Identifying Antibacterial Compounds in Black Walnuts (Juglans nigra) Using a Metabolomics Approach
Abstract
1. Introduction
2. Results
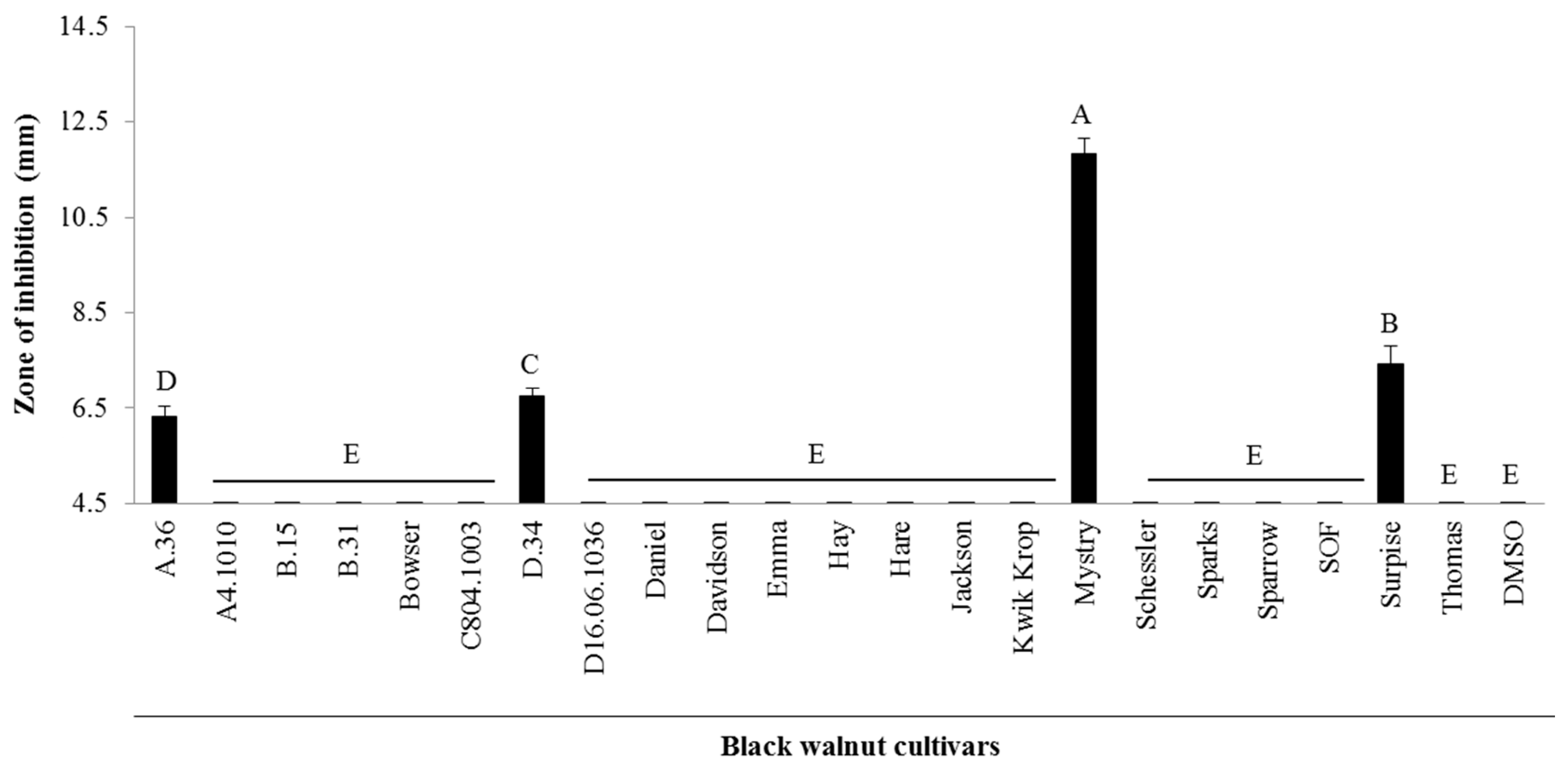
2.1. Antibacterial Activity from Twenty-Two Black Walnut Cultivars
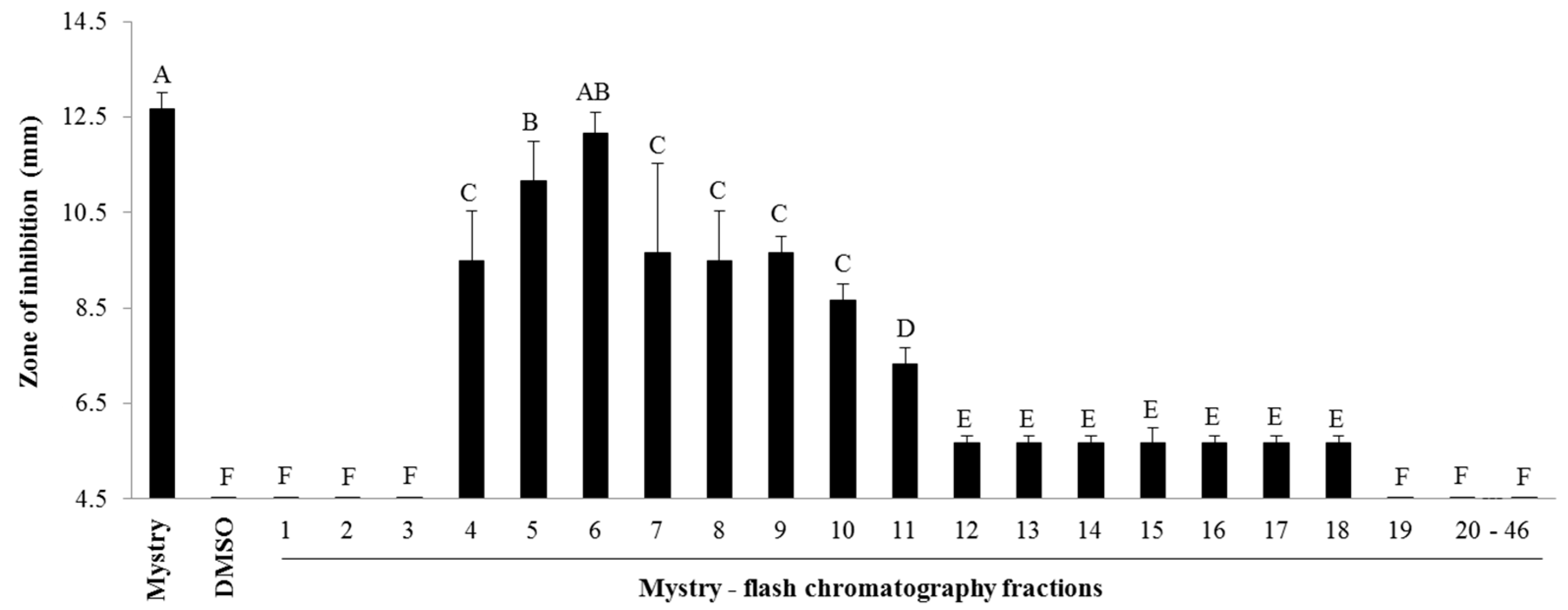
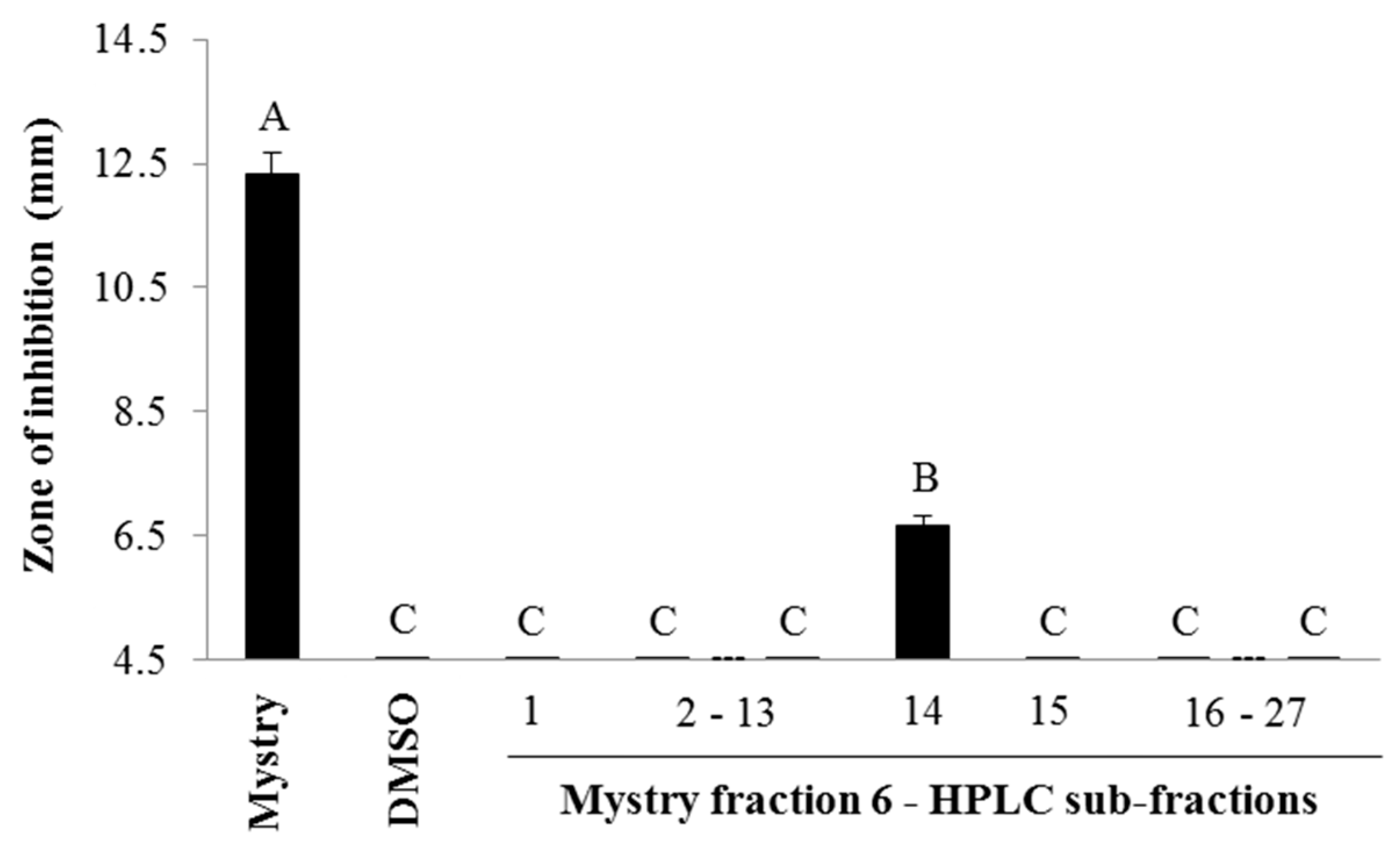
2.2. Identification of Antibacterial Compounds Derived from the Kernel Extract of Mystry
2.2.1. Column Chromatography
2.2.2. HPLC Analysis
2.2.3. UHPLC-QTOF-MS/MS Analysis to Identifying the Bioactive Compounds
3. Discussion
4. Materials and Methods
4.1. Black Walnut Cultivars
4.2. Extraction of Bioactive Compounds from the Kernels of Black Walnuts
4.3. Antibacterial Assay
4.4. Identification of Bioactive Compounds Using a Metabolic Approach
4.4.1. Column Chromatography
4.4.2. HPLC Analysis
4.4.3. UHPLC-QTOF-MS/MS Analysis
4.4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Randolph, K.C.; Rose, A.K.; Oswalt, C.M.; Brown, M.J. Status of black walnut (Juglans nigra L.) in the eastern United States in light of the discovery of thousand cankers disease. Castanea 2013, 78, 2–14. [Google Scholar] [CrossRef]
- McGranahan, G.; Leslie, C. Walnuts (Juglans). Acta Hortic. 1991, 290, 907–974. [Google Scholar] [CrossRef]
- Harlow, W.M.; Harrar, E.S. Textbook of Dendrology (American Forestry); Mcgraw-Hill Book Company Inc.: New York, NY, USA, 1968. [Google Scholar]
- Newton, L.; Fowler, G.; Neeley, A.D.; Schall, R.A.; Takeuchi, Y. Pathway Assessment: Geosmithia sp. and Pityophthorus juglandis Blackman Movement from the Western into the Eastern United States. Available online: https://agriculture.mo.gov/plants/pdf/tc_pathwayanalysis.pdf (accessed on 9 August 2018).
- Câmara, C.R.S.; Schlegel, V. A review on the potential human health benefits of the black walnut: A comparison with the English walnuts and other tree nuts. Int. J. Food Prop. 2016, 19, 2175–2189. [Google Scholar] [CrossRef]
- Zarger, T.G. Black walnuts as nut trees. In Handbook of North American Nut Trees; Jaynes, R.A., Ed.; Humphrey Press: Geneva, NY, USA, 1969; pp. 203–211. [Google Scholar]
- Williams, R.D. Juglans nigra L., black walnut. Silv. N. Am. 1990, 2, 391–399. [Google Scholar]
- Reid, W. Black walnuts. In Registry of New Fruit and Nut Varieties; Brooks, R., Olmo, H.P., Eds.; ASHS Press: Alexandria, VA, USA, 1997; pp. 156–160. [Google Scholar]
- Reid, W.; Coggeshall, M.V.; Hunt, K.L. Cultivar evaluation and development for black walnut orchards. In Proceedings of the 6th Walnut Council Research Symposium; Michler, C.H., Pijut, P.M., Van Sambeek, J.W., Coggeshall, M.V., Seifert, J., Woeste, K., Overton, R., Ponder, F., Jr., Eds.; North Central Research Station: Paul, MN, USA, 2004; pp. 18–24. [Google Scholar]
- Reid, W.; Coggeshall, M.V.; Garrett, H.E.; Van Sambeek, J.W. Growing Black Walnut for Nut Production. Available online: http://www.centerforagroforestry.org/pubs/walnutNuts.pdf (accessed on 29 August 2018).
- Vu, D.; Vo, P.; Coggeshall, M.; Lin, C. Identification and characterization of phenolic compounds in black walnut kernels. J. Agric. Food Chem. 2018, 66, 4503–4511. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.A.; Garcia, M.D.; Saenz, M.T. Antibacterial activity of the phenolic acids fractions of Scrophularia frutescens and Scrophularia sambucifolia. J. Ethnopharmacol. 1996, 53, 11–14. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tomiyama, D.; Yui, K.; Katsuki, M.; Ikeda, K.; Nonaka, A.; Miyamoto, T. Mechanism for the antibacterial action of epigallocatechin gallate (EGCg) on Bacillus subtilis. Biosci. Biotechnol. Biochem. 2015, 79, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The combination of catechin and epicatechin gallate from Fructus crataegi potentiates β-lactam antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) in vitro and in vivo. Int. J. Mol. Sci. 2013, 14, 1802–1821. [Google Scholar] [CrossRef] [PubMed]
- Watt, E.; Pretorius, J.C. Purification and identification of active antibacterial components in Carpobrotus edulis L. J. Ethnopharmacol. 2001, 76, 87–91. [Google Scholar] [CrossRef]
- Tsui, V.; Wong, R.; Rabie, A.M. The inhibitory effects of naringin on the growth of periodontal pathogens in vitro. Phytother. Res. 2008, 22, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Moori Bakhtiari, N.; Jamshidian, J.; Khalafi, E. Effect of Juglans regia L. stem bark hydroalcoholic extract on Methicillin Resistant Staphylococcus aureus. Jundishapur J. Nat. Pharm. Prod. 2016, 11, 1–5. [Google Scholar] [CrossRef]
- Blunt, J.W.; Calder, V.L.; Fenwick, G.D.; Lake, R.J.; McCombs, J.D.; Munro, M.H.; Perry, N.B. Reverse phase flash chromatography: A method for the rapid partitioning of natural product extracts. J. Nat. Prod. 1987, 50, 290–292. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Jing, L.; Qiu, F.; Zhang, H.; Huhman, D.; Zhou, Z.; Sumner, L.W. Construction of an ultrahigh pressure liquid chromatography-tandem mass spectral library of plant natural products and comparative spectral analyses. Anal. Chem. 2015, 87, 7373–7381. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Caboni, M.F.; Fernández-Gutiérrez, A. Development of a rapid method to determine phenolic and other polar compounds in walnut by capillary electrophoresis–electrospray ionization time-of-flight mass spectrometry. J. Chromatogr. A 2008, 1209, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Solar, A.; Veberic, R. Changes in phenolic profiles of red-colored pellicle walnut and hazelnut kernel during ripening. Food Chem. 2018, 252, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, X.; Qin, P.; Shan, F.; Ren, G. Flavonoid composition, antibacterial and antioxidant properties of tartary buckwheat bran extract. Ind. Crops Prod. 2013, 49, 312–317. [Google Scholar] [CrossRef]
- Chang, L.; Juang, L.; Wang, B.; Wang, M.; Tai, H.; Hung, W.; Chen, Y.; Huang, M. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food. Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.H.; Liu, G.R.; Liu, C.; Dong, Y.M. Isoquercitrin suppresses the expression of histamine and pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. Chin. J. Nat. Med. 2016, 14, 407–412. [Google Scholar] [CrossRef]
- Yun, J.; Lee, H.; Ko, H.J.; Woo, E.-R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim. Biophys. Acta 2015, 1848, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yao, Y.; Wang, Y.; Ren, G. Antidiabetic activity of isoquercetin in diabetic KK-A y mice. Nutr. Metab. 2011, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; Da Silva, E.V.G.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Predes, D.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Dudenhoeffer, A.C.; Borges, H.L.; Mendes, F.A.; Abreu, J.G. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J. Biol. Chem. 2014, 289, 35456–35467. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, X.; Yang, D.; Che, X.; Wang, J.; Li, X.; Zhang, Z.; Wang, Q.; Zheng, W.; Wang, L. Isoquercitrin inhibits bladder cancer progression in vivo and in vitro by regulating the PI3K/Akt and PKC signaling pathways. Oncol. Rep. 2016, 36, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.E.; Kuster, R.M.; Yamamoto, K.A.; Salles, T.S.; Campos, R.; de Meneses, M.D.; Soares, M.R.; Ferreira, D. Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasit. Vectors 2014, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.G.; Gasparotto, F.M.; Lourenço, E.L.B.; Crestani, S.; Stefanello, M.E.A.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.A.; Kassuya, C.A.L. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: evidence for the inhibition of angiotensin converting enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, J.; Wang, K.; Hao, X.; Ge, R.; Li, Q. Isoquercitrin inhibits hydrogen peroxide-induced apoptosis of EA. hy926 cells via the PI3K/Akt/GSK3β signaling pathway. Molecules 2016, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.G.; Prando, T.B.L.; Leme, T.d.S.V.; Gasparotto, F.M.; Lourenço, E.L.B.; Rattmann, Y.D.; Da Silva-Santos, J.E.; Kassuya, C.A.L.; Marques, M.C.A. Mechanisms underlying the diuretic effects of Tropaeolum majus L. extracts and its main component isoquercitrin. J. Ethnopharmacol. 2012, 141, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Veluri, R.; Weir, T.L.; Bais, H.P.; Stermitz, F.R.; Vivanco, J.M. Phytotoxic and antimicrobial activities of catechin derivatives. J. Agric. Food Chem. 2004, 52, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Hara-Kudo, Y.; Yamasaki, A.; Sasaki, M.; Okubo, T.; Minai, Y.; Haga, M.; Kondo, K.; Sugita-Konishi, Y. Antibacterial action on pathogenic bacterial spore by green tea catechins. J. Sci. Food Agric. 2005, 85, 2354–2361. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Saito, A.; Tanaka, A.; Nakajima, N.; Kuriyama, I.; Takemura, M.; Takeuchi, T.; Sugawara, F.; Yoshida, H. Structural analysis of catechin derivatives as mammalian DNA polymerase inhibitors. Biochem. Biophys. Res. Commun. 2005, 333, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Han, J.; Xiao, H.; Qiao, J.; Han, M. Effect of tea polyphenol compounds on anticancer drugs in terms of anti-tumor activity, toxicology, and pharmacokinetics. Nutrients 2016, 8, 762. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Park, K.D.; Lee, K.H.; Byun, Y.H.; Park, J.H.; Kim, S.H.; Kim, J.H.; Seong, B.L. Biological evaluation of anti-influenza viral activity of semi-synthetic catechin derivatives. Antiviral Res. 2007, 76, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Ren, D.; Wei, X.; Shi, H.; Zhang, X.; Perez, R.G.; Lou, H.; Lou, H. Eriodictyol-7-O-glucoside activates Nrf2 and protects against cerebral ischemic injury. Toxicol. Appl. Pharmacol. 2013, 273, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, C.-S.; Tu, T.H.; Kim, M.-S.; Goto, T.; Kawada, T.; Choi, M.-S.; Park, T.; Sung, M.-K.; Yun, J.W. Quercetin protects obesity-induced hypothalamic inflammation by reducing microglia-mediated inflammatory responses via HO-1 induction. Nutrients 2017, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, Y.; Tian, Y.; Ye, D. Quercetin, a bioflavonoid, attenuates mechanical allodynia in a rat model of cancer-induced bone pain via suppressing the PI3Kγ/Akt signaling pathway. J. Pain 2018, 19, S77. [Google Scholar] [CrossRef]
- Charnock, C.; Brudeli, B.; Klaveness, J. Evaluation of the antibacterial efficacy of diesters of azelaic acid. Eur. J. Pharm. Sci. 2004, 21, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, D.; Wei, Y.; Su, D.; Lu, C.; Hu, Y.; Zhou, F. Azelaic acid exerts antileukemic activity in acute myeloid leukemia. Front. Pharmacol. 2017, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.S. Azelaic acid: Potential as a general antitumoural agent. Med. Hypotheses 1999, 52, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Okuda, T.; Fukuda, T.; Hatano, T.; Yoshida, T. Two novel dicarboxylic acid derivatives and a new dimeric hydrolyzable tannin from walnuts. J. Agric. Food Chem. 2007, 55, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. HPLC-MSn identification and quantification of phenolic compounds in hazelnut kernels, oil and bagasse pellets. Food Res. Int. 2014, 64, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.-Y.O.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Raafat, K. Phytochemical analysis of Juglans regia oil and kernel exploring their antinociceptive and anti-inflammatory potentials utilizing combined bio-guided GC–FID, GC–MS and HPLC analyses. Rev. Bras. Farmacogn. 2018, 28, 358–368. [Google Scholar] [CrossRef]
- Ren, S.; Yan, X.; Ma, J.; Pan, Y.; Zhang, W.; Wang, D.; Fei, Z.; Liu, X. Defatted walnut powder extract reduces cholesterol gallstones formation in C57BL/6 mice by downregulating the levels of ABCG5/8 in the liver and NPC1L1 in the intestine. J. Funct. Foods 2018, 48, 85–91. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N. Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum Moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J. Agric. Food Chem. 2009, 57, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Inhibition of α-amylase and α-glucosidase activity by tea and grape seed extracts and their constituent catechins. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Puhl, I.; Stadler, F.; Treutter, D. Alterations of flavonoid biosynthesis in young grapevine (Vitis vinifera L.) leaves, flowers, and berries induced by the dioxygenase inhibitor prohexadione-Ca. J. Agric. Food Chem. 2008, 56, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Effect of hulling methods and roasting treatment on phenolic compounds and physicochemical properties of cultivars ‘Ohadi’and ‘Uzun’pistachios (Pistacia vera L.). Food Chem. 2018, 272, 418–426. [Google Scholar] [CrossRef]
- Chatzopoulou, A.; Karioti, A.; Gousiadou, C.; Lax Vivancos, V.; Kyriazopoulos, P.; Golegou, S.; Skaltsa, H. Depsides and other polar constituents from Origanum dictamnus L. and their in vitro antimicrobial activity in clinical strains. J. Agric. Food Chem. 2010, 58, 6064–6068. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.L.; Pandey, R.P.; Jung, N.; Jung, H.J.; Kim, E.-H.; Sohng, J.K. Hydroxylation of diverse flavonoids by CYP450 BM3 variants: Biosynthesis of eriodictyol from naringenin in whole cells and its biological activities. Microb. Cell Fact. 2016, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K. Anti-inflammatory effects of eriodictyol in lipopolysaccharidestimulated raw 264.7 murine macrophages. Arch. Pharm. Res. 2011, 34, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cho, Y.-Y.; Yao, K.; Nadas, J.; Kim, D.J.; Cho, E.-J.; Lee, M.-H.; Pugliese, A.; Zhang, J.; Bode, A.M. Eriodictyol inhibits RSK2-ATF1 signaling and suppresses EGF-induced neoplastic cell transformation. J. Biol. Chem. 2011, 286, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.F.; Trevisan, G.; Walker, C.I.B.; Klafke, J.Z.; de Oliveira, A.P.; Villarinho, J.G.; Zanon, R.B.; Royes, L.F.F.; Athayde, M.L.; Gomez, M.V. Eriodictyol: A flavonoid antagonist of the TRPV1 receptor with antioxidant activity. Biochem. Pharmacol. 2011, 81, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Orhan, I.; Ahsan, Z.; Aslan, S.; Gulfraz, M. Fatty acid composition of seed oil of different Sorghum bicolor varieties. Food Chem. 2008, 109, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Barri, T.; Hanhineva, K.; Juntunen, K.; Dragsted, L.O.; Mykkänen, H.; Poutanen, K. UPLC-QTOF/MS metabolic profiling unveils urinary changes in humans after a whole grain rye versus refined wheat bread intervention. Mol. Nutr. Food. Res. 2013, 57, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gover, M.D. Azelaic acid (15% gel) in the treatment of acne rosacea. Int. J. Dermatol. 2007, 46, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Hashim, P.W.; Chen, T.; Harper, J.C.; Kircik, L.H. The efficacy and safety of azelaic acid 15% foam in the treatment of facial acne vulgaris. J. Drugs Dermatol. 2018, 17, 641–645. [Google Scholar] [PubMed]
- Fitton, A.; Goa, K.L. Azelaic acid. Drugs 1991, 41, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Luo, H.; Xu, M.; Zhai, M.; Guo, Z.; Qiao, Y.; Wang, L. Dynamic changes in phenolics and antioxidant capacity during pecan (Carya illinoinensis) kernel ripening and its phenolics profiles. Molecules. 2018, 23, 435. [Google Scholar] [CrossRef] [PubMed]
- Bati, B.; Celik, I.; Dogan, A. Determination of hepatoprotective and antioxidant role of walnuts against ethanol-induced oxidative stress in rats. Cell Biochem. Biophys. 2015, 71, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, K.J.; Daly, N.L.; Craik, D.J. NMR of peptide toxins. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Elsevier: London, UK, 2009; Volume 68, pp. 89–147. [Google Scholar]
- Novick, R.P. The Staphylococcus as a molecular genetic system. In Handbook of Molecular Biology of the Staphylococci; Novick, R.P., Ed.; VCH Publishers: New York, NY, USA, 1990; pp. 1–40. [Google Scholar]
- Holder, I.A.; Boyce, S.T. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns 1994, 20, 426–429. [Google Scholar] [CrossRef]
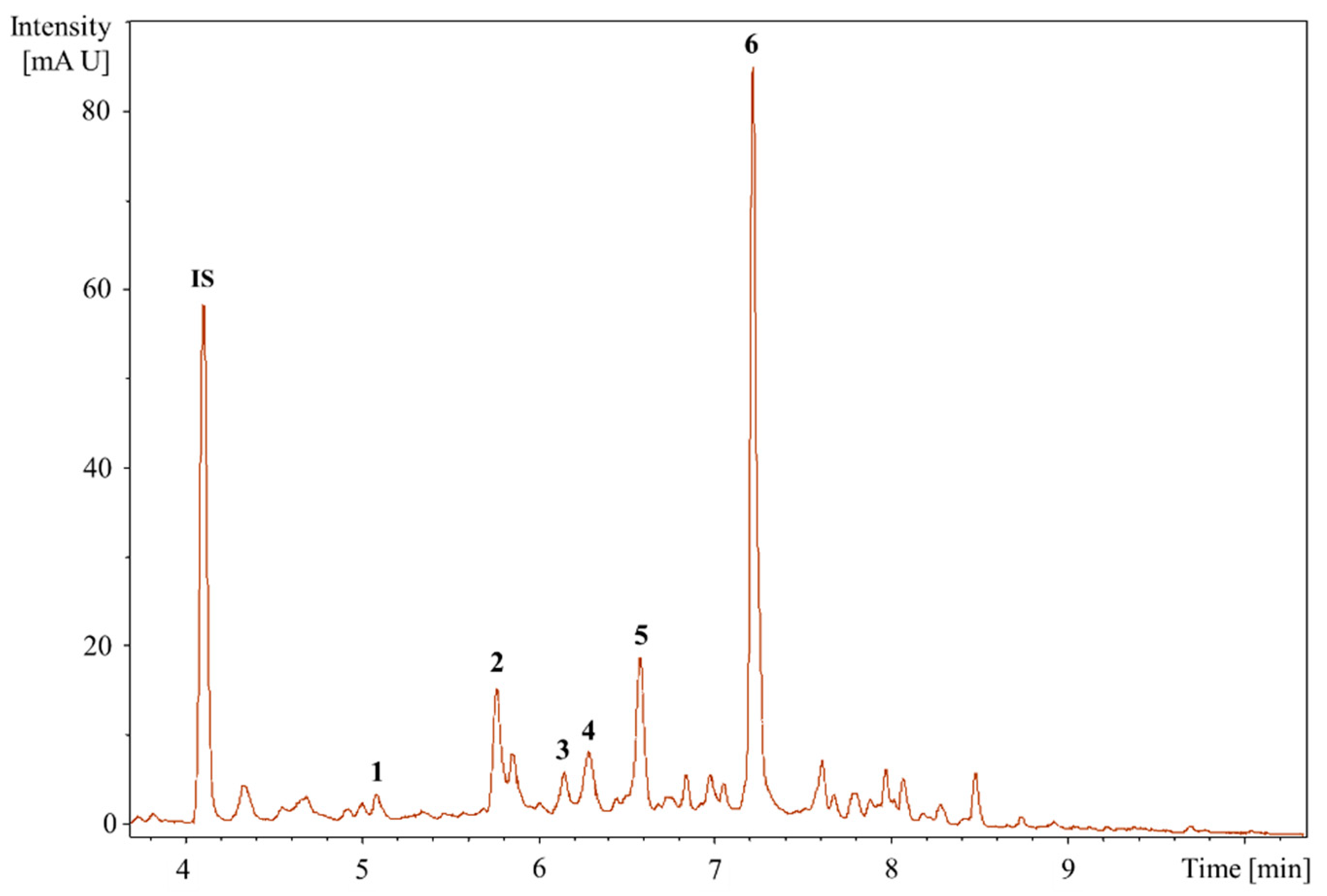
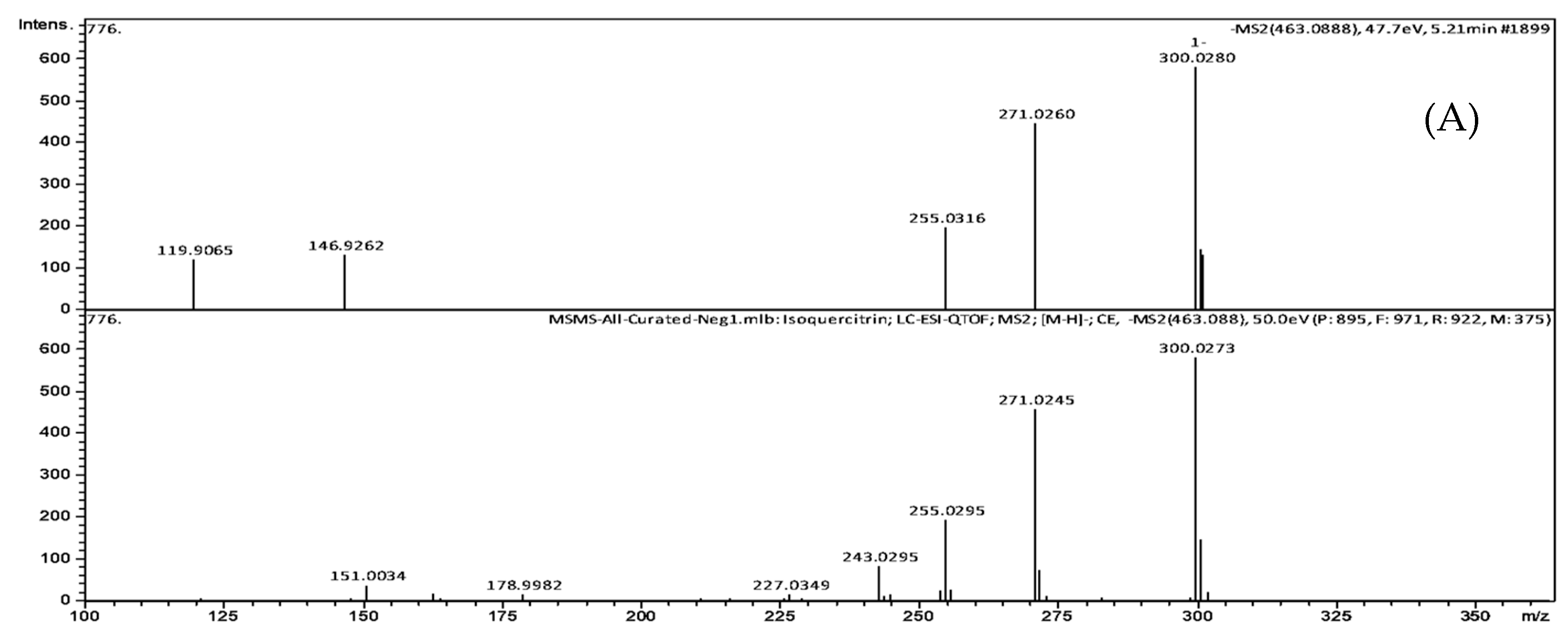
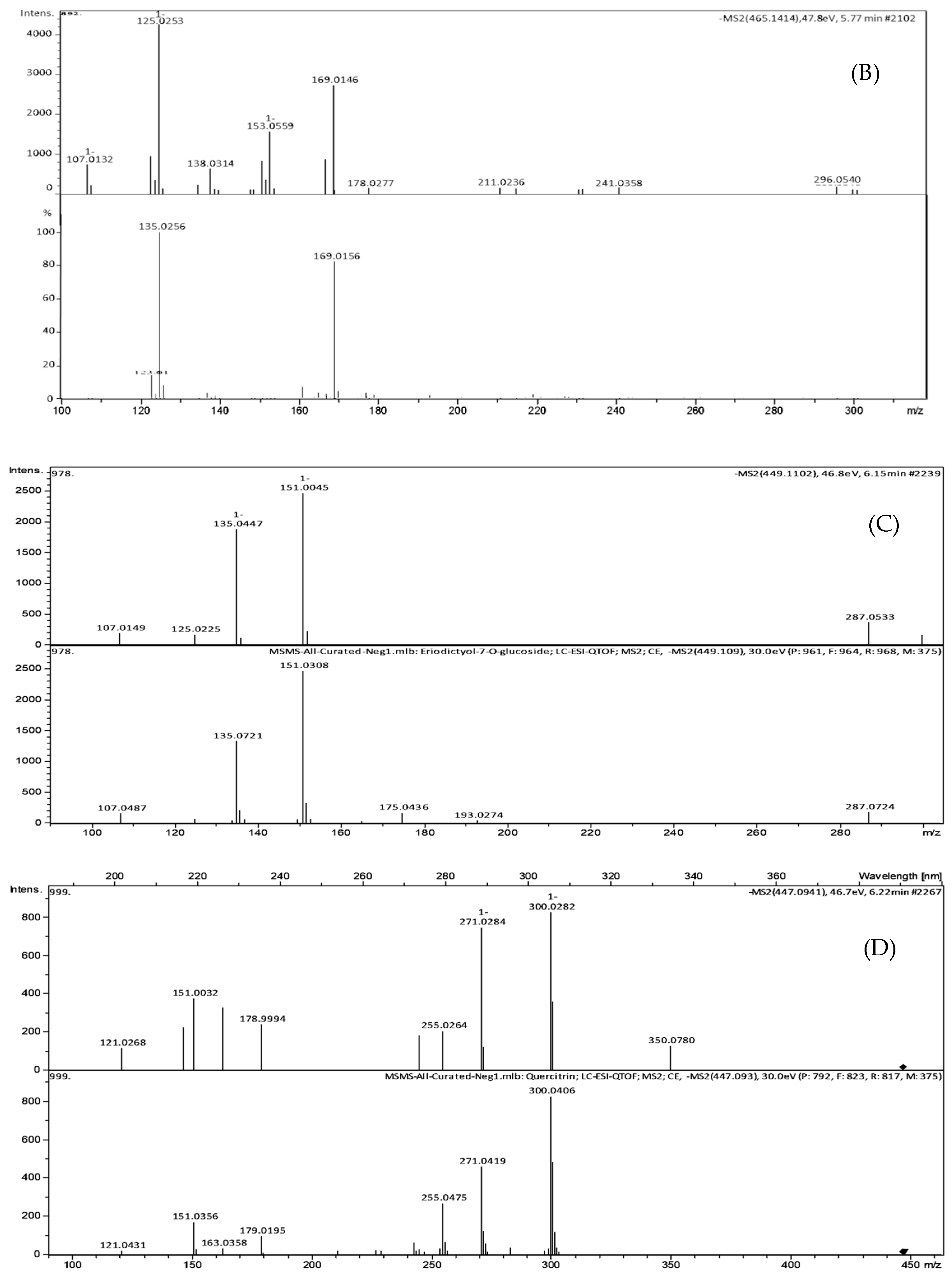
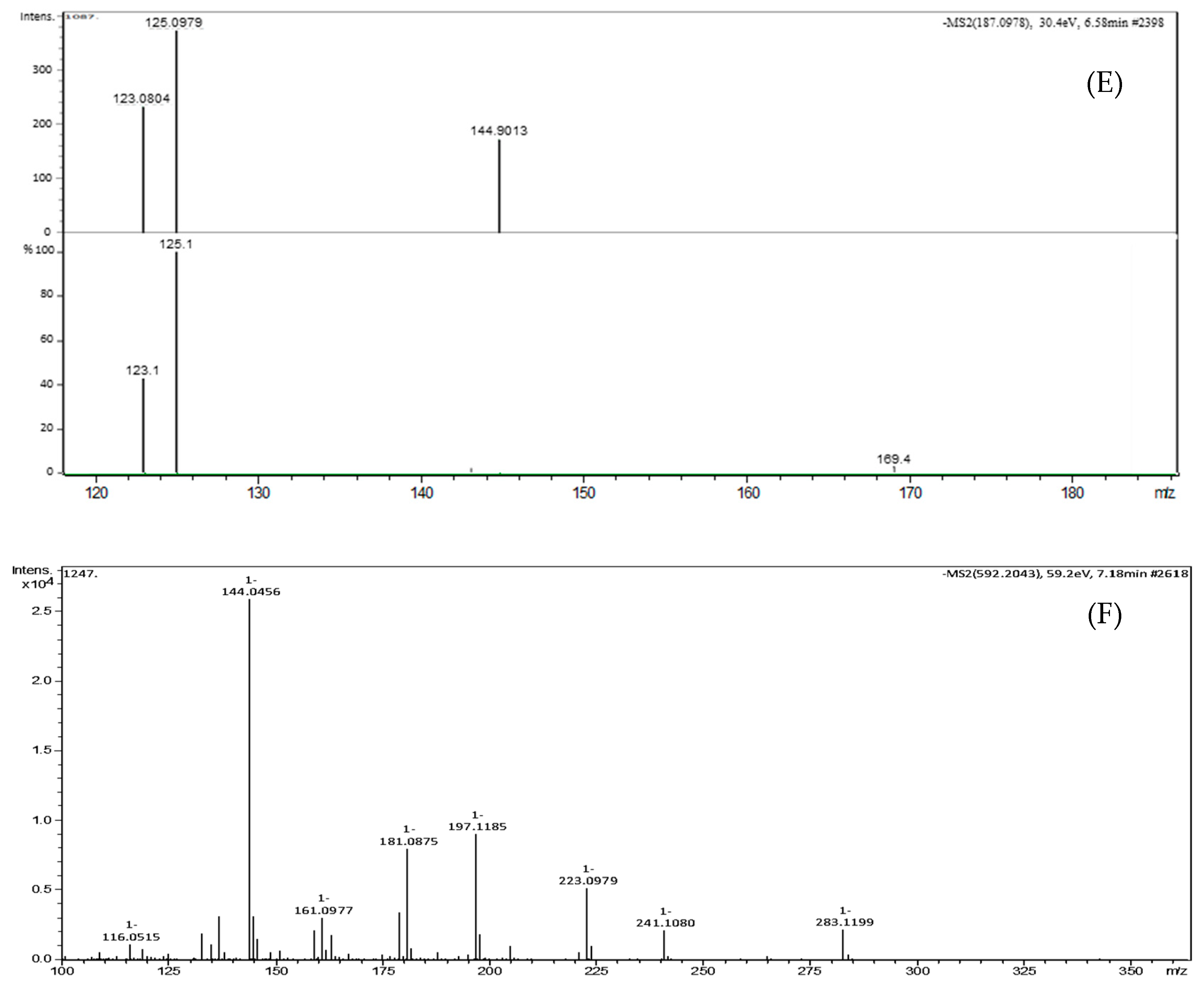
| Peak No. | TR (min) | [M-H]- (m/z) | Formula | Exact Mass | Δm (ppm) | MS/MS Fragments, m/z, Intensity (%) | Putative Identification * |
|---|---|---|---|---|---|---|---|
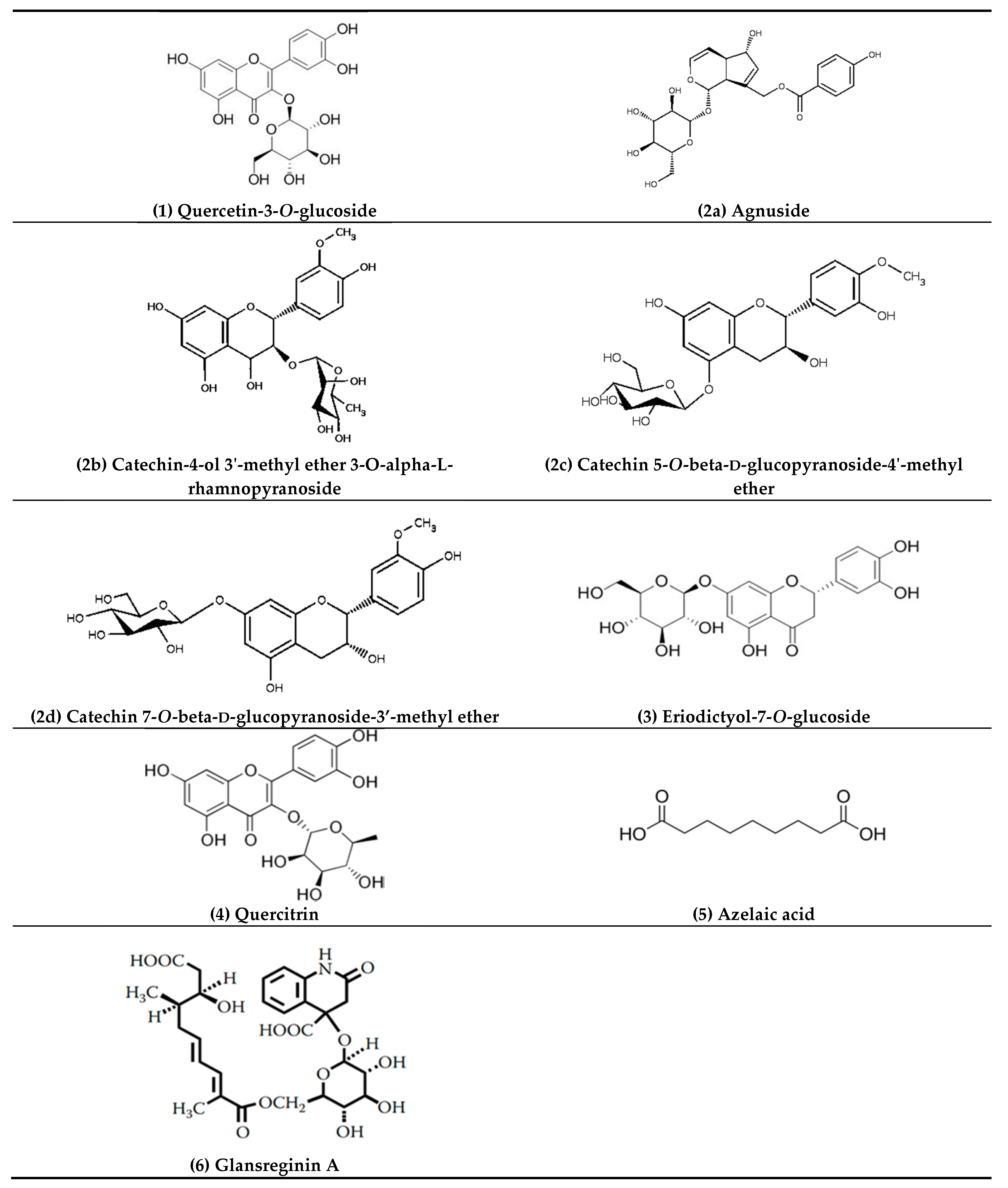
| 1 | 5.21 | 463.0888 | C21H20O12 | 464.0954 | 2.6 | 301.0308 (25.3), 300.0280 (100), 271.0260 (76.8), 255.0316 (34.3), 146.9262 (23.2), 119.9065 (21.1) | Quercetin-3-O-glucoside |
| 2 | 5.77 | 465.1414 | C22H18O11 | 458.0849 | 3.8 | 301.0147 (2.7), 300.0280 (3.0), 241.0358 (4.2), 169.0146 (64.2), 125.0253 (100), 107.0132 (17.5) | Agnuside Catechin-4-ol 3′-methyl ether 3-O-alpha-l-rhamnopyranoside Catechin 5-O-beta-d-glucopyranoside-4′-methyl ether Catechin 7-O-beta-d-glucopyranoside-3′-methyl ether |
| 3 | 6.15 | 449.1102 | C21H22O11 | 450.1162 | 4.0 | 299.9964 (12.4), 298.9994 (15.3), 255.0408 (12.4), 200.8817 (11.5), 174.9541 (17.1), 151.0039 (100), 135.0444 (77.5) | Eriodictyol-7-O-glucoside |
| 4 | 6.26 | 477.0941 | C21H20O11 | 448.1006 | 1.3 | 301.0373 (43.2), 300.0282 (100), 271.0284 (90.3), 255.0264 (24.8), 178.9994 (29.1), 151.0032 (45.4) | Quercitrin |
| 5 | 6.58 | 187.0977 | C9H14O4 | 186.0892 | 3.2 | 144.9013 (46.2), 125.0979 (100), 123.0804 (62.4), 97.0656 (47.8) | Azelaic acid |
| 6 | 7.18 | 592.2043 | C28H35NO13 | 593.2108 | 2.1 | 283.1199 (8.5), 241.1080 (8.2), 223.0979 (19.9), 197.1185 (34.5), 181.0875 (30.8), 144.0456 (100), 137.0972 (12.1) | Glansreginin A |
| No. | Compound | Bioactivities | References |
|---|---|---|---|
| 1 | Quercetin-3-O-glucoside | antimicrobial | Wang et al. [25] |
| antioxidant | Chang et al. [26] | ||
| anti-inflammatory | Li et al. [27] | ||
| anti-fungal | Yun et al. [28] | ||
| antidiabetic | Zhang et al. [29] | ||
| anti-allergic | Rogerio et al. [30] | ||
| antitumor | Amado et al. [31], Chen et al. [32] | ||
| antiviral | dos Santos et al. [33] | ||
| anti-hypertensive | Junior et al. [34] | ||
| anti-apoptoti | Zhu et al. [35] | ||
| diuretic effects | Junior et al. [36] | ||
| 2 | Catechin derivatives | antimicrobial | Veluri et al. [37], Hara-Kudo et al. [38] |
| antioxidant | Seeram et al. [39] | ||
| anti-inflammatory | Mizushina et al. [40] | ||
| antitumor | Cao et al. [41] | ||
| antiviral | Song et al. [42] | ||
| 3 | Eriodictyol-7-O-glucoside | antioxidant | Jing et al. [43] |
| 4 | Quercitrin | antimicrobial | Wang et al. [25] |
| antioxidant | Wang et al. [25] | ||
| anti-inflammatory | Yang et al. [44] | ||
| anti-allergic | Rogerio et al. [30] | ||
| antitumor | Liu et al. [45] | ||
| 5 | Azelaic acid | antimicrobial | Charnock et al. [46] |
| antitumor | Pan et al. [47], Breathnach [48] | ||
| 6 | Glansreginin A | antioxidant | Ito et al. [49], Slatnar et al. [50] |
| anti-inflammatory | Papoutsi et al. [51] | ||
| antiatherogenic effect | Berryman et al. [52] | ||
| antinociceptive effects | Raafat [53] | ||
| reduction of cholesterol absorption | Ren et al. [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, K.-V.; Lei, Z.; Sumner, L.W.; Coggeshall, M.V.; Hsieh, H.-Y.; Stewart, G.C.; Lin, C.-H. Identifying Antibacterial Compounds in Black Walnuts (Juglans nigra) Using a Metabolomics Approach. Metabolites 2018, 8, 58. https://doi.org/10.3390/metabo8040058
Ho K-V, Lei Z, Sumner LW, Coggeshall MV, Hsieh H-Y, Stewart GC, Lin C-H. Identifying Antibacterial Compounds in Black Walnuts (Juglans nigra) Using a Metabolomics Approach. Metabolites. 2018; 8(4):58. https://doi.org/10.3390/metabo8040058
Chicago/Turabian StyleHo, Khanh-Van, Zhentian Lei, Lloyd W. Sumner, Mark V. Coggeshall, Hsin-Yeh Hsieh, George C. Stewart, and Chung-Ho Lin. 2018. "Identifying Antibacterial Compounds in Black Walnuts (Juglans nigra) Using a Metabolomics Approach" Metabolites 8, no. 4: 58. https://doi.org/10.3390/metabo8040058
APA StyleHo, K.-V., Lei, Z., Sumner, L. W., Coggeshall, M. V., Hsieh, H.-Y., Stewart, G. C., & Lin, C.-H. (2018). Identifying Antibacterial Compounds in Black Walnuts (Juglans nigra) Using a Metabolomics Approach. Metabolites, 8(4), 58. https://doi.org/10.3390/metabo8040058






