Abstract
Cancer remains one of the most feared and dreaded diseases in this era of modern medicine, claiming the lives of many, and affecting the quality of life of several others around the globe despite major advances in the diagnosis, treatment, palliative care and the immense resources invested into cancer research. While research in cancer has largely focused on the neoplasm/tumor and the cancerous cells that make up the tumor, more recently, the existence, proliferation, differentiation, migration and invasion of cancer stem cells (CSCs) and the role that CSCs play in tumor initiation, progression, metastasis, drug resistance and relapse/recurrence of the disease has gained widespread interest in cancer research. Although the conventional therapeutic approaches such as surgery, chemotherapy and radiation therapy are effective cancer treatments, very often these treatment modalities fail to target the CSCs, which then later become the source of disease recurrence. A majority of the anti-cancer agents target rapidly dividing cancer cells and normal cells and hence, have side effects that are not expected. Targeting CSCs remains a challenge due to their deviant nature with a low proliferation rate and increased drug resistance mechanism. Ascorbic acid/Vitamin C (Vit.C), a potent antioxidant, is a cofactor for several biosynthetic and gene regulatory enzymes and a vital contributor to immune defense of the body, and was found to be deficient in patients with advanced stages of cancer. Vit.C has gained importance in the treatment of cancer due to its ability to modulate the redox status of the cell and influence epigenetic modifications and significant roles in HIF1α signaling. Studies have reported that intravenous administration of Vit.C at pharmacological doses selectively kills tumor cells and targets CSCs when administered along with chemotherapeutic drugs. In the current article, we provide an in-depth review of how Vit.C plays an important role in targeting CSCs and its possible use as an adjuvant, neoadjuvant or co-treatment in the treatment of cancers.
1. Introduction
According to World Health Organization (WHO) global statistics, cancer is the second main cause of death [1]. In 2018, it was estimated that around 9.6 million people worldwide died due to cancer. The most prevalent types of cancer in women include breast, colorectal, lung, cervical and thyroid cancer, while for men it includes lung, prostate, colorectal, stomach and liver cancer. Although great progress has been made in understanding the underlying pathophysiology of cancer, cancer detection and treatment strategies, a proper cure regime has still not yet been revealed [2]. Current treatment regimens result only in limited survival rates for most advanced stage cancers, as these treatments mainly target the tumor load and not cancer stem cells (CSCs) [3,4].CSCs are a smaller population of cells present in tumor loads of different types of cancers [5] like breast cancer, brain tumors, colorectal cancer, prostate cancer, lung cancer, and melanoma [6]. CSCs represent a minor subpopulation of about 0.001–0.1% of the whole tumor mass, but are considered as the key factor for cancer recurrence [6]. They are responsible for cancer reoccurrences as they reside in the tumor load [5]. They constantly adapt their energy metabolism to the surrounding micro-environmental change by expediently shifting their energy source or production from one pathway to another, or by attaining an intermediary metabolic phenotype [7].
Although, it’s known that the usual cancer therapies target the largely fast-growing neoplastic cells, failure in cancer therapies often urges the need to understand the debatable role of CSCs. It’s understood that CSCs exist during cancer progression and metastasis and that they have low proliferation rate and high drug resistance [2,8], which makes them easier to escape the existing cancer treatments. All conventional cancer therapies like hormonal therapy, surgery, immunotherapy and anti-angiogenesis therapy do not succeed in respect of the long-term effect for mainly two reasons. (1) All these treatments fail to target the CSCs, and (2) due to the unpredictable non-targeted toxic effect on the normal cells [2] (Figure 1). Recent studies have shown that intravenous administration of Vitamin C (Vit.C), along with the conventional cancer therapy, is successful in decreasing cancer progression and is a great hope for many cancer patients around the world [9]. This review briefs on the effect of Vit.C on cancer cells and their potential effect on CSCs.
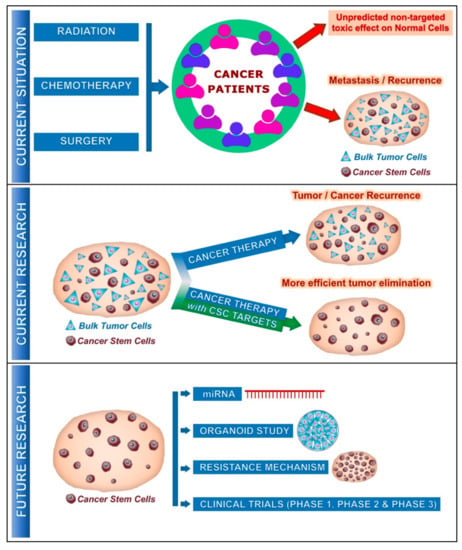
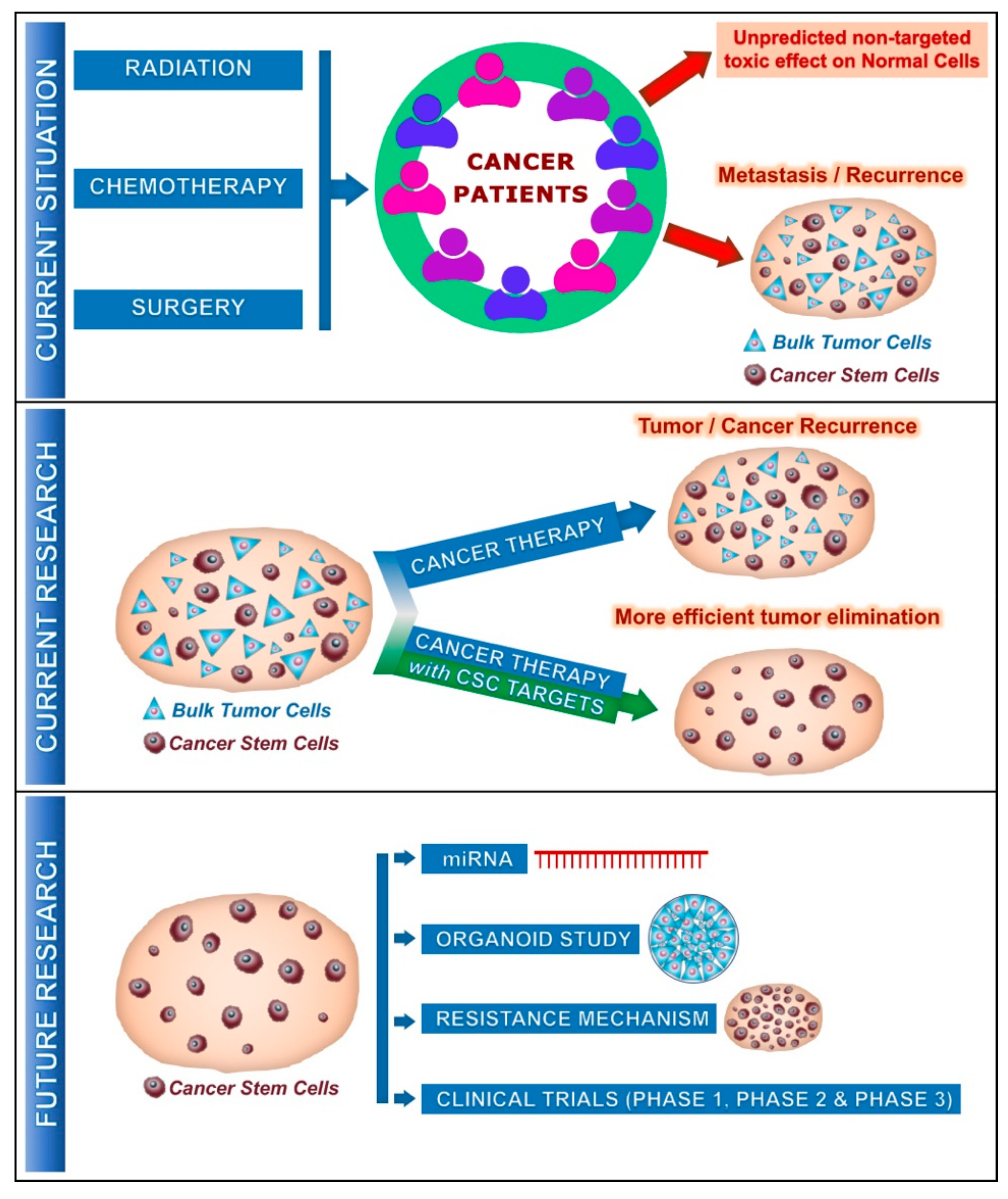
Figure 1.
Current and future involvement of cancer stem cells (CSCs) on cancer treatment: Current situation describes the effect of cancer therapy on unpredicted non-targeted effects on normal cells and metastasis/recurrence of cancer after several years due to the presence of CSCs along with tumor cells. Current research reveals that standard cancer therapy with CSC targets provides much more efficient outcomes on the tumor progression with elimination of CSCs. In the future, further studies could be focused on miRNA (microRNA), cancer organoid, resistance mechanism by CSCs and could enter the clinical phases, promising a better outcome for the cancer patients.
2. Cancer Stem Cell Metabolism
Similar to normal stem cells, CSCs exhibit characteristics like self-renewal, expression of markers, cluster of differentiation (CD), surface markers like CD24, CD133, CD44, CD49, CXCR4 (C-X-C chemokine receptor type 4) and Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) or intracellular markers like aldehyde dehydrogenase (ALDH) [6,10]. Table 1 shows the CSC markers present in various types of cancer. Major signaling pathways activated in CSCs include Wnt, JAK/STAT, Notch, PI3K/AKT and Hedgehog signaling [6,10]. CSCs are known to be in the resting G0 phase of the cell cycle and are in a quiescent state, and hence escape the cancer treatment that mainly targets the highly proliferating cancer cells [6,11]. Hence, cancer therapy must also target these potent small populations of CSCs for better treatment regimes. Identical to cancer cells, CSCs also undergo metabolic alterations. CSCs are managed by bioenergetic signaling pathways such as fatty acid metabolism, glutamine metabolism and the AKT-mTOR pathway. They undergo metabolic alterations in lipid metabolism, glycolytic activity and mitochondrial respiration. In addition, hypoxia—the crucial factor in malignancy, chemo resistance and poor survival rate of cancer patients—leads to the maintenance of an undifferentiated state that affects the proliferation and fate of the normal stem cells [6]. This opens the scope for focusing on CSC metabolism strategy for self-renewal, quiescence and cell division to develop a more robust and effectual cancer therapy with lesser chances of metastasis and recurrence [6,11,12].

Table 1.
Current research status on the effect of Vit.C on various cancer types. It also represents the various cancer stem cells (CSC) markers and miRNA associated with various cancer types.
Studies have also shown that non-coding RNAs like miRNAs (21–25 nucleotide long non-coding RNAs that regulate the gene expression at the post-transcriptional levels) play a role in various cancer types and are involved in the regulation of CSCs, which includes properties such as CSC cell division, tumorigenesis and CSC drug resistance [13,14]. Various types of miRNA that are present in CSCs are briefed in Table 1.
3. Vitamin C/Ascorbic Acid—Metabolism and Chemistry of Vitamin C
Vit.C, also known as ascorbic acid, is a water-soluble vitamin present naturally in many foods. All plants and most animals synthesize Vit.C, whilst for humans and some primates, it needs to be supplemented via diet [76]. A nutritional site (https://www.healthline.com/nutrition/vitamin-c-foods#section21) claims 90 mg to be the daily value of Vit.C required for humans. Some of the most common, rich sources of Vit.C include green chili peppers (242 mg/100 g), guavas (228 mg/100 g), sweet yellow papers (183 mg/100 g), black current (181 mg/100 g), thyme (160 mg/100 g), parsley (133 mg/100 g), kiwis (93 mg/100 g) and lemon (77 mg/100 g).
In 1928, Albert Szent-Gyorgyi was one of the first people who first isolated Vit.C, while in 1932, Szent-Gyorgyi and King discovered its antiscorbutic factor [76,77,78]. Vit.C deficiency could lead to a fatal disease called Scurvy if left untreated, and could only be cured by Vit.C administration [76].
Vit.C is a potent electron donor (reducing agent) and these released electrons play the central role in its physiological effects [76]. As these electrons (two electrons) released from Vit.C can reduce oxidants/oxidized species, they are categorized as an antioxidant, but with a twist. Vit.C released electrons could reduce metals such as iron and copper, and release superoxide and hydrogen peroxide which in turn leads to the production of reactive oxidants. Thus, in certain conditions, the end product of Vit.C action as a reducing agent would produce oxidants [76]. This was observed in both in vitro conditions, when physiological concentration of Vit.C is present along with the metals, and in vivo conditions, when pharmacological concentration (millimolar range) of Vit.C are achieved in extracellular fluids and in plasma [76,79].
Under physiological conditions, Vit.C exists in the state as ascorbate anion and donates two electrons from the double bonds at carbon two and three. Oxidation of ascorbate anion through the loss of the first electron releases the free radical-ascorbate radical/semi-dehydroascorbic acid, and is a reversible process. In animals, Vit.C catabolism products further enter the pentose phosphate pathway or other pathways of carbohydrate metabolism [19], hydrogen peroxide can be formed, while ascorbic acid is found in millimolar concentrations, together with the presence of metal ions.
The putative enzymatic role of Vit.C causes it to act as a cofactor to several enzymatic reactions [80]. It acts as an electron donor in numerous enzymatic reactions such as peptide hormone amidation, collagen hydroxylation (adding hydroxyl groups to proline or lysine residues to strengthen the collagen triple helix) and norepinephrine synthesis. During this enzymatic reaction, ascorbic acid retains the prosthetic metal ions of these enzymes (i.e., ferrous ion (Fe2+) and cupric ion (Cu2+) in their reduced forms [80,81].
4. Transporters of Vit.C
In vivo, Vit.C transport occurs via special transporters called sodium dependent Vit.C transporter (SVCT) 1 and 2 [76,82,83,84,85]. They belong to a nucleobase transporter family and are highly conserved. Studies on the mRNA expression of these transporter proteins revealed the distribution of them in humans and animals [76]. SVCT1 and SVCT2 transport ascorbic acid, but not dehydroascorbic acid (DHA) into the cells. Transportation of DHA to the cells is mainly done by glucose transporters—GLUTs (GLUTs 1–4 and 8) [76,86,87,88,89]. Certain GLUTs show a higher affinity to DHAs over glucose [76]. However, the transporter that transports Vit.C byproducts from cells to the extracellular fluid or plasma is still not understood. Mature red blood cells are the only ascorbate containing cells that lack SVCT transporters and this area needs to be further investigated. Red blood cells attain their ascorbate by the transportation of DHA and are internally immediately reduced. In humans, GLUT1 transports DHA into the red blood cells, while in mice, DHA is transported by GLUT 3 and/or 4 [76,90]. SVCT1 is mainly expressed in absorptive tissues like intestinal epithelium, in kidneys—at the proximal convoluted tubules and the descending part of the loop of Henle. SVCT1 is also present in skin, the liver and the lungs. However, SVCT2 and GLUTs are present in most body tissues, including the brain, pituitary gland, thyroid, heart, adrenals, skeletal muscles, spleen, stomach, pancreas, bladder, ovaries and testis in addition to the tissues where SVCT1 is present.
5. Evidence for Vit.C Effect in Cancer Treatment
In the general population, Vit.C deficiency is a rare condition, however its commonly observed in advanced cancer patients [91,92] This may be due to the insufficient oral intake of Vit.C, lower bioavailability, increased tissue utilization and increased oxidative stress [93].Vit.C is known for its vital role for many elemental biochemical processes, and as a source for reduced iron which is an imperative factor for proper functioning of the epigenetic regulators which in turn instigate the DNA and histones demethylation [91]. Epigenetic modification is a crucial mediator in cancer as it triggers and maintains the malignant phenotype characteristics of cancer [91,94]. Several invitro studies confirmed that physiological concentrations of Vit.C along with a hypomethylation compound would have a synergistic effect directly or indirectly on DNA demethylation [91]. In addition, recent studies confirmed that the intravenous administration of Vit.C at pharmacological doses selectively kills tumor cells [91,93].
In the human body, apart from the individual’s health status, bioavailability of Vit.C is controlled by intestinal and renal absorption, tissue stores and renal excretion. Levels of Vit.C range between 300 mg–2 g in the human body, with 300 mg during conditions such as scurvy. The normal range for ascorbate in human blood plasma is 0.70–1.4 mg/dL, while oral intake of Vit.C generates maximum serum levels of 1.3–4.0 mg/dL (73.8–227.1 μmol/L) whilst the intravenous administration of Vit.C maximizes the levels to 350 mg/dL (19.873 mm mol/L). The unit conversion for Vit.C levels is 1 mg/dL = 56.78 μmol/L [9]. Studies have confirmed that the high plasma concentrations of Vit.C could only be achieved via intravenous administration as the intestinal absorption of Vit.C is limited with oral intake. Vit.C exerts its antioxidant and pro-oxidant activity at low and high concentrations, respectively [95]. Vit.C acts as an electron donor and protects the body from oxidative stress via different routes. It protects plasma lipids from peroxyl radicals and protects the body against aqueous radicals that are present in the blood [95,96]. Vit.C exerts its pro-oxidant properties via intracellular reactive oxygen species (ROS) levels, induction of endoplasmic stress, inhibiting and suppressing the production of the angiogenic factor and insulin-like growth factors, respectively [97].
Recent research trends in cancer therapy have focused on in vitro and in vivo studies where the effect of high-dose Vit.C alone or in combination with radiation, chemotherapy (paclitaxel, cisplatin, carboplatin, azacitidine) or other drugs (doxycycline, azithromycin) is researched. The study details are briefed in Table 1.
6. Vit.C and Its Anticancer Mechanism
Several studies during the past decade confirmed that pharmacological concentrations of Vit.C in the millimolar range are effective in in vitro studies as they kill cancer cells, and in vivo by slowing down the tumor growth [9,98]. However, the mechanism behind the sensitivity of cancer cells to Vit.C and resistance of normal cells to Vit.C is still not clearly understood and needs further investigation. Since Vit.C controls various processes, the activity of Vit.C is also dependent on several different factors like cancer type and signaling pathways included in the tumor development. Ngo et al. proposed three different characteristics in cancer that the pharmacological levels of Vit.C could target to exert its effect on cancer metabolism [99]. These include Vit.C targeting the redox imbalance, epigenetic regulators and HIF1 signaling.
6.1. Redox Imbalance
In cancer cells, due to defective mitochondria and increased metabolic rate, oxidative stress is more in comparison to the normal cells. [99,100]. As known, ROS could increase the imbalance in the genetic make-up that facilitates tumor growth and also increase cell proliferation. Increased levels of ROS could be a danger to the same cancer cells. To escape this effect of ROS, and to compensate the ROS effect, cancer cells increase or improve other signaling pathways [99,101]. Hence, as ROS encourages the development of cancer, antioxidant treatments have to be considered as an anticancer treatment strategy, and more studies are needed to confirm the antioxidant induced cancer suppression. Conversely, a reverse effect of antioxidant therapy has been observed in both in vivo and clinical studies, with an increase in cancer growth in mouse models of melanoma and lung adenocarcinoma, and in lung and prostate cancer patients, respectively. [99,102,103]. Hence, it is assumed that certain types of cancer would be favored by antioxidants and would be susceptible to pro-oxidant therapy. Pro-oxidants induce oxidative stress either by ROS production or by inhibiting the antioxidant systems [99,104], and include therapies like radiation for pro-oxidant anticancer therapy. Despite of all these improvements in cancer treatments, the pro-oxidant therapy has also not been found to be completely effective and could lead to reduced effects of therapeutics [99,105]. Vit.C could potentially evade this issue due to two common characteristic features of cancer cells, which include the increased levels of labile metals like iron [99,106] and increased uptake of glucose and glycolysis (DHA via GLUT1) [99,107]. These two mechanisms could occur simultaneously in cancer cells, thereby inducing a synergistic effect on Vit.C on cancer cells.
6.1.1. Increased Levels of Labile Metals Like Iron
Vit.C induce its pro-oxidant activity in the vicinity of redox-active metals like iron. In cytosol and mitochondria, the labile iron as ferrous iron (Fe2+) (reduced state) (1 µM in humans), reacts with H2O2 (Hydrogen peroxide) releasing hydroxyl radical (OH) by Fenton reaction [99]. Vit.C helps in this reaction by donating electrons to the oxidized state of iron-ferric iron (Fe3+) to generate Fe2+, resulting in ROS production and hence cell death. Iron containing enzymes and haem containing enzymes play a vital role in DNA synthesis, epigenetics, cell cycles and cellular respiration, and hence cancer cells highly depend on labile Fe2+ state iron for their growth and survival. Breast cancer cells have almost twice the storage capacity of intracellular labile Fe2+ state iron than normal breast epithelial cells, and this is also similar in prostate and lymphoma [99,108,109]. Thus it is evident that compared to the normal cells, as cancer cells produce more H2O2 and OH, they become more susceptible to Vit.C [99].
6.1.2. DHA Uptake Instead of Glucose
The Warburg effect that explained the dependency of cancer cells to glucose and glycolysis was described as a fundamental mechanism for the proliferation and survival of cancer cells [99,107,110]. Mutations of proto-oncogenic genes—KRAS (Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, encoding a small GTPase superfamily protein) and BRAF (encoding a protein belonging to the RAF family of serine/threonine protein kinases which plays a key role in regulating the MAP kinase/ERK signaling pathway) also induce the Warburg effect along with GLUT1 up regulation [99,111]. A recent study in KRAS and BRAF mutant colorectal cancer cells, showed the effect of a high dose of Vit.C on exploiting the vulnerability of cancer cells on glucose and glycolysis. Due to the structural similarity of DHA—an oxidized form of Vit.C/ascorbic acid and glucose—they are taken up by KRAS and BRAF mutant cells, and the DHA is then reduced back to ascorbic acid by glutathione (GSH) and nicotinamide adenine dinucleotide phosphate (NADPH) in the intracellular environment, which leads to a decrease in intracellular antioxidants and increased ROS levels. The increased levels of ROS deactivate glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme, and activate poly (ADP-ribose) polymerase (PARP) [99,112], which in turn leads to the reduction of NAD+ (Nicotinamide adenine dinucleotide), a cofactor of GAPDH, and hence inhibits GAPDH further [99,113] (Figure 2). Thus, the inhibition of GAPDH in highly glycolysis dependent KRAS or BRAF mutant cells leads to a state of energy crisis and hence results in cell death, when compared to wild type cells.
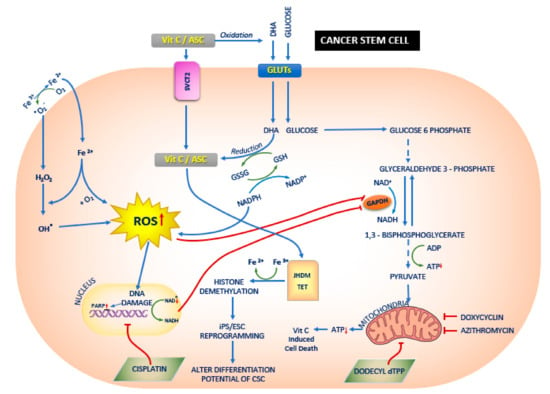
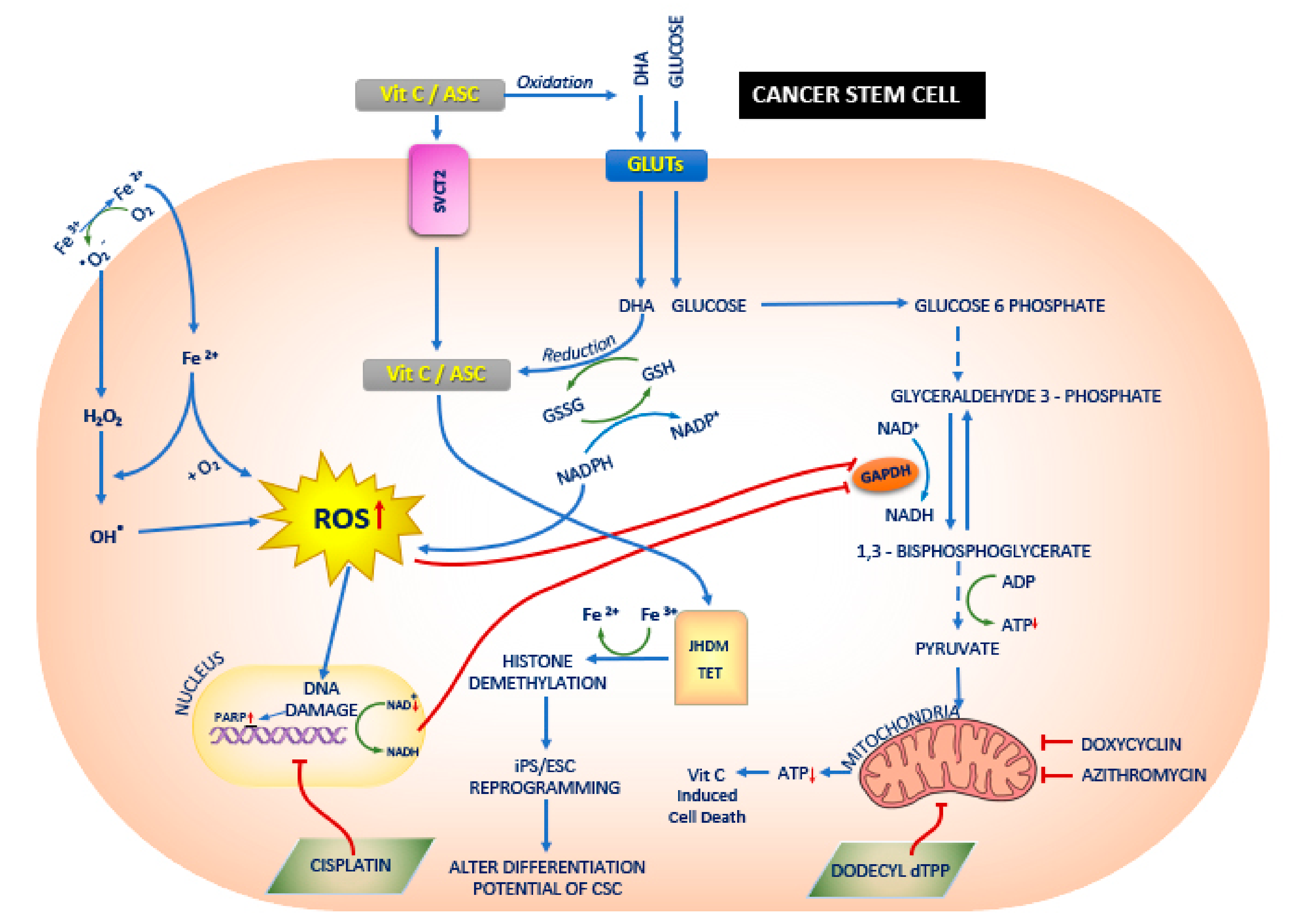
Figure 2.
The effect of Vitamin C (Vit.C) on cancer stem cells (CSCs). Vit.C along with the conventional cancer therapy has a synergistic effect on the treatment of cancers. Vit.C enters the CSC via sodium dependent Vit.C transporter 2 (SVCT2) or glucose transporters GLUTs, and thereby alters jumonji-C domain-containing histone demethylases (JHDM)/ Ten eleven translocation (TET) and reactive oxygen species (ROS) respectively. This leads to mitochondrial dysfunction and also alters the differentiation potential of the CSCs. In CSCs [117], glucose enters the cells via GLUTs [111] and a series of downstream processes occurs: ROS generation is increased via regulation of Glutathione (GSH) and Nicotinamide adenine dinucleotide phosphate (NADPH); DNA damage and increase in poly (ADP-ribose) polymerase (PARP) occurs; both increase in ROS and increase in PARP leads to the inhibition of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [111]. Vit.C enters the CSCs via SVCT2 [98] and increases JHDM and TET [118], leading to histone demethylation [119] and Induced pluripotent stem cells (iPS)/ embryonic stem cells (ESCs) reprogramming [118]. In addition, Ferrous (Fe3+) ion reduces to ferric (Fe2+) ion and enters the CSCs as Fe2+ and hydrogen peroxide (H2O2), thereby increasing the ROS production. Studies have shown the doxycyclin [117,120], azithromycin [20] and dodecyl tri-phenyl-phosphonium (dTPP) [121] inhibit mitochondrial activity which in turn leads to Vit.C induced cell death.
6.2. Ten Eleven Translocation (TET)—Cancer and Effect of Vit.C (Targeting Epigenetic Regulators)
Ten eleven translocation (TET) proteins are 180–230 kDa large multidomain enzymes. All TET proteins are conserved with a cysteine-rich domain, double-stranded β-helix (DSBH) domain and cofactor binding sites for Fe(II) and 2-oxoglutarate (2-OG) which together form the main catalytic domain in the C-terminus [114]. Structural studies confirmed that these catalytic domains specifically bind to cytosines in CG dinucleotides in DNA, known as CpG sites and do not bind to other DNA bases and have almost no specificity to the flanking DNA sequences [114]. The three family members of the TET family—TET1, TET2 and TE3—catalyze the successive hydroxylation of DNA methyl cytosine 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC), 5-formylcytosine (5-fC) and 5-carboxycytosine (5-caC) [115]. These 5-mC oxidation products 5-fC- and 5-caC-modified cytosine are the intermediates that are formed during the conversion of 5-mC to unmodified cytosine, impending for the first step for active DNA demethylation pathway [114,115]. De novo 5′methylation of cytosine is induced by DNA methyltransferase 3A (DNMT3A) and DNMT3B: 5-mC is maintained by methyltransferase DNMT1 [116].
The dysregulation of epigenetics, including DNA methylation, is a characteristic feature of cancer [114,122]. Recent studies have confirmed that in most cancers, DNA methyl cytosine (5-mC) are highly disturbed [122,123,124,125]. Several studies focused on the roles of the epigenetic regulators like DNMTs, TETs and isocitrate dehydrogenase (IDH) enzymes in gene expression, development, cellular development and transformation [122,126] and interestingly TET enzymes were noticed as a vital tumor suppressor mechanism in cancer [114]. TET2 is categorized as one of the most frequently categorized malignancies, and TET2 alteration is considered as an early onset of cancers [114]. The studies show that in various cancer types, mutation of all three TET genes, with reduced expression and impaired activity of the proteins, are present. Thus, it is concluded that defined regulation of DNA methylation patterns are partially regulated by TET enzymes which are vital for the normal development and fundamental protection against cellular transformation [114].
Enzymatic activity of TET enzymes is increased by Vit.C. Several in vitro studies confirmed the effect of Vit.C on increasing the DNA methylation in a TET dependence manner [116,127,128]. The stimulatory effect of Vit.C of TET activity is proposed by two mechanisms. The first mechanism proposes the role of Vit.C as an enzyme cofactor that directly binds to the catalytic domain of the TET enzyme which would increase enzymatic activity of these enzymes along with promoting the TET folding to improve the Fe(II) recycling [129]. In addition, in vitro studies also found that only Vit.C, and no other antioxidants, shows an effect of TET enzyme activity [128,129,130]. A second mechanism proposes the role of Vit.C as a stimulant on the TET enzymatic activity and is associated with the ability of Vit.C to promote reduction of Fe3+ to Fe2+ [119]. This mechanism is supported by similar effects, by compounds like redox-active quinone stimulating TET activity, by reducing iron-free Fe3+ to Fe2+ [119].
In human melanoma cell lines, physiological Vit.C concentration of 100 μM increased the concentration of 5-hmC to comparable levels of normal melanocytes and further reduced the malignant effect of these cells, without interfering the proliferation of these cells [116,131]. These low and physiological Vit.C concentrations of 100–200 μM induce apoptosis in certain melanoma cells via epigenetic downregulation of clusterin, leading to Bax activation and Bcl-XL sequestering in the mitochondria, which in turn result in apoptosis [116,132].
6.3. Hypoxia-Inducible Factor (HIF)—Cancer and Effect of Vit.C
Hypoxic conditions are often observed in solid tumors [116]. Hypoxia-inducible factor (HIF) is a heterodimeric transcription factor that hallmarks many cancers [133]. HIF intervene on several cancer progression processes, like epithelial-mesenchymal transition (EMT), angiogenesis, maintenance of stem cells, invasion and metastasis of cancer cells, resistance of cancer cells to chemotherapy and radiation therapy [116,134].
Under normal oxygen levels, Vit.C down regulates the HIF-1α unit via a Vit.C dependent hydroxylases, while on the other hand, under hypoxic conditions this process is reversed. Under hypoxic conditions, suppression of HIF-1α, hydroxylation is observed which leads to an amplification in HIF dependent gene transcription, neo-angiogenesis, tumor development and progression [116,135]. However, it’s interesting to notice that lower levels of Vit.C advance tumor growth and progression by reducing HIF-1α hydroxylation, thereby stabilizing the HIF-1α levels. Moreover, higher levels of HIF make cancer cells more sensitive to toxicity induced by Vit.C [116,135].
7. Synergetic Effect of Vit.C on Energy Metabolism in Cancer Stem Cells
A possible effect of Vit.C on CSCs propagation has drawn attention due to two mechanism of action of Vit.C in cancer cells [117] (Figure 2). Firstly, due to its potent pro-oxidant role that depletes the glutathione levels causing cellular oxidative stress and apoptosis, and secondly, due to its inhibitory role on glycolysis via targeting GAPDH, the key enzyme in the glycolytic pathway. These two mechanisms only gives us little knowledge in understanding the effect of Vit.C on CSC propagation, and hence, it is essential that new doors are unlocked and further research in this area is undertaken [117]. 3D mammosphere formation of 3 spheroid culture in breast cancer cell line MCF7 confirmed that a glycolysis inhibitor like Vit.C reduces stemness with an IC-50 of 1 mM [117]. This study also showed the NADH auto-fluorescence as a new biomarker for identifying CSCs [117]. Furthermore, the studies demonstrated the synergistic effect of Doxycycline and Vit.C as an effective combination therapy for eradicating CSCs, as Doxycycline inhibits the mitochondrial biogenesis and OXPHOS while Vit.C inhibits the glycolysis pathway via inhibiting GAPDH. Thus a combination therapy has a much greater effect in CSC eradication [120].
A triple combination therapy using lower doses of two clinically approved drugs—Doxycycline and Azithromycin—at a concentration of 1 μM and a dosage of Vit.C of 250 μM, showed a complete eradication of CSC propagation [20] (Figure 2). The synergistic effect of these compounds on the breast cancer cell line MCF7 were induced by the inhibition of two key targets, namely the large (39 s) and small mitochondrial ribosomes (28 s). Although Vit.C generally acts as an antioxidant, due to its relative concentration and cellular localization, they acted as a mild pro-oxidant and stimulated free radical production leading to mitochondrial biogenesis in addition to mitochondrial oxidative stress. However, in this study no Vit.C inhibiting glycolysis via inhibiting glycolytic enzyme GAPDH was observed [20].
A recently published article reemphasized the effect of Vit.C on eradicating CSCs. The effect of the integrated metabolic strategy to exterminate CSCs was validated [121]. Breast cancer cells MCF-7 and MDA- MB-231 were treated with mitochondria—a targeted small non-toxic compound Tri-Phenyl-Phosphonium (TPP) derivative called Dodecyl—TPP (d-TPP) (Figure 2). This TPP derivative is a powerful compound to block the mitochondrial function and reduces the cells viability of CSCs in a dose and time-dependent manner. The study also proves that d-TPP inhibits the formation of 3D mammosphere formation, a read-out for the CSC activity and proliferation. Metabolic flux analysis using Seahorse analyzer confirmed the inhibitory effect of d-TPP on mitochondrial basal respiration and ATP production and exhibited a transition to the glycolytic pathway to complement the effect on mitochondria. However, it was also observed that not only the d-TPP switches the energy pathway in these breast cancer cell lines, but also the sensitivity to other metabolic inhibitors like Vit.C and 2-DG (glycolysis inhibitors) and Doxycycline, Niclosamide, and Berberine (OXPHOS inhibitors). Thus this study conferred a synergistic effect, a “Two-Hit” approach by metabolic inhibitors on the CSC propagation, and has proven that Vit.C is a potential compound to target the glycolysis pathway for this effect on CSCs [121].
In vivo assay confers the effect of Vit.C on hepatocellular carcinoma (HCC) by showing a reduced effect on the self-renewal capacity, survival, CSC associated gene expression on CSC upon Vit.C treatment [98]. They showed the effect of Vit.C on HCC cells via DNA damage and depletion of ATP leading to the activation of cyclin-dependent kinase inhibitor p21 which in turn leads to the G2/M phase cell cycle arrest and caspase-dependent apoptosis. The study also focused on the synergistic effect on killing HCC cells of Vit.C along with cisplatin, a chemotherapeutic drug on both in vitro and in vivo conditions. Despite DNA damage caused by Vit.C induced ROS, cisplatin induced DNA damage via the reaction of platinum molecule at the nucleophilic sites and hence combined effect of both cisplatin and Vit.C had an extended effect on inducing DNA damage in HCC than single effect [98] (Figure 2). Furthermore, it was confirmed that in liver CSCs, SVCT-2 is highly expressed and could be used as a biological marker for the liver CSCs and the effect of Vit.C would be via SVCT-2 [98].
8. Role of Vit.C in Cancer Epigenome Regulation
DNA methylation, covalent addition of methyl group at cytosine within CpG dinucleotides at CpG islands is associated with regulation of gene expression and gene silencing to regulate the genome functioning by histone modification. It is understood that hypermethylation at the promoter regions off certain tumor-suppressor genes leads to its inactivation and gene silencing in different types of cancer [118]. In cancer cells, reinstating the TET function by Vit.C, in combination with certain epigenetic targeted therapies and hypomethylation compounds would help to expunge the epigenetic memory of the cancer cell state. Hence, the epigenome for these cells would resume its normal potential to differentiation and expression of tumor suppression gene [136].
The biological network of cell development is altered by Vit.C by reprogramming epigenome commitment in a reverse effect, by inhibiting senescence and by maintaining the differential potential. Cells epigenome is hypermethylated during normal growth environment with low levels of Vit.C, as the activity of a-ketoglutarate dependent dioxygenases (a-KGDDs) including Jumonji-C domain-containing histone demethylases (JHDMs) and TET proteins are inhibited (Figure 2). With an increase in Vit.C concentration, the enzymatic activity of JHDM and TET is increased, and this in-turn leads to the loss of histone and DNA methylation. This could facilitate the differentiation of embryonic stem cells (ESCs) to a blastocyst-like ESC state, somatic cell reprogramming, differentiation or cancer cell death and guard the adult cells from senescence and aging. Hence, it is concluded that Vit.C manipulates the epigenetic memory and alter the differentiation potential of cancer cells and cancer stem cells.
9. Future Studies on Vit.C on Cancer Stem Cells
Effective combination treatment strategy with Vit.C and chemotherapeutic drug is currently showing a good perspective in treating various cancers and hence the survival time of cancer affected patients could get better. However, this effect needs to be further investigated as only a little is being studied so far (Table 1). Certain miRNAs and their role in CSCs function is understood in several cancers like breast cancer, prostate cancer, osteosarcoma, liver cancer etc. (Table 1) [18,22]. Further research should focus on the effect of Vit.C on miRNAs associated with CSCs. In addition, organoid study on the CSCs isolated from peripheral blood mononuclear cells (PBMCs) [137] could be used to understand the effect of Vit.C on cancer stem cell progression in several cancer types and could give much better knowledge on the same (Figure 1). In addition, research should also focus on the possible resistance mechanism that CSC could develop to escape the Vit.C combination therapy (Figure 1).
10. Conclusions
Relapse of cancer on cancer patients is a challenge in cancer treatment and makes the cancer medical care challenging and greatly expensive. Researchers observed that conventional therapies fail in cancer treatment when they do not target CSC. Moreover, normal cells show toxic effects. Administration of Vit.C along with other chemotherapeutic drug proves to be an additional and effective treatment mechanism. Vit.C induces its effect via interfering the energy metabolism or cancer epigenome regulation in cancer stem cells. Several studies confirmed that combination therapy of Vit.C along with conventional therapy has a greater impact on cancer growth or progression and should be considered as a future treatment strategy. Further studies on their effect of miRNAs, organoid cultures and possible resistance mechanism will help to better understand the treatment strategies of combination therapy using Vit.C for eradiating cancer stem cell progression in various types of cancers. This will not only save money, but will also reduce the suffering of cancer patients and their families.
Author Contributions
The authors contributed as follows: Conceptualization, N.J.S. and D.B.; literature review and resources, N.J.S.; writing—original draft preparation, N.J.S.; writing—review and editing, N.J.S.; S.M.S. and D.B.; figure preparation and editing, N.J.S.; visualization, N.J.S. and D.B.; supervision, D.B.; project administration, D.B.; funding acquisition, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Priorities Research Program grant (NPRP 11S-1214-170101; awarded to Dietrich Büsselberg, June 2019—current) from the Qatar National Research Fund (QNRF, a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors.
Acknowledgments
We acknowledge Faizal Sherif for the preparation of the figures.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the article.
Abbreviations
| OH | Hydroxyl radical |
| α-KGDDs | alpha-ketoglutarate dependent dioxygenases |
| 5-caC | 5-carboxycytosine |
| 5-fC | 5-formylcytosine |
| 5-hmC | 5-hydroxymethylcytosine |
| 5-mC | 5-methylcytosine |
| AKT | Protein kinase B |
| ALDH | Aldehyde dehydrogenase |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| CD | Cluster of differentiation |
| CSCs | Cancer stem cells |
| Cu2+ | Cupric ion |
| CXCR4 | C-X-C chemokine receptor type 4 |
| d-TPP | Dodecyl—tri-phenyl-phosphonium |
| DHA | Dehydroascorbic acid |
| DNMT | DNA methyltransferase |
| EMT | Epithelial-mesenchymal transition |
| ESCs | Embryonic stem cells |
| Fe2+ | Ferrous ion |
| GAPDH | Glyceraldehyde-phosphate dehydrogenase |
| GLUT | Glucose transporter |
| H2O2 | Hydrogen peroxide |
| HCC | Hepatocellular carcinoma |
| HIF1α | Hypoxia-inducible factor 1 alpha |
| IC-50 | Inhibitory concentration |
| IDH | Isocitrate dehydrogenase |
| iPS | Induced pluripotent stem cells |
| JAK | Janus kinase |
| JHDM | Jumonji-C domain-containing histone demethylases |
| KRAS | KRAS proto-oncogene, GTPase |
| LGR5 | Leucine-rich repeat-containing G-protein coupled receptor 5 |
| miRNA | MicroRNA |
| mTOR | Mammalian target of rapamycin |
| NAD+ | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| PARP | Poly (ADP-ribose) polymerase |
| PBMC | Peripheral blood mononuclear cells |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| ROS | Reactive oxygen species |
| SCVT1/2 | Sodium dependent Vit.C transporter 1/2 |
| STAT | Signal transducer and activator of transcription |
| TET | Ten eleven translocation |
| TPP | Tri-phenyl-phosphonium |
| Vit.C | Vitamin C |
References
- Ghosh, D.; Venkataramani, P.; Nandi, S.; Bhattacharjee, S. CRISPR-Cas9 a boon or bane: The bumpy road ahead to cancer therapeutics. Cancer Cell Int. 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Fu, L. Targeting cancer stem cells: A new therapy to cure cancer patients. Am. J. Cancer Res. 2012, 2, 340–356. [Google Scholar] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.; Amiji, M.M. Cancer stem cell-targeted therapeutics and delivery strategies. Expert Opin. Drug Deliv. 2017, 14, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.C.; Kim, J.H. Cancer stem cell metabolism: Target for cancer therapy. BMB Rep. 2018, 51, 319–326. [Google Scholar] [CrossRef]
- Peiris-Pages, M.; Martinez-Outschoorn, U.E.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer stem cell metabolism. Breast Cancer Res. 2016, 18, 55. [Google Scholar] [CrossRef]
- Jones, R.J.; Matsui, W.H.; Smith, B.D. Cancer stem cells: Are we missing the target? J. Natl. Cancer Inst. 2004, 96, 583–585. [Google Scholar] [CrossRef]
- Carr, A.C.; Cook, J. Intravenous Vitamin C for Cancer Therapy—Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Busselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef]
- Rafalski, V.A.; Mancini, E.; Brunet, A. Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate. J. Cell Sci. 2012, 125, 5597–5608. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.R.; Junejo, K.; Steinhoff, M.; Uddin, S. Role of miRNA-Regulated Cancer Stem Cells in the Pathogenesis of Human Malignancies. Cells 2019, 8, 840. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.U.; Miyazaki, H.; Ochiya, T. The role of microRNAs in the regulation of cancer stem cells. Front. Genet. 2014, 4, 295. [Google Scholar] [CrossRef]
- Nechuta, S.; Lu, W.; Chen, Z.; Zheng, Y.; Gu, K.; Cai, H.; Zheng, W.; Shu, X.O. Vitamin supplement use during breast cancer treatment and survival: A prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 262–271. [Google Scholar] [CrossRef]
- Hao, J.; Zhao, S.; Zhang, Y.; Zhao, Z.; Ye, R.; Wen, J.; Li, J. Emerging role of microRNAs in cancer and cancer stem cells. J. Cell. Biochem. 2014, 115, 605–610. [Google Scholar] [CrossRef]
- Croker, A.K.; Goodale, D.; Chu, J.; Postenka, C.; Hedley, B.D.; Hess, D.A.; Allan, A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 2009, 13, 2236–2252. [Google Scholar] [CrossRef]
- Garg, M. Emerging role of microRNAs in cancer stem cells: Implications in cancer therapy. World J. Stem Cells 2015, 7, 1078–1089. [Google Scholar] [CrossRef]
- Hwang-Verslues, W.W.; Chang, P.H.; Wei, P.C.; Yang, C.Y.; Huang, C.K.; Kuo, W.H.; Shew, J.Y.; Chang, K.J.; Lee, E.Y.; Lee, W.H. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene 2011, 30, 2463–2474. [Google Scholar] [CrossRef]
- Fiorillo, M.; Toth, F.; Sotgia, F.; Lisanti, M.P. Doxycycline, Azithromycin and Vitamin C (DAV): A potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs). Aging 2019, 11, 2202–2216. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, Z.; Mansoori, B.; Mohammadi, A.; Aghajani, M.; Haji-Asgarzadeh, K.; Safarzadeh, E.; Mokhtarzadeh, A.; Duijf, P.H.G.; Baradaran, B. microRNAs in cancer stem cells: Biology, pathways, and therapeutic opportunities. J. Cell. Physiol. 2019, 234, 10002–10017. [Google Scholar] [CrossRef] [PubMed]
- Okuda, H.; Xing, F.; Pandey, P.R.; Sharma, S.; Watabe, M.; Pai, S.K.; Mo, Y.Y.; Iiizumi-Gairani, M.; Hirota, S.; Liu, Y.; et al. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013, 73, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xiao, G.G.; Mao, J.; Lu, Y.; Song, B.; Wang, L.; Fan, S.; Fan, P.; Hou, Z.; Li, J.; et al. Dysregulation of the miR-34a-SIRT1 axis inhibits breast cancer stemness. Oncotarget 2015, 6, 10432–10444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Y.; Tsuyada, A.; Ren, X.; Wu, X.; Stubblefield, K.; Rankin-Gee, E.K.; Wang, S.E. Transforming growth factor-beta regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene 2011, 30, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Ph 2 Trial of Vitamin C & G-FLIP (Low Doses Gemcitabine, 5FU, Leucovorin, Irinotecan, Oxaliplatin) for Pancreatic Cancer). Available online: https://clinicaltrials.gov/ct2/show/NCT01905150?term=vitamin+c&recrs=ade&cond=Pancreatic+Cancer&cntry=US&rank=1 (accessed on 2 October 2019).
- Lechner, A.; Leech, C.A.; Abraham, E.J.; Nolan, A.L.; Habener, J.F. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem. Biophys. Res. Commun. 2002, 293, 670–674. [Google Scholar] [CrossRef]
- Hasegawa, S.; Eguchi, H.; Nagano, H.; Konno, M.; Tomimaru, Y.; Wada, H.; Hama, N.; Kawamoto, K.; Kobayashi, S.; Nishida, N.; et al. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br. J. Cancer 2014, 111, 1572–1580. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Trial of Ascorbic Acid (AA) + Nanoparticle Paclitaxel Protein Bound + Cisplatin + Gemcitabine (AA NABPLAGEM) (AA NABPLAGEM). Available online: https://clinicaltrials.gov/ct2/show/NCT03410030?term=vitamin+c&recrs=ade&cond=Pancreatic+Cancer&cntry=US&phase=123&rank=2 (accessed on 2 October 2019).
- Tomuleasa, C.; Mosteanu, O.; Susman, S.; Cristea, V. ALDH as a tumor marker for pancreatic cancer. J. Gastrointest. Liver Dis. 2011, 20, 443–444, author reply 444. [Google Scholar]
- Bao, B.; Ali, S.; Ahmad, A.; Azmi, A.S.; Li, Y.; Banerjee, S.; Kong, D.; Sethi, S.; Aboukameel, A.; Padhye, S.B.; et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS ONE 2012, 7, e50165. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. High Dose Vitamin C Intravenous Infusion in Patients with Resectable or Metastatic Solid Tumor Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT03146962?term=vitamin+c&recrs=ade&cond=Pancreatic+Cancer&cntry=US&phase=123&rank=3 (accessed on 2 October 2019).
- Li, C.; Heidt, D.G.; Dalerba, P.; Burant, C.F.; Zhang, L.; Adsay, V.; Wicha, M.; Clarke, M.F.; Simeone, D.M. Identification of pancreatic cancer stem cells. Cancer Res. 2007, 67, 1030–1037. [Google Scholar] [CrossRef]
- Sureban, S.M.; May, R.; Qu, D.; Weygant, N.; Chandrakesan, P.; Ali, N.; Lightfoot, S.A.; Pantazis, P.; Rao, C.V.; Postier, R.G.; et al. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS ONE 2013, 8, e73940. [Google Scholar] [CrossRef]
- Immervoll, H.; Hoem, D.; Sakariassen, P.O.; Steffensen, O.J.; Molven, A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer 2008, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, J.; Li, X.; Zhu, H.; Fan, X.; Zhu, S.; Wang, Y.; Guo, Q.; Wang, L.; Huang, Y.; et al. MiR-200a inhibits epithelial-mesenchymal transition of pancreatic cancer stem cell. BMC Cancer 2014, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, D.; Campbell, N.R.; Karikari, C.; Chivukula, R.; Kent, O.A.; Mendell, J.T.; Maitra, A. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol. Cancer Ther. 2011, 10, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Corney, D.C.; Flesken-Nikitin, A.; Godwin, A.K.; Wang, W.; Nikitin, A.Y. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007, 67, 8433–8438. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Treatment of Newly Diagnosed Ovarian Cancer with Antioxidants. Available online: https://clinicaltrials.gov/ct2/show/NCT00228319?term=vitamin+c&recrs=ade&cond=Ovarian+cancer&cntry=US&phase=123&rank=1 (accessed on 2 October 2019).
- Dou, J.; Jiang, C.; Wang, J.; Zhang, X.; Zhao, F.; Hu, W.; He, X.; Li, X.; Zou, D.; Gu, N. Using ABCG2-molecule-expressing side population cells to identify cancer stem-like cells in a human ovarian cell line. Cell Biol. Int. 2011, 35, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.M.; Shaw, P.A.; Gedye, C.; Bernardini, M.Q.; Neel, B.G.; Ailles, L.E. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2011, 108, 6468–6473. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Phase 2 Trial of High-Dose Ascorbate in Glioblastoma Multiforme. Available online: https://clinicaltrials.gov/ct2/show/NCT02344355?term=vitamin+c&recrs=ade&cond=Glioblastoma&cntry=US&phase=123&rank=1 (accessed on 2 October 2019).
- Cui, S.Y.; Wang, R.; Chen, L.B. MicroRNA-145: A potent tumour suppressor that regulates multiple cellular pathways. J. Cell. Mol. Med. 2014, 18, 1913–1926. [Google Scholar] [CrossRef]
- Shang, C.; Guo, Y.; Hong, Y.; Liu, Y.H.; Xue, Y.X. MiR-21 up-regulation mediates glioblastoma cancer stem cells apoptosis and proliferation by targeting FASLG. Mol. Biol. Rep. 2015, 42, 721–727. [Google Scholar] [CrossRef]
- Turchi, L.; Debruyne, D.N.; Almairac, F.; Virolle, V.; Fareh, M.; Neirijnck, Y.; Burel-Vandenbos, F.; Paquis, P.; Junier, M.P.; Van Obberghen-Schilling, E.; et al. Tumorigenic potential of miR-18A* in glioma initiating cells requires NOTCH-1 signaling. Stem Cells 2013, 31, 1252–1265. [Google Scholar] [CrossRef]
- Ying, Z.; Li, Y.; Wu, J.; Zhu, X.; Yang, Y.; Tian, H.; Li, W.; Hu, B.; Cheng, S.Y.; Li, M. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res. 2013, 73, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Nowicki, M.O.; Bronisz, A.; Williams, S.; Otsuki, A.; Nuovo, G.; Raychaudhury, A.; Newton, H.B.; Chiocca, E.A.; Lawler, S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008, 68, 9125–9130. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Luo, H.; Pu, Y.; Zhou, Z.; Wu, X.; Xu, W.; Yang, Z. Methylation mediated silencing of miR-23b expression and its role in glioma stem cells. Neurosci. Lett. 2012, 528, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Summer, R.; Kotton, D.N.; Sun, X.; Ma, B.; Fitzsimmons, K.; Fine, A. Side population cells and Bcrp1 expression in lung. Am. J. Physiol Lung Cell. Mol. Physiol. 2003, 285, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Qiu, M.; Jiang, F.; Zhang, S.; Yang, X.; Wang, J.; Xu, L.; Yin, R. MiR-145 regulates cancer stem-like properties and epithelial-to-mesenchymal transition in lung adenocarcinoma-initiating cells. Tumour Biol. 2014, 35, 8953–8961. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Pharmacological Ascorbate for Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02420314?term=vitamin+c&recrs=ade&cond=lung+cancer&cntry=US&phase=123&rank=1 (accessed on 2 October 2019).
- Jiang, F.; Qiu, Q.; Khanna, A.; Todd, N.W.; Deepak, J.; Xing, L.; Wang, H.; Liu, Z.; Su, Y.; Stass, S.A.; et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 2009, 7, 330–338. [Google Scholar] [CrossRef]
- Xu, W.; Ji, J.; Xu, Y.; Liu, Y.; Shi, L.; Liu, Y.; Lu, X.; Zhao, Y.; Luo, F.; Wang, B.; et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol. Carcinog. 2015, 54, 148–161. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Phase 2 Study Adding Ascorbate to Chemotherapy and Radiation Therapy for NSCLC (XACT-LUNG). Available online: https://clinicaltrials.gov/ct2/show/NCT02905591?term=vitamin+c&recrs=ade&cond=lung+cancer&cntry=US&phase=123&rank=2 (accessed on 2 October 2019).
- Xi, S.; Xu, H.; Shan, J.; Tao, Y.; Hong, J.A.; Inchauste, S.; Zhang, M.; Kunst, T.F.; Mercedes, L.; Schrump, D.S. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J. Clin. Investig. 2013, 123, 1241–1261. [Google Scholar] [CrossRef]
- Bertolini, G.; Roz, L.; Perego, P.; Tortoreto, M.; Fontanella, E.; Gatti, L.; Pratesi, G.; Fabbri, A.; Andriani, F.; Tinelli, S.; et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 16281–16286. [Google Scholar] [CrossRef] [PubMed]
- King, C.E.; Cuatrecasas, M.; Castells, A.; Sepulveda, A.R.; Lee, J.S.; Rustgi, A.K. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011, 71, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.H.; Hynes, M.J.; Zhang, T.; Ginestier, C.; Dontu, G.; Appelman, H.; Fields, J.Z.; Wicha, M.S.; Boman, B.M. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009, 69, 3382–3389. [Google Scholar] [CrossRef] [PubMed]
- Dalerba, P.; Dylla, S.J.; Park, I.K.; Liu, R.; Wang, X.; Cho, R.W.; Hoey, T.; Gurney, A.; Huang, E.H.; Simeone, D.M.; et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Jaksch, M.; Munera, J.; Bajpai, R.; Terskikh, A.; Oshima, R.G. Cell cycle-dependent variation of a CD133 epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res. 2008, 68, 7882–7886. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. TET2 Mutations in Myelodysplastic Syndromes and Acute Myeloid Leukemia with Azacitidine + Ascorbic Acid. Available online: https://clinicaltrials.gov/ct2/show/NCT03397173?term=vitamin+c&recrs=ade&cond=Leukemia&cntry=US&phase=123&rank=1 (accessed on 2 October 2019).
- Scheibner, K.A.; Teaboldt, B.; Hauer, M.C.; Chen, X.; Cherukuri, S.; Guo, Y.; Kelley, S.M.; Liu, Z.; Baer, M.R.; Heimfeld, S.; et al. MiR-27a functions as a tumor suppressor in acute leukemia by regulating 14-3-3theta. PLoS ONE 2012, 7, e50895. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Therapeutic Use of Intravenous Vitamin C in Allogeneic Stem Cell Transplant Recipients. Available online: https://clinicaltrials.gov/ct2/show/NCT03613727?term=vitamin+c&recrs=ade&cond=Leukemia&cntry=US&phase=123&rank=2 (accessed on 2 October 2019).
- ClinicalTrials.gov. Ascorbic Acid and Combination Chemotherapy in Treating Patients with Relapsed or Refractory Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03418038?term=vitamin+c&recrs=ade&cond=Lymphoma&cntry=US&phase=123&rank=1 (accessed on 2 October 2019).
- Ma, S.; Tang, K.H.; Chan, Y.P.; Lee, T.K.; Kwan, P.S.; Castilho, A.; Ng, I.; Man, K.; Wong, N.; To, K.F.; et al. miR-130b Promotes CD133+ liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell 2010, 7, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.C.; Park, C.Y.; Bhagat, G.; Zhang, J.; Wang, Y.; Fan, J.B.; Liu, M.; Zou, Y.; Weissman, I.L.; Gu, H. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J. Exp. Med. 2010, 207, 475–489. [Google Scholar] [CrossRef]
- Babashah, S.; Sadeghizadeh, M.; Hajifathali, A.; Tavirani, M.R.; Zomorod, M.S.; Ghadiani, M.; Soleimani, M. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int. J. Cancer 2013, 133, 579–589. [Google Scholar] [CrossRef]
- Morris, V.A.; Zhang, A.; Yang, T.; Stirewalt, D.L.; Ramamurthy, R.; Meshinchi, S.; Oehler, V.G. MicroRNA-150 expression induces myeloid differentiation of human acute leukemia cells and normal hematopoietic progenitors. PLoS ONE 2013, 8, e75815. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Docetaxel with or without Ascorbic Acid in Treating Patients with Metastatic Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02516670?term=vitamin+c&recrs=ade&cond=Prostate+cancer&cntry=US&phase=123&rank=1 (accessed on 2 October 2019).
- Hellsten, R.; Johansson, M.; Dahlman, A.; Sterner, O.; Bjartell, A. Galiellalactone inhibits stem cell-like ALDH-positive prostate cancer cells. PLoS ONE 2011, 6, e22118. [Google Scholar] [CrossRef]
- Chang, Y.L.; Zhou, P.J.; Wei, L.; Li, W.; Ji, Z.; Fang, Y.X.; Gao, W.Q. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget 2015, 6, 24017–24031. [Google Scholar] [CrossRef]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kelnar, K.; Liu, B.; Chen, X.; Calhoun-Davis, T.; Li, H.; Patrawala, L.; Yan, H.; Jeter, C.; Honorio, S.; et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011, 17, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Vander Griend, D.J.; Karthaus, W.L.; Dalrymple, S.; Meeker, A.; DeMarzo, A.M.; Isaacs, J.T. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008, 68, 9703–9711. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, K.; Okada, M. Retarded nuclear migration in Drosophila embryos with aberrant F-actin reorganization caused by maternal mutations and by cytochalasin treatment. Development 1991, 111, 909–920. [Google Scholar]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Svirbely, J.L.; Szent-Gyorgyi, A. The chemical nature of vitamin C. Biochem. J. 1932, 26, 865–870. [Google Scholar] [CrossRef]
- King, C.G.; Waugh, W.A. The Chemical Nature of Vitamin C. Science 1932, 75, 357–358. [Google Scholar] [CrossRef]
- Parrow, N.L.; Leshin, J.A.; Levine, M. Parenteral ascorbate as a cancer therapeutic: A reassessment based on pharmacokinetics. Antioxid. Redox Signal. 2013, 19, 2141–2156. [Google Scholar] [CrossRef]
- Burzle, M.; Hediger, M.A. Functional and physiological role of vitamin C transporters. Curr. Top. Membr. 2012, 70, 357–375. [Google Scholar] [CrossRef]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dutta, B.; Huang, W.; Devoe, L.D.; Leibach, F.H.; Ganapathy, V.; Prasad, P.D. Human Na+-dependent vitamin C transporter 1 (hSVCT1): Primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim. Biophys. Acta Biomembr. 1999, 1461, 1–9. [Google Scholar] [CrossRef]
- Daruwala, R.; Song, J.; Koh, W.S.; Rumsey, S.C.; Levine, M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999, 460, 480–484. [Google Scholar] [CrossRef]
- Wang, Y.; Mackenzie, B.; Tsukaguchi, H.; Weremowicz, S.; Morton, C.C.; Hediger, M.A. Human vitamin C (L-ascorbic acid) transporter SVCT1. Biochem. Biophys. Res. Commun. 2000, 267, 488–494. [Google Scholar] [CrossRef]
- Corpe, C.P.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101. [Google Scholar] [CrossRef]
- Rumsey, S.C.; Kwon, O.; Xu, G.W.; Burant, C.F.; Simpson, I.; Levine, M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997, 272, 18982–18989. [Google Scholar] [CrossRef]
- Rumsey, S.C.; Daruwala, R.; Al-Hasani, H.; Zarnowski, M.J.; Simpson, I.A.; Levine, M. Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. J. Biol. Chem. 2000, 275, 28246–28253. [Google Scholar] [CrossRef]
- Vera, J.C.; Rivas, C.I.; Fischbarg, J.; Golde, D.W. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 1993, 364, 79–82. [Google Scholar] [CrossRef]
- Tu, H.; Li, H.; Wang, Y.; Niyyati, M.; Wang, Y.; Leshin, J.; Levine, M. Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascorbic Acid. EBioMedicine 2015, 2, 1735–1750. [Google Scholar] [CrossRef]
- Gillberg, L.; Orskov, A.D.; Liu, M.; Harslof, L.B.S.; Jones, P.A.; Gronbaek, K. Vitamin C—A new player in regulation of the cancer epigenome. Semin. Cancer Biol. 2018, 51, 59–67. [Google Scholar] [CrossRef]
- Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin C deficiency in cancer patients. Palliat. Med. 2005, 19, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Klimant, E.; Wright, H.; Rubin, D.; Seely, D.; Markman, M. Intravenous vitamin C in the supportive care of cancer patients: A review and rational approach. Curr. Oncol. 2018, 25, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kagohara, L.T.; Stein-O’Brien, G.L.; Kelley, D.; Flam, E.; Wick, H.C.; Danilova, L.V.; Easwaran, H.; Favorov, A.V.; Qian, J.; Gaykalova, D.A.; et al. Epigenetic regulation of gene expression in cancer: Techniques, resources and analysis. Brief. Funct. Genom. 2018, 17, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Wilson, M.K.; Baguley, B.C.; Wall, C.; Jameson, M.B.; Findlay, M.P. Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac. J. Clin. Oncol. 2014, 10, 22–37. [Google Scholar] [CrossRef]
- Lee, W.J. The prospects of vitamin C in cancer therapy. Immune Netw. 2009, 9, 147–152. [Google Scholar] [CrossRef]
- Lv, H.; Wang, C.; Fang, T.; Li, T.; Lv, G.; Han, Q.; Yang, W.; Wang, H. Vitamin C preferentially kills cancer stem cells in hepatocellular carcinoma via SVCT-2. NPJ Precis. Oncol. 2018, 2, 1. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Tuveson, D.A. ROS in Cancer: The Burning Question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Wondrak, G.T. Redox-directed cancer therapeutics: Molecular mechanisms and opportunities. Antioxid. Redox Signal. 2009, 11, 3013–3069. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Rychtarcikova, Z.; Lettlova, S.; Tomkova, V.; Korenkova, V.; Langerova, L.; Simonova, E.; Zjablovskaja, P.; Alberich-Jorda, M.; Neuzil, J.; Truksa, J. Tumor-initiating cells of breast and prostate origin show alterations in the expression of genes related to iron metabolism. Oncotarget 2017, 8, 6376–6398. [Google Scholar] [CrossRef]
- Kiessling, M.K.; Klemke, C.D.; Kaminski, M.M.; Galani, I.E.; Krammer, P.H.; Gulow, K. Inhibition of constitutively activated nuclear factor-kappaB induces reactive oxygen species- and iron-dependent cell death in cutaneous T-cell lymphoma. Cancer Res. 2009, 69, 2365–2374. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. Correction to: ‘The Warburg Effect: How Does it Benefit Cancer Cells?’: [Trends in Biochemical Sciences, 41 (2016) 211]. Trends Biochem. Sci. 2016, 41, 287. [Google Scholar] [CrossRef]
- Yun, J.; Rago, C.; Cheong, I.; Pagliarini, R.; Angenendt, P.; Rajagopalan, H.; Schmidt, K.; Willson, J.K.; Markowitz, S.; Zhou, S.; et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 2009, 325, 1555–1559. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Nandi, S. Rare Genetic Diseases with Defects in DNA Repair: Opportunities and Challenges in Orphan Drug Development for Targeted Cancer Therapy. Cancers 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Nandi, S. Synthetic lethality in DNA repair network: A novel avenue in targeted cancer therapy and combination therapeutics. IUBMB Life 2017, 69, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Melamed, P.; Yosefzon, Y.; David, C.; Tsukerman, A.; Pnueli, L. Tet Enzymes, Variants, and Differential Effects on Function. Front. Cell Dev. Biol. 2018, 6, 22. [Google Scholar] [CrossRef]
- Mastrangelo, D.; Pelosi, E.; Castelli, G.; Lo-Coco, F.; Testa, U. Mechanisms of anti-cancer effects of ascorbate: Cytotoxic activity and epigenetic modulation. Blood Cells Mol. Dis. 2018, 69, 57–64. [Google Scholar] [CrossRef]
- Bonuccelli, G.; De Francesco, E.M.; de Boer, R.; Tanowitz, H.B.; Lisanti, M.P. NADH autofluorescence, a new metabolic biomarker for cancer stem cells: Identification of Vitamin C and CAPE as natural products targeting “stemness”. Oncotarget 2017, 8, 20667–20678. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef]
- Hore, T.A.; von Meyenn, F.; Ravichandran, M.; Bachman, M.; Ficz, G.; Oxley, D.; Santos, F.; Balasubramanian, S.; Jurkowski, T.P.; Reik, W. Retinol and ascorbate drive erasure of epigenetic memory and enhance reprogramming to naive pluripotency by complementary mechanisms. Proc. Natl. Acad. Sci. USA 2016, 113, 12202–12207. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Bonuccelli, G.; Maggiolini, M.; Sotgia, F.; Lisanti, M.P. Vitamin C and Doxycycline: A synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs). Oncotarget 2017, 8, 67269–67286. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Ozsvari, B.; Sotgia, F.; Lisanti, M.P. Dodecyl-TPP Targets Mitochondria and Potently Eradicates Cancer Stem Cells (CSCs): Synergy With FDA-Approved Drugs and Natural Compounds (Vitamin C and Berberine). Front. Oncol. 2019, 9, 615. [Google Scholar] [CrossRef]
- An, J.; Rao, A.; Ko, M. TET family dioxygenases and DNA demethylation in stem cells and cancers. Exp. Mol. Med. 2017, 49, e323. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, L.; Abdel-Wahab, O.; Levine, R.L.; Aifantis, I. TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell 2011, 9, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rao, A. Connections between TET proteins and aberrant DNA modification in cancer. Trends Genet. 2014, 30, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; An, J.; Pastor, W.A.; Koralov, S.B.; Rajewsky, K.; Rao, A. TET proteins and 5-methylcytosine oxidation in hematological cancers. Immunol. Rev. 2015, 263, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Guillamot, M.; Cimmino, L.; Aifantis, I. The Impact of DNA Methylation in Hematopoietic Malignancies. Trends Cancer 2016, 2, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.L.; Brena, R.M.; Kolle, G.; Grimmond, S.M.; Berman, B.P.; Laird, P.W.; Pera, M.F.; Wolvetang, E.J. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells 2010, 28, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Yin, R.; Mao, S.Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef]
- Gustafson, C.B.; Yang, C.; Dickson, K.M.; Shao, H.; Van Booven, D.; Harbour, J.W.; Liu, Z.J.; Wang, G. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin. Epigenet. 2015, 7, 51. [Google Scholar] [CrossRef]
- Mustafi, S.; Sant, D.W.; Liu, Z.J.; Wang, G. Ascorbate induces apoptosis in melanoma cells by suppressing Clusterin expression. Sci. Rep. 2017, 7, 3671. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H.J.; Raval, R.R.; Harris, A.L.; Ratcliffe, P.J. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003, 63, 1764–1768. [Google Scholar] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Vissers, M.C. Ascorbate as a co-factor for Fe- and 2-Oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014, 4, 359. [Google Scholar] [CrossRef]
- Lee Chong, T.; Ahearn, E.L.; Cimmino, L. Reprogramming the Epigenome With Vitamin C. Front. Cell Dev. Biol. 2019, 7, 128. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.C.; Liu, L.P.; Zhang, H.M.; Tong, S. Circulating Tumor Cells and Tumor Stem Cells Detection in the Peripheral Blood Mononuclear Cells of Breast Cancer. J. Clin. Lab. Anal. 2016, 30, 616–622. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).