Metabolic Profiling of Fish Meat by GC-MS Analysis, and Correlations with Taste Attributes Obtained Using an Electronic Tongue
Abstract
:1. Introduction
2. Results and Discussion
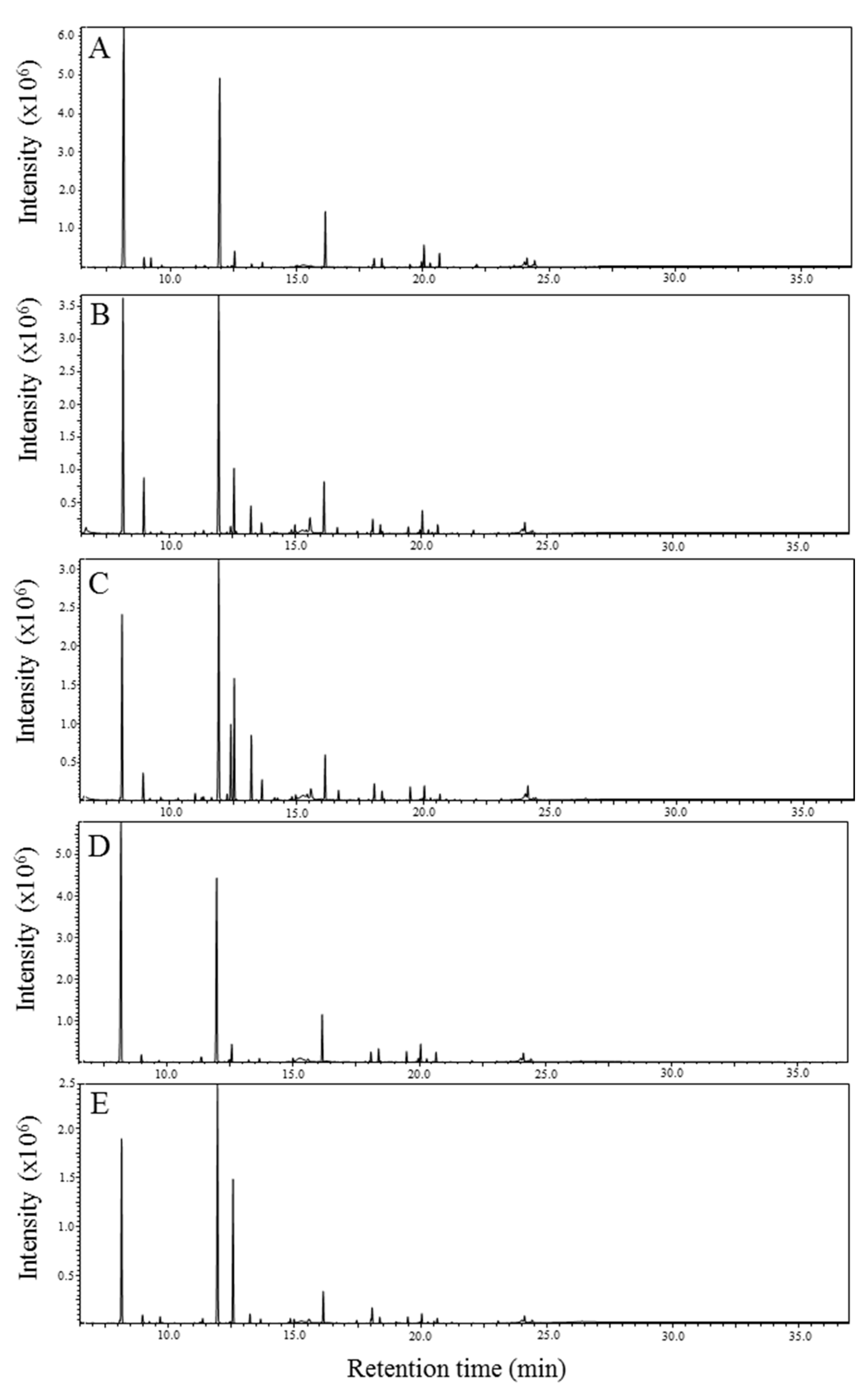
2.1. GC-MS Analysis
2.2. Electronic Tongue
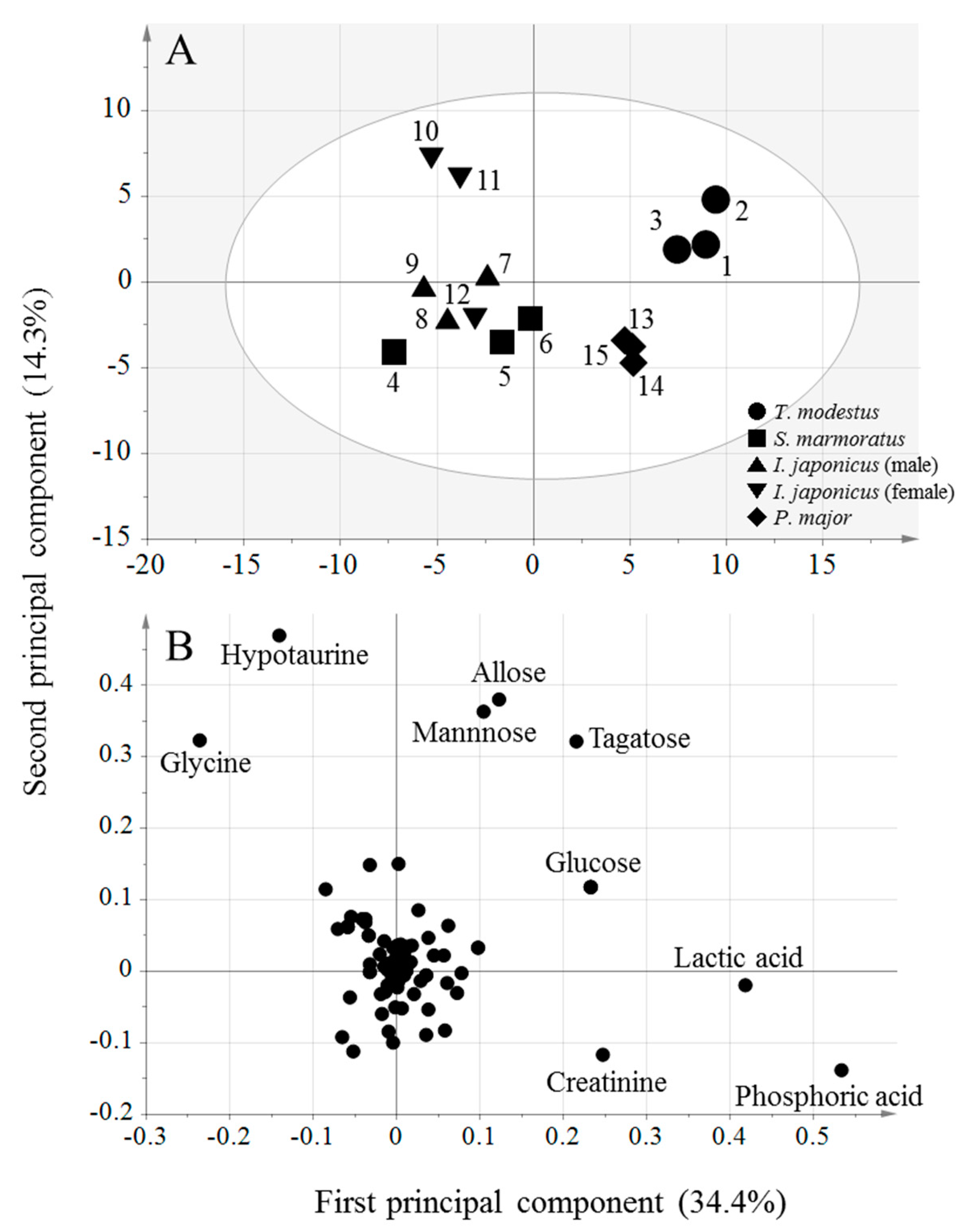
2.3. OPLS Analysis
3. Materials and Methods
3.1. Experimental Samples
3.2. GC-MS Analysis
3.2.1. Pretreatment
3.2.2. Analytical Conditions
3.2.3. Data Processing
3.3. Electronic Tongue
3.3.1. Sample Preparation
3.3.2. Method of Measurement
3.3.3. Statistical Analysis
3.4. OPLS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Fisheries Agency. FY2016 Trends in Fisheries FY2017 Fisheries Policy White Paper on Fisheries; Association of Agriculture and Forestry Statistics: Tokyo, Japan, 2017; pp. 114–133. ISBN 978-4-541-04153-1.
- Fuke, S.; Konosu, S. Taste-active components in some foods: A review of Japanese research. Physiol. Behav. 1991, 49, 863–868. [Google Scholar] [CrossRef]
- Kubota, S.; Itoh, K.; Niizeki, N.; Song, X.A.; Okimoto, K.; Ando, M.; Murata, M.; Sakaguchi, M. Organic taste-active components in the hot-water extract of yellowtail muscle. Food Sci. Technol. Res. 2002, 8, 45–49. [Google Scholar] [CrossRef]
- Sakaguchi, M. Nitrogenous low-molecular-weight components and palatability of fish and shellfish. NIPPON SUISAN GAKK 2001, 67, 787–793. [Google Scholar] [CrossRef]
- Zhang, M.X.; Wang, X.C.; Liu, Y.; Xu, XL.; Zhou, G.H. Isolation and identification of flavour peptides from Puffer fish (Takifugu obscurus) muscle using an electronic tongue and MALDI-TOF/TOF MS/MS. Food Chem. 2012, 135, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Putri, S.P.; Yamamoto, S.; Tsugawa, H.; Bamba, T.; Fukusaki, E. Current metabolomics: Practical applications. J. Biosci. Bioeng. 2013, 115, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Putri, S.P.; Nakayama, Y.; Matsuda, F.; Uchikata, T.; Kobayashi, S.; Matsubara, A.; Fukusaki, E. Current metabolomics: Technological advances. J. Biosci. Bioeng. 2013, 116, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shiga, K.; Yamamoto, S.; Nakajima, A.; Kodama, Y.; Imamura, M.; Sato, T.; Uchida, R.; Obata, A.; Bamba, T.; Fukusaki, E. Metabolic profiling approach to explore the compounds related to the umami of soy sauce. J. Agric. Food Chem. 2014, 62, 7317–7322. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Toko, K. Electronic tongues–a review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Yokoyama, M.; Kaneniwa, M.; Sakaguchi, M. Metabolites of L-[35S]cysteine injected into the peritoneal cavity of rainbow trout. Fish. Sci. 1997, 63, 799–801. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Murata, M.; Kawai, A. Taurine levels in some tissues of yellowtail (Seriola quinqueradiata) and mackerel (Scomber japonicus). Agric. Biol. Chem. 1982, 46, 2857–2858. [Google Scholar] [CrossRef]
- Goto, T.; Tiba, K.; Sakurada, Y.; Takagi, S. Determination of hepatic cysteinesulfinate decarboxylase activity in fish by means of OPA-prelabeling and reverse-phase high-performance liquid chromatographic separation. Fish. Sci. 2001, 67, 553–555. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near Infrared Technology in the Agricultural and Food Industries; Amer Assn of Cereal Chemists: Denver, CO, USA, 1987; pp. 143–167. ISBN 978-0913250495. [Google Scholar]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Hiratsuka, S. Effects of drying on the volatile flavor compounds in the extracts from niboshi (soup stock). NIPPON SUISAN GAKK 2017, 83, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Murayama, F.; Sato, J.; Touhata, K.; Ishida, N. Taste evaluations of ovigerous and soft-shelled female swimming crab Portunus trituberculatus meat extracts. NPPON SUISAN GAKK 2018, 84, 425–433. [Google Scholar] [CrossRef]
- Karanova, M.V. Phosphoethanolamine in the brain of the eurythermal pond fish Perccottus glehni (Eleotridae, Perciformes, Dyb. 1877) as a phenomenon depending on temperature factor. Zh. Evol. Biokhim. Fiziol. 2013, 49, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, R.; Adachi, M.; Kikutani, H.; Tanimoto, S. Discriminant analysis of muscle tissue type in yellowtail Seriola quinqueradiata muscle based on metabolic component profiles. Food Sci. Technol. Res. 2018, 24, 883–891. [Google Scholar] [CrossRef]
- Anjiki, N.; Hosoe, J.; Fuchino, H.; Kiuchi, F.; Sekita, S.; Ikezaki, H.; Mikage, M.; Kawahara, N.; Goda, Y. Evaluation of the taste of crude drug and Kampo formula by a taste-sensing system (4): Taste of processed aconite root. J. Nat. Med. 2011, 65, 293–300. [Google Scholar] [CrossRef] [PubMed]
| Taste Attributes | Fish Species | ||||
|---|---|---|---|---|---|
| Thamnaconus modestus | Pagrus major | Inimicus japonicus (Male) | Inimicus japonicus (Female) | Sebastiscus marmoratus | |
| Sourness | −28.7 ± 1.03 a | −29.5 ± 1.09 a | −34.4 ± 0.92 b | −35.5 ± 1.24 b | −35.3 ± 1.39 b |
| Acidic bitterness | 5.99 ± 0.77 a | 5.39 ± 0.70 a | 8.61 ± 1.11 b | 8.53 ± 0.20 b | 8.83 ± 1.13 b |
| Irritant | −0.76 ± 0.03 | −0.83 ± 0.19 | −0.11 ± 0.17 | −0.30 ± 0.58 | −0.70 ± 0.21 |
| Umami | 11.0 ± 0.33 a | 11.6 ± 0.46 ab | 11.8 ± 0.10 ab | 12.3 ± 0.28 b | 12.4 ± 0.47 b |
| Saltiness | −17.4 ± 0.48 a | −18.3 ± 0.11 a | −18.1 ± 0.52 a | −18.6 ± 1.66 ab | −20.7 ± 0.42 b |
| Bitterness | −0.25 ± 0.08 | −0.48 ± 0.08 | −0.29 ± 0.34 | −0.40 ± 0.18 | −0.40 ± 0.19 |
| Astringency | −0.27 ± 0.03 | −0.27 ± 0.03 | −0.25 ± 0.03 | −0.25 ± 0.01 | −0.28 ± 0.00 |
| Richness | 1.13 ± 0.24 | 1.09 ± 0.25 | 0.96 ± 0.22 | 0.93 ± 0.18 | 0.85 ± 0.24 |
| Taste | Scaling | A a | N b | R2X | R2Y | Q2Y | y | R2 | RMSEE | RMSEcv |
|---|---|---|---|---|---|---|---|---|---|---|
| Sourness | UV | 1 + 0 + 0 | 15 | 0.344 | 0.907 | 0.874 | 0.996x − 0.384 | 0.91 * | 1.02 | 1.14 |
| None | 1 + 1 + 0 | 15 | 0.982 | 0.989 | 0.984 | 0.434x − 18.62 | 0.27 | - | - | |
| Par | 1 + 0 + 0 | 15 | 0.587 | 0.880 | 0.859 | 1.000x − 0.034 | 0.88 * | 1.16 | 1.17 | |
| Acidic bitterness | UV | 1 + 0 + 0 | 15 | 0.343 | 0.771 | 0.697 | 0.983x + 0.246 | 0.78 * | 0.83 | 0.91 |
| None | 1 + 1 + 0 | 15 | 0.982 | 0.678 | 0.595 | 0.729x + 2.095 | 0.44 | - | - | |
| Par | 1 + 0 + 0 | 15 | 0.586 | 0.742 | 0.698 | 0.998x + 0.034 | 0.74 * | 0.89 | 0.89 | |
| Irritant | UV | 1 + 1 + 0 | 15 | 0.471 | 0.872 | 0.661 | 0.972x + 0.006 | 0.88 * | 0.15 | 0.25 |
| None | 1 + 1 + 0 | 15 | 0.981 | 0.847 | 0.761 | 0.882x − 0.062 | 0.54 | - | - | |
| Par | 1 + 0 + 0 | 15 | 0.574 | 0.468 | 0.362 | - | - | - | - | |
| Umami | UV | 1 + 1 + 0 | 15 | 0.439 | 0.894 | 0.663 | 0.963x + 0.442 | 0.90 * | 0.21 | 0.41 |
| None | 1 + 1 + 0 | 15 | 0.982 | 0.989 | 0.985 | −0.179x + 13.92 | 0.08 | - | - | |
| Par | 1 + 0 + 0 | 15 | 0.582 | 0.55 | 0.435 | - | - | |||
| Saltiness | UV | 1 + 2 + 0 | 15 | 0.557 | 0.963 | 0.81 | 0.965x − 0.654 | 0.96 * | 0.29 | 0.67 |
| None | 1 + 1 + 0 | 15 | 0.982 | 0.988 | 0.983 | −0.152x − 21.42 | 0.03 | - | - | |
| Par | 1 + 1 + 0 | 15 | 0.697 | 0.854 | 0.599 | 0.998x + 0.013 | 0.86 * | 0.55 | 0.83 | |
| Bitterness | UV | - | - | - | - | - | - | - | - | - |
| None | 1 + 1 + 0 | 15 | 0.981 | 0.828 | 0.745 | 1.094x + 0.034 | 0.14 | 0.19 | 0.20 | |
| Par | - | - | - | - | - | - | - | - | - | |
| Astringency | UV | 1 + 0 + 0 | 15 | 0.23 | 0.537 | −0.086 | - | - | - | - |
| None | 1 + 1 + 0 | 15 | 0.982 | 0.991 | 0.988 | 0.379x − 0.164 | 0.12 | 0.03 | 0.03 | |
| Par | 1 + 0 + 0 | 15 | 0.52 | 0.192 | 0.0325 | - | - | - | - | |
| Richness | UV | 1 + 1 + 0 | 15 | 0.451 | 0.864 | 0.673 | 0.928x + 0.072 | 0.87 * | 0.09 | 0.16 |
| None | 1 + 1 + 0 | 15 | 0.982 | 0.979 | 0.969 | 1.338x − 0.339 | 0.55 | - | - | |
| Par | 1 + 0 + 0 | 15 | 0.576 | 0.382 | 0.215 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabuchi, R.; Ishimaru, A.; Tanaka, M.; Kawaguchi, O.; Tanimoto, S. Metabolic Profiling of Fish Meat by GC-MS Analysis, and Correlations with Taste Attributes Obtained Using an Electronic Tongue. Metabolites 2019, 9, 1. https://doi.org/10.3390/metabo9010001
Mabuchi R, Ishimaru A, Tanaka M, Kawaguchi O, Tanimoto S. Metabolic Profiling of Fish Meat by GC-MS Analysis, and Correlations with Taste Attributes Obtained Using an Electronic Tongue. Metabolites. 2019; 9(1):1. https://doi.org/10.3390/metabo9010001
Chicago/Turabian StyleMabuchi, Ryota, Ayaka Ishimaru, Mao Tanaka, Osamu Kawaguchi, and Shota Tanimoto. 2019. "Metabolic Profiling of Fish Meat by GC-MS Analysis, and Correlations with Taste Attributes Obtained Using an Electronic Tongue" Metabolites 9, no. 1: 1. https://doi.org/10.3390/metabo9010001





