LC-MS-Based Untargeted Metabolic Profiling in Plasma Following Dapagliflozin Administration in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Chemicals and Reagents
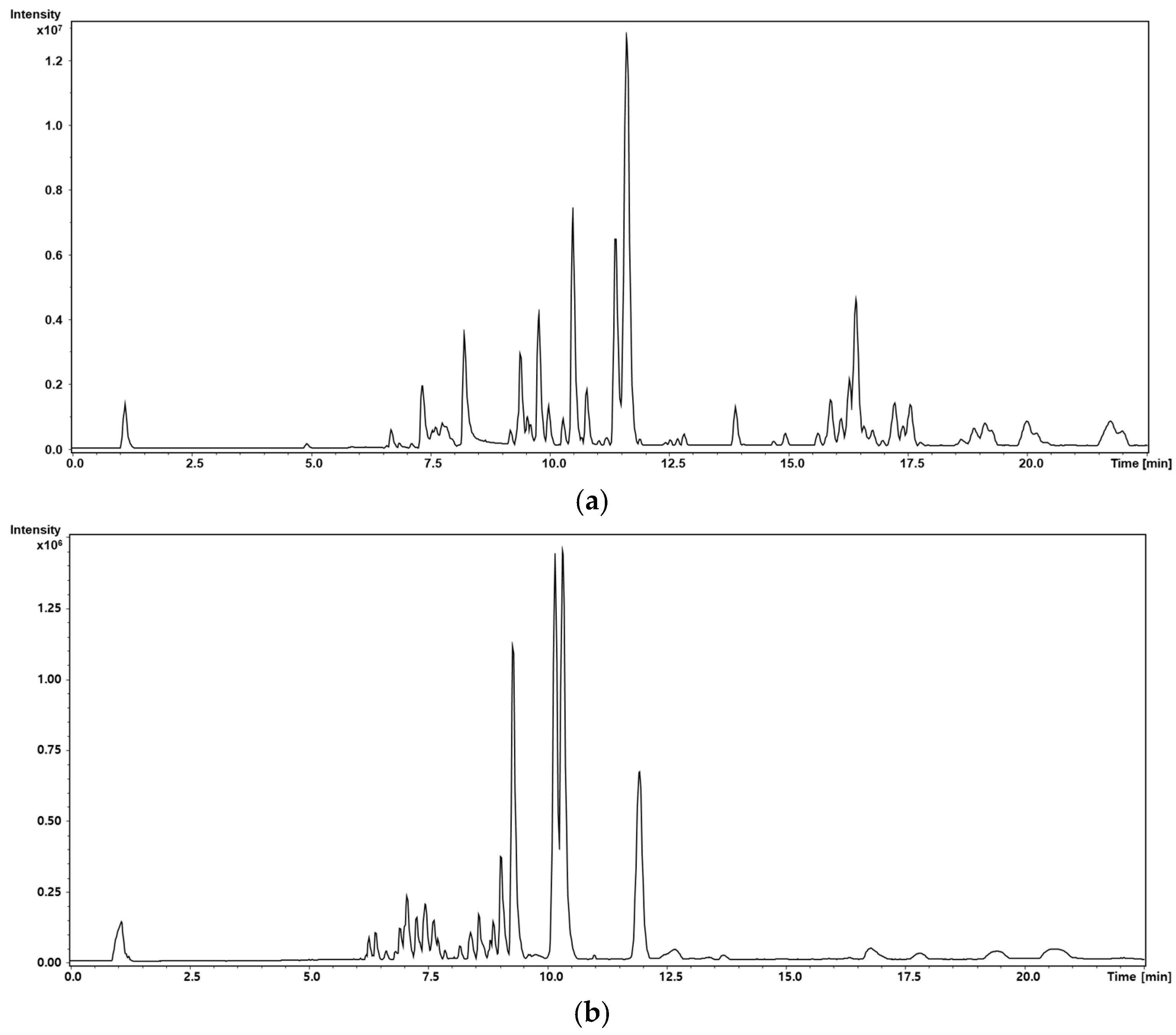
2.3. Untargeted Metabolic Profiling
2.3.1. Sample Preparation
2.3.2. Liquid Chromatography–Tandem MS Conditions
2.4. Data Analysis
2.5. Metabolite Annotation
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
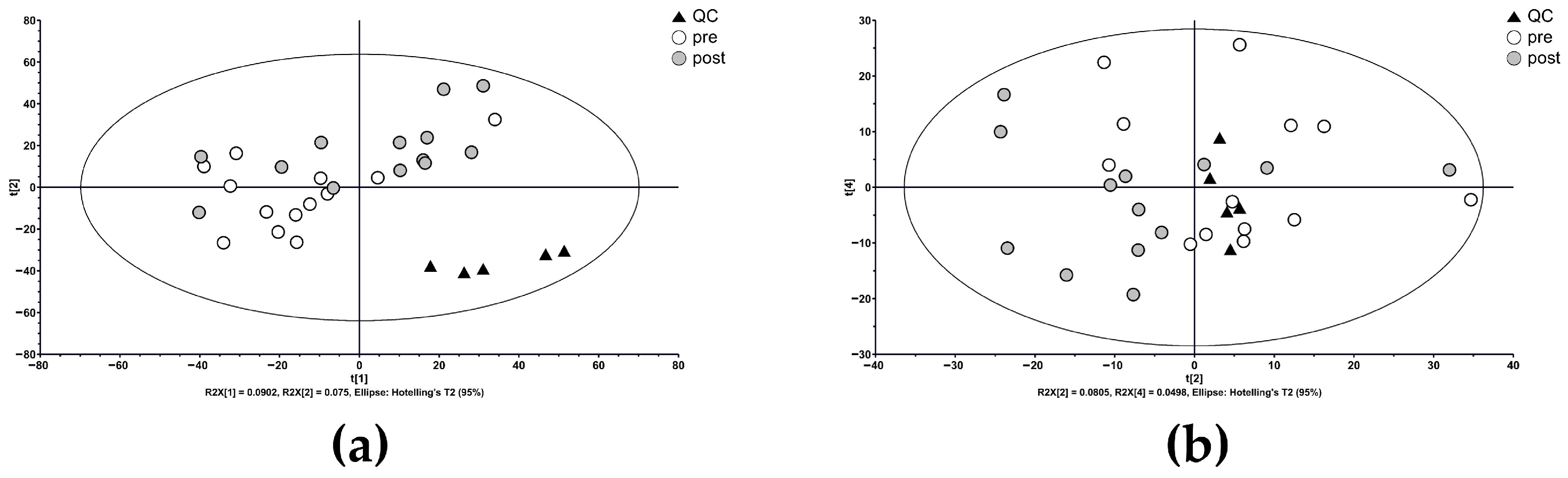
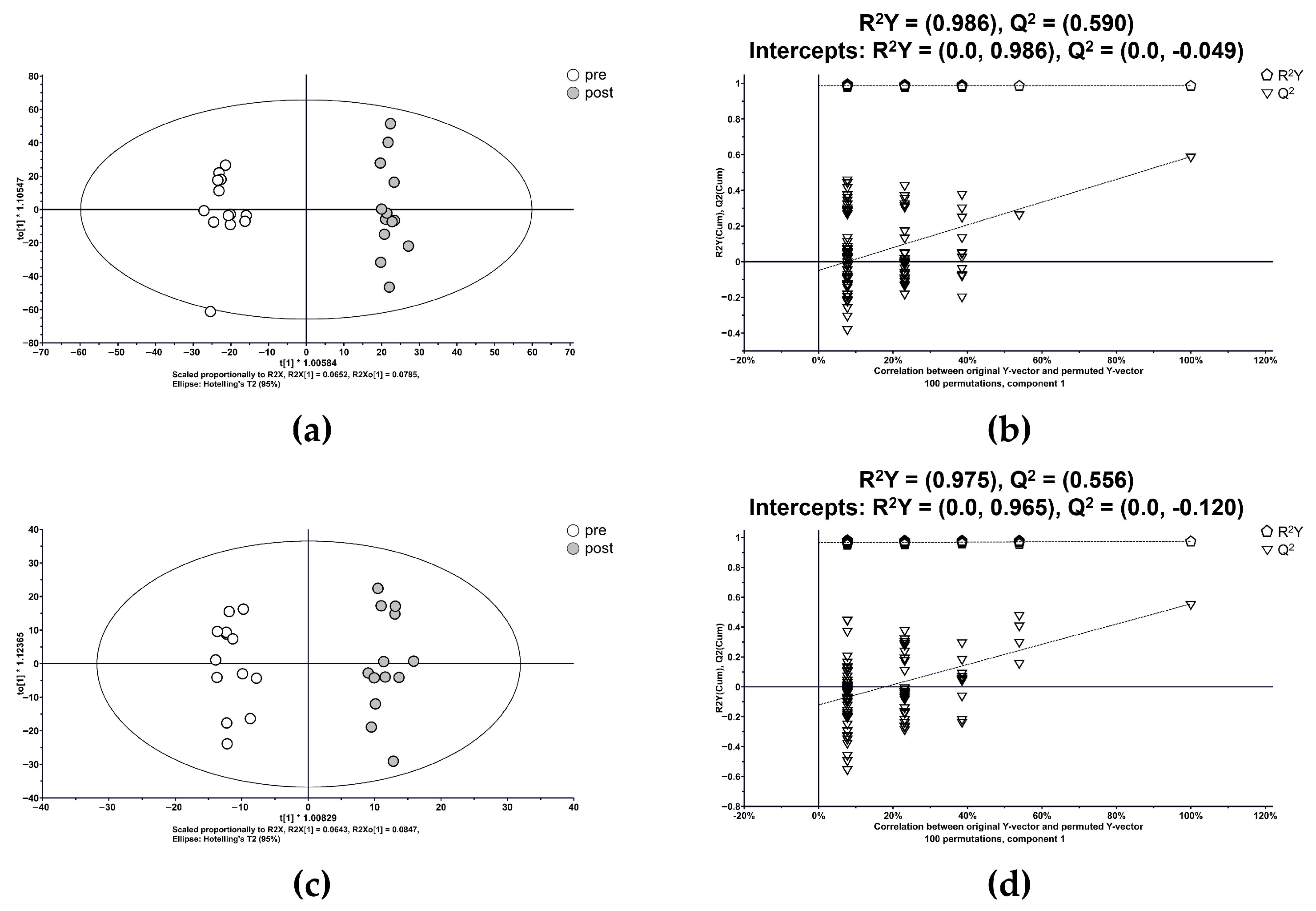
3.2. Metabolic Profiling and Multivariate Analysis
3.3. Metabolite Annotation
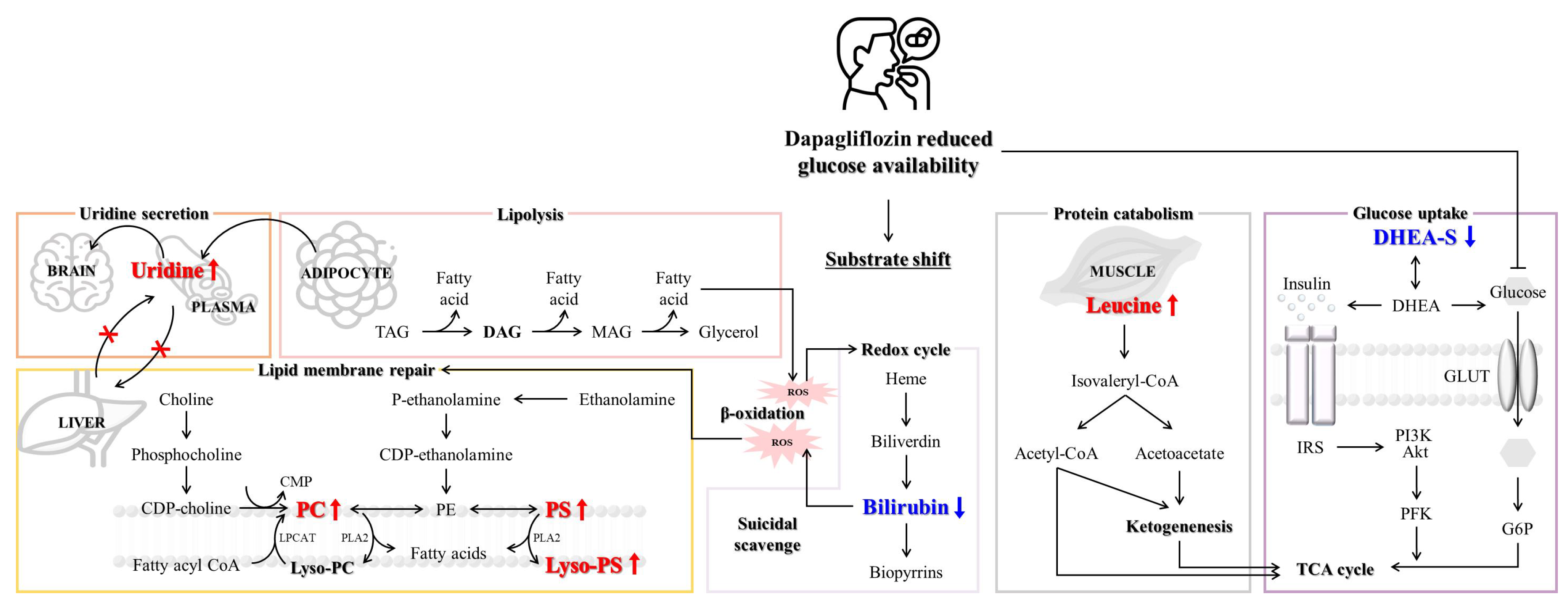
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| ALT | Alanine Transaminase |
| AST | Aspartate Transaminase |
| ATP | Adenosine Triphosphate |
| BMI | Body Mass Index |
| BUN | Blood Urea Nitrogen |
| CDP | Cytidine Diphosphate |
| ChEBI | Chemical Entities of Biological Interest |
| CMP | Cytidine Monophosphate |
| CoA | Coenzyme A |
| DAG | Diacylglycerol |
| DG | Diacylglycerol |
| DHEA | Dehydroepiandrosterone |
| DHEA-S | Dehydroepiandrosterone Sulfate |
| FC | Fold Change |
| FDR | False Discovery Rate |
| G6P | Glucose-6-Phosphate |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLUT | Glucose Transporter |
| HMDB | Human Metabolome Database |
| HPLC | High-Performance Liquid Chromatography |
| IRS | Insulin Receptor Substrate |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| LPCAT | Lysophosphatidylcholine Acyltransferase |
| Lyso-PC | Lysophosphatidylcholine |
| Lyso-PS | Lysophosphatidylserine |
| m/z | Mass-to-charge ratio |
| MAG | Monoacylglycerol |
| MS | Mass Spectrometry |
| MS/MS | Tandem Mass Spectrometry |
| NAGlySer | N-acyl glycyl serine |
| OPLS-DA | Orthogonal Partial Least Squares–Discriminant Analysis |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PFK | Phosphofructokinase |
| PI3K | Phosphoinositide 3-Kinase |
| PLA2 | Phospholipase A2 |
| PS | Phosphatidylserine |
| QC | Quality Control |
| ROS | Reactive Oxygen Species |
| RT | Retention Time |
| SGLT2 | Sodium–Glucose Cotransporter 2 |
| SM | Sphingomyelin |
| T2D | Type 2 Diabetes |
| TAG | Triacylglycerol |
| TCA cycle | Tricarboxylic Acid Cycle |
| UHPLC-QTOF/MS | Ultra-High-Performance Liquid Chromatography–quadrupole time-of-flight MS |
| VIP | Variable Importance in Projection |
| WBC | White Blood Cell Count |
References
- Yang, C.; Xiao, C.; Zhai, X.; Liu, J.; Yu, M. SGLT2 inhibitor improves kidney function and morphology by regulating renal metabolism in mice with diabetic kidney disease. J. Diabetes Complications 2024, 38, 108652. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.-J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Muto, S.; Fukuda, K.; Watanabe, M.; Ohara, K.; Koepsell, H.; Vallon, V.; Nagata, D. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol. Rep. 2020, 8, e14360. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. J. Atheroscler. Thromb. 2018, 25, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Edholm, T.; Degerblad, M.; Grybäck, P.; Hilsted, L.; Holst, J.J.; Jacobsson, H.; Efendic, S.; Schmidt, P.T.; Hellström, P.M. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol. Motil. 2010, 22, 1191-e315. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Chen, H.; Wen, S.; Yuan, Y.; Yang, L.; Li, Y.; Yuan, X.; Xu, D.; Zhou, L. The Neuronal and Non-Neuronal Pathways of Sodium-Glucose Cotransporter-2 Inhibitor on Body Weight-Loss and Insulin Resistance. Diabetes Metab. Syndr. Obes. 2023, 16, 425–435. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Pontiroli, A.E. Prevention of Diabetes and Cardiovascular Disease in Obesity. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Hanley, M.J.; Abernethy, D.R.; Greenblatt, D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 2010, 49, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Ribbenstedt, A.; Ziarrusta, H.; Benskin, J.P. Development, characterization and comparisons of targeted and non-targeted metabolomics methods. PLoS ONE 2018, 13, e0207082. [Google Scholar] [CrossRef] [PubMed]
- Sasse, M.; Rainer, M. Mass Spectrometric Methods for Non-Targeted Screening of Metabolites: A Future Perspective for the Identification of Unknown Compounds in Plant Extracts. Separations 2022, 9, 415. [Google Scholar] [CrossRef]
- Bletsa, E.; Filippas-Dekouan, S.; Kostara, C.; Dafopoulos, P.; Dimou, A.; Pappa, E.; Chasapi, S.; Spyroulias, G.; Koutsovasilis, A.; Bairaktari, E.; et al. Effect of Dapagliflozin on Urine Metabolome in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Andriessen, C.; Doligkeit, D.; Moonen-Kornips, E.; Mensink, M.; Hesselink, M.K.C.; Hoeks, J.; Schrauwen, P. The impact of prolonged fasting on 24h energy metabolism and its 24h rhythmicity in healthy, lean males: A randomized cross-over trial. Clin. Nutr. 2023, 42, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Modre-Osprian, R.; Osprian, I.; Tilg, B.; Schreier, G.; Weinberger, K.M.; Graber, A. Dynamic simulations on the mitochondrial fatty acid beta-oxidation network. BMC Syst. Biol. 2009, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.A.; Mashek, D.G. Mammalian triacylglycerol metabolism: Synthesis, lipolysis, and signaling. Chem. Rev. 2011, 111, 6359–6386. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Investig. 1999, 103, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Speijer, D. How the mitochondrion was shaped by radical differences in substrates: What carnitine shuttles and uncoupling tell us about mitochondrial evolution in response to ROS. Bioessays 2014, 36, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Zavadskiy, S.; Sologova, S.; Moldogazieva, N. Oxidative distress in aging and age-related diseases: Spatiotemporal dysregulation of protein oxidation and degradation. Biochimie 2022, 195, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Galam, L.; Failla, A.; Soundararajan, R.; Lockey, R.F.; Kolliputi, N. 4-hydroxynonenal regulates mitochondrial function in human small airway epithelial cells. Oncotarget 2015, 6, 41508–41521. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Compartmentalized regulation of lipid signaling in oxidative stress and inflammation: Plasmalogens, oxidized lipids and ferroptosis as new paradigms of bioactive lipid research. Prog. Lipid Res. 2023, 89, 101207. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.; Sanz, A.; Gómez, J.; Pamplona, R.; Portero-Otín, M.; Gredilla, R.; Barja, G. Effects of fasting on oxidative stress in rat liver mitochondria. Free Radic. Res. 2006, 40, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, T.O.; Lass, A. DAG tales: The multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling. Cell Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, V.A.; Yanamala, N.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Macphee, C.H.; Kagan, V.E. Specificity of lipoprotein-associated phospholipase A(2) toward oxidized phosphatidylserines: Liquid chromatography-electrospray ionization mass spectrometry characterization of products and computer modeling of interactions. Biochemistry 2012, 51, 9736–9750. [Google Scholar] [CrossRef] [PubMed]
- Ichu, T.A.; Reed, A.; Ogasawara, D.; Ulanovskaya, O.; Roberts, A.; Aguirre, C.A.; Bar-Peled, L.; Gao, J.; Germain, J.; Barbas, S.; et al. ABHD12 and LPCAT3 Interplay Regulates a Lyso-phosphatidylserine-C20:4 Phosphatidylserine Lipid Network Implicated in Neurological Disease. Biochemistry 2020, 59, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R. Membrane phospholipids, lipoxidative damage and molecular integrity: A causal role in aging and longevity. Biochim. Biophys. Acta 2008, 1777, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Vasavda, C.; Kothari, R.; Malla, A.P.; Tokhunts, R.; Lin, A.; Ji, M.; Ricco, C.; Xu, R.; Saavedra, H.G.; Sbodio, J.I.; et al. Bilirubin Links Heme Metabolism to Neuroprotection by Scavenging Superoxide. Cell Chem. Biol. 2019, 26, 1450–1460.e7. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Hortmann, M.; Oelze, M.; Opitz, B.; Steven, S.; Schell, R.; Knorr, M.; Karbach, S.; Schuhmacher, S.; Wenzel, P.; et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell Cardiol. 2010, 49, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Jiang, X.; Zhai, Y.Y.; Luo, L.Z.; Xu, H.L.; Xiao, J.; Kou, L.; Zhao, Y.-Z. Protective effects and mechanisms of bilirubin nanomedicine against acute pancreatitis. J. Control. Release 2020, 322, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.M.; Elliott, S.L.; Manton, K.J. Fasting increases serum bilirubin levels in clinically normal, healthy males but not females: A retrospective study from phase I clinical trial participants. J. Clin. Pathol. 2014, 67, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, H.; Teruya, T.; Yanagida, M. Metabolomics of human fasting: New insights about old questions. Open Biol. 2020, 10, 200176. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.G.; Kluge, H.; Hirche, F.; Kiowski, A.; Schutkowski, A.; Corrent, E.; Bartelt, J.; König, B.; Stangl, G.I.; Clelland, J.D. High Leucine Diets Stimulate Cerebral Branched-Chain Amino Acid Degradation and Modify Serotonin and Ketone Body Concentrations in a Pig Model. PLoS ONE 2016, 11, e0150376. [Google Scholar] [CrossRef] [PubMed]
- Grabacka, M.; Pierzchalska, M.; Dean, M.; Reiss, K. Regulation of Ketone Body Metabolism and the Role of PPARα. Int. J. Mol. Sci. 2016, 17, 2093. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M.; Mičuda, S. Amino acid concentrations and protein metabolism of two types of rat skeletal muscle in postprandial state and after brief starvation. Physiol. Res. 2017, 66, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Smith, T.J.; Wilson, M.A.; Bukhari, A.S.; Pasiakos, S.M.; McClung, H.L.; McClung, J.P.; Lieberman, H.R. Altered metabolic homeostasis is associated with appetite regulation during and following 48-h of severe energy deprivation in adults. Metabolism 2016, 65, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Idkowiak, J.; Taylor, A.E.; Subtil, S.; O’Neil, D.M.; Vijzelaar, R.; Dias, R.P.; Amin, R.; Barrett, T.G.; Shackleton, C.H.L.; Kirk, J.M.W.; et al. Steroid Sulfatase Deficiency and Androgen Activation Before and After Puberty. J. Clin. Endocrinol. Metab. 2016, 101, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Ge, C.; Yu, L.; Li, L.; Ma, H. Long-Term Administration of Dehydroepiandrosterone Accelerates Glucose Catabolism via Activation of PI3K/Akt-PFK-2 Signaling Pathway in Rats Fed a High-Fat Diet. PLoS ONE 2016, 11, e0159077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sano, H.; Eguez, L.; Teruel, M.N.; Fukuda, M.; Chuang, T.D.; Chavez, J.A.; Lienhard, G.E.; McGraw, T.E. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007, 5, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, M.; Sato, M.; Rutkowski, R.; Zawada, A.; Juchacz, A.; Mahadea, D.; Grzymisławski, M.; Dobrowolska, A.; Kawka, E.; Korybalska, K.; et al. Effect of the one-day fasting on cortisol and DHEA daily rhythm regarding sex, chronotype, and age among obese adults. Front. Nutr. 2023, 10, 1078508. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, Z.V.; Gordillo, R.; An, Y.; Zhang, C.; Liang, Q.; Yoshino, J.; Cautivo, K.M.; De Brabander, J.; Elmquist, J.K.; et al. An adipo-biliary-uridine axis that regulates energy homeostasis. Science 2017, 355, eaaf5375. [Google Scholar] [CrossRef] [PubMed]
- Zi, L.; Ma, W.; Zhang, L.; Qiao, B.; Qiu, Z.; Xu, J.; Zhang, J.; Ye, Y.; Yang, Y.; Dong, K.; et al. Uridine Inhibits Hepatocellular Carcinoma Cell Development by Inducing Ferroptosis. J. Clin. Med. 2023, 12, 3552. [Google Scholar] [CrossRef] [PubMed]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. GigaScience 2013, 2, 2047-17X. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean ± Standard Deviation |
|---|---|
| Age (years) | 29.6 ± 8.9 |
| Height (cm) | 174.9 ± 6.3 |
| Weight (kg) | 70.4 ± 8.7 |
| BMI (kg/m2) | 22.9 ± 1.9 |
| WBC (103/mL) | 6.2 ± 1.7 |
| Hemoglobin (g/mL) | 15.4 ± 0.7 |
| Hematocrit (%) | 46.1 ± 2.0 |
| Platelets (103/mL) | 266.8 ± 44.7 |
| Total protein (g/dL) | 7.6 ± 0.4 |
| Albumin (g/dL) | 4.9 ± 0.2 |
| Total bilirubin (mg/dL) | 0.8 ± 0.2 |
| AST (U/L) | 20.1 ± 5.4 |
| ALT (U/L) | 19.6 ± 9.1 |
| Alkaline phosphatase (U/L) | 79.7 ± 20.9 |
| Fasting glucose (mg/dL) | 97.5 ± 6.7 |
| BUN (mg/dL) | 12.5 ± 2.8 |
| Creatinine (mg/dL) | 0.9 ± 0.1 |
| Total cholesterol (mg/dL) | 181.2 ± 23.1 |
| Metabolite | VIP | RT (min) | m/z | Ion Type | Database | FC § | FDR ¶ |
|---|---|---|---|---|---|---|---|
| Fatty acyls | |||||||
| 13-Docosenamide | 2.27 | 12.65 | 360.3250 | [M + H]+ | HMDB0244507 | 0.965 | 0.153 |
| NAGlySer 31:1 | 2.10 | 17.68 | 639.4971 | [M + H]+ | Pubchem:164316244 | 0.974 | 0.219 |
| Octadecanoic acid | 1.78 | 14.62 | 355.2840 | [M − H]− | HMDB0000827 | 0.939 | 0.128 |
| 5-Hexyltetrahydro-2-furanoctanoic acid | 1.46 | 8.27 | 297.2418 | [M − H]− | HMDB0031127 | 0.978 | 0.280 |
| Glycerolipids | |||||||
| DG 40:6 | 2.04 | 19.55 | 669.5437 | [M + H]+ | ChEBI:85709 | 0.976 | 0.260 |
| DG 38:4 | 1.37 | 20.25 | 645.5444 | [M + H]+ | ChEBI:85705 | 0.985 | 0.247 |
| Phospholipids | |||||||
| PC O-36:5 | 2.73 | 16.29 | 766.5741 | [M + H]+ | HMDB0013415 | 1.066 | 0.038 |
| PC 36:4 | 2.22 | 15.80 | 782.5730 | [M + H]+ | ChEBI:64524 | 1.031 | 0.056 |
| PC 36:3 | 1.87 | 16.58 | 784.5912 | [M + H]+ | ChEBI:64523 | 1.021 | 0.019 |
| SM 51:9;2O | 1.84 | 21.74 | 925.7112 | [M + H]+ | PubChem:164450933 | 1.064 | 1.000 |
| PS 40:2 | 1.73 | 21.07 | 842.5909 | [M − H]− | ChEBI:141307 | 1.030 | 0.008 |
| PS 40:3 | 1.72 | 16.99 | 840.5778 | [M − H]− | ChEBI:141308 | 1.035 | 0.032 |
| PC 36:5 | 1.58 | 15.62 | 780.5553 | [M + H]+ | ChEBI:64504 | 1.022 | 0.057 |
| PS 36:1 | 1.52 | 16.09 | 788.5470 | [M − H]− | ChEBI:136256 | 1.039 | 0.048 |
| Lyso-PS 22:1 | 1.46 | 7.07 | 578.3479 | [M − H]− | ChEBI:72415 | 1.021 | 0.080 |
| Lyso-PS 22:1 | 1.42 | 6.92 | 578.3474 | [M − H]− | ChEBI:72415 | 1.019 | 0.022 |
| PS 40:4 | 1.35 | 16.35 | 838.5631 | [M − H]− | PubChem:138143401 | 1.033 | 0.024 |
| PC 42:9 | 1.33 | 17.18 | 856.5841 | [M + H]+ | ChEBI:136099 | 0.996 | 0.295 |
| Lyso-PC 22:6 | 1.32 | 9.26 | 568.3409 | [M + H]+ | HMDB0010404 | 0.996 | 0.402 |
| PC 34:3 | 1.13 | 15.49 | 756.5535 | [M + H]+ | ChEBI:64424 | 1.005 | 0.474 |
| PS 42:2 | 1.03 | 11.16 | 870.6242 | [M − H]− | PubChem:138212963 | 1.018 | 0.423 |
| PS 38:0 | 1.01 | 8.30 | 818.5938 | [M − H]− | PubChem:75958984 | 1.019 | 0.295 |
| Sterol lipids | |||||||
| DHEA-S | 2.54 | 12.33 | 367.1563 | [M − H]− | HMDB0001032 | 0.924 | 0.033 |
| Amino acids and nucleotides | |||||||
| Leucine | 1.44 | 1.98 | 132.1021 | [M + H]+ | HMDB0000687 | 1.024 | 0.051 |
| Uridine | 1.20 | 6.61 | 245.0788 | [M + H]+ | HMDB0000296 | 1.008 | 0.020 |
| Bile pigments | |||||||
| Bilirubin | 4.11 | 6.10 | 585.2711 | [M + H]+ | HMDB0000054 | 0.910 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Lee, J.H.; Park, J.S.; Park, J.J.; Lee, H.W.; Bunch, H.; Seong, S.J.; Gwon, M.-R.; Yoon, Y.-R. LC-MS-Based Untargeted Metabolic Profiling in Plasma Following Dapagliflozin Administration in Healthy Volunteers. Metabolites 2025, 15, 484. https://doi.org/10.3390/metabo15070484
Kim HJ, Lee JH, Park JS, Park JJ, Lee HW, Bunch H, Seong SJ, Gwon M-R, Yoon Y-R. LC-MS-Based Untargeted Metabolic Profiling in Plasma Following Dapagliflozin Administration in Healthy Volunteers. Metabolites. 2025; 15(7):484. https://doi.org/10.3390/metabo15070484
Chicago/Turabian StyleKim, Hyeon Ji, Jae Hwa Lee, Ji Seo Park, Jin Ju Park, Hae Won Lee, Heeyoun Bunch, Sook Jin Seong, Mi-Ri Gwon, and Young-Ran Yoon. 2025. "LC-MS-Based Untargeted Metabolic Profiling in Plasma Following Dapagliflozin Administration in Healthy Volunteers" Metabolites 15, no. 7: 484. https://doi.org/10.3390/metabo15070484
APA StyleKim, H. J., Lee, J. H., Park, J. S., Park, J. J., Lee, H. W., Bunch, H., Seong, S. J., Gwon, M.-R., & Yoon, Y.-R. (2025). LC-MS-Based Untargeted Metabolic Profiling in Plasma Following Dapagliflozin Administration in Healthy Volunteers. Metabolites, 15(7), 484. https://doi.org/10.3390/metabo15070484








