-Garawani, I.M.; Elkhateeb, W.A.; Zaghlol, G.M.; Almeer, R.S.; Ahmed, E.F.; Rateb, M.E.; Abdel Moneim, A.E. Candelariella vitellina extract triggers in vitro and in vivo cell death through induction of apoptosis: A novel anticancer agent. Food Chem. Toxicol. 2019, 127, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, H.Y.; Elsayed, H.E.; Mohyeldin, M.M.; Akl, M.R.; Bhattacharjee, J.; Egbert, S.; El Sayed, K.A. Norstictic Acid Inhibits Breast Cancer Cell Proliferation, Migration, Invasion, and In Vivo Invasive Growth Through Targeting C-Met: Norstictic Acid as Breast Cancer and c-Met Inhibitor. Phytother. Res. 2016, 30, 557–566. [Google Scholar] [CrossRef]
- Yang, Y.; Bae, W.K.; Lee, J.-Y.; Choi, Y.J.; Lee, K.H.; Park, M.-S.; Yu, Y.H.; Park, S.-Y.; Zhou, R.; Taş, İ.; et al. Potassium usnate, a water-soluble usnic acid salt, shows enhanced bioavailability and inhibits invasion and metastasis in colorectal cancer. Sci. Rep. 2018, 8, 16234. [Google Scholar] [CrossRef] [PubMed]
- Varol, M. Anti-breast cancer and anti-angiogenic potential of a lichen-derived small-molecule: Barbatolic acid. Cytotechnology 2018, 70, 1565–1573. [Google Scholar] [CrossRef]
- Su, Z.-Q.; Liu, Y.-H.; Guo, H.-Z.; Sun, C.-Y.; Xie, J.-H.; Li, Y.-C.; Chen, J.-N.; Lai, X.-P.; Su, Z.-R.; Chen, H.-M. Effect-enhancing and toxicity-reducing activity of usnic acid in ascitic tumor-bearing mice treated with bleomycin. Int. Immunopharmacol. 2017, 46, 146–155. [Google Scholar] [CrossRef]
- Kiliac, N.; Islakoglu, Y.O.; Buyuk, A.; Gur-Dedeoglu, B.; Cansaran-Duman, D. Determination of Usnic Acid Responsive miRNAs in Breast Cancer Cell Lines. ACAMC 2018, 18, 1463–1472. [Google Scholar]
- Reddy, S.D.; Siva, B.; Kumar, K.; Babu, V.S.P.; Sravanthi, V.; Boustie, J.; Nayak, V.L.; Tiwari, A.K.; Rao, C.V.; Sridhar, B.; et al. Comprehensive Analysis of Secondary Metabolites in Usnea longissima (Lichenized Ascomycetes, Parmeliaceae) Using UPLC-ESI-QTOF-MS/MS and Pro-Apoptotic Activity of Barbatic Acid. Molecules 2019, 24, 2270. [Google Scholar] [CrossRef]
- Sepulveda, B.; Chamy, M.C.; Piovano, M.; Areche, C. LICHENS: MIGHT BE CONSIDERED AS A SOURCE OF GASTROPROTECTIVE MOLECULES? J. Chil. Chem. Soc. 2013, 58, 1750–1752. [Google Scholar] [CrossRef]
- Demir, L.; Toğar, B.; Türkez, H.; Sozio, P.; Aslan, A.; Stefano, A.D.; Demir, L.; Toğar, B.; Türkez, H.; Sozio, P.; et al. The investigation of cytogenetic and oxidative effects of diffractaic acid on human lymphocyte cultures. Braz. Arch. Biol. Technol. 2015, 58, 75–81. [Google Scholar] [CrossRef]
- Morita, H.; Tsuchiya, T.; Kishibe, K.; Noya, S.; Shiro, M.; Hirasawa, Y. Antimitotic activity of lobaric acid and a new benzofuran, sakisacaulon A from Stereocaulon sasakii. Bioorg. Med. Chem. Lett. 2009, 19, 3679–3681. [Google Scholar] [CrossRef]
- Kim, T.K.; Kim, J.E.; Youn, U.J.; Han, S.J.; Kim, I.-C.; Cho, C.-G.; Yim, J.H. Total Syntheses of Lobaric Acid and Its Derivatives from the Antarctic Lichen Stereocaulon alpinum. J. Nat. Prod. 2018, 81, 1460–1467. [Google Scholar] [CrossRef]
- Emsen, B.; Aslan, A.; Togar, B.; Turkez, H. In Vitro antitumor activities of the lichen compounds olivetoric, physodic and psoromic acid in rat neuron and glioblastoma cells. Pharm. Biol. 2016, 54, 1748–1762. [Google Scholar] [CrossRef] [PubMed]
- Studzinska-Sroka, E.; Galanty, A.; Bylka, W. Atranorin—An Interesting Lichen Secondary Metabolite. Mini Rev. Med. Chem. 2017, 17, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Solár, P.; Hrčková, G.; Koptašíková, L.; Velebný, S.; Solárová, Z.; Bačkor, M. Murine breast carcinoma 4T1 cells are more sensitive to atranorin than normal epithelial NMuMG cells in vitro: Anticancer and hepatoprotective effects of atranorin in vivo. Chem. Biol. Interact. 2016, 250, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Dabagoglu Psav, S.; Avan, I.; Candan, M.; Sahinturk, V.; Koparal, A. Vulpinic acid, a lichen metabolite, emerges as a potential drug candidate in the therapy of oxidative stress–related diseases, such as atherosclerosis. Hum. Exp. Toxicol. 2019, 38, 675–684. [Google Scholar] [CrossRef]
- Kılıç, N.; Aras, S.; Cansaran-Duman, D. Determination of Vulpinic Acid Effect on Apoptosis and mRNA Expression Levels in Breast Cancer Cell Lines. ACAMC 2019, 18, 2032–2041. [Google Scholar] [CrossRef]
- Türk, H.; Yılmaz, M.; Tay, T.; Türk, A.Ö.; Kıvanç, M. Antimicrobial Activity of Extracts of Chemical Races of the Lichen Pseudevernia furfuracea and their Physodic Acid, Chloroatranorin, Atranorin, and Olivetoric Acid Constituents. Z. Für Nat. C 2006, 61, 499–507. [Google Scholar] [CrossRef]
- Stojanović, I.Z.; Najman, S.; Jovanović, O.; Petrović, G.; Najdanović, J.; Vasiljević, P.; Smelcerović, A. Effects of depsidones from Hypogymnia physodes on HeLa cell viability and growth. Folia Biol. (Praha) 2014, 60, 89–94. [Google Scholar]
- Emsen, B.; Turkez, H.; Togar, B.; Aslan, A. Evaluation of antioxidant and cytotoxic effects of olivetoric and physodic acid in cultured human amnion fibroblasts. Hum. Exp. Toxicol. 2017, 36, 376–385. [Google Scholar] [CrossRef]
- Behera, B.C.; Mahadik, N.; Morey, M. Antioxidative and cardiovascular-protective activities of metabolite usnic acid and psoromic acid produced by lichen species Usnea complanata under submerged fermentation. Pharm. Biol. 2012, 50, 968–979. [Google Scholar] [CrossRef]
- Nishanth, K.S.; Sreerag, R.S.; Deepa, I.; Mohandas, C.; Nambisan, B. Protocetraric acid: An excellent broad spectrum compound from the lichen Usnea albopunctata against medically important microbes. Nat. Prod. Res. 2015, 29, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Carlos, I.Z.; Carli, C.B.A.; Maia, D.C.G.; Benzatti, F.P.; Lopes, F.C.M.; Roese, F.M.; Watanabe, M.; Micheletti, A.C.; dos Santos, L.C.; Vilegas, W.; et al. Immunostimulatory effects of the phenolic compounds from lichens on nitric oxide and hydrogen peroxide production. Rev. Bras. De Farmacogn. 2009, 19, 847–852. [Google Scholar] [CrossRef]
- Ranković, B.; Kosanić, M.; Stanojković, T.; Vasiljević, P.; Manojlović, N. Biological Activities of Toninia candida and Usnea barbata Together with Their Norstictic Acid and Usnic Acid Constituents. IJMS 2012, 13, 14707–14722. [Google Scholar] [CrossRef] [PubMed]
- Ranković, B. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-16814-8. [Google Scholar]
- Oettl, S.K.; Gerstmeier, J.; Khan, S.Y.; Wiechmann, K.; Bauer, J.; Atanasov, A.G.; Malainer, C.; Awad, E.M.; Uhrin, P.; Heiss, E.H.; et al. Imbricaric Acid and Perlatolic Acid: Multi-Targeting Anti-Inflammatory Depsides from Cetrelia monachorum. PLoS ONE 2013, 8, e76929. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.G.; Veeraval, L.; Maitra, S.; Chollet-Krugler, M.; Tomasi, S.; Dévéhat, F.L.-L.; Boustie, J.; Chakravarty, S. Lichen-derived compounds show potential for central nervous system therapeutics. Phytomedicine 2016, 23, 1527–1534. [Google Scholar] [CrossRef]
- Çobanoğlu, G. Evaluation of the Antimicrobial Properties of Some Lichens. South West. J. Hortic. 2010, 6, 153–158. [Google Scholar]
- Luo, H.; Yamamoto, Y.; Kim, J.A.; Jung, J.S.; Koh, Y.J.; Hur, J.-S. Lecanoric acid, a secondary lichen substance with antioxidant properties from Umbilicaria antarctica in maritime Antarctica (King George Island). Polar. Biol. 2009, 32, 1033–1040. [Google Scholar] [CrossRef]
- Martins, M.C.B.; Lima, M.J.G.D.; Silva, F.P.; Azevedo-Ximenes, E.; Silva, N.H.D.; Pereira, E.C. Cladia aggregata (lichen) from Brazilian northeast: Chemical characterization and antimicrobial activity. Braz. Arch. Biol. Technol. 2010, 53, 115–122. [Google Scholar] [CrossRef]
- Verma, N.; Behera, B.C.; Sonone, A.; Makhija, U. Cell Aggregates Derived from Natural Lichen Thallus Fragments: Antioxidant Activities of Lichen Metabolites Developed In Vitro. Nat. Prod. Commun. 2008, 3, 1911–1918. [Google Scholar] [CrossRef]
- Honda, N.K.; Pavan, F.R.; Coelho, R.G.; de Andrade Leite, S.R.; Micheletti, A.C.; Lopes, T.I.B.; Misutsu, M.Y.; Beatriz, A.; Brum, R.L.; Leite, C.Q.F. Antimycobacterial activity of lichen substances. Phytomedicine 2010, 17, 328–332. [Google Scholar] [CrossRef]
- Manojlovic, N.T.; Vasiljevic, P.J.; Maskovic, P.Z.; Juskovic, M.; Bogdanovic-Dusanovic, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid. Based Complement. Altern. Med. 2012, 2012, 452431. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, S.; Cheng, A.; Wu, X.; Zhang, Y.; Lou, H. In vitro activities of retigeric acid B alone and in combination with azole antifungal agents against Candida albicans. Antimicrob. Agents Chemother. 2009, 53, 1586–1591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robertsdottir, A.R. Icelandic Herbs and Their Medicinal Uses; North Atlantic Books: Berkeley, CA, USA, 2016; ISBN 978-1-62317-023-3. [Google Scholar]
- Paudel, B.; Bhattarai, H.D.; Koh, H.Y.; Lee, S.G.; Han, S.J.; Lee, H.K.; Oh, H.; Shin, H.W.; Yim, J.H. Ramalin, a novel nontoxic antioxidant compound from the Antarctic lichen Ramalina terebrata. Phytomedicine 2011, 18, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.; Bhattarai, H.D.; Lee, J.S.; Hong, S.G.; Shin, H.W.; Yim, J.H. Antibacterial potential of Antarctic lichens against human pathogenic Gram-positive bacteria. Phytother. Res. 2008, 22, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hou, B.; Tang, C.; Liu, F.; Yang, J.; Pan, T.; Si, K.; Lu, D.; Wang, X.; Wang, J.; et al. (+)-Usnic Acid Inhibits Migration of c-KIT Positive Cells in Human Colorectal Cancer. Evid. Based Complement. Altern. Med. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, X.; Zhou, B.; Zhang, C.; Tu, J.; Chen, X.; Wang, J.; Gao, H.; Qin, G.; Pan, W. Usnic Acid Induces Cycle Arrest, Apoptosis, and Autophagy in Gastric Cancer Cells In Vitro and In Vivo. Med. Sci. Monit. 2018, 24, 556–566. [Google Scholar] [CrossRef]
- Bessadottir, M.; Egilsson, M.; Einarsdottir, E.; Magnusdottir, I.H.; Ogmundsdottir, M.H.; Omarsdottir, S.; Ogmundsdottir, H.M. Proton-Shuttling Lichen Compound Usnic Acid Affects Mitochondrial and Lysosomal Function in Cancer Cells. PLoS ONE 2012, 7, e51296. [Google Scholar] [CrossRef]
- Zuo, S.; Wang, L.; Zhang, Y.; Zhao, D.; Li, Q.; Shao, D.; Fang, X. Usnic acid induces apoptosis via an ROS-dependent mitochondrial pathway in human breast cancer cells in vitro and in vivo. RSC Adv. 2015, 5, 153–162. [Google Scholar] [CrossRef]
- Pyrczak-Felczykowska, A.; Narlawar, R.; Pawlik, A.; Guzow-Krzemińska, B.; Artymiuk, D.; Hać, A.; Ryś, K.; Rendina, L.M.; Reekie, T.A.; Herman-Antosiewicz, A.; et al. Synthesis of Usnic Acid Derivatives and Evaluation of Their Antiproliferative Activity against Cancer Cells. J. Nat. Prod. 2019, 82, 1768–1778. [Google Scholar] [CrossRef]
- Emsen, B.; Aslan, A.; Turkez, H.; Joughi, A.; Kaya, A. The anti-cancer efficacies of diffractaic, lobaric, and usnic acid: In vitro inhibition of glioma. J. Cancer Res. Ther. 2018, 14, 941. [Google Scholar] [CrossRef]
- Galanty, A.; Koczurkiewicz, P.; Wnuk, D.; Paw, M.; Karnas, E.; Podolak, I.; Węgrzyn, M.; Borusiewicz, M.; Madeja, Z.; Czyż, J.; et al. Usnic acid and atranorin exert selective cytostatic and anti-invasive effects on human prostate and melanoma cancer cells. Toxicol. Vitr. 2017, 40, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.T. Anti-angiogenic and antiproliferative properties of the lichen substances (-)—Usnic acid and vulpinic acid. Z. Nat. C J. Biosci. 2015, 70, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kılıc, N.; Derici, M.K.; Buyuk, I.; Aydın, S.S.; Aras, S.; Duman, D.C. Evaluation of in vitro Anticancer Activity of Vulpinic Acid and its Apoptotic Potential Using Gene Expression and Protein Analysis. IJPER 2018, 52, 626–634. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Kim, S.; Kim, J.H.; Youn, U.J.; Suh, S.-S. The Comprehensive Roles of ATRANORIN, A Secondary Metabolite from the Antarctic Lichen Stereocaulon caespitosum, in HCC Tumorigenesis. Molecules 2019, 24, 1414. [Google Scholar] [CrossRef] [PubMed]
- Brandão LF, G.; Alcantara, G.B.; Matos MD, F.C.; Bogo, D.; dos Santos Freitas, D.; Oyama, N.M.; Honda, N.K. Cytotoxic Evaluation of Phenolic Compounds from Lichens against Melanoma Cells. Chem. Pharm. Bull. 2013, 61, 176–183. [Google Scholar] [PubMed]
- Alexandrino, C.A.F.; Honda, N.K.; Matos, M.D.F.C.; Portugal, L.C.; Souza, P.R.B.D.; Perdomo, R.T.; Guimarães, R.D.C.A.; Kadri, M.C.T.; Silva, M.C.B.L.; Bogo, D. Antitumor effect of depsidones from lichens on tumor cell lines and experimental murine melanoma. Rev. Bras. De Farmacogn. 2019, 29, 449–456. [Google Scholar] [CrossRef]
- Manojlović, N.; Ranković, B.; Kosanić, M.; Vasiljević, P.; Stanojković, T. Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine 2012, 19, 1166–1172. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Hu, X.-Y.; Lu, T.; Cheng, Y.-N.; Young, C.Y.F.; Yuan, H.-Q.; Lou, H.-X. Retigeric Acid B Exhibits Antitumor Activity through Suppression of Nuclear Factor-κB Signaling in Prostate Cancer Cells in Vitro and in Vivo. PLoS ONE 2012, 7, e38000. [Google Scholar] [CrossRef]
- Brisdelli, F.; Perilli, M.; Sellitri, D.; Bellio, P.; Bozzi, A.; Amicosante, G.; Nicoletti, M.; Piovano, M.; Celenza, G. Protolichesterinic acid enhances doxorubicin-induced apoptosis in HeLa cells in vitro. Life Sci. 2016, 158, 89–97. [Google Scholar] [CrossRef]
- Bessadóttir, M.; Eiríksson, F.F.; Becker, S.; Ögmundsdóttir, M.H.; Ómarsdóttir, S.; Thorsteinsdóttir, M.; Ögmundsdóttir, H.M. Anti-proliferative and pro-apoptotic effects of lichen-derived compound protolichesterinic acid are not mediated by its lipoxygenase-inhibitory activity. Prostaglandins Leukot. Essent. Fat. Acids 2015, 98, 39–47. [Google Scholar] [CrossRef]
- Suh, S.-S.; Kim, T.; Kim, J.; Hong, J.-M.; Nguyen, T.; Han, S.; Youn, U.; Yim, J.; Kim, I.-C. Anticancer Activity of Ramalin, a Secondary Metabolite from the Antarctic Lichen Ramalina terebrata, against Colorectal Cancer Cells. Molecules 2017, 22, 1361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Park, S.-Y.; Nguyen, T.T.; Yu, Y.H.; Nguyen, T.V.; Sun, E.G.; Udeni, J.; Jeong, M.-H.; Pereira, I.; Moon, C.; et al. Lichen Secondary Metabolite, Physciosporin, Inhibits Lung Cancer Cell Motility. PLoS ONE 2015, 10, e0137889. [Google Scholar] [CrossRef] [PubMed]
- Tatipamula, V.B.; Vedula, G.S.; Sastry, A.V.S. Antarvedisides A-B from Manglicolous Lichen Dirinaria consimilis (Stirton) and their Pharmacological Profile. Asian J. Chem. 2019, 31, 805–812. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Yoon, S.; Yang, Y.; Lee, H.-B.; Oh, S.; Jeong, M.-H.; Kim, J.-J.; Yee, S.-T.; Crişan, F.; Moon, C.; et al. Lichen Secondary Metabolites in Flavocetraria cucullata Exhibit Anti-Cancer Effects on Human Cancer Cells through the Induction of Apoptosis and Suppression of Tumorigenic Potentials. PLoS ONE 2014, 9, e111575. [Google Scholar] [CrossRef] [PubMed]
- Zugic, A.; Jeremic, I.; Isakovic, A.; Arsic, I.; Savic, S.; Tadic, V. Evaluation of Anticancer and Antioxidant Activity of a Commercially Available CO2 Supercritical Extract of Old Man’s Beard (Usnea barbata). PLoS ONE 2016, 11, e0146342. [Google Scholar] [CrossRef]
- Kosanić, M.; Manojlović, N.; Janković, S.; Stanojković, T.; Ranković, B. Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef]
- Gonçalves, J.P.; Martins, M.C.B.; Buril, M.D.L.L.; Aguiar, J.D.S.; Da Silva, T.G.; Dos Santos Souza, T.G.; Santos, N.P.D.S.; Chagas, C.A.; Pereira, E.C.; Falcão, E.P.D.S.; et al. Antineoplastic Activity and Genotoxicity of Organic Extracts and Barbatic Acid Isolated from the Lichen Cladonia salzmannii Nyl. Int. Arch. Med. 2018, 11, 53. [Google Scholar] [CrossRef]
- Barroso Martins, M.C.; Alves Rocha, T.; Santos Silva, T.D.; Pacífico Cavalcanti-Neto, M.; Pereira da Silva Santos, N.; Gonçalves da Silva, T.; Amanajás Aguiar-Junior, F.C.; da Silva Falcão, E.P.; Pereira, E.C.; da Silva, N.H. In Vitro And In Vivo Antineoplastic Activity Of Barbatic Acid. Int. Arch. Med. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.; Bae, W.K.; Nam, S.-J.; Jeong, M.-H.; Zhou, R.; Park, S.-Y.; Taş, İ.; Hwang, Y.-H.; Park, M.-S.; Chung, I.J.; et al. Acetonic extracts of the endolichenic fungus EL002332 isolated from Endocarpon pusillum exhibits anticancer activity in human gastric cancer cells. Phytomedicine 2018, 40, 106–115. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Antioxidant, Antimicrobial, and Anticancer Activity of 3 Umbilicaria Species. J. Food Sci. 2012, 77, T20–T25. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Stošić, I.; Grujičić, D.; Milošević-Djordjević, O. Lasallia pustulata lichen as possible natural antigenotoxic, antioxidant, antimicrobial and anticancer agent. Cytotechnology 2016, 68, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.M.; Ranković, B.R.; Stanojković, T.P. Antioxidant, antimicrobial and anticancer activities of three Parmelia species. J. Sci. Food Agric. 2012, 92, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Ghate, N.B.; Chaudhuri, D.; Sarkar, R.; Sajem, A.L.; Panja, S.; Rout, J.; Mandal, N. An Antioxidant Extract of Tropical Lichen, Parmotrema reticulatum, Induces Cell Cycle Arrest and Apoptosis in Breast Carcinoma Cell Line MCF-7. PLoS ONE 2013, 8, e82293. [Google Scholar] [CrossRef] [PubMed]
- Grujičić, D.; Stošić, I.; Kosanić, M.; Stanojković, T.; Ranković, B.; Milošević-Djordjević, O. Evaluation of in vitro antioxidant, antimicrobial, genotoxic and anticancer activities of lichen Cetraria islandica. Cytotechnology 2014, 66, 803–813. [Google Scholar] [CrossRef]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T.; Vasiljevic, P.; Manojlovic, N. Biological activities and chemical composition of lichens from Serbia. EXCLI J. 2014, 13, 1226–1238. [Google Scholar]
- Kumar, J.; Dhar, P.; Tayade, A.B.; Gupta, D.; Chaurasia, O.P.; Upreti, D.K.; Arora, R.; Srivastava, R.B. Antioxidant Capacities, Phenolic Profile and Cytotoxic Effects of Saxicolous Lichens from Trans-Himalayan Cold Desert of Ladakh. PLoS ONE 2014, 9, e98696. [Google Scholar] [CrossRef]
- Ari, F.; Aztopal, N.; Oran, S.; Bozdemir, S.; Celikler, S.; Ozturk, S.; Ulukaya, E. Parmelia sulcata Taylor and Usnea filipendula Stirt induce apoptosis-like cell death and DNA damage in cancer cells. Cell Prolif. 2014, 47, 457–464. [Google Scholar] [CrossRef]
- Ari, F.; Celikler, S.; Oran, S.; Balikci, N.; Ozturk, S.; Ozel, M.Z.; Ozyurt, D.; Ulukaya, E. Genotoxic, cytotoxic, and apoptotic effects of Hypogymnia physodes (L.) Nyl. on breast cancer cells: GENOTOXIC, CYTOTOXIC, AND APOPTOTIC EFFECTS OF HYPOGYMNIA. Environ. Toxicol. 2014, 29, 804–813. [Google Scholar] [CrossRef]
- Coskun, Z.M.; Ersoz, M.; Acikgoz, B.; Karalti, I.; Cobanoglu, G.; Sesal, C. Anti-Proliferative and Apoptotic Effects of Methanolic Extracts from Different Cladonia Species on Human Breast Cancer Cells. Folia Biol. (Praha) 2015, 61, 97–103. [Google Scholar]
- Basile, A.; Rigano, D.; Loppi, S.; Di Santi, A.; Nebbioso, A.; Sorbo, S.; Conte, B.; Paoli, L.; De Ruberto, F.; Molinari, A.; et al. Antiproliferative, Antibacterial and Antifungal Activity of the Lichen Xanthoria parietina and Its Secondary Metabolite Parietin. IJMS 2015, 16, 7861–7875. [Google Scholar] [CrossRef]
- Ari, F.; Ulukaya, E.; Oran, S.; Celikler, S.; Ozturk, S.; Ozel, M.Z. Promising anticancer activity of a lichen, Parmelia sulcata Taylor, against breast cancer cell lines and genotoxic effect on human lymphocytes. Cytotechnology 2015, 67, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; El-Naggar, A.M.; Clair, L.L.S.; O’Neill, K.L. Anticancer Activities of Selected Species of North American Lichen Extracts: ANTICANCER ACTIVITIES OF LICHEN. Phytother. Res. 2015, 29, 100–107. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, E. Antibacterial, antioxidant, and cytotoxic activities of the corticolous lichens Canoparmelia aptata, Pannaria sp., and Parmotrema gardneri collected from Mt. Banahaw, Quezon, Philippines. CREAM 2016, 6, 173–183. [Google Scholar] [CrossRef]
- Açikgöz, B.; Karalti, I.; Ersöz, M.; Coşkun, Z.M.; Cobanoğlu, G.; Sesal, C. Screening of antimicrobial activity and cytotoxic effects of two Cladonia species. Z. Naturforsch. C. J. Biosci. 2013, 68, 191–197. [Google Scholar] [CrossRef]
- Felczykowska, A.; Pastuszak-Skrzypczak, A.; Pawlik, A.; Bogucka, K.; Herman-Antosiewicz, A.; Guzow-Krzemińska, B. Antibacterial and anticancer activities of acetone extracts from in vitro cultured lichen-forming fungi. BMC Complement. Altern. Med. 2017, 17, 300. [Google Scholar] [CrossRef]
- Ersoz, M.; Coskun, Z.M.; Acikgoz, B.; Karalti, I.; Cobanoglu, G.; Cesal, C. In vitro evaluation of cytotoxic, anti-proliferative, anti-oxidant, apoptotic, and anti-microbial activities of Cladonia pocillum. Cell. Mol. Biol. (Noisy-Le-Grand) 2017, 63, 69. [Google Scholar] [CrossRef]
- Delebassée, S.; Mambu, L.; Pinault, E.; Champavier, Y.; Liagre, B.; Millot, M. Cytochalasin E in the lichen Pleurosticta acetabulum. Anti-proliferative activity against human HT-29 colorectal cancer cells and quantitative variability. Fitoterapia 2017, 121, 146–151. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Zhang, Y.; Tu, Y.; Huang, C.; Tao, J.; Yang, M.; Yang, L. The Polysaccharide Extracted from Umbilicaria esculenta Inhibits Proliferation of Melanoma Cells through ROS-Activated Mitochondrial Apoptosis Pathway. Biol. Pharm. Bull. 2018, 41, 57–64. [Google Scholar] [CrossRef]
- Kosanić, M. EXTRACTS OF FIVE CLADONIA LICHENS AS SOURCES OF BIOLOGICALLY ACTIVE COMPOUNDS. Farmacia 2018, 66, 644–651. [Google Scholar] [CrossRef]
- Ozturk, S.; Erkisa, M.; Oran, S.; Ulukaya, E.; Celikler, S.; Ari, F. Lichens exerts an anti-proliferative effect on human breast and lung cancer cells through induction of apoptosis. Drug Chem. Toxicol. 2019, 1–9. [Google Scholar] [CrossRef]
- Lagarde, A.; Millot, M.; Pinon, A.; Liagre, B.; Girardot, M.; Imbert, C.; Ouk, T.S.; Jargeat, P.; Mambu, L. Antiproliferative and antibiofilm potentials of endolichenic fungi associated with the lichen Nephroma laevigatum. J. Appl. Microbiol. 2019, 126, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.S.; Pratoko, D.K.; Damayanti, Y.D.; Lestari, N.D.; Laksono, T.A.; Addy, H.S.; Untari, L.F.; Kusumawardani, B.; Wangchuk, P. Antibacterial and Anticancer Activities of Nine Lichens of Indonesian Java Island. J. Biol. Act. Prod. Nat. 2019, 9, 39–46. [Google Scholar] [CrossRef]
- Song, Y.; Dai, F.; Zhai, D.; Dong, Y.; Zhang, J.; Lu, B.; Luo, J.; Liu, M.; Yi, Z. Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated AKT and ERK1/2 signaling pathways. Angiogenesis 2012, 15, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, H.Y.; Akl, M.R.; Elsayed, H.E.; Hill, R.A.; El Sayed, K.A. Usnic Acid Benzylidene Analogues as Potent Mechanistic Target of Rapamycin Inhibitors for the Control of Breast Malignancies. J. Nat. Prod. 2017, 80, 932–952. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, R.; Suleyman, B.; Altuner, D.; Demirci, E.; Cetin, N.; Yilmaz, A.; Baykal, H.; Alpcan, H.; Turumtay, E.A.; Suleyman, H. Effect of ethyl acetate extract of usnea longissima on esophagogastric adenocarcinoma in rats. Acta Cir. Bras. 2019, 34, 3. [Google Scholar] [CrossRef] [PubMed]
- Poornima, S. Evaluation of Anti-Cancer Properties of Lichens Using <i>Albino wistar</i> Rats as an Animal Model. CRJ 2016, 4, 84. [Google Scholar]
- Karagoz, I.D.; Ozaslan, M.; Guler, I.; Uyar, C.; Yalim, T.; Kazanci, U.; Aslan, A.; Cakir, A. In vivo Antitumoral Effect of Diffractaic Acid from Lichen Metabolites on Swiss Albino Mice with Ehrlich Ascites Carcinoma: An Experimental Study. Int. J. Pharmacol. 2014, 10, 307–314. [Google Scholar]
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Baban, B.; Boniolo, G.; Wang, W.; Bubnov, R.; Kapalla, M.; Krapfenbauer, K.; Mozaffari, M.S.; Costigliola, V. Medicine in the early twenty-first century: Paradigm and anticipation—EPMA position paper 2016. EPMA J. 2016, 7, 23. [Google Scholar] [CrossRef]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef]
| Origin | Chemical Structure | Activities | References | |
|---|---|---|---|---|
Usnic acid (UA)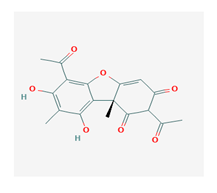 | Usnea diffracta, Cladonia arbuscula, | 2,6-diacetyl-7,9-dihydroxy-8,9b-dimethyl-1,3 | antimicrobial, antiprotozoal, antiviral, antiproliferate, anti-inflammatory, analgesic, antipyretic | [28,29] |
| CAS ID (125-46-2) | ||||
| Alectoria samentosa, | ||||
| Flavocetraria nivalis, | ||||
| Alectoria ochroleuca, | ||||
| Usnea florida | ||||
Diffractaic acid (DA)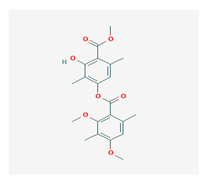 | Usnea longissimi, Usnea subcavata, | 4-[(2,4-dimethoxy-3,6-dimethylbenzoyl)oxy]-2-hydroxy-3,6-dimethylbenzoic acid | antioxidant, gastroprotective, analgesic, antiviral, | [38,39,40] |
| Protousnea magellanica | CAS ID (436-32-8) | |||
Lobaric acid (LA)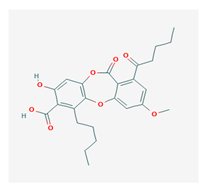 | Stereocaulon alpinum, Cladonia sp., Stereocaulon sasakii | 3-hydroxy-9-methoxy-6-oxo-7-(1-oxopentyl)-1-pentyl-2-benzo[b][1,4]benzodioxepincarboxylic acid | antibacterial, antioxidant, antimitotic | [41,42,43] |
| CAS ID (522-53-2) | ||||
Atranorin (ATR)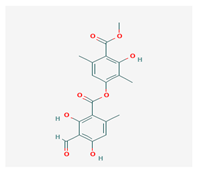 | Parmelia sulcate, Parmotrema stuppeum, Stereocaulon alpinum, Physcia aipolia | 3-hydroxy-4-methoxycarbonyl-2,5-dimethylphenyl | antimicrobial, antiprotozoal, antiviral, antifungal, antioxidant | [44,45] |
| CAS ID (479-20-9) | ||||
Vulpinic acid (VA)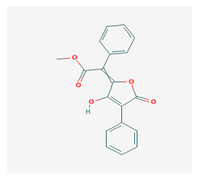 | Letharia vulpina | methyl (2E)-2-(3-hydroxy-5-oxo-4-phenylfuran-2-ylidene)-2-phenylacetate | antiproliferative, antimicrobial, antiangiogenic, | [46,47] |
| CAS ID (73622-57-8) | ||||
Physodic acid (PA)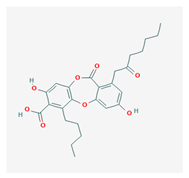 | Hypogymnia physodes | 3,9-dihydroxy-6-oxo-7-(2-oxoheptyl)-1-pentylbenzo[b][1,4]benzodioxepine-2-carboxylic acid | antimicrobial, antioxidant, immunoprotective | [48,49] |
| CAS ID (84-24-2) | ||||
Olivetoric acid (OA)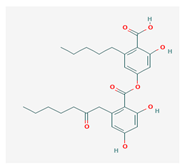 | Pseudevernia furfuracea | 4-[2,4-dihydroxy-6-(2-oxoheptyl)benzoyl]oxy-2-hydroxy-6-pentylbenzoic acid | antimicrobial, antioxidant | [48,50] |
| CAS ID (491-72-5) | ||||
Psoromic acid (PSA)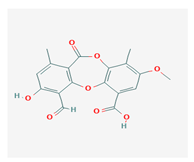 | Usnea camplanata | 10-formyl-9-hydroxy-3-methoxy-4,7-dimethyl-6-oxobenzo[b][1,4]benzodioxepine-1-carboxylic acid | cardioprotective | [51] |
| CAS ID (7299-11-8) | ||||
Protocetraric acid (PrA)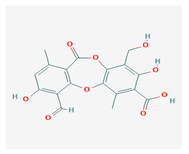 | Parmelia caperata, Usnea albopunctata, Parmelia saxatilis, Parmelia sulcate | 10-formyl-3,9-dihydroxy-4-(hydroxymethyl)-1,7-dimethyl-6-oxobenzo[b][1,4]benzodioxepine-2-carboxylic acid | antimicrobial, immunostimulatory | [52,53] |
| CAS ID (489-51-0) | ||||
Norstictic acid (NA)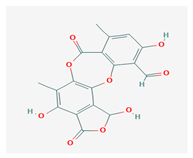 | Toninia candida | 1,3-Dihydro-1,4,10-trihydroxy-5,8-dimethyl-3,7-dioxo-7H-isobenzofuro(4,5-b)(1,4)benzodioxepin-11-carboxaldehyde | antioxidant, antibacterial | [54] |
| CAS ID (571-67-5) | ||||
Divaricatic acid (DiA)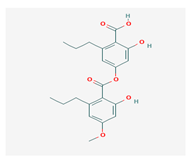 | Evernia mesomorpha | 2-hydroxy-4-[(2-hydroxy-4-methoxy-6-propylbenzoyl)oxy]-6-propylbenzoic acid | antimicrobial, antioxidant | [55] |
| CAS ID (491-62-3) | ||||
Perlatolic acid (PeA)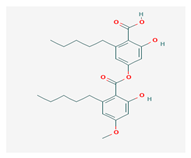 | Cetrelia monachorum | 2-Hydroxy-4-((2-hydroxy-4-methoxy-6-pentylbenzoyl)oxy)-6-pentylbenzoic acid | anti-inflammatory, anti-neurodegenerative | [56,57] |
| CAS ID (529-47-5) | ||||
Caperatic acid (CA)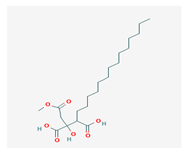 | Platismatia glauca | 2-hydroxy-2-(2-methoxy-2-oxoethyl)-3-tetradecylbutanedioic acid | fungitoxic | [58] |
| CAS ID (29227-64-3) | ||||
Lecanoric acid (LeA)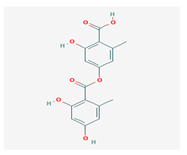 | Usnea subvacata Motyka, Parmotrema stuppuem, Parmotrema tinctorum and Parmotrema grayana | 4-(2,4-dihydroxy-6-methylbenzoyl)oxy-2-hydroxy-6-methylbenzoic acid | antioxidant | [59] |
| CAS ID (480-56-8) | ||||
Barbatic acid (BA)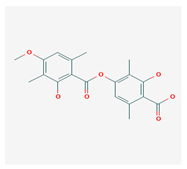 | Usnea longissima | 2-hydroxy-4-(2-hydroxy-4-methoxy-3,6-dimethylbenzoyl)oxy-3,6-dimethylbenzoic acid | antioxidant, antimicrobial | [60,61] |
| CAS ID (17636-16-7) | ||||
Barbatolic acid (BrA)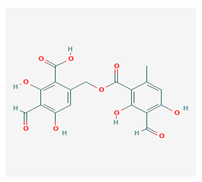 | Bryoria capillaris | 3-formyl-6-[(3-formyl-2,4-dihydroxy-6-methylbenzoyl)oxymethyl]-2,4-dihydroxybenzoic acid | antimicrobial | [35] |
| CAS ID (529-50-0) | ||||
Lobastin (LOB)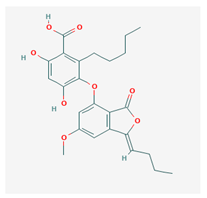 | Stereocaulon alpnum | 3-[[(1Z)-1-butylidene-6-methoxy-3-oxo-2-benzofuran-4-yl]oxy]-4,6-dihydroxy-2-pentylbenzoic acid | antibacterial, antioxidant | [30] |
Hypostictic acid (HA)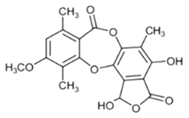 | Pseudoparmelia sphaerospora | (1,4-dihydroxy-10-methoxy-5,8,11-trimethyl-1H-benzo[e]furo[3′,4′:3,4]benzo[b][1,4]dioxepine-3,7-dione) | antimicrobial | [62] |
Salazinic acid (SA)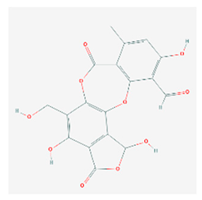 | Parmelia caperata | 5,13,17-trihydroxy-12-(hydroxymethyl)-7-methyl-9,15-dioxo-2,10,16-trioxatetracyclo[9.7.0.03,8.014,18]octadeca-1(11),3(8),4,6,12,14(18)-hexaene-4-carbaldehyde | antibacterial, antifungal, antioxidant, antiviral | [55,63] |
| CAS ID (521-39-1) | ||||
Retigeric acid B (RA-B)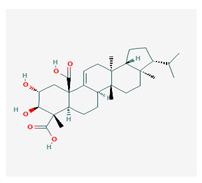 | Lobaria kurokawae | (3R,3aR,5aR,5bR,7aR,8S,9R,10R,11aR,13aS,13bR)-9,10-dihydroxy-3a,5a,8,13a-tetramethyl-3-propan-2-yl-1,2,3,4,5,5b,6,7,7a,9,10,11,13,13b-tetradecahydrocyclopenta[a]chrysene-8,11a-dicarboxylic acid | antifungal | [64] |
| CAS ID (38327-77-4) | ||||
Protolichesterinic acid (PLA)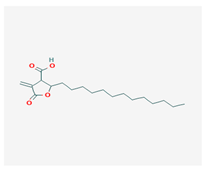 | Cornicularia aculeate, Centraria islandica | 4-methylidene-5-oxo-2-tridecyloxolane-3-carboxylic acid | antibacterial, anti-inflammatory | [65] |
| CAS ID (1448-96-0) | ||||
Ramalin (RAM)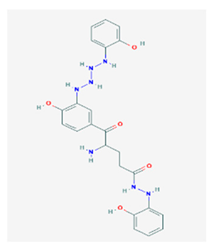 | Ramalina terebrata | γ-glutamyl-N′-(2-hydroxyphenyl)hydrazide | antioxidant, antibacterial | [66,67] |
Physciosporin (PHY)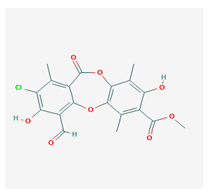 | Pseudocyphellaria granulate, Pseudocyphellaria coriacea | methyl 8-chloro-10-formyl-3,9-dihydroxy-1,4,7-trimethyl-6-oxobenzo[b][1,4]benzodioxepine-2-carboxylate | antiproliferative | [20] |
Sekikaic acid (SeA)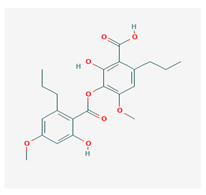 | Cladonia sp., Ramalina roesleri | 2-hydroxy-3-(2-hydroxy-4-methoxy-6-propylbenzoyl)oxy-4-methoxy-6-propylbenzoic acid | antioxidant, antibacterial, antidiabetic | [18] |
| CAS ID (607-11-4) |
| Lichen Acid/Species | Cell Lines | Effects | Reference |
|---|---|---|---|
| UA | CaCo2, HepG2, Hep2C, RD, Wehi, | ↑ cytotoxicity of CaCo2 (IC50 7.05 μM), HepG2 (IC50 15.4 μM), Hep2C (IC50 21.8 μM), RD (IC50 22.9 μM), Wehi (IC50 15.8 μM) | [29] |
| ↑ Bax ↓ Bcl-2 ↓ p53 | |||
| HCT116, LS174 | ↓ SCF-induced proliferation and migration of HCT116 and LS174 (c-KIT+) | [68] | |
| ↑ autophagy of HCT116 (via ↓ mTOR) | |||
| ↓ level of phosphorylated PKC-A, c-KIT of HCT116 | |||
| BGC823, SGC7901 | ↓ proliferation + G0/G1 and G2/M arrest of BGC823 (IC50 236.55 μM) and SGC7901 (IC50618.82 μM) | [69] | |
| → apoptosis, ↑ autophagy | |||
| ↑ Bax/Bcl-2 ratio | |||
| ↑ caspase-3, ↑ PARP | |||
| MCF-7, MDA-MB-231, BT-474 | MDA-MB-231: 67 UA-responsive miRNAs | [37] | |
| BT-474: 15 UA-responsive miRNAs | |||
| MCF-7: 8 UA-responsive miRNAs | |||
| HepG2, SNU-449 | → apoptosis and autophagy | [27] | |
| G0/G1, G2/M arrest | |||
| A549 | → apoptosis | [28] | |
| ↓ cell number | |||
| ↓ proliferation | |||
| ↓ expression CDK4, CDK6, cyclin D1 | |||
| ↑ expression of p21/cip1 protein | |||
| T47D and MCF-7 | Formation of autophagosome (H+ shuttling in mitochondria and lysosomes) | [70] | |
| Capan-2 | |||
| MCF-7 | → apoptosis | [71] | |
| A549, H460, H1650 and H1975 | ↓ motility of A549 | [19] | |
| ↓ invasion of H1650 and H1975 (↓β-catenin-mediated TOPFLASH and KITENIN-mediated AP-1 activity) | |||
| ↓ expression of CD44, c-myc and Cyclin D1 in all cell lines | |||
| ↓ GTP-Rac1 and RhoA | |||
| Synthetic derivatives of UA | MCF-7, PC-3, HeLa | Derivatives 2a, 2b: | [72] |
| ↓ proliferation of PC-3, MCF-7 (IC50 value 3 μM), HeLa (IC50 1 μM) | |||
| G0/G1 arrest + → apoptosis of MCF-7 | |||
| Activation of cytoplasmic vacuolisation | |||
| All active derivatives: | |||
| G0/G1 arrest + ↓ fraction in S and G2/M phase of HeLa | |||
| PU | HCT116, DLD1, SW480, HT29, SW620, Caco2, COLO320, CT26 | ↑ cytotoxicity (lower IC50 than UA, except of SW480 and CT26 cells) | [34] |
| ↓ invasion of Caco2 and HCT116 | |||
| ↓ Caco2 motility (↓CAPN1, CDC42, CFL1, IGF1, WASF1, WASL) | |||
| DA | U87MG-GBM, PRCC | LA: ↑cytotoxicity of GBM and PRCC (IC50 of LA, DA and UA 9.08, 122.26, 132.69 mg/L in PRCC and 5.77, 35.67 and 41.55 mg/L in U87MG) | [73] |
| LA | |||
| UA | |||
| UA | HTB-140, DU-145, PC-3 | ↓ proliferation, ↓ migration, ↓ actin organization | [74] |
| ATR | |||
| UA | HepG2, HUVEC, NS2OY | UA: ↑ cytotoxicity | [75] |
| VA | ↓ proliferation of NS2OY after VA- treatment | ||
| ↑ antiangiogenic effect | |||
| VA | MCF-7, MDA-MB-231, BT-474, SK-BR-3, MCF-12 A | ↑ cytotoxicity | [47] |
| → apoptosis | |||
| ↑ P53 in SK-BR-3 (vs MCF-12A) | |||
| CaCo2, HepG2 and Hep2C, RD, Wehi, L929, Vero | ↑ cytotoxicity of CaCo2 (IC50 13.7 μM), HepG2 (IC50 23.8 μM), Hep2C (IC50 25.3 μM), RD (IC50 34.4 μM), Wehi (IC50 38.6 μM) | [76] | |
| ↓ growth (HepG2, CaCo2, Hep2C, RD, Wehi) | |||
| ↑ Bax and p53 (HepG2, CaCo2, Hep2C, RD, Wehi) | |||
| ↓ Bcl-2 (HepG2, CaCo2, Hep2C, RD, Wehi) | |||
| ATR | 4T1, NMuMG | ↓ clonogenic potential of 4T1 cells; → caspase-3, PARP cleavage, depletion of Bcl xL (4T1) | [45] |
| ATR | SK-Hep1, Huh-7, SNU-182 | ↓ cancer cells growth (concentration, >10 μg/mL | [77] |
| ↑ necrotic cell death, ↓ migration and invasion (Sk-Hep1, Huh-7) | |||
| G2/M arrest (SK-Hep1) | |||
| ATR | A375 | PA (concentration, 6.25–50 μM): ↓ A375, ↑ apoptosis | [17] |
| GA | ATR and GA (high concentrations): ↓ A375 | ||
| OA | PRCC | ↑ cytotoxicity | [43] |
| PA | U87MG | ||
| PSA | |||
| ATR | UACC-62 | ↑ cytotoxicity (PrA, NA, PsA, DiA, PeA against UACC-62) | [78] |
| PrA | NIH/3T3 | ↑ selectivity of PrA, NA, PsA, DiA, PeA for UACC-62 | |
| UA | B16-F10 | ↑ effectivity of NA and DIA against B16-F10 | |
| DA | |||
| Li | |||
| NA | |||
| PeA | |||
| DiA | |||
| PSA | |||
| PA | HCT116, DLD-1, HaCaT | CA: strongest cytotoxic (concentration, 100 μM) | [26] |
| CA | CA (HCT116, DLD-1): ↓ β-catenin regulated expression of Axin2, ↓ migration | ||
| LeA | CA (HCT116): ↓ Axin2 | ||
| CA + PA (HaCat): ↓ MMP7, ↓ survivin | |||
| BA | HeLa, A549, MCF-7, DU-145, HEK293 | ↓ HeLa (IC50 3.2 μg/mL), A549 (IC50 1.8, 3.2 μg/mL), MCF-7 (IC50 3.2 μg/mL), DU-145 (IC50 9.0 μg/mL) | [38] |
| BA (concentration, 1 μM): | |||
| G0/G1 arrest, ↑ apoptosis, ↑ caspase-3 activity, PARP cleavage, annexin V staining and chromatin condensation (A549) | |||
| BrA | T-47D, HCC1428, HUVECs | ↓ endothelial tube formation | [35] |
| ↓ migration | |||
| LA | HeLa, HCT116 | ↓ viability (IC50 50 μM) | [30] |
| LOB | ↓ proliferation | ||
| → G2/M arrest | |||
| → apoptosis (↑ Annexin V-positivity and PARP cleavage, | |||
| ↓ Bcl-2) | |||
| HA, SA | B16-F10, 786-0, HT-29, K562 | HA (K562, B16-F10, 786-0): ↓ proliferation | [79] |
| SA (K562, HT-29, B16-F10): ↓ proliferation | |||
| SA, PA | FemX, LS174 | ↑ cytotoxicity | [80] |
| RA-B | PC-3, DU145 | → apoptosis | [81] |
| ↓ expression of Bcl-2, Bcl-XL, cyclin D1, and survivin | |||
| PLA | SK-BR-3, T-47D | ↓ proliferation of SK-BR-3 | [31] |
| ↑ expression of fatty acid synthase | |||
| ↓ expression of HER2 | |||
| ↓ ERK1/2 and AKT signalling | |||
| HeLa, SH-SY5Y, K562 | PLA with doxorubicin: synergic cytotoxic effect (HeLa) | [82] | |
| RPMI 8226 and U266 | ↓ proliferation | [83] | |
| AsPC-1 | → cell arrest of AsPC-1 | ||
| → apoptosis (RPMI 8226, U266) | |||
| ↑ cytotoxicity of U266 (IC50 3.5μg/mL), AsPC-1 (IC50 3.5μg/mL), RPMI 8226 (IC501.8 μg/mL) | |||
| RAM | HCT116 | ↓ proliferation (concentration, 50 - 100 μg/mL) | [84] |
| ↑ G2/M arrest (via ↑ TP53, ↑ p21, ↓ cyclin B1, ↓ CDK1) | |||
| → apoptotic cells (concentration, 100 μg/mL) | |||
| ↓ wound healing, invasion, migration | |||
| PHY | Caco2, DLD1, HCT116, SW620, CT26 | ↑ cytotoxicity of CT26 (IC50 11.5 μg/mL), SW620 (IC50 12.6 μg/mL), Caco2 (IC50 13.3 μg/mL), HCT116 (IC50 19.8 μg/mL) and DLD1 (IC50 24.9 μg/mL) | [22] |
| → apoptosis (PHY at toxic concentrations) | |||
| ↓ migration, invasion, colony formation (PHY at non-toxic doses) | |||
| ↓ downstream transcription factors and/or target genes of EM | |||
| ↓ KITENIN, ↓ β-catenin | |||
| ↓ actin-based cell motility | |||
| A549, H1650, H1975 | ↓ migration | [85] | |
| ↓ invasion | |||
| antarA | MCF-7, HeLa, A549, NHME | AntarB (concentration, 30 μg/mL): stronger growth inhibition (HeLa, MCF-7) vs doxorubicin (concentration, 10 μg/mL) | [86] |
| antarB | AntarB and 2′-O-methyl DiA: ↓ proliferation of A549 (IC50 values of 22.5 and 27.5 μg/mL, respectively) | ||
| SeA | All metabolites: ↓ toxicity against NHME vs cancer cells | ||
| ATR | |||
| DiA | |||
| 2′-O-methyl DiA |
| Lichen Acid/Species | Cell Lines | Effects | Reference |
|---|---|---|---|
| UA | HT29, AGS, A549, CWR22Rv-1 | ↑ selective cytotoxicity (acetone extract and UA) | [87] |
| Extract of Flavocetraria cucullata | ↓ tumorigenesis and motility | ||
| ↓ EMT and Akt phosphorylation | |||
| ↑ anticancer activity of extract vs. UA | |||
| Extract of Toninia candida, | FemX, LS174 | ↑ cytotoxicity | [54] |
| Extract of Usnea barbata | → apoptosis after UA treatment | ||
| NA, UA | |||
| SCE | B16, C6, HaCaT | ↑ cytotoxicity of B16 (IC50 31.21 μg/mL) and C6 (IC50 43.40 μg/mL) | [88] |
| ↑ apoptosis and/or autophagy in B16 and C6 | |||
| Low toxicity against HaCaT | |||
| Acetone extract of Evernia prunastri | FemX, LS174 | PA: ↑ cytotoxicity LS 174 and FemX | [89] |
| Acetone extracts of Pseudoevernia furfuraceae | ↓ FemX and LS174: S and G2/M arrest | ||
| PA | |||
| PA | MCF-7, T47D, MDA-MB-231, MCF-10A | PA: ↑ cytotoxicity of MCF-7 (IC50 72.4 μg/mL), T47D (IC50 75.4 IC50 μg/mL), MDA-MB-231 (IC50 93.9 μg/mL) | [24] |
| Acetone extract of Hypogymnia physodes | |||
| Ethanol extract of Usnea strigosa | MD-MB-231, MDA-MB-468, MCF-7, T-47D, BT-474, SK-BR-3, MCF-10A | U. strigosa extracts: ↑ cytotoxicity of MD-MB-231 (IC50 3.7 μg/mL) MDA-MB-468 (IC50 4.5 μg/mL), MCF-7 IC50 6.4 μg/mL), T-47D (IC50 9.6 μg/mL), BT-474 (IC50 7.9 μg/mL), SK-BR-3 IC50 7.5 μg/mL) | [33] |
| NA | NA: MD-MB-231(IC50 14.9 μg/mL), MDA-MB-468 (IC50 17.3 μg/mL) | ||
| ↓ proliferation | |||
| ↓ migration of MDA-MB-468 | |||
| ↓ invasion of MDA-MB-231 | |||
| ↓ c-Met, STAT3, paxillin/Rac-1and FAK phosphorylation in MDA-MB-231 | |||
| Acetone extracts of Melanelia subaurifera and Melanelia fuliginosa | HeLa, A549, LS174, MRC5 | Melanelia subaurifera extract: ↑ cytotoxicity of HeLa (IC50 9.88 μg/mL) A549 (IC50 31.25 μg/mL), LS174 (IC50 31.64 μg/mL); | [25] |
| LeA | Melanelia fuliginosa extract: HeLa (IC50 45.24 μg/mL) A549 (IC50 125.276 μg/mL), LS174 (IC50 142.87 μg/mL); | ||
| 2′-O-MA | |||
| Extract of Cladonia salzmannii | RAW 264.7, NCI-H292, HEp-2, MCF-7, HL-60 | Ether extract: cytotoxicity of HL-60 (IC50 3.59 μg/mL), HEP-2 (IC50 26.75 μg/mL), NCI-H292 (IC50 29.91 μg/mL), RAW-264.7 (IC50 36.54 μg/mL) | [90] |
| Acetone extract: cytotoxicity of MCF-7 (7.55 μg/mL) and NCI-H292 (16.60 μg/mL) | |||
| BA | Cytotoxicity of HEP-2 (IC50 15.79 μg/mL), MCF-7 (IC50 18.28 μg/mL), RAW-264.7 (IC50 20.79 μg/mL) | ||
| BA | HEp-2, NCI-H292, KB | Cytotoxicity of HEp-2 (IC50 6.25 μg/mL) | [91] |
| Acetone extracts of | A549 | ↓ migration (concentration, 10 μg/mL) | [20] |
| Everniastrum vexans | |||
| ATR | ↑ cytotoxicity (concentration, >5 μg/mL) | ||
| ↓ β-catenin-mediated TOPFLASH activity (via ↓ nuclear import of β-catenin, ↓ c-jun/AP-1) | |||
| ↓ mRNA expression of KITENIN | |||
| ↑ KAI1 mRNA | |||
| ↓ GTP-Cdc42, GTP-RhoA, STAT proteins | |||
| Extracts of endolichenic fungus EL002332 | AGS, TMK-1, CT26 | ↑ cytotoxicity (on AGS and CT26) | [92] |
| EL002332 + docetaxel: synergistic effects (on AGS and TMK-1) | |||
| myC | ↑ apoptosis (caspase activation, Bcl-2 family regulation) |
| Lichen Acid/species | Cell Lines | Effects | Reference |
|---|---|---|---|
| Extract of Umbilicaria crustulosa | FemX, LS174 | ↑ cytotoxicity (all tested extracts) | [93] |
| Extract of Umbilicaria cylindrica | |||
| Extract of Umbilicaria polyphylla | |||
| Methanol extract of Lasallia pustulata | FemX, LS174 | ↑ cytotoxicity: FemX (IC50 46.66 μg/mL); LS174 (IC50 71.71 μg/mL) | [94] |
| Extract of Parmelia caperata | FemX, LS174 | ↑ cytotoxicity (all tested extracts) | [95] |
| Extract of Parmelia sulcata | |||
| Extract of Parmelia saxatilis | |||
| Extract of Parmotrema reticulatum | MCF-7, A549, WI-38 | ↑ cytotoxicity | [96] |
| → cell cycle arrest | |||
| Methanol extract of Cetraria islandica | FemX, LS174 | ↑ cytotoxicity: FemX (IC50 22.68 μg/mL); LS174 (IC50 33.74 μg/mL) | [97] |
| Acetone extract of Parmelia arseneana | FemX, LS174, A549, K562 | ↑ cytotoxicity (IC50 11.61–47.06 μg/mL) | [98] |
| Water extracts of Dermatocarpon vellereum, Umbilicaria vellea, Xanthoria elegans and Melanelia disjuncta | HepG2, RKO | ↑ cytotoxicity (all extracts, mainly L. alphoplaca and M. disjuncta) | [99] |
| Methanol extracts of Melanelia disjuncta, Lobothallia alphoplaca and Xanthoparmelia stenophylla | |||
| Methanol extracts of Parmelia sulcata Taylor and Usnea filipendula Stirt | A549, PC-3, Hep3B | ↑ cytotoxicity (IC50 32.9–98.5 μg/mL) | [100] |
| Rat glioma C6 | |||
| → genotoxicity | |||
| → apoptotosis | |||
| Methanol extract of Hypogymnia physodes | MCF-7, MDA-MB-231 | ↑ anticancer and/or apoptosis-inducing (low concentration) effect | [101] |
| ↑ genotoxicity (high concentration) | |||
| Methanol extracts of Cladonia rangiformis and Cladonia convolute | MCF-7 | → apoptosis | [102] |
| ↓ proliferation | |||
| ↑ cytoxicity | |||
| Acetone extract of Xanthoria parietina | MCF-7, MDA-MB-231 | ↓ proliferation | [103] |
| ↓ cell cycle | |||
| ↑ apoptosis | |||
| Metanol extract of Parmelia sulcata | MCF-7, MDA-MB-231 | ↑ cytotoxicity MCF-7 (IC5039.1 μg/mL); MDA-MB-231 (IC50 16.5 μg/mL) | [104] |
| → apoptosis | |||
| Extracts of Xanthoparmelia chlorochroa and Tuckermannopsis ciliaris | Human Burkitt’s lymphoma (Raji) | → apoptosis | [105] |
| → cell arrest | |||
| ↑ p53 expression | |||
| Acetone extracts of | AGS, A549, MDCK | P. gardneri: ↑ cytotoxicity of AGS (IC50 39.1 μg/mL), A549 (IC50 20.24 μg/mL), MDCK (IC50 66.35 μg/mL); Canoparmelia aptata: AGS (IC50 167.9 μg/mL), A549 (IC50 200 μg/mL) | [106] |
| Parmotrema gardneri, Pannaria sp., and Canoparmelia aptata | |||
| Extract of Cladonia rangiformis | MCF-7 | ↑ cytotoxicity | [107] |
| Extract of Cladonia convoluta | |||
| Caloplaca pusilla (on G-LBM medium) | HeLa, MCF-7, PC-3 | ↓ viability of MCF-7 (IC50 7.29 μg/mL), PC-3 (IC50 7.96 μg/mL), HeLa (IC50 6.57 μg/mL) | [108] |
| → apoptosis | |||
| Xanthoria parietina (on PDA and G-LBM) | ↓ cancer cell viability of MCF-7, HeLa (IC50 about 8 μg/mL) | ||
| Methanol extract of Cladonia pocillumon | MCF-7 | → apoptosis (concentration-dependent) | [109] |
| Acetone extract of Pleurosticta acetabulum | HT-29 | ↑ cytotoxicity (IC50 after 48 h, 6 μg/mL) | [110] |
| (cytochalasin E) | ↓ proliferation | ||
| → apoptosis | |||
| Polysaccharide from Umbilicaria esculenta | A875, A375, HUVEC | ↑ cytotoxicity of A875 and A375 | [111] |
| ↑ Annexin-V positive and TUNEL positive A875 | |||
| → apoptosis of A875 (ROS generation followed by ↑ caspase-3 and -9) | |||
| Acetone extracts of Cladonia furcata and Cladonia foliacea | HeLa | Extract of C. foliacea: ↑ cytotoxicity of A549 (IC50 13.58 μg/mL), LS174 (IC50 28.98 μg/mL) | [112] |
| Human lung carcinoma A549 | Extract of C. furcata: ↑ cytotoxicity of HeLa (IC50 11.69 μg/mL) | ||
| Human colon carcinoma LS174 | |||
| Extract of Candelariella vitelline | Caco-2 | ↓ proliferation (Ki-67) | [32] |
| → apoptosis, ↑ necrosis (Caco-2, IC50 125 μg/mL) | |||
| ↓ Bcl-2 | |||
| ↑ Bax, ↑CASP3 protein level | |||
| ↑ Bax/Bcl-2 ratio | |||
| Methanol extract of Usnea intermedia | A549, H1299 MCF7, MDA-MB-231 | ↓ proliferation of H1299 (IC50 10.2 μg/mL) and MDA-MB-231 (IC50 3.0 μg/mL) | [113] |
| → apoptosis (phophatidylserine translocation, ↑ caspase 3/7 activity, loss of mitochondrial membrane potential, formation of pyknotic nuclei) | |||
| Nemania serpens and Nemania aenea var. aureolatum (isolates of endolichenic fungi associated with the lichen Nephroma laevigatum) | HT-29, HCT116, PC-3 and DU145 | ↑ anticancer efficacy (IC50 13–39 μg/mL) | [114] |
| → apoptosis (activated caspase 3, 8, PARP cleavage, chromatin fragmentation) | |||
| Physcia cf. Milegrana | HeLa, Vero | ↑ cytotoxicity of HeLa (IC50 137 μg/mL) | [115] |
| Lichen Metabolites/EXTRACS | Model | Effects | References |
|---|---|---|---|
| UA | Bcap-37 cells inoculated s.c. into C57BL/6 female nude mice; chick embryo chorioallantoic membrane assay; mouse corneal angiogenesis model | ↓ angiogenesis and VEGFR2 mediated ERK1/2 and AKT signaling; ↓ Bcap-37 cells growth; ↓ proliferation, migration, and tube formation and ↑ apoptosis of HUVEC cells | [116] |
| Human breast cancer MCF-7 cells inoculated s.c. into Balb/c nude mouse | ↓ tumor growth in dose dependent manner; any toxic effect in animals | [71] | |
| H22 cells inoculated into male Kunming mice | ↓ toxicity of bleomycin therapy; ↑ efficacy of combined therapy vs bleomycine alone-arrested tumor cells in G0/G1; ↑ caspase-3 and -8; ↓ levels of MDA, hydroxyproline, TNF-α, IL-1β, IL-6 and TGF-β1 and ↑ levels of SOD; ↓ p-Smad2/3; ↑ Smad7 proteins | [36] | |
| UA and its benzylidene analogue | Human breast cancer MDA-MB-231 and MCF-7 cells inoculated into athymic nude mice | ↑ anticancer activity on both xenograft models; ↑ autophagy; ↓ mTOR signaling | [117] |
| UA | Human gastric carcinoma BGC823 cells inoculated s.c. into the flank of female BALB/C nude mice | ↓ tumor volume and weight; ↑ tumor ratio of Bax/Bcl-2 compared to 5-FU | [69] |
| Flavocetraria cucullata extract, UA (F. cucullata), LiA (F. cucullata) | Human lung cancer A549 cells injected s.c. into the flank region of Balb/c nude mouse | tumor free survival number: F. cucullata group ˃ UA group ˃ LiA group. | [87] |
| UA and PU | Mouse colorectal cancer CT26-Fluc cells inoculated by intrasplenic injection of male BALB/c mice | ↓ tumor growth in orthotopic liver metastasis model; ↓ levels of EMT; PU without hepatotoxic effect in liver metastasis model | [34] |
| Ethyl acetate extract of Usnea longissimi | Gastric and esophageal adenocarcinomas of Albino Wistar male rats induced by oral N-methyl-N-nitro-N-nitrosoguanidin administration | ↓ tumor formation; extract concentrations of 50 and 100 mg/kg demonstrated selectivity to cancer tissue and low toxicity profile in animals | [118] |
| ATR | Mouse breast carcinoma 4T1 cells inoculated s.c. into BALB/c mice | ↑ survival time of tumor-bearing animals; ↓ tumor volume; ↑ apoptosis; ↓ oxidative stress in livers of tumor-bearing mice | [45] |
| Mouse Lewis lung carcinoma cells inoculated s.c. into the flanks of C57BL/6 mice | ↓ tumor volume and weight; ↓ Ki-67; ↓ KITENIN, CD44, STAT, and cyclin-D1 | [20] | |
| Extract of Rocella montagnei | Dalton’s lymphoma ascites cells inoculated into Albino Wistar rats and consequent cancer fluid aspiration from rat peritoneal cavity injected into new animals | ↓ tumor volume; effect comparable to Vincristine | [119] |
| Endolichenic fungus EL002332 (Endocarpon pusillum) | Mouse colorectal cancer CT26 cells inoculated s.c. into BALB/c syngeneic mice; TMK1 cells injected into the abdominal cavity of BALB/c mice (intraperitoneal xenografts) | ↓ tumor score and tumor volume in skin and intraperitoneal tumor-bearing animals | [92] |
| Extract of Candelariella vitelline | Ehrlich ascites carcinoma cells were injected i.p. and consequently transferred every 5 days into new female Swiss albino mice | ↓ tumor volume; ↓ tumor cell invasion and mitotic activity; ↑ formation of apoptotic bodies; ↑ ratio of Bax/Bcl-2 on both mRNA and protein levels | [32] |
| Physciosporin (Pseudocyphellaria granulata) | Mouse colorectal cancer cells CT26 implanted s.c. into male BALB/c mice | ↓ tumor volume and weight; without changes in body weight of animals | [22] |
| BA (Cladia aggregate) | Sarcoma-180 cells inoculated in the right axillary region of female albino Swiss mice | ↓ tumor weight; ↑ apoptosis (supposed mechanism) | [91] |
| HA (Pseudoparmelia sphaerospora) | Murine melanoma B16-F10 inoculated s.c. into male BALB/c mice | ↓ tumor volume in both acids; high cancer selectivity and low toxicity in both acids | [79] |
| SA (Parmotrema cetratum) | |||
| DA (Usnea longissima) | Ehrlich ascites carcinoma (EAC) cells inoculated i.p. to Balb/C male mice | anticancer effect on EAC cells; protective activity on different mouse organs | [120] |
| NA (Usnea strigosa) | Human breast cancer MDA-MB-231/GFP cells inoculated into female nude mice | ↓ tumor volume and weight; ↓ c-Met phosphorylation | [33] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solárová, Z.; Liskova, A.; Samec, M.; Kubatka, P.; Büsselberg, D.; Solár, P. Anticancer Potential of Lichens’ Secondary Metabolites. Biomolecules 2020, 10, 87. https://doi.org/10.3390/biom10010087
Solárová Z, Liskova A, Samec M, Kubatka P, Büsselberg D, Solár P. Anticancer Potential of Lichens’ Secondary Metabolites. Biomolecules. 2020; 10(1):87. https://doi.org/10.3390/biom10010087
Chicago/Turabian StyleSolárová, Zuzana, Alena Liskova, Marek Samec, Peter Kubatka, Dietrich Büsselberg, and Peter Solár. 2020. "Anticancer Potential of Lichens’ Secondary Metabolites" Biomolecules 10, no. 1: 87. https://doi.org/10.3390/biom10010087
APA StyleSolárová, Z., Liskova, A., Samec, M., Kubatka, P., Büsselberg, D., & Solár, P. (2020). Anticancer Potential of Lichens’ Secondary Metabolites. Biomolecules, 10(1), 87. https://doi.org/10.3390/biom10010087







