Prediagnostic Plasma Metabolomic Profiles Using NMR for Exfoliation Glaucoma Among US Health Professionals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Metabolite Profiling
2.3. Pilot Studies Examining the Stability and Reproducibility of the Metabolite Measures
2.4. Statistical Analysis
3. Results
3.1. Characteristics of XFG Cases and Matched Controls
3.2. Individual Metabolite Profiles
3.3. Metabolite Class and Subclass Analyses
3.4. Stratified Analyses by Sex and Analyses by XFG Severity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAA | Branched-chain amino acids |
| BMI | Body mass index |
| CI | Confidence interval |
| CV | Coefficient of variation |
| FDR | False discovery rate |
| GWAS | Genome-wide association studies |
| HDL | High-density lipoprotein |
| HPFS | Health Professionals Follow-up Study |
| ICC | Intraclass correlation coefficient |
| IDL | Intermediate-density lipoprotein |
| IOP | Intraocular pressure |
| LC-MS | Liquid chromatography tandem mass spectrometry |
| LDL | Low-density lipoprotein |
| LOXL1 | Lysyl oxidase-like 1 |
| MSEA | Metabolite Set Enrichment Analysis |
| NEF | Number of effective tests |
| NES | Normalized enrichment score |
| NHS | Nurses’ Health Study |
| NMR | Nuclear magnetic resonance |
| OR | Odds ratio |
| POAG | Primary open-angle glaucoma |
| SD | Standard deviation |
| VF | Visual field |
| VLDL | Very-low-density lipoprotein |
| XFG | Exfoliation glaucoma |
| XFS | Exfoliation syndrome |
References
- Schlötzer-Schrehardt, U.; Naumann, G.O.H. Ocular and systemic pseudoexfoliation syndrome. Am. J. Ophthalmol. 2006, 141, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Loomis, S.; Wiggs, J.L.; Stein, J.D.; Pasquale, L.R. Demographic and geographic features of exfoliation glaucoma in 2 United States-based prospective cohorts. Ophthalmology 2012, 119, 27–35. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Naumann, G.O. Trabecular meshwork in pseudoexfoliation syndrome with and without open-angle glaucoma. A morphometric, ultrastructural study. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1750–1764. [Google Scholar]
- Lee, R.K. The molecular pathophysiology of pseudoexfoliation glaucoma. Curr. Opin. Ophthalmol. 2008, 19, 95–101. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U. Molecular pathology of pseudoexfoliation syndrome/glaucoma—New insights from LOXL1 gene associations. Exp. Eye Res. 2009, 88, 776–785. [Google Scholar] [CrossRef]
- Stein, J.D.; Pasquale, L.R.; Talwar, N.; Kim, D.S.; Reed, D.M.; Nan, B.; Kang, J.H.; Wiggs, J.L.; Richards, J.E. Geographic and climatic factors associated with exfoliation syndrome. Arch. Ophthalmol. 2011, 129, 1053–1060. [Google Scholar] [CrossRef]
- Kang, J.H.; Loomis, S.J.; Wiggs, J.L.; Willett, W.C.; Pasquale, L.R. A prospective study of folate, vitamin B6, and vitamin B12 intake in relation to exfoliation glaucoma or suspected exfoliation glaucoma. JAMA Ophthalmol. 2014, 132, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Jiwani, A.Z.; Pasquale, L.R. Exfoliation Syndrome and Solar Exposure: New Epidemiological Insights Into the Pathophysiology of the Disease. Int. Ophthalmol. Clin. 2015, 55, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Ozaki, M.; Lee, M.C.; Schlötzer-Schrehardt, U.; Thorleifsson, G.; Mizoguchi, T.; Igo, R.P., Jr.; Haripriya, A.; Williams, S.E.; Astakhov, Y.S.; et al. Genetic association study of exfoliation syndrome identifies a protective rare variant at LOXL1 and five new susceptibility loci. Nat. Genet. 2017, 49, 993–1004. [Google Scholar] [CrossRef]
- Bikbov, M.M.; Zainullin, R.M.; Gilmanshin, T.R.; Kazakbaeva, G.M.; Yakupova, D.F.; Nuriev, I.F.; Zaynetdinov, A.F.; Khalimov, T.A.; Panda-Jonas, S.; Uzianbaeva, Y.V.; et al. Prevalence and Associated Factors of Pseudoexfoliation in a Russian Population: The Ural Eye and Medical Study. Am. J. Ophthalmol. 2020, 210, 158–166. [Google Scholar] [CrossRef]
- Wang, W.; He, M.; Zhou, M.; Zhang, X. Ocular pseudoexfoliation syndrome and vascular disease: A systematic review and meta-analysis. PLoS ONE 2014, 9, e92767. [Google Scholar] [CrossRef]
- Shrum, K.R.; Hattenhauer, M.G.; Hodge, D. Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am. J. Ophthalmol. 2000, 129, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, G.; Magnusson, K.P.; Sulem, P.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Jonasdottir, A.; Stefansdottir, G.; et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- McNally, S.; O’Brien, C.J. Metabolomics/Proteomics strategies used to identify biomarkers for exfoliation glaucoma. J. Glaucoma 2014, 23 (Suppl. S1), S51–S54. [Google Scholar] [CrossRef]
- Ritch, R. Ocular and systemic manifestations of exfoliation syndrome. J. Glaucoma 2014, 23 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef]
- Aung, T.; Ozaki, M.; Mizoguchi, T.; Allingham, R.R.; Li, Z.; Haripriya, A.; Nakano, S.; Uebe, S.; Harder, J.M.; Chan, A.S.Y.; et al. A common variant mapping to CACNA1A is associated with susceptibility to exfoliation syndrome. Nat. Genet. 2015, 47, 387–392. [Google Scholar] [CrossRef]
- Leruez, S.; Bresson, T.; de la Barca, J.M.C.; Marill, A.; de Saint Martin, G.; Buisset, A.; Muller, J.; Tessier, L.; Gadras, C.; Verny, C.; et al. A Plasma Metabolomic Signature of the Exfoliation Syndrome Involves Amino Acids, Acylcarnitines, and Polyamines. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1025–1032. [Google Scholar] [CrossRef]
- Myer, C.; Abdelrahman, L.; Banerjee, S.; Khattri, R.B.; Merritt, M.E.; Junk, A.K.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor metabolite profile of pseudoexfoliation glaucoma is distinctive. Mol. Omics 2020, 16, 425–435. [Google Scholar] [CrossRef]
- Kang, J.H.; Zeleznik, O.; Frueh, L.; Lasky-Su, J.; Eliassen, A.H.; Clish, C.; Rosner, B.A.; Pasquale, L.R.; Wiggs, J.L. Prediagnostic Plasma Metabolomics and the Risk of Exfoliation Glaucoma. Investig. Ophthalmol. Vis. Sci. 2022, 63, 15. [Google Scholar] [CrossRef]
- Collao, V.; Morris, J.; Chauhan, M.Z.; Abdelrahman, L.; Martínez-de-la-Casa, J.M.; Vidal-Villegas, B.; Burgos-Blasco, B.; Bhattacharya, S.K. Analyses of pseudoexfoliation aqueous humor lipidome. Mol. Omics 2022, 18, 387–396. [Google Scholar] [CrossRef]
- Mok, J.-H.; Park, D.Y.; Han, J.C. Differential protein expression and metabolite profiling in glaucoma: Insights from a multi-omics analysis. Biofactors 2024, 50, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Yokomichi, H.; Kashiwagi, K.; Kitamura, K.; Yoda, Y.; Tsuji, M.; Mochizuki, M.; Sato, M.; Shinohara, R.; Mizorogi, S.; Suzuki, K.; et al. Evaluation of the associations between changes in intraocular pressure and metabolic syndrome parameters: A retrospective cohort study in Japan. BMJ Open 2016, 6, e010360. [Google Scholar] [CrossRef]
- Wang, S.; Bao, X. Hyperlipidemia, Blood Lipid Level, and the Risk of Glaucoma: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1028–1043. [Google Scholar] [CrossRef]
- Pertl, L.; Mossböck, G.; Wedrich, A.; Weger, M.; Königsbrügge, O.; Silbernagel, G.; Posch, F. Triglycerides and Open Angle Glaucoma—A Meta-analysis with meta-regression. Sci. Rep. 2017, 7, 7829. [Google Scholar] [CrossRef]
- Zeleznik, O.A.; Kang, J.H.; Lasky-Su, J.; Eliassen, A.H.; Frueh, L.; Clish, C.B.; Rosner, B.A.; Elze, T.; Hysi, P.; Khawaja, A.; et al. Plasma metabolite profile for primary open-angle glaucoma in three US cohorts and the UK Biobank. Nat. Commun. 2023, 14, 2860. [Google Scholar] [CrossRef]
- Nusinovici, S.; Li, H.; Thakur, S.; Baskaran, M.; Tham, Y.C.; Zhou, L.; Sabanayagam, C.; Aung, T.; Silver, D.; Fan, Q.; et al. High-Density Lipoprotein 3 Cholesterol and Primary Open-Angle Glaucoma: Metabolomics and Mendelian Randomization Analyses. Ophthalmology 2022, 129, 285–294. [Google Scholar] [CrossRef]
- Xu, M.; Li, S.; Zhu, J.; Luo, D.; Song, W.; Zhou, M. Plasma lipid levels and risk of primary open angle glaucoma: A genetic study using Mendelian randomization. BMC Ophthalmol. 2020, 20, 390. [Google Scholar] [CrossRef]
- Türkyılmaz, K.; Öner, V.; Kırbas, A.; Sevim, M.S.; Sekeryapan, B.; Özgür, G.; Durmus, M. Serum YKL-40 levels as a novel marker of inflammation and endothelial dysfunction in patients with pseudoexfoliation syndrome. Eye 2013, 27, 854–859. [Google Scholar] [CrossRef]
- Janićijević, K.; Kocić, S.; Pajović, S.; Zdravković, N.; Šarenac-Vulović, T.; Janićijević-Petrović, M. The importance of developing atherosclerosis in pseudoexfoliation glaucoma. Vojnosanit. Pregl. 2017, 74, 8–12. [Google Scholar] [CrossRef]
- Mirza, E. Atherogenic indices in pseudoexfoliation syndrome. Eye 2019, 33, 1911–1915. [Google Scholar] [CrossRef]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Suna, T.; Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Bertoia, M.L.; Lenart, E.B.; Stampfer, M.J.; Willett, W.C.; Speizer, F.E.; Chavarro, J.E. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. Am. J. Public Health 2016, 106, 1573–1581. [Google Scholar] [CrossRef]
- Rimm, E.B.; Giovannucci, E.L.; Willett, W.C.; Colditz, G.A.; Ascherio, A.; Rosner, B.; Stampfer, M.J. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991, 338, 464–468. [Google Scholar] [PubMed]
- Hankinson, S.E.; Willett, W.C.; Manson, J.E.; Colditz, G.A.; Hunter, D.J.; Spiegelman, D.; Barbieri, R.L.; Speizer, F.E. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 1998, 90, 1292–1299. [Google Scholar] [CrossRef]
- Tworoger, S.S.; Hankinson, S.E. Use of biomarkers in epidemiologic studies: Minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control 2006, 17, 889–899. [Google Scholar] [CrossRef]
- Julkunen, H.; Cichońska, A.; Slagboom, P.E.; Würtz, P. Metabolic biomarker profiling for identification of susceptibility to severe pneumonia and COVID-19 in the general population. eLife 2021, 10, e63033. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Wiggs, J.L.; Pasquale, L.R. Relation between time spent outdoors and exfoliation glaucoma or exfoliation glaucoma suspect. Am. J. Ophthalmol. 2014, 158, 605–614.e1. [Google Scholar] [CrossRef]
- Kang, J.H.; VoPham, T.; Laden, F.; Rosner, B.A.; Wirostko, B.; Ritch, R.; Wiggs, J.L.; Qureshi, A.; Nan, H.; Pasquale, L.R. Cohort Study of Nonmelanoma Skin Cancer and the Risk of Exfoliation Glaucoma. J. Glaucoma 2020, 29, 448–455. [Google Scholar] [CrossRef]
- Taylor, H.R. Pseudoexfoliation, an environmental disease? Trans. Ophthalmol. Soc. 1979, 99, 302–307. [Google Scholar]
- Pasquale, L.R.; Wiggs, J.L.; Willett, W.C.; Kang, J.H. The Relationship between caffeine and coffee consumption and exfoliation glaucoma or glaucoma suspect: A prospective study in two cohorts. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6427–6433. [Google Scholar] [CrossRef]
- Hanyuda, A.; Rosner, B.A.; Wiggs, J.L.; Negishi, K.; Pasquale, L.R.; Kang, J.H. Long-term Alcohol Consumption and Risk of Exfoliation Glaucoma or Glaucoma Suspect Status among United States Health Professionals. Ophthalmology 2023, 130, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zehavi-Dorin, T.; Nahum, N.; Ben-Artsi, E.; Levkovitch-Verbin, H. Exfoliation syndrome: Association with systemic diseases-the Maccabi glaucoma study. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albr. Graefes Arch. Klin. Exp. Ophthalmol. 2021, 259, 3027–3034. [Google Scholar] [CrossRef]
- Razeghinejad, M.R.; Katz, L.J. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012, 47, 66–80. [Google Scholar] [CrossRef]
- Gao, X.; Starmer, J.; Martin, E.R. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet. Epidemiol. 2008, 32, 361–369. [Google Scholar] [CrossRef]
- Yamamoto, H.; Fujimori, T.; Sato, H.; Ishikawa, G.; Kami, K.; Ohashi, Y. Statistical hypothesis testing of factor loading in principal component analysis and its application to metabolite set enrichment analysis. BMC Bioinform. 2014, 15, 51. [Google Scholar] [CrossRef]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef]
- Kadoh, Y.; Takayanagi, Y.; Sasaki, J.; Tanito, M. Fingertip-Measured Skin Carotenoids and Advanced Glycation End Product Levels in Glaucoma. Antioxidants 2022, 11, 1138. [Google Scholar] [CrossRef] [PubMed]
- Tarkkanen, A.; Reunanen, A.; Kivelä, T. Frequency of systemic vascular diseases in patients with primary open-angle glaucoma and exfoliation glaucoma. Acta Ophthalmol. 2008, 86, 598–602. [Google Scholar] [CrossRef]
- Yu, M.; Hwang, H.H.; Wiggs, J.L.; Pasquale, L.R.; Kang, J.H. Association Between Diabetes and Exfoliation Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Ophthalmol. Sci. 2024, 4, 100436. [Google Scholar] [CrossRef]
- Mayro, E.L.; Ritch, R.; Pasquale, L.R. Early-Onset Exfoliation Syndrome: A Literature Synthesis. J. Glaucoma 2021, 30, e164–e168. [Google Scholar]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Fani, A.; Sofia, K.; Theodora, P.; Antonia, S. Pseudoexfoliation syndrome in diabetic patients: Transmission electron microscopy study of anterior lens epithelial cells. Rom. J. Ophthalmol. 2021, 65, 38–45. [Google Scholar] [CrossRef]
- Julkunen, H.; Cichońska, A.; Tiainen, M.; Koskela, H.; Nybo, K.; Mäkelä, V.; Nokso-Koivisto, J.; Kristiansson, K.; Perola, M.; Salomaa, V.; et al. Atlas of plasma NMR biomarkers for health and disease in 118,461 individuals from the UK Biobank. Nat. Commun. 2023, 14, 604. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Khawaja, A.P.; Wiggs, J.L.; Kim, J.; Hysi, P.; Elze, T.; Lasky-Su, J.; Kang, J.H.; Zeleznik, O.; UK Biobank Eye and Vision Consortium. Metabolite and Lipid Biomarkers Associated with Intraocular Pressure and Inner Retinal Morphology: 1H NMR Spectroscopy Results from the UK Biobank. Investig. Ophthalmol. Vis. Sci. 2023, 64, 11. [Google Scholar] [CrossRef]
- Mayers, J.R.; Wu, C.; Clish, C.B.; Kraft, P.; Torrence, M.E.; Fiske, B.P.; Yuan, C.; Bao, Y.; Townsend, M.K.; Tworoger, S.S.; et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 2014, 20, 1193–1198. [Google Scholar] [CrossRef]
- Zeleznik, O.A.; Balasubramanian, R.; Zhao, Y.; Frueh, L.; Jeanfavre, S.; Avila-Pacheco, J.; Clish, C.B.; Tworoger, S.S.; Eliassen, A.H. Circulating amino acids and amino acid-related metabolites and risk of breast cancer among predominantly premenopausal women. NPJ Breast Cancer 2021, 7, 54. [Google Scholar] [CrossRef]
- Hur, B.; Gupta, V.K.; Huang, H.; Wright, K.A.; Warrington, K.J.; Taneja, V.; Davis, J.M., 3rd; Sung, J. Plasma metabolomic profiling in patients with rheumatoid arthritis identifies biochemical features predictive of quantitative disease activity. Arthritis Res. Ther. 2021, 23, 164. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hazra, A.; Lawler, P.R.; Chandler, P.D.; Chasman, D.I.; Buring, J.E.; Lee, I.M.; Cheng, S.; Manson, J.E.; Mora, S. Circulating branched-chain amino acids and long-term risk of obesity-related cancers in women. Sci. Rep. 2020, 10, 16534. [Google Scholar] [CrossRef]
- Katagiri, R.; Goto, A.; Nakagawa, T.; Nishiumi, S.; Kobayashi, T.; Hidaka, A.; Budhathoki, S.; Yamaji, T.; Sawada, N.; Shimazu, T.; et al. Increased Levels of Branched-Chain Amino Acid Associated With Increased Risk of Pancreatic Cancer in a Prospective Case-Control Study of a Large Cohort. Gastroenterology 2018, 155, 1474–1482.e1. [Google Scholar] [CrossRef] [PubMed]
- Hiller, R.; Sperduto, R.D.; Krueger, D.E. Pseudoexfoliation, intraocular pressure, and senile lens changes in a population-based survey. Arch. Ophthalmol. 1982, 100, 1080–1082. [Google Scholar] [CrossRef] [PubMed]
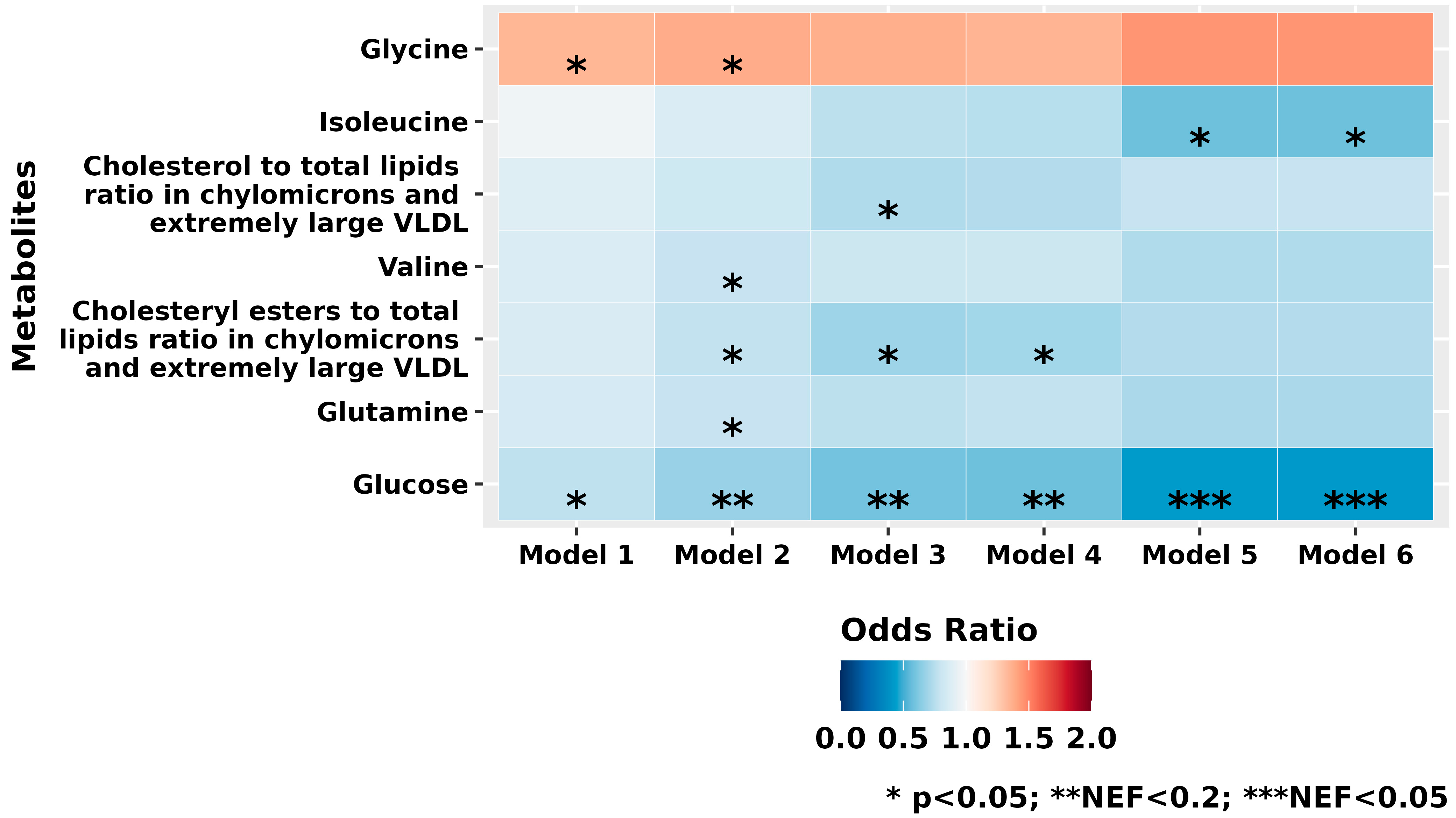
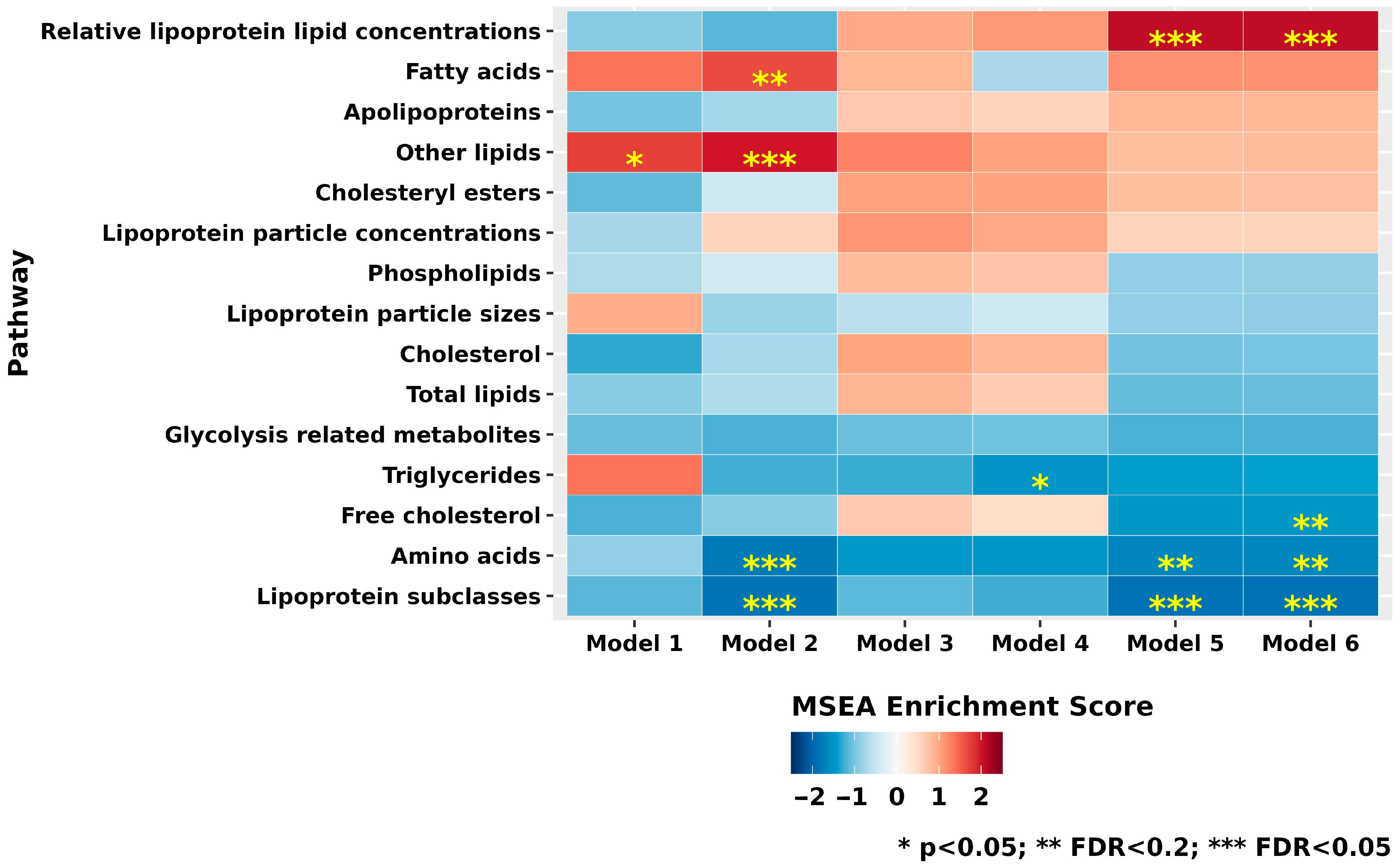
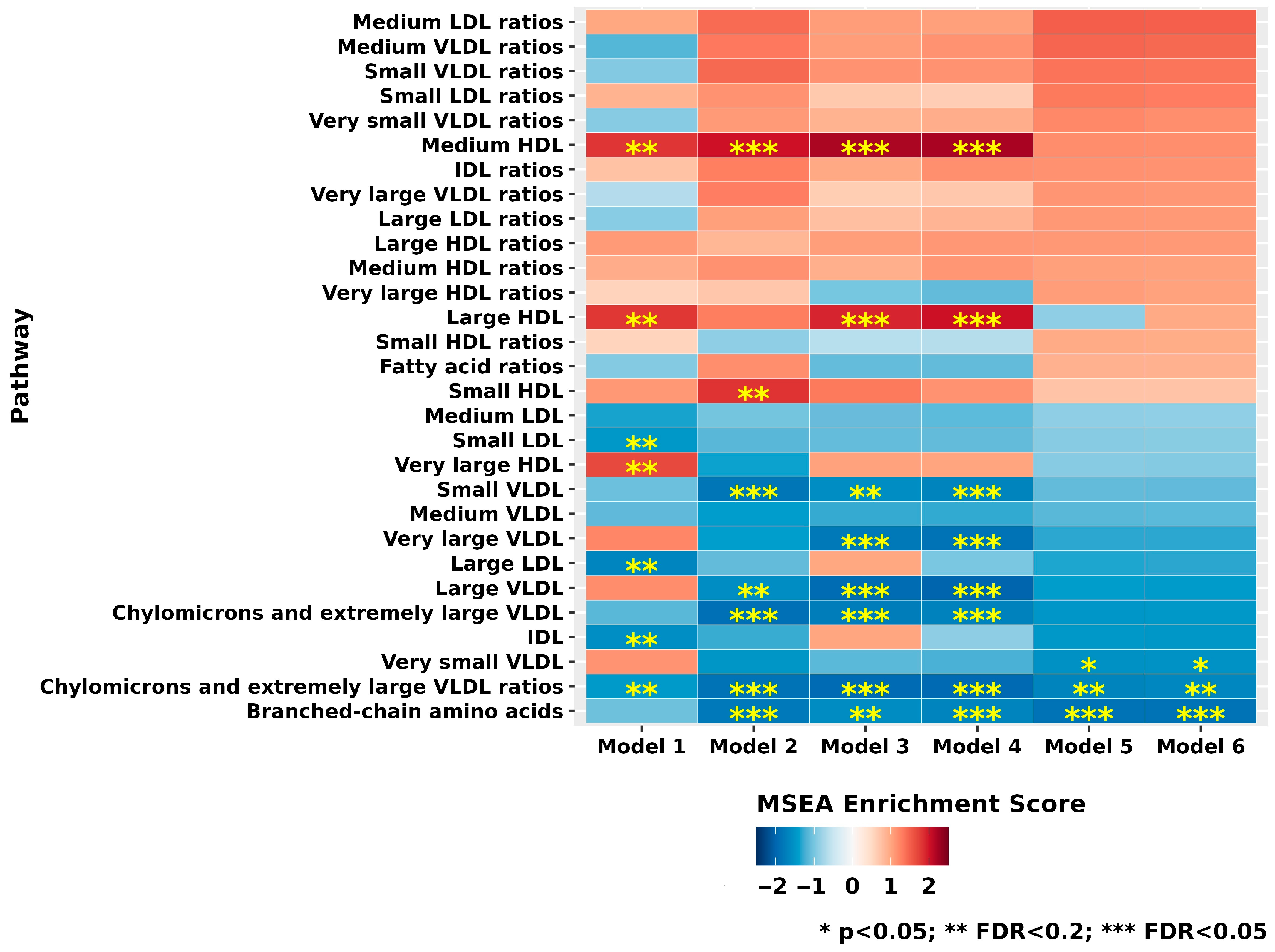
| Characteristics | XFG Cases (n = 217) | Controls (n = 217) | p-Value ** |
|---|---|---|---|
| Female, n (%) | 186 (85.7) | 186 (85.7) | 1.00 |
| Mean age at blood draw (SD), years | 58.6 (6.9) | 58.0 (6.3) | 0.40 |
| Race/ethnicity, n (%) | 0.30 | ||
| Other European | 166 (76.5) | 152 (70.0) | |
| Non-European | 2 (0.9) | 1 (0.5) | |
| Southern European | 34 (15.7) | 49 (22.6) | |
| Scandinavian European | 15 (6.9) | 15 (6.9) | |
| Fasting >8 h, n (%) | 149 (69.0) | 182 (83.9) | <0.01 |
| Mean age at diagnosis/index date of XFG (SD), years | 70.4 (7.8) | 69.8 (7.4) | 0.47 |
| Mean latitude of residence (SD), °N | 39.7 (4.3) | 39.6 (4.1) | 0.92 |
| Mean longitude of residence (SD), °W | −82.0 (13.3) | −82.2 (12.9) | 0.91 |
| Family history of glaucoma, n (%) | 53 (24.9) | 41 (19.4) | 0.22 |
| Mean caffeine intake (SD), mg/day | 294.7 (244.4) | 284.9 (246.7) | 0.68 |
| Mean folate intake (SD), mg/day | 436.1 (227.3) | 436.9 (249.0) | 0.98 |
| Outdoor sunlight exposure during summers in youth, n (%) | 0.69 | ||
| <1 h/week | 17 (8.7) | 15 (7.9) | |
| 1–5 h/week | 76 (38.8) | 85 (44.5) | |
| 6–10 h/week | 64 (32.7) | 59 (30.9) | |
| >11 h/week | 39 (19.9) | 32 (16.8) | |
| Non-melanoma skin cancer, n (%) | 28 (12.9) | 19 (8.8) | 0.22 |
| Mean alcohol intake (SD), g/day | 6.8 (10.0) | 6.3 (9.7) | 0.59 |
| Mean body mass index (SD), kg/m2 | 24.9 (4.4) | 24.9 (4.2) | 0.99 |
| Smoking status, n (%) | 0.29 | ||
| Never smoker | 104 (47.9) | 110 (50.7) | |
| Past smoker | 95 (43.8) | 97 (44.7) | |
| Current smoker | 18 (8.3) | 10 (4.6) | |
| Mean physical activity (SD), MET-h/week | 20.8 (24.7) | 19.6 (21.4) | 0.58 |
| Hypertension, n (%) | 3 (1.4) | 8 (3.7) | 0.22 |
| Hyperlipidemia, n (%) | 67 (30.9) | 81 (37.3) | 0.19 |
| Diabetes, n (%) | 5 (2.3) | 6 (2.8) | 1.00 |
| Among female individuals: | 0.96 | ||
| Missing, n (%) | 16 (8.6) | 13 (7.0) | |
| Postmenopausal and current PMH use, n (%) | 63 (33.9) | 66 (35.5) | |
| Postmenopausal and no PMH use, n (%) | 53 (28.5) | 56 (30.1) | |
| Postmenopausal and past PMH use, n (%) | 29 (15.6) | 26 (14.0) | |
| Premenopausal, n (%) *** | 25 (13.4) | 25 (13.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanyuda, A.; Zeleznik, O.A.; Raita, Y.; Haslam, D.E.; Sun, Q.; Negishi, K.; Pasquale, L.R.; Lasky-Su, J.; Wiggs, J.L.; Kang, J.H. Prediagnostic Plasma Metabolomic Profiles Using NMR for Exfoliation Glaucoma Among US Health Professionals. Metabolites 2025, 15, 469. https://doi.org/10.3390/metabo15070469
Hanyuda A, Zeleznik OA, Raita Y, Haslam DE, Sun Q, Negishi K, Pasquale LR, Lasky-Su J, Wiggs JL, Kang JH. Prediagnostic Plasma Metabolomic Profiles Using NMR for Exfoliation Glaucoma Among US Health Professionals. Metabolites. 2025; 15(7):469. https://doi.org/10.3390/metabo15070469
Chicago/Turabian StyleHanyuda, Akiko, Oana A. Zeleznik, Yoshihiko Raita, Danielle E. Haslam, Qi Sun, Kazuno Negishi, Louis R. Pasquale, Jessica Lasky-Su, Janey L. Wiggs, and Jae H. Kang. 2025. "Prediagnostic Plasma Metabolomic Profiles Using NMR for Exfoliation Glaucoma Among US Health Professionals" Metabolites 15, no. 7: 469. https://doi.org/10.3390/metabo15070469
APA StyleHanyuda, A., Zeleznik, O. A., Raita, Y., Haslam, D. E., Sun, Q., Negishi, K., Pasquale, L. R., Lasky-Su, J., Wiggs, J. L., & Kang, J. H. (2025). Prediagnostic Plasma Metabolomic Profiles Using NMR for Exfoliation Glaucoma Among US Health Professionals. Metabolites, 15(7), 469. https://doi.org/10.3390/metabo15070469








