Abstract
Stochastic Electrodynamics (SED) has had success modeling black body radiation, the harmonic oscillator, the Casimir effect, van der Waals forces, diamagnetism, and uniform acceleration of electrodynamic systems using the stochastic zero-point fluctuations of the electromagnetic field with classical mechanics. However the hydrogen atom, with its 1/r potential remains a critical challenge. Numerical calculations have shown that the SED field prevents the electron orbit from collapsing into the proton, but, eventually the atom becames ionized. We look at the issues of the H atom and SED from the perspective of symmetry of the quantum mechanical Hamiltonian, used to obtain the quantum mechanical results, and the Abraham-Lorentz equation, which is a force equation that includes the effects of radiation reaction, and is used to obtain the SED simulations. We contrast the physical computed effects of the quantized electromagnetic vacuum fluctuations with the role of the real stochastic electromagnetic field.
Keywords:
stochastic; electrodynamics; SED; hydrogen; symmetry; vacuum fluctuations; simulation; QED PACS:
11.10; 05.20; 05.30; 03.65
1. Introduction
The hydrogen atom has been the testing ground for theoretical atomic physics for over a hundred years. The original quantum mechanics was motivated to model the H atom and explain its spectrum [1]. That theory was refined into modern quantum mechanics by Bohr, Heisenberg and Schrodinger , Dirac, and others. Precision measurements of hydrogen energy levels by Willis Lamb in 1947 disagreed with the theory, which stimulated the development of Quantum Electrodynamics (QED), which included the effects of the vacuum fluctuations of the quantized electromagnetic field. The hydrogen atom is the fundamental two-body system and perhaps the most important tool of atomic physics and the challenge is to calculate its properties to the highest accuracy possible. The current QED theory is the most precise of any physical theory: The study of the hydrogen atom has been at the heart of the development of modern physics...theoretical calculations reach precision up to the 12th decimal place...high resolution laser spectroscopy experiments...reach to the 15th decimal place for the transition...The Rydberg constant is known to 6 parts in [2].
Stochastic electrodynamics (SED) represents an effort to explain quantum phenomena through classical physics done in the presence of a real stochastic electromagnetic field which is the sum of a portion for with spectral energy density , identical to that of the virtual zero-point vacuum fluctuations of the quantized electromagnetic field in QED, plus a Planckian spectrum for a finite temperature . In this paper, we focus on the component. SED has had success modeling the harmonic oscillator, and other linear systems. However, analytical efforts to address the H atom with SED have not been successful, so researchers have turned to numerical calculations to explore the H atom in SED and determine if SED can model the ground state [3,4]. The assertion of SED originally made by Boyer [5] is that the energy lost by radiation from the accelerating electron bound to the H nucleus is statistically compensated by the energy gained from the fluctuating vacuum field. In a variation of this work, Puthoff has shown that particularly for circular orbits, if a stable ground state of constant energy is achieved, then the energy provided by the stochastic vacuum field exactly compensates for the energy lost by radiation [6,7]. Others have asserted that no thermodynamic stable equilibrium will be achieved [8]. SED theory has been developed in several books by de la Pena and his collaborators [9] in which they have formulated an approach that claims to lead to the equations of standard quantum mechanics. In this paper, we will contrast the approach of quantum mechanics with that of SED, discussing the role of vacuum fluctuations in both theories. Emphasis is placed on the symmetry of the H atom since this plays such a pivotal role. For both theories we make some simplifying assumptions: non-relativistic mechanics, no spin, infinitely heavy proton. We discuss the most recent SED calculations which have shown stability for a limited number of orbits, but ionization for longer times [3,4].
2. Role of Vacuum Fluctuations in Quantum Mechanics and SED
In the nineteen-thirties, quantum theory had predicted zero-point vacuum fluctuations. Oppenheimer had calculated that the vacuum fluctuations gave an infinite and therefore unphysical energy shift to the free electron. The theory also predicted that the hydrogen and levels were degenerate. In 1947 Lamb and Retherford announced they had measured a shift of 1058 MHz at the Shelter Island Conference. After the conference, Hans Bethe, on the train ride to Schenectady, where he was consulting for GE, developed a new approach to include the vacuum fluctuations and QED was born. He, and others after him, applied renormalization methods, originally developed by Kramers and others, to deal successfully with the infinities predicted by prior approaches. Renormalization asserted that the vacuum fluctuations lead to the observed physical mass and charge of the electron.
In QED the vacuum field is typically expressed as a sum over an infinite number of plane waves with all possible momenta and directions with the restriction that the energy in each mode is . The vector potential is [10]
where the raising and lowering operators obey the commutation rules
and the two polarization vectors () are orthogonal to so , and
The quantization volume V is an artifice to avoid infinite volumes. In this box normalization , , and , with , and the integers , , and go from to The electric field is and .
To summarize the properties of the vacuum field in QED: no real photons are present, only random virtual photons of energy and momentum , with all possible momenta present consistent with Equation (1). The expectation values of the fields vanish but the variances do not. The fields are isotropic (invariant under rotations), invariant under space-time translations (homogeneous), and under boosts (Lorentz invariant). The energy density spectrum which is proportional to is also Lorentz invariant.
In QED, the zero-point quantum fluctuations are responsible for the mass and charge renormalization of particles. In this process one postulates the following:
bare point electron + vacuum fluctuations + radiative reaction →
electron with physical mass, charge and effective size about Compton wavelength.
electron with physical mass, charge and effective size about Compton wavelength.
A similar process occurs for an atom, in which the atom undergoes allowed virtual (energy conserving) transitions due to the vacuum field. These transitions can be seen as shifting the average energy of the atom. This is one picture (approximate) of radiative shifts, such as the Lamb shift [11]. The Lamb shift is a small shift, about 1 part in . It has been predicted correctly to about 1 part in by QED. In QED, radiative shifts are generally calculated using Feynmann diagrams, in which the atom is depicted as propagating in time, and it absorbs or emits a virtual photon changing its state correspondingly, then a short time later (consistent with the time-energy uncertainty principle) emits or absorbs the same virtual photon and returns to the initial state. This process arises from the term in the Hamiltonian and can involve all possible photons, but only photons of the resonant frequency will induce transitions. This model in a sense describes the interaction of the electron with its own radiation field. In QED this is equivalent to interacting with the ubiquitous virtual fluctuating vacuum field. The mass renormalization in QED is the state independent shift in energy for a bare electron given by the and the terms.
What makes Equation (1) for the vector potential unique to the quantized electromagnetic field is the presence of the operators and . The expression for the vector potential or the field can be recast with real (Hermitian) operators into a form which is most commonly adapted for use in SED:
where
and the expectation values with respect to the vacuum are and the variances are .
Equation (4) appears precisely the same as the equation often used in SED, with the differences that and are not operators but independent Gaussian variables with average 0 and variance 1. Also for each term the energy taken as the integral . In some cases a convergence factor for the energy may be used [4]. The SED field is real and is scaled so that the spectral energy density is identical to that of QED. The vacuum field in SED is also invariant under Lorentz transformations, which includes translations, rotations, and boosts.
Vacuum fluctuations are at the heart of SED. Because the expression for the vacuum field has an infinite number of plane waves, in SED calculations mathematical simplifications are employed to make calculations tractable. How the vacuum field is treated mathematically is probably one of the most critical aspects of SED modeling. A finite sum over discrete with a restricted range of is generally employed, which may alter the symmetry properties of the vacuum field. In the SED H atom calculations, the frequencies employed have been within one to four orders of magnitude of the rotational frequency of the electron in the atom [3,4]. The physical mass and charge of the electron are used, whereas in QED the higher frequency components of the vacuum fluctuations have the effect of giving a bare particle the physical mass and charge of the electron. These high frequency components are not explicitly considered in the SED calculations. Even with such simplifications, millions or billions of waves are included requiring extensive computing power. In SED, the vacuum fluctuations are the source of behavior characteristic of quantum systems, for example, for the harmonic oscillator. Indeed that is the basic contention of SED, that classical physics plus the vacuum field leads to the correct predictions for atomic systems, as does quantum mechanics:
classical mechanics + radiative reaction + point electron with physical mass +
real vacuum fluctuations → stable atom with correct behavior.
real vacuum fluctuations → stable atom with correct behavior.
In summary, the role of the vacuum field in SED is to stabilize the electron orbit by providing the energy lost to radiation from the term. Since the electron follows a classical orbit in SED, the orbit must be constantly changing to mimic wavelike behavior shown in quantum mechanics. Indeed from Earnshaw’s theorem there is no stable static arrangement of charges so fluctuations are to be expected. One interpretation of the orbital behavior is that when the electron loses energy and moves closer to the nucleus, its orbital frequency increases and it interacts more strongly with the higher frequency, higher energy waves in the vacuum field, causing it to accelerate, gain energy and move to a larger radius orbit. With the larger radius, the orbital frequency slows down, the electron interacts more strongly with the lower frequency waves, loses energy and moves to a smaller orbit. Thus the electron oscillates from smaller to larger radii [5,12].
3. The Hydrogen Atom in Quantum Mechanics and Classical Mechanics with No Radiative Reaction
In the quantum mechanical H atom there is no radiation from the electron due to the standing wave nature of the electron orbitals when the atom is in a stationary state. The wavefunctions and energy levels are determined solely by the symmetry of the Coulomb potential. The Hamiltonian is
We discuss the symmetry of H and its implication in the classical case with no radiation since SED is a classical theory. We also outline the very similar effects of the symmetry in the quantum mechanical system. Classically, the circular orbits about the Coulomb potential are stable orbits (assuming no radiation), meaning that a perturbation to the position of the electron in the orbit will lead to small oscillations. In the classical system there are orbits, in the quantum mechanical system there are wavefunctions. Solutions to Schrodinger’s equation are stationary energy eigenstates. The rotational symmetry of the atom implies that H is invariant under rotation. Rotations are generated by where the angular momentum , so . On the other hand so the angular momentum is conserved. For the classical orbit energy E and are also constant, and would be normal to the orbital plane. Symmetry operators of H are conserved in time. There is an additional symmetry operator of H, the Runge-Lenz vector :
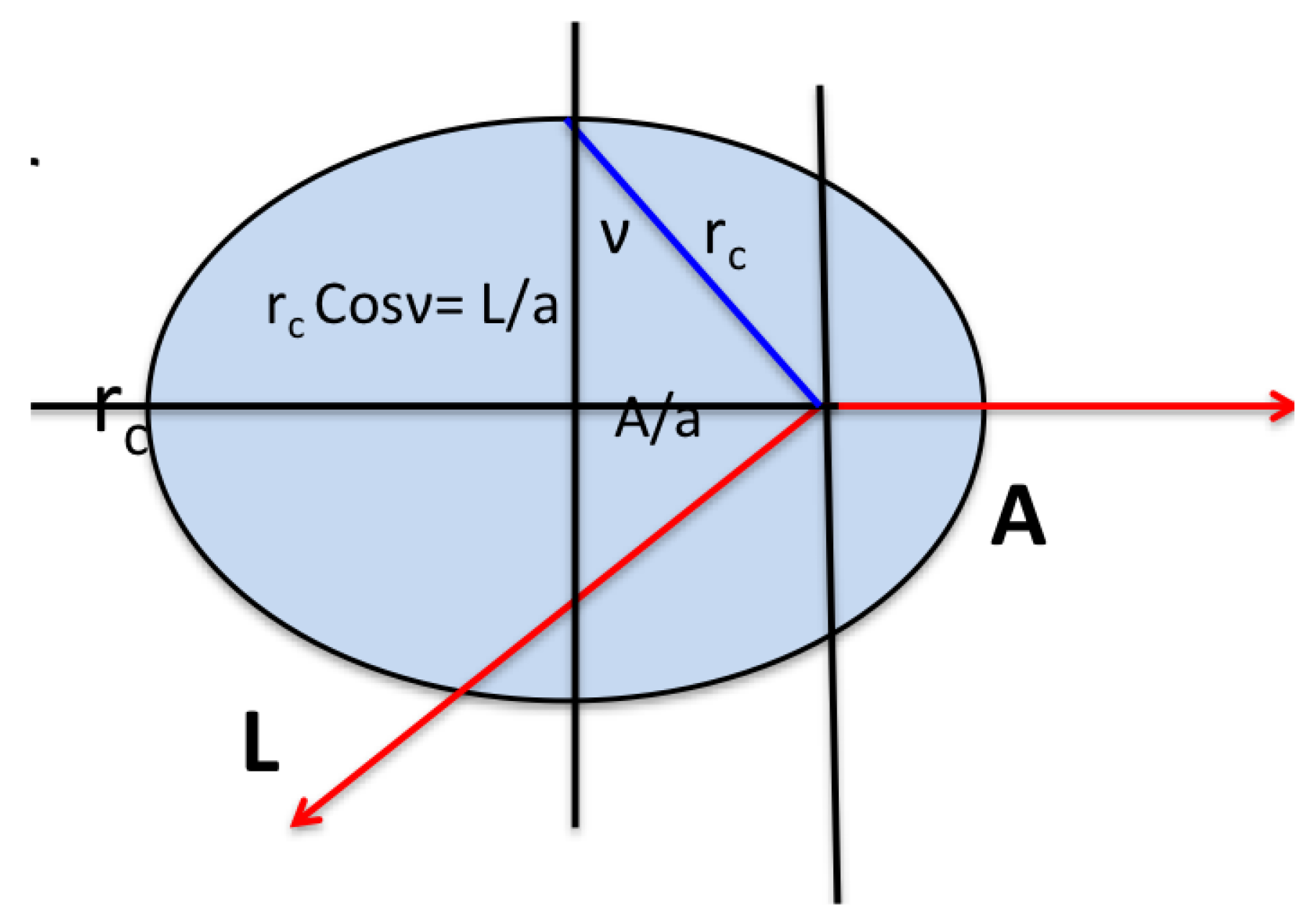
where , the effective momentum. The Runge-Lenz vector is constant, pointing along what would classically be the principal axis of the orbit, and is perpendicular to the angular momentum (see Figure 1). In the classical H atom is a generator of changes in the eccentricity of the orbit in such a way that the energy remains unchanged. In quantum mechanics, the Runge-Lenz vector generates a transformation that takes a state of given principal quantum number n and given L () into a superposition of the degenerate states with the same n.
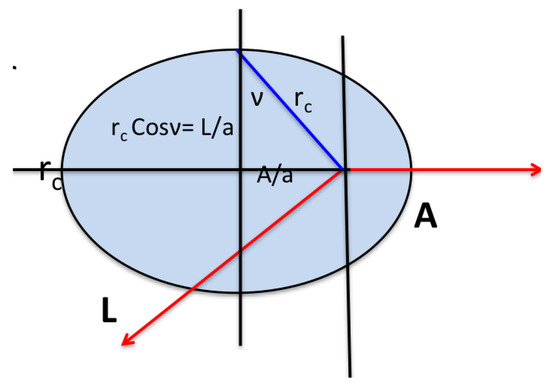
Figure 1.
The classical limit of the orbital is a non-precessing ellipse with constant and . The semi-major axis is and energy. The eccentricity and .
From the definitions of the two conserved vectors, it is trivial to obtain the equations for the classical orbits:
and substituting gives
which describes an ellipse with eccentricity . Computing and substituting the value for A gives
The semi-major axis is the average of the radii at the turning points. From the orbit equation, we find
The energy depends only on the length of the semi-major axis not on the eccentricity. This makes sense because changes the eccentricity but not the energy. Using the equations for and , with , we find
therefore,
Thus the values of A and L determine the energy E. A similar equation can be derived in quantum mechanics, and using the quantization of the operators and , we obtain the quantized energy levels as a function of the principal quantum number n. The commutators of and are
The generators form a Lie algebra that closes. To determine the corresponding symmetry group define the generators and which have the commutation properties of two disjoint groups :
The Lie algebra for M or N is the same as the familiar commutation relations for angular momentum . For angular momentum the allowed representations are characterized by the scalar , where The angular moment kets for this representation are generally chosen so , where . In analogy with the usual results for Thus we obtain
where n = 1, 2, 3,... is the principal quantum number and is the degree of degeneracy for the representation. The wavefunctions are eigenfunction of and . The symmetry of the wavefunctions is especially manifest in momentum space, where the wavefunctions are spherical harmonics in 4 dimensions. It is possible to enlarge the symmetry group from to in such a way that all states, scattering and bound, are included in the representation of the group and to use this larger group to derive a generating function for the radiative shifts for all atomic levels (n,L) [11].
In summary, the symmetry of the Hamiltonian for the H atom determines the energy levels, the orbits or wavefunctions, the degeneracy, the constants of the motion. For the ground state and and there is no degeneracy.
4. The Hydrogen Atom in SED
In SED, because of the radiative reaction, the energy of the electron is not conserved and the equation of motion generally used is the Abraham-Lorentz force equation [3,4,13]
The first term on the right of the equal sign is the Coulomb force, the next term is the radiative reaction, followed by the Lorentz force due to the vacuum fields and . The presence of the radiative reaction and the Lorentz force eliminates the symmetry of equation: angular momentum of the electron is not conserved, the Runge-Lenz vector is not constant and the electron energy is not constant. It should be mentioned that there is a fully relativistic form of Equation (17), the Abraham-Lorentz-Dirac equation, used, for example, in the study of charges accelerated uniformly through the vacuum [14].
The Abraham-Lorentz force is the non-relativistic recoil force for an accelerating charged particle caused by the particle emitting radiation which carries momentum, angular momentum and energy [15]. The radiation field from the particle is essentially exerting a force on itself, sometimes called a “self-field”, a phenomena which can leads to renormalization and a host of unsolved complications ultimately dealing with the nature of elementary particles [15,16]. Radiative reaction and vacuum fluctuations are intimately intertwined [10]. For example, in quantum mechanics, both are required to maintain the canonical commutation relations. The third order derivative for the Lorentz force requires the vacuum energy density to go as . Renormalization methods in classical physics and in QED have been developed to deal with these issues in an effective if not fundamental way. The Abraham-Lorentz equation is is a third order differential equation in time, which requires three initial conditions (the initial position, velocity and acceleration), and which leads to mathematical problems such as runaway solutions and acausal behavior and divergences (see [17] for a discussion).
In most calculations the magnetic field is dropped and a dipole approximation is used so the electric field is a function of time alone . The electron is assumed to move in a plane. Also often the third derivative is approximated by using the equation obtained when there is no radiative reaction or vacuum field so .
5. SED Simulations of the Ground State of the H Atom
Cole and Zou performed the first simulations of the hydrogen atom [3,12]. They retained the effect of the magnetic field, approximated the radiative term as discussed above, and did not use a dipole approximation. They used an adaptive time step Runge-Kutta 4th order algorithm, roughly equivalent to a net 5th order algorithm in dt to simulate 2 d orbits in a box 27 Å by 27 Å by 0.41 cm, where the normal to the plane of the orbit was in the larger Z direction. The advantage of choosing this geometry was that it reduced the number of plane waves they needed to consider to , with wavelengths from 0.1 Å to 900 Å and waves only in the Z direction, yet was able to capture the most important physical features [3]. They did 11 simulations, each starting at a radius of 0.53 Å but with different random amplitudes for the waves. They ran the simulations for about sec or roughly 100,000 orbits. In total these calculations took about 55 CPU days.
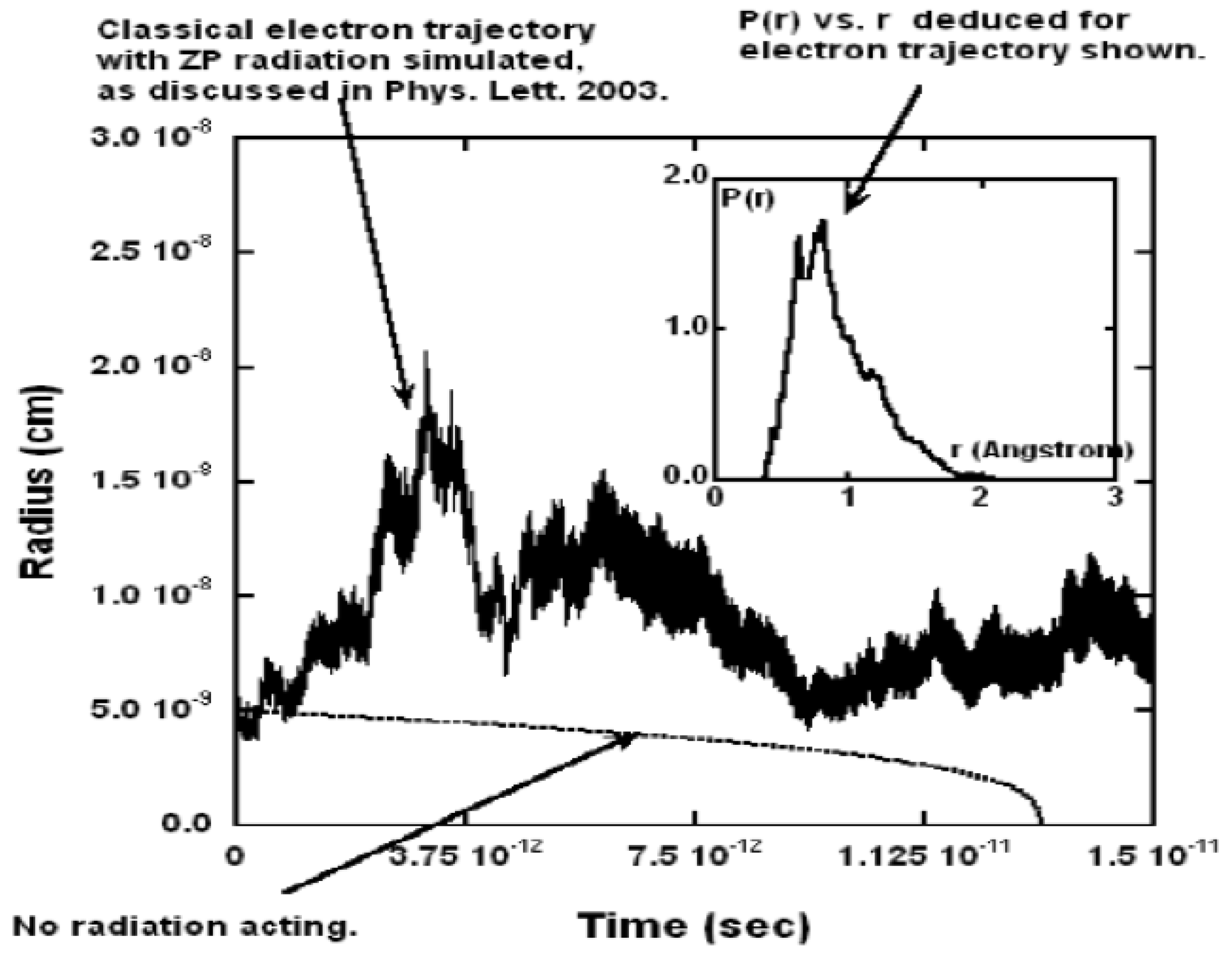
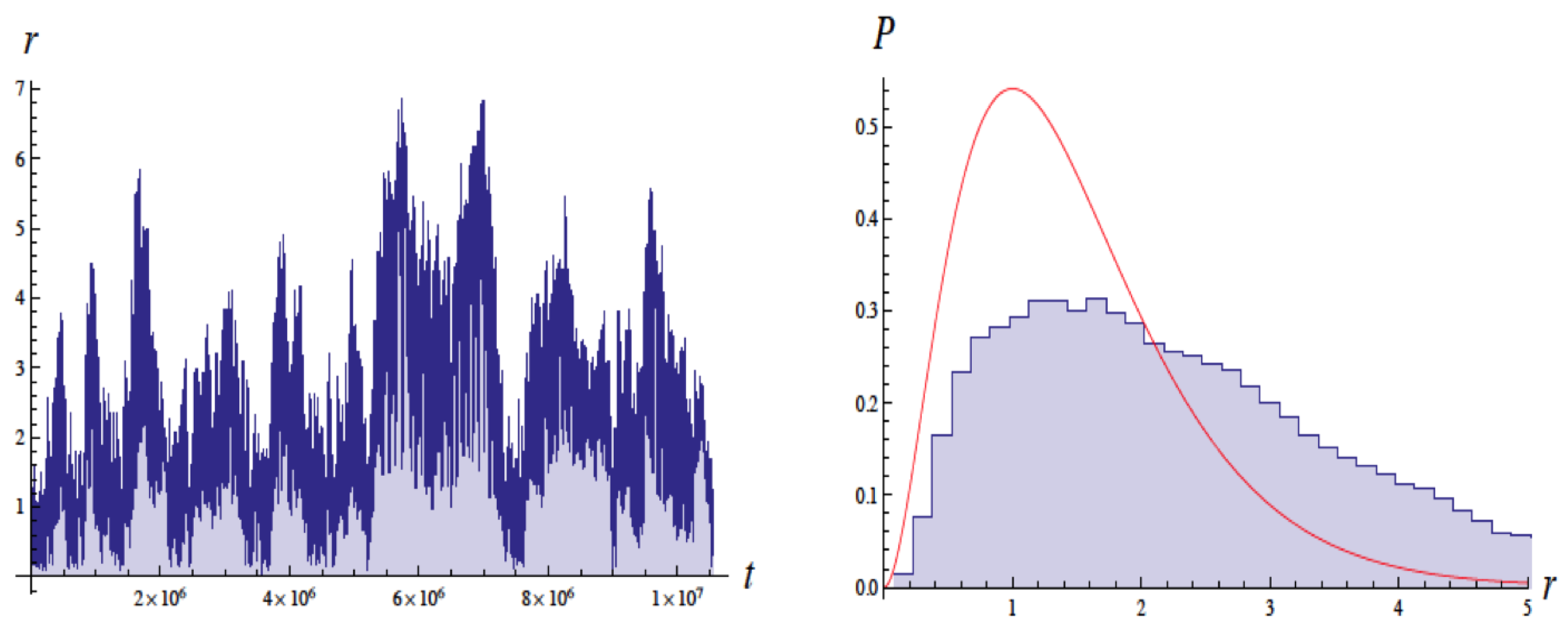
Figure 2 shows a typical trajectory as a function of time. It shows fluctuations in the radius by a factor of about 3 for roughly 100,000 orbits. As the inset shows, without the ZP field, the electron would have collapsed after about sec, so clearly the ZP field is preventing collapse during the interval shown.
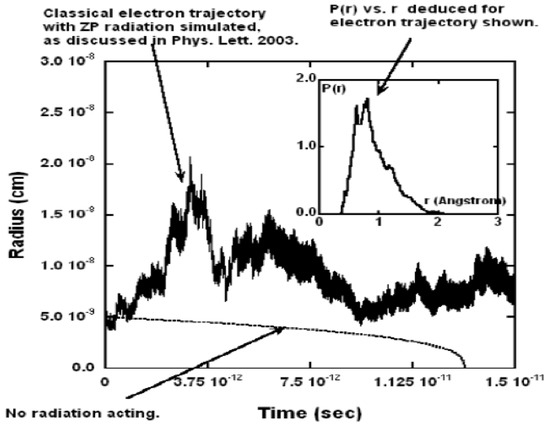
Figure 2.
Typical plot of r vs. time for one trajectory. The inset shows the probability density vs. r for this trajectory. A lower curve of r vs. t shows that if no zero-point radiation was present, then atomic collapse would occur in about sec. The starting trajectories began at 0.53 Å. Figure from [3] with permission.
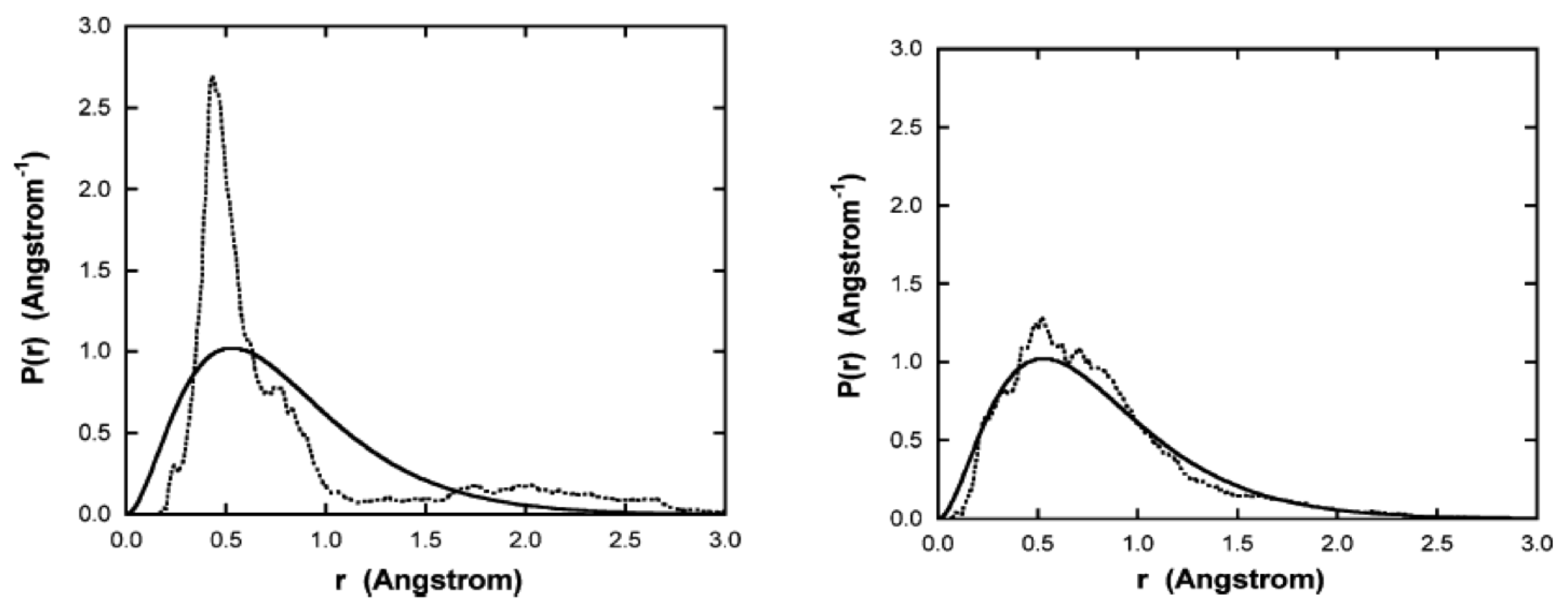
Figure 3 shows the radial probability distribution vs. radius, computed from a time average of the 11 simulations, up to the time indicated for each plot. Also shown is the computed from the ground state Schrodinger wavefunction. As time increases, the distribution approaches that predicted by quantum mechanics.
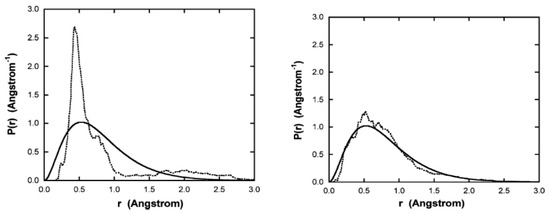
Figure 3.
Plots of the radial probability density vs. radius. The solid line was calculated from the ground state Schrodinger wavefunction. The dotted curves are simulation results, with starting trajectories began at 0.53 Å calculated as a time average for all 11 simulation runs from to the indicated time: left side, sec, right side, 7.25 × sec. Figure from [3] with permission.
The simulations of Cole and Zou typically ran for about sec or roughly 100,000 orbits and showed that as time increased, the radial distribution in r tended to look more like that predicted by quantum mechanics. In subsequent simulations using 30 PCs, Cole increased the span of wavelengths used for the applied zero-point field from to and found ionization occurred unless they increased the precision of the computation [12]. These preliminary results were only for about sec, about one tenth the time of the previous work and showed general agreement with the previous work. Cole and Zou also explored fundamental interactions between circularly polarized electromagnetic radiation and the electron initially in orbit about the proton, demonstrating that a stable circular orbit could be obtained by judicious choice of the amplitude and phase of a single CP normally incident wave with the same frequency as the orbital motion, and that a stable elliptical orbit could be obtained with a superposition of CP waves consisting of many harmonics of the orbital motion [18,19,20]. Cole and Zou [21] and Cole [22] did an ensemble of simulations with different initial positions of the electrons, and explored resonances that arise for certain orbital conditions, deepening understanding of the details of SED simulations and the classical H atom, and possibly paving the way for understanding transitions in H atom energy levels with SED.
The simulations by Nieuwenhuizen and Liska [4], done over a decade after the pioneering work of Cole and Zou [3] used the vastly improved computing power ( TFLOPS single precision floating point). They did a 3D simulation that included about plane waves. To simplify the computation they replaced the 3D sums over all by 1D sums over frequency with Gaussian amplitudes chosen such that they reproduce the same correlation function in the limit where the frequency mesh vanishes . They also computed orbits for times that were twice as long, about sec, with about orbits.
A 4th order Runge-Kutta algorithm was used with about 4000 iterations/orbit. The electric field was updated as a function of time ten times each orbit, with interpolation in between. Numerous methods were employed to check the computational accuracy of the results.
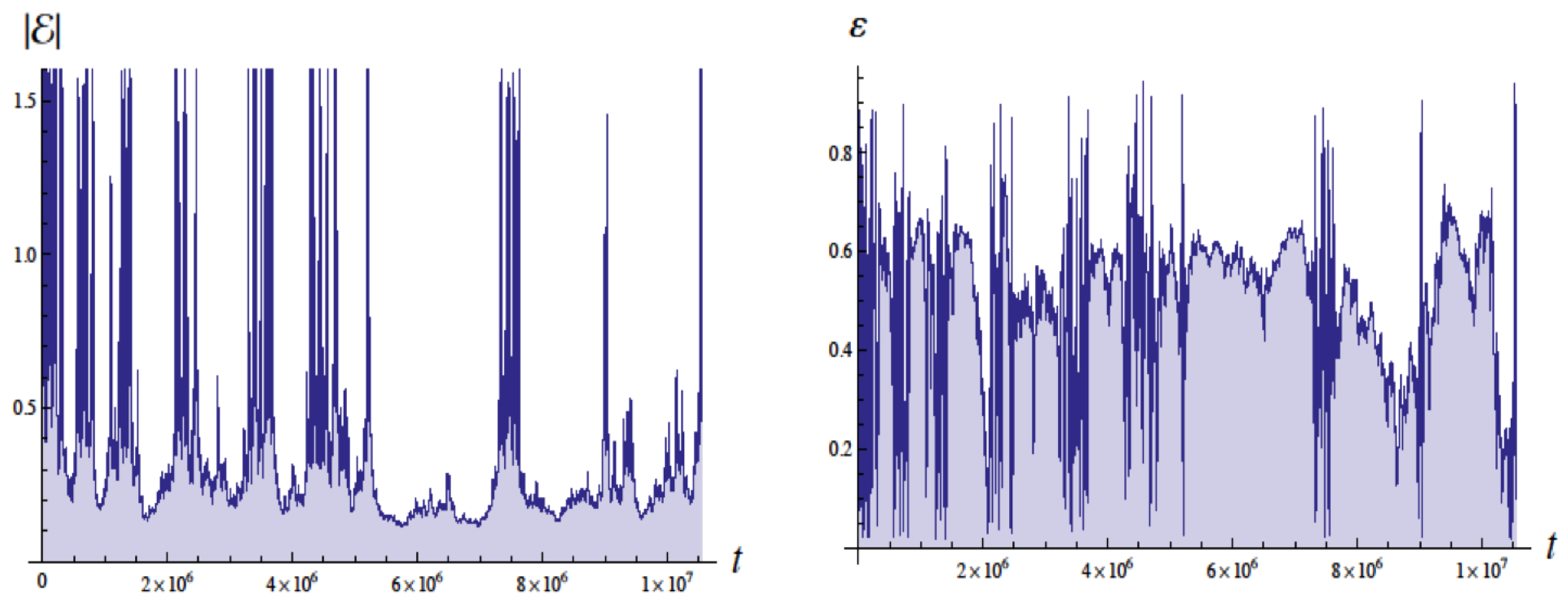
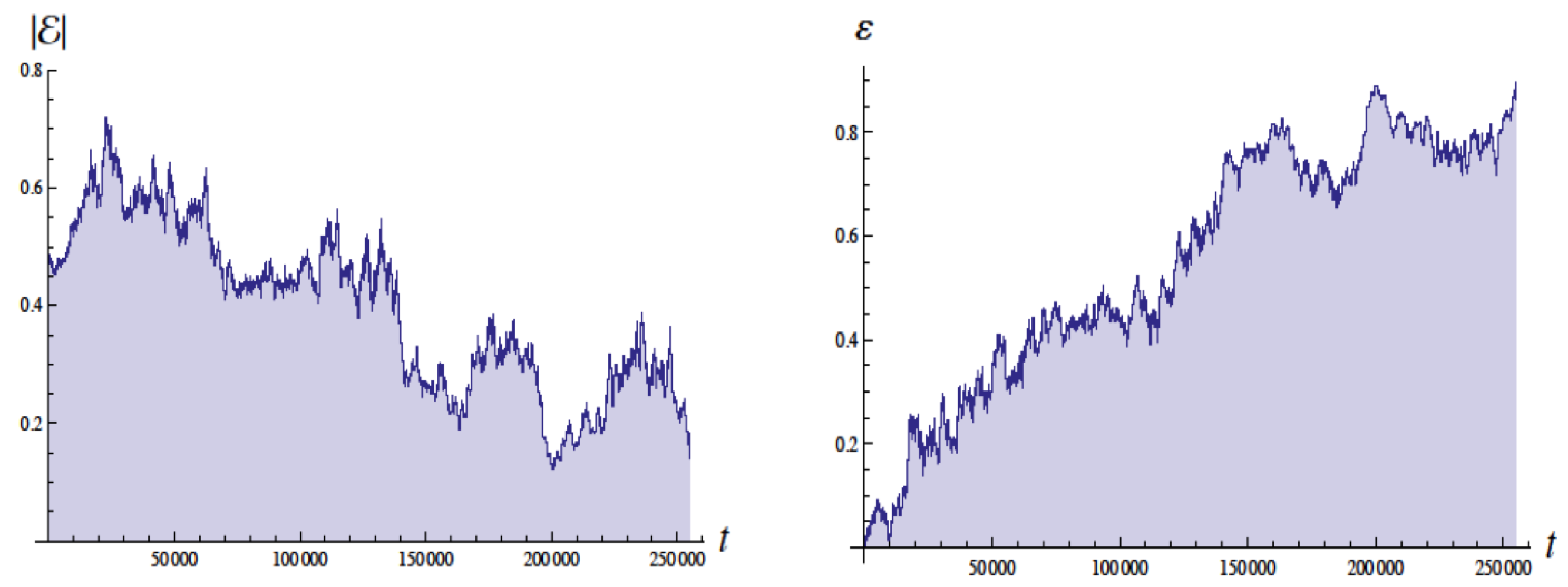
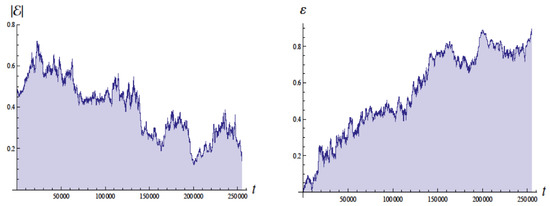
Figure 4 shows the energy in Bohr units and the eccentricity as a function of the time with units of , the Bohr time , which is of the QM orbital period. The energy of the ground state is . These simulations employed a moving cutoff equal to 2.5 times the orbital frequency. The energy varies from about to Bohr units (without radiative reaction the energy would be ). The eccentricity varies from about 0.05 to 0.95 (without radiative reaction would be 0 for a circular orbit).
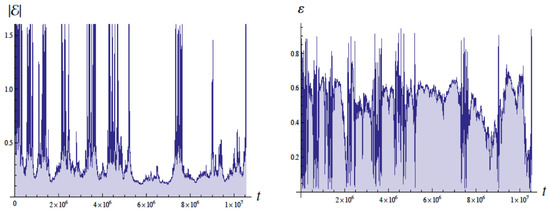
Figure 4.
Plots of the energy E (left side) and eccentricity (right side) as a function of time in units of for , for revolutions. The energy for the ground state in quantum mechanics would be with the scale used (Bohr units). Figure from [4] with permission.
Figure 5 shows the variation in the radius with time in units of and a corresponding histogram of the radius. The radius varies very rapidly from to 8 Bohr radii , while the eccentricity and energy remain somewhat more stable on longer timescales. Unlike Cole and Zou [3], they did not see a trend to reproduce the wavefunction, although their simulations suggested this might occur for shorter times scales.
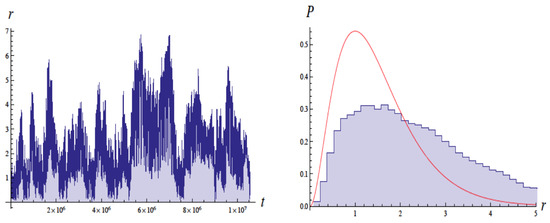
Figure 5.
Plots of the radius r of the electron orbit as a function of time in units of (left side) and a histogram of r (right side), for the same simulation as Figure 4. The smooth curve is the probability distribution expected from quantum mechanics. Figure from [4] with permission.
These plots suggest the possibility that the orbits may stabilize. However, when the simulations are done for longer times of the order of , instabilities developed which eventually lead to ionization as shown in Figure 6. When higher harmonics were included ( and ), ionization occurred earlier. Ionization was defined as the moment when the electron stayed above for at least . The moment of ionization is not shown in the subsequent plots [4].
With an upgrade in computing power, allowing double precision and a fixed cutoff of the frequency spectrum of the random fields, ionization occurred at an even earlier time. The energy of the electron tended to decrease to zero while the eccentricity increased leading to ionization. It is not clear precisely why these computational upgrades led to ionization at significantly earlier times.
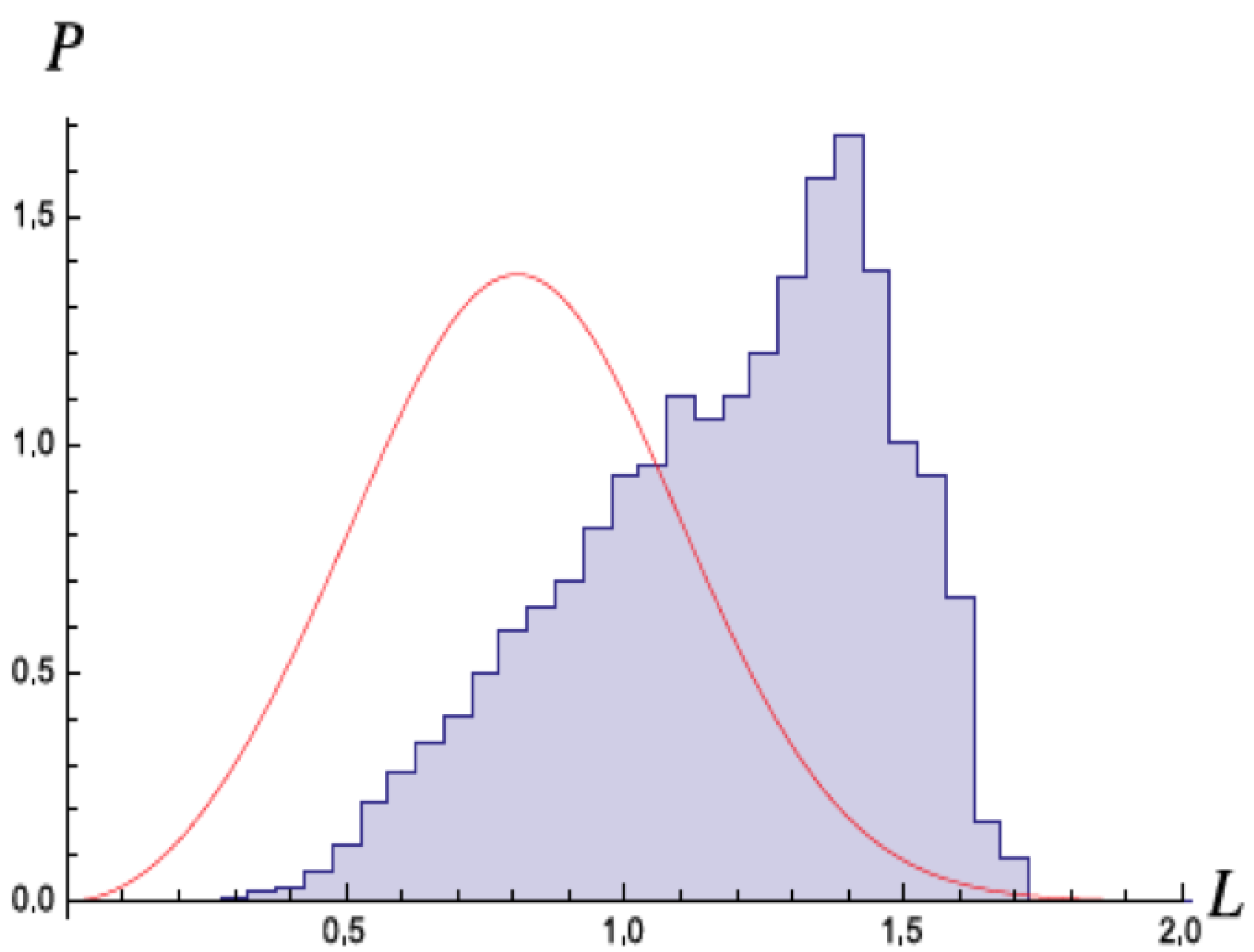
In a second set of simulations, Nieuwenhuizen and Liska included relativistic corrections and the effects of the magnetic fields [23]. Nevertheless, they obtained essentially the same results, ionization at early times. Niewenhuizen did a theoretical model of the H atom with the stochastic field, and computed the change in energy with each orbit of the electron [24]. If the energy gets very low and the angular momenta goes below , then the orbits are very eccentric, and the electron gains energy with each rotation, leading to self-ionization. In a simulation, as shown in Figure 7 they observed that the angular momentum seldom went below this critical value, in agreement with the predicted result.
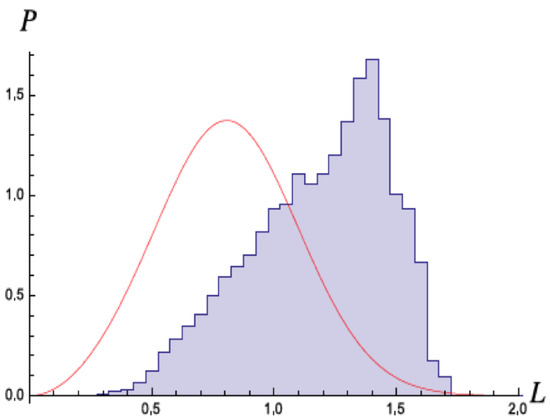
Figure 7.
Distribution of the angular momentum L (in units of ℏ) from the numerical simulation of the SED dynamics reported in [4]. Not much weight lies below , confirming that when such a value is reached at near-zero energy, self-ionisation may occur rapidly and the run is ended. Full curve: the distribution of L from the conjecture for the would-be stable ground state distribution from [24,25]. Figure from [24] with permission.
6. Discussion
SED researchers have made significant progress in the very complex task of modeling the ground state of the H atom, and shown that for short times corresponding to tens of thousands of orbits the real stochastic zero-point field can provide the energy lost to radiation and maintain the orbital motion. However, as time passes and more regions of phase space are explored, the orbits tend to become highly elliptical, with lower angular momentum, and self-ionization occurs. The question arises: is this instability due: (1) to the specific implementations of SED, including the approximations and numerical methods used, or (2) is this instability inherent in the SED approach to the H atom, or (3) is it more fundamental in nature, and perhaps reflects a characteristic chaos arising from non-linear potentials [13,22], as observed, for example, in comparisons of classical and quantum models of photo-dissociation with the Morse potential for diatomic molecules [26]. For a variety of non-linear systems there are regions of classical phase-space characterized by chaos. SED computations of the anharmonic oscillator might provide insight into the effect of a controllable non-linearity.
Contrasting the approach of quantum mechanics and quantum electrodynamics to that of SED is illuminating. In QED, the various physical phenomena, including the zero-point field, the fundamental properties of the electron, the presence of radiation, the properties of the Coulomb field, and the stability of atoms, are all parsed in such a way that the system is mathematically tractable and well behaved solutions, reflecting the symmetry of the potential, are found. On the other hand it appears that the current SED approach lumps all these phenomena together in the Abraham-Lorentz equation with the zero-point field, which leads to very challenging mathematical entanglements that mask the symmetry of the solutions. For example, in QED, the self interaction leads to a big effect on the electron: mass renormalization. The remaining radiative shift for the atom is very small. In SED, the radiative effects in the Abraham-Lorentz equation lead to weak but persistent forces, whereas the vacuum field forces are much larger and fluctuate greatly from positive to negative values, making it difficult to obtain a stable orbit.
There are many more issues that SED needs to address beyond the attainment of a stable orbit. Can SED develop a set of states for the H atom that are isomorphic to those predicted by QM, with characteristic quantized values of angular momentum and energy? with the same degree of degeneracy? Can SED predict the transitions between energy levels? Can SED predict the results of measurements on the atom? It is very difficult to see a path for attaining these objectives with the current SED approach. For a first step, for example, SED needs not only to obtain stable ground state orbit, but, to probably predict an orbit that is similar to the quantum ground state which has a spherically symmetric probability distribution with quantized angular momentum of zero. Perhaps this might be obtained with a superposition of suitable initial conditions. However, considering superpositions leads to ideas characteristic of quantum mechanics. This raises the question: does making SED predict the H atom correctly require the modification of SED so that it becomes essentially a reformulation of quantum mechanics? Perhaps SED, which predicts particles with definite orbits, has a relationship to Bohmian mechanics, which also describes definite orbits, has a non-local potential, and predicts measurements that agree with those of quantum mechanics [27].
Funding
I thank Hermann Batelaan and the organizers of Boston SED2018 for an excellent conference and for funding travel expenses and publication fees for this paper.
Acknowledgments
The author would like to thank Peter Milonni for his encouragement and his comments on this paper, and to thank Dan Cole for sharing references and research I was unaware of, and for his extraordinarily careful reading and numerous insightful suggestions and comments. Thanks to Elsevier for permission to include Figures 2 and 3 from [3]; thanks to IOP PUblishing to include Figures 1, 2, 3, 6, 9 from [4]; and thanks to Theo Niewenhuizen for permission to use Figure 1 from [24].
Conflicts of Interest
The author declares no conflict of interest.
References
- Hertzberg, G. Atomic Spectra and Atomic Structure; Dover Publications: New York, NY, USA, 1944. [Google Scholar]
- Beyer, A.; Maisenbacher, L.; Matveev, A.; Pohl, R.; Khabarova, K.; Grinin, A.; Lamour, T.; Yost, D.C.; Hänsch, T.W.; Kolachevsky, N.; et al. The Rydberg constant and proton size from atomic hydrogen. Science 2017, 358, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.; Zou, Y. Quantum mechanical ground state of hydrogen obtained from classical electrodynamics. Phys. Lett. A 2003, 317, 14–20. [Google Scholar] [CrossRef]
- Niewenhuizen, T.; Liska, M. Simulation of the hydrogen ground state in Stochastic Electrodynamics. Phys. Scr. 2015, T165, 014006. [Google Scholar] [CrossRef]
- Boyer, T.H. Random electrodynamics: The theory of classical electrodynamics with classical electromagnetic zero-point radiation. Phys. Rev. D 1975, 11, 790–808. [Google Scholar] [CrossRef]
- Puthoff, H. Ground state of hydrogen as a zero-point-fluctuation-determined state. Phys. Rev. D 1987, 35, 3266. [Google Scholar] [CrossRef]
- Puthoff, H. Quantum ground states as equilibrium particle-vacuum interaction states. Quantum Stud. Math. Found. 2016, 3, 5–10. [Google Scholar] [CrossRef]
- Claverie, P.; Soto, F. Nonrecurrence of the stochastic process for the hydrogen atom problem in stochastic electrodynamics. Math. Phys. 1982, 23, 753–759. [Google Scholar] [CrossRef]
- de la Pena, L.; Cetto, A. The Quantum Dice: An Introduction to Stochastic Electrodynamics; Kluwer Publishers: Dortrecht, The Netherlands, 1996. [Google Scholar]
- Milonni, P. An Introduction to Quantum Optics and Quantum Fluctuations; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Maclay, J. The Symmetry of the Energy Levels of the Hydrogen Atom and the Application of the Symmetry to the Calculation of Radiative Level Shifts. Ph.D. Thesis, Yale University, New Haven, CT, USA, 1972. [Google Scholar]
- Cole, D. Simulation Results Related to Stochastic Electrodynamics. AIP Conf. Proc. 2006, 810, 99–113. [Google Scholar] [CrossRef]
- Cole, D. Classical Electrodynamic Systems Interacting with Classical Electromagnetic Random Radiation. Found. Phys. 1989, 20, 225–240. [Google Scholar] [CrossRef]
- Cole, D. Properties of a Classical Charged Harmonic Oscillator Accelerated Through Classical Electromagnetic Zero-point Radiation. Phy. Rev. D 1985, 31, 1972. [Google Scholar] [CrossRef]
- Teitelboim, C. Splitting of the Maxwell Tensor: Radiation Reaction without Advanced Fields. Phys. Rev. D 1970, 1, 1572. [Google Scholar] [CrossRef]
- Jackson, D. Classical Electrodynamics; John Wiley and Sons: New York, NY, USA, 1962; p. 579. [Google Scholar]
- Milonni, P. The Quantum Vacuum, An Introduction to Electrodynamics; Academic Press: San Diego, CA, USA, 1994; p. 156. [Google Scholar]
- Cole, D.; Zou, Y. Perturbation Analysis and Simulation Study of the Effects of Phase on the Classical Hydrogen Atom Interacting with Circularly Polarized Electromagnetic Radiation. J. Sci. Comput. 2004, 21, 145–172. [Google Scholar] [CrossRef]
- Cole, D.; Zou, Y. Simulation Study of Aspects of the Classical Hydrogen Atom Interacting with Electromagnetic Radiation: Elliptical Orbits. J. Sci. Comput. 2004, 20, 379–404. [Google Scholar] [CrossRef]
- Cole, D.; Zou, Y. Simulation study of aspects of the classical hydrogen atom interacting with electromagnetic radiation: Circular orbits. J. Sci. Comput. 2003, 20, 43–68. [Google Scholar] [CrossRef]
- Cole, D.; Zou, Y. Subharmonic resonance behavior for the classical hydrogen atomic system. J. Sci. Comput. 2009, 39, 1–27. [Google Scholar] [CrossRef]
- Cole, D. Subharmonic resonance and critical eccentricity for the classical hydrogen atomic system. Eur. Phys. J. D 2018, 72, 200. [Google Scholar] [CrossRef]
- Niewenhuizen, T.; Liska, M. Simulation of the hydrogen ground state in Stochastic Electrodynamics-2. Found. Phys. 2016, 45, 1190. [Google Scholar] [CrossRef]
- Niewenhuizen, T. On the stability of classical orbits of the hydrogen ground state in Stochastic Electrodynamics. Entropy 2016, 18, 136. [Google Scholar] [CrossRef]
- Niewenhuizen, T. Classical phase space density for the relativistic hydrogen atom. AIP Conf. Proc. 2006, 810, 198. [Google Scholar]
- Goggin, M.; Milonni, P. Driven Morse oscillator: Classical chaos, quantum theory, and photodissociation. Phys. Rev. A 1988, 37, 796. [Google Scholar] [CrossRef]
- Holland, P. The Quantum Theory of Motion, An account of the de Broglie-Bohm Causal Interpretation of Quantum Mechanics; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).