5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5'-Monophosphate (AICAR), a Highly Conserved Purine Intermediate with Multiple Effects
Abstract
:1. Introduction
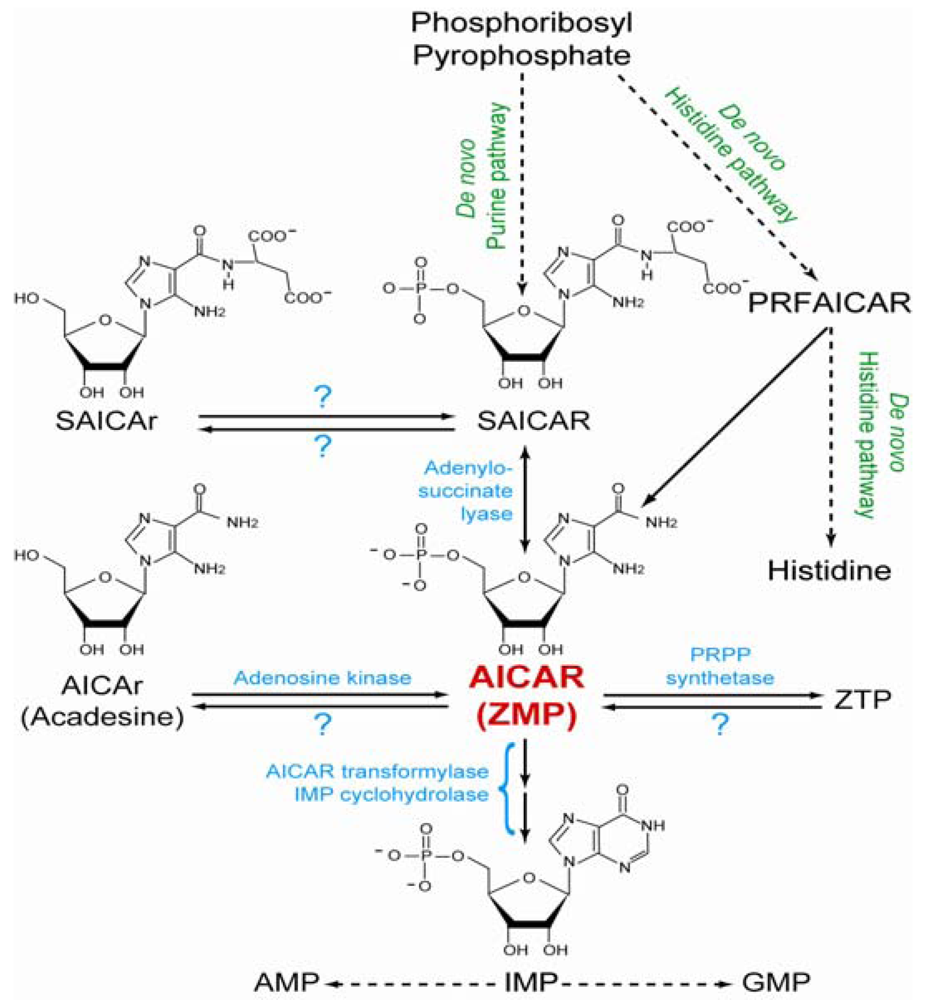
2. Metabolism of AICAR
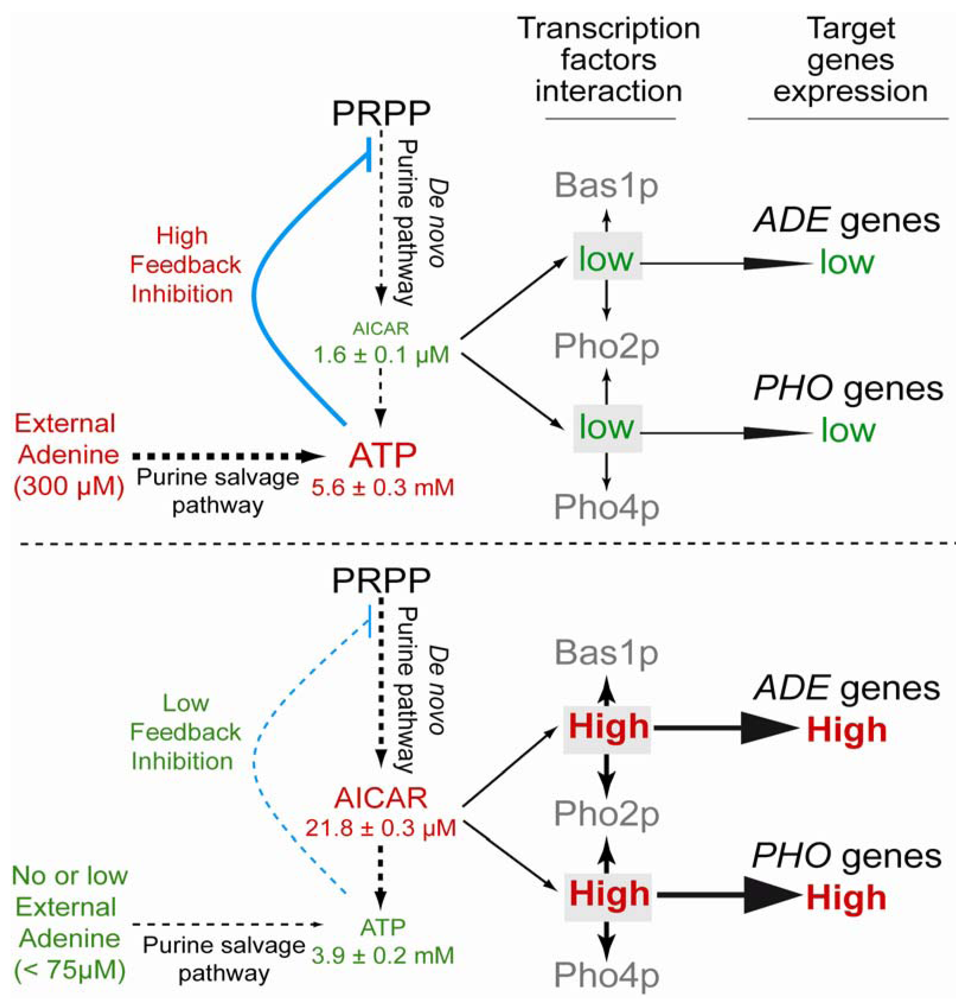
3. Roles of Physiologically Produced AICAR and Accumulation in Metabolic Diseases
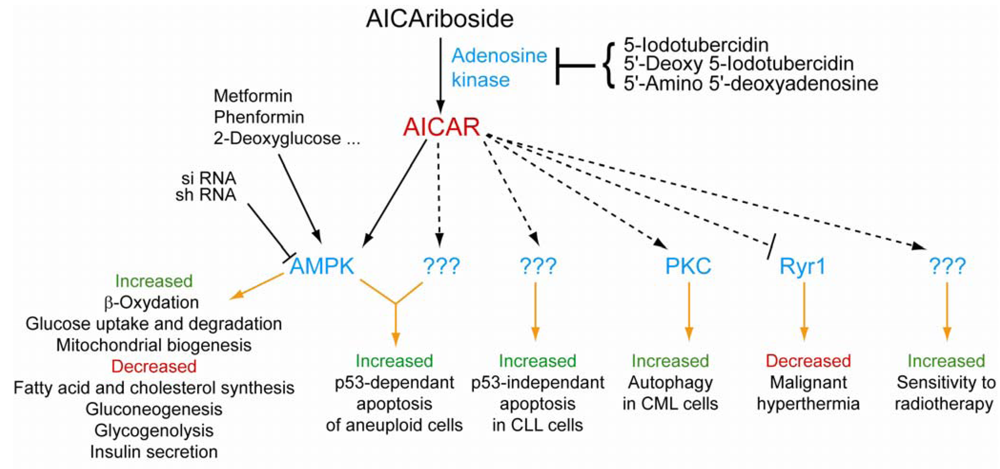
4. AICAR, a Potent Activator of AMP-Activated Protein Kinase
5. AMPK-Independent Effects of AICAR: Other Protein Targets
6. Effects of AICAR on Whole Organisms
7. Conclusion
Acknowledgments
Conflict of Interest
References and Notes
- Bochner, B.R.; Ames, B.N. ZTP (5-amino 4-imidazole carboxamide riboside 5'-triphosphate): a proposed alarmone for 10-formyl-tetrahydrofolate deficiency. Cell 1982, 29, 929–937. [Google Scholar] [CrossRef]
- Rebora, K.; Desmoucelles, C.; Borne, F.; Pinson, B.; Daignan-Fornier, B. Yeast AMP pathway genes respond to adenine through regulated synthesis of a metabolic intermediate. Mol. Cell Biol. 2001, 21, 7901–7912. [Google Scholar]
- Pinson, B.; Vaur, S.; Sagot, I.; Coulpier, F.; Lemoine, S.; Daignan-Fornier, B. Metabolic intermediates selectively stimulate transcription factor interaction and modulate phosphate and purine pathways. Genes Dev. 2009, 23, 1399–1407. [Google Scholar] [CrossRef]
- Marie, S.; Heron, B.; Bitoun, P.; Timmerman, T.; Van Den Berghe, G.; Vincent, M.F. AICA-ribosiduria: a novel, neurologically devastating inborn error of purine biosynthesis caused by mutation of ATIC. Am. J. Hum. Genet. 2004, 74, 1276–1281. [Google Scholar] [CrossRef]
- Sabina, R.L.; Patterson, D.; Holmes, E.W. 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J. Biol. Chem. 1985, 260, 6107–6114. [Google Scholar]
- Sabina, R.L.; Holmes, E.W.; Becker, M.A. The enzymatic synthesis of 5-amino-4-imidazolecarboxamide riboside triphosphate (ZTP). Science 1984, 223, 1193–1195. [Google Scholar]
- Rohlman, C.E.; Matthews, R.G. Role of purine biosynthetic intermediates in response to folate stress in Escherichia coli. J. Bacteriol. 1990, 172, 7200–7210. [Google Scholar]
- Rebora, K.; Laloo, B.; Daignan-Fornier, B. Revisiting purine-histidine cross-pathway regulation in Saccharomyces cerevisiae: a central role for a small molecule. Genetics 2005, 170, 61–70. [Google Scholar] [CrossRef]
- Daignan-Fornier, B.; Fink, G.R. Coregulation of purine and histidine biosynthesis by the transcriptional activators BAS1 and BAS2. Proc. Natl. Acad. Sci. USA 1992, 89, 6746–6750. [Google Scholar] [CrossRef]
- Denis, V.; Boucherie, H.; Monribot, C.; Daignan-Fornier, B. Role of the myb-like protein bas1p in Saccharomyces cerevisiae: a proteome analysis. Mol. Microbiol. 1998, 30, 557–566. [Google Scholar] [CrossRef]
- Denis, V.; Daignan-Fornier, B. Synthesis of glutamine, glycine and 10-formyl tetrahydrofolate is coregulated with purine biosynthesis in Saccharomyces cerevisiae. Mol. Gen. Genet. 1998, 259, 246–255. [Google Scholar] [CrossRef]
- Gauthier, S.; Coulpier, F.; Jourdren, L.; Merle, M.; Beck, S.; Konrad, M.; Daignan-Fornier, B.; Pinson, B. Co-regulation of yeast purine and phosphate pathways in response to adenylic nucleotide variations. Mol. Microbiol. 2008, 68, 1583–1594. [Google Scholar]
- Springer, C.; Kunzler, M.; Balmelli, T.; Braus, G.H. Amino acid and adenine cross-pathway regulation act through the same 5'-TGACTC-3' motif in the yeast HIS7 promoter. J. Biol. Chem. 1996, 271, 29637–29643. [Google Scholar]
- Tice-Baldwin, K.; Fink, G.R.; Arndt, K.T. BAS1 has a Myb motif and activates HIS4 transcription only in combination with BAS2. Science 1989, 246, 931–935. [Google Scholar]
- Hurlimann, H.C.; Laloo, B.; Simon-Kayser, B.; Saint-Marc, C.; Coulpier, F.; Lemoine, S.; Daignan-Fornier, B.; Pinson, B. Physiological and toxic effects of purine intermediate 5-amino-4-imidazolecarboxamide ribonucleotide (AICAR) in yeast. J. Biol. Chem. 2011, 286, 30994–31002. [Google Scholar]
- Pinson, B.; Kongsrud, T.L.; Ording, E.; Johansen, L.; Daignan-Fornier, B.; Gabrielsen, O.S. Signaling through regulated transcription factor interaction: mapping of a regulatory interaction domain in the Myb-related Bas1p. Nucleic. Acids Res. 2000, 28, 4665–4673. [Google Scholar]
- Sidi, Y.; Mitchell, B.S. Z-nucleotide accumulation in erythrocytes from Lesch-Nyhan patients. J. Clin. Invest. 1985, 76, 2416–2419. [Google Scholar]
- Lopez, J.M. Is ZMP the toxic metabolite in Lesch-Nyhan disease? Med. Hypotheses 2008, 71, 657–663. [Google Scholar] [CrossRef]
- Laporte, D.; Lebaudy, A.; Sahin, A.; Pinson, B.; Ceschin, J.; Daignan-Fornier, B.; Sagot, I. Metabolic status rather than cell cycle signals control quiescence entry and exit. J. Cell Biol. 2011, 192, 949–957. [Google Scholar]
- Sullivan, J.E.; Carey, F.; Carling, D.; Beri, R.K. Characterisation of 5'-AMP-activated protein kinase in human liver using specific peptide substrates and the effects of 5'-AMP analogues on enzyme activity. Biochem. Biophys. Res. Commun. 1994, 200, 1551–1556. [Google Scholar]
- Sullivan, J.E.; Brocklehurst, K.J.; Marley, A.E.; Carey, F.; Carling, D.; Beri, R.K. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994, 353, 33–36. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Guigas, B.; Bertrand, L.; Taleux, N.; Foretz, M.; Wiernsperger, N.; Vertommen, D.; Andreelli, F.; Viollet, B.; Hue, L. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes 2006, 55, 865–874. [Google Scholar]
- Mukhtar, M.H.; Payne, V.A.; Arden, C.; Harbottle, A.; Khan, S.; Lange, A.J.; Agius, L. Inhibition of glucokinase translocation by AMP-activated protein kinase is associated with phosphorylation of both GKRP and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R766–774. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hebrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta. Physiol. (Oxf.) 2009, 196, 81–98. [Google Scholar] [CrossRef]
- Day, P.; Sharff, A.; Parra, L.; Cleasby, A.; Williams, M.; Horer, S.; Nar, H.; Redemann, N.; Tickle, I.; Yon, J. Structure of a CBS-domain pair from the regulatory gamma1 subunit of human AMPK in complex with AMP and ZMP. Acta. Crystallog.r D. Biol. Crystallogr. 2007, 63, 587–596. [Google Scholar]
- Fryer, L.G.; Parbu-Patel, A.; Carling, D. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 2002, 531, 189–192. [Google Scholar] [CrossRef]
- Ugarkar, B.G.; DaRe, J.M.; Kopcho, J.J.; Browne, C.E., 3rd; Schanzer, J.M.; Wiesner, J.B.; Erion, M.D. Adenosine kinase inhibitors. 1. Synthesis, enzyme inhibition, and antiseizure activity of 5-iodotubercidin analogues. J. Med. Chem. 2000, 43, 2883–2893. [Google Scholar]
- Tang, Y.C.; Williams, B.R.; Siegel, J.J.; Amon, A. Identification of aneuploidy-selective antiproliferation compounds. Cell 2011, 144, 499–512. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell. Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Wong, A.K.; Howie, J.; Petrie, J.R.; Lang, C.C. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin. Sci. (Lond.) 2009, 116, 607–620. [Google Scholar] [CrossRef]
- Santidrian, A.F.; Gonzalez-Girones, D.M.; Iglesias-Serret, D.; Coll-Mulet, L.; Cosialls, A.M.; de Frias, M.; Campas, C.; Gonzalez-Barca, E.; Alonso, E.; Labi, V.; Viollet, B.; Benito, A.; Pons, G.; Villunger, A.; Gil, J. AICAR induces apoptosis independently of AMPK and p53 through up-regulation of the BH3-only proteins BIM and NOXA in chronic lymphocytic leukemia cells. Blood 2010, 116, 3023–3032. [Google Scholar]
- Lanner, J.T.; Georgiou, D.K.; Dagnino-Acosta, A.; Ainbinder, A.; Cheng, Q.; Joshi, A.D.; Chen, Z.; Yarotskyy, V.; Oakes, J.M.; Lee, C.S.; Monroe, T.O.; Santillan, A.; Dong, K.; Goodyear, L.; Ismailov, II; Rodney, G.G.; Dirksen, R.T.; Hamilton, S.L. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat. Med. 2012, 18, 244–251. [Google Scholar]
- Isebaert, S.F.; Swinnen, J.V.; McBride, W.H.; Begg, A.C.; Haustermans, K.M. 5-aminoimidazole-4-carboxamide riboside enhances effect of ionizing radiation in PC3 prostate cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1515–1523. [Google Scholar]
- Robert, G.; Ben Sahra, I.; Puissant, A.; Colosetti, P.; Belhacene, N.; Gounon, P.; Hofman, P.; Bost, F.; Cassuto, J.P.; Auberger, P. Acadesine kills chronic myelogenous leukemia (CML) cells through PKC-dependent induction of autophagic cell death. PLoS One 2009, 4, e7889. [Google Scholar]
- Guigas, B.; Taleux, N.; Foretz, M.; Detaille, D.; Andreelli, F.; Viollet, B.; Hue, L. AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem. J. 2007, 404, 499–507. [Google Scholar]
- Jacobs, R.L.; Lingrell, S.; Dyck, J.R.; Vance, D.E. Inhibition of hepatic phosphatidylcholine synthesis by 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside is independent of AMP-activated protein kinase activation. J. Biol. Chem. 2007, 282, 4516–4523. [Google Scholar]
- Kuo, C.L.; Ho, F.M.; Chang, M.Y.; Prakash, E.; Lin, W.W. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 5-aminoimidazole-4-carboxamide riboside is independent of AMP-activated protein kinase. J. Cell Biochem. 2008, 103, 931–940. [Google Scholar] [CrossRef]
- Lopez, J.M.; Santidrian, A.F.; Campas, C.; Gil, J. 5-Aminoimidazole-4-carboxamide riboside induces apoptosis in Jurkat cells, but the AMP-activated protein kinase is not involved. Biochem. J. 2003, 370, 1027–1032. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Mayer, F.V.; Heath, R.; Underwood, E.; Sanders, M.J.; Carmena, D.; McCartney, R.R.; Leiper, F.C.; Xiao, B.; Jing, C.; Walker, P.A.; Haire, L.F.; Ogrodowicz, R.; Martin, S.R.; Schmidt, M.C.; Gamblin, S.J.; Carling, D. ADP regulates SNF1, the Saccharomyces cerevisiae homolog of AMP-activated protein kinase. Cell Metab. 2011, 14, 707–714. [Google Scholar]
- Vincent, M.F.; Bontemps, F.; Van den Berghe, G. Inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside in isolated rat hepatocytes. Biochem. J. 1992, 281 ( Pt 1), 267–272. [Google Scholar]
- Shang, J.; Lehrman, M.A. Activation of glycogen phosphorylase with 5-aminoimidazole-4-carboxamide riboside (AICAR). Assessment of glycogen as a precursor of mannosyl residues in glycoconjugates. J. Biol. Chem. 2004, 279, 12076–12080. [Google Scholar]
- Javaux, F.; Vincent, M.F.; Wagner, D.R.; van den Berghe, G. Cell-type specificity of inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside. Lack of effect in rabbit cardiomyocytes and human erythrocytes, and inhibition in FTO-2B rat hepatoma cells. Biochem. J. 1995, 305 ( Pt 3), 913–919. [Google Scholar]
- Vincent, M.F.; Marangos, P.J.; Gruber, H.E.; Van den Berghe, G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes 1991, 40, 1259–1266. [Google Scholar]
- Meli, M.; Pennati, M.; Curto, M.; Daidone, M.G.; Plescia, J.; Toba, S.; Altieri, D.C.; Zaffaroni, N.; Colombo, G. Small-molecule targeting of heat shock protein 90 chaperone function: rational identification of a new anticancer lead. J. Med. Chem. 2006, 49, 7721–7730. [Google Scholar]
- Rattan, R.; Giri, S.; Singh, A.K.; Singh, I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J. Biol. Chem. 2005, 280, 39582–39593. [Google Scholar]
- Lemieux, G.A.; Liu, J.; Mayer, N.; Bainton, R.J.; Ashrafi, K.; Werb, Z. A whole-organism screen identifies new regulators of fat storage. Nat. Chem. Biol. 2011, 7, 206–213. [Google Scholar]
- Vigne, P.; Tauc, M.; Frelin, C. Strong dietary restrictions protect Drosophila against anoxia/reoxygenation injuries. PLoS One 2009, 4, e5422. [Google Scholar]
- Mullane, K. Acadesine: the prototype adenosine regulating agent for reducing myocardial ischaemic injury. Cardiovasc. Res. 1993, 27, 43–47. [Google Scholar] [CrossRef]
- Leung, J.M.; Stanley, T., 3rd; Mathew, J.; Curling, P.; Barash, P.; Salmenpera, M.; Reves, J.G.; Hollenberg, M.; Mangano, D.T. An initial multicenter, randomized controlled trial on the safety and efficacy of acadesine in patients undergoing coronary artery bypass graft surgery. SPI Research Group. Anesth. Analg. 1994, 78, 420–434. [Google Scholar]
- Vincent, M.F.; Erion, M.D.; Gruber, H.E.; Van den Berghe, G. Hypoglycaemic effect of AICAriboside in mice. Diabetologia 1996, 39, 1148–1155. [Google Scholar] [CrossRef]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; Kang, H.; Shaw, R.J.; Evans, R.M. AMPK and PPARdelta agonists are exercise mimetics. Cell 2008, 134, 405–415. [Google Scholar]
- Bosselaar, M.; Smits, P.; van Loon, L.J.; Tack, C.J. Intravenous AICAR during hyperinsulinemia induces systemic hemodynamic changes but has no local metabolic effect. J. Clin. Pharmacol. 2011, 51, 1449–1458. [Google Scholar] [CrossRef]
- Babraj, J.A.; Mustard, K.; Sutherland, C.; Towler, M.C.; Chen, S.; Smith, K.; Green, K.; Leese, G.; Hardie, D.G.; Rennie, M.J.; Cuthbertson, D.J. Blunting of AICAR-induced human skeletal muscle glucose uptake in type 2 diabetes is dependent on age rather than diabetic status. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1042–1048. [Google Scholar]
- Cuthbertson, D.J.; Babraj, J.A.; Mustard, K.J.; Towler, M.C.; Green, K.A.; Wackerhage, H.; Leese, G.P.; Baar, K.; Thomason-Hughes, M.; Sutherland, C.; Hardie, D.G.; Rennie, M.J. 5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes 2007, 56, 2078–2084. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Daignan-Fornier, B.; Pinson, B. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5'-Monophosphate (AICAR), a Highly Conserved Purine Intermediate with Multiple Effects. Metabolites 2012, 2, 292-302. https://doi.org/10.3390/metabo2020292
Daignan-Fornier B, Pinson B. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5'-Monophosphate (AICAR), a Highly Conserved Purine Intermediate with Multiple Effects. Metabolites. 2012; 2(2):292-302. https://doi.org/10.3390/metabo2020292
Chicago/Turabian StyleDaignan-Fornier, Bertrand, and Benoît Pinson. 2012. "5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5'-Monophosphate (AICAR), a Highly Conserved Purine Intermediate with Multiple Effects" Metabolites 2, no. 2: 292-302. https://doi.org/10.3390/metabo2020292
APA StyleDaignan-Fornier, B., & Pinson, B. (2012). 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5'-Monophosphate (AICAR), a Highly Conserved Purine Intermediate with Multiple Effects. Metabolites, 2(2), 292-302. https://doi.org/10.3390/metabo2020292