BEAMDB and MOLD—Databases at the Serbian Virtual Observatory for Collisional and Radiative Processes
Abstract
1. Introduction
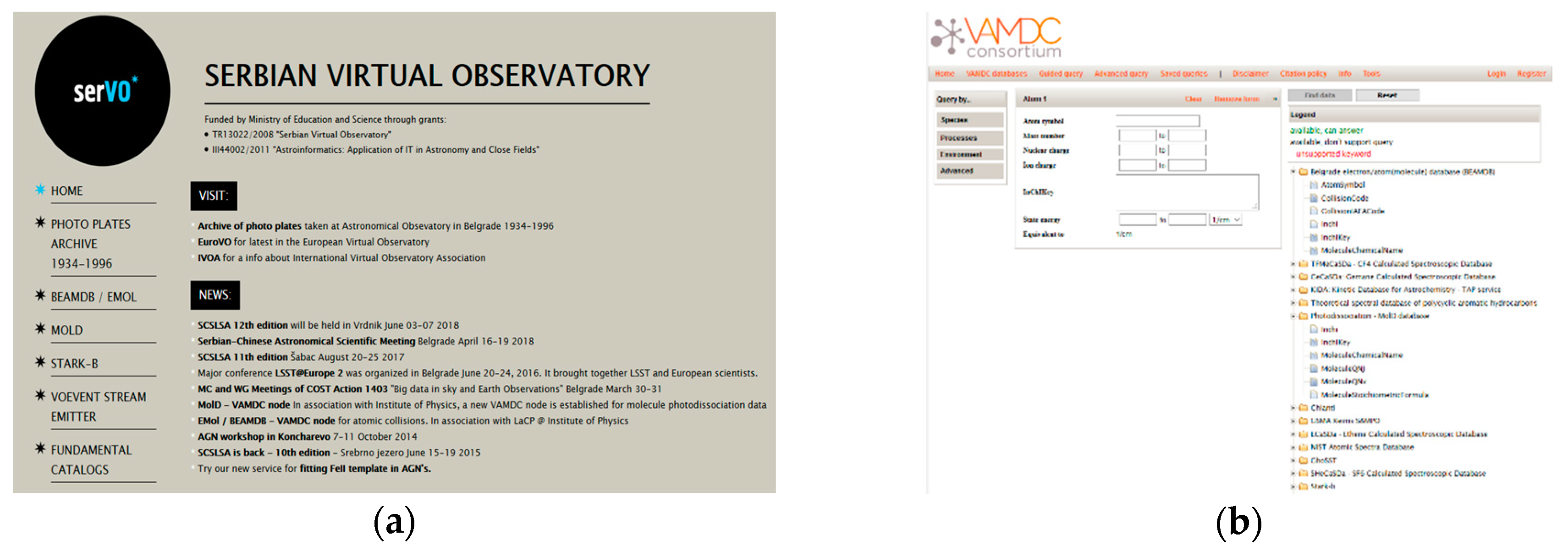
2. BEAMDB and MolD Database Nodes
2.1. BEAMDB—Belgrade Electron/Atom(Molecule) DataBase
2.1.1. Elastic Electron Scattering by Methane Molecule—Early Experiments
2.1.2. Elastic Electron Scattering by Methane Molecule—Modern Experiments and Calculations
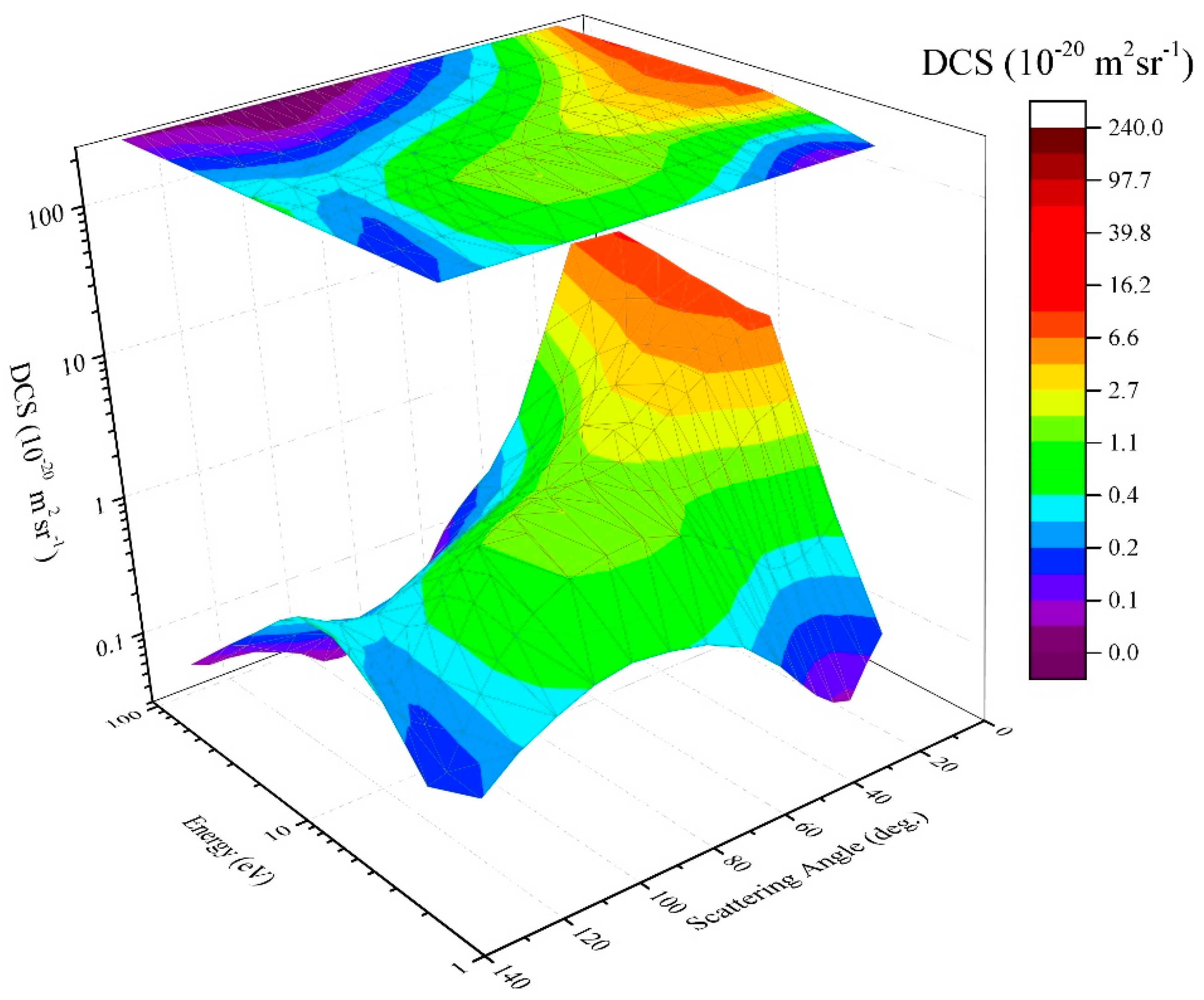
2.1.3. Elastic Electron Scattering by Methane Molecule—The Twenty-First Century Results
2.1.4. Elastic Electron Scattering by Methane Molecule—Coverage in the BEAMDB
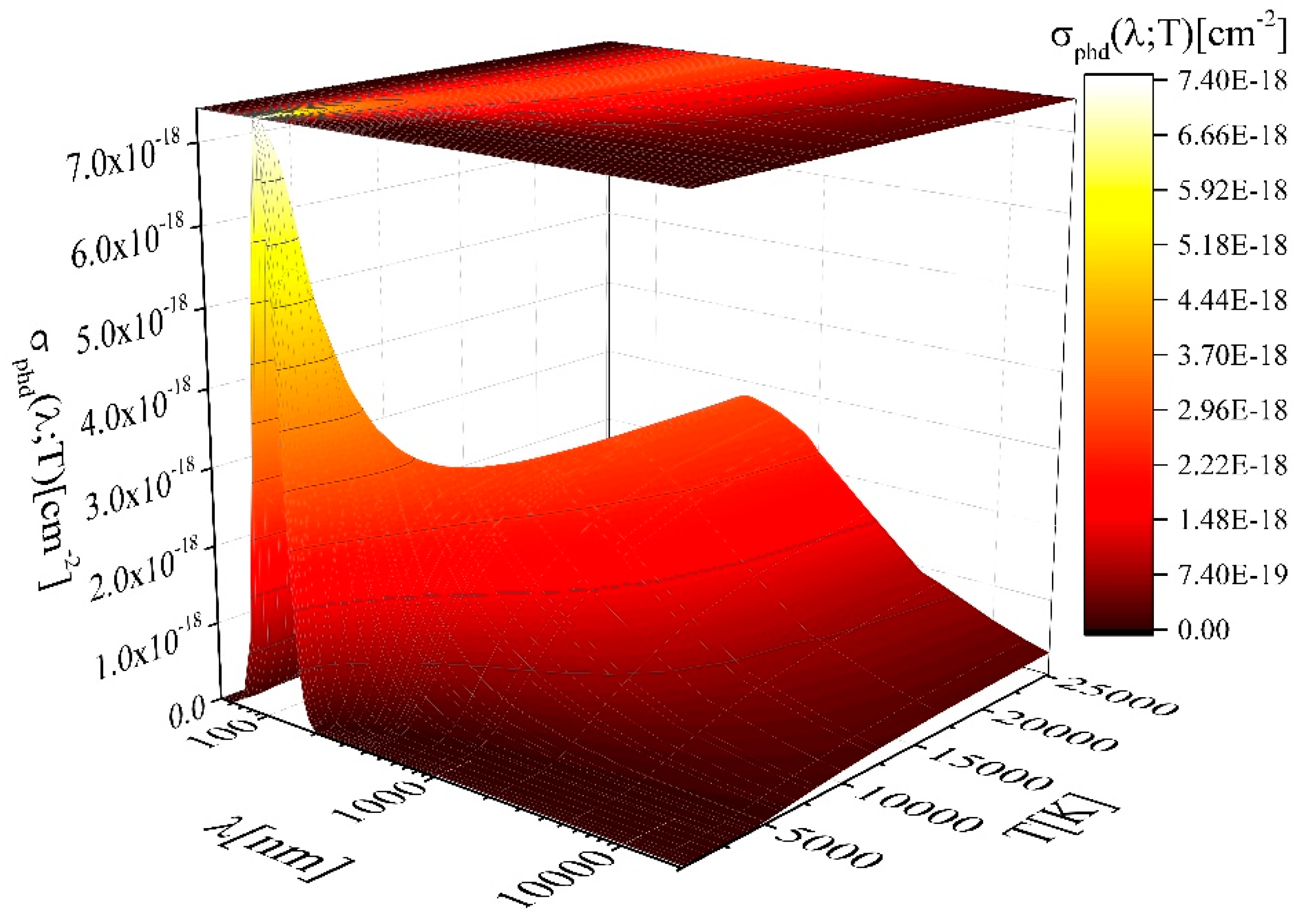
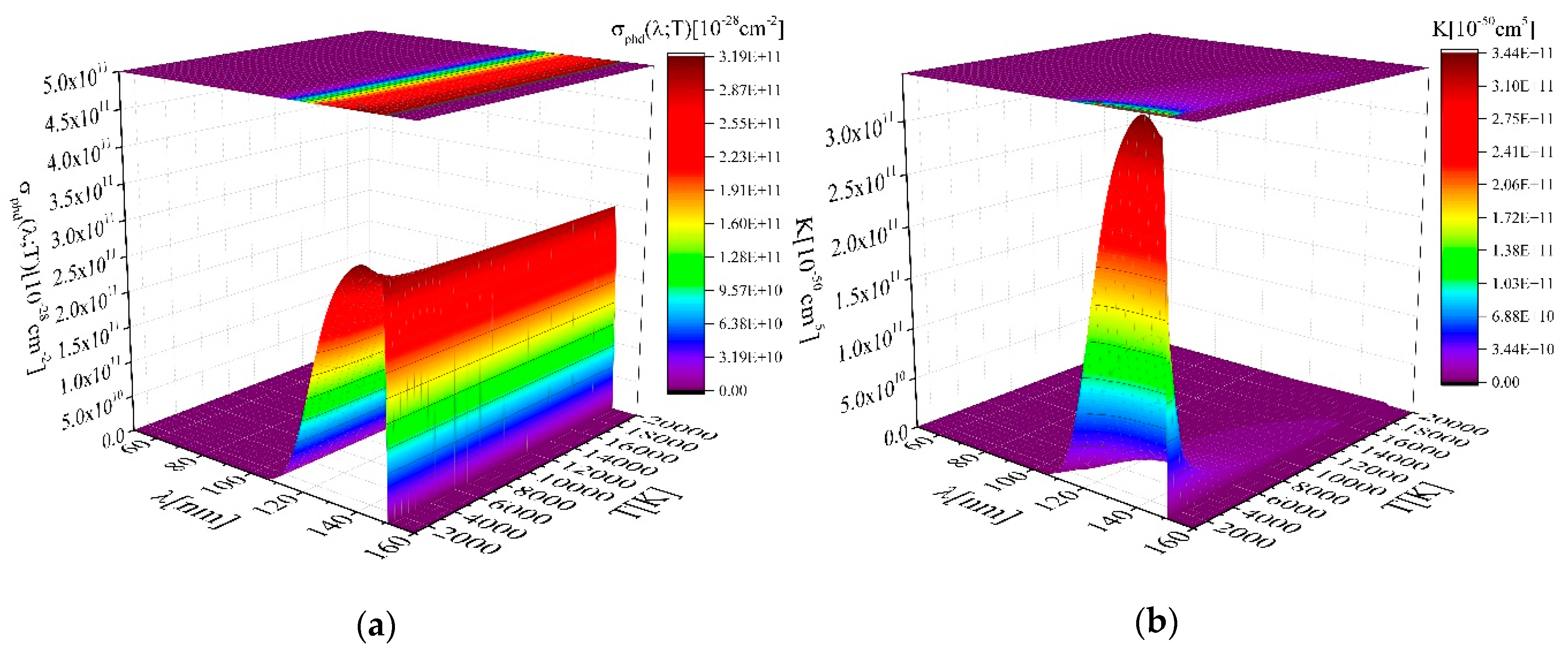
2.2. Photodissociation—The MolD Database
2.3. Node Maintenance
3. Conclusions
- Developing of the VAMDC Portal as a Major Enabler of Atomic and Molecular Data Citation;
- Python, Django updates;
- Installing the Query Store on VAMDC node that could have a plan store for holding the execution plan information, and a runtime stats store for carrying on the execution statistics information.
- XSAMS evolution to deal with Big Data (resources to be accessed by diverse client platforms across the network; generating and transferring data over a network without requiring human-to-human or human-to-computer; provide security and data quality; etc.).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mason, N.J. The status of the database for plasma processing. J. Phys. D 2009, 42, 194003. [Google Scholar] [CrossRef]
- Tennyson, J.; Rahimi, S.; Hill, C.; Tse, L.; Vibhakar, A.; Akello-Egwel, D.; Brown, D.B.; Dzarasova, A.; Hamilton, J.R.; Jaksch, D.; et al. QDB: A new database of plasma chemistries and reactions. Plasma Sources Sci. Technol. 2017, 26, 055014. [Google Scholar] [CrossRef]
- Sanche, L. Interaction of low energy electrons with DNA: Applications to cancer radiation therapy. Radiat. Phys. Chem. 2016, 128, 36–43. [Google Scholar] [CrossRef]
- Marinković, B.P.; Jevremović, D.; Srećković, V.A.; Vujčić, V.; Ignjatović, L.M.; Dimitrijević, M.S.; Mason, N.J. BEAMDB and MolD—Databases for atomic and molecular collisional and radiative processes: Belgrade nodes of VAMDC. Eur. Phys. J. D 2017, 71, 158. [Google Scholar] [CrossRef]
- Jevremović, D.; Dimitrijević, M.S.; Popović, L.Č.; Dačić, M.; Benišek, V.P.; Bon, E.; Gavrilović, N.; Kovačević, J.; Benišek, V.; Kovačević, A.; et al. The project of Serbian Virtual Observatory and data for stellar atmosphere modeling. New Astron. Rev. 2009, 53, 222–226. [Google Scholar] [CrossRef]
- Dubernet, M.L.; Antony, B.K.; Ba, Y.A.; Babikov, Y.L.; Bartschat, K.; Boudon, V.; Braams, B.J.; Chung, H.-K.; Daniel, F.; Delahaye, F.; et al. The virtual atomic and molecular data centre (VAMDC) consortium. J. Phys. B 2016, 49, 074003. [Google Scholar] [CrossRef]
- VAMDC Consortium—Active Databases. Available online: https://portal.vamdc.eu/vamdc_portal/home.seam (accessed on 26 October 2018).
- GitHub—VAMDC/NodeSoftware: Python/Django-Based Software for Running VAMDC Data Nodes. Available online: https://github.com/VAMDC/NodeSoftware (accessed on 26 October 2018).
- Django: The Web Framework for Perfectionists with Deadlines. Available online: https://www.djangoproject.com/ (accessed on 26 October 2018).
- Cvjetković, V.; Marinković, B.; Šević, D. Information System in Atomic Collision Physics. In Book Advances and Innovations in Systems, Computing Sciences and Software Engineering; Elleithy, K., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 485–490. ISBN 978-1-4020-6263-6. [Google Scholar] [CrossRef]
- Marinković, B.P.; Vujčić, V.; Sushko, G.; Vudragović, D.; Marinković, D.B.; Đorđević, S.; Ivanović, S.; Nešić, M.; Jevremović, D.; Solov’yov, A.V. Development of Collisional Data Base for Elementary Processes of Electron Scattering by Atoms and Molecules. Nucl. Instrum. Methods Phys. Res. B 2015, 354, 90–95. [Google Scholar] [CrossRef]
- Marinković, B.P.; Bredehöft, J.H.; Vujčić, V.; Jevremović, D.; Mason, N.J. Rosetta Mission: Electron Scattering Cross Sections—Data Needs and Coverage in BEAMDB Database. Atoms 2017, 5, 46. [Google Scholar] [CrossRef]
- Huo, W.M.; Kim, Y.K. Electron collision cross-section data for plasma modelling. IEEE Trans. Plasma Sci. 1999, 27, 1225–1240. [Google Scholar] [CrossRef]
- White, R.D.; Cocks, D.; Boyle, G.; Casey, M.; Garland, N.; Konovalov, D.; Philippa, B.; Stokes, P.; de Urquijo, J.; González-Magaña, O.; et al. Electron transport in biomolecular gaseous and liquid systems: Theory, experiment and self-consistent cross-sections. Plasma Sources Sci. Technol. 2018, 27, 053001. [Google Scholar] [CrossRef]
- Pitchford, L.C.; Alves, L.L.; Bartschat, K.; Biagi, S.F.; Bordage, M.-C.; Bray, I.; Brion, C.E.; Brunger, M.J.; Campbell, L.; Chachereau, A.; et al. LXCat: An Open-Access, Web-Based Platform for Data Needed for Modeling Low Temperature Plasmas. Plasma Process. Polym. 2017, 14, 1600098. [Google Scholar] [CrossRef]
- Falconer, I. JJ Thomson and the discovery of the electron. Phys. Educ. 1997, 32, 226–231. [Google Scholar] [CrossRef]
- Dymond, E.G.; Watson, E.E. Electron scattering in helium. Proc. R. Soc. Lond. A 1929, 122, 571–582. [Google Scholar] [CrossRef]
- Arnot, F.L. Electron Scattering in Mercury Vapour. Proc. R. Soc. Lond. A 1929, 125, 660–669. [Google Scholar] [CrossRef]
- Bullard, E.C.; Massey, H.S.W. The elastic scattering of slow electrons in argon. Proc. R. Soc. Lond. A 1931, 130, 579–590. [Google Scholar] [CrossRef]
- Mott, N.F. Elastic Collisions of Electrons with Helium. Nature 1929, 123, 717. [Google Scholar] [CrossRef]
- Mott, N.F.; Massey, H.S.W. The Theory of Atomic Collisions, 3rd ed.; Oxford University Press: Oxford, UK, 1965. [Google Scholar]
- Bartschat, K.; Tennyson, J.; Zatsarinny, O. Quantum-Mechanical Calculations of Cross Sections for Electron Collisions with Atoms and Molecules. Plasma Process. Polym. 2017, 14, 1600093. [Google Scholar] [CrossRef]
- Fursa, D.V.; Bray, I. Fully Relativistic Convergent Close-Coupling Method for Excitation and Ionization Processes in Electron Collisions with Atoms and Ions. Phys. Rev. Lett. 2008, 100, 113201. [Google Scholar] [CrossRef]
- Madison, D.H.; Al-Hagan, O. The Distorted-Wave Born Approach for Calculating Electron-Impact Ionization of Molecules. Hindawi J. At. Mol. Opt. Phys. 2010, 2010, 367180. [Google Scholar] [CrossRef]
- Blanco, F.; García, G. Improved non-empirical absorption potential for electron scattering at intermediate and high energies: 30–10000 eV. Phys. Lett. A 1999, 255, 147–153. [Google Scholar] [CrossRef]
- Das, T.; Stauffer, A.D.; Srivastava, R. A method to obtain static potentials for electron-molecule scattering. Eur. Phys. J. D 2014, 68, 102. [Google Scholar] [CrossRef]
- Tennyson, J. Electron–molecule collision calculations using the R-matrix method. Phys. Rep. 2010, 491, 29–76. [Google Scholar] [CrossRef]
- Boudon, V.; Pirali, O.; Roy, P.; Brubach, J.-B.; Manceron, L.; Vander Auwera, J. The high-resolution far-infrared spectrum of methane at the SOLEIL synchrotron. J. Quant. Spectrosc. Radiat. Trans. 2010, 111, 1117–1129. [Google Scholar] [CrossRef]
- Romani, P.N.; Atreya, S.K. Methane photochemistry and haze production on Neptune. Icarus 1988, 74, 424–445. [Google Scholar] [CrossRef]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Herzberg, G. Molecular Spectra and Molecular Structure. III. Electronic Spectra of Polyatomic Molecules; Van Nostrand Reinhold Company: New York, NY, USA, 1966; p. 392. ISBN 0-442-03387-7. [Google Scholar]
- Gianturco, F.A.; Thompson, D.G. The scattering of slow electrons by polyatomic molecules. A model study for CH4, H2O and H2S. J. Phys. B 1980, 13, 613–625. [Google Scholar] [CrossRef]
- Hollister, C.; Sinanoglu, O. Molecular Binding Energies. J. Am. Chem. Soc. 1966, 88, 13–21. [Google Scholar] [CrossRef]
- Arnot, F.L. The Diffraction of Electrons in Gases. Proc. R. Soc. Lond. A 1931, 133, 615–636. [Google Scholar] [CrossRef]
- Bullard, E.C.; Massey, H.S.W. The Elastic Scattering of Slow Electrons in Gases—II. Proc. R. Soc. Lond. A 1931, 133, 637–651. [Google Scholar] [CrossRef]
- Mohr, C.B.O.; Nicoll, F.H. The large angle scattering of electrons in gases—II. Proc. R. Soc. Lond. A 1932, 138, 469–478. [Google Scholar] [CrossRef]
- Hughes, A.L.; McMillen, J.H. Electron Scattering in Methane, Acetylene and Ethylene. Phys. Rev. 1933, 44, 876–882. [Google Scholar] [CrossRef]
- Dahl, S.S.; Srivastava, B.B.; Shingal, R. Elastic scattering of electrons by methane molecules at intermediate energies. J. Phys. B 1979, 12, 2727–2734. [Google Scholar] [CrossRef]
- Rohr, K. Cross beam experiment for the scattering of low-energy electrons from methane. J. Phys. B 1980, 13, 4897–4905. [Google Scholar] [CrossRef]
- Tanaka, H.; Okada, T.; Boesten, L.; Suzuki, T.; Yamamoto, T.; Kubo, M. Differential cross sections for elastic scattering of electrons by CH4 in the energy range of 3 to 20 eV. J. Phys. B 1982, 15, 3305–3319. [Google Scholar] [CrossRef]
- Boesten, L.; Tanaka, H. Elastic DCS for e+CH4 collisions, 1.5–100 eV. J. Phys. B 1991, 24, 821–832. [Google Scholar] [CrossRef]
- Vušković, L.; Trajmar, S. Electron impact excitation of methane. J. Chem. Phys. 1983, 78, 4947–4951. [Google Scholar] [CrossRef]
- Curry, P.J.; Newell, W.R.; Smith, A.C.H. Elastic and inelastic scattering of electrons by methane and ethane. J. Phys. B 1985, 18, 2303–2318. [Google Scholar] [CrossRef]
- Sohn, W.; Jung, K.; Ehrhardt, H. Threshold structures in the cross sections of low-energy electron scattering of methane. J. Phys. B 1983, 16, 891–901. [Google Scholar] [CrossRef]
- Müller, R.; Jung, K.; Kochem, K.-H.; Sohn, W.; Ehrhardt, H. Rotational excitation of CH4 by low-energy-electron collisions. J. Phys. B 1985, 18, 3971–3985. [Google Scholar] [CrossRef]
- Sohn, W.; Kochem, K.-H.; Scheuerlein, K.-M.; Jung, K.; Ehrhardt, H. Elastic electron scattering from CH4 for collision energies between 0.2 and 5 eV. J. Phys. B 1986, 19, 3625–3632. [Google Scholar] [CrossRef]
- Sakae, T.; Sumiyoshi, S.; Murakami, E.; Matsumoto, Y.; Ishibashi, K.; Katase, A. Scattering of electrons by CH4, CF4 and SF6 in the 75–700 eV range. J. Phys. B 1989, 22, 1385–1394. [Google Scholar] [CrossRef]
- Shyn, T.W.; Cravens, T.E. Angular distribution of electrons elastically scattered from CH4. J. Phys. B 1990, 23, 293–300. [Google Scholar] [CrossRef]
- Jain, A.; Thopmson, D.G. Elastic scattering of slow electrons by CH4 and H2O using a local exchange potential and new polarisation potential. J. Phys. B 1982, 15, L631–L637. [Google Scholar] [CrossRef]
- Abusalbi, N.; Eades, R.A.; Nam, T.; Thirumalai, D.; Dixon, D.A.; Truhlar, D.G. Electron scattering by methane: Elastic scattering and rotational excitation cross sections calculated with ab initio interaction potentials. J. Chem. Phys. 1983, 78, 1213–1227. [Google Scholar] [CrossRef]
- Jain, A. Total (elastic+absorption) cross sections for e-CH4 collisions in a spherical model at 0.10–500 eV. Phys. Rev. A 1986, 34, 3707–3722. [Google Scholar] [CrossRef]
- Gianturco, F.A.; Jain, A.; Pantano, L.C. Electron-methane scattering via a parameter-free model interaction. J. Phys. B 1987, 20, 571–586. [Google Scholar] [CrossRef]
- McNaughten, P.; Thompson, D.G.; Jain, A. Low-energy electron-CH4 collisions using exact exchange plus parameter-free polarisation potential. J. Phys. B 1990, 23, 2405S. [Google Scholar] [CrossRef]
- Lengsfield, B.H., III; Rescigno, T.N.; McCurdy, C.W. Ab initio study of low-energy electron-methane scattering. Phys. Rev. A 1991, 44, 4296–4308. [Google Scholar] [CrossRef]
- Gianturco, F.A.; Rodriguez-Ruiz, J.A.; Sanna, N. Elastic scattering of electrons by methane molecules. Phys. Rev. A 1995, 52, 1257–1265. [Google Scholar] [CrossRef]
- Nishimura, T.; Itikawa, Y. Elastic scattering of electrons by methane molecules. J. Phys. B 1994, 27, 2309–2316. [Google Scholar] [CrossRef]
- Nestmann, B.M.; Pfingst, K.; Peyerimhoff, S.D. R-matrix calculation for electron-methane scattering cross sections. J. Phys. B 1994, 27, 2297–2308. [Google Scholar] [CrossRef]
- Mapstone, B.; Newell, W.R. Elastic differential electron scattering from CH4, C2H4 and C2H6. J. Phys. B 1992, 25, 491–506. [Google Scholar] [CrossRef]
- Bundschu, C.T.; Gibson, J.C.; Gulley, R.J.; Brunger, M.J.; Buckman, S.J.; Sanna, N.; Gianturco, F.A. Low-energy electron scattering from methane. J. Phys. B 1997, 30, 2239–2259. [Google Scholar] [CrossRef]
- Przybyla, D.A.; Kauppila, W.E.; Kwan, C.K.; Smith, S.J.; Stein, T.S. Measurements of positron-methane differential scattering cross sections. Phys. Rev. A 1997, 55, 4244–4247. [Google Scholar] [CrossRef]
- Maji, S.; Basavaraju, G.; Bharathi, S.M.; Bhushan, K.G.; Khare, S.P. Elastic scattering of electrons by polyatomic molecules in the energy range 300–1300 eV: CO, CO2, CH4, C2H4 and C2H6. J. Phys. B 1998, 31, 4975–4990. [Google Scholar] [CrossRef]
- Basavaraju, G.; Bharathi, S.M.; Bhushan, K.G.; Maji, S.; Patil, S.H. A Unified Description of Elastic, High Energy Electron–Molecule Scattering. Phys. Scr. 1999, 60, 28–31. [Google Scholar] [CrossRef]
- Iga, I.; Lee, M.-T.; Homem, M.G.P.; Machado, L.E.; Brescansin, L.M. Elastic cross sections for CH4 collisions at intermediate energies. Phys. Rev. A 2000, 61, 022708. [Google Scholar] [CrossRef]
- Lee, M.-T.; Iga, I.; Machado, L.E.; Brescansin, L.M. Model absorption potential for electron-molecule scattering in the intermediate-energy range. Phys. Rev. A 2000, 62, 062710. [Google Scholar] [CrossRef]
- Bettega, M.H.F.; Varella, M.T. do, N.; Lima, M.A.P. Polarization effects in the elastic scattering of low-energy electrons by XH4 (X=C, Si, Ge, Sn, Pb). Phys. Rev. A 2003, 68, 012706. [Google Scholar] [CrossRef]
- Allan, M. Excitation of the four fundamental vibrations of CH4 by electron impact near threshold. J. Phys. B At. Mol. Opt. Phys. 2005, 38, 1679–1685. [Google Scholar] [CrossRef]
- Varambhia, H.N.; Munro, J.J.; Tennyson, J. R-matrix calculations of low-energy electron alkane collisions. Int. J. Mass Spectrom. 2008, 271, 1–7. [Google Scholar] [CrossRef]
- Brigg, W.J.; Tennyson, J.; Plummer, M. R-matrix calculations of low-energy electron collisions with methane. J. Phys. B 2014, 47, 185203. [Google Scholar] [CrossRef]
- Fedus, K.; Karwasz, G.P. Ramsauer-Townsend minimum in methane—Modified effective range analysis. Eur. Phys. J. D 2014, 68, 93. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Q.; Zhang, Y.; Li, H.; Feng, H.; Fan, Q. Predicting differential cross sections of electron scattering from polyatomic molecules. J. Phys. B 2015, 48, 125201. [Google Scholar] [CrossRef]
- Zammit, M.C.; Savage, J.S.; Colgan, J.; Fursa, D.V.; Kilcrease, D.P.; Bray, I.; Fontes, C.J.; Hakel, P.; Timmermans, E. State-resolved Photodissociation and Radiative Association Data for the Molecular Hydrogen Ion. Astrophys. J. 2017, 851, 64. [Google Scholar] [CrossRef]
- Mihajlov, A.A.; Sakan, N.M.; Srećković, V.A.; Vitel, Y. Modeling of continuous absorption of electromagnetic radiation in dense partially ionized plasmas. J. Phys. A. 2011, 44, 095502. [Google Scholar] [CrossRef]
- Ignjatović, L.M.; Mihajlov, A.A.; Srećković, V.A.; Dimitrijević, M.S. Absorption non-symmetric ion–atom processes in helium-rich white dwarf atmospheres. Mon. Not. R. Astron. Soc. 2014, 439, 2342–2350. [Google Scholar] [CrossRef]
- Heathcote, D.; Vallance, C. Total electron ionization cross-sections for neutral molecules relevant to astrochemistry. J. Phys. B 2018, 51, 195203. [Google Scholar] [CrossRef]
- Mihajlov, A.A.; Sakan, N.M.; Srećković, V.A.; Vitel, Y. Modeling of the continuous absorption of electromagnetic radiation in dense Hydrogen plasma. Baltic Astron. 2011, 20, 604–608. [Google Scholar] [CrossRef]
- Babb, J.F. State resolved data for radiative association of H and H+ and for Photodissociation of H2+. Astrophys. J. Suppl. Ser. 2015, 216, 21. [Google Scholar] [CrossRef]
- Coppola, C.M.; Galli, D.; Palla, F.; Longo, S.; Chluba, J. Non-thermal photons and H2 formation in the early Universe. Mon. Not. R. Astron. Soc. 2013, 434, 114–122. [Google Scholar] [CrossRef]
- Wen, Z.L.; Han, J.L. A sample of 1959 massive galaxy clusters at high redshifts. Mon. Not. R. Astron. Soc. 2018, 481, 4158–4168. [Google Scholar] [CrossRef]
- Ignjatović, L.M.; Mihajlov, A.A.; Srećković, V.A.; Dimitrijević, M.S. The ion–atom absorption processes as one of the factors of the influence on the sunspot opacity. Mon. Not. R. Astron. Soc. 2014, 441, 1504–1512. [Google Scholar] [CrossRef]
- Vujčič, V.; Jevremović, D.; Mihajlov, A.A.; Ignjatović, L.M.; Srećković, V.A.; Dimitrijević, M.S.; Malović, M. MOL-D: A Collisional Database and Web Service within the Virtual Atomic and Molecular Data Center. J. Astrophys. Astron. 2015, 36, 693–703. [Google Scholar] [CrossRef]
- Srećković, V.A.; Ignjatović, L.M.; Jevremović, D.; Vujčić, V.; Dimitrijević, M.S. Radiative and Collisional Molecular Data and Virtual Laboratory Astrophysics. Atoms 2017, 5, 31. [Google Scholar] [CrossRef]
- Pichler, G.; Makdisi, Y.; Kokaj, J.; Mathew, J.; Rakić, M.; Beuc, R. Superheating effects in line broadening of dense alkali vapors. J. Phys. Conf. Ser. 2017, 810, 012013. [Google Scholar] [CrossRef]
- Strobel, D.F.; Zhu, X.; Summers, M.E. On the vertical thermal structure of Io’s atmosphere. Icarus 1994, 111, 18–30. [Google Scholar] [CrossRef]
- Dalgarno, A.; Black, J.H. Molecule formation in the interstellar gas. Rep. Prog. Phys. 1976, 39, 573. [Google Scholar] [CrossRef]
- Zwölf, C.-M.; Moreau, N.; Dubernet, M.-L. New model for datasets citation and extraction reproducibility in VAMDC. J. Mol. Spectrosc. 2016, 327, 122–137. [Google Scholar] [CrossRef]
- Regandell, S.; Marquart, T.; Piskunov, N. Inside a VAMDC data node—Putting standards into practical software. Phys. Scr. 2018, 93, 035001. [Google Scholar] [CrossRef]
- Asmi, A.; Rauber, A.; Pröll, S.; van Uytvanck, D. Citing Dynamic Data-Research Data Alliance working group recommendations. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 17–22 April 2016; Volume 18. [Google Scholar]
- Moreau, N.; Zwolf, C.-M.; Ba, Y.-A.; Richard, C.; Boudon, V.; Dubernet, M.-L. The VAMDC Portal as a Major Enabler of Atomic and Molecular Data Citation. Galaxies 2018, 6, 105. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinković, B.P.; Srećković, V.A.; Vujčić, V.; Ivanović, S.; Uskoković, N.; Nešić, M.; Ignjatović, L.M.; Jevremović, D.; Dimitrijević, M.S.; Mason, N.J. BEAMDB and MOLD—Databases at the Serbian Virtual Observatory for Collisional and Radiative Processes. Atoms 2019, 7, 11. https://doi.org/10.3390/atoms7010011
Marinković BP, Srećković VA, Vujčić V, Ivanović S, Uskoković N, Nešić M, Ignjatović LM, Jevremović D, Dimitrijević MS, Mason NJ. BEAMDB and MOLD—Databases at the Serbian Virtual Observatory for Collisional and Radiative Processes. Atoms. 2019; 7(1):11. https://doi.org/10.3390/atoms7010011
Chicago/Turabian StyleMarinković, Bratislav P., Vladimir A. Srećković, Veljko Vujčić, Stefan Ivanović, Nebojša Uskoković, Milutin Nešić, Ljubinko M. Ignjatović, Darko Jevremović, Milan S. Dimitrijević, and Nigel J. Mason. 2019. "BEAMDB and MOLD—Databases at the Serbian Virtual Observatory for Collisional and Radiative Processes" Atoms 7, no. 1: 11. https://doi.org/10.3390/atoms7010011
APA StyleMarinković, B. P., Srećković, V. A., Vujčić, V., Ivanović, S., Uskoković, N., Nešić, M., Ignjatović, L. M., Jevremović, D., Dimitrijević, M. S., & Mason, N. J. (2019). BEAMDB and MOLD—Databases at the Serbian Virtual Observatory for Collisional and Radiative Processes. Atoms, 7(1), 11. https://doi.org/10.3390/atoms7010011







