Abscisic Acid Receptors Modulate Metabolite Levels and Phenotype in Arabidopsis Under Normal Growing Conditions
Abstract
1. Introduction
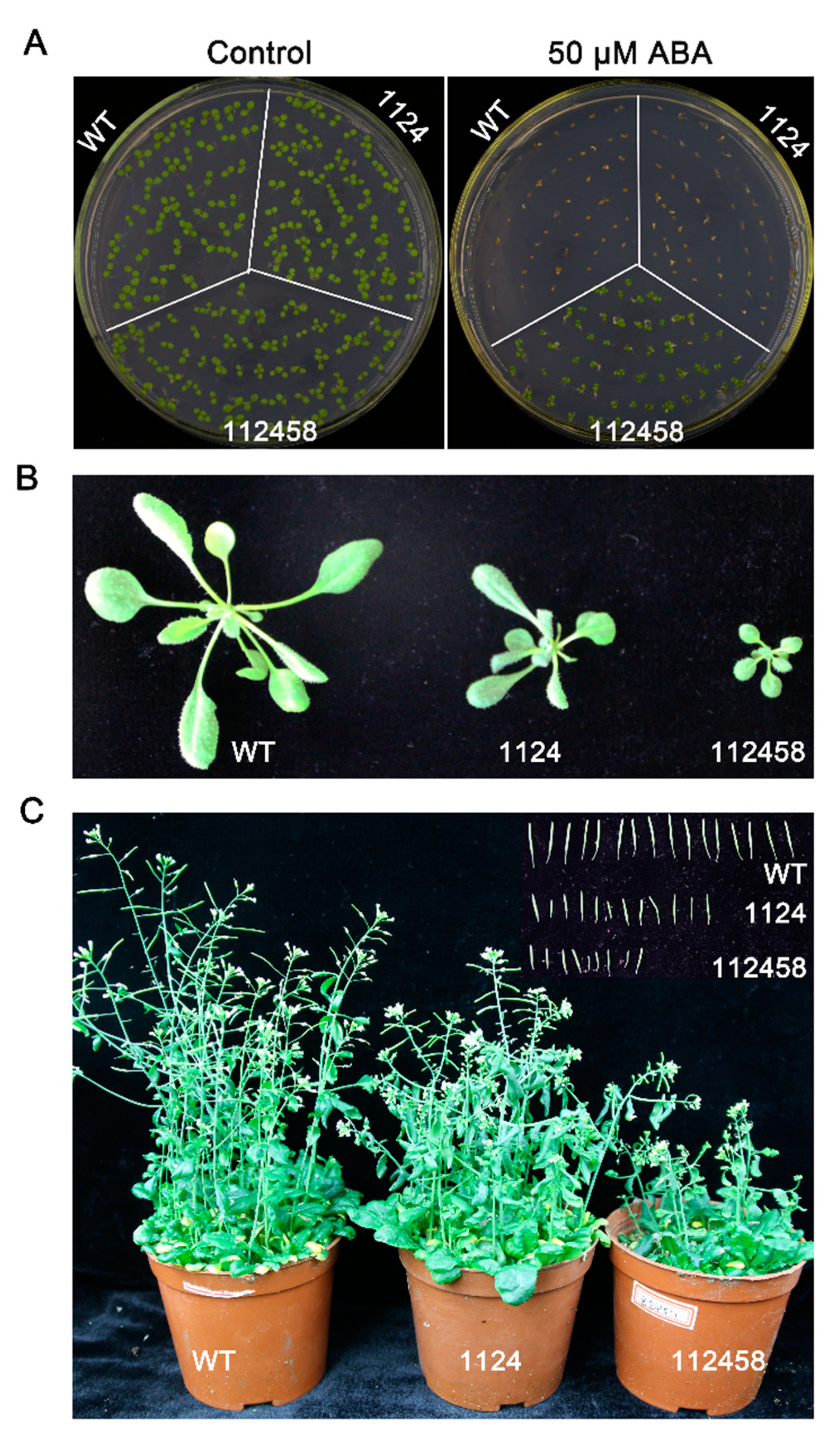
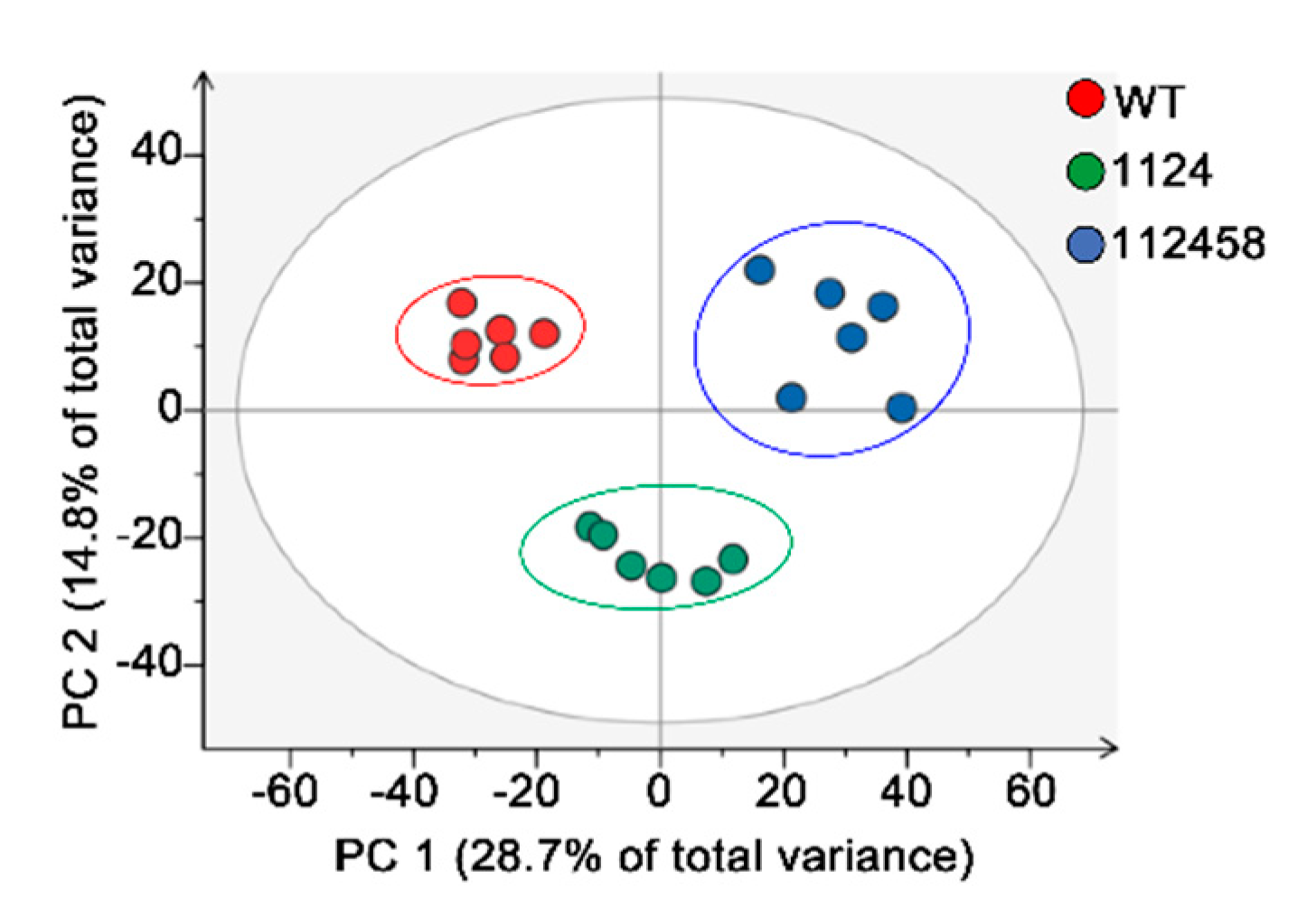
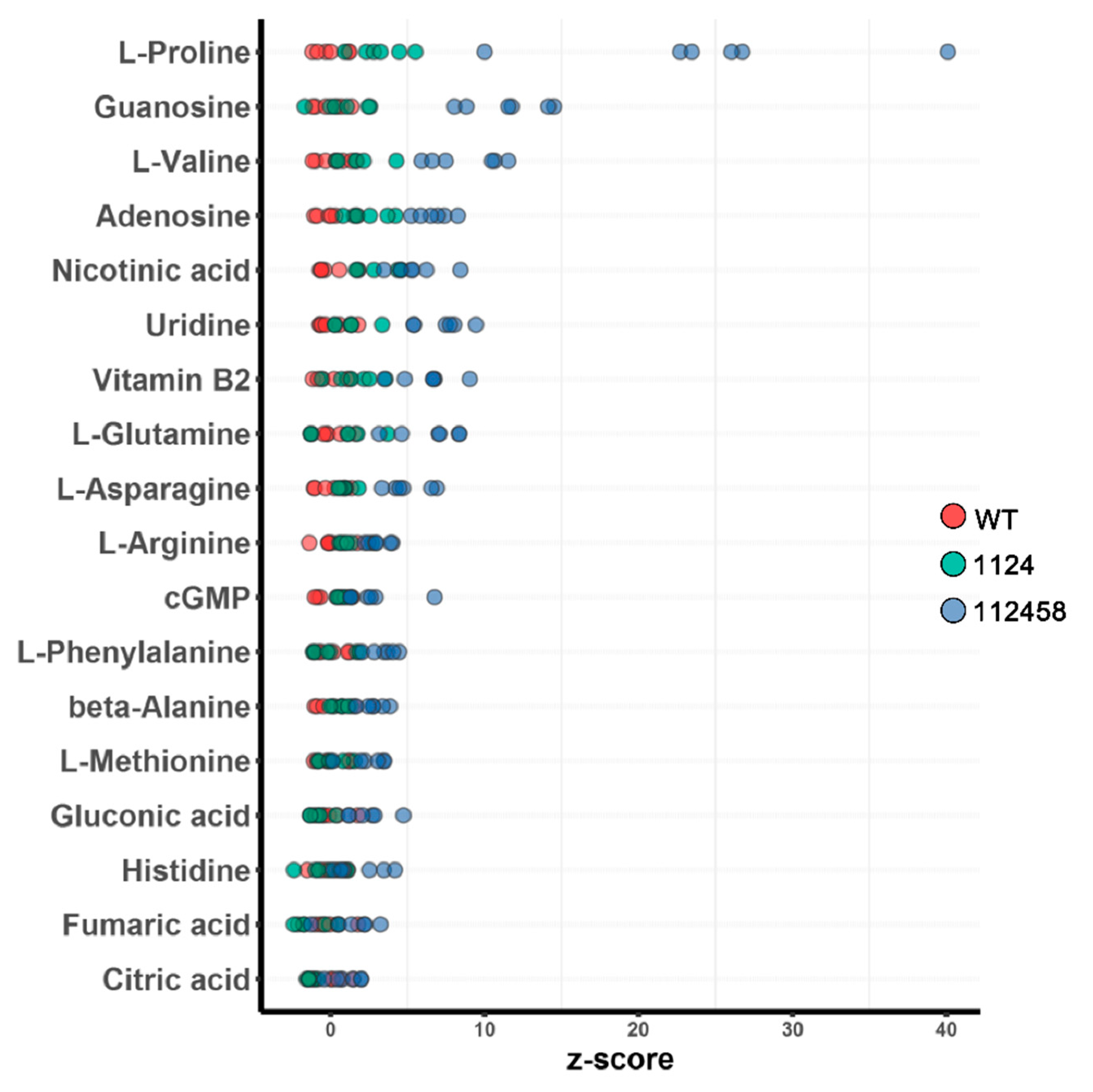
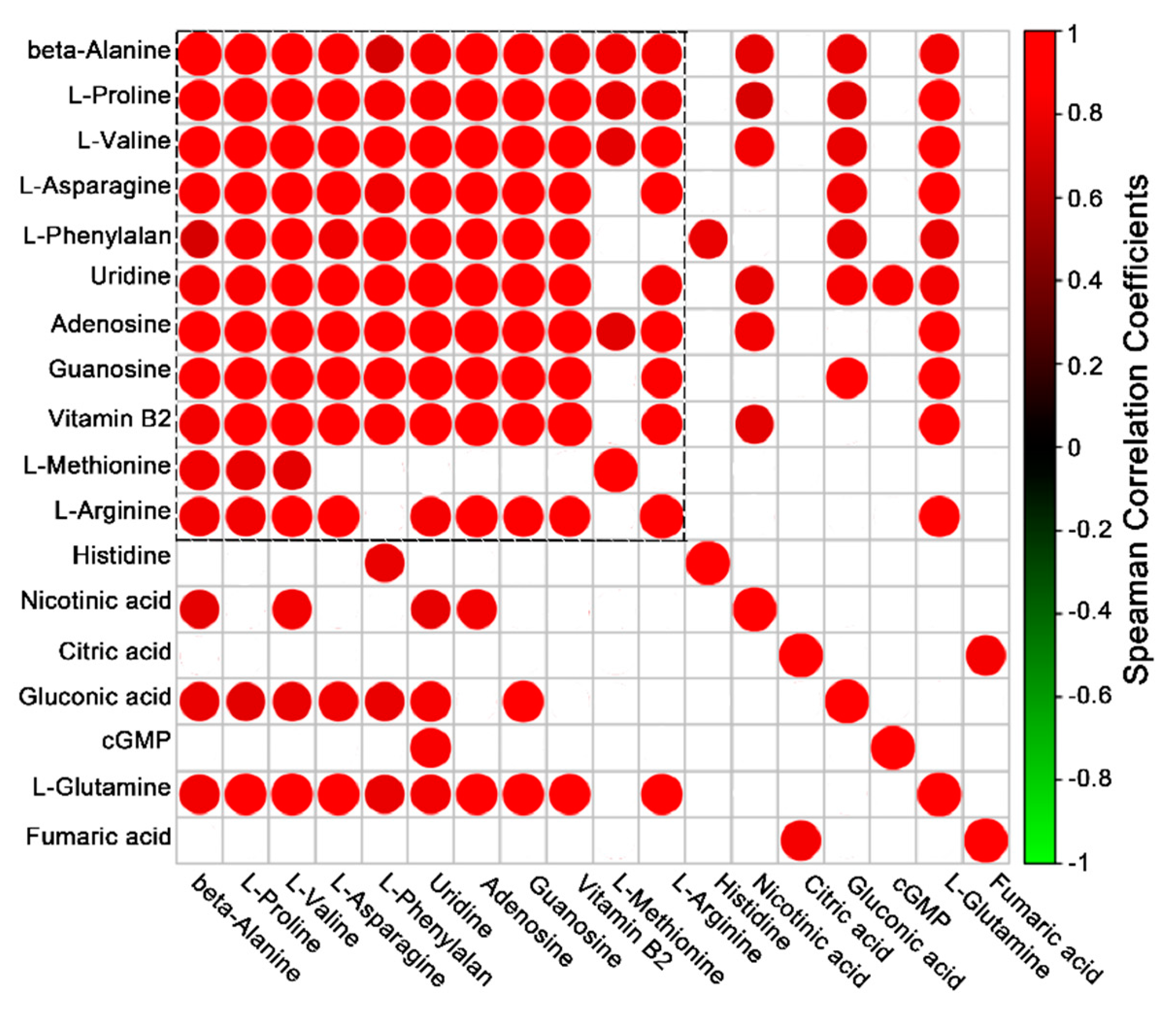
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Seedling Establishment Assay
4.3. Metabolite Extraction and Sample Preparation for Metabolomics
4.4. Metabolomics Data Processing and Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| WT | wild-type Arabidopsis thaliana |
| 1124 | pyr1pyl1pyl2pyl4 |
| 112458 | pyr1pyl1pyl2pyl4pyl5pyl8 |
| PYR/PYL/RCARs | PYRABACTINRESISTANT/PYRABACTIN RESISTANT-LIKE/REGULATORY COMPONENT OF ABA RECEPTORs |
| GC-MS | Gas chromatography-mass spectrometry |
References
- Leung, J.; Giraudat, J. Abscisic acid signal transduction. Annu. Rev. Plant Biol. 1998, 49, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA-Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic Acid: Emergence of a Core Signaling Network. Annu. Rev. Plant Boil. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef]
- Papacek, M.; Christmann, A.; Grill, E. Interaction network of ABA receptors in grey poplar. Plant J. 2017, 92, 199–210. [Google Scholar] [CrossRef]
- Pri-Tal, O.; Shaar-Moshe, L.; Wiseglass, G.; Peleg, Z.; Mosquna, A. Non-redundant functions of the dimeric ABA receptor BdPYL1 in the grass Brachypodium. Plant J. 2017, 92, 774–786. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.-Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.-K. In vitro reconstitution of an abscisic acid signalling pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef]
- Tischer, S.V.; Wunschel, C.; Papacek, M.; Kleigrewe, K.; Hofmann, T.; Christmann, A.; Grill, E. Combinatorial interaction network of abscisic acid receptors and coreceptors from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, 10280–10285. [Google Scholar] [CrossRef]
- Hao, Q.; Yin, P.; Li, W.; Wang, L.; Yan, C.; Lin, Z.; Wu, J.Z.; Wang, J.; Yan, S.F.; Yan, N. The Molecular Basis of ABA-Independent Inhibition of PP2Cs by a Subclass of PYL Proteins. Mol. Cell 2011, 42, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Dupeux, F.; Round, A.; Antoni, R.; Park, S.-Y.; Jamin, M.; Cutler, S.R.; Rodríguez, P.L.; Márquez, J.A. The abscisic acid receptor PYR1 in complex with abscisic acid. Nature 2009, 462, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Xin, Q.; Yu, L.; Wang, Z.; Wu, W.; Jiang, L.; Wang, G.; Tian, W.; Deng, Z.; et al. Complex Structures of the Abscisic Acid Receptor PYL3/RCAR13 Reveal a Unique Regulatory Mechanism. Structure 2012, 20, 780–790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belda-Palazon, B.; Gonzalez-Garcia, M.P.; Lozano-Juste, J.; Coego, A.; Antoni, R.; Julian, J.; Fernandez, M.A. PYL8 mediates ABA perception in the root through non-cell-autonomous and ligand-stabilization-based mechanisms. Proc. Natl. Acad. Sci. USA 2018, 115, E11857–E11863. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Hitomi, K.; Arvai, A.S.; Rambo, R.P.; Hitomi, C.; Cutler, S.R.; Schroeder, J.I.; Getzoff, E.D. Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science 2009, 326, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Guzman, M.; Pizzio, G.A.; Antoni, R.; Vera-Sirera, F.; Merilo, E.; Bassel, G.W.; Fernández, M.A.; Holdsworth, M.J.; Perez-Amador, M.A.; Kollist, H.; et al. Arabidopsis PYR/PYL/RCAR Receptors Play a Major Role in Quantitative Regulation of Stomatal Aperture and Transcriptional Response to Abscisic Acid. Plant Cell 2012, 24, 2483–2496. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Sarkeshik, A.; Nito, K.; Park, S.Y.; Wang, A.; Carvalho, P.C.; Lee, S.; Caddell, D.F.; Cutler, S.R.; Chory, J.; et al. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J. 2010, 61, 290–299. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef]
- Antoni, R.; GonzalezGuzman, M.; Rodriguez, L.; PeiratsLlobet, M.; Pizzio, G.A.; Winne, N.D.; Jaeger, G.D.; Dietrich, D.; Bennett, M.J.; Rodriguez, P.L. PYL8 plays an important role for regulation of ABA signaling in root. Plant Physiol. 2013, 16, 931–941. [Google Scholar] [CrossRef]
- Zhang, J.; Hafeez, M.T.; Di, D.; Wu, L.; Zhang, L. Precise control of ABA signaling through post-translational protein modification. Plant Growth Regul. 2019, 88, 99–111. [Google Scholar] [CrossRef]
- Benina, M.; Obata, T.; Mehterov, N.; Ivanov, I.; Petrov, V.; Toneva, V.; Fernie, A.R.; Gechev, T.S. Comparative metabolic profiling of Haberlea rhodopensis, Thellungiella halophyla, and Arabidopsis thaliana exposed to low temperature. Front. Plant Sci. 2013, 4, 499. [Google Scholar] [CrossRef]
- Miao, C.; Xiao, L.; Hua, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. USA 2018, 115, 6058–6063. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Wijnands, K.A.; Castermans, T.M.; Hommen, M.P.; Meesters, D.M.; Poeze, M. Arginine and Citrulline and the Immune Response in Sepsis. Nutrients 2015, 7, 1426–1463. [Google Scholar] [CrossRef]
- Castillo, M.-C.; Lozano-Juste, J.; González-Guzmán, M.; Rodriguez, L.; Rodriguez, P.L.; León, J. Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci. Signal. 2015, 8, ra89. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Beard, K.F.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Tcherkez, G.; Mahé, A.; Gauthier, P.; Mauve, C.; Gout, E.; Bligny, R.; Cornic, G.; Hodges, M. In Folio Respiratory Fluxomics Revealed by 13C Isotopic Labeling and H/D Isotope Effects Highlight the Noncyclic Nature of the Tricarboxylic Acid “Cycle” in Illuminated Leaves1[W]. Plant Physiol. 2009, 151, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Obata, T.; Feil, R.; Lunn, J.E.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Fernie, A.R. The Role of Abscisic Acid Signaling in Maintaining the Metabolic Balance Required for Arabidopsis Growth under Non-stress Conditions. Plant Cell 2019, 31, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Shin, D.; Jeon, B.W.; Kwak, J.M. ROS-Mediated ABA Signaling. J. Plant Biol. 2009, 52, 102–113. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, B.; Li, H.; Liu, R.; Kalia, R.K.; Zhu, J.K.; Chinnusamy, V. Arabidopsis proline-rich protein important for development and abiotic stress tolerance is involved in microRNA biogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 18198–18203. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yu, B.; Zhu, N.; Dufresne, C.; Chen, S. Metabolomics and Proteomics of Brassica napus Guard Cells in Response to Low CO2. Front. Mol. Biosci. 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Deng, L.; Zhang, L.; Yue, X.; Mao, J.; Ma, F.; Wang, X.; Zhang, Q.; Zhang, W.; Li, P. Comparative Metabolomic Analysis of Rapeseeds from Three Countries. Metabolites 2019, 9, 161. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wu, L.; Qiu, Y.; Wang, T.; Zhou, Q.; Zhang, Q.; Zhang, W.; Liu, Z. Abscisic Acid Receptors Modulate Metabolite Levels and Phenotype in Arabidopsis Under Normal Growing Conditions. Metabolites 2019, 9, 249. https://doi.org/10.3390/metabo9110249
Li X, Wu L, Qiu Y, Wang T, Zhou Q, Zhang Q, Zhang W, Liu Z. Abscisic Acid Receptors Modulate Metabolite Levels and Phenotype in Arabidopsis Under Normal Growing Conditions. Metabolites. 2019; 9(11):249. https://doi.org/10.3390/metabo9110249
Chicago/Turabian StyleLi, Xiaoyi, Lintao Wu, Yao Qiu, Tao Wang, Qin Zhou, Qian Zhang, Wei Zhang, and Zhibin Liu. 2019. "Abscisic Acid Receptors Modulate Metabolite Levels and Phenotype in Arabidopsis Under Normal Growing Conditions" Metabolites 9, no. 11: 249. https://doi.org/10.3390/metabo9110249
APA StyleLi, X., Wu, L., Qiu, Y., Wang, T., Zhou, Q., Zhang, Q., Zhang, W., & Liu, Z. (2019). Abscisic Acid Receptors Modulate Metabolite Levels and Phenotype in Arabidopsis Under Normal Growing Conditions. Metabolites, 9(11), 249. https://doi.org/10.3390/metabo9110249



