A Historical Overview of Natural Products in Drug Discovery
Abstract
:1. Introduction
1.1. Natural Products in History
1.2. Medicinal Plants in Folklore
1.3. Medicinal Natural Products from Other Sources Used in Folklore
1.4. Primary and Secondary Metabolites (Natural Products)
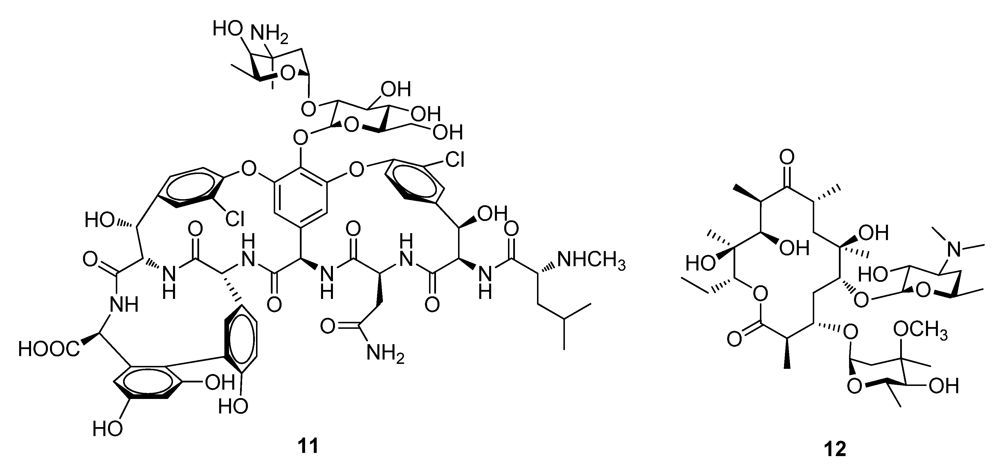
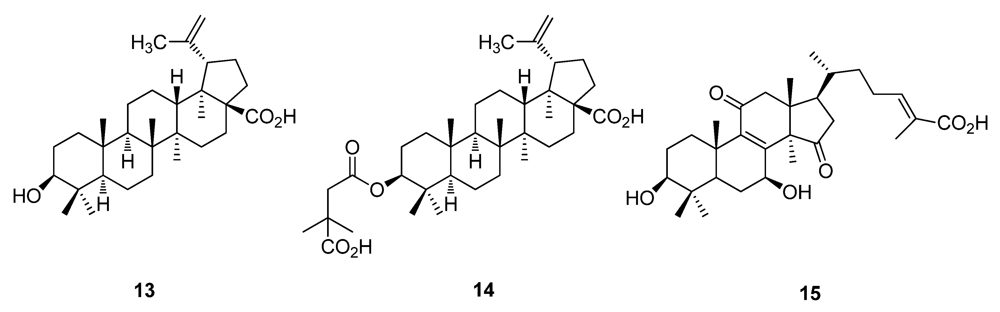
2. Historically Important Natural Products
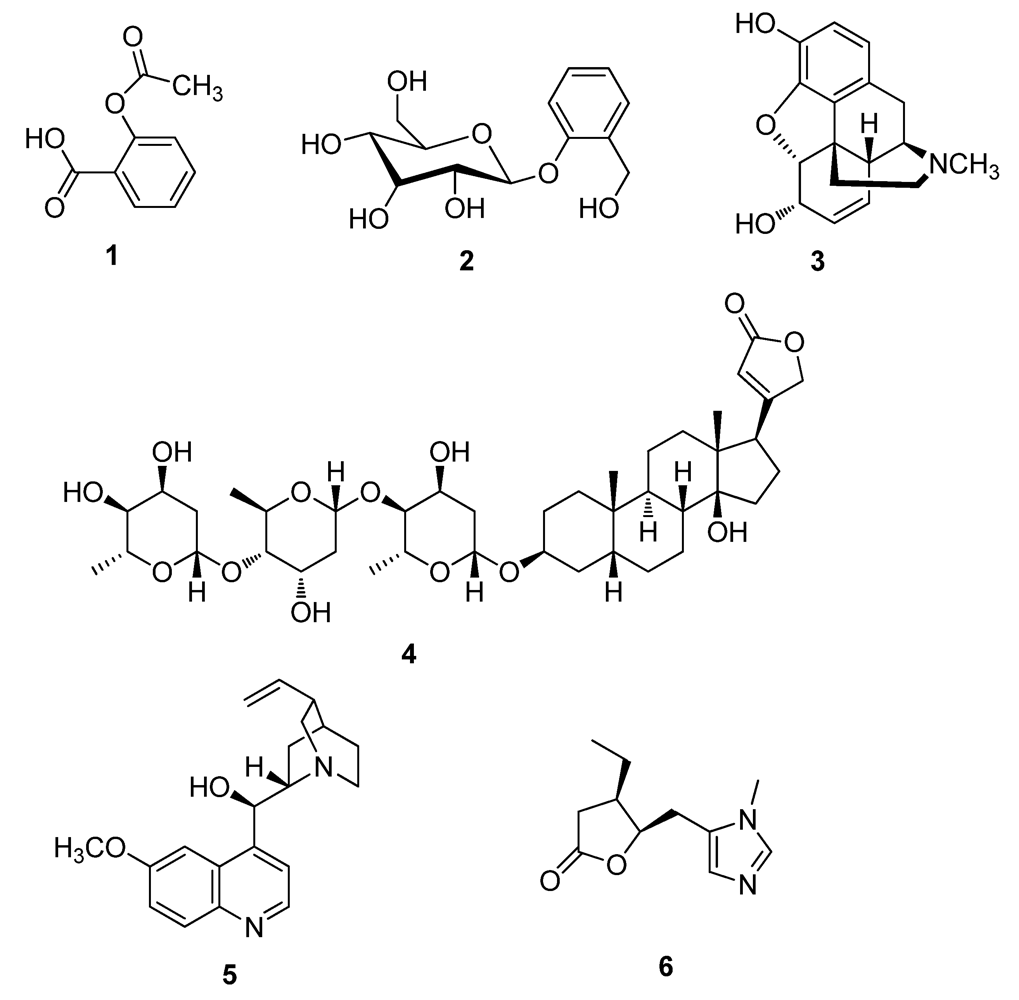
2.1. Natural Products from Fungi
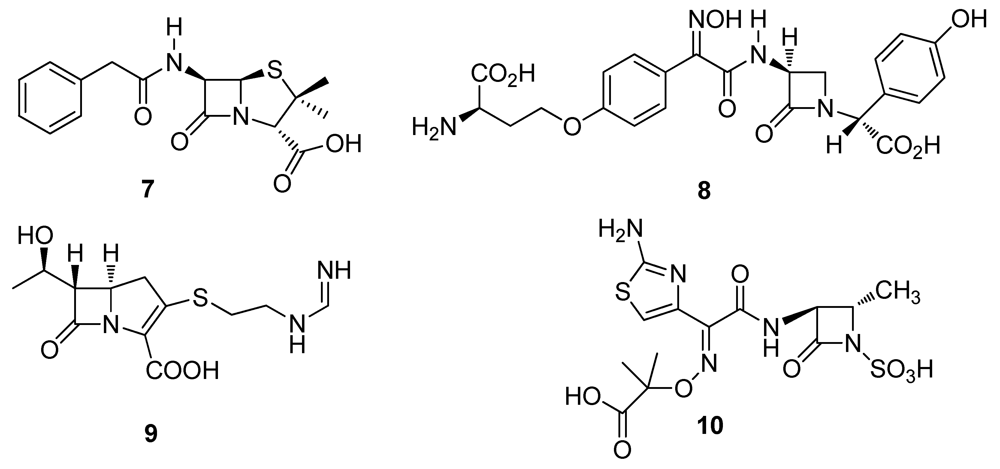
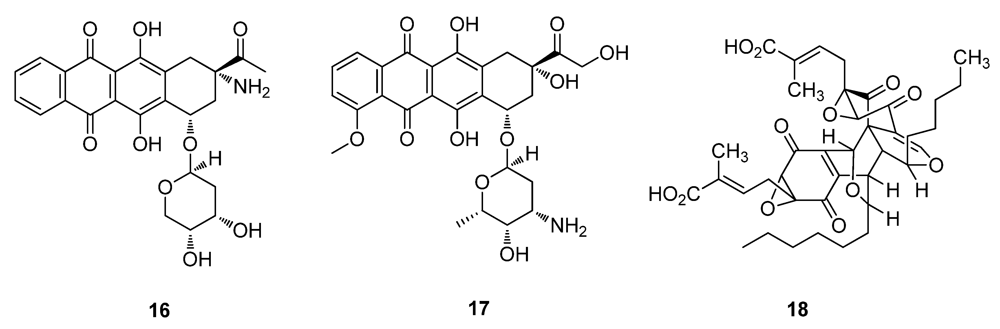
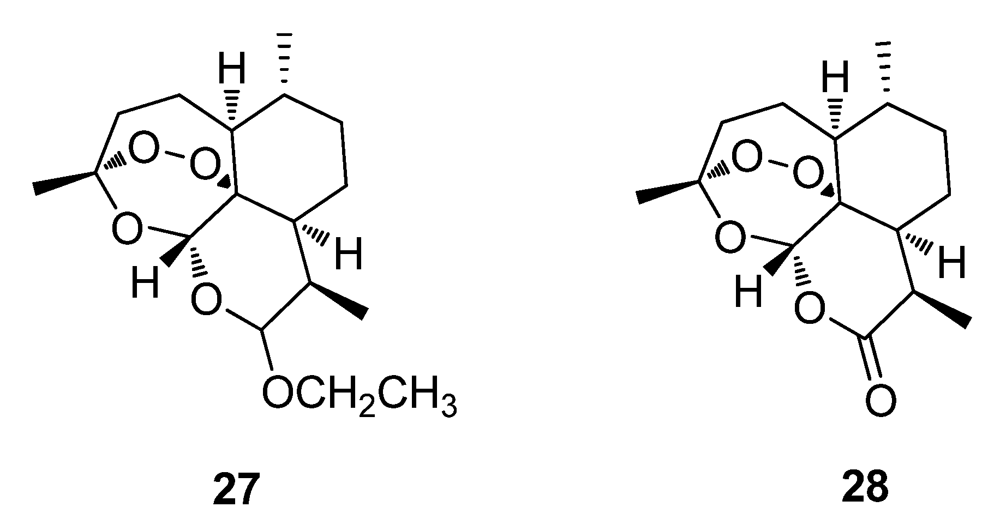
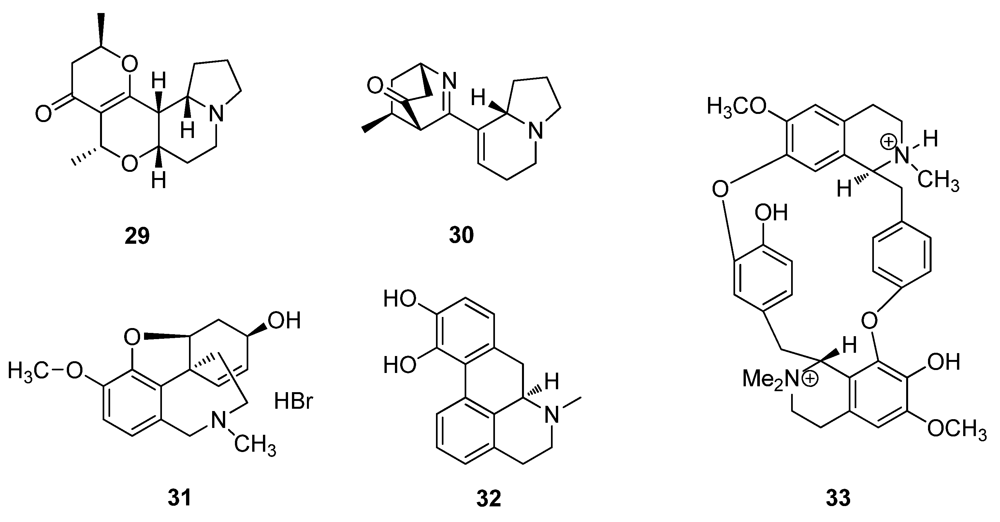
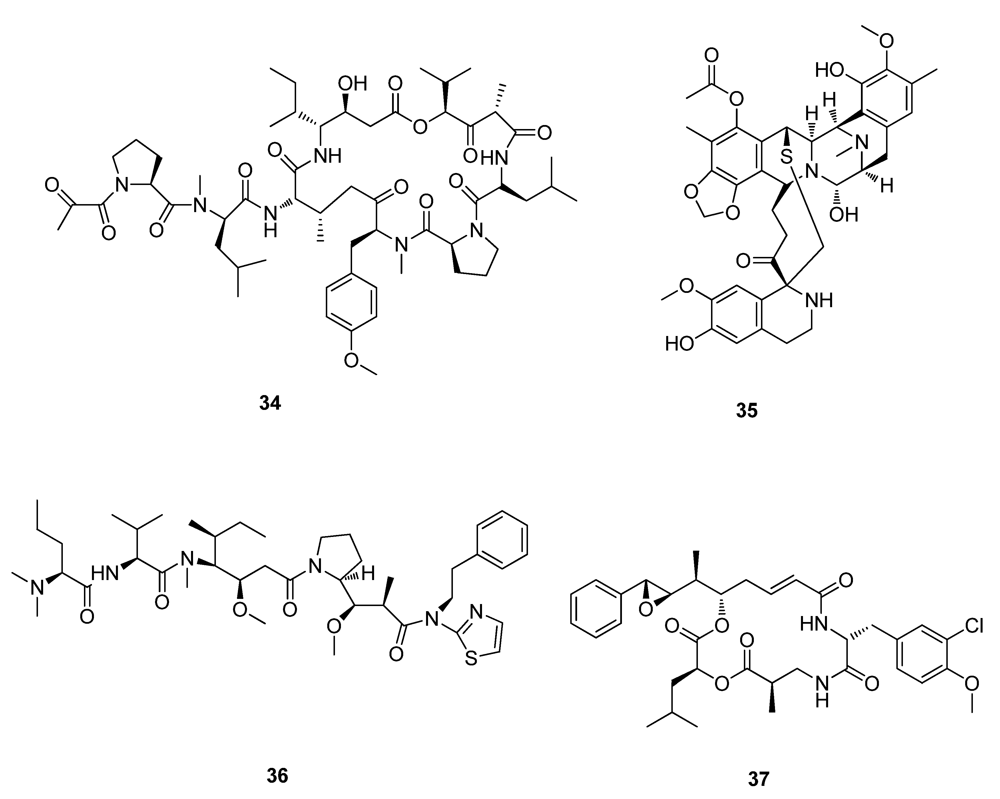
2.2. Natural Products from Plants
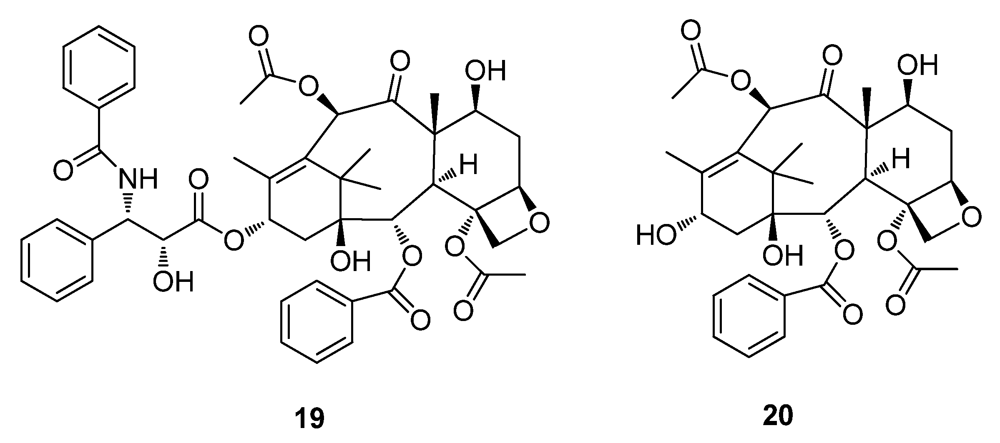
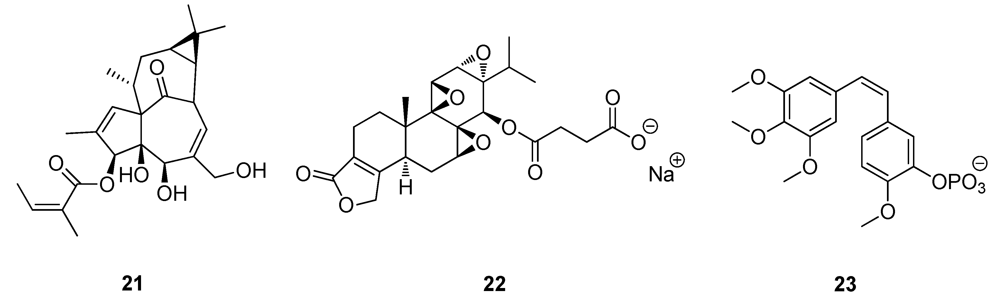
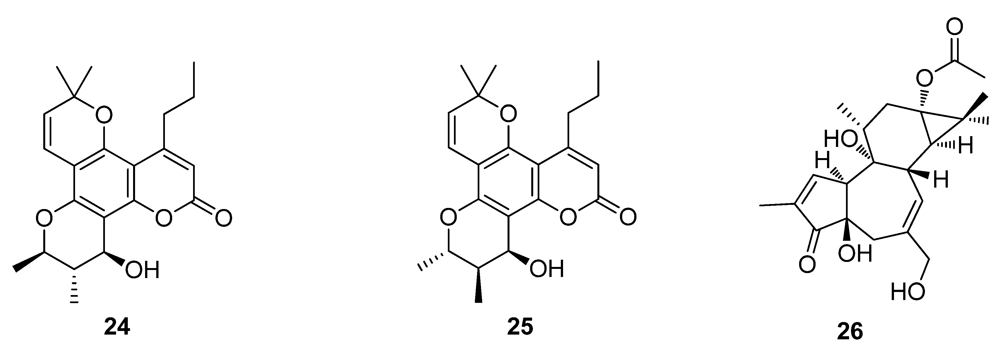
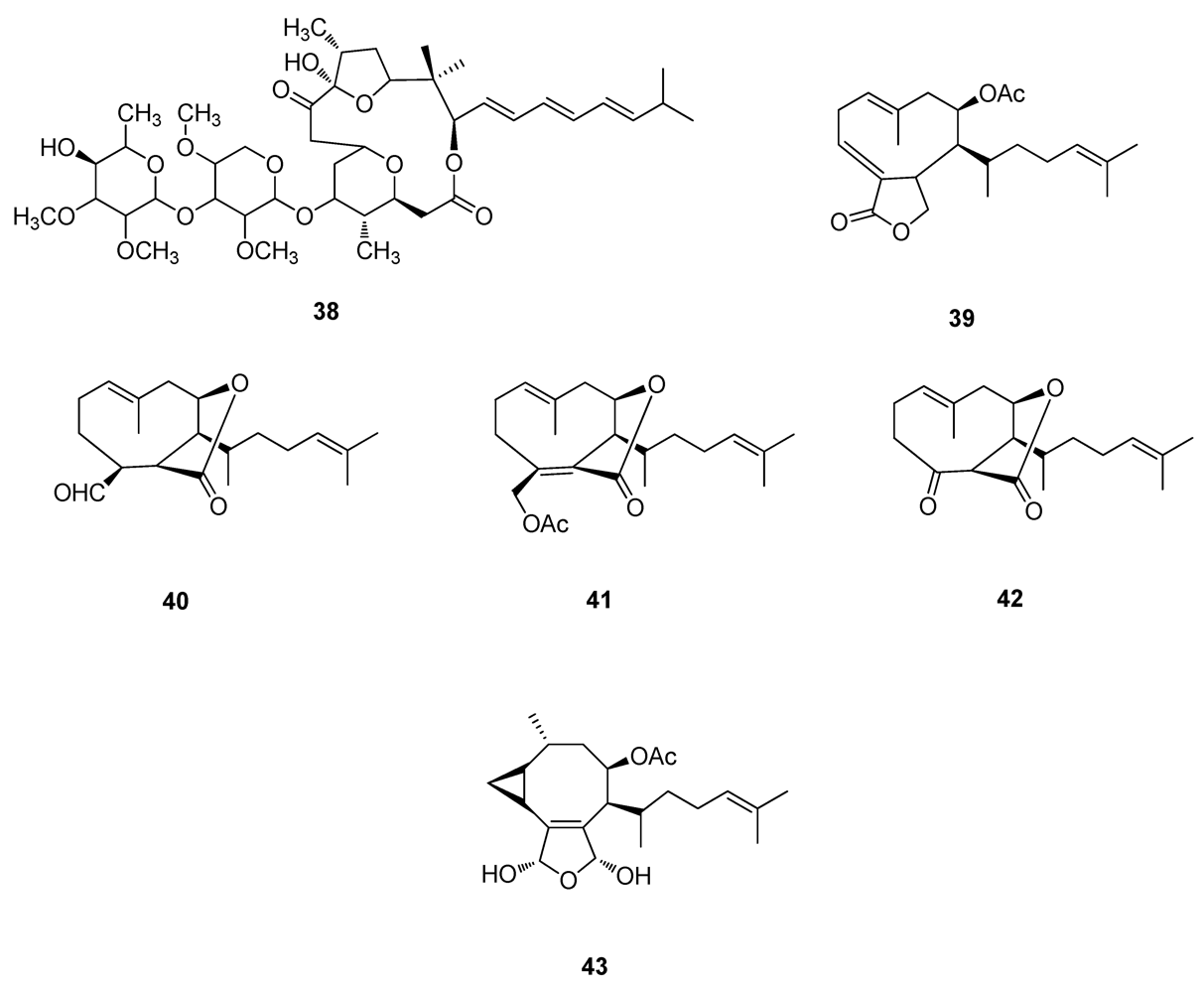
2.3. Natural Products from the Marine Environment
2.4. Natural Products from Marine Algae
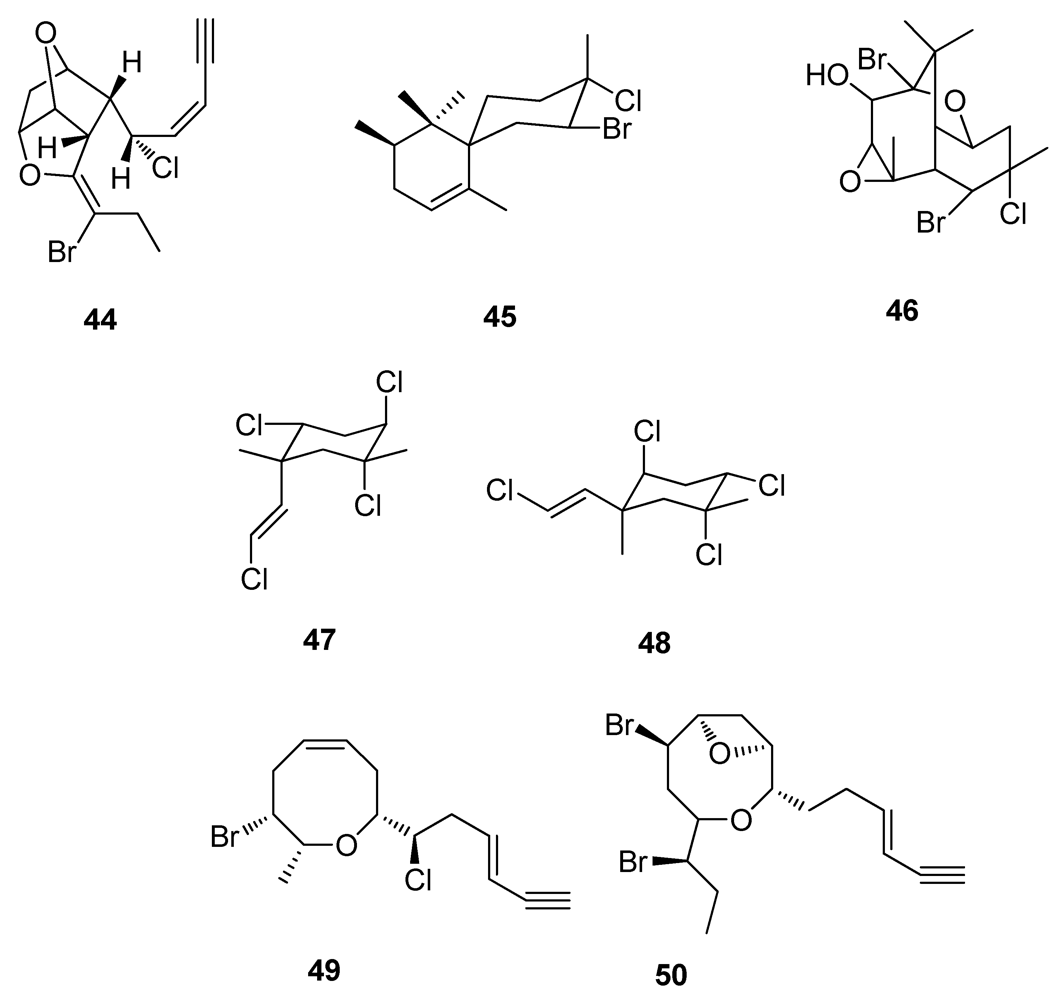
2.5. Natural Products from Marine Sponges
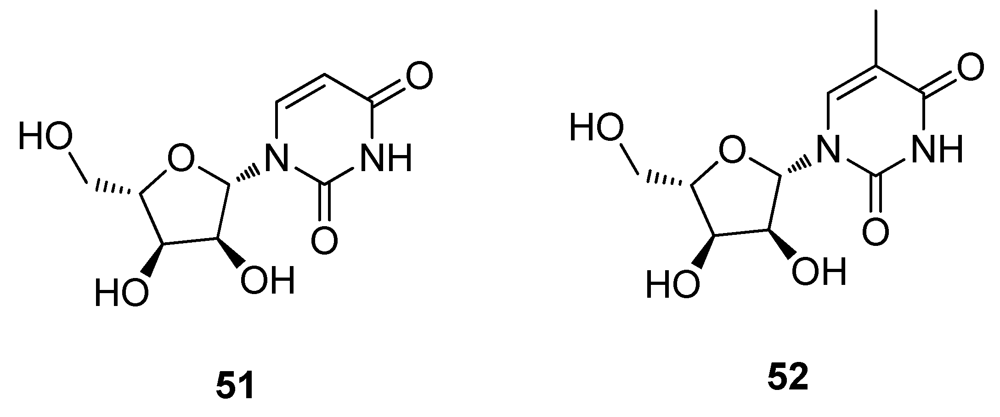
2.6. Natural Products from other Marine Sources
3. Drug Discovery: Natural Product Chemistry versus Combinatorial Chemistry
3.1. Dereplication
3.2. Dereplication Methods
3.3. Database Searching
- Have there been any previous literature reports on the target organism (terrestrial or marine?)
- Is there potential to isolate novel compounds (geographical or seasonal variations?)
- What kind of compound classes has been isolated from the species and if not from the species, then the genus or family?
- Is there incomplete or poor NMR spectroscopic data for previously uncharacterized natural products?
- Are there any new biological activities for known compounds that have been overlooked?
3.4. Hyphenated Instrumentation “Classical versus Hyphenated (on-line) Approaches”
4. Combining Natural Product Chemistry and Metabolomics Approaches in Drug Discovery
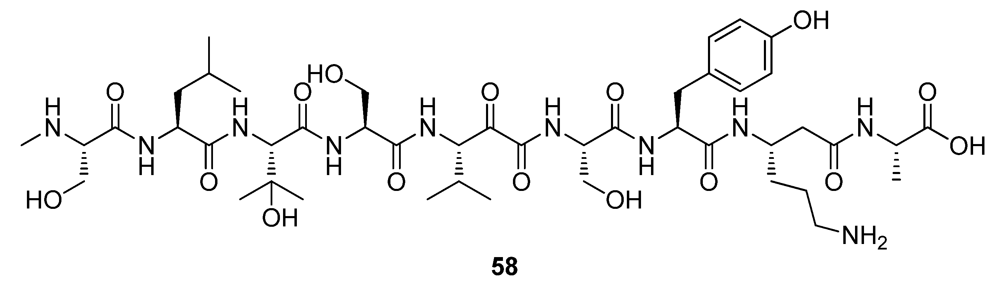
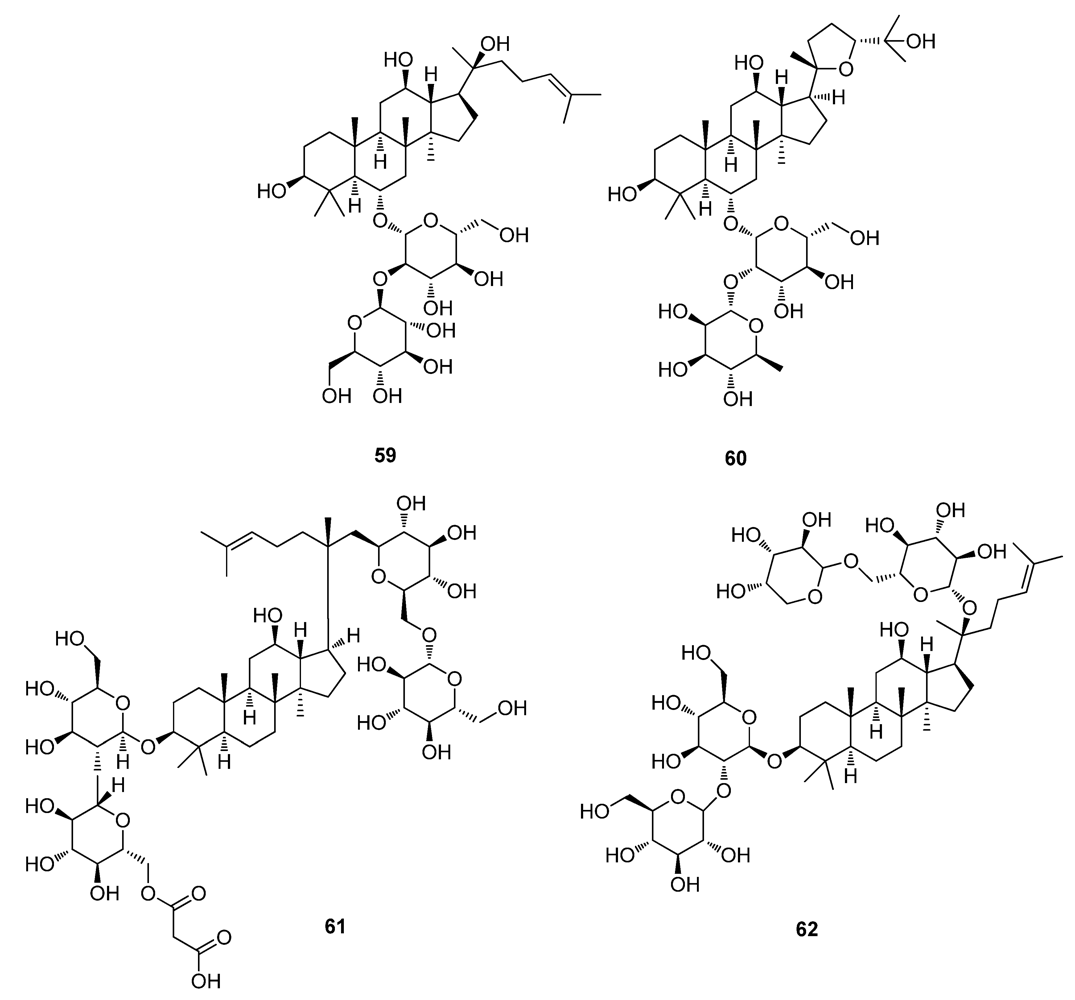
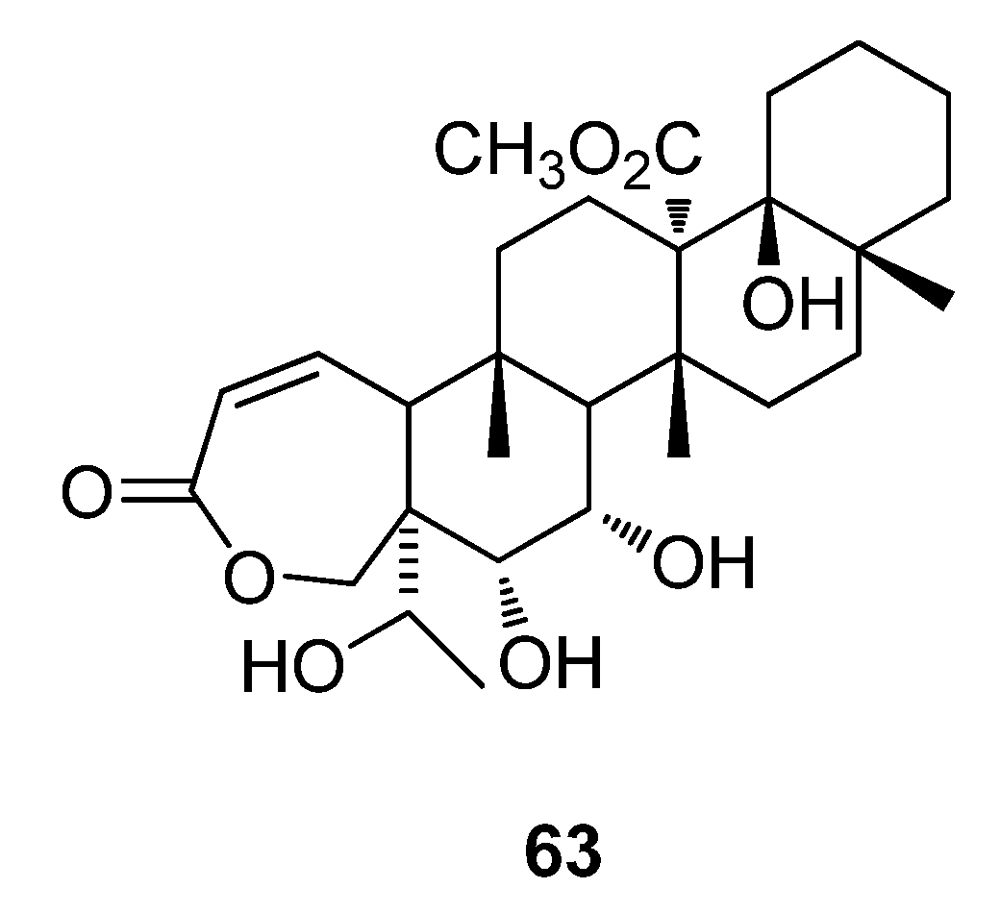
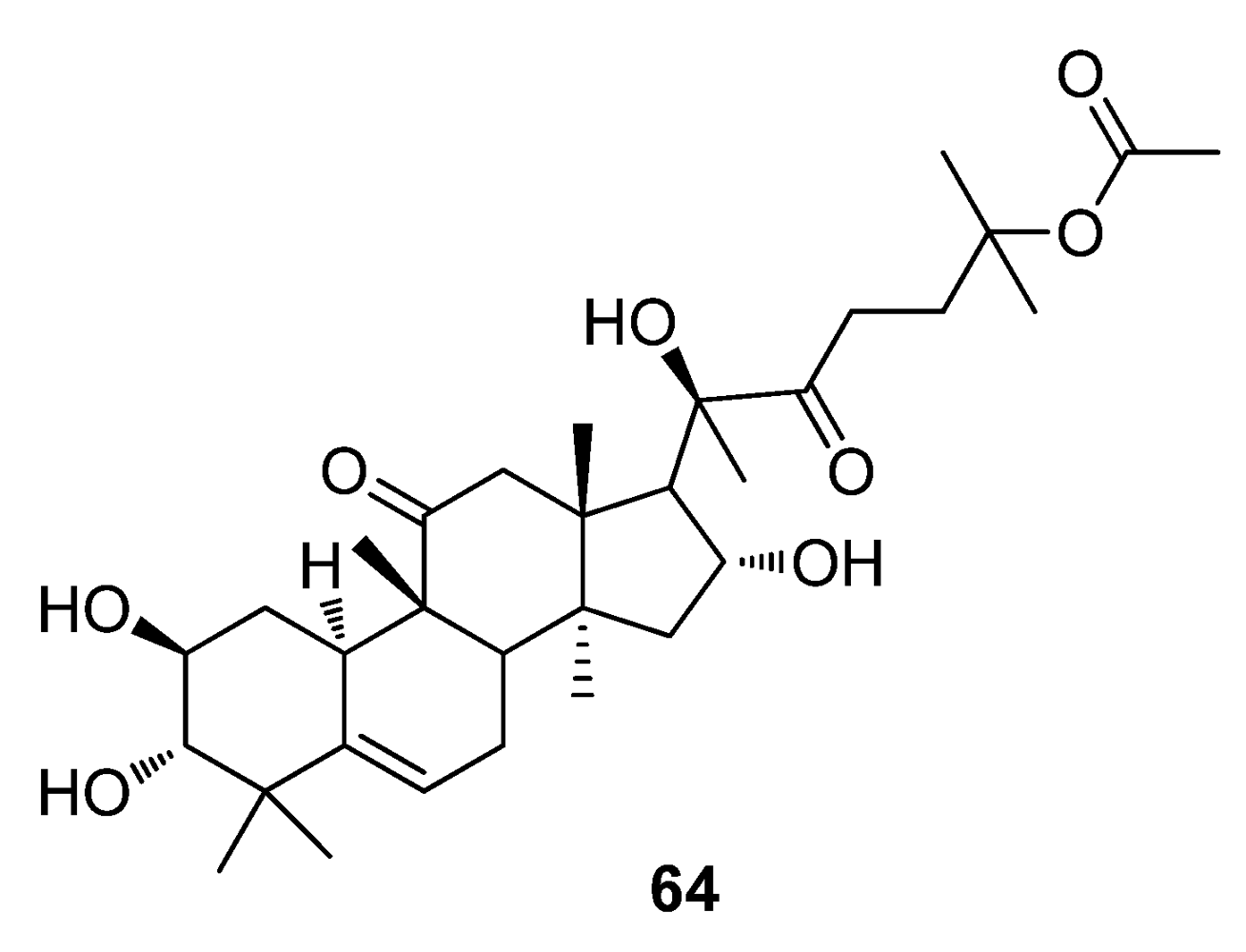
5. Conclusions
Conflict of Interest
References
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- Rey-Ladino, J.; Ross, A.G.; Cripps, A.W.; McManus, D.P.; Quinn, R. Natural products and the search for novel vaccine adjuvants. Vaccine 2011, 29, 6464–6471. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Biodiversity: A continuing source of novel drug leads. Pure Appl. Chem. 2005, 77, 7–24. [Google Scholar]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Butler, M.S. The role of natural product in chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Hicks, S. Desert Plants and People, 1st ed; Naylor Co.: San Antonio, TX, USA; p. 75. [Green Version]
- Kinghorn, A.D.; Pan, L.; Fletcher, J.N.; Chai, H. The relevance of higher plants in lead compound discovery programs. J. Nat. Prod. 2011, 74, 1539–1555. [Google Scholar]
- Duke, J.A.; Duke, P.A.K.; du Cellier, J.L. Duke's Handbook of Medicinal Plants of the Bible; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2008; p. 552. [Google Scholar] [Green Version]
- Dillenius, J.J. Synopsis Methodica Stirpium Britannicarum, 3rd ed; G. and J. Innys: London, UK, 1724; p. 482. [Google Scholar] [Green Version]
- Martin, M. A Description of the Western Isles of Scotland, 4th; Macleod, D.J., Ed.; Stirling: Eneas Mackay: Cornhill, UK, 1934. [Google Scholar] [Green Version]
- Lightfoot, J. Flora Scotica; Benjamin White: London, UK, 1977; Volume 2. [Google Scholar] [Green Version]
- Beith, M. Healing Threads: Traditional Medicines of the Highlands and Islands Edinburgh; Polygon: Edinburgh, UK, 1999. [Google Scholar] [Green Version]
- London, UK. Indberetninger fra en Reise I Færøe 1781 og 1782; Selskabet til Udgivelse af Færøske Kildeskrifter og Studier: Copenhagen, Denmark, 1959; p. 497. [Google Scholar] [Green Version]
- Allen, D.E.; Hatfield, G. Medicinal Plants in Folk Tradition: An Ethnobotany of Britain and Ireland; Timber Press: Cambridge, UK, 2004; p. 431. [Google Scholar] [Green Version]
- Swanton, E.W. Economic and folklore notes. Trans. Br. Mycol. Soc. 1915, 5, 408–409. [Google Scholar] [CrossRef]
- Swanton, E.W. Sussex County Magazine; T.R. Beckett: Eastbourne, UK, 1932; Volume 6, p. 709. [Google Scholar] [Green Version]
- Hatfield, G. Country Remedies: Traditional East Anglian Plant Remedies in the Twentieth Century; Boydell Press: Woodbridge, UK, 2005. [Google Scholar] [Green Version]
- Müller, K. Pharmaceutically relevant metabolites from lichens. Appl. Microbiol. Biotechnol. 2001, 56, 9. [Google Scholar]
- Purvis, W. Lichens; Natural History Museum, London/Smithsonian Institution: Washington D.C., USA, 2000; p. 112. [Google Scholar] [Green Version]
- MacFarlane, A.M. Gaelic names of plants: Study of their uses and lore. Trans. Gaelic Soc. Inverness 1929, 32, 1–48. [Google Scholar]
- Cameron, J. The Gaelic names of plants (Scottish, Irish and Mnax) Collected and Artanged in Scientific Order, with Notes on Their Etymology, Uses, Plant Superstitions, etc., among the Celts, with Copious Gaelic, English and Scientific Indices; John Mackay: Glasgow, Scotland, UK, 1900; p. 160. [Google Scholar] [Green Version]
- Vickery, R. A Dictionary of Plant-Lore; Oxford University Press: Oxford, UK, 1995. [Google Scholar] [Green Version]
- Moloney, M.F. Irish Ethno-botany and the Evolution of Medicine in Ireland; Dublin, M.H., Ed.; Gill and Son: Dublin, Ireland, 1919. [Google Scholar] [Green Version]
- Borlase, W. The Natural History of Cornwall; E. & W. Books: Oxford, UK, 1758. [Google Scholar] [Green Version]
- Ó hEithir, R. Folk Medical Beliefs and Practices in the Aran Islands. Master’s thesis, National University of Ireland: Galway, Ireland, 1983. [Google Scholar] [Green Version]
- Dewick, P.M. Medicinal Natural Products: A Biosynthentic Approach, 2nd ed; John Wiley and Son: West Sussex, UK, 2002; p. 520. [Google Scholar] [Green Version]
- Maplestone, R.A.; Stone, M.J.; Williams, D.H. The evolutionary role of secondary metabolites—A review. Gene 1992, 115, 151–157. [Google Scholar] [CrossRef]
- Colegate, S.M.; Molyneux, R.J. Bioactive Natural Products: Detection, Isolation and Structure Determination; CRC Press: Boca Raton, FL, USA, 2008; pp. 421–437. [Google Scholar] [Green Version]
- Sarker, S.D.; Latif, Z.; Gray, A.I. Methods in Biotechnology: Natural Product Isolation; Satyajit, D., Ed.; Human Press Inc: Totowa, NJ, USA, 2006; p. 528. [Google Scholar] [Green Version]
- Der Marderosian, A.; Beutler, J.A. The Review of Natural Products, 2nd ed; Facts and Comparisons: Seattle, WA, USA, 2003; pp. 13–43. [Google Scholar] [Green Version]
- Aniszewski, T. Alkaloids—Secrets of Life. In Alkaloid Chemistry, Biological Significance, Applications and Ecological Role; Elsevier Science: Amsterdam, The Netherlands, 2007; p. 334. [Google Scholar] [Green Version]
- Lorenzen, K.; Anke, T. Basidiomycetes as a source for new bioactive natural products. Curr. Org. Chem. 1996, 2, 329–364. [Google Scholar]
- Mann, J. Murder, Magic, and Medicine; Oxford University Press: New York, NY, USA, 1994; pp. 164–170. [Google Scholar] [Green Version]
- Abraham, E.P.; Chain, E.; Fletcher, C.M. Further observations on penicillin. Lancet 1941, 16, 177–189. [Google Scholar]
- Alder, A.L. The History of Penicillin Production; American Institute of Chemical Engineers: New York, NY, USA, 1970. [Google Scholar] [Green Version]
- Lax, E. The Mold in Dr. Florey’s Coat: The Story of the Penicillian Miracle; John Macrae/Henry Hol: New York, NY, USA, 2004; p. 308. [Google Scholar] [Green Version]
- Wainwright, M. Miracle Cure: The Story of Penicillin and the Golden Age of Antibiotics; Blackwell Scientific: Oxford, UK, 1990; p. 57. [Google Scholar] [Green Version]
- Mann, J. The Elusive Magic Bullet: The Search for the Perfect Drug; Oxford University Press: New York, NY, USA, 1999. [Google Scholar] [Green Version]
- Williams, J.D. β-lactamases and β-lactamase inhibitor. Int. J. Antimicrob. Agents 1999, 12, S2–S7, (Suppl. 1). [Google Scholar]
- Buss, A.D.; Waigh, R.D. Antiparasitic drugs. In Burger's Medicinal Chemistry and Drug Discovery, 5th; Wolff, M.E., Ed.; Wiley-Interscience: New York, NY, USA, 1995; Volume 1, pp. 1021–1028. [Google Scholar]
- Fabbretti, A.; Gualerzi, C.O.; Brandi, L. How to cope with the quest for new antibiotics. FEBS Lett. 2011, 585, 1673–1681. [Google Scholar] [CrossRef]
- Zjawiony, J.K. Biologically active compounds from aphyllophorales (Polypore) fungi. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar] [CrossRef]
- Stamets, P. Novel Antimicrobials from mushrooms. Herbal Gram 2002, 54, 28–33. [Google Scholar]
- Gwinn, K.D.; Schardl, C.L.; Friburg, A. Southern regional information exchange group (SRIEG-37) on the tall fescue endophyte. J. Prod. Agric. 1992, 5, 189–190. [Google Scholar]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites (1987 to 2000). Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Petrini, O. Taxonomy of endophytic fungi of aerial plant tissues. In Microbiology of Phyllosphere; Fokkema, N.J., van den Heuvel, J., Eds.; Cambridge University Press: Cambridge, UK, 1986; pp. 175–187. [Google Scholar] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Martin, D.E.; Blum, R.; Wilton, J. Safety and pharmacokinetics of beririmat (PA-457) a novel inhibitor of human immunodeficiency virus maturation, in healthy volunteers. Antimicrob. Agents Chemother. 2007, 51, 3063–3066. [Google Scholar]
- Kashiwada, Y.; Hashimoto, F.; Cosentino, L.M. Betulinic acid and dihydrobetulinic acid derivatives as potent HIV agents. J. Med. Chem. 1996, 39, 1016–1017. [Google Scholar]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 763–771. [Google Scholar]
- Heider, D.; Verheyen, J.; Hoffman, D. Predicting Bevirimat resistance of HIV-1 from genotype. BMC Bioinformatics 2010, 11, 1–9. [Google Scholar] [CrossRef]
- Min, B.-S.; Nakamura, N.; Miyashiro, H.; Bae, K.-W.; Hattori, M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998, 46, 1607–1612. [Google Scholar] [CrossRef]
- Lee, J.C.; Strobel, G.A.; Lobkovsky, E.; Clardy, J.C. Torreyanic acid: A selectively cytotoxic quinone dimer from the endophytic fungus Pestalotiopsis microspora. J. Org. Chem. 1996, 61, 3232–3233. [Google Scholar] [CrossRef]
- Li, C.; Johnson, R.P.; Porco, J.A. Total synthesis of the quinine epoxide dimer (+)-torreyanic acid: application of a biomimetic oxidation/electrocyclization/Diels-Alder dimerization cascade. J. Am. Chem. Soc. 2003, 125, 5059–5106. [Google Scholar]
- McRae, J.; Yang, Q.; Crawford, R.; Palombo, W. Review of the methods used for isolating pharmaceutical lead compounds from traditional medicinal plants. Environmentalist 2007, 27, 165–174. [Google Scholar] [CrossRef]
- Fellows, L.; Scofield, A. Chemical diversity in plants. In Intellectual Property Rights and Biodiversity Conservation—An Interdisciplinary Analysis of the Values of Medicinal Plants; University Press: Cambridge, UK, 1995. [Google Scholar] [Green Version]
- Farnsworth, N.R.; Akerele, R.O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal Plants in Therapy. Bull. WHO 1985, 63, 965–981. [Google Scholar]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Cragg, G.M. Paclitaxel (Taxol): A success story with valuable lessons for natural product drug discovery and development. Med. Res. Rev. 1998, 18, 315–331. [Google Scholar] [CrossRef]
- Cseke, L.J.; Kirakosyan, A.; Kaufmann, P.B.; Warber, S.L.; Duke, J.A.; Brielmann, H.L. Natural Products from Plants, 2nd ed; CRC, Taylor and Francis: Boca Raton, FL, USA, 2006; p. 640. [Google Scholar] [Green Version]
- Nicolaou, K.C.; Yang, Z.; Liu, J.J.; Ueno, H.; Nantermet, P.G.; Guy, R.K.; Claiborne, C.F.; Renaud, J.; Couladouros, E.A.; Paulvannan, K.; Sorensen, E.J. Total synthesis of taxol. Nature 1994, 367, 630–634. [Google Scholar]
- Kedei, N.; Lundberg, D.J.; Toth, A.; Welburn, P.; Garfield, S.H.; Blumberg, P.M. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004, 64, 3243–3255. [Google Scholar]
- Ogbourne, S.M.; Suhrbier, A.; Jones, B. Antitumour activity of ingenol 3-angelate: Plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004, 64, 2833–2839. [Google Scholar] [CrossRef]
- Kiviharju, T.M.; Lecane, P.S.; Sellers, R.G.; Peehl, D.M. Antiproliferative and proapoptic of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin. Cancer. Res. 2002, 8, 2666–2674. [Google Scholar]
- Fidler, J.M.; Li, K.; Chung, C. PG490-88, a derivative of triptolide, casues tumor regression and sensitizes tumors to chemotherapy. Mol. Cancer. Ther. 2003, 2, 855–862. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Drug Discovery, Therapeutics, and Preventive Medicine; Zhang, L., Fleming, A., Demain, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2005; p. 74. [Google Scholar] [Green Version]
- Holwell, S.E.; Cooper, P.A.; Grosios, J.W.; Lippert, J.W., III; Pettit, G.R.; Snyder, S.D.; Bibby, M.C. Combretastatin A-1 phosphate, a novel tubulin-binding agent with in-vivo anti-vascular effects in experimental tumors. Anticancer Res. 2002, 22, 707–712. [Google Scholar]
- Kashman, Y.; Gustafson, K.R.; Fuller, R.W.; Cardellin, J.H., II; McMahon, J.B.; Currens, M.J.; Buckheist, R.W.; Hughes, S.H.; Cragg, G.M.; Boyd, M.R. The Calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, calophyllum lanigerum. J. Med. Chem. 1992, 35, 2735–2743. [Google Scholar]
- Gustafson, K.R.; Cardellin, J.H., II; McMahon, J.B.; Gulakowski, R.J.; Ishitoya, J.; Szallasi, Z.; Lewin, N.E.; Blumberg, P.M.; Weislow, O.S.; Beutler, J.A.; et al. A nonpromoting phorbol from the Samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV-1. J. Med. Chem. 1992, 35, 1978–1986. [Google Scholar]
- Cox, P.A. Saving the Ethnopharmacological Heritage of Samoa. Pharm. Biol. 2001, 39, 33–40. [Google Scholar]
- Davidson, R.N.; den Boer, M.; Ritmeijer, K. Paromomycin. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 653–660. [Google Scholar]
- Carroll, A.R.; Arumugan, G.; Quinn, R.J.; Redburn, J.; Guymer, G.; Grimshaw, P. Grandisine A and B, novel indolizidine alkaloids with -opioid receptor binding affinity from the leaves of the human australian rainforest tree Elaeocarpus grandis. J. Org. Chem. 2005, 70, 1889–1892. [Google Scholar]
- Howes, M.-J.R.; Perry, N.S.L.; Houghton, P.J. Plants with traditional uses and activitities, relevant to the management of Alzheimer's disease and other cognitive disorders. Phytother. Res. 2003, 17, 1–18. [Google Scholar]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop-the development of a modern drug against Alzheimer's disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. [Google Scholar] [CrossRef]
- Deleu, D.; Hanssens, Y.; Northway, M.G. Subcutaneous apomorphine: An evidence-based review of its use in Parkinson's disease. Drugs Aging 2004, 21, 687–709. [Google Scholar] [CrossRef]
- Marris, E. Marine natural products: Drugs from the deep. Nature 2006, 443, 904–905. [Google Scholar] [CrossRef]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Alejandro, M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharm. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Lithgow-Bertelloni, A.M. Novel antiviral and cytotoxic agent, dehydrodidemnin B. PCT Int. Pat. Appl. 1991, 15, 248086q. [Google Scholar]
- Urdiales, J.L.; Morata, P.; De Castro, I.N.; Sanchez-Jimenez, F. Anti-proliferative effect of dehydrodidemnin B (DDB), a depsipeptide isolated from Mediterranean tunicates. Cancer Lett. 1996, 102, 31–37. [Google Scholar] [CrossRef]
- Henríquez, R.; Faircloth, G.; Cuevas, C. In Ecteinascidin 743 (ET-743, Yondelis), aplidin, and kahalalide F. In Anticancer Agents from Natural Products; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2005; p. 215. [Google Scholar] [Green Version]
- Rinehart, K.L.; Holt, T.G.; Fregeau, N.L.; Stroh, J.G.; Keifer, P.A.; Sun, F.; Li, L.H.; Martin, D.G. Ecteinascidins 729, 743, 745, 759A, 759B, and 770: Potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4512–4515. [Google Scholar]
- Wright, A.E.; Forleo, D.A.; Gunawardana, G.P.; Gunasekera, S.P.; Koehn, F.E.; McConnell, O.J. Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinata. J. Org. Chem. 1990, 55, 4508–4512. [Google Scholar] [CrossRef]
- Manzanares, I.; Cuevas, C.; Garcia-Nieto, R.; Marco, E.; Gago, F. Advances in the chemistry and pharmacology of ecteinascidins, a promising new class of anticancer agents. Curr. Med. Chem. Anticancer Agents 2001, 1, 257. [Google Scholar] [CrossRef]
- Cuevas, C.; Francesch, A. Development of Yondelis® (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep. 2009, 26, 322–337. [Google Scholar] [CrossRef]
- Alvarez-Miranda, M.; Rodriguez-Gonzalez, A.; Ptero, G.; Lacal, J.C. Characterization of the mechanism of action of ES-285, a novel antitumor drug from Mactomeris polynyma. Clin. Cancer Res. 2003, 9. Abstract C17. [Google Scholar] [Green Version]
- Cuadros, R.; Montejo de Garcini, E.; Wandosell, F.; Faircloth, G.; Fernandez-Sousa, J.M.; Avila, J. The marine compound spisulosine, an inhibitor of cell proliferation, promotes the disassembly of actin stress fibers. Cancer Lett. 2000, 152, 23. [Google Scholar] [CrossRef]
- Salcedo, M.; Cuevas, C.; Otero, G.; Sanchez-Puelles, J.M.; Fernandez-Sousa, J.M.; Avila, J.; Wandosell, F. The marine antitmor compound ES 285 activates EGD receptors. Clin. Cancer Res. 2003, 9. Abstract C24. [Google Scholar] [Green Version]
- Salcedo, M.; Cuevas, C.; Sanchez-Puelles, J.M.; Otero, G.; Sousa, J.M.F.; Avila, J.; Wandosell, F. ES-285, a novel antitumoral compound, interacts with EDG receptors. In Proceedings of American Association for Cancer Research 94th Meeting, Washington, 11–14 July, 2003. Abstract 3649. [Green Version]
- Trimurtulu, G.; Ohtani, I.; Patterson, G.M.L.; Moore, R.E.; Corbett, T.H.; Valeriote, F.A.; Demchik, L. Total structures of cryptophycins, potent antitumor depsipeptides from the blue-green alga Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1994, 116, 4729–4737. [Google Scholar]
- Faulkner, D.J. Marine natural products. J. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar]
- Bhakuni, D.S.; Rawat, D.S. Bioactive Marine Natural Products, 1st ed; Springer-Verlag: New Delhi, India, 2005; p. 404. [Google Scholar] [Green Version]
- Baslow, M.N. Marine Pharmacology; A Study of Toxins and Other Biologically Active Substances of Marine Origin; Williams & Wilkins Co: Baltimore, MD, USA, 1969; p. 286. [Google Scholar] [Green Version]
- Yotsu-Yamashita, M.; Haddock, R.L.; Yasumoto, T. Polycavernoside A: a novel glycosidic macrolide from the red alga Polycavernosa tsudai (Gracilaria edulis). J. Am. Chem. Soc. 1993, 115, 1147–1148. [Google Scholar]
- White, J.D.; Blakemore, P.R.; Browder, C.C.; Hong, J.; Lincoln, C.M.; Nagornyy, P.A.; Robarge, L.A.; Wardrop, D.J. Total synthesis of the marine toxin polycavernoside A via selective macrolactonization of a trihydroxy carboxylic acid. J. Am. Chem. Soc. 2001, 123, 8593–8595. [Google Scholar]
- Paquette, L.A.; Barriault, L.; Pissarnitski, D.; Johnston, J.N. Stereocontrolled elaboration of natural (-)-Polycavernoside A, a powerfully toxic metabolite of the red alga Polycavernosa tsudai. J. Am. Chem. Soc. 2000, 122, 619–631. [Google Scholar] [CrossRef]
- Ishitsuka, M.O.; Kusumi, T.K.H. Antitumor xenicane and norxenicane lactones from the brown alga Dictyota dichotoma. J. Org. Chem. 1988, 53, 5010–5013. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1988, 20, 269–309. [Google Scholar]
- Tringali, C.; Oriente, G.; Piattelli, M.; Geraci, C.; Nicolosi, G.; Breitmaier, E. Crenuladial, an antimicrobial diterpenoid from the brown alga Dilophus ligulatus. Can. J. Chem. 1988, 66, 2799–2802. [Google Scholar] [CrossRef]
- San-Martin, A.; Darias, J.; Soto, H.; Contreras, C.; Herrera, J.S.; Rovirosa, J. A new C15 acetogenin from the marine alga Laurencia claviformis. Nat. Prod. Lett. 1997, 10, 303–311. [Google Scholar] [CrossRef]
- Kimura, J.; Kamada, N.; Yoshikazu, T. Fifteen chamigrane derivatives from the red alga Laurencia nidifica. Bull. Chem. Soc. Jpn. 1999, 72, 289–88292. [Google Scholar] [CrossRef]
- Elsworth, J.M. A new chamigrane from Laurenciaglomerata. J. Nat. Prod. 1989, 52, 893–895. [Google Scholar] [CrossRef]
- Dias, D.A.; White, J.M.; Urban, S. Laurenciafiliformis: Phytochemical profiling by conventional and HPLC-NMR approaches. Nat. Prod. Commun. 2009, 4, 157–172. [Google Scholar]
- Dias, D.A.; Urban, S. Phytochemical studies of the southern Australian marine alga, Laurenciaelata. Phytochemistry 2011, 72, 2081–2089. [Google Scholar]
- Duke, S.O.; Menn, J.J.; Plimmer, J.R. Pest Control with Enhanced Environmental Safety; ACS Symposium Series No. 514; Duke, S.O., Menn, J.J., Plimmer, J.R., Eds.; American Chemical Society: Washington, DC, USA, 1993. [Google Scholar] [Green Version]
- Georghiou, G.P. Overview of insecticide resistance. In Managing Resistance to Agrochemicals: From Fundamental Research to Practical Strategies; Green, M.B., Le Baron, H.M., Moberg, W.K., Eds.; ACS Symp. Ser. 421. Am. Chem. Soc: Washington, DC, USA, 1990; pp. 18–41. [Google Scholar] [Green Version]
- El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Greenplate, J.T.; Wideman, M.A. Marine natural products as prototype insecticidal agents. J. Agric. Food Chem. 1997, 45, 2735–2739. [Google Scholar]
- San-Martin, A.; Negrete, R.; Rovirosa, J. Insecticide and acaricide activities of polyhalogenated monoterpenes from Chilean Plocamium cartilagineum. Phytochemistry 1991, 30, 2165–2169. [Google Scholar]
- Fukuzawa, A.; Masamune, T. Laurepinnacin and isolaurepinnacin, new acetylenic cyclic ethers from the marine red alga Laurencia pinnata Yamada. Tetrahedron Lett. 1981, 22, 4081–4084. [Google Scholar] [CrossRef]
- Watanabe, K.; Umeda, K.; Miyakado, M. Isolation and identification of three insecticidal principles from the red alga Laurencia nipponica Yamada. Agric. Biol. Chem. 1989, 53, 2513–2515. [Google Scholar] [CrossRef]
- McConnell, O.; Longley, R.E.; Koehn, F.E. The Discovery of Natural Products with Therapeutic Potential; Gullo, V.P., Ed.; Butterworth-Heinemann: Boston, MA, USA, 1994; pp. 109–174. [Google Scholar] [Green Version]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products on drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar]
- Chin, Y.-W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, 239–253. [Google Scholar]
- Uemura, D.; Takahashi, K.; Yamamoto, T.; Katayama, C.; Tanaka, J.; Okumura, Y.; Hirata, Y. Norhalichondrin A: An antitumor polyether macrolide from a marine sponge. J. Am. Chem. Soc. 1985, 107, 4796–4798. [Google Scholar] [CrossRef]
- Pettit, G.R.; Herald, C.L.; Boyd, M.R.; Leet, J.E.; Dufresne, C.; Doubek, D.L.; Schmidt, J.M.; Cerny, R.L.; Hooper, J.N.A.; Rutzler, K.C. Antineoplastic agents. 219. Isolation and structure of the cell growth inhibitory constituents from the western Pacific marine sponge Axinella sp. J. Med. Chem. 1991, 34, 3339–3340. [Google Scholar] [CrossRef]
- Pettit, G.R.; Srirangam, J.K.; Herald, D.L.; Erickson, K.L.; Doubek, D.L.; Schmidt, J.M.; Tackett, L.P.; Bakus, G.J. Antineoplastic agents. 251. Isolation and structure of stylostatin 1 from the Papua New Guinea marine sponge Stylotella sp. J. Org. Chem. 1993, 58, 3222. [Google Scholar]
- Litaudon, M.; Hart, J.B.; Blunt, J.W.; Lake, R.J.; Munro, M.H.G. Isohomohalichondrin B, a new antitumour polyether macrolide from the New Zealand deep-water sponge, Lyssodendoryx sp. Tetrahedron Lett. 1994, 35, 9435. [Google Scholar]
- Aicher, T.D.; Buszek, K.R.; Fang, F.G.; Forsyth, C.J.; Jung, S.H.; Kishi, Y.; Matelich, M.C.; Scola, P.M.; Spero, D.M.; Yoon, S.K. Total synthesis of halichondrin B and norhalichondrin B. J. Am. Chem.Soc. 1992, 114, 3162–3164. [Google Scholar]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef]
- Ojima, I. Modern natural products chemistry and drug discovery. J. Med. Chem. 2008, 51, 2587–2588. [Google Scholar] [CrossRef]
- Nussbaum, F.V.; Brands, M.; Hinzen, B.; Weigand, S.; Habich, D. Antibacterial natural products in medicinal chemistry - exodus or revival? Angew.Chem. Int. Ed. 2006, 45, 5072–5129. [Google Scholar]
- Luzhetskyy, A.; Pelzer, S.; Bechthold, A. The future of natural products as a source of new antibiotics. Curr. Opin. Investig. Drugs 2007, 8, 608–613. [Google Scholar]
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J.Med. Chem. 2008, 51, 2589–2599. [Google Scholar]
- Ramakrishna, N.V.S.; Nadkarni, S.R.; Bhat, R.G.; Naker, S.D.; Kumar, E.K.S.V.; Lal, B. Screening of natural product extracts for antibacterial activity: Early identification and elimination of known compounds by dereplication. Ind. J. Chem. 1999, 38B, 1384–1387. [Google Scholar]
- Sashidhara, K.V.; Rosaiah, J.N. Various dereplication strategies using LC-MS for rapid natural product lead identification and drug discovery. Nat. Prod. Commun. 2007, 2, 193–202. [Google Scholar]
- Cordell, G.A.; Shin, Y.G. Finding the needle in the haystack. The dereplication of natural product extracts. Pure Appl. Chem. 1999, 71, 1089–1094. [Google Scholar] [CrossRef]
- Brkljaca, R.; Urban, S. Recent advances in HPLC-NMR and applications for natural product profiling and identification. J. Liq. Chroma. Rel. Technol. 2011, 34, 1063–1076. [Google Scholar] [CrossRef]
- Blunt, J.W.; Munro, M.H.G. Dictionary of Marine Natural Products. Available online: http://dmnp.chemnetbase.com/ (accessed on 21 July 2009). [Green Version]
- Buckingham, J. The Dictionary of Natural Products. Available online: http://dmnp.chemnetbase.com/ (accessed on 22 August 2011). [Green Version]
- MarinLit. Available online: http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml (accessed on 23 June 2011). [Green Version]
- Blunt, J.W.; Munro, M.H.G.; Laatsch, H. AntiMarin Database. Available online: http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml (accessed on 10 April 2012). [Green Version]
- Lang, G.; Mayhudin, N.A.; Mitova, M.I.; Sun, L.; van der Sar, S.; Blunt, J.W.; Cole, A.L.J.; Ellis, G.; Laatsch, H.; Munro, M.H.G. Evolving trends in the dereplication of natural product extracts: New methodology for rapid, small-scale investigation of natural product extracts. J. Nat. Prod. 2008, 19, 1595–1599. [Google Scholar]
- SciFinder Scholar. Available online: http://www.cas.org/SCIFINDER/SCHOLAR/ (accessed on 2 July 2011). [Green Version]
- SCOPUS. Available online: http://www.scopus.com/home.url (accessed on 25 August 2011). [Green Version]
- Napralert. Available online: http://www.napralert.org/ (accessed on 3 May 2011). [Green Version]
- Urban, S.; Separovic, F. Developments in hyphenated spectroscopic methods in natural product profiling. Front. Drug Des. Discov. 2005, 1, 113–166. [Google Scholar]
- Wolfender, J.-L.; Ndjoko, K.; Hostettman, K. Liquid chromatography with ultraviolet absorbance-mass spectrometric detection and with nuclear magnetic resonance spectroscopy: A powerful combination for the on-line structural investigation of plant metabolites. J. Chromatogr. A 2003, 1000, 437–455. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Queiroz, E.F.; Hostettman, K. The importance of hyphenated techniques in the discovery of new lead compounds from nature. Expert Opin. Drug Dis. 2006, 1, 237–260. [Google Scholar] [CrossRef]
- Schroeder, F.C.; Gronquist, M. Extending the scope of NMR spectroscopy with microcoil probes. Angew. Chem. Int. Ed. 2006, 45, 7122–7131. [Google Scholar] [CrossRef]
- Lewis, R.J.; Bernstein, M.A.; Duncan, S.J.; Sleigh, C.J. A comparison of capillary-scale LC-NMR with alternative techniques: Spectroscopic and practical considerations. Magn. Reson. Chem. 2005, 43, 783–789. [Google Scholar]
- Dias, D.; Urban, S. Phytochemical analysis of the southern australian marine alga, Plocamium mertensii using HPLC-NMR. Phytochem. Anal. 2008, 19, 453–470. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S. Application of HPLC-NMR for the rapid chemical profiling of a Southern Australian Sponge, Dactylospongia sp. J. Sep. Sci. 2009, 32, 542–548. [Google Scholar] [CrossRef]
- Lin, Y.; Schiavo, S.; Orjala, J.; Vouros, P.; Kautz, R. Microscale LC-MS-NMR platform applied to the identification of active cyanobacterial metabolites. Anal. Chem. 2008, 80, 8045–8054. [Google Scholar]
- Sun Lin, J.; Mahyudin, N.A.; Chamyuang, S.; Blunt, J.W.; Cole, T.; Lang, G.; Mitova, M.I.; van der Sar, S.; Munro, M.H.G. Less is more: Dereplication and discovery using CapNMR techniques. In Proceedings of Manapro XII: 12th International Symposium of Marine Natural Products, Queenstown, New Zealand, Feb 4th–9th, 2007. [Green Version]
- Clarkson, C.; Stærk, D.; Hansen, S.H.; Smith, P.J.; Jaroszewski, J.W. Discovering new natural products directly from crude extracts by HPLC-SPE-NMR: Chinane diterpenes Harpagophytum procumbens. J. Nat. Prod. 2006, 69, 527–530. [Google Scholar] [CrossRef]
- Cogne, A.-L.; Queiroz, E.F.; Marston, A.; Wolfender, J.L.; Mavi, S.; Hostettmann, K. On-line identification of unstable iridoids from Jamesbrittenia fodina by HPLC-MS and HPLC-NMR. Phytochem. Anal. 2005, 16, 429–439. [Google Scholar] [CrossRef]
- Zschocke, S.; Klaiber, I.; Bauer, R.; Vogler, B. HPLC-coupled spectroscopic techniques (UV, MS, NMR) for the structure elucidation of phthalides in Ligusticum chuanxiong. Mol. Divers. 2005, 9, 33–39. [Google Scholar]
- Roessner, U.; Beckles, D.M. Metabolite measurements. In Plant Metabolic Networks; Junker, B., Schwender, J., Eds.; Springer: Heidelberg, Germany, 2009. [Google Scholar] [Green Version]
- Roessner, U.; Nahid, A.; Hunter, A.; Bellgard, M. Metabolomics—The combination of analytical chemistry, biology and informatics. In Comprehensive Biotechnology, 2nd; Moo-Young, M., Ed.; Springer: Heidelberg, Germany, 2011; Volume 1, pp. 447–459. [Google Scholar]
- Beckles, D.M.; Roessner, U. Plant Metabolomics—Applications and opportunities for agricultural biotechnology. In Plant Biotechnology and Agriculture: Prospects for the 21st Century; Altmann, A., Hasegawa, P.M., Eds.; Elsevier/Academic Press: Boston, MA, USA, 2011. [Google Scholar] [Green Version]
- Rochfort, S. Metabolomics reviewed: A new “Omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod. 2005, 68, 1813–1820. [Google Scholar] [CrossRef]
- Cortina, N.S.; Krug, D.; Plaza, A.; Revermann, O.; Muller, R. Myxoprincomide: A natural product from Myxococcus xanthus discovered by comprehensive analysis of the secondary metabolome. Angew. Chem. Int. Ed. 2012, 51, 811–816. [Google Scholar]
- Guoxiang, X.; Robert, P.; Mingming, S.; Zhaohui, X.; Aihua, Z.; Mingfeng, Q.; Xiangbao, L.; Zhong, L.; Wei, J. Ultra-performance LC/TOF MS analysis of medicinal Panax herbs for metabolomic research. J. Sep. Sci. 2008, 31, 1015–1026. [Google Scholar] [CrossRef]
- Politi, M.; Peschel, W.; Wilson, N.; Zloh, M.; Prieto, J.M.; Heinrich, M. Cannabis water extracts and tinctures analysed by NMR spectroscopy; different strategies to reduce the content of D9-THC. Phytochemistry 2008, 69, 562–570. [Google Scholar]
- Verpoorte, R.; Choi, Y.H.; Kim, H.K. Ethnopharmacology and systems biology: A perfect holistic match. J. Ethnopharmacol. 2005, 100, 53–56. [Google Scholar] [CrossRef]
- Wang, M.; Lamers, R.J.A.N.; Korthout, H.A.; Van Nesselrooij, J.H.J.; Witkamp, R.F.; Van der Heijden, R. Metabolomics in the context of systems biology: Bridging traditional Chinese medicine and molecular pharmacology. Phytother. Res. 2005, 3, 173–182. [Google Scholar]
- Cardoso-Taketa, A.T.; Pereda-Miranda, R.; Choi, Y.H.; Verpoorte, R.; Villarreal, M.L. Metabolic profiling of the Mexican anxiolytic and sedative plant Galphimia glauca using nuclear magnetic resonance spectroscopy and multivariate data analysis. Planta Med. 2008, 74, 1295–1301. [Google Scholar] [CrossRef]
- Biao-Yi, Z.; Yan Yu, Y.; Zeng-Liang, Y. Investigation of antimicrobial model of Hemsleya pengxianensis W.J. Chang and its main active component by metabolomics technique. J. Ethnopharmacol. 2008, 116, 89–95. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]
- Deyrupa, S.T.; Eckman, L.E.; McCarthy, P.H.; Smedley, S.R.; Meinwald, J.; Schroeder, F.C. 2D NMR-spectroscopic screening reveals polyketides in ladybugs. Proc. Natl. Acad. Soc. USA 2011, 108, 9753–9758. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303-336. https://doi.org/10.3390/metabo2020303
Dias DA, Urban S, Roessner U. A Historical Overview of Natural Products in Drug Discovery. Metabolites. 2012; 2(2):303-336. https://doi.org/10.3390/metabo2020303
Chicago/Turabian StyleDias, Daniel A., Sylvia Urban, and Ute Roessner. 2012. "A Historical Overview of Natural Products in Drug Discovery" Metabolites 2, no. 2: 303-336. https://doi.org/10.3390/metabo2020303
APA StyleDias, D. A., Urban, S., & Roessner, U. (2012). A Historical Overview of Natural Products in Drug Discovery. Metabolites, 2(2), 303-336. https://doi.org/10.3390/metabo2020303



